Abstract
PURPOSE
Adults with diabetes are at a high risk of developing coronary heart disease. The purpose of this study was to assess coronary artery vascular function non-invasively in individuals with and without Type 2 diabetes and to compare these coronary responses to another microvascular bed (i.e. retina). We hypothesized that individuals with diabetes would have impaired coronary reactivity and that these impairments would be associated with impairments in retinal reactivity.
METHODS
Coronary blood velocity (Transthoracic Doppler Echocardiography) and retinal diameters (Dynamic Vessel Analyzer) were measured continuously during five minutes of breathing 100% oxygen (i.e. hyperoxia) in 15 persons with Type 2 diabetes and 15 age-matched control subjects. Using fundus photographs, retinal vascular calibers were also measured (central retinal arteriole and venule equivalents).
RESULTS
Individuals with diabetes compared to controls had impaired coronary (−2.34 ± 16.64% vs. −14.27 ± 10.58%, P =0.03) and retinal (arteriole: −0.04 ± 3.34% vs. −3.65 ± 5.07%, P = 0.03; venule: −1.65 ± 3.68% vs. −5.23 ± 5.47%, P = 0.05) vasoconstrictor responses to hyperoxia, and smaller central arteriole-venule equivalent ratios (0.83 ± 0.07 vs. 0.90 ± 0.07, P = 0.014). Coronary reactivity was associated with central retinal arteriole equivalents (r = −0.516, P = 0.005) and retinal venular reactivity (r = 0.387, P = 0.034).
CONCLUSION
Diabetes impairs coronary and retinal microvascular function to hyperoxia. Impaired vasoconstrictor responses may be part of a systemic diabetic vasculopathy, which may contribute to adverse cardiovascular events in individuals with diabetes.
Introduction
Adults with diabetes are two to four times more likely to have heart disease than adults without diabetes (Centers for Disease Control and Prevention (CDC), 2014). Endothelial vascular dysfunction is the initiating process in the development of heart disease (Caballero, 2003). Abnormalities of coronary vascular vasodilation using pharmacological methods or sympathetic stimulation (cold pressor test) have been reported in patients with diabetes even in the presence of normal coronary arteries (Akasaka et al., 1997; Di Carli et al., 2003; Nahser et al., 1995; Nitenberg et al., 1993; Prior et al., 2005; Yokoyama et al., 1997). However, these techniques involve methodologies, which were invasive (i.e. angiograms) and/or expensive (i.e. pharmacological adenosine and positron emission tomography (PET)). Yet vascular function in the coronary arteries can be studied in other ways. Transthoracic Doppler echocardiography (TTDE) may be a good non-invasive and low cost alternative for examining coronary reactivity to a variety of stimuli in individuals who are healthy as well as those with diabetes (Atar et al., 2012). We were interested in using a robust systemic stimulus in which the coronary vascular bed and another microvascular bed could be examined under similar conditions.
The administration of 100% oxygen (i.e. hyperoxia) has been shown to be a potent non-invasive stimulus to measure vascular function (i.e. reactivity) in the coronary vascular bed during angiograms in individuals with coronary artery disease (McNulty et al., 2005; McNulty et al., 2007). The underlying mechanism of hyperoxia-induced vasoconstriction is believed to be due in part to an increased oxidative degradation of endothelial-derived nitric oxide (Jamieson et al., 1986; Rubanyi and Vanhoutte, 1986b); However, animal studies suggest that other factors such as increases in arachidonic acid and the endothelin-1 may also play a role in hyperoxic vasoconstriction (Zhu et al., 1998). Thus, regardless of the mechanism, hyperoxia can be used as potent vascular vasoconstrictor. Our laboratory has used this stimulus to measure changes in peak coronary blood velocity with TTDE in healthy subjects (Gao et al., 2012; Momen et al., 2009). However, there has been a lack of studies using hyperoxia to examine the impact of diabetes on coronary blood flow dynamics measured by TTDE and to examine whether vascular function in other microvascular beds such as retinal vascular bed are equally affected.
The purpose of this study was to assess the effect of diabetes on epicardial coronary blood velocity responses to hyperoxia measured by TTDE. We hypothesized that individuals with Type 2 diabetes would have impaired coronary reactivity. In addition, we compared the magnitude of change of coronary blood velocity responses to a change in another microvascular bed (i.e. the retinal blood vessels) to hyperoxia. We hypothesized that the magnitude of change to hyperoxia would be similar between the coronary and retinal vascular beds.
Material and Methods
Subjects
Thirty of 44 enrolled subjects recruited by study research flyers had complete retinal and coronary studies. Individuals with Type 2 diabetes (n=15) ranged in age from 38 to 73 years and were non-smokers. Control subjects (n=15) were age and body mass index (BMI) matched similar to the diabetic group. The diagnosis of diabetes was based on hemoglobin A1C (HbA1C) levels using the American Diabetes Association criteria of diabetes to be HbA1C ≥ 6.5% and non-diabetic to be HbA1C < 5.7% (2010). All participants signed an informed consent that was institutionally approved by the Penn State College of Medicine Institutional Review Board prior to testing under the Declaration of Helsinki. All individuals had a physical examination and eye screening and were excluded if they had any history of cardiac, cerebrovascular, or pulmonary disease, uncontrolled hypertension, intraocular pressures greater than 21 mmHg, diabetic retinopathy or other eye diseases, seizures, were currently pregnant or breast-feeding, had a BMI greater than 45 kg/m2 or were unable to fixate using the Dynamic Vessel Analyzer (DVA).
Study Design & Protocol
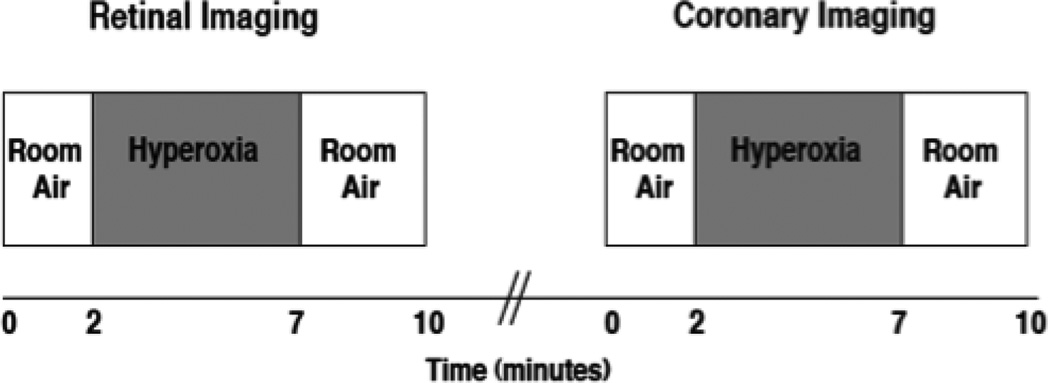
This explorative cross-sectional design assessed coronary and retinal reactivity to hyperoxia in individuals with Type 2 diabetes compared to healthy controls. Twenty-four hours prior to testing, subjects avoided exercise, caffeine or alcohol containing products, and non-steroidal anti-inflammatory agents. Individuals with diabetes held all their medications except for antihypertensive medications on the morning of the study. Measurements were performed in a dimly lit room at room temperature (21° C). Subjects arrived in a fasted state and had blood samples of glucose, insulin, lipid panel and HbA1c drawn from the antecubital vein. The eye with the best visual acuity was dilated with tropicamide (1%) and if needed, phenylephrine (2.5%) was added to obtain optimal dilation. Subjects were seated upright for the DVA examination and were positioned supine for the coronary vessel part of the study. After a 15 minute rest period, fundus photographs were initially taken using the DVA. Using a standardized hyperoxia protocol (Lott et al., 2012), coronary blood velocity was measured by TTDE (Momen et al., 2009) and retinal diameters by the DVA (Garhofer et al., 2010). While the study participants inhaled room air, continuous measurements of the retinal diameters were taken to establish baseline data. Oxygen (100%) was then administered via a mouthpiece and a Douglas bag set-up for five minutes followed by inhalation of room air. After a 30 minute rest period, the hyperoxia protocol was repeated for coronary blood velocity measurements. Heart rate, blood pressure, end tidal carbon dioxide (CO2ET) and systemic oxygen saturation were measured continuously (see Figure 1 for Study schematic).
Figure 1. Experimental protocol.
After baseline measurements (one minute) at room air, hyperoxia stimulus was applied for five minutes and then returned back to room air for three minutes of recovery. Retinal imaging with hyperoxia occurred first and then after 30 minutes of rest, coronary imaging during the hyperoxia was performed.
Coronary blood velocity
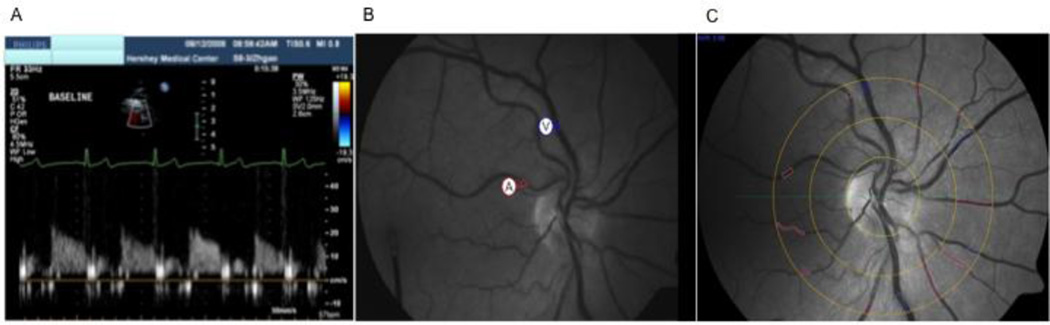
We externally insonated the left descending coronary artery using pulsed ultrasound TTDE (S8-3 MHz probe, IE33, Phillips Healthcare, Andover, MA). For imaging over the apical region of the heart, the probe was manually adjusted and color enhancement was used to locate the artery. Coronary blood velocity was measured continuously (Figure 2A) and post analysis of peak coronary diastolic velocity was calculated using Prosolve software (Momen et al., 2009). It has been reported that changes in coronary blood velocity reflect changes in absolute coronary blood flow, thus it is a surrogate for coronary blood flow (Doucette et al., 1992).
Figure 2. Coronary and Retinal Imaging.
TTDE is a novel non-invasive technique used to measure coronary flow velocity in the distal epicardial coronary artery (A). The Dynamic Vessel Analyzer is able to provide continuous imaging of the retinal blood vessels (B). The retinal arteriole and venule are noted by the circled (A) and (V). Static fundus photographs using special Visualis software calculated a total average equivalent of the all the marked arteriole (red) and venule (blue) calibers (C).
Retinal arteriole and venule reactivity
Dynamic measurements were obtained using the DVA (Imedos Inc., Germany), which consists of a fundus camera (FF450 Zeiss, Jena, Germany), a charge-coupled device (CCD)-measuring video camera, and a computer (Seifertl and Vilser, 2002). The DVA analyzes the brightness profile of the retinal blood vessels using two optical pathways, and light reflected by the retina back to the CCD video camera. This optical display is viewed on a computer system and, using specialized software, vessel diameters can be measured. On the computerized screen, two regions of interest are marked over a superior or inferior temporal retinal arteriole and venule between one to two optic disc diameters from the optic nerve disc margin (Figure 2B). Eye-tracking technology in the DVA compensates for small eye movements. All images were stored on a videotape recorder for off line measurements by one observer using the DVA software. The coefficient of variability for measuring retinal blood vessels ranges between 1.5% and 2.8% (Seifertl and Vilser, 2002) and we have similar standards established in our laboratory.
Static fundus photographs
The diameters of all the arterioles and venules traversing a specified area of rings positioned 1 to 2 disc distances from the optic disc were measured using Imedos Visualis software (see Figure 2C). This software program derives two indices that are estimates of the averaged retinal arteriole and venule calibers taking into consideration branching patterns: central retinal arteriole equivalents and central retinal venular equivalents. A ratio of these two measurements known as the central arteriole-venule equivalent ratio is also calculated (Hubbard et al., 1999; Ikram et al., 2006; Liew et al., 2008; Wong et al., 2002). Smaller arterioles, larger venules, and lower ratio are associated with chronic diseases (Kifley et al., 2008; Nguyen et al., 2008; Wong et al., 2002). Reproducibility for these measurements has been previously reported to be between 0.78 to 0.99 (Hubbard et al., 1999; Wong et al., 2006).
Hemodynamic measurements
Heart rate was calculated from electrocardiogram recordings. Blood pressure was measured throughout each trial using the Finapres device (model 2300, Ohmeda, Boulder, CO) stabilized at heart level and corrected based on measurements from an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL) taken in duplicate before and after each trial.
Blood Plasma
Glucose, insulin, HbA1C, and fasting lipid panels were obtained. Insulin sensitivity was calculated using the quantitative insulin-sensitivity check index (QUICKI) method (Muniyappa et al., 2008).
Respiratory measurements
CO2ET concentration was measured using a respiratory gas monitor (RGM 5250; Ohmeda, Madison, WI, USA). Systemic oxygen saturation was measured by finger pulse oximetry (Model Biox 3740; Ohmeda, Louisville, CO, USA).
Data analysis
Baseline measurements were averaged over a period of one minute. Diameter and velocity measurements in the last 30 to 60 seconds of hyperoxia were averaged at one, three, and five minutes. These measurements were compared to baseline diameters or velocities and presented as percent change (%). Coronary vascular resistance was calculated using the formula mean artery pressure/coronary blood velocity. From previously published retinal studies in healthy and diabetic populations (Blum et al., 2003; Jean-Louis et al., 2005), power was calculated for a two group comparison (control vs. diabetic groups) for the main outcome measure, a change in mean retinal diameter. An average percentage decrease in control and diabetic retinal diameters was assumed to be 11.2% vs. 6.2% with SD of 3.25 (Blum et al., 2003; Jean-Louis et al., 2005). Thus, power for detecting a difference using a t-test with alpha=.05 and n=18 per group is greater than 95%. Using the Statistical Package for the Social Sciences (SPSS), paired t-tests were used to assess percent change in reactivity between groups. If homogeneity of variance was violated (i.e. data sets not normally distributed), the non-parametric Kruskal-Wallis test was performed. For examining temporal diameter or velocity patterns, repeated analysis of variance was used in a subgroup of subjects who had data at all 3 time points (1, 3, and 5 minutes of hyperoxia) for coronary reactivity (n = 8/group) and retinal reactivity (n = 15/group). To examine the associations between the two vascular beds using Pearson correlations, all groups were combined (N=30) unless otherwise stated. Statistical significance was accepted at P < 0.05. All data are reported as mean ± SD.
Results
Subject demographics
Diabetic and control groups were similar in gender, age, BMI, and lipid profile (see Table 1). The individuals with Type 2 diabetes had this disease for 6.1 ± 4.9 years. As expected, individuals with diabetes had higher HbA1C and fasting blood glucoses and lower insulin sensitivity compared to the control group. Individuals with diabetes were on oral diabetic medications (73% - eleven individuals) which included one or more of biguanidine (64%), sulfonylureas (55%), thiazolidinediones (27%), and sitagliptins (27%) and/or insulin (20% - three individuals) (i.e. short acting (100%) and long acting (33%)). Individuals with diabetes were also on statins (73%), and antihypertensive (89%) medications. Anti-hypertensive therapy included: thiazides (13%), angiotensin II receptor antagonists (20%), beta blockers (27%), calcium channel blockers (40%), and angiotensin-converting-enzyme inhibitors (73%). Healthy controls were on no lipid or vasodilator medications. Individuals with diabetes had significantly higher resting heart rates and higher blood pressures than healthy controls.
Table 1.
Participant characteristics
| Controls (n=15) |
Type 2 Diabetes (n=15) |
P valve | |
|---|---|---|---|
|
Demographics & anthropometric measurements |
|||
| Gender (Male/Female) | 6/9 | 6/9 | .645 |
| Age (yrs.) | 52 ± 10 | 56 ± 9 | .272 |
| Height (cm) | 171 ± 11 | 171 ± 9 | .890 |
| Weight (kg) | 85 ± 19 | 92 ± 18 | .326 |
| Body mass index (kg/m2) | 28.7 ± 4.8 | 31.4 ± 5.5 | .169 |
| Laboratory Findings | |||
| HbA1C (%) | 5.3 ± 0.3 | 7.4 ± 1.4* | .000 |
| Fasting glucose (mg/dl) | 85 ± 10 | 113 ± 32* | .004 |
| Fasting insulin (mmol/L) | 4.2 ± 3.6 | 18.6 ± 32.6 | .100 |
| Insulin sensitivity (QUICKI) | 0.416 ±0.048 | 0.346 ± 0.058* | .001 |
| Total cholesterol (mg/dl) | 195 ± 35 | 180 ± 43 | .291 |
| Low density lipoprotein (mg/dl) | 123 ± 29 | 105 ± 38 | .172 |
| High density lipoprotein (mg/dl) | 54 ± 19 | 47 ± 11 | .236 |
| Triglycerides (mg/dl) | 87 ± 38 | 134 ± 70* | .028 |
| Resting Hemodynamics | |||
| Heart rate (bpm) | 61 ± 7 | 71 ± 12* | .008 |
| Mean arterial blood pressure (mmHg) | 88 ± 8 | 95 ± 8* | .028 |
| Systolic blood pressure (mmHg) | 116 ± 10 | 127 ± 14* | .013 |
| Diastolic blood pressure (mmHg) | 74 ± 8 | 79 ± 6 | .084 |
| Intraocular pressure (mmHg) | 15.3 ± 2.9 | 16.3 ± 2.4 | .304 |
HbA1C = hemoglobin A1C; QUICKI = quantitative insulin sensitivity check index;
Significantly different than controls (P<0.05).
Coronary reactivity to hyperoxia
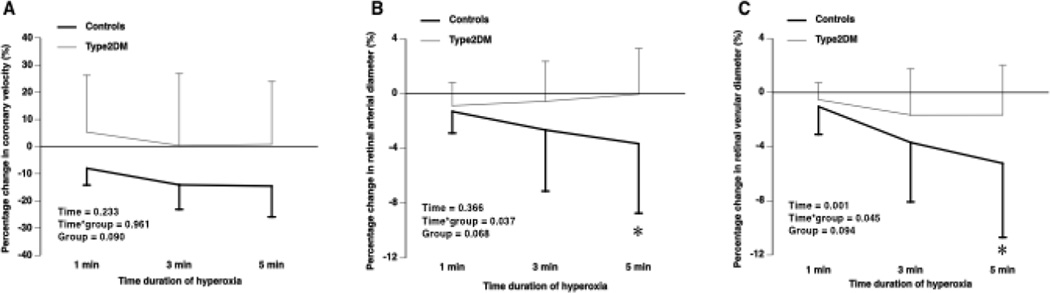
Hyperoxia significantly increased systemic oxygen saturation in both groups with no significant changes on CO2ET, heart rate or blood pressure (data not shown). Although individuals with diabetes compared to controls had similar resting coronary blood velocities, they had significantly impaired coronary vasoconstrictor responses at five minutes of hyperoxia (−2.34 ± 16.64% versus −14.27 ± 10.58%, n = 30, P = 0.026). Examination of the temporal pattern response of coronary blood velocities between a subgroup of healthy and Type 2 diabetes individuals, demonstrated a group effect where individuals with diabetes had impaired coronary responses throughout the five minutes of hyperoxia; however, this group effect was non-significant (P = 0.09; Figure 3A).
Figure 3. Temporal retinal and coronary responses to hyperoxia.
Individuals with diabetes compared to healthy controls had impaired coronary artery vasoconstrictor responses to hyperoxia throughout hyperoxia (one, three, and five minutes) (A). The percent changes in retinal arteriolar diameter responses to hyperoxia were attenuated in individuals with diabetes compared to healthy controls with significant differences observed at five minutes of hyperoxia (B). Similar responses were seen in the retinal venules (C). Type2DM =Type 2 Diabetics; * P<0.05 significant difference between individuals with diabetes compared to healthy controls.
Coronary and retinal reactivity to hyperoxia
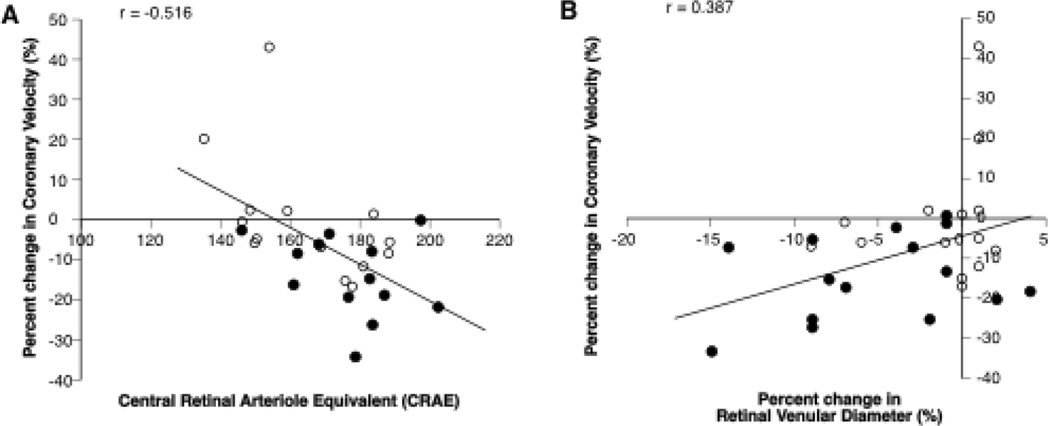
Retinal arteriolar and venular vasoconstrictor responses to hyperoxia were significantly attenuated in individuals with Type 2 diabetes compared to healthy controls (Arterioles: −0.04 ± 3.34 versus −3.65 ± 5.07%, P = 0.030; Venules: −1.65 ± 3.68 versus −5.23 ± 5.47%, P = 0.045) and the temporal pattern response of the retinal arterial and venule blood vessels in individuals with Type 2 diabetes (n = 15) and healthy controls (n = 15) confirmed group differences at five minutes of hyperoxia (Interaction Effect for arterioles P = 0.037 and venule P = 0.045; Figures 3 B and C, Table 2). With all groups combined, a significant correlation between changes in coronary blood velocity and retinal venular reactivity to hyperoxia was observed (r = 0.387, P = 0.034) (Figure 4A). The same was observed between changes in coronary vascular resistance and retinal venular reactivity to hyperoxia (r = −.420, P = 0.021). However, there was not a correlation between changes in coronary velocity and changes in retinal arteriole reactivity (r = 0.282, P = 0.132).
Table 2.
Resting and Hyperoxia effects on the coronary and retinal vasculature
| Controls (n=15) |
Type 2 Diabetic (n=15) |
P valve | |
|---|---|---|---|
| Coronary blood velocity | |||
| Resting (cm/s) | 16.88 ± 3.78 | 16.83 ± 3.23 | .970 |
| Percent change after 5 min of hyperoxia (%) | −14.27 ± 10.58 | −2.34 ± 16.64* | .026 |
| Coronary vascular resistance | |||
| Resting (cm/s) | 5.48 ± 1.33 | 5.90 ± 1.55 | .415 |
| Percent change after 5 min of hyperoxia (%) | 21.0 ± 18.0 | 6.80 ± 15.90* | .031 |
| Retinal blood vessel calibers | |||
| Central retinal arteriole equivalents | 177 ± 15 | 166 ± 17 | .080 |
| Central retinal venular equivalents | 198 ± 19 | 201 ± 30 | .779 |
| Arteriole-venule ratio | 0.90 ± 0.07 | 0.83 ± 0.07* | .024 |
| Retinal blood vessel diameters | |||
| Resting arteriolar diameter (µm) | 116 ± 12 | 120 ± 17 | .375 |
| Percent change after 5 min of hyperoxia (%) | −3.65 ± 5.07 | −0.04 ± 3.34* | .029 |
| Retinal venular diameter (µm) | 135 ± 19 | 152 ± 24* | .037 |
| Percent change after 5 min of hyperoxia (%) | −5.23 ± 5.47 | −1.65 ± 3.68* | .045 |
Significantly different than controls (P<0.05).
Figure 4. Retinal and coronary reactivity correlations.
With all the groups combined, smaller retinal central arteriole equivalents were associated with attenuated coronary vasoconstriction to hyperoxia (A). Attenuated venular reactivity was associated with attenuated coronary reactivity in response to hyperoxia (B). Black circles = controls; Open circles = Type 2 Diabetics.
Coronary reactivity and static retinal calibers
Individuals with diabetes compared to controls had significantly smaller central arteriole-venule equivalent ratios (0.83 ± 0.07 versus 0.90 ± 0.07; P = 0.024, n = 28) and a trend for smaller central retinal arteriole equivalents (166 ± 17 versus 177 ± 15; P = 0.080, n = 28). Central retinal arteriole equivalents were significantly correlated with coronary reactivity and coronary vascular resistance to hyperoxia (r = −0.516, P = 0.005, n = 28, Figures 4B and r = 0.544, P = 0.003, respectively). There were no correlations between changes in coronary reactivity and the other static retinal parameters (data not shown).
Discussion
This is the first study to reveal that coronary reactivity to hyperoxia measured by TTDE is impaired in individuals with Type 2 diabetes compared to healthy controls. Our findings also demonstrate a modest correlation between coronary and retinal vasoconstrictor reactivity to hyperoxia as well between coronary vasoconstrictor reactivity to hyperoxia and retinal arteriolar calibers. These findings are suggestive of systemic microvascular impairments in Type 2 diabetes, which may contribute to the later development of coronary heart disease.
Since individuals with diabetes are at a higher risk of developing coronary artery disease and diabetic retinopathy (Centers for Disease Control and Prevention (CDC), 2014), it is important to detect disease in its early stages. Endothelial vascular dysfunction (i.e. altered vasoconstriction and/or vasodilation) is known to be the initiating event in the development of atherosclerosis in the body’s blood vessels (Caballero, 2003). Thus, evaluation of endothelial vascular function may be helpful in early detection of disease.
While the stimulus of hyperoxia is not physiologic per se, its vasoconstrictor responses may yield important information on vascular function. Hyperoxia is predominately a metabolic stimulus that operates independently from sympathomimetic influences. Prior studies using hyperoxia have shown vasoconstrictor responses in the peripheral vascular beds (Crawford et al., 1997; Mak et al., 2002) and retinal microvasculature (Jean-Louis et al., 2005; Wimpissinger et al., 2005). Only a few studies have examined the effects of hyperoxia on coronary arteries (Ganz et al., 1972; Mak et al., 2001; McNulty et al., 2005; McNulty et al., 2007). While patients with normal coronary arteries demonstrated a reduction in blood flow (17.1%) (Ganz et al., 1972), patients with coronary artery disease and heart failure have demonstrated impaired reductions in coronary sinus blood flow and coronary epicardial blood flow from 8% to 29% in response to hyperoxia using thermodilution or intravascular Doppler ultrasound during invasive angiograms (Farquhar et al., 2009)(Review). McNulty (2007) observed that the degree of coronary blood velocity impairment to hyperoxia in individuals with coronary artery disease was associated with lower venous concentrations of nitric oxide metabolites as measured by nitrotyrosine, nitrite and nitrate (McNulty et al., 2007) suggesting an association between the degree of vasoconstriction and level of basal nitric oxide. In healthy subjects, our laboratory has demonstrated that coronary artery blood velocity measured by TTDE could be reduced 15 ± 3% in response to hyperoxia (Momen et al., 2009). Our findings agree with this prior study using TTDE in that coronary blood velocity had a robust reduction (14.27%) in response to hyperoxia in healthy controls. However, our study is the first to show that individuals with diabetes have attenuated coronary blood velocity reductions (2.75%) after five minutes of hyperoxia. Although not significant, the impaired coronary vasoconstriction in individuals with diabetes occurred throughout the 5 minutes of hyperoxia.
The impairment of endothelial-independent vasodilation as reflected by a reduction in coronary flow reserve using pharmacological infusions in individuals with diabetes has been well documented using a variety of methods such as angiograms (Akasaka et al., 1997; Nahser et al., 1995; Picchi et al., 2011) and positron emission tomography (Di Carli et al., 2003; Momose et al., 2002; Prior et al., 2005; Schindler et al., 2004; Yokoyama et al., 1997). Thus, it appears that the vascular smooth muscle of the coronary arteries is dysfunctional. Using these diagnostic and imaging methods, diabetes has been also shown to impair endothelial-dependent vasodilation using pharmacological methods of acetylcholine infusions as well as sympathetic stimulation using cold pressor tests (Di Carli et al., 2003; Momose et al., 2002; Nitenberg et al., 1993; Prior et al., 2005; Schindler et al., 2004). These studies suggest that the ability of the endothelium to release vasoactive substances such as nitric oxide and prostaglandins is impaired in diabetes. Recently, impaired coronary flow reserve (~23% lower) measured by TTDE using a pharmacological infusion was shown in Type 2 diabetics compared to healthy controls (Atar et al., 2012; Erdogan et al., 2013). Thus, these studies using TTDE confirm that diabetes impairs coronary both endothelial-dependent and independent vasodilation. Since we hypothesize that individuals with diabetes would also be insensitive to the vasoconstrictor effect of hyperoxia which is likely due to primarily reduced availability of nitric oxide, our findings of impaired coronary vasoconstrictor responses to hyperoxia in individuals with diabetes agrees with the prior vascular studies using other stimuli and imaging techniques.
The use of hyperoxia has also been frequently used in ophthalmology and has shown pronounced retinal arteriole and venule vasoconstrictor effects (6% to 12 %) in healthy young individuals (Jean-Louis et al., 2005; Wimpissinger et al., 2005). While our healthy controls also exhibited vasoconstrictor responses in retinal arterioles and venules (−3.65% and −5.23%, respectively), the magnitude of the response was lower than the previous ocular studies. The differences in the magnitude of vasoconstriction is likely due to differences in protocols used and that our subjects were older and thus their vessels may have been stiffer. Prior studies have shown that individuals with diabetes (mixture of type 1 and 2 diabetes) without diabetic retinopathy (mean aged range 33 to 52 years) have impaired arteriolar vasoconstrictor responses to hyperoxia ranging from −2.5% to −4% (Gilmore et al., 2007; Justesen et al., 2010). Our study confirmed a greater impairment of retinal vasoconstrictor responses in individuals with Type 2 diabetes. Since we showed no significant change in blood pressure or CO2 levels during hyperoxia, which could have attenuated the vascular responses, it is reasonable to postulate that the impaired vasoconstriction effect was coming primarily from the oxygen effects on the retinal vessel and not due to the effects of blood pressure or CO2. Differences in the magnitude of impairment in individuals with diabetes between previous retinal studies and our study may be due to different study protocols used to deliver hyperoxia, age of the subjects (our subjects were older), medications used, and years with diabetes. Our study further examined the temporal responses of each vascular bed to hyperoxia. Prior studies have shown that retinal vasoconstriction to hyperoxia in healthy subjects is achieved within five to six minutes (Kiss et al., 2002), thus we used the duration of five minutes for the delivery of oxygen. It was only at the five minute mark that individuals with diabetes had significantly attenuated or absent vasoconstrictor response compared to the robust vasoconstrictor effects observed in healthy controls. Thus, the time delay in significant vasoconstrictor differences being observed suggest that metabolic mechanisms may be involved such as altered nitric oxide levels.
While the specific underlying mechanism of hyperoxia on blood vessels is not clearly understood, hyperoxia is suggested to be due to in part by an inactivation or degradation of nitric oxide synthase or nitric oxide co-factors through an increase in oxidative stress and/or reactive oxygen species (Crawford et al., 1997; Daly and Bondurant, 1962; Jaimes et al., 2001; Kolodjaschna et al., 2008; McNulty et al., 2005; McNulty et al., 2007; Rubanyi and Vanhoutte, 1986a). Thus, the more nitric oxide inactivated or quenched by hyperoxia, the greater the vasoconstrictor response. McNulty and colleagues (2007) demonstrated that an infusion of vitamin C prior to hyperoxia, prevented hyperoxia-induced coronary constriction in 12 individuals with coronary artery disease (McNulty et al., 2007). However, the administration of vitamin C did not alter retinal vasoconstriction during hyperoxia in healthy individuals (Weigert et al., 2009). Differences between these study’s findings may be due to populations studies and the dose of the vitamin C infusions. The impairment in coronary and retinal vascular function in individuals with diabetes may also be influenced by other factors besides lower levels of nitric oxide. Retinal vascular studies in healthy animal and human subjects have suggested that hyperoxia may lead to the increased production of arachidonic acid metabolites (i.e. cyclooxygenase and substance P450) and endothelin-1 (Dallinger et al., 2000; Higgins et al., 1998; Takagi et al., 1996; Zhu et al., 1998). In addition, nitric oxide may be involved with the regulation of retinal endothelin-1 (Izumi et al., 2008). Diabetes has also been associated with elevated endothelin-1 levels, which may lead to vascular remodeling promoting stiffness of the vessels limiting blood vessels responsiveness (Feng et al., 2011; Harris et al., 2005). However, despite higher endothlin-1 levels, diabetes may lead to a desensitization of the endothelin-1 receptor contributing to an attenuated vasoconstrictor response (Bursell et al., 1995). Lastly, diabetes may impair wall thickness, vascular smooth muscle cells, pericytes, and endothelial cells in the coronary and retinal beds, which contributes to the impaired responses (Fein et al., 1985; Reusch and Watson, 2004; Srivastava, 2002). Further studies will be needed to evaluate these factors with coronary and retinal reactivity in diabetes.
Several prior studies have shown weak (r = 0.31) to moderate (r = 0.78) correlations between coronary reactivity using pharmacological vasoactive infusions and peripheral reactivity also using vasoactive infusions or brachial flow-mediated vasodilation. Subjects used for these studies were individuals undergoing angiograms to evaluate for coronary artery disease who also had several risk factors for heart disease (Anderson et al., 1995; Takase et al., 2005; Takase et al., 1998; Teragawa et al., 2005). Although the coronary circulation has direct sympathetic innervation whereas the retinal vessels may not, both the retinal and coronary vascular beds use similar local autoregulatory mechanisms such as metabolic and myogenic mechanisms to help control blood flow. Our findings showed a correlation between coronary artery and retinal venular reactivity (r = −0.387) using the same stimulus of hyperoxia in individuals with and without Type 2 diabetes but no history of coronary artery disease. Although this retinal and coronary reactivity correlation was modest, it is the first study to show that endothelial function in the coronary arteries parallels the smaller retinal blood vessels in diabetes. The abnormalities of retinal reactivity in response to oxygen may provide a simple way to identify early abnormalities in coronary reactivity in individuals with diabetes.
Changes in retinal vascular calibers, as measured by central arteriole-venule equivalent ratio, central retinal arteriole equivalents and central retinal venular equivalents, have been associated with risk factors for cardiovascular disease such as hypertension (Wong et al., 2004b), diabetes (Nguyen et al., 2008; Wong et al., 2006), metabolic syndrome (Wong et al., 2004a) and incidence of coronary heart disease (Wong et al., 2002). The narrowing of retinal arterioles has been associated with risk factors for heart disease, known coronary artery disease, and increased mortality (Wang et al., 2007; Wong et al., 2004b; Wong et al., 2002). Altered retinal calibers have also been associated with other indices of early subclinical findings of atherosclerosis such as reduction in carotid intima thickness and increases in coronary calcium scores (Cheung et al., 2007; Ojaimi et al., 2011). Thus, we explored the relationship between the coronary vasoreactivity and retinal vascular structures in the diabetic and healthy subjects. We observed that smaller central retinal arteriole equivalents were associated with impaired coronary vasoconstriction to hyperoxia. Thus, changes in retinal arteriole calibers are associated with impairments in vascular function of the coronary arteries, which may be a sign of early atherosclerosis. Further studies examining retinal calibers and coronary function in a larger population is needed to confirm these results.
There are several limitations to the study. The first limitation of the study involves the comparison of two measurements of vascular function (i.e. blood velocity and diameters). To control for the use of different measurement parameters in the coronary and retinal vessels, we presented the data as percent change in both of these measurements to normalize them. Some of our subjects with diabetes (52%) required additional dilation with the use of phenylephrine, which theoretically could elevate mean arterial pressure through increasing sympathetic stimulation; however, our findings did not change when these individuals were excluded from the analysis. Thus, we do not feel that the use of phenylephrine affected our study results. In addition, the majority of the diabetic patients were taking statins, which could affect flow responses. However, the vasoconstrictor responses were still attenuated in these diabetics. Thus, we do not feel that being on statins affected the vascular comparisons. Third, the sample size of this exploratory study was derived from power calculations from previous retinal vascular responses (Blum et al., 2003; Jean-Louis et al., 2005) and not coronary responses. We originally had projected 18 subjects/group. However, we only had complete data of retinal and coronary in 15/group. Even with a reduction in sample size, we still had greater than 95% power to detect differences between groups using our original estimates based on data from Blum and others 2003 and Jean-Louis and others 2005. However, a larger sample size may had lead to stronger coronary findings.
Conclusions
In conclusion, diabetes impairs coronary and retinal microvascular reactivity to hyperoxia. Impaired vasoconstrictor responses to hyperoxia may be part of a systemic diabetic vasculopathy, which may contribute to adverse cardiovascular events in individuals with diabetes.
Highlights.
We compared the effects of hyperoxia on coronary and retinal vascular function.
Individuals with diabetes exhibited impaired coronary and retinal reactivity.
Retinal structure and reactivity modestly correlated to coronary reactivity.
Impaired vasoconstrictor responses may be part of a systemic diabetic vasculopathy.
Acknowledgments
We would like to thank Bruce Smith, Vikram Shivkumar, Michael Herr, Surju Patel, Cheryl Blaha and Jessica Mast for their help with the study. We would like to thank Allen Kunselman in Department for Public Health Sciences for his assistance with power and sample size calculations. We would like to thank the individuals who participated in the studies, as well as the nursing staff of the Clinical Research Center. Support was provided by a grant from Pennsylvania Tobacco Settlement Funds. The DVA was partially funded by the Penn State Diabetes and Obesity Institute Equipment Grant and the Pennsylvania Lions Sight Conservation and Eye Research Foundation and the Jack and Nancy Turner Professorship at Penn State supported Dr. Gardner. Dr. Gardner is currently supported by the A. Alfred Taubman Medical Research Institute at the University of Michigan, a Research to Prevent Blindness Physician-Scientist Award and EY20582 and DK094292. The studies were performed in Penn State’s Clinical Research Center, which was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
Author Contributions:
| Task | ML | JS | ZG | RG | DQ | TG | K B |
|---|---|---|---|---|---|---|---|
| Conception and Design | X | X | X | X | |||
| Performed Experiments | X | X | X | X | X | X | |
| Analyze Data & Interpret Results | X | X | X | ||||
| Prepare Figures | X | ||||||
| Draft Manuscript | X | ||||||
| Edit/revise Manuscript | X | X | X | X | X | X | X |
| Approved Manuscript | X | X | X | X | X | X | X |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasaka T, et al. Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol. 1997;30:935–941. doi: 10.1016/s0735-1097(97)00242-8. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Atar AI, et al. Coronary flow reserve in patients with diabetes mellitus and prediabetes. Echocardiography. 2012;29:634–640. doi: 10.1111/j.1540-8175.2012.01668.x. [DOI] [PubMed] [Google Scholar]
- Blum M, et al. Vasoconstriction of retinal arterioles with oxygen breathing in diabetic retinopathy. Ophthalmologe. 2003;100:306–309. doi: 10.1007/s00347-002-0731-9. [DOI] [PubMed] [Google Scholar]
- Bursell SE, et al. The in vivo effect of endothelins on retinal circulation in nondiabetic and diabetic rats. Invest Ophthalmol Vis Sci. 1995;36:596–607. [PubMed] [Google Scholar]
- Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Diabetes Statistics Report, 2014. 2014 http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- Cheung N, et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50:617–622. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- Crawford P, et al. Effects of supplemental oxygen on forearm vasodilation in humans. J Appl Physiol. 1997;82:1601–1606. doi: 10.1152/jappl.1997.82.5.1601. [DOI] [PubMed] [Google Scholar]
- Dallinger S, et al. Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Invest Ophthalmol Vis Sci. 2000;41:864–869. [PubMed] [Google Scholar]
- Daly WJ, Bondurant S. Effects of oxygen breathing on the heart rate, blood pressure and cardiac index of normal men-resting, with reactive hyperemia, and after atropine. J Clin Invest. 1962;41:126–132. doi: 10.1172/JCI104454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carli MF, et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- Doucette JW, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- Erdogan D, et al. Effects of prediabetes and diabetes on left ventricular and coronary microvascular functions. Metabolism. 2013;62:1123–1130. doi: 10.1016/j.metabol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Farquhar H, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158:371–377. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Fein FS, et al. Altered myocardial mechanics in diabetic rabbits. Am J Physiol Heart Circ Physiol. 1985;248:H729–H736. doi: 10.1152/ajpheart.1985.248.5.H729. [DOI] [PubMed] [Google Scholar]
- Feng J, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–252. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz W, et al. Coronary hemodynamics and myocardial oxygen metabolism during oxygen breathing in patients with and without coronary artery disease. Circulation. 1972;45:763–768. doi: 10.1161/01.cir.45.4.763. [DOI] [PubMed] [Google Scholar]
- Gao Z, et al. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012;112:483–492. doi: 10.1007/s00421-011-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garhofer G, et al. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010;88:717–722. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Gilmore ED, et al. Retinal arteriolar diameter, blood velocity, and blood flow response to an isocapnic hyperoxic provocation in early sight-threatening diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:1744–1750. doi: 10.1167/iovs.06-1016. [DOI] [PubMed] [Google Scholar]
- Harris AK, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- Higgins RD, et al. Hyperoxia stimulates endothelin-1 secretion from endothelial cells; modulation by captopril and nifedipine. Curr Eye Res. 1998;17:487–493. doi: 10.1076/ceyr.17.5.487.5190. [DOI] [PubMed] [Google Scholar]
- Hubbard LD, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- Ikram MK, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- Izumi N, et al. Role of nitric oxide in regulation of retinal blood flow in response to hyperoxia in cats. Invest Ophthalmol Vis Sci. 2008;49:4595–4603. doi: 10.1167/iovs.07-1667. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, et al. Effects of the reactive oxygen species hydrogen peroxide and hypochlorite on endothelial nitric oxide production. Hypertension. 2001;38:877–883. [PubMed] [Google Scholar]
- Jamieson D, et al. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Jean-Louis S, et al. Systemic hyperoxia and retinal vasomotor responses. Invest Ophthalmol Vis Sci. 2005;46:1714–1720. doi: 10.1167/iovs.04-1216. [DOI] [PubMed] [Google Scholar]
- Justesen BL, et al. Retinal arterioles have impaired reactivity to hyperoxia in type 1 diabetes. Acta Ophthalmol. 2010;88:453–457. doi: 10.1111/j.1755-3768.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- Kifley A, et al. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: the Blue Mountains Eye Study. Microcirculation. 2008;15:373–377. doi: 10.1080/10739680701812220. [DOI] [PubMed] [Google Scholar]
- Kiss B, et al. Retinal blood flow during hyperoxia in humans revisited: concerted results using different measurement techniques. Microvasc Res. 2002;64:75–85. doi: 10.1006/mvre.2002.2402. [DOI] [PubMed] [Google Scholar]
- Kolodjaschna J, et al. Reactivity of retinal blood flow to 100% oxygen breathing after lipopolysaccharide administration in healthy subjects. Exp Eye Res. 2008;87:131–136. doi: 10.1016/j.exer.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Liew G, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott ME, et al. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol. 2012;90:e434–e441. doi: 10.1111/j.1755-3768.2012.02445.x. [DOI] [PubMed] [Google Scholar]
- Mak S, et al. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- Mak S, et al. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414–H2421. doi: 10.1152/ajpheart.00947.2001. [DOI] [PubMed] [Google Scholar]
- McNulty PH, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–H1062. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- McNulty PH, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102:2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- Momen A, et al. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296:H854–H861. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose M, et al. Dysregulation of coronary microvascular reactivity in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2002;29:1675–1679. doi: 10.1007/s00259-002-0977-0. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Nahser PJ, Jr, et al. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, et al. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–539. doi: 10.2337/db07-1376. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–1025. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- Ojaimi E, et al. Retinopathy signs in people without diabetes: the multi-ethnic study of atherosclerosis. Ophthalmology. 2011;118:656–662. doi: 10.1016/j.ophtha.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchi A, et al. Increased basal coronary blood flow as a cause of reduced coronary flow reserve in diabetic patients. Am J Physiol Heart Circ Physiol. 2011;301:H2279–H2284. doi: 10.1152/ajpheart.00615.2011. [DOI] [PubMed] [Google Scholar]
- Prior JO, et al. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- Reusch JE, Watson PA. Loss of CREB regulation of vascular smooth muscle cell quiescence in diabetes. Rev Endocr Metab Disord. 2004;5:209–219. doi: 10.1023/B:REMD.0000032409.13963.bc. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 1986a;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986b;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schindler TH, et al. Chronic inflammation and impaired coronary vasoreactivity in patients with coronary risk factors. Circulation. 2004;110:1069–1075. doi: 10.1161/01.CIR.0000140264.56496.76. [DOI] [PubMed] [Google Scholar]
- Seifertl BU, Vilser W. Retinal Vessel Analyzer (RVA)--design and function. Biomed Tech (Berl) 2002;47(Suppl 1 Pt 2):678–681. doi: 10.1515/bmte.2002.47.s1b.678. [DOI] [PubMed] [Google Scholar]
- Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: a potential role in the pathogenesis of vascular dysfunction in diabetes (review) Int J Mol Med. 2002;9:85–89. [PubMed] [Google Scholar]
- Takagi C, et al. Endothelin-1 action via endothelin receptors is a primary mechanism modulating retinal circulatory response to hyperoxia. Invest Ophthalmol Vis Sci. 1996;37:2099–2109. [PubMed] [Google Scholar]
- Takase B, et al. Close relationship between the vasodilator response to acetylcholine in the brachial and coronary artery in suspected coronary artery disease. Int J Cardiol. 2005;105:58–66. doi: 10.1016/j.ijcard.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Takase B, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- Teragawa H, et al. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460–466. doi: 10.1002/clc.4960281004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28:1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- Weigert G, et al. Effects of vitamin C on hyperoxia-induced reduction of retinal blood flow. Microvasc Res. 2009;77:256–259. doi: 10.1016/j.mvr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Wimpissinger B, et al. Response of retinal blood flow to systemic hyperoxia in smokers and nonsmokers. Graefes Arch Clin Exp Ophthalmol. 2005;243:646–652. doi: 10.1007/s00417-004-1083-8. [DOI] [PubMed] [Google Scholar]
- Wong TY, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004a;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- Wong TY, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004b;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- Wong TY, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- Yokoyama I, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–1477. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. Mechanisms of hyperoxia-induced reductions in retinal blood flow in newborn pig. Exp Eye Res. 1998;67:357–369. doi: 10.1006/exer.1998.0535. [DOI] [PubMed] [Google Scholar]