Abstract
Behavioral genetic studies have provided insights into why early substance use initiation is associated with increased risk for disorder. Few genetically-informative studies, however, have operationalized initiation as the timing of first use and simultaneously modeled the timing of initiation and problematic use of multiple substances. Such research can help capture the risk associated with early initiation and determine the extent to which genetic and environmental risk generalizes across substances. This study utilized a behavior genetic approach to examine the relation between the age of substance use initiation and symptoms of substance use disorder. Participants were 7,285 monozygotic and dizygotic twins (40% male, mean age at interview=30.6) from the Australian Twin Registry who reported on their ages of tobacco, alcohol, and cannabis initiation and symptoms of DSM-IV nicotine dependence, alcohol use disorder, and cannabis use disorder. Biometric modeling was conducted to (a) determine the structure of genetic and environmental influences on initiation and disorder and (b) examine their genetic and environmental overlap. The latent structure of initiation differed across men and women. The familial covariance between initiation and disorder was genetic among men and genetic and environmental among women, suggesting that the relation between first substance use and disorder is partly explained by a shared liability. After accounting for familial overlap, significant unique environmental correlations were observed, indicating that the age of initiation of multiple drugs may directly increase risk for substance-related problems. Results support the utility of conceptualizing initiation in terms of age and of adopting a multivariate approach.
Keywords: Age of initiation, substance use disorder, twins, sex differences
Early first use of substances including tobacco (e.g., Hu, Davies, & Kandel, 2006), alcohol (e.g., Grant & Dawson, 1997), and cannabis (e.g., Lynskey et al., 2003) is robustly associated with increased risk for substance use disorder. For instance, results from nationally representative surveys indicate that individuals who start drinking before age 15 are approximately 1.4 times more likely to develop alcohol dependence (AD) than those who start later (Dawson, Goldstein, Chou, Ruan, & Grant, 2008). Debate exists, however, as to whether early initiation is a direct risk factor for disorder or rather a marker for liability to problems. Results from genetically informed studies have varied by investigation and substance. It has been reported that much of the relation between early alcohol initiation and AD (Prescott & Kendler, 1999; Sartor et al., 2009) and early cannabis use and nicotine dependence (Agrawal et al., 2008) is accounted for by common genetic factors. Others have found that these factors do not fully explain the relation between early cannabis use and later illicit drug use (Lynskey, Vink, & Boomsma, 2006) and drug use disorders (Lynskey et al., 2003), providing evidence that early substance use uptake directly increases risk for problems. Further, although substance use initiation and problems are influenced by distinct genetic and environmental influences, much of the variation in initiation and progression may also be attributable to a shared liability (Fowler et al., 2007; Maes et al., 2004).
Behavior genetic studies have largely conceptualized substance use initiation as a dichotomous measure of lifetime use, or “ever use.” These investigations have found that the family environment explains a notable proportion of the variance in initiation, with genetic factors increasing and family environmental factors decreasing in influence as individuals progress toward problems (Dick, Prescott, & McGue, 2009; Rhee et al., 2003). Far fewer genetically informative studies, however, have conceptualized substance use initiation as the timing of first use or examined the genetic and environmental overlap between age at first use and later problems. Such studies are necessary to capture the unique risk associated with early initiation. They can also allow for rigorous tests of a potentially causal association between early initiation and disorder (Turkheimer & Harden, 2014). Initiation and problems may be explained by a common inherited liability, in which case their association will be mediated by genetic factors. Similarly, their relation may be explained by aspects of the family environment that relate to risk for both outcomes. If, however, early substance use uptake exerts some direct influence on risk for disorder, one would expect to observe the relation after controlling for common heritable and environmental influences. Thus, significant overlap in the unique environmental influences on initiation and disorder is suggestive of a “quasi-causal” relation (Turkheimer & Harden, 2014). The few behavioral genetic studies to examine the timing of substance use uptake have detected notable genetic overlap between initiation and problems, providing evidence that their relation is at least partly explained by a shared liability. Prescott and Kendler (1999) found that nearly all of the correlation between age at first drink and AD was explained by genetic sources, and Sartor and colleagues (2009) obtained a genetic correlation of 0.59 between the timing of first alcohol use and AD. We are aware of no studies that have quantified the genetic and environmental overlap between the timing of first tobacco use and nicotine dependence or the timing of first cannabis use and cannabis dependence.
Previous genetically informative studies (e.g., Grant et al., 2015; Han, McGue, & Iacono, 1999; Kendler, Myers, & Prescott, 2007; Kendler, Schmitt, Aggen, & Prescott, 2008; Palmer et al., 2012; Xian et al., 2008) have modeled cigarette, alcohol, and cannabis use in combination; however, they have only examined measures of consumption, lifetime use, and disorder. Examining the timing of initiation of multiple drugs can help elucidate important relations between substances. For instance, a prior analysis by our group found that the order in which individuals initiated use of tobacco, alcohol, and cannabis influenced the degree to which their timing of first use impacted later uptake (Richmond-Rakerd et al., 2015). To our knowledge, however, only one study has evaluated the overlap between genetic and environmental influences on the ages of initiation of multiple drugs. Employing a sample of African-American women, Sartor and colleagues (2009) found that genetic correlations across substances ranged from 0.25–0.70. We are aware of no replication studies and no multivariate behavior genetic studies that have assessed the timing of initiation and problematic use of multiple substances simultaneously. Doing so will help clarify substance-specific and common liability factors for the age of initiation and its relation to disorder. Integrating findings across multiple substances of abuse – rather than examining individual substances in isolation – can help identify shared pathways of risk for substance use uptake and progression toward problems. This may inform more parsimonious theoretical models of substance involvement, and also assist in the direction of intervention efforts so as to have the most widespread effects on polysubstance use.
The current study used data from a large twin sample to conduct a multivariate behavior genetic analysis of the age of substance use initiation and symptoms of substance use disorder. We had three primary aims. First, we aimed to establish the multivariate latent structure of the timing of substance use initiation. Specifically, we explored whether genetic and environmental influences common to the ages of tobacco, alcohol, and cannabis initiation were mediated through a single factor. If so, this would suggest common mechanisms of risk for early initiation across substances. Second, we aimed to determine the structure of common genetic and environmental risk factors for substance use disorder. Measures included symptoms of nicotine dependence, alcohol use disorder, and cannabis use disorder. Finally, we modeled initiation and disorder simultaneously to examine the degree of overlap in their genetic and environmental influences. Significant overlap in the genetic and/or shared environmental contributions to initiation and disorder would provide evidence that a shared familial liability explains their association. A significant relation between their unique environmental influences would provide evidence that the propensity to initiate substance use at a younger age may exert some direct influence on the general risk for disorder.
Method
Participants
Participants were 7,285 monozygotic (MZ) and same-sex dizygotic (DZ) twins from the Australian Twin Registry Cohort II and Cohort III. These individuals were drawn from the larger sample of 7,334 MZ and same-sex DZ twins, and consisted of individuals who (a) reported having ever used tobacco, alcohol, and/or cannabis in their lifetime, or (b) were lifetime abstainers, but had a co-twin who reported having ever used tobacco, alcohol, and/or cannabis (for more information about participants, see Knopik et al., 2004; Hines et al., 2015; and Lynskey et al., 2012). Table 1 displays the sample characteristics.1
Table 1.
Cohort Characteristics
| Cohort II
|
Cohort III
|
Full Sample
|
|
|---|---|---|---|
| Males | 2051 | 890 | 2941 |
| Females | 2608 | 1736 | 4344 |
| Total N | 4659 | 2626 | 7285 |
| Complete Pairs | 2086 | 1036 | 3122 |
| N twins from Incomplete Pairs | 487 | 554 | 1041 |
| Monozygotic Male Twins | 1089 | 507 | 1596 |
| Monozygotic Female Twins | 1465 | 984 | 2449 |
| Dizygotic Male Twins | 962 | 383 | 1345 |
| Dizygotic Female Twins | 1143 | 752 | 1895 |
| Years of birth | 1964–1971a | 1972–1979b | 1964–1979 |
| Years surveyed | 1996–2000 | 2005–2009 | 1996–2009 |
| Mean Age at Interview (Range) | 29.9 (24–36) | 31.8 (27–40) | 30.6 (24–40) |
Notes. This sample of 7,285 individuals was drawn from the larger sample of 7,334 monozygotic and same-sex dizygotic twins, and consisted of individuals who (a) reported having ever used tobacco, alcohol, and/or cannabis in their lifetime, or (b) were lifetime abstainers, but had a co-twin who reported having ever used tobacco, alcohol, and/or cannabis
In Cohort II, four respondents were born before 1964 and four were born after 1971.
In Cohort III, one respondent was born before 1972 and two were born after 1979.
Procedure
Both cohorts completed interviews based on the Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al., 1994). The Cohort II assessment was administered by telephone and the Cohort III assessment was administered using a computer-assisted telephone interview. Retest data were collected 3.7 years (standard deviation (SD)=0.4, range=1.1–4.3) after the interview for a subsample of twins (n=216) from Cohort II. Interviews were conducted by trained lay-interviewers who were blind to the status of the co-twin. Informed consent was obtained from participants and the study was approved by the Institutional Review Boards at Washington University School of Medicine, the University of Missouri, and the Queensland Institute of Medical Research.
Measures
Age of tobacco initiation
Lifetime abstainers from cigarette smoking constituted 11.9% of the sample. In Cohort II, non-abstainers were asked, “How old were you when you tried your first cigarette?” Cohort III was queried, “How old were you the first time you smoked even a puff of a cigarette?” Responses for tobacco initiation ranged from 2 to 33 years. To reduce the influence of low values, individuals who reported ages below 5 years were equated to 5 years. The variable reasonably approximated a univariate normal distribution (skewness=0.22, kurtosis=1.35). Reported ages of smoking initiation were 14.0 years in Cohort II and 14.3 years in Cohort III. In the current combined sample, mean ages of smoking initiation for men and women were 13.7 years (SD=3.4) and 14.4 years (SD=3.4), respectively. Retest data demonstrated good reliability in respondent recall (r(148)=.72, p<.0001).2 Potential age-related bias was examined by correlating individuals' ages with their reported ages of cigarette initiation. The correlation of −.01 suggested minimal bias. Associations between the age of cigarette initiation and birth year (r=.06) and year of assessment (r=.05) were low.
Age of alcohol initiation
Lifetime abstainers from alcohol were 0.8% of the sample. In Cohort II, non-abstainers were asked, “How old were you the first time you had more than just a sip of beer, wine or spirits?” Cohort III was asked, “How old were you the first time you had a full drink of beer, wine or spirits?” Responses ranged from 1 to 35 years. To reduce the influence of low values, individuals reporting ages below 5 years were equated to 5 years. The variable was minimally skewed and moderately kurtotic (skewness=−0.34, kurtosis=5.00). Transformation of the variable was ineffective in reducing kurtosis; therefore, the raw variable was used and an estimator robust to deviations from normality was employed in all analyses (see Statistical Analysis). Reported ages of drinking initiation were 15.9 years in Cohort II and 15.9 years in Cohort III. In the combined sample, mean ages of drinking initiation for men and women were 15.4 years (SD=2.5) and 16.2 years (SD=2.5), respectively. These data demonstrated excellent retest reliability in recall (r(161)=.82, p<.0001) and age-related bias was minimal (r=.03). The age of alcohol initiation was minimally associated with year of birth (r=−.01) and year of assessment (r=.01).
Age of cannabis initiation
37.5% of the sample reported having never tried cannabis. Non-abstainers in both cohorts were queried, “How old were you the first time you used cannabis?” Responses ranged from 6 to 34 years. The variable reasonably approximated a normal distribution (skewness=0.99, kurtosis=1.25). Reported ages of cannabis initiation were 18.9 years in Cohort II and 17.9 years in Cohort III. Mean ages of first cannabis use in Cohort II were 18.7 years (SD=3.2) for men and 19.2 years (SD=3.5) for women, and in Cohort III were 17.6 years (SD=3.2) for men and 18.1 years (SD=3.5) for women.3 Retest data indicated good reliability in recall (r(117)=.80, p<.0001). The correlation between age and reported age at first cannabis use (r=.08) was slightly higher than for the ages of tobacco and alcohol initiation, but still small, suggesting limited age-related bias. The age of cannabis initiation was negatively associated with birth year (r=−.19) and assessment year (r=−.13).
Nicotine dependence (ND)
Nicotine dependence was defined conditionally, such that (a) lifetime DSM-IV ND items were combined into a seven-item symptom count for respondents who endorsed having ever used cigarettes, and (b) abstainers were coded as missing. Following Lessov and colleagues (2004), tolerance to nicotine was operationalized as having ever smoked 20 or more cigarettes in one day. The normality parameters for the symptom count were within acceptable limits (skewness=0.62, kurtosis=−1.01). The number of ND symptoms ranged from 0 to 7 in men (M=1.85, SD=1.97) and women (M=1.65, SD=1.96) and the average number of symptoms in the sample was 1.74 (SD=1.97). The lifetime prevalence of ND among men, women, and the sample was 35.2%, 30.3%, and 32.3%, respectively. The symptom count demonstrated good internal consistency reliability (Cronbach’s α=0.81) and good test-retest reliability (r(147)=.84, p<.0001). It was correlated modestly with birth year (r=−.06) and assessment year (r=−.06).
Alcohol use disorder (AUD)
Alcohol use disorder was defined conditionally, such that (a) lifetime DSM-IV alcohol abuse and dependence items were combined into an 11-item symptom count for respondents who endorsed having ever used alcohol, and (b) abstainers were coded as missing. The variable was skewed and kurtotic and was log-transformed to approximate normality (after transformation: skewness=0.56, kurtosis=−0.77). The number of symptoms ranged from 0 to 10 in men (M=1.99, SD=2.15) and women (M=1.07, SD=1.55) and the average number of symptoms in the sample was 1.44 (SD=1.87). Lifetime prevalence of alcohol abuse among men, women, and the sample was 40.0%, 18.6%, and 27.2%, respectively. Lifetime prevalence of alcohol dependence among men, women, and the sample was 16.2%, 7.5%, and 11.0%, respectively. The symptom count demonstrated acceptable internal consistency (Cronbach’s α=0.77) and the test-retest reliability (r(161)=.64) was lower than for the ND and CUD symptom counts. The correlations with birth year (r=.09) and assessment year (r=.07) were low.
Cannabis use disorder (CUD)
The Cohort III interview included a complete assessment of lifetime DSM-IV cannabis abuse and dependence. The Cohort II interview included an abbreviated assessment containing two abuse criteria (use in physically hazardous situations, use interfering with role obligations) and four dependence criteria (using more frequently or for longer periods than intended, tolerance, continued use despite use causing emotional problems, recurrent desire to cut down on use). This abbreviated assessment has been previously utilized (see Lynskey et al., 2006). To maintain consistency across cohorts, a six-item symptom count using the restricted CUD criteria was created. Cannabis use disorder was defined conditionally, such that (a) the symptom count was constructed for respondents who endorsed having ever used cannabis, and (b) abstainers were coded as missing. The variable was skewed and kurtotic and was log-transformed (after transformation: skewness=1.23, kurtosis=−0.04). The number of symptoms ranged from 0 to 6 in men (M=1.09, SD=1.71) and women (M=0.70, SD=1.43) and the average number of symptoms in the sample was 0.88 (SD=1.58). The prevalence of individuals endorsing one or more symptoms was 38.1%, 25.5%, and 31.2% for men, women, and the sample, respectively. The symptom count exhibited good internal consistency (Cronbach’s α=0.84) and good test-retest reliability (r(118)=.82, p<.0001). It was minimally associated with birth year (r=−.02) and assessment year (r=−.05).
Measurement invariance
We tested for measurement invariance of the symptom counts across sex by fitting multiple group confirmatory factor analysis models within cohort. Results indicated that the loadings and thresholds could not be equated across sex for any of the symptom counts in Cohort II or Cohort III (Cohort II: p=.004-p<.0001, Cohort III: p=.002-p<.0001). Follow-up tests for evidence of differential item functioning (DIF) were conducted using MIMIC models (Jöreskog & Goldberger, 1975) and items within all three symptom counts showed evidence of DIF. Complete results of these tests are available in Tables S1–S3 in supplemental materials. Results of tests for DIF were used to inform the multivariate model-fitting (see Results).
Censoring
It is possible that respondents in the present study (particularly those in Cohort II) may not have passed through the age period of risk for initiation of substance use. This is of minimal concern for alcohol, as 99.2% of the sample had used alcohol in their lifetime. However, this remains a possibility for tobacco and cannabis use. We therefore explored the potential impact of censoring. With regard to cannabis, in Cohort II, the cumulative initiation probability by age 24 (.51) was 84% of the initiation probability by age 36 (.61). In Cohort III, the initiation probability by age 24 (.61) was 90% of the initiation probability by age 36 (.68). For tobacco, in Cohort II, the cumulative initiation probability by age 24 (.87) was 98% of the initiation probability by age 36 (.89). In Cohort III, the initiation probability by age 24 (.85) was 98% of the initiation probability by age 36 (.87). The percentage of cannabis abstainers in both cohorts differed by only 8.2% (Cohort II: abstention rate=40.4%, Cohort III: rate=32.2%), and the percentage of tobacco abstainers differed by only 1.1% (Cohort II: abstention rate=11.5% Cohort III: rate=12.6%). Further, the median age of first tobacco use was 14 among both men and women, and the median age of first cannabis use was 18 among both men and women. By contrast, the median age at interview among abstainers from tobacco was 30 for men and 31 for women, and the median age among abstainers from cannabis was 30 for men and 31 for women. Taken together, these data suggest that most abstainers had passed through their period of risk and are likely to remain abstainers.
Statistical Analysis
Initiation versus experimentation
We investigated the percentage of individuals who progressed beyond first substance use. Because of slight differences in the assessment of substance use across cohorts, we only evaluated experimentation in Cohort II. In Cohort II, 69.5% of lifetime tobacco users reported having smoked cigarettes more than “one or two times ‘just to try’”; 93.1% of lifetime alcohol users reported having become regular drinkers (drinking at least once a month for six months or more) and/or having gotten drunk after their first experience with alcohol; and 79.4% of lifetime cannabis users reported having used cannabis three or more times. Therefore, it appears that for the majority of participants, age at first use was not one-time experimentation.
Adjustment for cohort
Although participants in Cohorts II and III were interviewed at similar ages, assessments were conducted approximately 10 years apart (Table 1). This might lead to differences in substance use across groups that reflect a changing social milieu. We therefore controlled for cohort membership by regressing participant birth year out of all variables prior to analysis.
Phenotypic analyses
Descriptive analyses, including measures of association between the age of substance use initiation and symptoms of substance use disorder, were calculated using survey analysis procedures in SAS version 9.4 (SAS Institute, Cary, NC). Data were treated as clustered in all analyses, with the family number for each twin pair specified as the clustering variable.
Twin modeling
Although the distributions of the variables reasonably approximated normality, some variables remained mildly-moderately skewed and/or kurtotic following transformation. Therefore, biometric models were fitted by method of maximum likelihood with robust standard errors (MLR) to the raw twin data using Mplus version 7 (Muthén & Muthén, 1998–2012). Univariate models were constructed to partition the variation in the ages of tobacco, alcohol, and cannabis initiation – each considered continuously – into additive genetic (A), shared environmental (C), and unique environmental (E) influences, and test for quantitative sex differences. Evidence for sex differences was tested by comparing the fits of models that allowed parameter estimates for men and women to vary with the fits of models that equated estimates. Because the MLR estimator was employed, nested models were compared using the Satorra-Bentler scaled chi-square difference test.
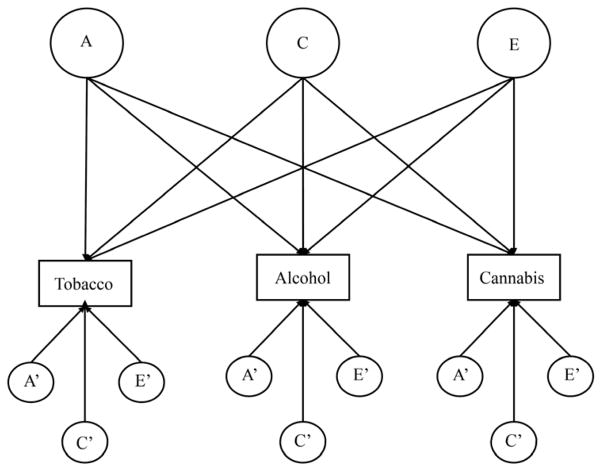
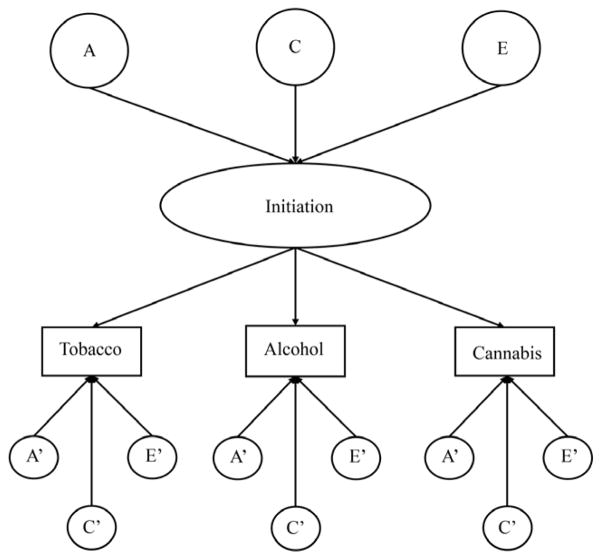
To further investigate the nature of the relations between the ages of tobacco, alcohol, and cannabis initiation, two types of multivariate models were fit to the data: an independent pathway model (IPM; Figure 1) and a common pathway model (CPM; Figure 2). The IPM specifies common genetic, common shared environmental, and common unique environmental factors that load onto the ages of tobacco, alcohol, and cannabis initiation, as well as genetic and environmental factors specific to each substance. The CPM, by contrast, specifies that all common genetic and environmental factors are mediated through a latent phenotype (indicated here as “initiation”). In the IPM, the common genetic and environmental factors influencing an outcome are allowed to have different variances and different loadings. In the CPM, these factors have different variances, but their loadings are the same. The CPM has fewer parameters than the IPM and is considered more parsimonious than the IPM, if it fits as well to the data. Because the CPM is nested within the IPM, the fits of the two models can be formally compared. Two indices were used to determine the best-fitting multivariate model: the Satorra-Bentler scaled chi-square difference test and the Akaike information criterion (AIC). The AIC represents a balance between model fit and parsimony, with lower values of AIC indicating the more suitable model.
Figure 1.
Independent pathway model for the ages of tobacco, alcohol, and cannabis initiation. For ease of presentation, this path diagram represents only one twin in a pair. Unprimed factors are common to all three substances and primed factors are specific to each substance. A=additive genetic, C=shared environment, E=unique environment.
Figure 2.
Common pathway model for the ages of tobacco, alcohol, and cannabis initiation. For ease of presentation, this path diagram represents only one twin in a pair. Unprimed factors are common to all three substances and primed factors are specific to each substance. A=additive genetic, C=shared environment, E=unique environment.
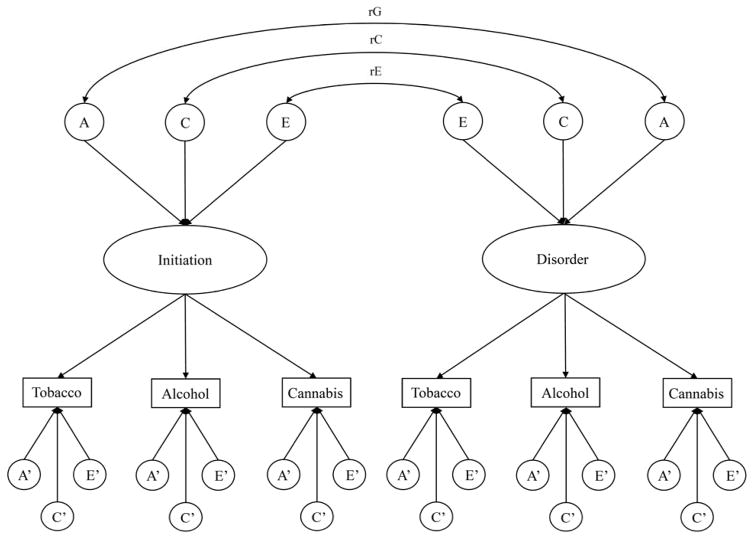
After fitting univariate and multivariate biometric models to the data for the age of first substance use, the same univariate and multivariate modeling procedures were applied to the ND, AUD, and CUD symptom counts. Subsequently, we fit a bivariate factor model (Figure 3). In this model, the first factor loads on the substance use initiation measures, and the second loads on the disorder measures. Consistent with the CPM, sources of genetic and environmental influence are modeled at the factor and residual levels. The relation between the two factors is modeled in terms of correlations in underlying additive genetic (rG), shared environmental (rC), and unique environmental (rE) influences. These correlations indicate the degree to which the genetic, shared environmental, and unique environmental influences on the age of substance use initiation are the same as the genetic, shared environmental, and unique environmental influences on substance use disorder.
Figure 3.
Bivariate factor model for the ages of tobacco, alcohol, and cannabis initiation and nicotine dependence, alcohol use disorder, and cannabis use disorder. For ease of presentation, this path diagram represents only one twin in a pair. Unprimed factors are common to all three substances and primed factors are specific to each substance. A=additive genetic, C=shared environment, E=unique environment. rG=genetic correlation, rC=shared environmental correlation, rE=unique environmental correlation.
Inclusion of abstainers
We were primarily interested in examining the association between first substance use and problematic use, which is contingent on having used a substance. Thus, respondents who had never used a substance were coded as missing for both their age of initiation and the symptom count for that substance. Despite this, all abstainers contributed to the multivariate analyses. Multivariate models included all twin pairs where at least one twin reported having used at least one substance, as well as singletons who had used at least one substance. Maximum likelihood procedures were employed to account for missing data.
Results
Descriptive Analyses
4,385 individuals (60.2% of the sample) reported having used all three substances. Of these individuals, the most common sequence of initiation was tobacco prior to alcohol prior to cannabis (46.6%), and 2.7% of individuals reported first using all three substances at the same age. The ages of initiation of all three substances were modestly correlated (men: rs=0.25–0.34, women: rs=0.25–0.29), as were the symptom counts (men: rs=0.32–0.33, women: rs=0.28–0.35). Modest relations were also observed between the ages of first use and symptom counts (men: rs=−0.10 – −0.31, women: rs=−0.12 – −0.28).
Twin Correlations
Inspection of Table 2 reveals the following: (1) The within-trait MZ twin correlations were larger than the DZ twin correlations (shaded cells), indicating genetic influences on all phenotypes studied; (2) the within-trait DZ correlations were closer in magnitude to the MZ correlations for initiation than disorder, providing greater evidence for shared environmental contributions to initiation; (3) most of the cross-trait MZ correlations were larger than the DZ correlations, implicating genetic factors in the covariation between the age of substance use initiation and substance use disorder; and (4) the pattern of MZ and DZ correlations differed somewhat across males and females, providing some evidence for quantitative sex differences. These observations were rigorously tested in the biometric model-fitting.
Table 2.
Within-Trait and Cross-Trait Twin Correlations Between the Age of Substance Use Initiation and Symptoms of Substance Use Disorder
| Twin 1 phenotype | Twin 2 phenotype
|
Twin 2 phenotype
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monozygotic men (665 pairs)
|
Monozygotic women (1,099 pairs)
|
|||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| 1. Age of Tobacco Initiation | 0.40 | 0.54 | ||||||||||
| 2. Age of Alcohol Initiation | 0.20 | 0.45 | 0.19 | 0.44 | ||||||||
| 3. Age of Cannabis Initiation | 0.17 | 0.22 | 0.56 | 0.23 | 0.27 | 0.52 | ||||||
| 4. Nicotine Dependence | 0.12 | 0.15 | 0.24 | 0.61 | 0.17 | 0.14 | 0.26 | 0.58 | ||||
| 5. Alcohol Use Disorder | 0.16 | 0.20 | 0.18 | 0.29 | 0.49 | 0.11 | 0.22 | 0.15 | 0.26 | 0.41 | ||
| 6. Cannabis Use Disorder | 0.09 | 0.19 | 0.27 | 0.31 | 0.28 | 0.53 | 0.18 | 0.19 | 0.29 | 0.26 | 0.19 | 0.43 |
| Twin 1 phenotype | Twin 2 phenotype
|
Twin 2 phenotype
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dizygotic men (532 pairs)
|
Dizygotic women (826 pairs)
|
|||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| 1. Age of Tobacco Initiation | 0.29 | 0.37 | ||||||||||
| 2. Age of Alcohol Initiation | 0.18 | 0.30 | 0.14 | 0.33 | ||||||||
| 3. Age of Cannabis Initiation | 0.14 | 0.11 | 0.43 | 0.25 | 0.20 | 0.42 | ||||||
| 4. Nicotine Dependence | 0.01 | 0.05 | 0.18 | 0.31 | 0.15 | 0.10 | 0.18 | 0.31 | ||||
| 5. Alcohol Use Disorder | 0.02 | 0.06 | 0.09 | 0.14 | 0.30 | 0.11 | 0.11 | 0.12 | 0.17 | 0.17 | ||
| 6. Cannabis Use Disorder | 0.01 | 0.14 | 0.27 | 0.19 | 0.13 | 0.33 | 0.12 | 0.12 | 0.19 | 0.18 | 0.11 | 0.29 |
Notes. Also included in the analyses were data from 1,041 single twins: 266 monozygotic men, 251 monozygotic women, 281 dizygotic men, and 243 dizygotic women. The positive correlations between age of initiation and disorder are due to reverse scoring the age of initiation variables.
Biometric Model Fitting: Age of Substance Use Initiation
Univariate models
Univariate model-fitting results are presented in Table S4 in supplemental materials and standardized full model estimates of A, C, and E for the full sample, men, and women are presented in Table 3. Inspection reveals that: (1) shared environmental influences explained a significant proportion of the variation in all substances; (2) genetic influences on the age of tobacco initiation were significant among women (.32 [.12, .53]), but not men (.19 [−.09, .47]); (3) genetic influences on the age of alcohol initiation were significant among men (.29 [.01, .58]), but not women (.21 [−.04, .45]); and (4) genetic influences on the age of cannabis initiation were not significant in men (.22 [−.07, .51]) or women (.18 [−.09, .45]).
Table 3.
Univariate Model Results for the Age of Substance Use Initiation and Substance Use Disorder
| Age of Substance Use Initiation
|
Substance Use Disorder
|
|||||
|---|---|---|---|---|---|---|
| Tobacco
|
Alcohol
|
Cannabis
|
Tobacco
|
Alcohol
|
Cannabis
|
|
| Full Sample | ||||||
|
| ||||||
| Proportion of Variation | ||||||
| Additive Genetic | .27 [.10, .44] | .24 [.05, .43] | .19 [−.004, .39] | .57 [.43, .71] | .43 [.30, .57] | .35 [.15, .55] |
| Shared Environment | .21 [.06, .35] | .20 [.04, .36] | .33 [.17, .50] | .02 [−.10, .14] | .02 [−.10, .13] | .11 [−.05, .28] |
| Unique Environment | .52 [.48, .58] | .56 [.51, .62] | .48 [.41, .54] | .41 [.37, .45] | .55 [.51, .59] | .54 [.48, .60] |
|
| ||||||
| Men | ||||||
|
| ||||||
| Proportion of Variation | ||||||
| Additive Genetic | .19 [−.09, .47] | .29 [.01, .58] | .22 [−.07, .51] | .60 [.54, .66] | .39 [.19, .59] | .45 [.18, .72] |
| Shared Environment | .19 [−.04, .43] | .15 [−.09, .39] | .33 [.09, .57] | .00 [.00, .00] | .10 [−.07, .28] | .07 [−.16, .29] |
| Unique Environment | .62 [.53, .70] | .56 [.48, .64] | .45 [.36, .54] | .40 [.34, .46] | .51 [.45, .56] | .48 [.39, .57] |
|
| ||||||
| Women | ||||||
|
| ||||||
| Proportion of Variation | ||||||
| Additive Genetic | .32 [.12, .53] | .21 [−.04, .45] | .18 [−.09, .45] | .54 [.36, .72] | .40 [.35, .46] | .25 [−.04, .53] |
| Shared Environment | .22 [.03, .40] | .22 [.02, .43] | .33 [.11, .55] | .04 [−.12, .20] | .00 [.00, .00] | .15 [−.08, .40] |
| Unique Environment | .46 [.40, .52] | .57 [.49, .64] | .49 [.40, .58] | .42 [.37, .48] | .60 [.55, .66] | .60 [.51, .69] |
Notes. 95% confidence intervals are presented in brackets.
Multivariate models
There was no significant decrement in fit when the CPM was compared to the IPM (Δχ2=9.24, df=8, p=.32). Further, a lower AIC value was observed for the CPM, indicating that it provided a superior balance of fit and parsimony (IPM: AIC=88601.29, CPM: AIC=88592.79). Therefore, it appeared that in these data, the genetic and environmental influences on the ages of tobacco, alcohol, and cannabis initiation were mediated through a single latent factor. Full model estimates for the IPM are presented in Table S5 in supplemental materials.
Within the CPM, we attempted to equate the paths from the latent factor to the ages of tobacco, alcohol, and cannabis initiation across men and women. The paths to alcohol and cannabis could not be equated (alcohol: Δχ2=8.35, df=1, p=.004; cannabis: Δχ2=17.73, df=1, p<.0001). Alcohol was more strongly related to the latent factor among men (standardized loading=.64, 95% CI [.56, .71]) than women (.50 [.43, .56]), and cannabis was more strongly related among women (.62 [.55, .69]) than men (.46 [.40, .52]). Given this information – as well as the evidence for some sex-specific effects in the univariate models – subsequent multivariate model-fitting was conducted separately among males and females. Table 4a displays the model-fitting results. For the men, the best-fitting model (I) was a full model. Although genetic and shared environmental influences could be dropped individually at the common and specific levels, dropping them simultaneously resulted in a significant decrement in fit. For the women, the best-fitting model (IV) contained common sources of shared environmental and unique environmental influence, as well as specific genetic and unique environmental factors. Table S6 in supplemental materials indicates the proportion of variation in the age of substance use initiation due to common and substance-specific sources of genetic and environmental variance within the full common pathway model.
Table 4a.
Common Pathway Model-Fitting Results for the Age of Substance Use Initiation
| Δχ2
|
df
|
p-value
|
AIC
|
|
|---|---|---|---|---|
| Men
| ||||
| I. Full common pathway model | -- | 37 | -- | 37186.58 |
| II. Model I without common C | 3.34 | 38 | .0676 | 37187.92 |
| III. Model I without common A | 3.33 | 38 | .0681 | 37187.91 |
| IV. Model I without common C or A | 55.4 | 39 | <.0001 | 37293.4 |
| V. Model I without specific C | 2.28 | 40 | .5170 | 37187.41 |
| VI. Model I without specific A | 2.47 | 40 | .4809 | 37187.99 |
| VII. Model I without specific C or A | 37.13 | 43 | <.0001 | 37397.37 |
| Women
| ||||
| I. Full common pathway model | -- | 37 | -- | 51406.21 |
| II. Model I without common C | 16.2 | 38 | .0001 | 51420.41 |
| III. Model I without common A | 1.88 | 38 | .1698 | 51406.09 |
| IV. Model III without specific C | 0.50 | 41 | .9735 | 51401.59 |
| V. Model III without specific A | 10.33 | 41 | .0352 | 51431.09 |
Notes. Bold type indicates the best-fitting model. Nested models were compared using the Satorra-Bentler scaled chi-square difference test. A=additive genetic, C=shared environment.
Biometric Model Fitting: Substance Use Disorder
Univariate models
Univariate model-fitting results are presented in Table S7 in supplemental materials and standardized full model estimates of A, C, and E for the full sample, men and women are presented in Table 3. Inspection reveals that: (1) additive genetic factors explained a significant proportion of the variation in all three symptom counts, (2) the pattern of estimates for ND was very similar across men and women, and (3) shared environmental influences were minimal for ND and AUD, but explained a modest (although non-significant) proportion of the variation in CUD (.11 [−.05, .28]).
Multivariate models
There was a significant change in fit when the CPM was compared against the IPM (Δχ2=17.47, df=8, p=.03). However, we proceeded with fitting the CPM because: (1) we aimed to be consistent with the modeling approach taken for the age of substance use initiation, and (2) this was necessary to facilitate model-fitting for the primary goal of this analysis: to examine the extent of overlap in the genetic and environmental influences on initiation and disorder. Furthermore, additional fit indices indicated that the CPM fit the data very well (RMSEA=.01, CFI=.994, TLI=.995), and the AIC value was slightly lower for the CPM than the IPM (CPM: AIC=46211.44, IPM: AIC=46212.21). Therefore, we fit a CPM to the data. Full model estimates for the IPM are presented in Table S8 in supplemental materials.
Subsequent model-fitting was conducted separately by sex for the following reasons: (1) results of univariate model-fitting indicated the possibility of sex-specific effects for AUD and CUD, and (2) there was evidence for a lack of measurement invariance of the symptom counts across men and women (see Measurement invariance).
The best-fitting model structure was the same in men and women: both common and specific family environmental influences could be constrained to zero (see Table 4b). Table S9 in supplemental materials displays the proportion of variation in substance use disorder attributable to common and substance-specific sources of genetic and environmental variance within the full CPM.
Table 4b.
Common Pathway Model-Fitting Results for Substance Use Disorder
| Δχ2
|
df
|
p-value
|
AIC
|
|
|---|---|---|---|---|
| Men
| ||||
| I. Full common pathway model | -- | 37 | -- | 20008.99 |
| II. Model I without common A | 26.79 | 38 | <.0001 | 20042.22 |
| III. Model I without common C | 0.00 | 38 | .9776 | 20006.99 |
| IV. Model III without specific A | 17.35 | 41 | .0006 | 20017.20 |
| V. Model III without specific C | 5.87 | 41 | .1181 | 20005.95 |
| Women
| ||||
| I. Full common pathway model | -- | 37 | -- | 26202.44 |
| II. Model I without common A | 11.71 | 38 | .0006 | 26260.48 |
| III. Model I without common C | 1.97 | 38 | .1609 | 26203.03 |
| IV. Model III without specific A | 75.72 | 41 | <.0001 | 26232.42 |
| V. Model III without specific C | 3.08 | 41 | .3788 | 26198.48 |
Notes. Bold type indicates the best-fitting model. Nested models were compared using the Satorra-Bentler scaled chi-square difference test. A=additive genetic, C=shared environment.
Biometric Model-Fitting: Bivariate Factor Model
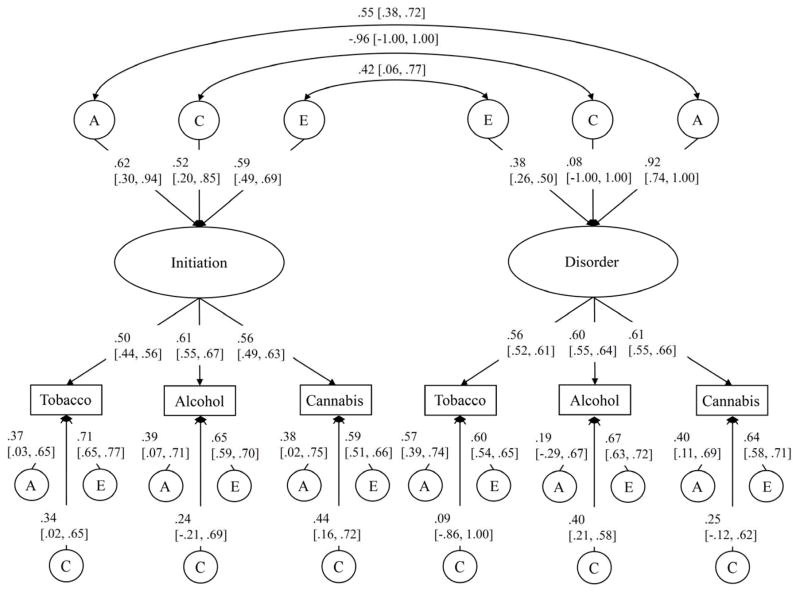
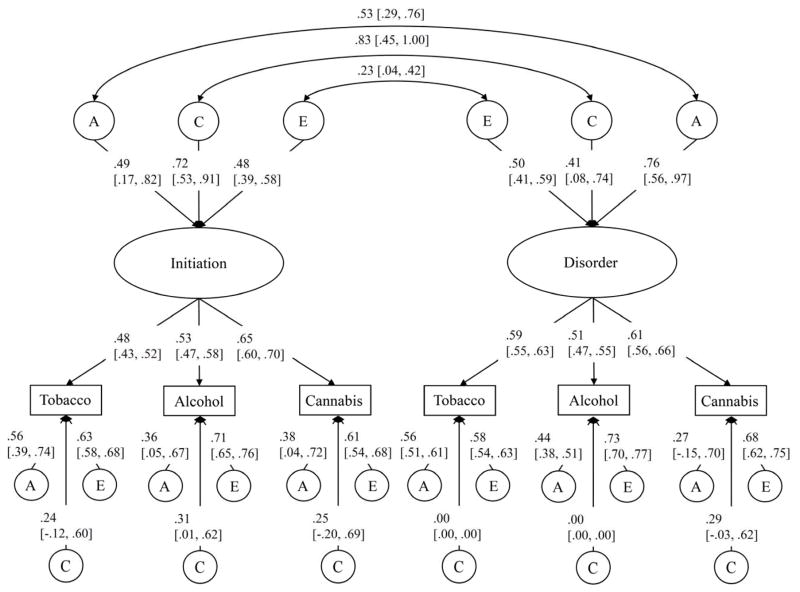
Estimates from the full bivariate factor models for men and women are depicted in Figures 4 and 5, respectively. The genetic correlations between the age of substance use initiation and substance use disorder were significant in both men (rG=.55 [.38, .72]) and women (rG=.53 [.29, .76]). Further, although shared environmental influences explained only a modest proportion (19%) of the variation in disorder in women, these influences were very highly correlated with the shared environmental influences on initiation (rC=.83 [.45, 1.00]). Notably, after accounting for overlap in the familial influences on initiation and disorder, significant unique environmental correlations were observed (men: rE=.42 [.06, .77], women: rE=.23 [.04, .42]). Descriptively, this suggests that a member of an identical twin pair who starts using substances earlier than their co-twin will be more likely to develop a substance use disorder than their co-twin. This is consistent with the hypothesis that early substance use uptake causally influences risk for disorder: if the relation were fully mediated by familial influences, no remaining association would be observed among identical twins after accounting for genetic and family environmental factors.
Figure 4.
Standardized parameter estimates for the bivariate factor model for men. The positive correlations between the factors are due to reverse scoring the age of initiation variables. Note that although the shared environmental correlation looks high, it is not statistically significant. 95% confidence limits presented in brackets. A=additive genetic, C=shared environment, E=unique environment. rG=genetic correlation, rC=shared environmental correlation, rE=unique environmental correlation.
Figure 5.
Standardized parameter estimates for the bivariate factor model for women. The positive correlations between the factors are due to reverse scoring the age of initiation variables. 95% confidence limits presented in brackets. A=additive genetic, C=shared environment, E=unique environment. rG=genetic correlation, rC=shared environmental correlation, rE=unique environmental correlation.
Sensitivity analyses
Our primary aim was to characterize the overlap in genetic and environmental influences on initiation and disorder that were common across substances. Thus, we focused on estimating the common factor correlations. However, it should be noted that failing to correlate the residual ACE estimates might inflate the covariances between the common factors. In particular, failing to account for correlated measurement error may bias genetic and shared environmental effects (Lessov et al., 2004). When we estimated these covariances, we observed attenuation of the unique environmental correlations in men and women. The genetic and shared environmental correlations remained largely unchanged. (The shared environmental correlation among men became positive; however, the correlation obtained prior to accounting for residual variation was highly unreliable (rC=−.96 [−1.00, 1.00])). The residual unique environmental estimates include environmental variation and error that is (a) specific to each substance and (b) common to pairs of substances, but not all three. Accounting for this variation appeared to explain part – but not all – of the potentially direct association between the timing of tobacco, alcohol, and cannabis initiation and later abuse/dependence on these substances.
Discussion
This study employed a sample of twins who reported on their ages of tobacco, alcohol, and cannabis initiation, as well as symptoms of DSM-IV nicotine dependence, alcohol use disorder, and cannabis use disorder. Using behavior genetic modeling, we aimed to (a) establish the multivariate structure of genetic and environmental influences on early substance use initiation, (b) determine the multivariate structure of genetic and environmental risk for substance use disorder, and (c) evaluate the degree of overlap between the genetic and environmental contributions to initiation and disorder. Analyses identified significant shared environmental contributions to the timing of first substance use. There was strong support for a common pathway structure for the age of substance use initiation, and the multivariate structure of initiation differed across men and women. Significant overlap was found between the genetic influences on initiation and disorder, indicating that these processes partially stem from a common inherited liability. After accounting for genetic and shared environmental overlap, significant unique environmental correlations were observed, suggesting that early substance use uptake may exert some causal influence on risk for disorder.
Shared Family Environment
Shared environmental influences explained a significant proportion of the variation in the ages of first use of all three substances. By contrast, these influences could be dropped from all models of disorder. This replicates prior research indicating that shared environmental factors explain a notable proportion of the variance in early-initiation phenotypes, and decrease in influence while genetic influences increase as individuals progress toward problems (Dick et al., 2009; Rhee et al., 2003). Recent research, however, demonstrates that this trend may occur to varying degrees across drugs. In particular, the shared family environment explains a significant proportion of the variation in heavy and problematic use of cannabis. A meta-analysis of studies of problematic cannabis use (Verweij et al., 2010) obtained weighted average estimates of the influence of the shared environment of .20 in males and .15 in females. (Notably, we also obtained a family environmental estimate of .15 for CUD in women). Why might family environmental factors contribute more strongly to cannabis-related problems than other substance use disorders? This may be partly due to availability and access, which has been shown to moderate the family environmental influences on substance involvement. For instance, the heritability estimates for alcohol consumption are increased and the shared environmental estimates are decreased in urban compared with rural areas, which may be attributable to the higher number of alcohol outlets in these regions (Rose, Dick, Viken, & Kaprio, 2001). With regard to tobacco consumption, it has been shown that the genetic influences on smoking declined significantly following legislation prohibiting smoking in public places (Boardman, Blalock, & Pampel, 2010). Gillespie and colleagues (2009) demonstrated that nearly all of the shared environmental influence on cannabis abuse was explained by perceived availability of cannabis, and that the influence of availability on abuse was mediated through cannabis initiation. Given that cannabis is an illicit drug, shared environmental estimates may be increased because individuals have less access to cannabis than licit substances such as tobacco or alcohol.
Recent research (e.g., Baker, Maes, & Kendler, 2012; Samek, Keyes, Iacono, & McGue, 2013) has started to investigate specific variables, such as parental attitudes toward substance use and peer deviance, that may account for the shared environmental influence on early substance involvement. To date, the focus has primarily been general adolescent substance involvement or adolescent use of multiple drugs (e.g., Walden, McGue, Iacono, Burt, & Elkins, 2004). Notable exceptions are studies that have found parental separation (Waldron et al., 2014a; Waldron et al., 2014b) and childhood sexual abuse (Nelson et al., 2006; Sartor et al., 2013) predict early substance use. Research examining additional measured shared environmental contributions to the timing of initiation of multiple drugs will be necessary to (a) determine the most important proximal influences on substance use uptake and (b) clarify to what degree these factors exert common and specific influence across substances.
Multivariate Structure of Initiation and Disorder
The current study is the first to investigate the multivariate latent structure of the timing of substance use initiation. A large degree of genetic and environmental variation was substance-specific, which is to be expected given the modest correlations among the age of initiation measures (men: rs=0.25–0.34, women: rs=0.25–0.29). The variation common to all three substances, however, was mediated through a single phenotype. With regard to substance use disorder, there was a significant decrement in fit when the common pathway model was compared to the independent pathway model, but the CPM still fit the data very well. Previous multivariate analyses of substance use in adolescent and adult samples (e.g., Han et al., 1999; Hettema, Corey, & Kendler, 1999; Karkowski, Prescott, & Kendler, 2000) have found support for a common pathway structure. Further, two prior analyses of nicotine, alcohol, and cannabis dependence (Palmer et al., 2012; Xian et al., 2008) found support for a common pathway model. Differences in phenotype definition and sample characteristics exist between these studies and the current report, which may explain the slight difference in our findings.
There was evidence that the latent structure of the age of substance use initiation differed across men and women, and this was largely accounted for by differences in the strength of the relation between alcohol and cannabis initiation and the latent factor. Further, multivariate model-fitting supported retaining a full model among men, but dropping common sources of genetic influence and specific sources of shared environmental influence among women. Thus, these data suggest differences in the contribution of familial influences to first use of multiple substances in males and females. There have been no prior multivariate studies of the timing of first substance use that employed both men and women; therefore, current findings regarding sex differences in initiation require replication. Concerning disorder, results differ somewhat from a previous study of tobacco, alcohol, and cannabis dependence (Palmer et al., 2012), in which the best-fitting common pathway model differed across men and women due to differences in substance-specific estimates. As mentioned above, however, this study differed with respect to phenotype definition and sample characteristics. Also, strong inferences regarding sex differences in CPM structure should not be drawn from the present report, as this model did not fit significantly better than the IPM. Prior analyses of the multivariate structure of substance abuse/dependence in adults (Kendler et al., 2003; Tsuang et al., 1998; Xian et al., 2008) have largely been conducted with male-only or female-only samples. Future multivariate studies that (a) employ both men and women and (b) examine substance use disorder in relation to other phenotypes will help clarify the nature of sex differences in polysubstance use across various stages of involvement.
Relation Between Initiation and Disorder
There was evidence for overlap in the familial influences on the timing of substance use initiation and symptoms of substance use disorder. Genetic influences were significantly correlated in both men and women. Further, although shared family environmental influences only explained a modest (and non-significant) proportion of the variation in disorder among women, these influences were highly correlated with the family environmental influences on initiation. The overlap in familial influences on first use and disorder is in accord with previous studies (e.g., Agrawal et al., 2008; Prescott & Kendler, 1999; Sartor et al., 2009).
After accounting for the genetic and shared environmental overlap between substance use initiation and substance use disorder, we obtained significant unique environmental correlations among men and women. This indicates that familial factors could not entirely explain the association between the timing of substance use initiation and later disorder, and are suggestive of a “quasi-causal” relation (Turkheimer & Harden, 2014). Specifically, this suggests that the propensity to initiate substance use at a younger age may exert some direct – or phenotypic – influence on the general risk for disorder. As reviewed above, evidence for a causal impact of early substance use initiation on disorder is mixed. To our knowledge, however, no prior studies have addressed this question from a multivariate perspective. That is, they have not examined whether the general tendency for earlier initiation of substance use might convey risk for later general susceptibility to develop a substance use disorder. The current data support further examination of early polysubstance initiation as a potential risk factor for later comorbid substance use problems. We emphasize, however, that strong causal conclusions cannot be drawn from the present findings, as (a) they concern cross-sectional data, (b) the association between early substance use initiation and disorder may be confounded by variables that differ within twin pairs and relate to both first use and problems, and (c) common measurement error related to the assessment of substance use initiation and disorder may contribute to their unique environmental correlation. Future research employing prospective data and measured confounds will help clarify the nature of the relation between early first substance use and later abuse/dependence. Longitudinal prevention studies evaluating the impact of delaying first substance use may be particularly informative.
Results have implications for prevention efforts. We obtained notable overlap in the liability for early initiation and substance use disorder, suggesting that appropriately targeted interventions may reduce the risk for early uptake and problematic use of multiple substances. Significant genetic overlap was observed among both men and women, indicating the importance of addressing heritable, trait-level factors that confer vulnerability to substance misuse broadly (e.g., behavioral disinhibition and childhood externalizing behavior (McGue, Iacono, & Krueger, 2006)). Further, the high shared environmental correlation obtained among women suggests that interventions targeting parent and peer factors may be particularly relevant for this group. Although there was no overlap in the family environmental influences on initiation and disorder among men, this does not suggest that environmental factors are unimportant. Traits such as conduct disorder may confer risk for substance misuse through active genotype-environment correlation (Scarr & McCartney, 1983), in which individuals seek out environments (e.g., substance-using peers) that facilitate expression of this vulnerability. Furthermore, gene-environment interaction may influence risk for substance misuse, such that heritable influences on adolescent involvement are increased in more “facilitative” environments (for instance, those characterized by low parental monitoring; Dick, 2011). Finally, our finding of significant unique environmental overlap across initiation and disorder suggests a potentially causal relation between these outcomes, indicating that delaying early substance initiation may help directly reduce risk for disorder.
It should be noted that the associations between the ages of substance use initiation and symptoms of substance use disorder were modest (men: rs=−0.10 – −0.31, women: rs=−0.12 – −0.28). Thus, addressing shared mechanisms of liability for early initiation and disorder and delaying the timing of first use will likely reduce only a small amount of the risk for substance use problems. This is not unexpected, however, given that substance use disorders have been associated with a number of biological and psychosocial factors, many of which may confer only a small degree of liability. To generate a more complete profile of risk for substance-related problems, the present findings should be integrated with those from genetically-informed studies of additional risk factors and associated behaviors.
Limitations
This study has limitations. The first concerns generalizability, as the current sample is largely Caucasian. Further, this sample has a very low alcohol abstinence rate and findings may not generalize to groups with more limited alcohol involvement. Second, in Australia, cannabis-containing joints often include tobacco (Bélanger, Akre, Kuntsche, Gmel, & Suris, 2011), and cannabis and tobacco have a common route of administration. Both of these factors may help explain some of the genetic and environmental overlap between the substances. Thus, cannabis- or tobacco-“specific” estimates may reflect some cross-substance processes. Studies of polysubstance involvement employing samples that do not co-use cannabis with tobacco will be important to examine the replicability of the current findings. Third, we employed a restricted CUD measure. This was necessary to harmonize the Cohort II and Cohort III assessments. Therefore, current results may not generalize to analyses employing complete DSM-IV CUD symptom counts. However, it should be noted that when analyses were re-run in Cohort III using the 11-item symptom count, the results were very comparable to those obtained with the restricted variable. Fourth, Cohorts II and III were assessed in different years. We regressed birth year out of all variables to control for potential cohort differences; however, it remains possible that this did not fully account for all potential secular trends in substance involvement. Fifth, we ignored the issue of censoring, i.e., the fact that particularly in Cohort II, the youngest members may not have passed through the age period of risk for initiation of substance use. However, survival analyses indicated that censoring likely did not significantly impact results. Sixth, we may have had greater statistical power to detect effects among women than men, as the present sample was 60% female. However, this “imbalance” in relative power was likely reduced by the higher prevalence of substance use disorders among men. Seventh, individuals who reported abstaining from a substance did not contribute data concerning their age of initiation for that substance. Thus, conclusions regarding the relative contributions of genetic and environmental factors to the age at first substance use may not generalize to the phenotype of any lifetime use. Finally, participants reported on their ages of first substance use in adulthood, which may decrease reliability. For instance, individuals have been shown to report progressively later ages of initiation over time (Parra, O’Neill, & Sher, 2003). Despite this, correlations between respondents’ ages at interview and their reported ages of initiation were minimal (rs = −.01 to .08), suggesting limited age-related bias. Nevertheless, accuracy in reporting may be increased via prospective assessment.
Conclusions
Notwithstanding limitations, this study offers a significant advance in our understanding of the relation between the age of substance use initiation and symptoms of later substance use disorder. Findings provide evidence of overlap in both the familial and unique environmental liability to early substance involvement and later disorder, suggesting that there are shared pathways of risk for nicotine, alcohol, and cannabis. Further, some of this risk may be transmitted via causal mechanisms. Continued investigation of substance-specific and cross-substance processes will allow for a more complete understanding of the mechanisms underlying polysubstance involvement and comorbid disorders.
Supplementary Material
General Scientific Summary.
Previous studies have detected a robust relation between early substance use initiation and increased risk for substance use disorder. The present study found that this association is attributable to genetic and environmental factors that are common across multiple substances of abuse. Results suggest that the relation between the timing of first substance use and symptoms of substance use disorder is explained by both a shared familial liability and a potentially direct influence of initiation on problems.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants AA023419 (LR), AA21235 (AA), and AA007728 (AH), and National Institute on Drug Abuse grants DA18267 (ML), DA32573 (AA), and DA23668 (AA). We thank Bronwyn Morris, Megan Fergusson, David Smyth, Olivia Zheng, Harry Beeby, Anjali Henders, Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville for data collection and data management. We thank the Australian Twin Registry twins for their continued participation. Gratitude is also expressed to Paige Harden and Phillip Wood for providing consultation regarding the analytic approach.
Footnotes
Opposite-sex twins were excluded from the analyses as (a) tests for measurement invariance of the substance use disorder symptom counts across sex indicated a lack of invariance (see Measurement invariance), and (b) the factor structure for the timing of substance use initiation differed across men and women (see Statistical Analysis). Thus, there was evidence that the constructs under consideration operated differently in men and women, and combining opposite-sex and same-sex twins might lead to incorrect inferences regarding sex differences.
For all variables, separate test-retest correlations were calculated for men and women and pooled via a Fisher's z-transformation to compute the overall correlation coefficient.
Because there was heterogeneity in the age of cannabis initiation across cohorts, mean ages for men and women are reported separately by cohort rather than within the full sample.
There are no conflicts of interest to declare.
References
- Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, Madden PAF. Early cannabis use and DSM-IV nicotine dependence: A twin study. Addiction. 2008;103:1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Kendler KS. Shared environmental contributions to substance use. Behavior Genetics. 2012;42:345–353. doi: 10.1007/s10519-011-9516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger RE, Akre C, Kuntsche E, Gmel G, Suris JC. Adding tobacco to cannabis – its frequency and likely implications. Nicotine & Tobacco Research. 2011;13:746–750. doi: 10.1093/ntr/ntr043. [DOI] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC. Trends in the genetic influences on smoking. Journal of Health and Social Behavior. 2010;51:108–123. doi: 10.1177/0022146509361195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JL, … Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol and Drugs. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. Gene-environment interplay in psychological traits and disorders. Annual Review of Clinical Psychology. 2011;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Prescott C, McGue M. The genetics of substance use and substance use disorders. In: Kim Y-K, editor. Handbook of behavior genetics. 1. New York, NY: Springer; 2009. pp. 443–453. [DOI] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice R, Thapar A, Neale MC, … van den Bree MBM. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;101:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: A multistage model from cannabis availability, cannabis initiation, and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant JD, Lynskey MT, Madden PAF, Nelson EC, Few LR, Bucholz KK, … Agrawal A. The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders. Psychological Medicine. 2015 doi: 10.1017/S0033291715001518. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: Univariate and bivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug and Alcohol Dependence. 1999;57:69–78. doi: 10.1016/S0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Strang J, Agrawal A, Nelson EC, Statham D, … Lynskey MT. The association between speed of transition from initiation to subsequent use of cannabis and later problematic cannabis use, abuse and dependence. Addiction. 2015;110:1311–1320. doi: 10.1111/add.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöreskog KG, Goldberger AS. Estimation of a model with multiple indicators and multiple causes of a single latent variable. Journal of the American Statistical Association. 1975;70:631–639. doi: 10.1080/01621459.1975.10482485. [DOI] [Google Scholar]
- Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:665–670. doi: 10.1002/1096-8628(20001009)96:5%3C665::AID-AJMG13%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. American Journal of Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PAF, Bucholz KK, Slutske WS, Nelson EC, … Martin NG. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;8:1519–1530. doi: 10.1017/S0033291704002922. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, … Madden PAF. Defining nicotine dependence for genetic research: Evidence from Australian twins. Psychological Medicine. 2004;34:865–879. doi: 10.1017/S0033291703001582. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A, Bucholz KK, Nelson EC, Madden PAF, Todorov AA, … Heath AC. Subtypes of illicit drug users: A latent class analysis of data from an Australian twin sample. Twin Research and Human Genetics. 2006;9:523–530. doi: 10.1375/twin.9.4.523. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A, Henders A, Nelson EC, Madden PAF, Martin NG. An Australian twin study of cannabis and other illicit drug use and misuse, and other psychopathology. Twin Research and Human Genetics. 2012;15:631–641. doi: 10.1017/thg.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, … Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behavior Genetics. 2006;36:195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychological Medicine. 2004;34:1–11. doi: 10.1017/S0033291704002405. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: A twin study. Psychological Medicine. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Palmer RHC, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, … Hewitt JK. Genetic etiology of the common liability to drug dependence: Evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug and Alcohol Dependence. 2012;123S1:S23–S32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra GR, O’Neill SE, Sher KJ. Reliability of self-reported age of substance involvement onset. Psychology of Addictive Behaviors. 2003;17:211–218. doi: 10.1037/0893-164X.17.3.211. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcoholism: Clinical and Experimental Research. 1999;23:101–107. doi: 10.1097/00000374-199901000-00014. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Deutsch AR, Lynskey MT, Agrawal A, Madden PAF, … Martin NG. Progression in Substance Use Initiation: A Multilevel Discordant Monozygotic Twin Design. Journal of Abnormal Psychology. 2015;124:596–605. doi: 10.1037/abn0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. doi: 10.1111/j.1530-0277.2001.tb02261.x. [DOI] [PubMed] [Google Scholar]
- Samek DR, Keyes MA, Iacono WG, McGue M. Peer deviance, alcohol expectancies, and adolescent alcohol use: Explaining shared and nonshared environmental effects using an adoptive sibling pair design. Behavior Genetics. 2013;43:286–296. doi: 10.1007/s10519-013-9595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: Evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Waldron M, Duncan AE, Grant JD, McCutcheon VV, Nelson EC, … Heath AC. Childhood sexual abuse and early substance use in adolescent girls: The role of familial influences. Addiction. 2013;108:993–1000. doi: 10.1111/add.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. doi: dx.doi.org/10.2307/1129703. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, … Eaves L. Co-occurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Harden KP. Behavior genetic research methods: Testing quasi-causal hypotheses using multivariate twin data. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Personality and Social Psychology. 2. Cambridge, UK: Cambridge University Press; 2014. pp. 159–187. [DOI] [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden B, Mcgue M, Iacono W, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: The respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Waldron M, Grant JD, Bucholz KK, Lynskey MT, Slutske WS, Glowinski AL, … Heath AC. Parental separation and early substance involvement: Results from children of alcoholic and cannabis dependent twins. Drug and Alcohol Dependence. 2014a;134:78–84. doi: 10.1016/j.drugalcdep.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M, Vaughan EL, Bucholz KK, Lynskey MT, Sartor CE, Duncan AE, … Heath AC. Risks for early substance involvement associated with parental alcoholism and parental separation in an adolescent female cohort. Drug and Alcohol Dependence. 2014b;138:130–136. doi: 10.1016/j.drugalcdep.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Scherrer JE, Grant JD, Eisen SA, True WR, Jacob W, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.