Abstract
The high toxicity of organophosphorus compounds originates from covalent inhibition of acetylcholinesterase (AChE), an essential enzyme in cholinergic neurotransmission. Poisonings that lead to life-threatening toxic manifestations require immediate treatment that combines administration of anticholinergic drugs and an aldoxime as a reactivator of AChE. An alternative approach to reduce the in vivo toxicity of OPs focuses on the use of bioscavengers against the parent organophosphate. Our previous research showed that AChE mutagenesis can enable aldoximes to substantially accelerate the reactivation of OP-enzyme conjugates, while dramatically slowing down rates of OP-conjugate dealkylation (aging). Herein, we demonstrate an efficient HI-6-assisted VX detoxification, both ex vivo in human blood and in vivo in mice by hAChE mutants modified at the choline binding site (Y337A and Y337A/F338A). The catalytic scavenging of VX in mice improved therapeutic outcomes preventing lethality and resulted in a delayed onset of toxicity symptoms.
Keywords: antidotes, cholinesterase, nerve agents, organophosphates, oximes, reactivation, 2-PAM
1. Introduction
Organophosphates (OPs), such as the nerve agents (VX, tabun, soman), represent a threatening means of potential terrorism, warfare deployment as well as OP pesticide exposure to humans and wildlife, due to their ability to covalently inhibit acetylcholinesterase (AChE, EC 3.1.1.7), leading to morbidity and mortality. Current treatment relies on the anticholinergic drug atropine to reduce the effects of accumulating acetylcholine and an oxime that acts as a reactivator of inhibited AChE [1,2]. Nevertheless, this therapy is insufficient and new means of treatment such as bioscavengers are a particular focus of current research. Stoichiometric, catalytic or oxime-assisted catalytic bioscavengers are directed toward inactivating OP compounds before they react with the target AChE [3-6]. So far, the administration of butyrylcholinesterase (BChE, EC 3.1.1.8) purified from human plasma has been indicated as the most promising prophylaxis [7-10]. However, high dosing requirements for the large protein of BChE in the field [5,7] for stoichiometric scavenging, coupled with its laborious production [11-13], may be minimized by catalytic mutant human AChE (hAChE) based bioscavengers assisted by oximes [14-18].
AChE crystallographic [19,20] and kinetic studies [21-23] suggest that the orientation of the oxime and conjugated organophosphate within the narrow confines of the gorge as well as the rate of nucleophilic displacement of phosphorus moiety by oxime are critical determinants for the reactivation mechanism. However, alterations of the active site configuration could improve not only the reactivation of phosphylated AChE assisted by an oxime, but also the rate of phosphylation to form OP-conjugates and their subsequent rate of ageing [18,21]. Our kinetic studies have demonstrated that mutations around the choline binding site in human AChE (hAChE), such as Y337A and Y337A/F338A possess enhanced capacity for the reactivation of their phosphylated conjugates [15,18,22-25]. In addition, the dealkylation (aging) of OP-conjugated Y337A/F338A hAChE is dramatically slowed, allowing efficient oxime assisted catalytic turnover for those OPs that rapidly dealkylate. Molecular modeling revealed that replacement of large aromatic amino acids in the choline binding site with alanine facilitated HI-6 access to the phosphorus atom allowing a more efficient nucleophilic attack [24].
In this study, based on enhanced in vitro reactivation rates of VX-Y337A/F338A hAChE conjugates by HI-6 [24], we investigate VX detoxification with hAChE Y337A and F338A mutations ex vivo and in vivo to enhance bioscavenging of VX by AChE mutants.
2. Materials and Methods
2.1. Chemicals
Oxime HI-6 (US Biological, Swampscott, MA, USA) and 2-PAM (Sigma Chemical Co., St. Louis, MO, USA) stock solutions were prepared in water and diluted in sodium phosphate buffer just before use. For in vivo studies, stock solutions of HI-6 were prepared in water for i.m application with atropine or in saline for i.v. application with the hAChE mutant Y337A/F338A.
VX was purchased from NC Laboratory, Spiez, Switzerland. It was diluted in isopropyl alcohol and further dilutions in water (or saline for in vivo) were made before use.
The substrate, acetylthiocholine iodide (ATCh), and thiol reactive reagent 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma Chemical Co., St. Louis, MO, USA. Final concentration of ATCh in in vitro and ex vivo experiments was 1.0 mM and of DTNB 0.3 mM.
2.2. Enzymes
Recombinant hAChE wild type and hAChE mutants Y337A and Y337A/F338A were prepared as described earlier [24]. Human whole blood (hWB) was collected from a young female donor at the Institute for Medical Research and Occupational Health, Zagreb, Croatia. The blood was collected from the antecubital vein into heparin-coated vacutainers (Becton Dickenson, UK). This study was reviewed and approved by the institutional Ethics Committee and a written informed consent was obtained from the donor.
2.3. In vitro enzyme activity assays
For reactivation kinetics in vitro, hAChE and its mutants were incubated with VX to achieve at least 90 % inhibition. The inhibited enzyme was passed through a Sephadex G-50 spin column (Roche Diagnostic GmbH, Mannheim, Germany) to remove excess of unconjugated VX. Then, the enzyme fraction was incubated with an oxime (0.01 – 1.0 mM), and at specified time intervals, an aliquot was diluted for activity measurement. An equivalent sample of uninhibited enzyme was passed through a parallel column, diluted to the same extent as the inhibition mixture, and control activity was measured in the presence of oxime. Both activities of control and reactivation mixture were corrected for oxime-induced hydrolysis of ATCh. Kinetic parameters of reactivation, constants kmax (maximal first order reactivation rate constant), Kox (Michaelis type phosphorylated enzyme-oxime interaction constant) and kr (second-order rate constant of reactivation), were calculated from the observed first order rate constant of reactivation, kobs and experimental data obtained in at least three experiments by equations described earlier [22]. kobs was determined from the kinetics of reactivation at given oxime concentrations.
Progressive inhibition of wild-type hAChE and its mutants by VX was measured after a given time of enzyme incubation (up to 30 min) with VX (0.5-50 nM). The second-order rate constant of inhibition by VX (ki) was calculated as described earlier [21].
For the VX detoxification ex vivo, hWB was supplemented with the hAChE mutant and incubated with 10- or 50-excess of VX. After 1 h of inhibition (achieving 95-100 % inhibition), HI-6 (0.10 or 1.0 mM) was added to the mixture. At specified time intervals, an aliquot was diluted for enzyme activity measurements, and detoxification of VX in WB was expressed as percentage of control activity.
All enzyme activity measurements (inhibition, reactivation, and detoxification) were performed by the Ellman method [26] in 0.1 M sodium phosphate buffer containing 0.01 % BSA, pH 7.4, with temperature control at 25 °C. Spectrophotometric measurements were made at 412 nm (or 436 nm with whole blood experiments), on a CARY 300 spectrophotometer (Varian Inc., Australia).
2.4. Detoxification of VX-exposed mice by combining the human AChE mutant Y337A/F338A with HI-6
Male CD-1 mice of 25-30 g body weight (purchased from Ruđer Bošković Institute, Zagreb, Croatia) were fed with a standard diet, had free access to water and were kept in Macrolone cages at 21 °C, exchanging light and dark cycles every 12 h. Mice were divided into groups of four mice for each dose. Acute toxicity (LD50) was based upon 24 h mortality rates and calculated according to Thompson [27] and Weil [28]. Antidotal efficacy of HI-6 therapy alone against VX poisoning was tested by administering HI-6 intramuscularly to mice (112.5 mg/kg, a dose equal to 25% of HI-6 LD50 where no antidote toxic signs were observed) together with atropine sulfate (10 mg/kg) one minute after subcutaneous VX exposure as described previously [29]. To test the catalytic detoxification of VX-exposed mice in vivo, a combination of pretreatment and therapy was applied [30,31]. Mice were pretreated intravenously with HI-6 (70.7 mg/kg) and Y337A/F338A (1.0 mg/kg) 5 min prior to VX exposure and then treated by HI-6 (112.5 mg/kg) in atropine (10 mg/kg). Time of 5 min for pretreatment was chosen due to relatively short biological half-life of HI-6 (t1/2=8.8 min after i.v. application)[32] and non-pegylated enzyme (t1/2=8 min, mean residence time was 120 min for non-pegylated recombinant F338A-AChE) [7]. The antidotal efficacy of treatments was expressed as a protective index (PI) with 95 % confidence limits and maximal dose of poison (MDP). The PI was the ratio of LD50 between VX with treatment and VX given alone. The MDP was the maximal dose of the VX LD50 that was fully counteracted by the treatment applied. The mice were treated in accordance with the approval of the Ethics Committee of the Institute for Medical Research and Occupational Health in Zagreb, Croatia.
3. Results and Discussion
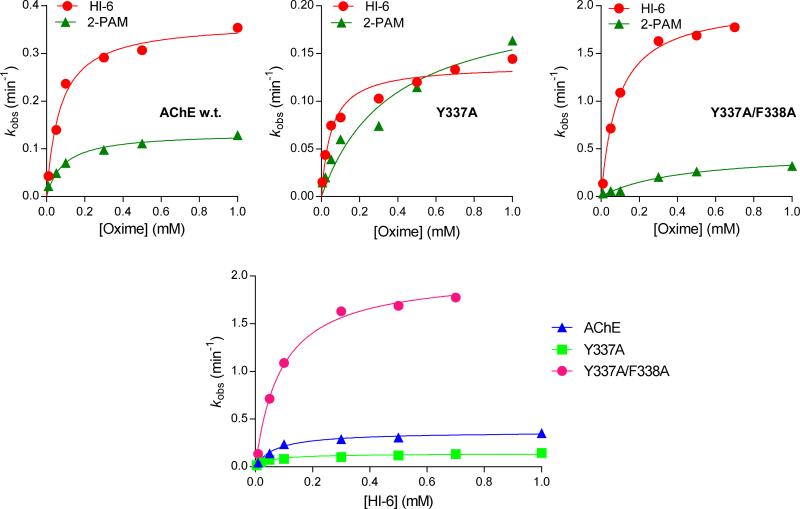
Following our recent findings indicating that HI-6 is a potent reactivator of AChE and its double mutant when inhibited by a VX-analog forming an identical conjugate to VX [24], we tested the HI-6-assisted reactivation of the hAChE and two hAChE mutants (Y337A and Y337A/F338A), when inhibited by the actual nerve agent, VX. If compared with the VX analog, in vitro results showed that the reactivation of VX-enzyme conjugates by HI-6 was efficient (Table 1) despite a substantially lower maximal reactivation rate reflected in the kmax (0.61 and 3.20 min−1 for AChE w.t. and Y337A/F338A, respectively, [24]) as one might expect due to different experimental conditions (previous reactivation experiments were done at 37 °C). Nevertheless, similar to the VX-analog, the overall second-order reactivation rate constant (kr) was enhanced (Table 1) primarily due to the higher affinity of HI-6 for the VX conjugates (i.e. 3-5 times lower KOX).
Table 1.
Detailed kinetic analysis of reactivation of VX-inhibited cholinesterases by HI-6 and 2-PAM at 25 °C. The maximal first-order reactivation rate constant (kmax), the dissociation constant of the phosphylated enzyme-oxime reversible complex (KOX), and overall second-order reactivation rate constant (kr), maximal reactivation (Reactmax, %) and time in that maximal reactivation was reached (tmax) were determined from at least 3 experiments.
| Enzyme | Oxime | kmax (min−1) | KOX (μM) | kr (min−1M−1) | Reactmax (%) | tmax (min) |
|---|---|---|---|---|---|---|
| AChE w.t. | HI-6 | 0.33 ± 0.02 | 58 ± 11 | 5740 ± 1120 | 90 | 15-60 |
| 2-PAM | 0.12 ± 0.01 | 58 ± 15 | 2000 ± 530 | 40-80 | 30-90 | |
| Y337A | HI-6 | 0.14 ± 0.02 | 40 ± 5 | 3530 ± 720 | 80 | 25-90 |
| 2-PAM | 0.15 ± 0.02 | 110 ± 45 | 1400 ± 640 | 10-65 | 20-40 | |
| Y337A/F338A | HI-6 | 1.85 ± 0.07 | 58 ± 9 | 31900 ± 5100 | 95 | 1-15 |
| 2-PAM | 0.41± 0.04 | 317 ± 72 | 1300 ± 320 | 20-80 | 15-40 |
Our results also confirmed the HI-6 superiority over 2-PAM (Figure 1); the latter reactivated the wild type and two AChE mutants generally with slower maximal reactivation rates, lower affinities reflected in KOX, and reactivation up to a maximum of 80% activity (Table 1). Moreover, maximal percentage of reactivation with 2PAM is strongly dependent on oxime concentration, and therefore Reactmax and tmax are presented as ranges.
Figure 1.
Reactivation kinetics of VX-inhibited human AChE w.t. and its mutants Y337A and Y337A/F338A by oximes HI-6 and 2-PAM. Means of at least three experiments are presented. Reactivation constants were determined by nonlinear regression of experimental data and are presented in Table 1.
The two choline binding site mutations had a positive synergistic effect on the HI-6-assisted reactivation of VX conjugates (Table 1, Figure 1) despite of the structural observation that Y337 is closest to the gorge wall. While the single Y337A mutation compromised the relatively fast reactivation rate of the wild-type hAChE, the double Y337A/F338A mutation enhanced the rate of nucleophilic displacement of the phosphonylmoiety from the active site serine (kmax), some 5.5-fold, when compared to the wild type enzyme. Accordingly, the second order rate constant, kr, was larger than the wild-type enzyme demonstrating that the VX-conjugated AChE double mutant retains its binding affinity toward the HI-6 and its capacity to enable the nucleophilic oxime reaction.
We further analyzed phosphonylation kinetics by VX, and as given in Table 2 the second-rate inhibition, ki values, for mutations at the choline binding site were slightly increased. This ensured that phosphonylation of mutants will not compromise the oxime-assisted catalytic scavenging of VX.
Table 2.
The second-rate constant of enzyme inhibition with VX at 25 °C.
| Enzyme | ki (106 M−1min−1) |
|---|---|
| AChE w.t. | 11.1 ± 1.1 |
| BChE w.t. | 4.0 ± 0.1 |
| Y337A | 44.8 ± 0.6 |
| Y337A/F338A | 14.4 ± 1.6 |
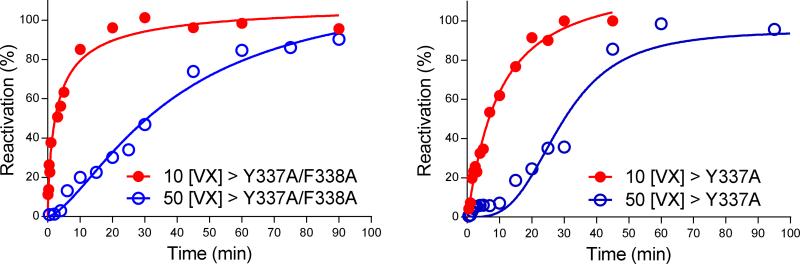
Based on these results with the hAChE mutants that indicated potent HI-6-assisted reactivation and uncompromised phosphonylation by VX, we then tested the bioscavenger potential of the mutant AChEs ex vivo. Human whole blood (hWB) was supplemented with Y337A or Y337A/F338A and inhibited by a 10- and 50-fold excess of VX. As seen in Figure 2, VX detoxification set in quickly after adding HI-6 (1.0 or 0.1 mM – not shown) and resulted in a 95 % recovery of total cholinesterase activity with respect to VX excess. No recovery of activity was observed when hWB was supplemented only with the mutant enzymes. In the presence of HI-6 in hWB (without any supplemental mutant enzyme) the maximal recovery of activity was 50% and 20% with 1 mM HI-6 (20% and 5% with 0.1 mM HI-6) in case of 3.4 and 17 μM VX, respectively. The most rapid VX detoxification was observed within the initial 15 min of oxime-assisted catalysis, when hWB was supplemented with the double mutant Y337A/F338A. Accordingly, VX was degraded by cycles of re-inhibition and reactivation, and then the catalytic activity of the mutant increased to its maximum as a result of total VX detoxification by the mutant and its reactivator. The efficient VX detoxification by the single mutant Y337A probably emphasized the importance of the phosphorylation rate especially in the case of efficient reactivation. It seems that the rate-limiting step for bioscavenging then is the rate of phosphylation. Nevertheless, our results confirmed that the rate-limiting step for bioscavenging may depend on both the rate of phosphylation and subsequent reactivation of AChE as the enzyme cycles through its phosphonylated and unconjugated species to clear excess VX.
Figure 2.
Ex vivo detoxification of VX in whole blood supplemented by AChE mutants Y337A/F338A (0.5 μM) and Y337A (0.34 μM) and oxime HI-6 (1.0 mM).
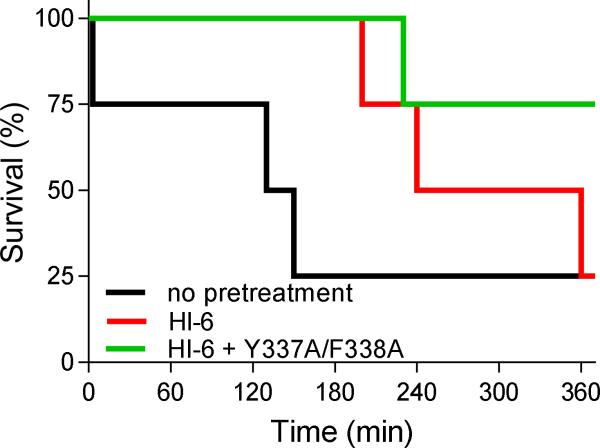
Extending these in vitro and ex vivo findings, catalytic scavenging of the Y337A/F338A mutant, assisted by HI-6 to degrade VX, was assayed in vivo in mice. The acute toxicity of HI-6 for mice was 450 mg/kg for i.m. and 280 mg/kg for i.v. application, classifying HI-6 as a reactivator of lower toxicity and confirming our previous results [18,29]. No toxicity symptoms were observed upon i.v. administration of human AChE mutant Y337A/F338A (1 mg/kg). The antidotal efficacy of tested scavenging system in terms of protective index (PI) and maximal dose of VX (MDP) is shown in Table 3. Post-exposure therapy with HI-6 alone ensured 140-fold protection of mice, while pretreatment of mice with HI-6 additionally improved the antidotal efficacy by 10 %. Furthermore, the combined 5 min pretreatment of mutant enzyme with HI-6 increased the Protection Index by 20 % compared to the protection observed with only HI-6 therapy. Although the maximal dose of VX (MDP) survived by all of the mice also increased modestly from 100 to 126 LD50 of VX, the symptoms of toxicity in mice pretreated with a combination of mutant and HI-6 were significantly less intense; e.g. tremor, convulsions, breathing, and locomotion disturbances. Moreover, even at the highest dose of VX (200 × LD50), a delay in the time of lethality was noticeable when mice were pretreated with the Y337A/F338A plus HI-6 combination (Figure 3).
Table 3.
Antidotal/scavenging efficacy of HI-6 and human AChE mutant Y337A/F338A in VX-exposed mice (s.c.) presented in terms of the protective index (95 % confident limits are given in parentheses) and the maximal dose of poison.a,b
| Pretreatment (i.v.) 5 min before VX | Therapy (i.m.) 1 min after VX | Protective index | MDP |
|---|---|---|---|
| 112.5 mg/kg HI-6 with atropine | 141 (120–167) | 100 | |
| 70.7 mg/kg HI-6 | 112.5 mg/kg HI-6 with atropine | 159 (68–369) | <100 |
| 1 mg/kg mutant +70.7 mg/kg HI-6 | 112.5 mg/kg HI-6 with atropine | 171 (135–219) | 126 |
LD50 (s.c.) of VX was 30 μg/kg, while LD50 of HI-6 was 450 and 283 mg/kg for i.m. and i.v. administration, respectively.
MDP (maximal dose of poison) is the maximal dose of the VX LD50 that was fully counteracted by the treatment applied.
Figure 3.
Survival plots for mice pretreated with HI-6 or HI-6 plus human AChE mutant Y337A/F338A 5 min prior to exposure to 200 × LD50 of VX (6000 μg/kg). Doses in pretreatment were 1 mg/kg AChE mutant and 70.7 mg/kg HI-6. All mice received HI-6 (112.5 mg/kg) in atropine (10 mg/kg) 1 min after VX exposure. Experiment utilized four mice in each group.
The investigation presented here was guided by our recent results on soman that the administration of a mixture of the aging-resistant human AChE mutant Y337A/F338A and an oxime as a reactivator could provide considerable improvement in soman exposure treatment creating a unique scavenging system in vivo in soman-exposed mice. Here, again, using a combined administration regimen of HI-6 and mutant AChE (pretreatment/post-exposure therapy), we observed a significant delay in symptoms of toxicity and time of death. However, the HI-6 antidotal regimen is likely restricted to the peripheral tissues as shown in our recent study with an analog of HI-6 [33]. Nevertheless, we assume that attenuated symptoms such as tremor, breathing, and locomotor disturbances in mice pretreated with mutant and HI-6 reflected an oxime-assisted the catalytic bioscavenging in vivo. Our observations are in accordance with previous studies conducted after percutaneous exposure of guinea pigs to VX. Although maximum blood levels of VX were not reached until several hours after exposure followed by a slow elimination [34], blood levels of VX in guinea pigs that received pretreatment appeared lower, than the VX levels in untreated animals. These investigators presumed that BChE was reactivated by obidoxime, thereby releasing binding sites for circulated and tissue localized VX [35]. This implicates that an oxime-assisted catalytic scavenging of OP could be a possible treatment regimen for detoxification of OP after percutaneous exposure as well. Moreover, as proposed by Sidel et al. [36] the appearance of the most severe signs leading to death implicates that penetration of VX into critical organs, such as the brain and muscle, will not occur until the entire scavenging pool for VX in blood is inhibited. This result also confirms that degradation of OP in circulation by cycles of reactivation and re-inhibition from tissue stores can improve clinical outcomes. In situations of exposure to multiple organophosphates, the oxime could assist scavenging in the blood as well as serve as a conventional antidote in target tissues.
Our study has demonstrated through a combination of in vitro, ex vivo, and in vivo approaches, a feasible development sequence for an oxime-assisted catalytic bioscavenger of VX, based on hAChE mutant in combination with a paired efficient reactivator. Further bioscavenging developments should consider not only optimization of mutant AChE/oxime doses applied but also adjunct therapy to slow oxime clearance in blood. The latter would extend pretreatment times and increase efficiency of scavenging in the plasma before an organophosphate distributes and/or crosses the blood-brain barrier. One advantage of the cholinesterases as bioscavengers is that stereoselective preference for the organophosphorus enantiomer for inactivation likely matches the stereo preference for reactivation, as we have shown in several earlier studies [22,23]. Hence the more toxic enantiomer formed with excess organophosphate is also most susceptible to reactivation and detoxification.
In summary, the double hAChE mutant Y337A/F338A, in combination with the standard oxime, HI-6, carries the potential for bioscavenging a broad spectrum of organophosphates. Very slow aging makes it effective as a soman bioscavenger [18] and, as described here, VX toxicity and concentrations are also efficiently curtailed.
Supplementary Material
Highlights.
VX detoxification is proved ex vivo in blood and in vivo in mice by hAChE mutants.
Aging resistant Y337A/F338A mutant with HI-6 improves therapy in VX exposed mice.
Cycles of reactivation and re-inhibition attenuate symptoms of poisoning.
Acknowledgements
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS) (Grants number R21NS072086 and R21NS084904) and by the Croatian Science Foundation (Project 4307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no conflicts of interest associated with this work.
References
- 1.Gray AP. Design and structure-activity relationships of antidote to organophosphorus anticholinesterase agents. Drug. Metab. Rev. 1984;15:557–589. doi: 10.3109/03602538409029973. [DOI] [PubMed] [Google Scholar]
- 2.Dawson RM. Review of oximes available for the treatment of nerve agent poisoning. J. Appl. Toxicol. 1994;14:317–331. doi: 10.1002/jat.2550140502. [DOI] [PubMed] [Google Scholar]
- 3.Cerasoli DM, Griffiths EM, Doctor BP, Saxena A, Fedorko JM, Greig NH, Yu QS, Huang Y, Wilgus H, Karatzas CN, Koplovitz I, E Lenz D. In vitro and in vivo characterization of recombinant human butyrylcholinesterase (Protexia) as a potential nerve agent bioscavenger. Chem.-Biol. Interact. 2005;157-158:363–365. doi: 10.1016/j.cbi.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 4.Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem.-Biol. Interact. 2005;157-158:167–171. doi: 10.1016/j.cbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 2007;233:31–39. doi: 10.1016/j.tox.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 6.Nachon F, Brazzolotto X, Trovaslet M, Masson P. Progress in the development of enzyme-based nerve agent bioscavengers. Chem.-Biol. Interact. 2013;206:536–544. doi: 10.1016/j.cbi.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Raveh L, Grauer E, Grunwald J, Cohen E, Ashani Y. The stoichiometry of protection against soman and VX toxicity in monkeys pretreated with human butyrylcholinesterase. Toxicol. Appl. Pharmacol. 1997;145:43–53. doi: 10.1006/taap.1997.8160. [DOI] [PubMed] [Google Scholar]
- 8.Lenz DE, Clarkson ED, Schulz SM, Cerasoli DM. Butyrylcholinesterase as a therapeutic drug for protection against percutaneous VX. Chem. Biol. Interact. 2010;187:249–252. doi: 10.1016/j.cbi.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem. Pharmacol. 2011;81:164–169. doi: 10.1016/j.bcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Vučinić S, Zlatkovič M, Antonijević B, Ćurčić M, Bošković B. Fresh frozen plasma as a successful antidotal supplement in acute organophosphate poisoning. Arh. Hig. Rada Toksikol. 2013;64:273–277. doi: 10.2478/10004-1254-64-2013-2378. [DOI] [PubMed] [Google Scholar]
- 11.Rochu D, Chabrière E, Masson P. Human paraoxonase: a promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology. 2007;233:47–59. doi: 10.1016/j.tox.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Masson P, Rochu D. Catalytic bioscavengers against toxic esters, an alternative approach for prophylaxis and treatments of poisonings. Acta Naturae. 2009;1:68–79. [PMC free article] [PubMed] [Google Scholar]
- 13.Renault F, Carus T, Barraud C, Elias M, Chabriere E, Masson P, Rochu D. Integrative analytical approach by capillary electrophoresis under high pressure optimized for deciphering intrinsic and extrinsic cofactors that modulate activity and stability of paraoxonase. J. Chromatogr. B. 2010;878:1346–1355. doi: 10.1016/j.jchromb.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Saxena A, Maxwell DM, Quinn DM, Radić Z, Taylor P, Doctor BP. Mutant acetylcholinesterases as potential detoxification agents for organophosphate poisoning. Biochem. Pharmacol. 1997;54:269–274. doi: 10.1016/s0006-2952(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 15.Kovarik Z, Radić Z, Berman HA, Taylor P. Mutation of acetylcholinesterase to enhance oxime-assisted catalytic turnover of methylphosphonates. Toxicology. 2007;233:79–84. doi: 10.1016/j.tox.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Taylor P, Reiner E, Kovarik Z, Radić Z. Application of recombinant DNA methods for production of cholinesterases as organophosphate antidotes and detectors. Arh. Hig. Rada Toksikol. 2007;58:339–345. doi: 10.2478/v10004-007-0027-1. [DOI] [PubMed] [Google Scholar]
- 17.Mazor O, Cohen O, Kronman C, Raveh L, Stein D, Ordentlich A, Shafferman A. Aging-resistant organophosphate bioscavenger based on polyethylene glycol-conjugated F338A human acetylcholinesterase. Mol. Pharmacol. 2008;74:755–763. doi: 10.1124/mol.108.047449. [DOI] [PubMed] [Google Scholar]
- 18.Kovarik Z, Maček Hrvat N, Katalinić M, Sit RK, Paradyse A, Žunec S, Musilek K, Fokin VV, Taylor P, Radić Z. Catalytic soman scavenging by Y337A/F338A acetylcholinesterase mutant assisted with novel site-directed aldoximes. Chem. Res. Toxicol. 2015;28:1036–1044. doi: 10.1021/acs.chemrestox.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millard CB, Koellner G, Ordentlich A, Shafferman A, Silman I, Sussman JL. Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad. J. Am. Chem. Soc. 1999;121:9883–9884. [Google Scholar]
- 20.Ekström F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry. 2006;45:74–81. doi: 10.1021/bi051286t. [DOI] [PubMed] [Google Scholar]
- 21.Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem. J. 2003;373:33–40. doi: 10.1042/BJ20021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry. 2004;43:3222–3229. doi: 10.1021/bi036191a. [DOI] [PubMed] [Google Scholar]
- 23.Kovarik Z, Ciban N, Radic Z, Simeon-Rudolf V, Taylor P. Active site mutant acetylcholinesterase interactions with 2-PAM, HI-6, and DDVP. Biochemical and Biophysical Research Communications. 2006;342:973–978. doi: 10.1016/j.bbrc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 24.Cochran R, Kalisiak J, Kucukkilinc T, Radic Z, Garcia E, Zhang L, Ho KY, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. Oxime-assisted acetylcholinesterase catalytic scavengers of organophosphates that resist aging. J. Biol. Chem. 2011;286:29718–29724. doi: 10.1074/jbc.M111.264739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovarik Z, Maček N, Sit RK, Radić Z, Fokin VV, Sharpless KB, Taylor P. Centrally acting oximes in reactivation of tabun-phosphoramidated AChE. Chem. Biol. Interact. 2013;203:77–80. doi: 10.1016/j.cbi.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellman GL, Courtney KD, Jr V, Andres RM. Featherstone, New and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Thompson WR. Use of moving averages and interpolation to estimate median-effective dose. Bacteriol. Rev. 1947;11:115–145. [PubMed] [Google Scholar]
- 28.Weil CS. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instruction in their use. Biometrics. 1952;8:249–263. [Google Scholar]
- 29.Čalić M, Lucić Vrdoljak A, Radić B, Jelić D, Jun D, Kuča K, Kovarik Z. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology. 2006;219:85–96. doi: 10.1016/j.tox.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Berend S, Katalinić M, Lucić Vrdoljak A, Kovarik Z, Kuča K, Radić B. In vivo experimental approach to treatment against tabun poisoning. J. Enzyme Inhib. Med. Chem. 2010;25:531–536. doi: 10.3109/14756360903357593. [DOI] [PubMed] [Google Scholar]
- 31.Radić Z, Dale T, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Duggan BM, Ajami D, Rebek J, Taylor P. Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem. J. 2013;450:231–242. doi: 10.1042/BJ20121612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milić B, Maksimović M, Nedeljković M. Trimeoxime and HI-6: Kinetic comparison after intravenous administration to mice. Pharmacol Toxicol. 1996;78:269–272. doi: 10.1111/j.1600-0773.1996.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 33.Katalinić M, Maček Hrvat N, Žďárová Karasová J, Misik J, Kovarik Z. Translation of in vitro to in vivo pyridinium oxime potential in tabun poisoning. Arh. Hig. Rada Toksikol. 2015;66:291–298. doi: 10.1515/aiht-2015-66-2740. [DOI] [PubMed] [Google Scholar]
- 34.van der Schans MJ, Lander BJ, van der WH, Langenberg JP, Benschop HP. Toxicokinetics of the nerve agent (+/−)-VX in anesthetized and atropinized hairless guinea pigs and marmosets after intravenous and percutaneous administration Toxicol. Appl. Pharmacol. 2003;191:48–62. doi: 10.1016/s0041-008x(03)00216-3. [DOI] [PubMed] [Google Scholar]
- 35.Joosen MJA, van der Schans MJ, van Helden HPM. Percutaneous exposure to the nerve agent VX: Efficacy of combined atropine, obidoxime and diazepam treatment. Chem.-Biol. Interact. 2010;188:255–263. doi: 10.1016/j.cbi.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Sidel FR, Takafuji ET, Franz DR. Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare. Office of the Surgeon General; Washington DC: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.