Abstract
Background
Observational studies and a few clinical trials suggest that use of low nitrosamine smokeless tobacco (snus) can facilitate smoking cessation. To better understand the real-world impact of snus on smoking behavior, a large-scale, long-term clinical trial of naturalistic snus use among smokers is needed.
Study Design
A nationwide clinical trial compared abstinence outcomes among smokers who were randomized to receive free samples of snus vs. not. Participants (N=1236) were recruited throughout the U.S. and assessed for one year following a six-week naturalistic sampling period, with high retention throughout. Primary outcomes included self reported quit attempts, floating abstinence (any 7-day period of non-smoking) and 7-day point prevalence abstinence at 6 and 12 months. Secondary outcomes were changes in smoking, motivation and confidence to quit, and adverse events. No tobacco industry support was provided.
Results
Within snus group, 82% used at least once, and 16% were using regularly at end of sampling period. Compared to control participants, smokers in the snus group were less likely to make any quit attempt (RR =0.83; 95% CI: 0.70 – 1.00), and any 24-hour quit attempt (RR = 0.77; 95% CI: 0.63 – 0.95). There were no group differences on any measure of abstinence.
Conclusions
Provision of snus in a naturalistic context resulted in minimal uptake, and as a whole, undermined quit attempts and did not increase smoking abstinence. Results do not support the unguided, free provision of snus among smokers not motivated to quit as a means to facilitate quit attempts.
Trial Registration
clinicaltrials.gov Identifier: NCT01509586
Keywords: potentially reduced exposure products (PREP)s, low nitrosamine smokeless tobacco, harm reduction, smoking cessation
INTRODUCTION
The significant increase of alternative tobacco products has given smokers many more options in addition to combustible cigarettes. Low nitrosamine smokeless tobacco, i.e., snus, is one such product, widely used in Scandinavian countries. Compared to conventional cigarettes and conventional smokeless tobacco products, snus is associated with reduced harm to individual users [1–7]. For example, in Sweden where snus is widely prominent, rates of lung cancer have dramatically declined [8] without increased risk of other cancers [9–12].
The population impact of snus depends on if and how it is used among smokers, and particularly whether it is adopted as a substitute for cigarettes. Snus could conceivably help smokers to quit cigarettes, or, alternatively, it could maintain dependence, allowing them to circumvent smoking restrictions and thus undermine smoking cessation. Scandinavian studies [13–20] show an association between snus use and increased quitting of conventional cigarettes. However, most of these studies have been observational, either cross-sectional or cohort studies, and therefore are limited by self-selection of participants.
The optimal test of this question is a randomized trial, of which there have been four. Two such studies were cessation trials, both from Europe. Danish smokers wanting to quit were randomized to receive snus or not [21]. Abstinence rates were significantly higher at 7 weeks of follow-up (36% vs. 21%; Odds Ratio/OR = 2.1) but did not persist after six months (23% vs. 21%; OR = 1.3). A larger, longer and placebo-controlled study from Serbia found a 3-fold increase in abstinence at 6 months (9.5% vs. 3.7%, Adjusted Odds Ratio/AOR = 2.7), and a nearly 2-fold increase at one year (15.8% vs. 9.3%, AOR = 1.9 [22]. Among smokers wanting to stop smoking, this evidence suggests snus might be an effective substitute for cigarettes. However, no randomized studies have investigated the impact on smoking behavior of providing snus to smokers in a naturalistic setting among smokers not motivated to quit.
There have also been two large-scale clinical trials of snus within the US. The first was a placebo controlled trial in which 250 smokers wanting to quit were randomized to receive active/placebo snus for 14 weeks, with brief adjunctive behavioral support [23]. Abstinence rates were low overall, and point prevalence rates were higher among smokers receiving active vs. placebo snus at 6-weeks (18% vs. 9%; OR = 2.3), 16-weeks (18% vs. 8%; OR = 2.4), and 28- weeks of follow-up (13% vs. 7%; OR = 1.9). A more recent study compared effects of snus vs. nicotine gum (an active comparative, in contrast to above studies) among 391 smokers who were “interested in completely switching” [24]. Abstinence from cigarettes was nearly identical after 3 months of follow-up (27 vs. 25%; independently calculated Relative Risk = 1.1). A separate meta-analysis of only the Fagerström (US) [23] and Joksić (Serbian) [22] RCTs suggested a three-fold increase in abstinence in favor of snus [25].
The existing evidence leads to the following inferences: 1) only a small number of randomized trials have tested the impact of snus on smoking behavior, 2) most studies suggest positive (or sometimes non-significant) effects from snus; no published study has shown negative effects on quitting, and 3) all randomized trials to date have been abstinence focused, recruiting smokers wanting to quit and providing snus with explicit instructions to do so. To better understand the regulatory issues surrounding snus, a study is needed that is both naturalistic (unguided use of snus) and randomized (avoiding biases of self-selection). Additionally, since at any time the majority of smokers are not motivated to quit [26, 27], it is also important to assess how smokers across a broader motivational continuum respond to snus. The present randomized trial was designed to examine the impact of snus use within a naturalistic, non-cessation context. It is the largest and longest trial of snus use among US smokers.
METHODS
Study Overview
Smokers across the U.S. (N=1236) who did not want to quit were randomized to receive a six-week supply of snus or not, after which everyone was advised to quit all tobacco products and then followed for 12 months. The primary outcome was incidence and duration of quit attempts. Secondary outcomes were point prevalence abstinence from cigarette smoking, at 6 and 12 months, smoking reduction, and associated measures of quitting. No support from the tobacco industry was provided. Given our naturalistic focus, we were granted a waiver of IND from the FDA. All procedures were approved by local (Medical University of South Carolina) IRB review.
Participant Recruitment, Eligibility, and Randomization
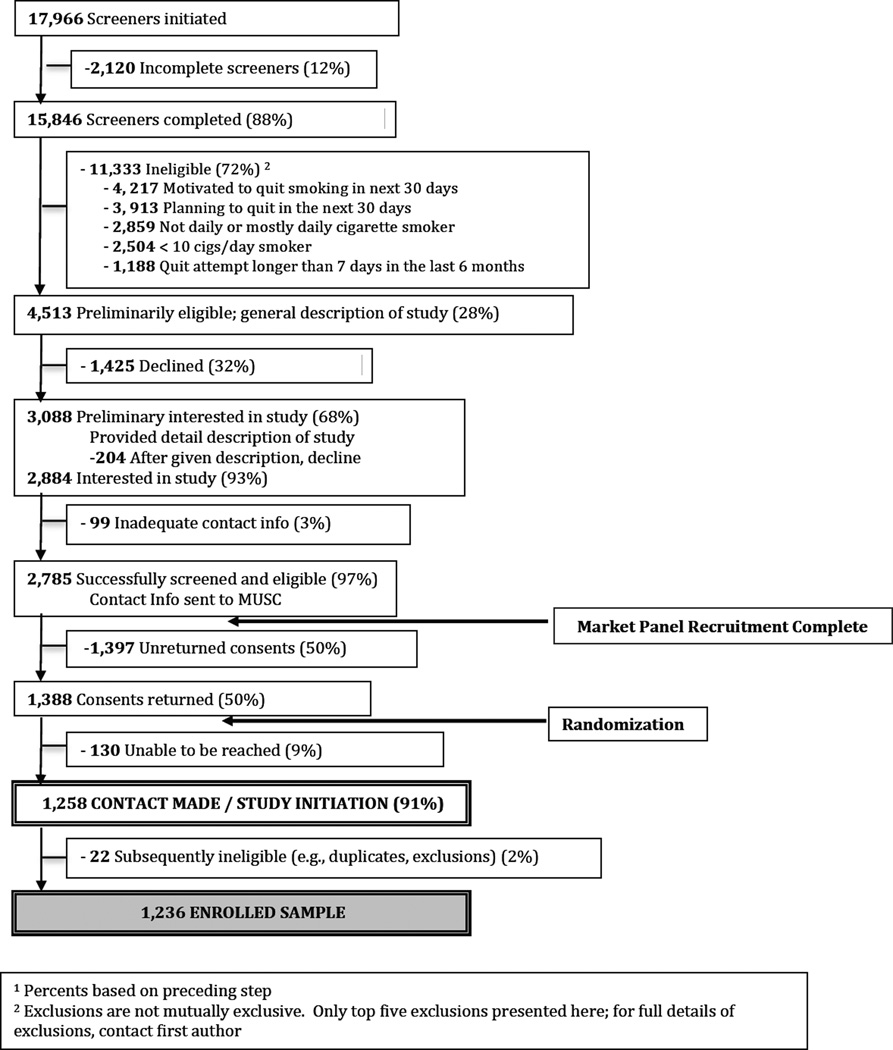
A CONSORT flow of recruitment is shown in Figure 1. Participants were recruited via national market research panels (Knowledge Networks [KN], now GfK). KN emailed to potential study participants a link to a brief study description, and interested individuals then completed a brief online screener to determine eligibility (criteria below). A more complete study description was offered to individuals who were eligible and interested in study participation. This description did not explicitly mention any product by name, but rather stated that the study was a test of a “new, potentially safer tobacco product. While there is no such thing as a safe tobacco product, some evidence suggests that the product we will be testing could be safer than conventional cigarettes.” This recruitment step also emphasized that a) smokers would be randomized to receive the new tobacco product or not, b) product use would be entirely self-determined, and c) there would be no requirement to quit. Those who affirmed interest provided name and full contact information, which was forwarded to the central study office. Study personnel then mailed to each individual a packet consisting of: a) two copies of the consent form, b) a pre-paid, pre-addressed return envelope, and c) baseline questionnaire. Many individuals failed to respond to this mailing. Upon receipt of signed consent at the central study office, individuals were contacted for the first study call, formally commencing study participation. Thus, the final sample of enrolled participants consisted of those who were eligible, returned consent, and completed the initial study call (N=1236). Recruitment took place between 10/2011 and 8/2013. Among those who were eligible for study participation, there were no significant differences between those who did and did not return consent in terms of age, gender, race, or cigarettes smoked per day.
Figure 1.
CONSORT Recruitment Flow1
1 Percents based on preceding step
2 Exclusions are not mutually exclusive. Only top five exclusions presented here; for full details of exclusions, contact first author
Participants met the following eligibility criteria: a) age ≥ 19yrs, b) daily cigarette smoker of ≥ 10 cigarettes per day, c) English speaking, d) live in the contiguous U.S., e) non-use of smokeless tobacco or other reduced exposure products in prior 6 months, f) not breastfeeding, pregnant, or planning a pregnancy, g) no cardiovascular trauma (stroke, myocardial infarction, uncontrolled hypertension) in past 6 months, h) no quit attempt of ≥ 1 week in past 6 months, i) no use of cessation pharmacotherapy in past 3 months, and j) unmotivated to quit smoking in next 30 days, operationalized as 1) ≤ 7 on a 0–10 contemplation ladder [28] and 2) no intention to quit in the next 30 days (asked yes/no) [29].
Participants were allocated to group using mixed block randomization (2–8 participants; NQuery Advisor 6.0), stratifying on state-specific cigarette tax (high vs. medium vs. low) under the notion that uptake of snus and its impact on cessation might vary by state-specific tobacco policies [30, 31]. Given our large sample size, we did not stratify on individual-level predictors of quit attempts (e.g., dependence, smoking history).
Sampling Period and Follow-Up
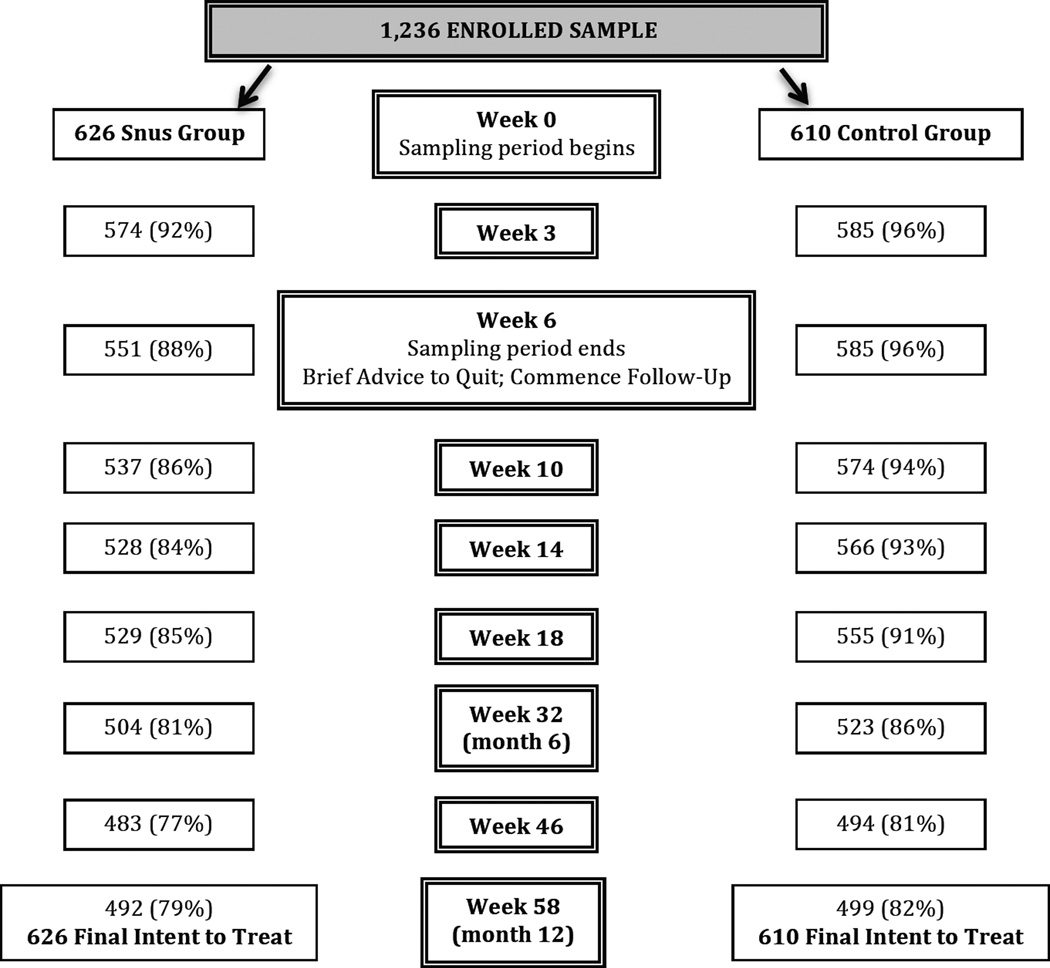
During the initial call participants completed a brief assessment to verify smoking status. Participants were then informed of group status (snus vs. control), and briefed on upcoming calls and reimbursement schedule. The sampling period lasted six weeks, with an interim call at Week 3 (Figure 2). The rationale for a six week sampling period was to provide 1) participants with sufficient time to sample snus and get adjusted to it and 2) the research team with sufficient time to mail product. At the end of the sampling period (Week 6), participants in both groups were given a brief prompt to quit all tobacco products, including snus if still using. All participants were given a referral to their state quitline. Calls to snus participants were slightly longer in duration during the sampling period as compared to control group (mean 17.8 vs. 13.8 minutes), but did not meaningfully differ for follow-up calls (mean 9.0 vs. 8.3 minutes for snus, control groups respectively).
Figure 2.
Study Retention
All participants were followed for an additional 12 months, for a total of 8 follow-up calls (see Figure 2 for study retention). Across all 9,888 (8 × 1236) follow-up calls, 87% were completed (84% snus vs. 90% control; p<.01).
Snus Group
During the first call, participants randomly assigned to receive snus were given a brief product overview, how it differentiates from traditional smokeless tobacco, and why it might be considered safer than cigarettes. No explicit usage instructions were given, though participants were told they could use snus to cut down and/or quit smoking (i.e., completely switch to snus), and/or get through smoking restrictions. A repeated theme was that choice and manner of use was entirely self-determined. In all mailings and phone contacts, participants were reminded that snus should a) not be shared with anyone, b) be kept away from children, and c) not be used by anyone pregnant or who could be pregnant.
We considered but opted against a menu of snus products from which to choose, and rather chose Camel Snus for several reasons. A menu of options would create unwanted variance and need for larger sample size. Evidence available at the time of study initiation [32] and since [33] suggested greatest relief of craving and high favorability for Camel Snus. It was also marketed the most aggressively and was at the time of study initiation the only major smokeless tobacco product with its own dedicated website (camel.com).
Participants were given choice of flavor (Winterchill or Robust), 2.5–2.6 mg nicotine per pouch [24, 34], and were mailed 10 tins (15 pouches per tin; 150 total pouches) to use over the next 3 weeks, split into two mailings. We knowingly oversupplied smokers with snus so that amount of uptake would not be artificially suppressed. At the interim-sampling phone call (week 3), use of snus was assessed in addition to all other outcomes, and again participants were asked if they wished to receive additional product. Another mailing of snus (up to 10 tins) based on preferred flavor was sent, again split across two shipments. Snus was provided free of charge to all participants.
Control Group
Participants were matched on all call schedules, including the sampling period during which they were instructed to smoke/reduce/quit cigarettes as they wish. To equate for free product, these participants received extra reimbursement.
Assessments
The primary outcome was the incidence of quit attempts, both any self-defined and any lasting at least 24 hours, per CDC definition [35]. We also assessed cessation, defined as 7-day point prevalence abstinence, at both 6 and 12 months (i.e., 32 and 58 weeks), and in all cases report abstinence among the entire sample. In addition, we assessed floating abstinence, i.e., any 7-day period of non-smoking at any point during the study. Floating abstinence is a common outcome in cessation induction studies, allowing for the possibility that successful abstinence may occur at any time [36, 37]. Amount of smoking was based on the average number of cigarettes smoked in the last 7 days. Motivation and confidence to quit was assessed via standard (0–10) ladders [28, 38], each asked in reference to quitting within next month, and each with verbal anchors at polar ends only to minimize clustering around labels (0 = “very definitely no” vs. 10 = “very definitely yes”).
Statistical Considerations and Data Analysis
The study was designed to detect a difference in quit attempts between snus and control groups. With a sample size of 1250, the study was adequately powered (82%) to detect an 8% group difference in the prevalence of quit attempts (e.g., 32% vs. 40%) with a two-sided alpha of 0.05. The 32% base rate for quit attempts was based on our prior nationwide study of unmotivated, untreated smokers [39], conservatively proportioned over a 1yr follow-up. All analyses are based on intent-to-treat. Using an intent-to-treat approach, participants with missing data were presumed to neither have made a quit attempt nor be abstinent. For the primary outcomes of quit attempts, 24-hour quit attempts, 7-day point prevalence abstinence at six and 12 months, and floating abstinence, chi-square tests were used to compare the rates between groups. Mixed models with repeated measures were used to evaluate outcomes measured multiple times during the study such as motivation and confidence to quit smoking. Each of these models included group, visit, and the group by visit interaction.
RESULTS
Participant Characteristics (Table 1)
Table 1.
Sample Characteristics
| Snus Group n=626 |
Control Group n=610 |
p-value | |
|---|---|---|---|
| Age, mean (SD) | 48.7 (12.5) | 48.7 (12.6) | .9 |
| % Female | 70% | 65% | .1 |
| Race | .7 | ||
| % Caucasian | 89% | 87% | |
| % African American | 9% | 10% | |
| % Other | 2% | 3% | |
| % Married or Within a Couple | 47% | 51% | .2 |
| % Full/Part Employment | 42% | 45% | .3 |
| Education | .4 | ||
| % HS | 32% | 32% | |
| % > HS | 64% | 62% | |
| % Very/Somewhat Concerned of health | 74% | 74% | .9 |
| Age began smoking | 16.9 (3.7) | 17.0 (4.4) | .5 |
| Cigarettes per Day | 20.1 (8.7) | 19.9 (8.4) | .7 |
| Heaviness of Smoking Index | 3.5 (1.2) | 3.5 (1.2) | .9 |
| % Quit Attempt, past year | 9% | 8% | .6 |
| % Live with other smoker | 45% | 47% | .5 |
| % Allow smoking indoors at home | 55% | 50% | .01 |
| % Heard of low nitrosamine SLT1 | 76% | 75% | .6 |
| % Prior use of low nitrosamine SLT, lifetime1 | 7% | 8% | .4 |
| % Heard of Camel Snus | 70% | 68% | .7 |
| % Prior use of Camel Snus, lifetime | 5% | 5% | .4 |
| Intend to quit smoking, next month | 1.4 (2.3) | 1.3 (2.4) | .8 |
| Confidence to quit smoking | 2.6 (3.1) | 2.7 (3.0) | .4 |
Notes:
HS=high school; SLT=smokeless tobacco;
low nitrosamine SLT: Ariva, Revel, Exalt, Stonewall, Camel Snus, Marlboro Snus (exclusive of both e-cigarettes and traditional SLT products)
Participants were generally female, self-identified Caucasian, and middle-aged. Most were concerned of the health effects of smoking. By design, participants were minimally motivated to quit smoking, and by expectation were minimally confident to quit and had limited quit history in the past year. With the exception of home smoking policies, there were no differences between groups on baseline characteristics.
General Snus Use
Within the snus group, 96% opted to receive snus at least once during the sampling period, and 82% used it at least once. Forty-two percent reported use at the end of the sampling period: 165 (26%) using irregularly (1–5 days of preceding week) and 98 (16%) using regularly (6–7 days). Less than half (40%) reported snus use during the 1-year follow-up period but only 11% reported independent purchase. A small number of participants reported past-week use at 6 and 12 month follow-up (8% and 4%, respectively). Within the control group, <1% of participants reported any snus use at any given follow-up week, on their own accord.
Quit Attempts and Abstinence
Snus participants were significantly less likely than control participants to report a quit attempt of any kind during the course of the study (Table 2). During the six-week sampling period, there were no differences in rates of any quit attempt (6.6% vs. 5.3% in snus and control groups, respectively) or in rates of any 24-hour quit attempts (3.7% vs. 3.0%). Restricting analyses to the follow-up period alone, those in the snus group were significantly less likely to make any quit attempt (23.2% vs. 29.5%; RR = 0.78; 95% CI: 0.65 – 0.95) and any 24-hour quit attempt (18.2% vs. 24.4%; RR = 0.75; 95% CI: 0.60 – 0.93). There were no differences in rates of floating or point prevalence abstinence, either at 6 or 12-month follow-up (Table 2). Within the snus group alone, rates of cigarette-only vs. all tobacco (cigarettes + snus) abstinence were nearly identical: 6% vs. 5% at 6 months, and 7% vs. 7% at 12 months. Among those who made a quit attempt, the longest duration of abstinence also did not vary between snus (61.1 days; SD=98.4) and control (62.3 days; SD=97.3) participants.
Table 2.
Quit Attempts and Abstinence
| No. (%) of Participants | |||||
|---|---|---|---|---|---|
| Snus (n=626) |
Control (n=610) |
Relative Risk (95% CI) |
Risk Difference (95% CI) |
p-value | |
| Any Quit Attempt, ever within study | 161 (25.7) | 188 (30.8) | 0.83 (0.70 – 1.00) | .05 (0.0008 – 0.10) | .05 |
| Any 24-hr Quit Attempt, ever within study | 124 (19.8) | 156 (25.6) | 0.77 (0.63 – 0.95) | .06 (0.01 – 0.10) | .02 |
| Floating Abstinencea | 93 (14.9) | 99 (16.2) | 0.92 (0.71 – 1.19) | .01 (−0.03 – 0.05) | .5 |
| Week 32 PPAb | 39 (6.2) | 34 (5.6) | 1.12 (0.72 – 1.75) | .006 (−0.02 – 0.03 | .6 |
| Week 58 PPAb | 32 (5.1) | 34 (5.6) | 0.92 (0.27 – 1.47) | .005 (−0.02 – 0.03) | .7 |
Notes:
Floating Abstinence: Any 7-day period of non-smoking, ever within study
PPA: Point prevalence
Use of cessation medication at any time during the study was significantly lower among snus participants: 9% vs. 13% (RR = 0.69; 95% CI: 0.50 – 0.96). Use of behavioral treatment for smoking cessation (physician advice, counseling, classes, quitlines) was comparable between groups (25% snus vs. 24% control), as was use of any cessation support, either medication or behavioral treatment (28% vs. 27%).
Smoking Reduction
Participants in both groups reduced the number of cigarettes smoked per day by 23% from baseline to the 1-yr follow-up (p < 0.0001). There were no between-group differences: 22.4% and 22.9% of snus and control participants, respectively, achieved a 50% reduction in smoking level.
Motivation / Confidence to Quit
In parallel with above, there were significant increases over time in both motivation and confidence to quit smoking in the next 30 days (both p<.0001), but no significant between-group differences and motivation to quit remained low both at the end of the sampling period (mean snus group: 2.0 vs. control: 1.9) and at final follow-up (mean snus group: 3.0 vs. control: 3.3).
Sub-group Comparisons
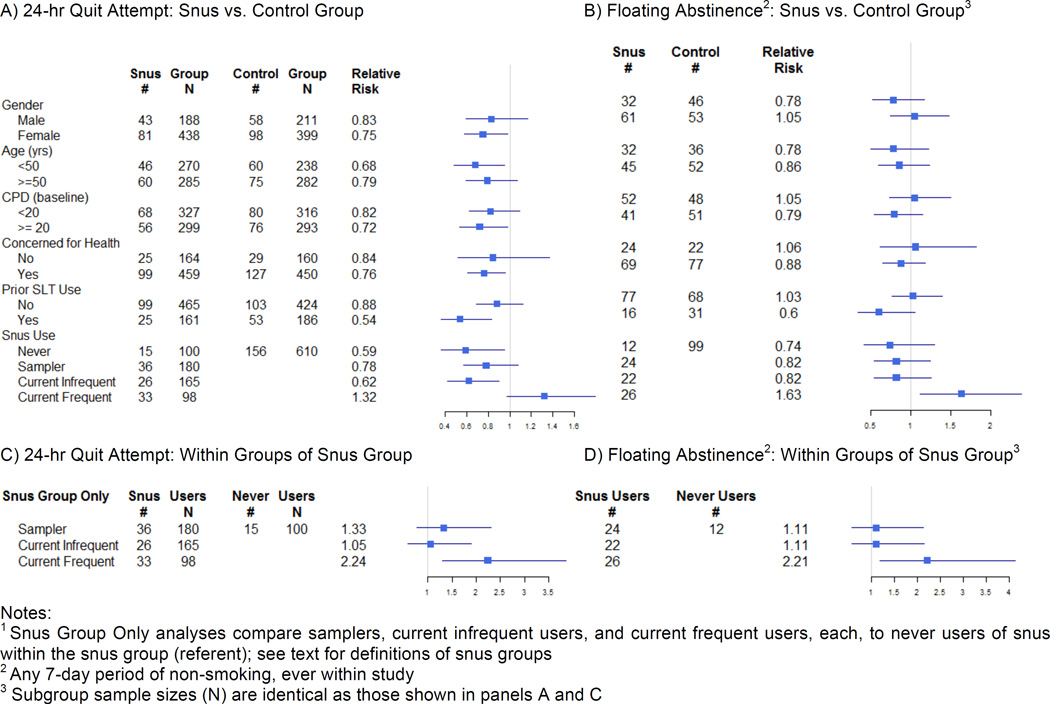
Sensitivity analyses separately examined incidence of a) any 24-hour quit attempt (Figure 3A), and b) floating abstinence (Figure 3B), each as measured across the entire study duration inclusive of the sampling period. Provision of snus significantly undermined quit attempts for participants who were: female; younger than 50 years of age; smoking ≥ 20 cigarettes per day; participants with tobacco-related health concerns; and who had a history of smokeless tobacco use. There were no differences in rates of floating abstinence within any subgroups tested.
Figure 3.
Subgroup Comparisons for Snus vs. Control Group: A) Incidence of 24-hr, and B) Floating Abstinence, and Within Snus Group Alone1: C) Incidence of 24-hr, and D) Floating Abstinence
Notes:
1 Snus Group Only analyses compare samplers, current infrequent users, and current frequent users, each, to never users of snus within the snus group (referent); see text for definitions of snus groups
2 Any 7-day period of non-smoking, ever within study
3 Subgroup sample sizes (N) are identical as those shown in panels A and C
We next compared sub-groups of snus participants who a) never used snus, b) tried product during sampling period but discontinued use (i.e., “samplers”), c) reported current infrequent use at end of sampling period, and d) reported current frequent use at end of sampling period. Compared to control group participants, never and current infrequent users were less likely to make a quit attempt. However, current frequent users of snus at the end of the sampling period were marginally more likely to make a quit attempt as compared to control group participants (RR = 1.32; 95%CI: 0.99 – 1.83), and were significantly more likely to achieve floating abstinence during the study (RR = 1.67; 95%CI: 1.15 – 2.43). Within the snus group alone (Figures 3C, 3D), with never users as a referent, participants who become current frequent users at the end of the sampling period were more likely to try (RR = 2.24; 95%CI: 1.30 – 3.86) and succeed (at any point) in quitting (RR = 2.21; 95%CI: 1.18 – 4.13).
Adverse Events
During the sampling period alone, 240 snus participants (38%) reported a total of 412 adverse events compared with 187 control participants (31%) who reported a total of 300 adverse events (p = 0.005). The between-group self-ratings of symptom severity in the snus versus control group were very similar. There were no snus-related, FDA-defined instances of serious adverse events. The most common side effects reported were nausea (12%), burning in throat or mouth (10%) and heartburn (8%) in the snus group and headache (16%), nausea (10%) and dry mouth (8%) in the control group.
DISCUSSION
We report herein on 1-yr outcomes from a large randomized trial of naturalistic snus use among US smokers, based on smokers not ready to quit, with the notion that assessment of naturalistic use, but under randomized conditions, would best guide policy evaluation of snus. Snus was provided in a manner that optimized the opportunity to observe benefit; i.e., removing all barriers to use by providing it at no cost and with home delivery. Our results do not point toward any benefits of snus for quitting, and in contrast to prior studies, suggest snus may undermine quit attempts, at least among a sample of smokers not ready to quit. We did not find any impact – positive or negative – of snus on abstinence.
The present study contrasts with several prior studies that suggest use of snus may facilitate quitting [21–25, 33, 40]. Snus may prompt smoking abstinence only when provided to smokers who endorse that intention and who use snus, with accompanying instruction/support to quit. In our study, a small number of snus participants who used the product with regularity showed increased quitting behavior. On the whole however, and within all other subgroups tested, our results show that providing snus to smokers who do not wish to quit, devoid of any instruction on how to use it, could be harmful in that it undermines quit attempts, though it has no impact on abstinence.
Within our snus group, there were a small number of participants (n=98; 16%) who, through the sampling period, became frequent snus users. For this subset of participants, outcomes were favorable in terms of inducing quit attempts and abstinence. This subset represents a self-selected sample of participants who were favorable of snus. Separate analyses, presented elsewhere, will differentiate those participants who did vs. did not use snus, and will also determine the long-term trajectory of snus use beyond the sampling period. Nonetheless, snus may offer individual benefits to a small group of smokers who go on to adopt snus.
Though our methods focus on one product alone, we have no reason to suspect that the results for other snus products would not generalize. Of greater concern for generalizability is that our study sample consisted primarily of Caucasian women. While both men and women reported fewer quit attempts within snus group, this was only significant among women, and this may have influenced our negative results. Additionally, our focus on smokers not wanting to quit may create the impression that it was methodologically biased against snus. We did not believe it ethically appropriate to provide a tobacco product without instructions or support to smokers wanting to quit; anyone with such interest was referred to their state quitline. However, using identical naturalistic sampling methods we have previously shown that provision of nicotine replacement therapy to smokers who do not want to quit effectively prompts quitting [37, 41]. Thus, the results reported herein are specific to snus and are not attributable to the low motivational status of our sample. One study limitation is the lack of biochemical verification of abstinence, though national guidelines suggest no such need within studies of minimal intervention [42]. Few biomarkers exist that differentiate smoked vs. smokeless tobacco, and collection of carbon monoxide breathalyzer was not possible given geographically dispersed sample. Finally, though retention rates favored the control group, the slight differences make it doubtful that this would materially influence the results.
Conclusions
In a randomized trial of naturalistic snus use among smokers not wanting to quit, provision of snus led to decreased incidence of quit attempts and had no impact on smoking abstinence. Uptake of snus was generally poor, though a minority of smokers did adopt frequent use, with beneficial impact on quitting. Notwithstanding the potential for snus to lower individual health risk, the overall adverse impact of providing snus in an unguided context to smokers on quit attempts provides clinical and regulatory caution against snus as an inducement or aid for smoking cessation, at least among smokers not yet ready to quit.
WHAT THIS PAPER ADDS.
What is already known on this subject
-
➢
A number of cross-sectional and retrospective surveys, many of which come from Scandinavian settings, suggest an association between use of low nitrosamine smokeless tobacco (i.e., snus) and smoking abstinence.
-
➢
A small number of randomized clinical trials have examined snus for purposes of quitting, with generally suggestive or positive results; i.e., snus promotes abstinence.
What important gaps in knowledge exist on this topic
-
➢
The literature lacks a large-scale clinical trial of naturalistic snus use and impact, particularly among smokers not interested in cessation. Such a trial would inform the regulatory debate on snus as a means of harm reduction and/or cessation induction.
What this study adds
-
➢
Provision of snus to smokers not yet ready to quit decreased the incidence of quit attempts over a one year follow-up period. There was no impact of snus on subsequent abstinence.
-
➢
Uptake of snus was generally poor and the negative impact on quit attempts provides caution against wide scale, unguided use of snus as an aid to cessation.
Acknowledgments
Dr. Carpenter received funding from NCI was overall responsible for study conduct, including data analyses and manuscript preparation. Mrs. Wahlquist was responsible for primary data analyses, and edited the manuscript. Dr. Burris assisted in study coordination and edited the manuscript. Dr. Gray provided medical oversight and edited the manuscript. Dr. Garrett-Mayer provided senior statistical oversight and edited the manuscript. Drs. Cumming and Alberg provide scientific input on study design and edited the manuscript.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors thank the study coordinator, Amy Boatright, as well the principle research assistants who managed study procedures: Nichols Mabry and Caitlyn Hood.
FUNDING
This work was supported by the National Cancer Institute of the National Institutes of Health (grant numbers R01 CA154992 to M.J.C., K07 CA181351 to J.L.B., and P30 CA138313 to A.J.A.), and by the National Center for Advancing Translational Science of the National Institutes of Health (grant number UL1 TR000062).
Footnotes
COMPETING INTERESTS
Drs. Alberg, Burris, Carpenter, Garrett-Mayer, Wahlquist report no conflicts of interests. Dr. Gray has received support from Merck Inc. and Supernus Pharmaceuticals for unrelated research. Dr. Cummings has received support from Pfizer for the evaluation of a hospital based cessation program, and has received payment as an expert witness in litigation against cigarette companies.
REFERENCES
- 1.Hatsukami DK, Jensen J, Anderson A, et al. Oral tobacco products: Preference and effects among smokers. Drug and Alcohol Dependence. 2011;118:230–236. doi: 10.1016/j.drugalcdep.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krautter GR, Chen PX, Borgerding MF. Consumption patterns and biomarkers of exposure in cigarette smokers switched to Snus, various dissovable tobacco products, Dual use, or tobacco abstinence. Regulatory Toxicology and Pharmacology. 2015;71:186–197. doi: 10.1016/j.yrtph.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:2035–2042. [PubMed] [Google Scholar]
- 4.Levy DT, Mumford EA, Cummings KM, et al. The potential impact of a low-nitrosamine smokeless tobacco product on cigarette smoking in the United States: Estimates of a panel of experts. Addictive Behaviors. 2006;31:1190–1200. doi: 10.1016/j.addbeh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Stepanov I, Biener L, Yershova K, et al. Monitoring tobacco-specific n-nitrosamines and nicotine in novel smokeless tobacco products: Findings from round II of the New Product Watch. Nicotine & Tobacco Research. 2014;16:1070–1078. doi: 10.1093/ntr/ntu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepanov I, Jensen J, Hatsukami D, et al. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanov I, Jensen J, Hatsukami D, et al. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- 8.Foulds J, Ramstrom L, Burke M, et al. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee PN. Summary of the epidemiological evidence relating snus to health. Regulatory Toxicology and Pharmacology. 2011;59:197–214. doi: 10.1016/j.yrtph.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Lee PN. Health risks related to dual use of cigarettes and snus - A systematic review. Regulatory Toxicology and Pharmacology. 2014;69:125–134. doi: 10.1016/j.yrtph.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Medicine. 2009;7:36. doi: 10.1186/1741-7015-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo J, Ye W, Zendehdel K, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. Lancet. 2007;369:2015–2020. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- 13.Furberg H, Bulik C, Lerman C, et al. Is Swedish snus associated with smoking initiation or smoking cessation? Tobacco Control. 2005;14:422–424. doi: 10.1136/tc.2005.012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furberg H, Lichtenstein P, Pedersen NL, et al. Snus use and other correlates of smoking cessation in the Swedish Twin Registry. Psychological Medicine. 2007;38:1299–1308. doi: 10.1017/S0033291707002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilljam H, Galanti MR. Role of snus (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction. 2003;98:1183–1189. doi: 10.1046/j.1360-0443.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- 16.Lund KE. Association between willingness to use snus to quit smoking and perception of relative risk between snus and cigarettes. Nicotine and Tobacco Research. 2012;14:1221–1228. doi: 10.1093/ntr/nts077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund KE, Scheffels J, McNeill A. The association between use of snus and quit rates for smoking: results from seven Norwegian cross-sectional studies. Addiction. 2011;106:162–167. doi: 10.1111/j.1360-0443.2010.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund M, Lund KE, Halkjelsvik T. Contrasting smokers' and snus users' perceptions of personal tobacco behavior in Norway. Nicotine and Tobacco Research. 2014;16:1577–1585. doi: 10.1093/ntr/ntu109. [DOI] [PubMed] [Google Scholar]
- 19.Lundqvist G, Sandström H, Öhman A, et al. Patterns of tobacco use: A 10-year follow-up study of smoking and snus habits in a middle-aged Swedish population. Scandinavian Journal of Public Health. 2009;37:161–167. doi: 10.1177/1403494808096169. [DOI] [PubMed] [Google Scholar]
- 20.Ramström LM, Foulds J. Role of snus in initiation and cessation of tobacco smoking in Sweden. Tobacco Control. 2006;15:210–214. doi: 10.1136/tc.2005.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonnesen P, Mikkelsen K, Bremann L. Smoking cessation with smokeless tobacco and group therapy: An open, randomized, controlled trial. Nicotine & Tobacco Research. 2008;10:1365–1372. doi: 10.1080/14622200802238969. [DOI] [PubMed] [Google Scholar]
- 22.Joksić G, Spasojević-Tišma V, Antić R, et al. Randomized, placebo-controlled, double- blind trial of Swedish snus for smoking reduction and cessation. Harm Reduction Journal. 2011;8:25. doi: 10.1186/1477-7517-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagerstrom K, Rutqvist LE, Hughes JR. Snus as a smoking cessation aid: A randomized placebo-controlled trial. Nicotine & Tobacco Research. 2012;14:306–312. doi: 10.1093/ntr/ntr214. [DOI] [PubMed] [Google Scholar]
- 24.Hatsukami DK, Severson H, Anderson A, et al. Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching. Tobacco Control. doi: 10.1136/tobaccocontrol-2014-052080. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutqvist LE, Fry JS, Lee PN. Systematic review of Swedish snus for smoking cessation based on primary subject data from randmised clinical trials. Journal of Smoking Cessation. 2013;8:33–44. [Google Scholar]
- 26.Wewers ME, Stillman FA, Hartman AM, et al. Distribution of daily smokers by stage of change: Current Population Survey results. Preventive Medicine. 2003;36:710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhu SH, Lee M, Zhuang YL, et al. Interventions to increase smoking cessation at the population level: how much progress has been made in the last two decades? Tobacco Control. 2012;21:110–118. doi: 10.1136/tobaccocontrol-2011-050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 29.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. American Psychologist. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 30.Chaloupka FJ, Cummings KM, Morley CP, et al. Tax, price and cigarette smoking: Evidence from the tobacco documents and implications for tobacco company marketing strategies. Tobacco Control. 2002;11:i62–i72. doi: 10.1136/tc.11.suppl_1.i62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franz GA. Price effects on the smoking behaviour of adult age groups. Public Health. 2008;122(12):1343–1348. doi: 10.1016/j.puhe.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Kotlyar M, Mendoza-Baumgart MI, Li Z, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tobacco Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldwell B, Burgess C, Crane J. Randomized crossover trial of the acceptability of snus, nicotine gum, and Zonnic therapy for smoking reduction in heavy smokers. Nicotine & Tobacco Research. 2010;12:179–183. doi: 10.1093/ntr/ntp189. [DOI] [PubMed] [Google Scholar]
- 34.Stepanov I, Biener L, Knezevich A, et al. Monitoring tobacco-specific n-nitrosamines and nicotine in novel marlboro and camel smokeless tobacco products: findings from round 1 of the New Product Watch. Nicotine & Tobacco Research. 2012;14:274–281. doi: 10.1093/ntr/ntr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.USDHS. Cigarette smoking—United States, 1965–2008. MMWR. 2011;60:109–113. [PubMed] [Google Scholar]
- 36.Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: Floating prolonged abstinence. Nicotine & Tobacco Research. 2009;11:475–480. doi: 10.1093/ntr/ntp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter MJ, Hughes JR, Gray KM, et al. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: A randomized clinical trial. Archives of Internal Medicine. 2011;171:1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burris JL, Heckman BW, Mathew AR, et al. A mechanistic test of nicotine replacement therapy sampling for smoking cessation induction. Psychology of Addictive Behaviors. 2015;29:392–399. doi: 10.1037/adb0000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter MJ, Hughes JR, Solomon LJ, et al. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 40.Kotlyar M, Hertsgaard LA, Lindgren BR, et al. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: A feasibility study. Cancer Epidemiology, Biomarkers and Prevention. 2011;20:91–100. doi: 10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jardin BF, Cropsey KL, Wahlquist AE, et al. Evaluating the effect of access to free medication to quit smoking: A clinical trial testing the role of motivation. Nicotine & Tobacco Research. 2014;16:992–999. doi: 10.1093/ntr/ntu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes J, Keely J, Niaura R, et al. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5(1):13–26. [PubMed] [Google Scholar]