Abstract
Introduction
High mobility group box 1 protein (HMGB-1) has been implicated in the pathogenesis of lupus nephritis (LN). There is increased HMGB-1 expression in the kidneys and increased levels are observed in serum and urine of patients with LN. This study was performed to determine whether the increased urinary HMGB-1 was specific for active lupus or secondary to renal damage.
Methods
Urine from 61 lupus patients (32 had active LN and 29 had SLE with no evidence of LN) and 14 control proteinuric patients (all with hypertension and 8 also with diabetes) were included in this study. HMGB-1 was detected by western blot. Urine protein was normalized to urine creatinine to account for volume of the specimen.
Results
Median normalized urine HMGB-1 levels were significantly elevated in LN patients compared to lupus patients without kidney disease (53.81 vs. 9.46, p<0.001). A difference in median levels was seen between LN classes; with a significant difference between proliferative and membranous disease (33.4 vs. 138.8, p=0.003). Urine protein to urine creatinine ratio (P/C) correlated with urinary HMGB-1 (r=0.52, p<0.001), but across the classes this was true only for membranous disease (r=0.71, p=0.022, proliferative, p=0.63; mixed, p=0.34).
Conclusions
HMGB-1 is elevated in the urine of patients with active LN. Levels are associated with LN class and higher levels of urinary HMGB-1 are seen in patients with class V when compared to both proliferative and mixed classes. Therefore, urinary HMGB-1 may be suggestive of membranous LN and warrants further evaluation in large lupus cohort and control disease.
Keywords: HMGB-1, Lupus nephritis, Membranous nephritis
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect multiple organ systems. Its hallmark is the presence of auto-antibodies that are primarily directed against nuclear antigens (1). Lupus glomerulonephritis (LN) is among the most devastating manifestations of lupus and ranks high as a cause of morbidity and mortality in SLE patients. The pathological manifestations of LN are very diverse and have been reclassified (2). Of the six classes defined by the International Society of Nephrology/Renal Pathology Society, necrotic lesions characterize class III, while class V can have evidence of sclerosis and even necrosis in the setting of mixed lesions (2), suggesting a role for necrosis and danger signals released by necrosis in the pathology.
HMGB-1 is a highly abundant and conserved nuclear protein that maintains chromatin structure and stability, and regulates transcription (3). It is also a critical component of DNA-containing immune complexes and has been suggested to play a role in LN pathogenesis (4). Indeed, HMGB-1 can be released into the extracellular space during both apoptosis and necrosis and can act as a “danger signal” (5). Once outside the cell, HMGB-1 simulates cytokine secretion and cell migration. These functions are often mediated through multiple receptors namely: TLR2, TLR4, TLR9, RAGE (Receptor of Advanced Glycation End Products) and CD24 (5).
HMGB-1 can be released in the extracellular space either passively by necrosis or by active secretion by monocytes. HMGB-1 released by each mechanism has distinctive functions due to the different post-translational modifications and possibly different redox states (6, 7). Acetylation of HMGB-1 is required for cytoplasmic localization of HMGB-1 and therefore precedes active secretion (7). HMGB-1 released during necrosis, however, is nuclear and therefore non-acetylated. The post-translational modifications of HMGB-1 can define the receptor ligation (5, 7). HMGB-1 containing a disulfide bond between two of the three cysteines (with the third cysteine as thiol) induces cytokine production while the all-thiol form (reduced) acts as a chemoattractant (6, 7).
HMGB-1 has been found to be associated with multiple autoimmune diseases including SLE. It has been previously shown that serum HMGB-1 levels are increased in patients with active lupus as compared to healthy controls and are associated with disease activity (8, 9). HMGB-1 has also been implicated in LN, possibly due to the release of HMGB-1 by resident macrophages during LN (5). Moreover, HMGB-1/anti-DNA antibody complexes are known to be pathogenic (4, 10). Recently, HMGB-1 has been found to be over-expressed in the kidneys of patients with LN (9, 10). Abdulahad et al. reported that urinary HMGB-1 levels are significantly increased in LN patients with active renal disease as compared to both healthy controls and in SLE patients with active disease but no history of LN (11).
These results suggest that HMGB-1 could in the future become a non-invasive biomarker in LN, a pathology that currently requires the invasive renal biopsy procedure to follow disease activity. In LN diagnosis and management, it is important to be able to determine the class of LN. The report by Abdulahad et al. did not address whether urinary HMGB-1 can distinguish among LN classes. In the present study we aimed at determining whether urinary HMGB-1 may be suggestive of a specific class of LN. To this end, we performed a cross-sectional analysis and compared the HMGB-1 levels in urine from active LN patients to patients with SLE and no evidence of LN and importantly to disease controls. Our results show that despite comparable proteinuria, urinary HMGB-1 levels were significantly higher in SLE patients with active class V LN, when compared to disease controls and those with either proliferative or mixed class LN, suggesting that measurements of urinary HMGB-1 warrants further investigation in large lupus cohorts to support and, possibly in the future, substitute renal biopsy as diagnostic tool to classify LN.
Methods
Patients
Urine samples from 61 SLE patients randomly selected from the Einstein Lupus Cohort (ELC) were included in this study. The ELC is an IRB approved prospective cohort of lupus patients followed in the Montefiore and Jacobi Medical Centers lupus clinics. At each clinic visit each ELC participant has a complete history and physical exam. Medication use and disease activity indices, including the SELENA-SLEDAI, are recorded. Serum and urine are collected from each patient and evaluated for general labs including electrolytes, dsDNA antibody levels, C3/C4 levels as well as urinalysis and a spot protein to creatinine ratio (P/C). Remaining sera and urine are stored in a central core facility at −80°C. Of the 61 patients in this study, thirty-two had active, biopsy-proven LN (fifteen had class III or IV, seven had mixed class III/IV+V, ten had class V), and all had renal SLEDAI≥4. All urine samples were collected after the renal biopsy, at different time points (0 to 6 months) but with still active renal disease. Twenty-nine patients had inactive, non-LN SLE with overall SLEDAI≤2). Urine samples from 14 control patients with non-SLE renal disease were collected in the nephrology clinic at Temple University Hospital. All controls had hypertension (HTN) and 8 had diabetes mellitus (DM). These were selected based on known history of proteinuria unrelated to autoimmune disease. Urine was spun at 1200 rpm for 5 minutes. The supernatants were aliquoted and stored at −80°C. Patients were consented and enrolled during regular scheduled visits. All patients signed an informed consent. This study was approved by Institutional Review Boards of Temple University and Albert Einstein College of Medicine.
Measurement of HMGB-1
Urine samples were concentrated using Amicon Ultra Centrifugal Filters (EMD Millipore, Billerica, MA) and separated on a 10% denaturing SDS PAGE gel. The proteins were transferred to nitrocellulose membranes, blocked with 5% non-fat dry milk for 1h at room temperature, and incubated overnight with polyclonal antibody against HMGB-1 (Abcam, Cambridge, MA). A DM patient sample was included on each gel as standard positive control. HMGB-1 on membranes was detected by ECL system (Pierce Thermo Scientific, Rockford, IL). Band intensities were measured using ImageJ software and normalized to the intensity of positive control. The numbers were normalized to urine creatinine to account for volume of the specimen. Levels of HMGB-1 were correlated with LN classes. Furthermore HMGB-1 levels were correlated with the presence of necrotic cells, podocyte effacement, mesangial proliferation and complement deposition. The analysis was based on the official reports from the renal biopsies.
Statistical analysis
All data was analyzed using STATA 10.1 (College Station, TX). Since data were not normally distributed, non-parametric methods were used. We then performed linear regression modeling to better understand the relationship between the LN classes and proteinuria.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. The median age of SLE population was 39 years, and was statistically lower when compared to controls (39 years (IQR 28–49) vs 59.5 (45–70), p<0.001). Most of the patients were either African American or Hispanic. This was expected given that SLE has a higher prevalence in these races and hospitals are located within and draw patients from predominantly minority communities.
Table 1.
Patient demographics
| Patients | Non active (29) | Active LN (32) | HTN/DM (14) | p value |
|---|---|---|---|---|
|
| ||||
| Age (median years, IQR) | 46 (32–52) | 35 (27.5–43) | 59.5 (45–70) | <0.001 |
| Gender (%Female) | 100% | 81% | 81% | <0.001 |
|
| ||||
| Race/Ethnicity (%) | ||||
|
| ||||
| African American | 44.8 | 50 | 35.7 | |
| Hispanic | 41.4 | 50 | 57.1 | |
| Other | 13.8 | - | 7.1 | 0.228 |
|
| ||||
| SLEDAI (median score, IQR) | 0 (0-0) | 8 (6–13) | - | <0.001 |
| Urine Protein/Creatinine (median mg/mg, IQR) | 0.11 (0.07–0.17) | 1.2 (0.55–1.64) | 1.27 (0.54–2.91) | <0.001 |
| dsDNA level (median IU, IQR) | 24.2 (7.0–138.5) | 82.3 (5.7–144.2) | ND | 0.69 |
| Complement C3 level (median mg/dL, (IQR) | 111 (96–147) | 109 (78–132) | ND | 0.21 |
| Complement C4 level, (median mg/dL, (IQR) | 21 (16–35.5) | 23 (17–32.2) | ND | 0.94 |
| Disease duration (median years, IQR) | 8.5 (4–19.5) | 7 (3–11.5) | 0.25 | |
|
| ||||
| Medications (% usage) | ||||
|
| ||||
| Plaquenil | 75% | 65% | NA | 0.49 |
| Immunosuppressant | 40% | 75% | NA | 0.03 |
|
| ||||
| Renal characteristics of LN patients | ||||
|
| ||||
| Proliferative | Mixed | Membranous | p value | |
|
| ||||
| Serum Creatinine (median mg/dL (IQR) | 1.1 (0.8) | 0.8 (0.5) | 0.65 (0.3) | 0.04 |
| Serum Albumin(median g/dL, IQR) | 3.7 (0.8) | 3.6 (1) | 3.2 (1.5) | 0.41 |
| Renal SLEDAI (median units, (IQR) | 4 (4) | 4 (4) | 4 (8) | 0.73 |
| GFR (median ml/min/1.73m2, IQR) | 62.9 (65.4) | 109.0 (43.5) | 124.5 (50.5) | 0.04 |
ND: Not determined, NA: Not available. The numbers are represented as median, IQR
The overall disease duration of SLE patients was 7.5 years. Those with active LN had higher levels of anti-dsDNA antibody levels but the difference was not statistically significant. There was similar usage of anti-malarials between the two groups; however, those with active LN were more likely to be on an immunosuppressive agent including mycophenolate mofetil and azathioprine.
HMGB-1 urine levels are higher in lupus patients
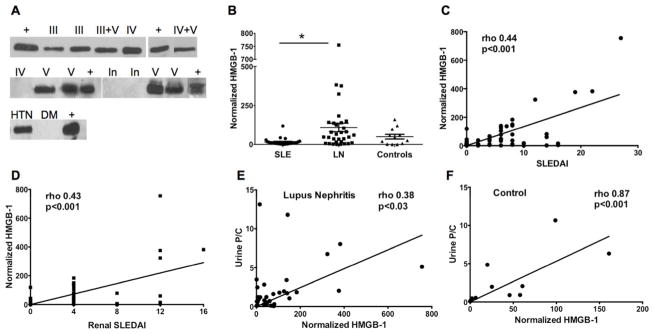
We analyzed HMGB-1 by western blot (WB). Figure 1A shows several examples of WB from lupus and control patients. HMGB-1 was detected in the urine of most samples, however, the levels of HMGB-1 in the urine from patients with active LN were significantly higher (Figure 1B; p=0.0014).
Figure 1. Urine HMGB-1 levels are higher in LN patients.
A) Representative blots for urine HMGB-1. III: class III LN, IV: class IV LN, V: class V LN, III+V: mixed (class III+V) LN, IV+V: mixed (class IV+V), In: inactive lupus, HTN: hypertension, DM: diabetes mellitus, +: Positive control. B) Urine HMGB-1 levels were significantly higher in LN group (p=0.0014). Horizontal lines represent median. LN: SLE patients with active LN, SLE: patients with inactive non LN SLE, controls: DM/HTN patients. C) Urine HMGB-1 levels correlate positively with general SLEDAI (rho 0.44, p <0.001) and D) renal SLEDAI (rho 0.43, p<0.001). E) Urine P/C positively correlates with HMGB-1 levels in SLE/LN group (rho 0.38, p <0.03) and F) DM/HTN (control) group (rho 0.87, p <0.001).
HMGB-1 levels correlate with disease activity
The levels of HMGB-1 in the lupus group correlated positively with SLE disease activity as measured by the overall SLEDAI score (rho 0.44, p <0.001) and the renal SLEDAI score (rho 0.43, p<0.001; Figure 1C, D). Surprisingly, no correlation was observed between urine HMGB-1 and serum creatinine, dsDNA or C3, C4, although complement deposition did correlate with urinary levels for LN Class V (p=0.025, not shown). Interestingly, the urine P/C ratio correlated with HMGB-1 levels in both lupus (rho 0.38, p<0.03) and non-lupus DM/HTN groups (rho 0.87, p<0.001), although the levels of HMGB-1 were significantly lower in the control disease group (Figure 1E, 1F). The low levels of HMGB-1 detected in the control disease group may be due to a mild chronic renal inflammation.
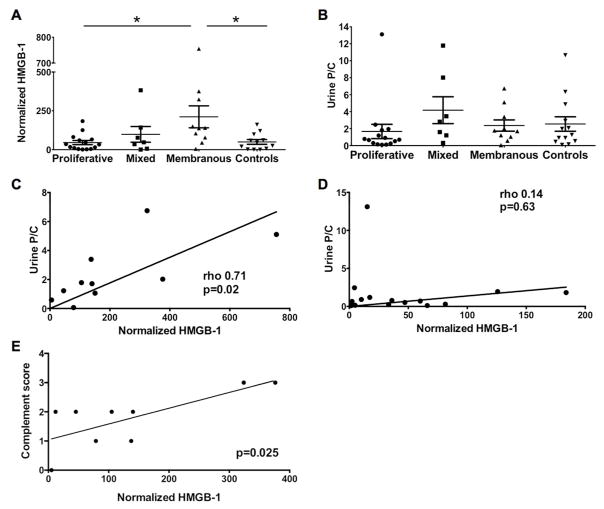
HMGB-1 levels are higher in membranous LN and correlate with proteinuria and complement deposition
We further analyzed HMGB-1 levels within the biopsy classes. Figure 2A shows that the levels of HMGB-1 in membranous LN were significantly higher than those in other classes of LN or in diseased controls (p=0.03). However, there was no statistically significant difference between the urine P/C ratio between these 4 groups (p=0.13). Although urine P/C ratio correlate with HMGB-1 levels across all patients (rho 0.55, p<0.001, Fig. 1E), urine P/C ratio correlated with HMGB-1 only in membranous LN (rho 0.71, p=0.02, Fig. 2C, p=n.s. Fig. 2D).
Figure 2. Urine HMGB-1 levels are higher in membranous disease when compared within LN classes.
A) HMGB-1 in membranous LN were significantly higher than those in other classes of LN or in diseased controls (p =0.03). B) Urine P/C ratio between the 4 groups were not significantly different (p =0.13). C) Urine P/C ratio correlated positively with HMGB-1 in membranous LN (rho 0.71, p =0.02). D) Urine P/C ratio does not correlate with HMGB-1 in mixed LN (Rho=0.43, p=0.33). E) Complement deposition in renal biopsies was scored from 0–4 based on biopsy report. Urinary HMGB-1 correlated positively with complement deposition score in membranous LN (p=0.025).
To further investigate the relationship between proteinuria and HMGB-1 levels, we performed a linear regression model. In univariate analyses, HMGB-1 was again significantly associated with the P/C ratio (β=18.1, p<0.001), and with membranous nephritis as compared to the other classes and controls (β=161.8, p=0.004). Interestingly, when we controlled for the P/C ratio in our multivariate models we found that membranous nephritis was still significantly associated with HMGB-1 levels despite controlling for the proteinuria (β=172.1, p=0.002). Finally we investigated possible mechanisms of passive and active release of HMGB-1 and correlated the levels of urinary HMGB-1 in LN Class V and the presence of necrotic cells, podocyte effacement, mesangial proliferation and complement deposition based on the official histology and electron microscopy reports for 31 renal biopsies. We found that only complement deposition correlated with the levels of HMGB-1 (p=0.025 and data not shown). Therefore, the elevated urinary HMGB-1 in membranous LN patients is not solely explained by passive excretion secondary to elevated proteinuria, but is likely secondary to a mechanism inherent to class V LN such as complement activation.
Discussion
In the present study we show that urinary HMGB-1 was increased in proteinuric patients. Moreover, the levels of HMGB-1 were higher in active LN patients compared to non-active and hypertension and/or DM patients despite similar urine P/C ratio. The levels of HMGB-1 correlated with disease activity indices as well as P/C ratio. We also show that within LN classes, the HMGB-1 levels were significantly higher in membranous lupus nephritis and that those levels correlated with urine P/C ratio in this class. HMGB-1 also correlated with complement deposition but not with podocyte effacement, necrotic cell death or mesangial proliferation. Finally, the association between HMGB-1 and membranous LN persisted when proteinuria was controlled for.
HMGB-1 is an inflammatory protein bound to auto-antibodies, and has been suggested to indicate active disease and damage (10). An increase in HMGB-1 serum and urine levels in nephritic patients has been reported previously (9, 11).
The present study was carried out to better characterize the relevance of HMGB-1 in LN. Specifically we aimed at determining whether during nephritis HMGB-1 actively participates in inflammation or is passively released as a result of the tissue damage. In order to differentiate between those two, we used disease controls with high urine P/C ratios, comparable to the active lupus nephritis group. Our data show that HMGB-1 is significantly higher in lupus nephritis compared to disease controls, suggesting that the increase is not merely due to compromised renal function.
Renal tissue with proliferative LN shows massive infiltration of leukocytes, which so far are the best-characterized sources of actively secreted HMGB-1. Surprisingly, within LN classes, we observed increased HMGB-1 in membranous LN rather than proliferative disease. Although infiltrating leukocytes and endothelial cells are thought to be the source of HMGB-1 in the kidney, the exact source remains to be determined. Our data suggest that a distinct mechanism in membranous lupus nephritis may contribute to the release of HMGB-1.
Membranous lupus nephritis is characterized by sub-epithelial immune-complex deposition and the damage to the tissue is believed to be due to activation of the classical complement cascade (12). The cell death resulting from complement activation leading to membrane attack complex would typically be necrosis, due to loss in membrane permeability (13), which would also release HMGB-1 from those dying cells. This mechanism could explain our findings. We did not find a correlation with necrotic cells, nevertheless the complement deposition did correlate with the levels of urinary HMGB-1 and complement activation itself has been previously linked to HMGB-1 release during inflammation (14).
Renal cells known to express the HMGB-1 receptor RAGE include mesangial cells, podocytes and endothelial cells. Podocytes participate in renal inflammation and due to their pivotal role in glomerular filtration, disruption of podocyte function leads to glomerular injury and proteinuria. Stimulation with pro-inflammatory cytokines increases their expression of TLR-1, 2, and 4 (15) and contributes to their death. Ligation of RAGE is also suggested to induce apoptosis in podocytes and contribute to proteinuria (16). Therefore HMGB-1 released in the kidney during LN may be due to complement activation and induce podocyte activation resulting in proteinuria.
We therefore propose that during membranous LN, HMGB-1 is released by a combination of mechanisms such as passive by cell death and active due to complement deposition and activation. The latter leads to macrophage activation and release of HMGB-1 but also to podocyte death and proteinuria. Moreover, the activation and death of podocytes further releases HMGB-1.
Overall, our data suggest that urinary HMGB-1 in LN does not simply follow albumin in the urine due to the compromised renal function, and that its release in the urine specifically correlates with membranous LN. The presence of unique lesions in membranous LN compared to other classes partly supports our working hypothesis that the mechanism of release and target cell type of HMGB-1 differs in different classes of LN. Our results indirectly suggest so as the levels of HMGB-1 not only correlated with membranous LN but also with potential mechanisms of HMGB-1 production, i.e. complement deposition (14). It would therefore be interesting to determine whether HMGB-1 co-localizes with podocytes or other cell types in renal biopsies from membranous LN patients, although previous studies did not find such correlation (9). Our data suggest that increase in urine HMGB-1 is a consequence of the upstream inflammatory trigger specific for membranous LN, and therefore can be indicative of membranous LN. Moreover, HMGB-1 may also prove to be suggestive of non-lupus associated membranous nephritis. However further studies and replication in larger cohorts are required to establish HMGB-1 as a biomarker for membranous LN.
In this paper we have show for the first time that urinary levels of HMGB-1 protein may prove to be a non-invasive diagnostic tool to distinguish membranous LN from all the other LNs. Future studies will determine whether these urinary levels of HMGB-1 change with the progression of the membranous LN, how they reflect flares and recoveries and therefore whether urinary levels of HMGB-1 can be used to monitor severity of kidney damage and response to treatment in this specific form of LN.
Acknowledgments
We thank Drs. Philip L. Cohen, Stefania Gallucci and Michael Madaio for critically reading the manuscript and for their numerous suggestions.
Funding Acknowledgement
This work was supported by a Mentored Clinical and Translational Research Career Development Award supported through an National Institutes of Health Clinical and Translational Science Award (CTSA) grant, [grant number KL2RR025749 to I.B.], and the National Institute of Health- National Institute of Arthritis and Musculoskeletal and Skin Diseases NIH-NIAMS [grant number R01-AR061569-01A1] to R.C.
List of abbreviations
- DM
Diabetes Mellitus
- HMGB-1
High Mobility Group Box -1
- HTN
Hypertension
- LN
Lupus nephritis
- RAGE
Receptor of Advanced Glycation End Products
- SLE
Systemic Lupus Erythematosus
- SELENA-SLEDAI
Safety of Estrogens in Lupus Erythematosus National Assessment- SLE Disease Activity Index
- TLR
Toll Like Receptor
- urine P/C ratio
urine protein/creatinine ratio
Footnotes
Conflict of interest statement
The Authors declare that there is no conflict of interest
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–90. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084–91. [PubMed] [Google Scholar]
- 4.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 5.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 6.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–73. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulahad DA, Westra J, Bijzet J, et al. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zickert A, Palmblad K, Sundelin B, et al. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qing X, Pitashny M, Thomas DB, et al. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121:61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdulahad DA, Westra J, Bijzet J, et al. Urine levels of HMGB1 in Systemic Lupus Erythematosus patients with and without renal manifestations. Arthritis Res Ther. 2012;14:R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai N, Cimbaluk D, Lewis EJ, Whittier WL. Proteinuria in membranous lupus nephritis: the pathology is in the podocyte. Lupus. 2013;22:461–8. doi: 10.1177/0961203313477225. [DOI] [PubMed] [Google Scholar]
- 13.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 14.Erces D, Nogrady M, Nagy E, et al. Complement C5A antagonist treatment improves the acute circulatory and inflammatory consequences of experimental cardiac tamponade. Crit Care Med. 2013;41:e344–51. doi: 10.1097/CCM.0b013e31828a6768. [DOI] [PubMed] [Google Scholar]
- 15.Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–13. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–70. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]