Abstract
Objective
To compare the efficacy of Mindfulness-Based Addiction Treatment (MBAT) to a Cognitive Behavioral Treatment (CBT) that matched MBAT on treatment contact time, and a Usual Care (UC) condition that comprised brief individual counseling.
Method
Participants (N=412) were 48.2% African-American, 41.5% non-Latino White, 5.4% Latino and 4.9% other, and 57.6% reported a total annual household income < $30,000. The majority of participants were female (54.9%). Mean cigarettes per day was 19.9 (SD= 10.1). Following the baseline visit, participants were randomized to UC (n = 103), CBT (n = 155), or MBAT (n = 154). All participants were given self-help materials and nicotine patch therapy. CBT and MBAT groups received eight two-hour in person group counseling sessions. UC participants received four brief individual counseling sessions. Biochemically verified smoking abstinence was assessed 4 and 26 weeks after the quit date.
Results
Logistic random effects model analyses over time indicated no overall significant treatment effects, (completers only: F(2,236) = 0.29, p=.749; intent-to-treat: F(2,401) = 0.9, p=.407). Among participants classified as smoking at the last treatment session, analyses examining the recovery of abstinence revealed a significant overall treatment effect, F(2,103)=4.41, p=.015 (MBAT vs. CBT: OR=4.94, 95% CI: 1.47 to 16.59, p=.010, Effect Size =.88; MBAT vs. UC: OR=4.18, 95% CI: 1.04 to 16.75, p=.043, Effect Size =.79).
Conclusions
Although there were no overall significant effects of treatment on abstinence, MBAT may be more effective than CBT or UC in promoting recovery from lapses.
Keywords: mindfulness, tobacco treatment, group therapy, nicotine dependence
The prevalence of smoking in the US, although declining, remains high at 17.8% (Jamal et al., 2014). Most smokers want to quit, and nearly half of all smokers attempt to quit each year (CDC, 2009), but only about 5% of all smokers successfully quit each year (Cohen et al., 1989). These low quit rates are not surprising given that cessation is associated with increased levels of negative affect and stress that can persist for months, as assessed by both self-report and asymmetries in brain activity (Gilbert et al., 2002; Piasecki, Fiore, & Baker, 1998). This phenomenon is further complicated by a plethora of evidence indicating that stress, negative affect, and depression strongly predict and are setting events for relapse (Baker, Brandon, & Chassin, 2004; Borrelli, Bock, King, Pinto, & Marcus, 1996; Brandon, 1994; Correa-Fernandez et al., 2012; Glassman et al., 1990; Niaura et al., 1999; Shiffman, 2005; Welsch et al., 1999). Thus, an important goal for intervention development research is to carefully target these aversive emotional consequences of quitting smoking in an effort to enhance cessation rates and ultimately prevent relapse. One factor found to be broadly and consistently linked with enhanced emotional regulation is mindfulness, and mindfulness-based treatments may be particularly well-suited for treating nicotine dependence and other substance use disorders.
Definition of Mindfulness
Mindfulness has been defined as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” (Kabat-Zinn, 1994) and as “bringing one's complete attention to the present experience on a moment-to-moment basis” (Marlatt & Kristeller, 1999). All approaches that include mindfulness note that it should be practiced nonjudgmentally, meaning that to the extent possible, phenomena entering awareness should not be labeled as true or false, good or bad, etc. (Kabat-Zinn, 1994; Linehan, 1994; Segal, Teasdale, Williams, & Gemar, 2002). A key characteristic of mindfulness is that by simply noticing emotions, cognitions, perceptions, and sensations in a nonjudgmental manner, individuals learn over time that these phenomena are transient and do not demand impulsive action (Heppner, Adams, Vidrine, & Wetter, In press). Thus, flexible, adaptive responding is fostered when awareness is brought to the present moment (Roemer & Orsillo, 2003; Teasdale, 1997).
Evidence Broadly Supporting Mindfulness-Based Treatments
The two most prominent explicitly mindfulness-based treatments are Mindfulness-Based Stress Reduction (MBSR; Kabat-Zinn, 1990) and Mindfulness-Based Cognitive Therapy (MBCT; Segal, Vincent, & Levitt, 2002). MBSR was initially targeted at stress and pain-related disorders, and MBCT was developed to treat chronic or recurrent depressive disorders. Both of these approaches use meditation as the principal means of teaching mindfulness. Numerous meta-analyses have concluded that mindfulness-based treatments are effective across a wide range of conditions and disorders (e.g., stress, pain, anxiety-related disorders, eating disorders, depressive relapse, psychological and physiological outcomes for individuals with vascular disease, multiple sclerosis, fibromyalgia, somatization and mental health disorders; Abbott et al., 2014; Aucoin, Lalonde-Parsi, & Cooley, 2014; Baer, 2003; Godfrey, Gallo, & Afari, 2014; Hatchard, Lepage, Hutton, Skidmore, & Poulin, 2014; Kabat-Zinn, 1982; Kabat-Zinn, Lipworth, Burncy, & Sellers, 1987; Kabat-Zinn, Lipworth, & Burney, 1985; Khoury, Lecomte, Fortin, et al., 2013; Khoury, Lecomte, Gaudiano, & Paquin, 2013; Kim et al., 2013; Kristeller & Hallett, 1999; Lakhan & Schofield, 2013; Lauche, Cramer, Dobos, Langhorst, & Schmidt, 2013; Speca, Carlson, Goodey, & Angen, 2000). In addition, multiple reviews of the meditation literature have concluded that MBSR and meditation were effective not only across numerous disorders and populations, but that in many cases, MBSR was effective when the individual treatment groups themselves were heterogeneous with respect to the condition/disorder being treated (Baer, 2003; Chiesa & Serretti, 2009, 2011, 2014; Hofmann, Sawyer, Witt, & Oh, 2010). Importantly, mindfulness-based treatments lead to improvements in both anxious and depressive mood states (Goyal et al., 2014), and mindfulness/metacognitive awareness appears to a key mechanism of action. Furthermore, MBCT has demonstrated strong efficacy in preventing relapse to depression compared to alternative approaches (Brown & Ryan, 2003; Teasdale et al., 2002).
There is a rapidly growing body of published studies that have evaluated the efficacy of mindfulness-based treatments for nicotine dependence and other substance use disorders. Outcomes evaluated have included tobacco and other substance use (Bowen et al., 2009; Bowen & Marlatt, 2009; Brewer et al., 2011; Brewer et al., 2009; Davis, Fleming, Bonus, & Baker, 2007; Davis, Goldberg, et al., 2014), psychological distress, craving, mindfulness (Davis et al., 2007; Davis, Manley, Goldberg, Smith, & Jorenby, 2014), and treatment dropout (Marcus et al., 2007). Although these studies have generally been small with varied outcomes, the results have been promising.
To date, at least six studies have evaluated the efficacy of mindfulness-based treatments for nicotine dependence. Four of the studies (Bowen & Marlatt, 2009; Brewer et al., 2011; Davis, Manley, et al., 2014; Davis et al., 2013) found that the mindfulness-based intervention evaluated produced significantly higher smoking abstinence rates than the control treatment. The study conducted by Brewer and colleagues (2011) compared a Mindfulness Training (MT) intervention for smoking cessation to the American Lung Association's Freedom From Smoking (FFS) treatment (N=88). Both interventions were delivered in a group format over a 4-week period, twice per week. Smoking abstinence was assessed at the end of treatment and 13 weeks following the end of treatment. MT participants had slightly (although not significantly) higher abstinence rates at the end of treatment (i.e., 36% vs. 15%, p=.06), and significantly higher abstinence rates 13 weeks following the end of treatment (i.e., 31% vs. 6%, p=.01). One of these studies found that although mindfulness-based treatment was not associated with significantly higher abstinence rates compared to standard treatment (25.0% vs. 17.9%), mindfulness-based treatment participants reported significantly greater decreases in smoking urges, perceived stress, and experiential avoidance, and significantly greater increases in mindfulness (Davis, Manley, et al., 2014). The remaining study was very small (n=18) and uncontrolled, but found that an 8-week group mindfulness-based intervention yielded a 7-day biochemically confirmed point prevalence abstinence rate of 56% at six weeks following the quit date (Davis et al., 2007). Results further indicated that compliance with mindfulness meditation was positively associated with decreases in stress and affective distress. In addition, compliance with mindfulness meditation was also positively associated with smoking abstinence.
At least three studies have evaluated the efficacy of mindfulness-based treatments for other substance use. Two of these studies found that the mindfulness-based treatments evaluated were associated with significantly lower rates of substance use (Bowen et al., 2009; Bowen et al., 2014), whereas the other study found no differences in substance use outcomes between a mindfulness-based treatment and a standard CBT-based control condition (Brewer et al., 2009). In addition, the Bowen and colleagues study described above (2009) found that mindfulness-based treatment was associated with significantly greater increases in acceptance and acting with awareness, and significantly greater decreases in craving.
Mindfulness-Based Addiction Treatment (MBAT)
Given that many smokers have a history of failed quit attempts, high levels of nicotine dependence, and/or other comorbidities (Irvin & Brandon, 2000), there is a critical need for new behavioral treatments. Mindfulness-based treatments may add an innovative and important intervention option to the clinical end of the treatment continuum for nicotine dependence. Mindfulness-based treatments may be particularly appropriate given the efficacy of mindfulness-based interventions in reducing emotional distress across exceedingly diverse conditions and populations, and evidence that greater trait mindfulness is associated with higher smoking cessation rates, greater ability to recover from a smoking lapse, and a plethora of beneficial factors (Heppner et al., In press; Heppner et al., In press.; Vidrine et al., 2009; Waters et al., 2009). Furthermore, mindfulness researchers have noted that future rigorous tests of mindfulness-based interventions should include adequate control groups and sufficient power (Baer, 2003; Dimidjian & Linehan, 2003; Roemer & Orsillo, 2003; Teasdale, Segal, & Williams, 1995).
The current study was specifically designed to be responsive to these issues and to build upon the foundation of the studies described above, as well as other previous studies. To the best of our knowledge, the current study is the largest randomized clinical trial to evaluate a mindfulness-based treatment for nicotine dependence or other substance use disorder. This trial was adequately powered to support a rigorous evaluation of the efficacy of Mindfulness-Based Addiction Treatment (MBAT) compared to two control conditions, a Cognitive Behavioral Treatment (CBT) condition that matched MBAT on treatment contact time (i.e., number and length of counseling sessions) and a Usual Care (UC) condition comprised of brief individual counseling sessions based on the Treating Tobacco Use and Dependence Clinical Practice Guideline (Fiore et al., 2008). Although CBT and MBAT were matched on treatment contact time, MBAT included homework assignments whereas CBT did not include such assignments. Given our prior research suggesting that trait mindfulness is associated with significantly higher rates of abstinence and recovery of abstinence following a lapse (Heppner et al., In press.), we hypothesized that individuals randomized to MB AT (versus CBT or UC) would be more likely to achieve abstinence and to recover abstinence following a smoking lapse.
Method
Participants
Participants were recruited from the Houston metropolitan area via local print media. Inclusion criteria included: ≥18 years of age, current smoker with an average of at least 5 cigarettes per day for the past year, motivated to quit smoking within the next 30 days, had a viable home address and phone number, able to read and write in English, an expired air CO level of ≥ 8 ppm, and provided collateral contact information. Exclusion criteria included: contraindication for nicotine patch use, regular use of tobacco products other than cigarettes, use of bupropion or nicotine replacement products other than the study patches, pregnancy or lactation, another household member enrolled in the study, active substance dependence, current psychiatric disorder or use of psychotropic medications, and participation in a smoking cessation treatment program in the previous 90 days. Study advertisements asked if individuals wanted help with quitting smoking, indicated that counseling and nicotine patches would be provided, and stated that participants would be compensated for their time. All data were collected between January 2007 and February 2010. The study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center and informed consent was obtained from all participants.
Procedures
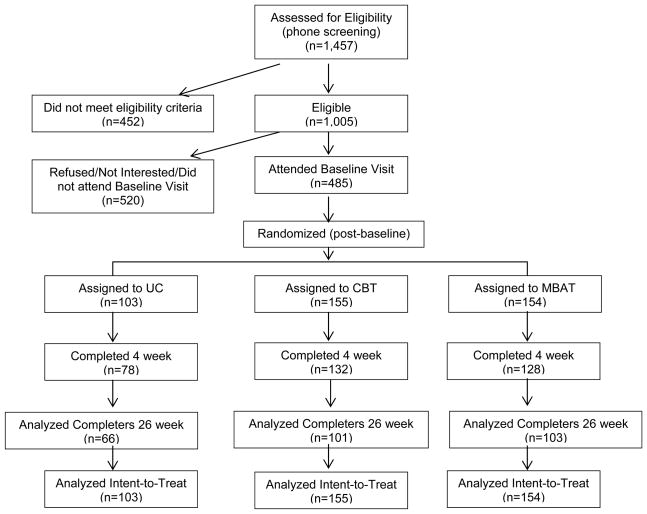
Following the baseline visit, participants were randomized into UC (n = 103), CBT (n = 155), or MB AT (n = 154) using a form of adaptive randomization called minimization. Randomization was based on age, education, race/ethnicity, depression history, and cigarettes per day. Participants and research personnel were not blinded to treatment condition following randomization. Fewer participants were randomized to UC (vs. MBAT and CBT) because we expected that there would be a larger difference in abstinence between MBAT and UC than between MBAT and CBT, and a power analysis revealed that a smaller sample size in the UC group yielded sufficient power. Participant flow through the study is detailed in Figure 1. All groups were given self-help materials and nicotine patch therapy. Patch therapy for participants who smoked >10 cigarettes per day consisted of 4 weeks of 21 mg patches, 1 week of 14 mg patches, and 1 week of 7 mg patches. Patch therapy for participants who smoked 5 to10 cigarettes per day consisted of 4 weeks of 14 mg patches and 2 weeks of 7 mg patches. Self-help materials consisted of the consumer products developed for the 2008 update of the Treating Tobacco Use and Dependence Clinical Practice Guideline (Fiore et al., 2008). The CBT and MBAT groups received eight two-hour in person group counseling sessions. UC participants received four 5- to-10 minute Guideline-based individual counseling sessions (Fiore et al., 2008).
Figure 1.
CONSORT flowchart for recruitment, enrollment, and follow-up assessments.
MBAT Intervention
MBCT represents an adaptation of MBSR that includes techniques from cognitive behavioral therapy. Because MBAT integrates MBSR with a cognitive behavioral/relapse prevention theory based approach to smoking cessation, MBAT is closely modeled on MBCT. The rationale and session-by-session instructions for MBCT have been published by Segal and colleagues (2002; Segal, Williams, & Teasdale, 2002). MBAT closely follows the MBCT treatment procedures, but replaces the depression-related material with nicotine dependence-related material. MBAT utilizes the same structure, within and across sessions, as does MBCT.
The core aims of MBAT are derived from MBCT. Those aims are to help individuals: 1) become more aware of thoughts, feelings, and sensations from moment to moment, 2) develop a different way of relating to thoughts, feelings, and sensations, and 3) increase the ability to disengage attention and choose skillful responses to any thoughts, feelings, or situations that arise. Therefore, sessions 1-4 of MBAT concentrated on learning how to direct and focus attention. Participants were taught to become aware of how little attention is usually paid to what they are doing in their daily life (i.e., how much of their daily lives are spent on “automatic pilot”). In addition, they were taught to become aware of how rapidly the mind shifts between topics. Next, they learned how to not only notice that the mind is wandering, but to bring it back to a single focus on the breath. Furthermore, participants learned how a wandering mind can increase negative thoughts and feelings. For example, fantasies about smoking can lead to feelings of anger about being deprived of cigarettes. Engaging in these thoughts can easily escalate craving such that it becomes more difficult to enact a purposeful, adaptive response. By bringing attention back to the present moment however, one can disengage from this cascade of thoughts and deal with the situation much more flexibly. For example, one could note that the craving is a sensation or mental event (as opposed to an imperative) and simply notice the sensations nonjudgmentally until they pass, choose to engage in a coping behavior, or bring one's attention back to the breath, which is designed to refocus attention on the present moment.
It is important to note that MBAT is also similar to MBRP in many respects (Bowen et al., 2009; e.g., teaching mindfulness-based strategies for coping with cravings), but differs in key ways. First, MBRP has been evaluated primarily as an aftercare program, whereas MBAT is intended to serve as a primary treatment approach. Second, MBRP opens with at least 20 minutes of meditation, whereas MBAT incorporates meditation practice later within each treatment session. We created a manual of the MBAT program for use in this trial (Wetter et al., 2007), with content that paralleled that of CBT (described below) in addition to the mindfulness-based techniques.
The scheduled quit day was on session 5 for participants randomized to MBAT. Sessions 5-8 focused on continuing to develop awareness of the present moment, along with an expansion of techniques for dealing with problematic thoughts, feelings, and situations. To provide an example, one technique is a “breathing space.” A breathing space involves three steps: 1) bringing attention into the present moment and becoming aware of one's current experience (thoughts, feelings, and bodily sensations), 2) gathering one's full attention so that it can be redirected to breathing and using the breath as a tool to anchor oneself in the present moment, 3) followed by expanding the field of awareness around breathing to the entire body. The breathing space occupies a central role in both MBCT and MBAT, can be used in virtually any situation, and is a technique for stepping out of automatic pilot by bringing attention to the present moment. Importantly, the breathing space is a method of generalizing the practice of mindfulness that is developed with formal meditation practices to one's daily life. Participants were taught that they can utilize a breathing space whenever they become aware of urges, stressful situations, or other problematic phenomena.
Cognitive Behavioral Treatment (CBT)
CBT utilized a fairly standard problem-solving/coping skills training approach based on relapse prevention theory (Marlatt & Gordon, 1985) and the Guideline (Fiore et al., 2008). The treatment is manualized and the manual provides a detailed overview of each session including time estimates for each activity and notes to the therapist highlighting potential participant issues and possible responses/probes. All activities are geared toward promoting smoking cessation and the maintenance of abstinence. Each session has specific objectives, and each activity coincides with a minimum of at least one objective. Salient issues covered include nicotine replacement therapy, commitment to abstinence, social/peer pressure, health issues, motivation to change, commitment to change, and coping with stress. Major topics covered in the eight group sessions included: 1) planning to quit smoking; smoking patterns; tools to quit; 2) nicotine addiction; using the nicotine patch; health impact of smoking; triggers; 3) adjusting the stop smoking plan; 4) stress management tools; 5) nutrition and exercise; 6) coping skills; 7) social factors influencing smoking; costs/benefits of quitting; and 8) tapering off the patch; maintaining abstinence; and review of skills from the program. The scheduled quit day was on session 5 for participants randomized to CBT in order to match MBAT.
We chose CBT as our control condition because it is an empirically supported and recommended treatment for smoking cessation (Fiore et al., 2000; Fiore et al., 2008). Treatment contact time and assessments were identical in CBT and MBAT, and therapists were completely crossed with treatment group (i.e., CBT and MBAT groups differed only with respect to counseling content). This study design was intended to allow us to carefully delineate MBAT mechanisms and effects from the effects of an empirically supported cessation treatment that was matched on treatment delivery modality (i.e., group), clinical contact (i.e., number of sessions and duration of each session), and therapists.
Usual Care (UC) Intervention
UC participants received four 5-to-10 minute individual counseling sessions based on the Guideline (Fiore et al., 2008). UC was intended to be equivalent to the intervention a smoker might receive when asking a health care provider for help. The content of the sessions emphasized problem-solving and coping skills training. The scheduled quit day was on session three for participants randomized to UC.
Treatment Delivery and Integrity
The CBT and MBAT groups were led by two masters-level therapists, both of whom were skilled in delivering MBSR and one of whom held a certification in MBSR awarded by the University of Massachusetts Medical Center. Both therapists had extensive personal mindfulness practices and completed approximately 15 hours of training on the components of treatment related to smoking cessation. All groups were led individually by a single therapist. To ensure that any potential treatment group differences would not be attributable to therapist effects, UC was also delivered by the same two therapists that delivered MBAT and CBT. Therapists were completely crossed with treatments such that each counselor delivered equal numbers of MBAT and CBT groups. Therefore, therapist effects were not controlled for in the analyses.
Overall, 37.7% of participants in MBAT completed all 8 group counseling sessions, 53.2% completed between 4 and 7 sessions, and 9.1% completed between 1 and 3 sessions. In CBT, 34.8% of participants completed all 8 group counseling sessions, 52.9% completed between 4 and 7 sessions, and 12.3% completed between 1 and 3 sessions. Of those in UC, 53.4% completed all four in-person individual counseling sessions, 30.1% completed 3 of the sessions, and 16.5% completed 1 or 2 sessions.
Measures
All questionnaires were administered and completed via computer. The measures and variables examined are described below.
Demographics
Demographic variables collected at baseline included age, gender, race/ethnicity, partner status, total annual household income, and educational level.
Nicotine Dependence was assessed at baseline using the Heaviness of Smoking Index (HSI) (Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994). The HSI comprises the two items from the Fagerstrom Test for Nicotine Dependence (FTND) that most strongly predict smoking relapse, cigarettes per day (CPD) and minutes to the first cigarette after waking. Given that the HSI comprises only two items, and has demonstrated psychometric equivalence to the FTND in multiple studies (Borland, Yong, O'Connor, Hyland, & Thompson, 2010; Chabrol, Niezborala, Chastan, & de Leon, 2005; Hymowitz et al., 1997; Kozlowski et al., 1994; Schnoll, Goren, Annunziata, & Suaya, 2013), it was chosen to reduce participant burden.
Mindfulness technique practice during treatment
Among participants randomized to the MBAT condition, their practice of mindfulness techniques was assessed weekly during the course of treatment. Participants were asked to report 1) the average number of days spent engaging in any of the mindfulness techniques learned during the MBAT treatment during the previous week, and 2) the average number of days spent during the previous week engaging in specific mindfulness techniques taught during treatment (i.e., sitting meditation, body scan, walking meditation, yoga, and awareness of the breath). This measure was administered at 7 time points (i.e., weeks 2, 3, 4, 5, 6, 7 and 8), and self-reported days spent practicing were averaged across the weeks to generate composite variables that reflected average days per week spent practicing mindfulness techniques in general as well as for each of the specific mindfulness techniques.
Smoking Abstinence
Seven-day point prevalence abstinence from smoking was assessed at two time points, 4 weeks following the quit day and 26 weeks following the quit day. Because participants were expected to be using the nicotine patch at the assessment that occurred 4 weeks following the quit day, self-reported abstinence was biochemically confirmed using a CO level < 6 ppm. We elected to use a CO cutoff of < 6 ppm rather than a cutoff of < 10 ppm because some research has indicated that a cutoff of < 10 ppm may be too high, and may ultimately result in the misclassification of a proportion of smokers as nonsmokers (Javors, Hatch, & Lamb, 2005). It is important to note that we analyzed our data using both cutoff points, and no statistically significant differences in outcomes were observed. Specifically, at the 4-week assessment, point-prevalence abstinence was defined as self-report of complete abstinence from smoking for the previous 7 days and an expired CO level < 6 ppm. Continuous abstinence was defined as self-report of complete abstinence from smoking since the quit day, and an expired CO level < 6 ppm.
At the 26-week assessment, point-prevalence abstinence was defined as self-report of complete abstinence from smoking for the previous 7 days and CO level < 6 ppm. Continuous abstinence was defined as self-report of complete abstinence from smoking since the quit day, and a CO level < 6 ppm. However, those participants who did not attend the in-person 26-week assessment visit were asked to provide a saliva cotinine sample via mail. For those participants who provided a saliva sample (n=29), a saliva cotinine level cutoff of < 20 ng/ml was utilized to biochemically confirm self-reported abstinence from smoking. Of the participants at the 26-week assessment who self-reported abstinence and lacked CO data, none reported use of any nicotine replacement products. Therefore, saliva cotinine levels should not have been affected by therapeutic nicotine use. This collection method has been validated in prior research (McBride et al., 1999).
Lapse Recovery
Recovery from a lapse was assessed by examining biochemically confirmed (CO<6ppm), 7-day point prevalence abstinence rates at 26 weeks post quit day among participants who were classified as smoking at the end of treatment.
Data Analysis
Chi-square tests and one-way ANOVA tests were used to evaluate differences between MBAT, CBT, and UC at baseline on demographics, smoking rate, and levels of trait mindfulness. Biochemically-confirmed 7-day point prevalence and continuous abstinence assessments were conducted 4 weeks and 26 weeks post quit day. Logistic random effects modeling examined 7-day point prevalence abstinence over time (i.e., at 4 and 26 weeks post quit day) and continuous ratio logit models examined continuous abstinence over time. Both unadjusted and adjusted analyses (controlling for age, education, gender, race/ethnicity, partner status, and HSI scores) were conducted. Because there were no differences between unadjusted and adjusted analyses, only adjusted analyses are reported. In addition, the time indicator (week 4 and week 26) was included as a covariate in the logistic random effects and continuous ratio logit models. Consistent with standard practice in smoking cessation trials, completers-only and intent-to-treat analyses (whereby participants lost to follow-up were coded as relapsed) were conducted. In addition, because single imputation methods may be more biased than other approaches to missing data, attrition analyses were conducted to ascertain whether there were any systematic differences between those with complete data versus those lost to follow-up. To further investigate this question, a sensitivity analysis was conducted to examine the effects of varying missing data assumptions.
Because behaviors within groups are often dependent (i.e., influenced by other members of the group), failure to take group effects into account in statistical analyses may lead to inaccurate inferences, particularly in the form of Type I errors (Herzog et al., 2002; Kapson, McDonald, & Haaga, 2012). Therefore, all models examined controlled for group effects. Group effects were controlled for by including a random intercept of treatment group membership to the model to account for the nested structure of the data (i.e., participants being nested in groups). The ICC was 0.005 for the group effect model. Due to the considerably reduced sample size of the lapse recovery group, this estimate of the covariance parameter was numerically unstable. Therefore, we did not calculate the ICC for this model.
Treatment effects of MBAT (vs. CBT and UC) in helping individuals to recover from lapses were also examined. Specifically, logistic random effect modeling was used to examine group differences in 7-day point prevalence abstinence rates over time among participants who were classified as smoking at the end of treatment (adjusting for age, education, gender, race/ethnicity, partner status, and HSI scores). Finally, associations of mindfulness practice with smoking cessation outcomes were examined among individuals in MBAT.
Results
Participant Characteristics
Participants (N=412) were racially/ethnically diverse (48.2% African-American, 41.5% non-Latino White, 5.4% Latino and 4.9% other) and most reported a total annual household income of less than $30,000 (57.6%). The majority of participants were female (54.9%) and were not married or living with a significant other (70.0%). Approximately one third of participants had less than or equal to a high school education or GED. Average smoking rate was 19.9 (SD= 10.1) cigarettes per day, and 38.6% of participants reported smoking their first cigarette within 5 minutes of waking (see Table 1).
Table 1. Participant Baseline Demographics, Dependence and Mindfulness by Treatment Group.
| Demographics | Overall Sample n=412 |
MBAT n=154 |
CBT n=155 |
UC n=103 |
p-value |
|---|---|---|---|---|---|
| Age | 48.7 (11.9) | 48.4 (11.7) | 48.8 (11.9) | 49.0 (12.5) | 0.93 |
| Gender (% female) | 54.9 | 54.6 | 54.2 | 56.3 | 0.94 |
| Race/Ethnicity (%) | 0.99 | ||||
| Non-Latino white | 41.5 | 39.7 | 42.4 | 42.7 | |
| African American | 48.2 | 49.7 | 47.0 | 47.6 | |
| Latino | 5.4 | 5.3 | 6.0 | 4.9 | |
| Other | 4.9 | 5.3 | 4.6 | 4.9 | |
| Partner Status (%) | 0.46 | ||||
| No Partner | 70.0 | 70.8 | 66.7 | 73.8 | |
| Total Household Income (%) | 0.94 | ||||
| <$30,000/year | 57.6 | 56.7 | 57.6 | 59.0 | |
| ≥$30,000/year | 42.4 | 43.3 | 42.4 | 41.0 | |
| Education (%) | 0.57 | ||||
| < High School | 9.0 | 9.1 | 8.5 | 9.7 | |
| High School/GED | 24.6 | 25.3 | 24.2 | 24.3 | |
| Some College | 47.3 | 43.5 | 49.0 | 50.5 | |
| 4-year College Degree | 13.4 | 18.2 | 11.1 | 9.7 | |
| > 4-year College Degree | 5.6 | 3.9 | 7.2 | 5.8 | |
| Mindfulness | |||||
| KIMS Total Score | 32.0(3.8) | 32.0(3.8) | 31.9(3.6) | 31.9(4.0) | 0.92 |
| MAAS Total Score | 4.3(0.9) | 4.3(1.0) | 4.3(0.9) | 4.2(0.9) | 0.71 |
| Nicotine Dependence | |||||
| Cigarettes per day | 19.9 (10.1) | 20.3 (11.7) | 19.9 (9.3) | 19.3 (8.6) | 0.70 |
| 1st cig. w/in 5 min. of waking | 38.6 | 38.6 | 39.2 | 37.9 | 0.98 |
| History of Depression (%) | 17.3 | 14.3 | 17.5 | 21.6 | 0.32 |
MAAS = Mindfulness Attention Awareness Scale; KIMS = Kentucky Inventory of Mindfulness
Baseline Differences
No significant baseline differences in demographics, nicotine dependence, or levels of trait mindfulness emerged among the three groups (Table 1).
Overall Treatment Effects on Cessation Outcomes
Biochemically verified 7-day point prevalence abstinence rates based on a completers-only approach were 32.5% in UC, 39.1% in CBT, and 42.1% in MBAT at 4 weeks post quit day (one week following the end of treatment) and 19.1% in UC, 23.8% in CBT, and 19.4% in MBAT 26 weeks post quit day. Using an intent-to-treat approach, 7-day point prevalence abstinence rates were 24.3% in UC, 32.3% in CBT, and 34.4% in MBAT 4 weeks post quit day and 11.7% UC, 15.5% in CBT, and 13.0% in MBAT 26 weeks post quit day (see Figure 2).
Figure 2.
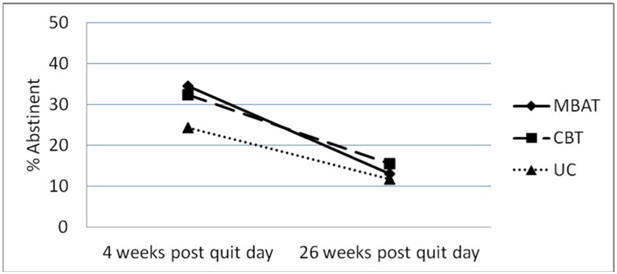
7-day point prevalence abstinence rates by treatment group at 4 and 26 weeks post quit day (intent to treat). N=412.
Logistic random effects model analyses that compared the efficacy of UC, CBT, and MBAT over time yielded no overall significant treatment effects, (F(2,236) = 0.29, p=.749; intent-to-treat: F(2,401) = 0.9, p=.407). Consistent with the point prevalence analyses, the main effect of treatment on continuous abstinence was not significant over time using an intent-to-treat or a completers only approach.
MBAT vs. CBT
To examine differences in 7-day point prevalence abstinence between MBAT and CBT, we conducted a separate set of analyses that included only these two treatment groups. A logistic random effects model indicated that there were no significant differences between these two conditions over time (completers only: OR=1.09, 95% CI: .64 to 1.86, p=.750, Effect Size =.05; intent-to-treat: OR=1.09, 95% CI: .64 to 1.85, p=.755, Effect Size =.05). Consistent with the point prevalence analyses, the main effect of treatment on continuous abstinence over time was not significant.
MBAT vs. UC
To examine differences in 7-day point prevalence abstinence between MBAT and UC, we conducted a separate set of analyses that included only these two treatment groups. Over time analyses indicated that the difference between MBAT and UC was not significant (completers only: OR=1.32, 95% CI: .67 to 2.60, p=.427, Effect Size =.15; intent-to-treat: OR=1.58, 95% CI: .84 to 2.99, p=.159, Effect Size =.25). Consistent with the point prevalence analyses, the main effect of treatment on continuous abstinence was not significant over time.
Effects of MBAT in Facilitating Recovery from a Lapse
Among participants classified as smoking on the last treatment session (completers only; n=145), 14.7% in UC,7.0% in CBT, and 27.8% in MBAT had recovered abstinence one week following the end of treatment. Twenty-six weeks following the quit day, 0% in UC, 5.0% in CBT and 10.3% in MBAT had recovered abstinence (completers only; n=110). Logistic random effects model analyses that examined the effect of treatment on 7-day point prevalence abstinence over time revealed a significant overall treatment effect, F(2,103)=4.41, p=.015. Post-hoc tests revealed significant differences between MBAT and CBT (MBAT vs. CBT: OR=4.94, 95% CI: 1.47 to 16.59, p=.010, Effect Size =.88) and between MBAT and UC (MBAT vs. UC: OR=4.18, 95% CI: 1.04 to 16.75, p=.043, Effect Size =.79).
Intent-to-treat analyses of recovery from a lapse revealed a similar pattern of results (n=151). Among participants classified as smoking at the last treatment session, 13.2% in UC, 7.0% in CBT, and 26.8% in MBAT regained abstinence one week following the end of treatment, and 0% in UC, 3.5% in CBT, and 7.1% in MBAT regained abstinence by 26 weeks post quit day. Logistic random effect modeling analyses examining 7-day point prevalence abstinence over time indicated a significant overall treatment effect (F(2,146)=4.57, p=.012) among participants classified as smoking at the last treatment session. Post-hoc tests revealed a significant treatment effect between MBAT and CBT (MBAT vs. CBT: OR=4.34, 95% CI: 1.35 to 13.99, p=.014, Effect Size=.81) and between MBAT and UC (MBAT vs. UC: OR=4.82, 95% CI: 1.25 to 118.57, p=.023, Effect Size =.87; see Figure 3).
Figure 3.
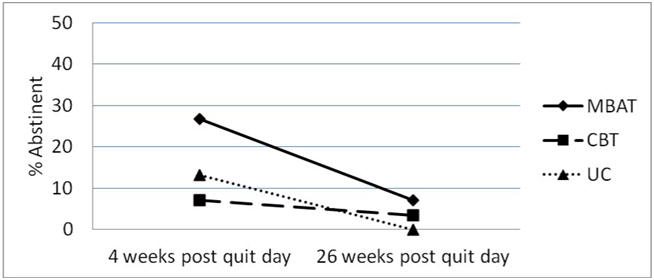
7-day point prevalence abstinence rates by treatment group at 4 and 26 weeks post quit day among individuals classified as smoking on the last treatment session (intent to treat). N=151.
Associations of Mindfulness Practice Dosage during Treatment with Abstinence
Among individuals randomized to MBAT, self-reported mindfulness practice during treatment was examined. Specifically, six items assessed the average number of days over the previous week spent practicing the following activities: sitting meditation, body scan, walking meditation, yoga, awareness of the breath during the day, and the exercises in the workbook. These six items were administered at seven times points (i.e., at treatment sessions two through eight). For each mindfulness practice technique at each time point, the association with abstinence was examined at the end of treatment, 4 weeks following the quit day, 26 weeks following the quit day, and over time (i.e., a total of 4 analyses for each of the 6 items). This resulted in 168 statistical comparisons (i.e., 6 practice technique items × 7 assessment time points × 4 abstinence measures = 168 tests of association). Analyses yielded 6 significant findings encompassing 4 different constructs out of 168 tests. Given that there are actually fewer significant results than would be expected by chance, and the fact that the significant results were not consistent with respect to identifying particular constructs or patterns that might be important, we do not report these results.
Association of Prior Meditation Experience with Abstinence
Associations between experience with meditation prior to study entry and abstinence were also examined. Results indicated that experience with meditation was not associated with smoking abstinence in the overall sample (p's>.184), and previous experience with meditation did not interact significantly with treatment condition to predict smoking abstinence (p's>.221). Among participants classified as smoking at the last treatment session, those who had (vs. did not have) previous experience with meditation were more likely to recover abstinence from smoking across the two follow-up assessment points (OR=3.61, 95%: 1.21 to 10.74, p=0.022, Effect Size =.71 for completers and OR=3.34, 95%: 1.16 to 9.58, p=0.025, Effect Size =.67 for intent-to-treat analyses). However, previous experience with meditation was not found to interact with treatment condition to predict abstinence recovery among individuals classified as smoking on the last treatment session (ps>860). Finally, associations between total number of treatment sessions completed and smoking abstinence were examined. The analyses examining this association with smoking abstinence over time or in single time point analyses were not statistically significant (all ps≥.051).
Attrition and Sensitivity Analyses
Differences in abstinence rates at four weeks post quit day were examined between participants with complete data versus those lost to follow-up on demographics (age, gender, race/ethnicity, education, income, marital status, employment status), nicotine dependence, psychosocial factors (perceived stress, negative affect, positive affect, history of depression), and treatment group. No significant differences were found. Similarly, with regard to differences in attrition rates by treatment group, a Chi-square analysis indicated that there were no significant differences, χ2(2)= 2.738, p=0.254, when examined four weeks following the quit day (MBAT = 6.8 %; ST = 6.6 %; UC =6.3). However, participants with complete data and those with missing data at 26 weeks post quit day differed significantly in race/ethnicity (p = 0.004), marital status (p = 0.027), and positive affect at baseline (p = 0.037). Those lost to follow-up at 26 weeks post quit day were more likely to be non-Hispanic White (as opposed to African American), married or living with a partner, and have lower positive affect scores. Consistent with the examination of treatment group differences at four weeks post quit day, there were no significant differences in attrition rates by treatment group at the 26-week assessment (MBAT=12.4%; ST=13.1%; UC=9.7%), χ2(2)= 0.899, p=0.638. Associations between perceived stress, negative affect, and positive affect at 4 weeks post quit day, and missingness at 26 weeks post quit day, were examined. No significant associations were found.
To address potential bias arising from missing data, sensitivity analyses were conducted to examine the effect of varying missing data assumptions using a multiple imputation approach for treatment effects on 7-day point prevalence abstinence outcomes. Pattern-mixture models were used to generate inferences for various scenarios under the MNAR assumption, with a shift parameter chosen as 0.5, 1, 5, -0.5, -1, or -5 to reflect different degrees of departure of the missing data mechanism from MAR (page 5100, chapter 63: The MI procedure, SAS 9.4 documentation). Results obtained from separate analyses of MBAT vs. CBT, and MBAT vs. UC, using the multiple imputation of treatment effects on 7-day point prevalence abstinence were similar to completers only or intent-to-treat analysis approaches (details of those nonsignificant results are not shown). The conclusions obtained under the missing not at random (MNAR) assumptions were similar to the ones under missing at random (MAR) in that the nonsignificant results remained the same. Therefore, we are confident that our study findings of treatment effects on 7-day point prevalence abstinence outcomes were robust.
Significant findings supporting the analyses examining the effect of MBAT in facilitating recovery from a lapse (MBAT vs. CBT) remained the same across all methods: multiple imputation, intent-to-treat, and completers only (OR=3.22 to 5.01, p-value=0.010 to 0.020, see table below). However, the MBAT vs. UC analysis result was slightly different in the multiple imputation approach (OR=2.72, p=0.123) compared with the other two methods (intent-to-treat OR=4.82, p=0.023, completers only: OR=4.18, p=0.043). Sensitivity analyses of the lapse recovery results (MBAT vs. UC) using multiple imputation with the MNAR assumption revealed similar results compared with the MAR assumption. Therefore, the significant finding based on the ITT analysis (missing = relapsed) in this case needs to be interpreted with caution.
Discussion
This randomized clinical trial was designed to evaluate the efficacy of MBAT compared to CBT and UC with respect to both smoking cessation and recovery from a lapse. Results indicated that there were no significant overall differences in abstinence rates across the three treatments. The results were surprising for several reasons. First, several recent randomized controlled trials have indicated that mindfulness-based treatments for tobacco dependence improve abstinence outcomes compared to standard smoking cessation treatments (Brewer et al., 2011; Davis, Goldberg, et al., 2014). Second, both MBAT and CBT were more intensive therapies than was UC, and treatment intensity has been strongly associated with greater efficacy (Fiore et al., 2008). However, MBAT did show benefits over and above CBT and UC in promoting recovery from a lapse, consistent with findings on the efficacy of MBRP for relapse prevention among individuals with substance use disorders (Bowen et al., 2014). Specifically, among participants who were not abstinent at the end of treatment, those randomized to MBAT appeared to be more likely to recover abstinence post treatment. Thus, although MBAT did not produce superior abstinence rates compared to UC or CBT, MBAT may be effective for preventing early lapses from transitioning to full-blown relapse.
There may be several potential reasons why we failed to find a significant effect of MBAT over the control conditions on abstinence. The 7-day point prevalence abstinence rate for our MBAT group one week post-treatment using an intent-to-treat approach was very similar to that found by both Brewer and colleagues (2011) and Davis and colleagues (2014) at the end of treatment (i.e., 38% in MBAT, 36% in the MT trial conducted by Brewer, and 25.7% in the MTS trial conducted by Davis). However, abstinence rates in our two control groups at the end of treatment were substantially higher than those observed in the Brewer and Davis trials (i.e., 38.1% in CBT and 27.2% in UC one week post treatment compared to 15% at the end of treatment in Brewer et al. and 17.6% at the end of treatment in Davis et al.). Our comparison of MBAT to CBT was also extremely rigorous given that: 1) CBT represents the current state of the science approach, and 2) CBT and MBAT were matched on treatment duration, contact time, and therapists. Nevertheless, MBAT did not improve overall cessation rates as hypothesized. Another possibility is that the MBAT intervention may have unintentionally reduced nicotine patch use relative to the other two treatments. Such a scenario could potentially have led to an overall failure to find overall treatment group differences.
The finding that MBAT appeared to improve lapse recovery is consistent with theoretical and empirical work on mindfulness. Specifically, mindfulness is hypothesized to promote a “decentered perspective” which reduces the tendency for automatic emotional reactions, and this enhanced emotional regulation is in turn, thought to attenuate the likelihood of relapse. Mindfulness is also thought to moderate the association between negative affect and relapse such that in the face of negative affect, individuals with higher levels of mindfulness should have a lower likelihood of relapse compared to individuals with lower levels of mindfulness (i.e., the linkage between negative affect and relapse is weakened among individuals with higher levels of mindfulness; Roemer & Orsillo, 2003; Teasdale, 1997; Teasdale et al., 2002). Recent research has been supportive of both effects with respect to relations among mindfulness, negative affect, and alcohol problems (i.e., that mindfulness both reduces negative affect and reduces the strength of the association between negative affect and alcohol problems; Adams et al., 2014). Neurological studies also provide support that mindfulness training reduces both the severity of negative emotions and reactivity to those emotions (Brown, Goodman, & Inzlicht, 2012; Farb, Anderson, & Segal, 2012; Goldin & Gross, 2010; van den Hurk, Janssen, Giommi, Barendregt, & Gielen, 2010). Thus, MBAT may have improved recovery from a lapse by lessening the negative emotional response to a lapse, and/or by weakening the association between the negative emotional response to a lapse and the likelihood of future lapses.
The fact that MBAT may have some promise in helping smokers recover from early lapses has important implications given that existing treatments designed to prevent relapse and promote recovery from lapses have generally not demonstrated superior efficacy relative to other treatment approaches (Carroll, 1996; Lichtenstein & Glasgow, 1992). Our results suggest that incorporating mindfulness-based techniques into existing smoking cessation treatments could potentially improve the recovery of abstinence after lapses. For example, treatments that increase mindfulness might simply lessen the impact of a lapse when it does occur as noted above, and it also possible that mindfulness strategies could be strategically employed in response to lapses. In particular, briefer meditative practices and other “non-meditation” mindfulness practices might be well-suited to acute lapse-recovery situations. Other possibilities are that mindfulness-based interventions might be particularly effective for more recalcitrant smokers who are likely to lapse early in a quit attempt, or that such interventions could improve cessation rates over a longer course of time in which smokers make multiple quit attempts, lapse, and attempt to regain abstinence. In addition, researchers have suggested that smokers with high anxiety sensitivity related to mental concerns (e.g., fear that having difficulty concentrating means that one is going crazy) might particularly benefit from mindfulness training (Guillot, Zvolensky, & Leventhal, 2015). Finally, MBAT may have important utility as a relapse prevention intervention that is delivered after the achievement of initial abstinence from smoking. That is, mindfulness practice may have particular efficacy in mitigating the impact of lapses leading to full-blown relapse as opposed to facilitating initial cessation success. Further research evaluating the efficacy of mindfulness-based techniques in relapse prevention/recovery is warranted, as is research examining whether such approaches are particularly effective for certain people.
It is unclear why mindfulness practice was not related to overall abstinence in the current study. However, formal mindfulness practice rates were low, and the association of mindfulness practices with cessation could have been attenuated by a restriction in range in the practice variables. Along these lines, it may simply be that a greater amount of mindfulness practice that occurs outside treatment sessions is needed to meaningfully impact cessation outcomes. Another possibility is that our measures of mindfulness practice were crude and may not accurately capture the amount of practice, and they did not capture the quality of practice, which may be essential. In addition, more informal mindfulness practices that occur throughout the day (e.g., 3-minute breathing space, mindful attention to thoughts or feelings) were not assessed, and these more in-the-moment practices may be important in influencing cessation outcomes.
A marked strength of the current study was the inclusion of two control groups representing different levels of treatment intensity. Our CBT treatment was delivered in a group format and matched the MBAT treatment on contact time and intensity. Our UC group was delivered individually and was comparable to standard Guideline-based treatment that might be delivered in the community. Our use of the same two therapists to deliver the three treatment conditions in the current study was an important study design consideration. We chose to use the same therapists to help ensure that any potential differences that emerged between the treatment conditions would be attributable to the treatment rather than to therapist characteristics.
Another considerable strength is our community-based sample. Participants were racially/ethnically diverse, relatively low-income, just over half were female, and more than two-thirds were without a partner. The current findings indicate that MBAT yielded similar abstinence rates compared to more traditional Guideline-based treatments among a diverse and relatively low SES sample of smokers, suggesting that MBAT may be a viable treatment option for such individuals.
Some important limitations should also be acknowledged. Given that MBAT requires specialized and intensive training on the part of therapists and a high level of engagement on the part of individuals enrolled in the treatment, MBAT is not likely to be broadly disseminable in its current format. This is an important limitation from a public health perspective, and a critically important goal for future research should be to examine ways to enhance the disseminability of mindfulness-based strategies. Second, it is important to acknowledge that the use of the same two therapists to deliver all of the study treatments may have resulted in a phenomenon known as “treatment diffusion bias” (Kazdin, 1992). Treatment diffusion bias threatens internal validity, and this phenomenon may have contributed to the absence of significant differences between treatment conditions in the current study. One potential mechanism that may have contributed to treatment diffusion bias is the warmth and compassion expressed by therapists proficient in the delivery of mindfulness-based interventions. For example, modeling of self-compassion may have occurred in all three treatment conditions and may have served as a mechanism facilitating cessation. Such modeling has been suggested to be an active ingredient in mindfulness-based treatments (van der Velden et al., 2015).
A third important limitation is a lack of data to establish fidelity by study interventionists. The treatments were manualized and included specific checklists of topics and activities for each therapy approach and each session. MBAT included very specific activities that were major components of treatment with respect to both content and time spent in therapy that were clearly not part of the CBT or UC treatments. Thus descriptively the interventions differed in important and meaningful ways. However, interventionists were not rated for fidelity to each intervention. In addition, the assessment of treatment fidelity decreases the likelihood of treatment diffusion bias. Thus, the absence of treatment fidelity assessment is an important study limitation. Future research should incorporate ratings to establish that MBAT is conducted with fidelity. A fourth limitation is that rates of compliance with formal meditative practices were low in the current study. Thus, strategies that increase the acceptability of meditation-based practices, or the inclusion of more acceptable non-meditation practices, are clearly needed when reaching out to the general population of smokers. A fifth limitation is that information on use of the nicotine patch was not collected during the study. Because patch use was not tracked, it was not possible to examine potential interactions between MBAT, patch use, and abstinence. A sixth limitation is that our definition of “lapse recovery” among individuals who were smoking at the end of treatment did not differentiate between individuals who never quit versus those individuals who achieved some period of abstinence during the treatment period. Finally, participant attrition is an important study limitation that should be acknowledged.
In summary, the results of this large RCT, at least with respect to comparison with other mindfulness based treatment studies, indicate that MBAT yielded abstinence rates that were similar to two standard Guideline-based treatments of varying intensity among a diverse and relatively low SES sample of smokers. Furthermore, compared to the two control conditions, MBAT may have greater efficacy than CBT and UC in helping individuals recover from lapses. This finding has both clinical and theoretical implications, and future research should examine both replicability and the mechanisms underlying this effect. Future studies should also examine the efficacy of “booster” treatment sessions delivered during the follow-up period. Investigating the efficacy of mindfulness treatment approaches that do not utilize meditation as a primary technique is another important direction for future research. Finally, given that the population of remaining smokers appears to be becoming increasingly recalcitrant (Irvin & Brandon, 2000; Irvin, Hendricks, & Brandon, 2003), specialized, intensive treatments such as MBAT are likely to be needed for certain subgroups of smokers who may have particular difficulty quitting. As such, studies should examine individual differences as potential moderators of the efficacy of MBAT.
Table 2. Average self-reported days spent practicing MBAT techniques across the 8 weeks of treatment among individuals randomized to MBAT.
| Mindfulness Techniques | Average days spent practicing |
|---|---|
| M(SD) | |
| Across 8 weeks of treatment | |
| Any Technique | 3.43 (1.54) |
| Sitting Meditation | 2.17 (1.55) |
| Body Scan | 1.61 (1.43) |
| Walking Meditation | 1.55 (1.70) |
| Yoga | 0.92 (1.34) |
| Awareness of the Breath | 2.84 (1.89) |
| Exercises in Workbook | 0.55 (1.01) |
| Up to the quit day | |
| Any Technique | 3.42 (1.58) |
| Sitting Meditation | 1.90 (1.58) |
| Body Scan | 1.77 (1.58) |
| Walking Meditation | 0.97 (1.62) |
| Yoga | 1.08 (1.60) |
| Awareness of the Breath | 2.27 (1.95) |
| Exercises in Workbook | 0.56 (1.12) |
Table 3. Treatment Content and Timeline.
| MBAT | CBT | UC | |
|---|---|---|---|
|
Questionnaire Assessment Schedule: Baseline, all treatment sessions, and 26 weeks post quit day Smoking Abstinence Assessment Schedule: 4 and 26 weeks post quit day |
|||
| Session 1 | Orientation and Introductions Mindfulness versus automatic pilot Raisin Exercise Body Scan Meditation Typical smoking day Reasons people smoke oping: ACE strategies Daily practice assignment |
Orientation and Introductions Typical smoking day Reasons people smoke Coping: ACE strategies Enhancing self-efficacy Homework Assignment |
Benefits of quitting Reasons people smoke Enhancing self-efficacy Coping: ACE strategies |
| Session 2 | Daily practice review Barriers to mindfulness practice Thoughts and Feelings exercise 20-minute Sitting Meditation Problem solving, high-risk situations Awareness of pleasant events Daily practice assignment |
Problem-solving, high risk situations Learning from former relapses Strengthening motivation Links between activity and mood Emotions, thoughts and smoking Enlisting support |
Enlisting Support General Health and Well-being Problem-solving High risk situations Quit Day preparation |
| Session 3 | Brief “seeing” or “hearing” exercise. Sitting Meditation Daily practice review and assignment Stress, affect, and smoking 3-Minute Breathing Space Mindful Yoga Thoughts and feelings about quitting Priority of cessation, weight gain |
Pros and cons of quitting Benefits of quitting Stress, affect and smoking Feelings of loss about quitting Making quitting top priority Weight gain |
Quit Day Handling Withdrawal Symptoms Identifying Barriers Territory of lapse/relapse Education on the nicotine patch Distribution nicotine patches |
| Session 4 | 5-minute “seeing” or “hearing” exercise Sitting Meditation Daily practice review and assignment Automatic Thoughts 3-Minute Breathing Space Mindful Walking Substitutes for smoking Education on the nicotine patch Quit Day preparation |
Clearing the environment Substitutes for smoking Coping: ACE strategies Managing stress, negative affect Stress management Worry Quit Day preparation Education on the nicotine patch Distribution nicotine patches |
Managing stress, negative affect Weight gain Threats to relapse Distribution nicotine patches |
| Session 5 |
Quit Day Sitting Meditation “Allowing” and “letting be” Daily practice review and assignment Review quit day 3-Minute Breathing Space: Coping Surfing the Urge Clearing environment progress Distribution nicotine patches |
Quit Day Review quit day Getting through withdrawal Territory of Lapse/Relapse Enhancing self-efficacy Coping: ACE strategies Surfing the Urge Coping with negative emotions Distribution nicotine patches |
|
| Session 6 | Sitting Meditation Progress Report (lapse issues) 3-Minute Breathing Space Long term urges Personal high-risk situations and coping Distribution nicotine patches Daily practice assignment |
Progress Report (lapse issues) Long-term urges Personal high-risk situations, coping Enlisting support (review) Stress management (review) Preparing for end of group Distribution nicotine patches |
|
| Session 7 | Mindful Yoga and Walking Meditation Progress Report, discussion of lapses Daily practice review and assignment 3-Minute Breathing Threats to relapse, continued vigilance Distribution of nicotine patches |
Progress Report, discussion of lapses Reflecting on previous quit attempts Strengthening motivation Threats to relapse, continued vigilance Distribution of nicotine patches |
|
| Session 8 | Body Scan Meditation Progress report: achievements Review whole course Maintaining mindfulness practice 3-minute Breathing Space Responding to lapses Distribution of patches, tapering off Concluding meditation |
Progress report: achievements Review any lapses Substitutes for smoking: review Enlisting support: review Predictors of relapse: review How to handle future lapses Distribution of patches, tapering off |
|
Public Health Significance.
Although there were no significant differences in overall abstinence between Mindfulness Based Addiction Treatment (MBAT) and traditional Guideline-based treatments within a diverse and relatively low SES sample of smokers, MBAT may be more efficacious than CBT or UC in facilitating lapse recovery.
Acknowledgments
This research and preparation of this manuscript were supported by grants from the National Institute on Drug Abuse, the Centers for Disease Control and Prevention, the National Cancer Institute, the National Center for Complementary and Integrative Health, and the Oklahoma Tobacco Settlement Endowment Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- Abbott RA, Whear R, Rodgers LR, Bethel A, Thompson Coon J, Kuyken W, et al. Dickens C. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. J Psychosom Res. 2014;76(5):341–351. doi: 10.1016/j.jpsychores.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Adams CE, Chen M, Guo L, Lam CY, Stewart DW, Correa-Fernandez V, et al. Wetter DW. Mindfulness predicts lower affective volatility among African Americans during smoking cessation. Psychol Addict Behav. 2014;28(2):580–585. doi: 10.1037/a0036512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucoin M, Lalonde-Parsi MJ, Cooley K. Mindfulness-based therapies in the treatment of functional gastrointestinal disorders: a meta-analysis. Evid Based Complement Alternat Med. 2014;2014:140724. doi: 10.1155/2014/140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–143. [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Borland R, Yong HH, O'Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12 Suppl:S45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. Am J Prev Med. 1996;12(5):378–387. [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, et al. Marlatt A. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295–305. doi: 10.1080/08897070903250084. doi:916712090[pii]10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Marlatt A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol Addict Behav. 2009;23(4):666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry. 2014;71(5):547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3:33–37. [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Rounsaville BJ. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1-2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, et al. Rounsaville BJ. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–317. doi: 10.1080/08897070903250241. doi:916725438[pii]10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Goodman RJ, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4(1):46–54. [Google Scholar]
- CDC. Cigarette Smoking Among Adults and Trends in Smoking Cessation --- United States, 2008. Morbidity and Mortality Weekly Report. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- Chabrol H, Niezborala M, Chastan E, de Leon J. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addict Behav. 2005;30(7):1474–1477. doi: 10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Subst Use Misuse. 2014;49(5):492–512. doi: 10.3109/10826084.2013.770027. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E, Prochaska JO, Rossi JS, Gritz ER, Carr CR, et al. Debunking myths about self-quitting. Evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. Am Psychol. 1989;44(11):1355–1365. doi: 10.1037//0003-066x.44.11.1355. [DOI] [PubMed] [Google Scholar]
- Correa-Fernandez V, Ji L, Castro Y, Heppner WL, Vidrine JI, Costello TJ, et al. Wetter DW. Mediators of the association of major depressive syndrome and anxiety syndrome with postpartum smoking relapse. J Consult Clin Psychol. 2012;80(4):636–648. doi: 10.1037/a0027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. BMC Complement Altern Med. 2007;7:2. doi: 10.1186/1472-6882-7-2. doi:1472-6882-7-2[pii]10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB. Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Subst Use Misuse. 2014;49(5):571–585. doi: 10.3109/10826084.2013.770025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE. Randomized trial comparing mindfulness training for smokers to a matched control. J Subst Abuse Treat. 2014;47(3):213–221. doi: 10.1016/j.jsat.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS. Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complement Altern Med. 2013;13:215. doi: 10.1186/1472-6882-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Linehan M. Defining an agenda for future research on the clinical application of mindfulness practice. Clinical Psychology: Science and Practice. 2003;10:166–171. [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry. 2012;57(2):70–77. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Wewers ME. Treating Tobacco Use and Dependence: Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services (USDHHS), Public Health Service (PHS); 2000. [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NJ, Curry SJ, et al. Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services (USDHHS), Public Health Service (PHS); 2008. [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, Sly KF. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J Consult Clin Psychol. 2002;70(1):142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. Journal of the American Medical Association. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- Godfrey KM, Gallo LC, Afari N. Mindfulness-based interventions for binge eating: a systematic review and meta-analysis. J Behav Med. 2014 doi: 10.1007/s10865-014-9610-5. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al. Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Zvolensky MJ, Leventhal AM. Differential associations between components of anxiety sensitivity and smoking-related characteristics. Addict Behav. 2015;40:39–44. doi: 10.1016/j.addbeh.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchard T, Lepage C, Hutton B, Skidmore B, Poulin PA. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain disorders: protocol for a systematic review and meta-analysis with indirect comparisons. Syst Rev. 2014;3(1):134. doi: 10.1186/2046-4053-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner W, Adams C, Vidrine J, Wetter D. Mindfulness and emotion regulation. In: Ostafin B, Robinson M, Meier B, editors. Handbook of mindfulness and self-regulation. New York: Springer; In press. [Google Scholar]
- Heppner WL, Adams CE, Correa-Fernández V, Castro Y, Li Y, Guo B, et al. Wetter DW. Dispositional mindfulness predicts enhanced smoking cessation and smoking lapse recovery. Annals of Behavioral Medicine. doi: 10.1007/s12160-015-9759-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog T, Lazev A, Irvin J, Juliano L, Greenbaum P, Brandon T. Testing for group membership effects during and after treatment: The example of group therapy for smoking cessation. Behavior Therapy. 2002;33:29–43. [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine Tob Res. 2000;2(1):79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine Tob Res. 2003;5(1):27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Jamal A, Agaku IT, O'Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults--United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–1112. [PMC free article] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Dell; 1990. [Google Scholar]
- Kabat-Zinn J. Wherever you go, there you are: Mindfulness meditation in everyday life. New York: Hyperion; 1994. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burncy R, Sellers W. Four-Year Follow-Up of a Meditation-Based Program for the Self-Regulation of Chronic Pain: Treatment Outcomes and Compliance. Clinical Journal of Pain. 1987;2(3):159–173. [Google Scholar]
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- Kapson HS, McDonald DO, Haaga DAF. The effect of unanimous first session attendance on psychoeducational smoking cessation groups. Group Dynamics: Theory, Research, and Practice. 2012;16(2):148–158. [Google Scholar]
- Kazdin A. Research Design in Clinical Psychology 2nd Edition. 2nd. Boston: Allyn & Bacon; 1992. [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Hofmann SG. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev. 2013;33(6):763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Gaudiano BA, Paquin K. Mindfulness interventions for psychosis: a meta-analysis. Schizophr Res. 2013;150(1):176–184. doi: 10.1016/j.schres.2013.07.055. [DOI] [PubMed] [Google Scholar]
- Kim SH, Schneider SM, Bevans M, Kravitz L, Mermier C, Qualls C, Burge MR. PTSD symptom reduction with mindfulness-based stretching and deep breathing exercise: randomized controlled clinical trial of efficacy. J Clin Endocrinol Metab. 2013;98(7):2984–2992. doi: 10.1210/jc.2012-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34(3):211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Hallett CB. An Exploratory Study of a Meditation-based Intervention for Binge Eating Disorder. J Health Psychol. 1999;4(3):357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Schofield KL. Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PLoS One. 2013;8(8):e71834. doi: 10.1371/journal.pone.0071834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75(6):500–510. doi: 10.1016/j.jpsychores.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Glasgow RE. Smoking cessation: what have we learned over the past decade? Journal of Consulting and Clinical Psychology. 1992;60(4):518–527. doi: 10.1037//0022-006x.60.4.518. [DOI] [PubMed] [Google Scholar]
- Linehan M. Acceptance and change: The central dialectic in psychotherapy. In: Hayes SC, Jacobson NS, Follette VM, Dougher M, editors. Acceptance and Change: Content and Context in Psychotherapy. Reno, NV: Context Press; 1994. pp. 73–86. [Google Scholar]
- Marcus MT, Liehr PR, Schmitz J, Moeller FG, Swank P, Fine M, et al. Carroll DD. Behavioral therapies trials: a case example. Nurs Res. 2007;56(3):210–216. doi: 10.1097/01.NNR.0000270024.52242.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Marlatt GA, Kristeller JL. Mindfulness and meditation. In: Miller WR, editor. Integrating Spirituality into Treatment: Resources for Practitioners. Washington, D.C: American Psychological Association; 1999. pp. 67–84. [Google Scholar]
- McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. Am J Public Health. 1999;89(5):706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Borrelli B, Shadel WG, Abrams DB, Goldstein MG. History and symptoms of depression among smokers during a self-initiated quit attempt. Nicotine Tob Res. 1999;1(3):251–257. doi: 10.1080/14622299050011371. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology. 1998;107(2):238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Roemer L, Orsillo SM. Mindfulness: A promising intervention strategy in need of further study. Clinical Psychology: Science and Practice. 2003;10:172–178. [Google Scholar]
- Schnoll RA, Goren A, Annunziata K, Suaya JA. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction. 2013;108(11):1989–2000. doi: 10.1111/add.12285. [DOI] [PubMed] [Google Scholar]
- Segal Z, Vincent P, Levitt A. Efficacy of combined, sequential and crossover psychotherapy and pharmacotherapy in improving outcomes in depression. J Psychiatry Neurosci. 2002;27(4):281–290. [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Teasdale JD, Williams JM, Gemar MC. The mindfulness-based cognitive therapy adherence scale: inter-rater reliability, adherence to protocol and treatment distinctiveness. Clinical Psychology & Psychotherapy. 2002;9(2):131–138. doi: 10.1002/cpp.320. [DOI] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York, NY: Guilford Press; 2002. [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. J Pers. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62(5):613–622. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. The relationship between cognition and emotion: the mind-in-place in mood disorders. In: Clark DM, Fairburn CG, editors. Science and Practice of Cognitive Behaviour Therapy. Oxford: Oxford University Press; 1997. pp. 67–93. [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JM. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther. 1995;33(1):25–39. doi: 10.1016/0005-7967(94)e0011-7. doi:0005-7967(94)E0011-7 [pii] [DOI] [PubMed] [Google Scholar]
- van den Hurk PA, Janssen BH, Giommi F, Barendregt HP, Gielen SC. Mindfulness meditation associated with alterations in bottom-up processing: psychophysiological evidence for reduced reactivity. Int J Psychophysiol. 2010;78(2):151–157. doi: 10.1016/j.ijpsycho.2010.07.002. [DOI] [PubMed] [Google Scholar]
- van der Velden AM, Kuyken W, Wattar U, Crane C, Pallesen KJ, Dahlgaard J, et al. Piet J. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin Psychol Rev. 2015;37:26–39. doi: 10.1016/j.cpr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Businelle MS, Cinciripini P, Li Y, Marcus MT, Waters AJ, et al. Wetter DW. Associations of mindfulness with nicotine dependence, withdrawal, and agency. Subst Abus. 2009;30(4):318–327. doi: 10.1080/08897070903252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Reitzel LR, Cinciripini P, Li Y, Marcus MT, Vidrine JI, Wetter DW. Associations between mindfulness and implicit cognition and self-reported affect. Subst Abus. 2009;30(4):328–337. doi: 10.1080/08897070903252080. doi:916722039[pii]10.1080/08897070903252080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter D, Vidrine J, Fine M, Rowan P, Reitzel L, Tindle H. Mindfulness-Based Addiction Treatment (MBAT) Manual 2007 [Google Scholar]