Abstract
None of the proposed mechanisms of Alzheimer’s disease (AD) fully explains the distribution patterns of the neuropathological changes at the cellular and regional levels, and their clinical correlates. One aspect of this problem lies in the complex genetic, epigenetic, and environmental landscape of AD: early-onset AD is often familial with autosomal dominant inheritance, while the vast majority of AD cases are late-onset, with the ε4 variant of the gene encoding apolipoprotein E (APOE) known to confer a 5–20 fold increased risk with partial penetrance. Mechanisms by which genetic variants and environmental factors influence the development of AD pathological changes, especially neurofibrillary degeneration, are not yet known. Here we review current knowledge of the involvement of the monoaminergic systems in AD. The changes in the serotonergic, noradrenergic, dopaminergic, histaminergic, and melatonergic systems in AD are briefly described. We also summarize the possibilities for monoamine-based treatment in AD. Besides neuropathologic AD criteria that include the noradrenergic locus coeruleus (LC), special emphasis is given to the serotonergic dorsal raphe nucleus (DRN). Both of these brainstem nuclei are among the first to be affected by tau protein abnormalities in the course of sporadic AD, causing behavioral and cognitive symptoms of variable severity. The possibility that most of the tangle-bearing neurons of the LC and DRN may release amyloid β as well as soluble monomeric or oligomeric tau protein trans-synaptically by their diffuse projections to the cerebral cortex emphasizes their selective vulnerability and warrants further investigations of the monoaminergic systems in AD.
Keywords: 5-hydroxytryptamine (serotonin), Alzheimer's disease, amyloid beta (Aβ) protein, blood-brain barrier, cerebrospinal fluid, epigenetics, locus coeruleus, metals, monoamines, neurofibrillary degeneration, non-cognitive symptoms, nucleus raphe dorsalis, phosphorylation, sleep-wake cycle, tau protein
1. Clinical and neuropathological criteria for AD diagnosis
Alzheimer’s disease (AD) accounts for 60–70% of cases of dementia (World Health Organization, WHO Fact Sheet No. 362, March 2015). The report of Alzheimer’s Disease International (ADI, Alzheimer World Report, 2015) showed that nearly 35.6 million people suffered from dementia in 2012. It is estimated that this number will quadruple by 2050. Therefore, the WHO in 2012 declared AD a global public health priority. There is still no effective treatment to prevent or cure AD. Currently, approved drugs only temporarily alleviate some of the disease’s symptoms to a limited extent. Cholinomimetics (tacrine, rivastigmine, donepezil, and galantamine) do so by enhancing the cholinergic neurotransmission, whereas memantine (a non-competitive antagonist of N-methyl-d-aspartate receptors, NMDAR) is considered to have protective activity against glutamate-induced excitotoxic neuronal death (Yiannopoulou and Papageorgiou, 2013).
1.1. Clues to the etiology of AD
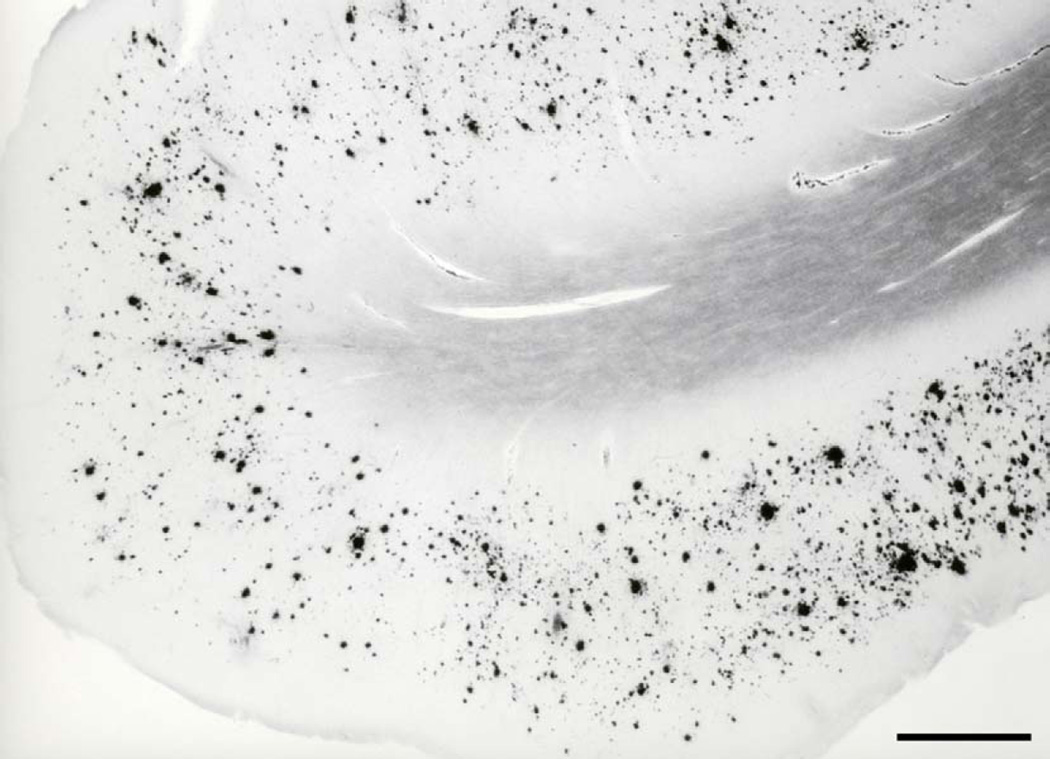
After the milestone discoveries that cerebrovascular amyloid (due to cerebral amyloid angiopathy, CAA) and senile plaques (SP) are composed of amyloid β (Aβ) protein, that the same antigenic determinants (Glenner and Wong, 1984a; Wong et al., 1985) are shared in both AD and Down’s syndrome (Glenner and Wong, 1984b), and that the Val717Ile missense (“London”) mutation in the amyloid precursor protein (APP) gene on chromosome 21 was found to be causally related to the early-onset autosomal-dominant familial AD (Goate et al., 1991), Hardy, Selkoe and colleagues (Hardy and Allsop, 1991; Selkoe, 1991; Hardy and Higgins, 1992) formulated the amyloid cascade hypothesis, which has become a dominant view of AD pathogenesis ever since. An illustration of amyloid plaques in the brain of an AD case is given in Figure 1. According to the amyloid theory, excessive production of Aβ (which exists in monomeric, oligomeric, and aggregated forms as SP) via serial cleavage of the larger amyloid precursor protein (APP) molecule by β-secretase (β-site APP cleaving enzyme, BACE, encoded by the BACE1 gene) and γ-secretase (multiprotein complex now known to minimally consist of 4 individual proteins: presenilin, nicastrin, anterior pharynx-defective 1, APH-1, and presenilin enhancer 2, PEN-2) (Blanquet et al. 1987; Robakis et al., 1987; St George-Hyslop et al., 1987; Shoji et al., 1992; Citron et al., 1992), is the key pathological event which drives all other pathological changes. These pathological changes include altered calcium homeostasis, microglial activation/inflammation, astrocytosis, an upregulated production of nitric oxide and DNA damage (Šimić et al., 2000), dysregulation of energy metabolism and cell cycle control, a significant increase in the full-length mitochondrial DNA (mtDNA) accompanied by extensive fragmentation of the unamplified mtDNA (Diana et al., 2008), the development of neurofibrillary tangles (NFT), synaptic loss, excitotoxicity, neuronal death, and dementia, not only in early-onset cases (EOAD) but also in late-onset cases of AD (LOAD). In 1987 Goldgaber and collaborators isolated APP and localized its gene to chromosome 21 (Goldgaber et al., 1987). Interestingly, the first APP mutation discovered, a G to C mutation at codon 693 (APP Glu693Gln) was not causing AD, but instead coused hereditary cerebral hemorrhage with amyloidosis – Dutch type (HCHWA-D; Van Broeckhoven et al., 1990; Levy et al., 1990). This is most probably due to the fact that affected individuals died from cerebral bleeding at a younger age before developing clinical AD. Interestingly enough, two out of four other known mutations within the Aβ-coding part of APP (exons 16 and 17) also cause fatal hemorrhages due to amyloid angiopathy (APP Cys692Gly – Flemish, and APP Glu693Lys – Italian), while only the rare “Arctic” (APP Glu693Gly) and Osaka (APP Glu693Δ) mutations cause EOAD.
Fig. 1.
Amyloid plaques, as revealed by Campbell-Switzer-Martin's method. The anterior part of the parahippocampal gyrus of an 84-year-old woman who died 3.5 years after the clinical diagnosis of AD was made. Scale bar = 1 mm.
The long-known fact that there are many families in which AD has an early onset (before age of 60) and is inherited in an autosomal dominant manner (Lowenberg and Waggoner, 1934) could therefore not be explained by a very small number of AD families with APP mutations. This question had been resolved by the discovery of mutations in the presenilin 1 (PSEN1) gene on chromosome 14 (St. George-Hyslop et al., 1992; Sherrington et al., 1995) and homologous gene PSEN2, on chromosome 1 (Schellenberg et al., 1992; Levy-Lahad et al., 1995). PSEN1 and PSEN2 are components of the γ-secretase complex, which can cleave APP at several points resulting in Aβ of various lengths. The peptides associated with AD are 40 and 42 amino acid-long, with Aβ42 more likely to aggregate to form SP in the brain than Aβ40. All PSEN mutations lead to an increase in the Aβ42:Aβ40 ratio, although the total quantity of Aβ produced remains constant (Citron et al., 1997; Czech et al., 2000). Whether PSEN mutations correspond to a gain or loss of function is still controversial, although PS1 mutations were expressed at normal levels, they impaired γ-secretase activity but not γ-secretase-independent functions of PS1 (Woodruff et al., 2013). Thus, PS1 mutations do not act as simple loss of PS1 function, but instead dominantly as gain of PS1 activity toxic to some, but not all conditions. Presenilins are also implicated in the processing of Notch (Okochi et al., 2002; De Strooper et al., 2012), an important developmental protein. PS1 knockout mice die early in development from abnormalities similar to those found when Notch is disrupted (Shen et al., 1997). APP can also be cleaved by α-secretases such as a disintegrin and metalloprotease 10 (ADAM10) and tumor necrosis factor alpha (TNF-α) converting enzyme (TACE), but this cleavage does not result in Aβ, instead generating a neurotrophic and neuroprotective fragment APPs-α (Corrigan et al., 2011; for review see Endres and Fahrenholz, 2012).
1.1.1. The role of amyloid β protein (Aβ)
Most researchers still held the view, usually unwritten, that Aβ was just a waste product of APP metabolism, while some others have suggested that Aβ1–42 may have an acute “protective” role in sealing microhemorrhages in the extensive network of blood vessels that meanders for more than 600 km through the human brain (Atwood et al., 2003; Hardy, 2007; Hardy, 2009). The upregulation of N-terminal fragment of APP would, under such a scheme, be part of the attempt to prevent clotting in the hemorrhaged region caused by blood contact with the brain tissue (if the coagulation cascade would exist in the brain, vascular blockage would lead to ischemic stroke and permanent neuronal death because the brain, unlike other tissues, has close to zero ability to replace terminally differentiated neurons), whereas Aβ would be a vascular sealant, anticoagulant and remodeling molecule (Atwood et al., 2003). This would also explain close and intimate relationships between plaques and blood vessels in transgenic mouse models (Kumar-Singh et al., 2005), the presence of iron in every plaque (Falangola et al., 2005), and the association of APOE ε2 genotype with cerebral hemorrhage (McCarron and Nicoll, 2000; Loehrer et al., 2014; Charidimou et al., 2015). Unlike microbleeds in the deep and infratentorial regions, which are thought to reflect hypertensive arteriopathy, lobar microbleeds are associated clinically with CAA, and frequently observed in seemingly asymptomatic populations (Loehrer et al., 2014). If the above concept is true, it would also be useful to explain the fact why about around 40% of AD patients have normotensive hydrocephalus (because CAA would prevent the proper exchange of water and ions through the blood-brain barrier, BBB), and why about 40% of adult patients with idiopathic chronic normal pressure hydrocephalus have histological lesions characteristic of AD, as revealed from cortical biopsies (Golomb et al., 2000). Alternatively, the role of Aβ in maintaining vascular homeostasis (mediated by sealing the BBB) may be related to restraining periarterial drainage in order to prevent the elimination of high molecular weight substances from the brain, as such a drainage of brain antigens from brain’s interstitial fluid to cervical lymph nodes would cause autoimmune encephalomyelitis and multiple sclerosis (Weller, 1998). These insights may also explain the root cause of the encephalomyelitis suffered by individuals in immunotherapy trials as being directly associated with removal of Aβ from the vasculature, as immunological responses to Aβ vaccination do not discriminate between vascular deposits of Aβ and deposits of Aβ in SP (Lambracht-Washington and Rosenberg, 2012), which has been confirmed in a mouse model (Furlan et al., 2003). Furthermore, it would also fit well to in vivo evidence that the removal of deposited Aβ from the vasculature leads to increased cerebral hemorrhages (Uro-Coste et al., 2010), again strongly supporting the above mentioned concept of APP/Aβ functions as sealant, anticoagulant and remodeling molecule (Atwood et al., 2003, Hardy, 2009).
1.1.2. Genetics of AD
Collectively, the genetic etiology of AD is very complex. EOAD (less than 1% of cases) is often familial (fAD), with autosomal dominant and fully penetrant inheritance and can be caused by any of more than 200 pathogenic mutations in APP (33 mutations, duplication), PSEN1 (185 mutations) and PSEN2 (13 mutations; http://www.molgen.ua.ac.be/ADmutations). A rare mutation in the APP gene that protects against AD and cognitive decline in the elderly without AD was also reported (Jonsson et al., 2012). Most AD cases (over 99%) however are sporadic, late-onset (sAD, LOAD) and have few evident genetic components. The ε4 variant of the gene encoding apolipoprotein E (APOE) is known to confer increased risk for LOAD (Strittmatter et al., 1993; Saunders et al., 1993) with partial penetrance. Based on 320 meta-analyses of 1395 studies in which 695 genes and their 2973 polymorphisms have been tested as late-onset AD candidate genes, over 30 yield positive evidence for association. The number one gene is APOE, with a Bayes factor (BF) > 50. Using APOE genotype ε3/ε3 as a neutral benchmark for comparison, individuals with a single copy of ε4 allele manifest a 5-fold increased risk of developing LOAD, while those with two copies have an estimated 20-fold increased risk (Strittmatter, 2012). It seems that different APOE alleles are not associated with an increase in Aβ production, but with an inability to clear Aβ from the brain (Mawuenyega et al., 2010; Castellano et al., 2011). This may be related to the reduced production of Aβ auto-antibodies in AD subjects (Qu et al., 2014).
The next nine genes with the highest association with LOAD are: BIN1 (BF = 23.4) which encodes several isoforms of a nucleoplasmic adaptor protein, one of which was identified as MYC-interacting protein; CLU (BF = 20.1), which encodes apolipoprotein J, ABCA7 (BF = 18.8) for ATP-binding cassette transporter, subfamily A [ABC1], member 7, CR1 (BF = 18.1) for complement component receptor 1; PICALM (BF = 17.3), for phosphatidylinositol binding clathrin assembly protein; MS4A6A (BF = 8.7), CD33 (BF = 7.7) for a transmembrane receptor expressed on cells of myeloid lineage – cluster of differentiation 33; MS4A4E (BF = 6.9), coding for protein membrane-spanning 4-domains, subfamily A, member 4E, and CD2AP (BF = 6.6) which codes for a scaffolding molecule that regulates the actin cytoskeleton (according to www.alzgene.org assessed in December 2015). Genetic variants of all of these genes have a relatively minor influence on AD progression when altered (Cacabelos, 2007). Although their influence on the development and course of sAD remain largely unknown (Hollingworth et al., 2011; Naj et al., 2011; Lardenoije et al., 2015), most of them are presumably involved in the metabolism of Aβ. Some of them, such as APOE and ABCA7, are known to be also centrally involved in cholesterol transport and metabolism; both of these genes are targets of transcription factors and nuclear receptors called liver-X receptors (LXR) (Štefulj et al., 2013). Most recently, rare mutations of TREM2 (Jonsson et al., 2013, Guerreiro et al., 2013) and PLD3 (Cruchaga et al., 2014) were proposed to confer a much larger increase in risk for LOAD than the aforementioned common sequence variants. However, a role for PLD3 rare variants in AD could not be confirmed in a European Consortium Cohort (Cacace et al., 2015). Recent evidence also suggests that, besides mutations causing EOAD, there are novel, rare additional variants in APP, PSEN1, PSEN2, and ADAM10 that alter the risk for LOAD (Karch and Goate, 2015). For example, rare variants in APP may increase (e.g., APP Asn660Tyr), decrease (e.g., APP Ala673Thr), or have no effect on risk (e.g., APP Glu599Lys), whereas PSEN1 polymorphism Glu318Gly (Benitez et al., 2013) and ADAM10 risk variants Gln170His and Arg181Gly (Kim et al., 2009) are associated with a significant increase in LOAD risk. In addition to increasing Aβ levels in vitro (Kim et al., 2009), in one of the best characterized mouse models for AD, the Tg2576, the two aforementioned ADAM risk variants were also shown to disrupt α-secretase activity and shift APP processing toward amyloidogenic cleavage, thus yielding increased plaque load (Suh et al., 2013).
1.1.3. The role of tau protein
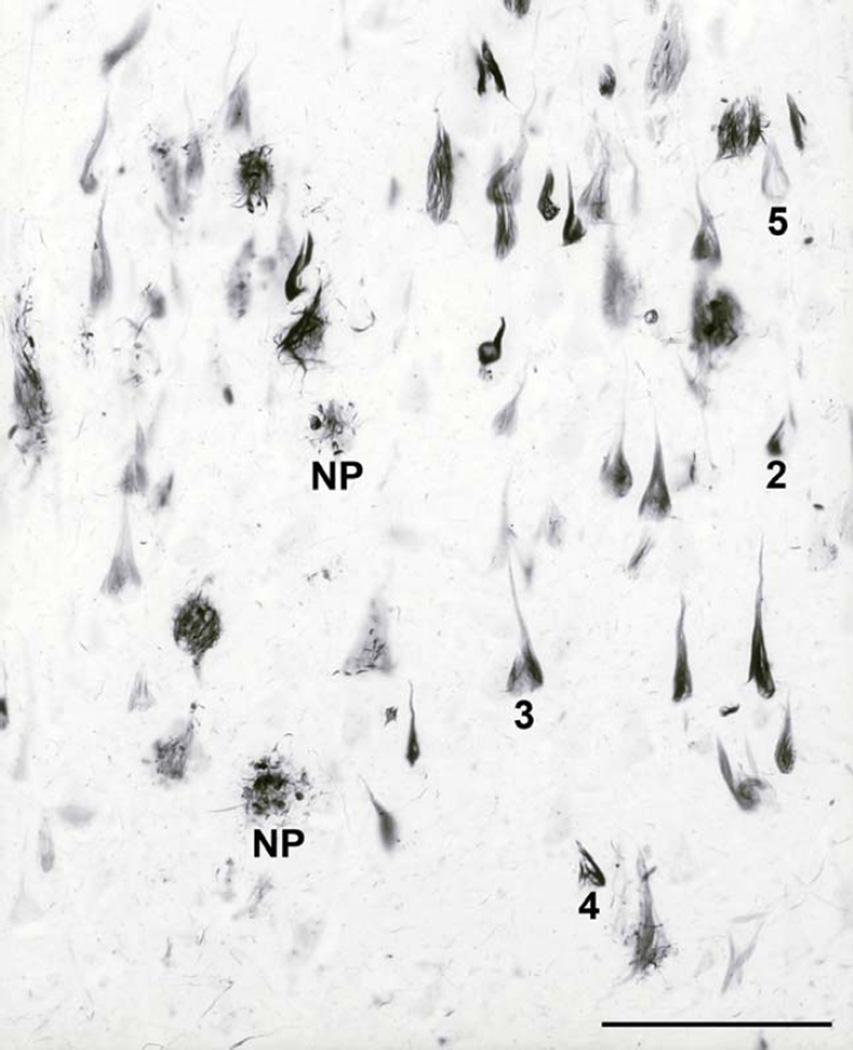
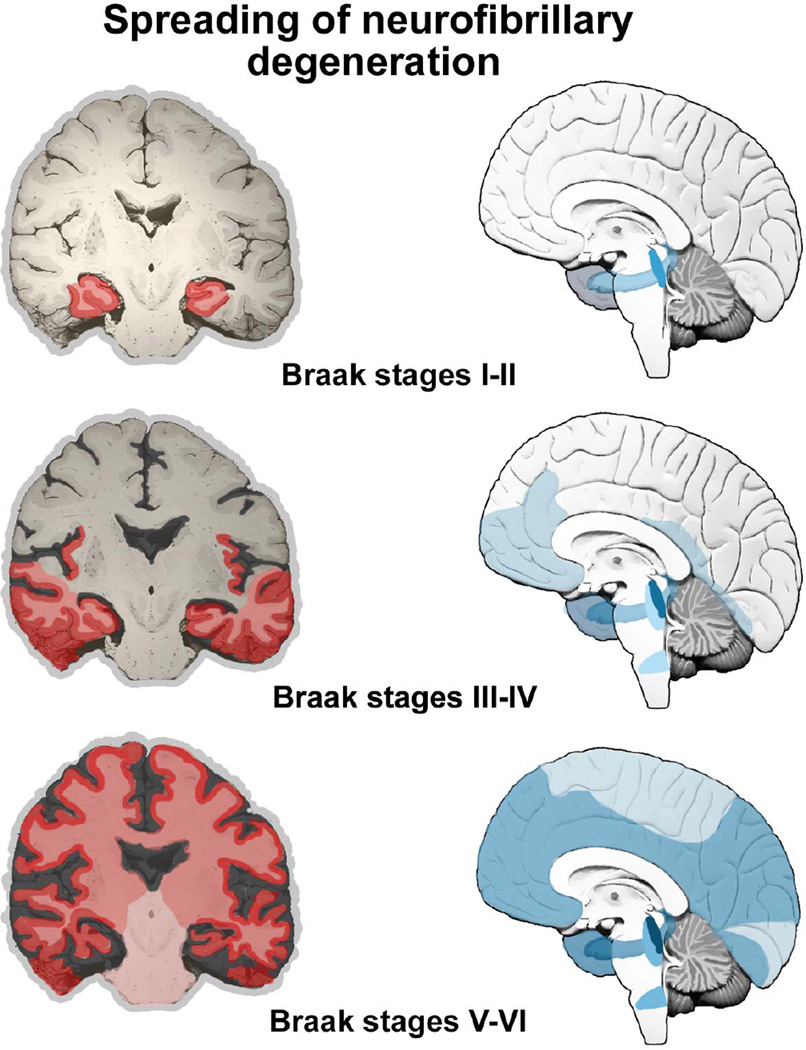
The significance of tau protein, essential for microtubule (MT) assembly (Weingarten et al., 1975), in the pathogenesis of AD remained in the shadow of the amyloid theory during the late 1980s and early 1990s. However, the distribution pattern and overall quantity of Aβ turned out to be of limited significance for pathological staging of AD progression and symptom severity. After detailed studies of the maturation and distribution of NFT showing correlation with the degree of cognitive decline and memory impairment in AD using classical silver staining (Braak and Braak, 1991; Fig. 2) and immunohistochemical staining for hyperphosphorylated tau (Braak et al., 2006; see example in Fig. 3), a neuropathological staging of tau deposition in the brain, including NFT and neuropil threads (NT) in neurites, was proposed (Fig. 4). The possibility that the burden of NFT provides a better association with cognitive impairment was soon confirmed (Arriagada et al. 1992; Bierer et al., 1995), supporting a significant role for tau pathology in the disease. As shown in Figure 4, the Braak’s staging system classifies the topographic progression of AD neurofibrillary degeneration in six stages. Spreading from the transentorhinal region to the hippocampal formation (initial stages I and II) clinically correlate with subjective or objective impairment of memory for recent events and mild spatial disorientation, but with preservation of general cognitive functioning with or without minimum impairment of daily living activities (Braak and Braak, 1991; Šimić et al., 2005; Šimić et al., 2009). Further spread to the temporal, frontal, and parietal neocortex (intermediate stages III and IV) correlates with impaired recall, delayed word recall and word finding difficulties, disorientation in time and space, and impaired concentration, comprehension, and conceptualization, among other symptoms of dementia. Finally, neurofibrillary degeneration affects unimodal and primary sensory and motor areas of the neocortex (late stages V and VI), which roughly correlates with disturbances in object recognition, and other perceptual and motor skills.
Fig. 2.
Neurofibrillary changes in AD. Modified silver staining of the CA1 field from the body of the hippocampus of an 84-year-old woman, who died 3.5 years after clinical diagnosis of AD was made. NP = neuritic plaque. Numbers designate groups or "classes" of neurons with neurofibrillary changes, as defined and described in Braak et al., 1994b: 2 = early rod-like argyrophilic inclusions in the soma, 3 = typical developed NFT, which fills almost the whole cytoplasm and therefore acquires the shape of the neuron („flame-like“ appearance in the case of this pyramidal neuron), 4 = early extracellular NFT (such tangles are called „tombstone“ or „ghost“ tangles because the neurons have died and only the NFT remain), 5 = late extracellular tangle. Scale bar = 100 µm.
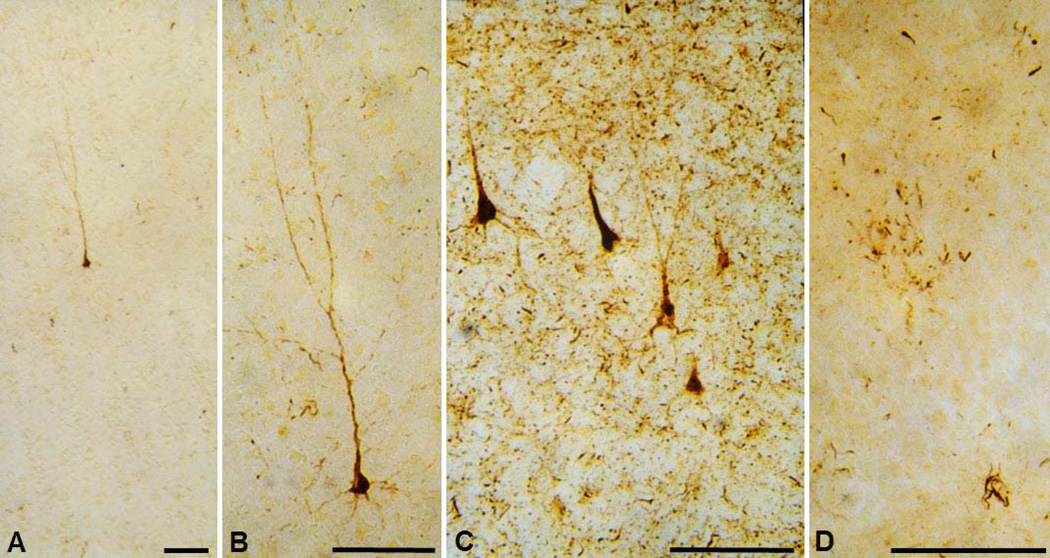
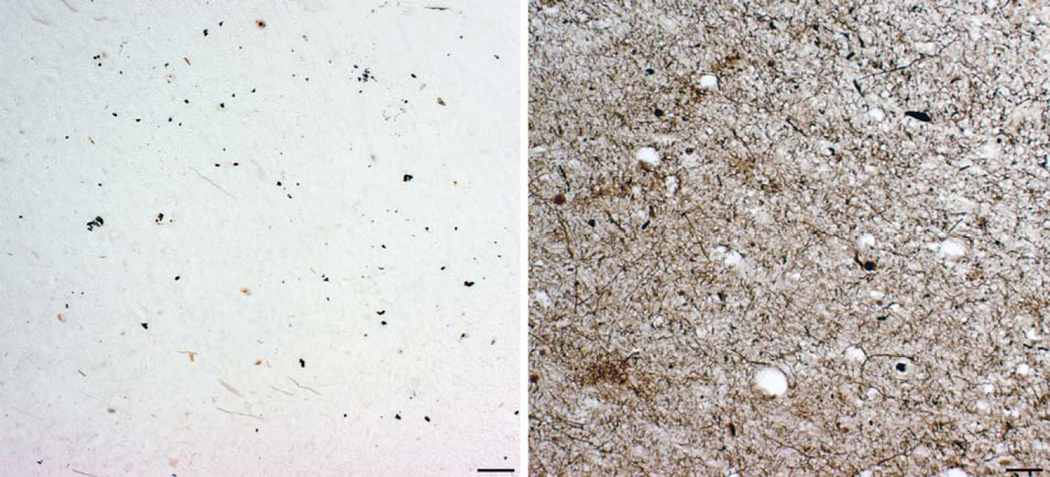
Figure 3.
Vizualization of the hyperphosphorylated tau protein by using antibody AT8. A. The earliest detectable changes: hyperphosphorylated tau is localized in somatodendritic compartment of an isolated layer III pyramidal neuron in the transentorhinal cortex of a cognitively normal 59-year-old adult person. According to the criteria put forward in Braak et al. 1994b, such neurons belong to the group 1 neurons (they cannot be revealed by silver staining as they are bearing no tangle, just containing hyperphosphorylated tau). It is not known whether this change is reversible. B. Enlarged image from A. Note evenly distributed AT8-immunoreactive material in soma and all neuronal processes as well as grossly normal neuronal morphology. C. A group of temporal cortex pyramidal neurons in advanced stages of neurofibrillary degenerative changes in the brain of a 73-year old subject with a 7-year history of AD. A spectrum of conspicuous cytoskeletal alterations is visible in all five neurons (belonging to all groups/“classes“ neurons according to Braak et al., 1994, except group 5 end-stage neurons when their AT8 immunoreactivity is gone). All of these neurons also show argyrophilia, meaning that their neurofibrillary tangles can be revealed by using silver stainging methods. D. AT8 - immunoreactivity in 'granules' and tortuous apical and basal dendrites of granule cells of the hippocampal dentate gyrus of the same subject as in C. Perikarya of granule cells are rarely AT8-positive and do not usually contain typical NFT (even in cases with long-lasting history of AD), perhaps because this special neuronal type does not express MAPT mRNAs containing exon 10 (4R isoforms), supposedly conferring their resistance to neurofibrillary changes. Scale bars = 100 µm.
Fig. 4.
The Braak’s staging system (Braak and Braak, 1991). The topographic progression of AD classifies neurofibrillary degeneration in 6 stages, spreading from the transentorhinal region to the hippocampal formation (initial stages I and II, which clinically correlates with subjective or objective impairment of memory for recent events and mild spatial disorientation, but with preservation of general cognitive functioning without or with minimum impairment of activities of daily living), then to the temporal, frontal, and parietal neocortex (intermediate stages III and IV, which correlates with impaired recall, delayed word recall and word finding difficulties, disorientation in time and space, and impaired concentration, comprehension and conceptualization, among other symptoms of dementia), and finally to unimodal and primary sensory and motor areas of the neocortex (late stages V and VI, which roughly correlates with disturbances in object recognition, and other perceptual and motor skills). Braak staging system can be reduced to four with improved inter-rater reliability (Nagy et al., 1998): B0: no NFT, B1: Braak stages I/II, with NFT predominantly in entorhinal cortex and closely related areas, B2: stages III/IV, with NFTs more abundant in hippocampus and amygdala while extending slightly into the association cortex, and B3: stages V/VI, with NFT, neuropil threads and dystrophic neurites widely distributed throughout the neocortex and ultimately involving primary motor and sensory areas.
One explanation for early AD changes in the hippocampus, entorhinal cortex, and temporal neocortex can be an age-dependent BBB breakdown in the hippocampus, as recently revealed by an advanced dynamic contrast-enhanced magnetic resonance imaging (MRI) protocol with high spatial and temporal resolutions to quantify regional BBB permeability in the living human brain (Montagne et al., 2015). Indeed, the BBB breakdown in the hippocampus and dentate gyrus worsened with MCI that correlated with injury to BBB-associated pericytes, as shown by CSF analysis (Montagne et al., 2015).
Neurons in layers II and III of the transentorhinal and entorhinal cortex are consistently affected by neurofibrillary degeneration, either during normal aging or in primary age-related tauopathy, PART (Braak and Braak, 1991; Šimić et al., 2005; Crary et al., 2014; Jellinger et al., 2015). Stereologic estimates showed a 43.5% average neuron loss in 32–83 year old subjects (Šimić et al., 2005). Hof and collaborators showed that a considerable proportion (73–77%) of entorhinal layer II neurons affected by neurofibrillary degeneration might preserve some function even at stages with a Clinical Dementia Rating (CDR) score of 3 (Hof et al., 2003). As long as elderly patients do not suffer from AD, they appear neuropathologically quite comparable as a group (Hof et al., 2003). It is therefore not surprising that significant neuron loss due solely to aging cannot be revealed without younger adult cases included in the regressions. On the other hand, when neuronal loss attributable to aging is superimposed to an unbiased estimate of the number of NFT in AD, regions like the entorhinal cortex and hippocampal formation may display neuronal loss larger than that accounted for by NFT counts alone (Šimić et al., 1998; Krill et al., 2002). Thus, the pattern of neuron loss does not necessarily match the pattern of NFT formation, due to mechanisms other than neurofibrillary degeneration (Šimić et al., 1998a, 1998b; Hof et al., 2003; Andrade-Moraes et al., 2013). Based on the notion that NFT evolved from an accumulation of abnormally hyperphosphorylated tau without PHF formation (described as the ‘pretangle’ stage, Bancher et al., 1989), Braak and others also demonstrated that hyperphosphorylation is probably a crucial step leading to the formation of both soluble and insoluble tau filaments (Braak et al., 1994), that neuronal damage in AD actually begins many years before any clinical symptoms and signs (Braak and Del Tredici, 2015), and that, unlike Aβ, the distribution of tau pathology is associated with the clinical progression of AD (Bierer et al., 1995). In contrast to the amyloid cascade hypothesis of AD, which implies that tau pathology is a secondary, downstream phenomenon, the neuropathological findings of Braak and collaborators have fueled a significant controversy concerning the importance or contributions of Aβ burden-induced damage compared to that caused by tau pathology, particularly in LOAD. Additionally, the pathological Aβ and tau proteins mutually interact and are influenced by many other factors, such as epigenetic (Lardenoije et al., 2015), inflammatory (Joshi and Praticò, 2014), vascular, and possibly direct environmental causes (metals, metalloids, pollutants, various compounds in food), as well as compensatory neuroplastic response to counteract neural injury associated with neurodegenerative processes (Wang et al., 2011), all of which may promote cognitive and behavioral decline.
Compelling evidence that tau malfunction or dysregulation alone can be sufficient to cause neurodegeneration came from the identification of mutations in the tau-encoding MAPT gene on chromosome 17, which cause frontotemporal dementia with parkinsonism (FTDP-17; Hutton et al., 1998). This finding strengthens cytoskeletal abnormalities as a possible pivotal mechanism in neurodegeneration in AD (Terry, 1996; Šimić et al., 1998a), and positioned AD as the most important secondary tauopathy (as the tau-coding MAPT geneitself is not mutated), while mutations in the MAPT gene subsequently identified into a new group of diseases now called primary tauopathies. In the years to follow, both in vitro and in vivo studies have shown that reducing endogeneous tau ameliorates Aβ-induced deficits (Roberson et al., 2007; Bhatia and Hall, 2013; for review see Wang and Mandelkow, 2016), which provided compelling evidence that tau is sufficient and necessary for Aβ-induced neurodegeneration.
Genetic studies, including genome-wide association (GWAS), have demonstrated the importance of both the inversion polymorphism and haplotype-specific polymorphisms (the common haplotype clades marking the majority and inverted sequences are termed H1 and H2, respectively) of MAPT in various tauopathies (Anaya et al., 2011; for review, see Trabzuni et al., 2012). More specifically, abnormal phosphorylation, aggregation, and proteolysis of the tau protein in a “pre-tangle” stage of neurofibrillary degeneration have been neuropathologically documented to be an early and crucial event in the pathogenesis of AD, but also other sporadic tauopathies, such as progressive supranuclear palsy (PSP) (Luk et al., 2010) and argyrophilic grain disease (AgD) (Šimić, 2002; Williams, 2006; Murray, 2014), confirming involvement of tau in common pathogenetic pathways. Based on the tau isoforms found in the aggregates, tauopathies are classified into three groups: 4R tauopathies (including PSP, AgD, and cortico-basal degeneration, CBD), 3R tauopathies (e.g. frontotemporal lobar degeneration with tau inclusions, FTLD-tau, previously known as Pick’s disease) and 3R/4R tauopathies (e.g. AD). Owing to an additional repeat microtubule-binding domain (R2), 4R tau isoforms show higher affinity for microtubules than 3R isoforms. Tau repeat domains bind at the interface between α- and β-tubulin heterodimers, suggesting that there is competition between their physiological interaction with tubulin and pathogenic misfolding (Kadavath et al., 2015). Most recently, Huntington’s disease has been confirmed as 4R tauopathy (Fernández-Nogales et al., 2014).
1.1.3.1. Phoshorylation of tau protein
Phosphorylation plays a crucial role in regulating functions of tau, including it binding to microtubules. The longest brain isoform of tau, tau1–441, has about 80 Ser/Thr and 5 Tyr residues that can be phosphorylated by various protein kinases encoded by 518 protein kinase genes in the human genome (Buée et al., 2000; Manning et al., 2002; Šimić et al., 2016). Immunolabeling with phopho-dependent antibodies raised against various tau phosphorylation sites, as well as spectrometric analysis, revealed that over 40 Ser/Thr and 2 Tyr residues are phosphorylated in PHF (Buée et al., 2000; Iqbal et al., 2016; Šimić et al., 2016). In AD, Ser/Thr residues followed by Pro are the most frequently phosphorylated sites, accounting for about half of phosphorylated residues. These sites are outside the microtubule-binding domain and are phosphorylated by proline-directed protein kinases (PDPK). The main PDPK are glycogen synthase kinase-3β (GSK-3β), mitogen-activated protein kinase (MAPK), JNK (c-Jun N-terminal kinase), cyclin-dependent-like kinase 5 (CDCK5) and dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A; Buée et al., 2000; Iqbal et al., 2016). Ser/Thr residues that are not followed by Pro (i.e. non-proline-directed sites of tau) are phosphorylated by non-PDPK. Non-PDPK are directed toward KXGS-motif and some of the most well known are: calcium/calmodulin-activated protein kinase II (CaMKII), microtubule-affinity-regulated kinase 110 (MARK p110), protein kinase A (PKA), and kasein kinase 1 (CK1; Buée et al., 2000; Iqbal et al., 2016). Phosphorylation of KXGS motifs in the repeat domain of tau (particularly Ser262) reduce the affinity of tau to microtubules and, together with phosphorylation of Ser212 Thr231, trigger the detachment of tau from microtubules (Wang and Mandelkow, 2016), similarly as MAPT mutations affecting tau protein near the microtubule-binding domain (e.g., Gly272Val, Asn279Lys, ΔLys280, Pro301Leu, Val337Met and Arg406Trp) reduce its affinity for microtubules and increase tendency for aggregation (Hong et al., 1998).
Regardless of the large number of various protein kinases that can phosphorylate tau, the phosphorylation state of a protein is the net sum of the activities of both its kinases and phosphatases. The main regulator of tau dephosphorylation is protein phosphatase 2A (PP2A), which accounts for about 70% of the total tau phosphatase activity in the human brain (Liu et al., 2005). PP2A regulates dephosphorylation of tau directly and indirectly, by regulating the activities of CaMKII, PKA, CDK5, and GSK-3β; Iqbal et al., 2005; Jazvinšćak Jembrek et al., 2013). As it has been well known that PP2A activity is compromised in AD (Gong et al., 1995), PP2A represents one of the most important therapeutic targets. Targeting PP2A for potential treatment of AD has gained even more attention after finding that dietary supplementation with a minor component of coffee unrelated to caffeine, eicosanoyl-5-hydroxytryptamide (EHT), provided protection in a rat model of AD (Basurto-Islas et al., 2014). The effect of EHT was due to its ability to inhibit demethylation of the PP2A catalytic C subunit (PP2Ac), thereby preventing a decline in PP2A activity (Basurto-Islas et al., 2014). A similar effect of enhancing PP2A activity had also been observed for metformin and sodium selenite, which are currently under development for inhibition of tau phosphorylation (Iqbal et al., 2016).
Normal tau protein is thought to have a paper clip-like form, where its C- and N-termini fold over the microtubule-binding domains as short and long ‘arms’, respectively, probably preventing the protein from self-aggregation (Mandelkow et al., 2007). According to Luna-Muñoz and collaborators, at least five different events take place in the “pre-tangle” stage: 1) C-terminal truncation of tau species (Glu-391); 2) a cascade of specific phosphorylations of tau protein in the N-terminus; 3) C-terminal truncation by the action of caspase-3; 4) oligomerization and aggregation of tau; and 5) assembly of tau into PHF (Luna-Muñoz et al., 2013; reviewed in Šimić et al., 2016). However, which form of tau is the most toxic (aggregated misfolded/fibrillar, soluble hyperphosphorylated/mislocalized, or both) and whether that toxicity represents a gain or loss of function continues to be debated. The hypothesis that soluble forms of tau are more toxic to neuronal and synaptic function is increasingly gaining favor, implying that the formation of NFT may protect neurons acutely from the effects of toxic soluble tau (Kopeikina et al., 2012). This hypothesis is supported by the observation that neuron loss in the cerebral cortex of the superior temporal sulcus (Gómez-Isla et al., 1997), as well as entorhinal cortex and hippocampal formation exceeds the number of NFT in AD (Šimić et al., 1998b; Krill et al., 2002).
As tau AD-like hyperphosphorylation occurs in vivo during animal hibernation (Arendt et al., 2003) and in anaesthesia-induced hypothermia (as a consequence of the fact that hypothermia inhibits phosphatases exponentially, but inhibits protein kinases linearly, Planel et al., 2004), it is still not known whether hyperphosphorylation alone is sufficient for tau aggregation. Conversely, aggregation of tau can be induced in vitro e.g. by heparin or other polyanions (Goedert et al., 1996), regardless tau phosphorylation status. Thus, besides hyperphosphorylation, the truncation of tau also seems to be of paramount importance as it promotes tau aggregation through oligomerization of the microtubule-binding repeats (Iqbal et al., 2016; Wang and Mandelkow, 2016). However, although the phosphorylation of tau is in general considered to increase chances of tau for aggregation, phosphorylation of tau at some specific sites seem to be protective, e.g. phosphorylation at Ser422 inhibits the cleavage of tau by caspase-3 at Asp421 (Guillozet-Bongaards et al., 2006), illustrating insufficient knowledge of precise sequence of early molecular events that lead to tau aggregation. What is much better known is that microtubule-binding repeat region R3, which is common to all six tau isoforms, and R2, which is an extra repeat in 4R tau, contain the two hydrophobic hexapeptide motifs with β-sheet structure (VQIINK and VQIVYK, respectively, see Fig. 2 in Šimić et al., 2016), which are responsible for downstream tau aggregation by a nucleation-elongation mechanism (von Bergen et al., 2000) and PHF formation (for review, see Šimić et al., 2016). It is believed that generation of tau transgenic mouse strains with ‘pro-aggregant’ (e.g. ΔK280 mutation in the R2 domain) and ‘anti-aggregant’ (e.g. by adding proline substitutions in the hexapeptide motifs) it will enable better understanding of the importance of tau aggregation for neurodegeneration. Aggregation of tau can experimentally be accelerated in mouse models by adding external seeds from preformed PHF (see below). The third most important post-translational modification of tau is probably O-GlcNAcylation, because it depends highly on intracellular glucose metabolism (Knezović et al., 2015).
1.1.3.2. Acetylation of tau protein
Since its discovery, the role of acetylation of tau pathology has been controversial. First, it has been proposed that tau protein acetylation may be responsible for tau aggregation in AD. On the contrary, however, it was recently shown that the acetylation of tau on KXGS motifs inhibits phosphorylation of the same motif, consequently also preventing tau polymerization and aggregation (Cook et al., 2014). Namely, using a site-specific antibody to detect acetylation of KXGS motifs, it has been found that these sites are hypoacetylated in AD patients as well as in a mouse tauopathy model, suggesting that loss of acetylation on KXGS motifs may be an early event in AD (and that augmenting acetylation of the KXGS motifs would probably decrease tau seeding capacity) (Cook et al., 2014). The first antibody developed to detect acetylation of tau at Lys280 (Irwin et al., 2012) showed that tau acetylated at this epitope colocalized with other classical markers of tau pathology (most prominently in moderate to severe disease stages), and is therefore rather a response to than a cause of the disease process (Cook et al., 2014). Strikingly, subsequent usage of the second antibody developed to dectect acetylation at Lys274 residue of tau, has shown that that acetylation of this epitope is a very early change in AD brains (Min et al., 2015), which occurs even before tangles are detectable (Grinberg et al., 2013). Interestingly enough, acetylation of tau at Lys274 was detected in all tauopathies (both primary and secondary), except in AgD (Grinberg et al., 2013). Argyrophilic grain disease is a common sporadic 4R tauopathy. The term ‘argyrophilic grains’ is derived from their strong staining with the Gallyas silver iodide method, although not all silver methods permit their visualization. In combination with AD or alone, AgD significantly contributes to dementia in older age subjects and alone accounts for about 5% of all dementia cases (Braak and Braak, 1998; Šimić, 2002). Due to the fact that AgD pathological changes are mostly confined to the CA1 subfield of the cornu ammonis, entorhinal and transentorhinal cortices, the amygdala, and the hypothalamic lateral tuberal nuclei (Šimić, 2002), it has been hypothesized that tau acetylation at Lys274 could also promote spreading of tau pathology (whereas in AgD it could have a protective role in this respect) (Cook et al., 2014). The acetylation of tau protein, however, seems to be much more complex than described here due to the fact that, besides Lys274 and Lys280, there are many self-acetylation (including Lys280 site, Luo et al., 2014) and sites acetylated by the CBP (cAMP response element binding protein) and P300 acetyltransferase (Kamah et al., 2014). Lysin residues acetylated by CBP may be deacetylated by histone deacetylase 6 (HDAC6), whereas P300 acetyltransferase sites can be deacetylated by sirtuin 1 (SIRT1) (Cook et al., 2014). In conclusion, depending on the sites involved, the acetylation of tau could both inhibit its degradation (lysine residues 163, 280, 281, and 369) or facilitate its degradation, at the same time suppressing its phosphorylation and aggregation (lysine residues within the KXGS motifs 259, 290, 321, 353, according to the numbering of the longest isoform; acetylation of these sites is reduced in AD and rTg4510 transgenic mice). Targeting specific lysine residues through specific binding of the molecular tweezer molecule CLR01 has been shown to inhibit both tau (Sinha et al., 2011) and Aβ aggregation and fibrillogenesis in vitro (Attar et al., 2013).
1.1.3.3. Propagation of tau protein pathology
Since 2009, the evidence has been mounting that tau protein can also be directly involved in spreading of AD pathology to neighbouring neurons, however, strong evidence supporting this hierarchical progression (“prion-like behavior of misprocessed tau”) is still missing (Hall and Patuto, 2012). Data support the hypotesis that tau hyperphosphorylation alone or in combination with other post-translational tau modifications of tau protein, such as truncation, acetylation, ubiquitination, glycation, N-glycosylation, O-GlcNAcylation, nitration, lipoperoxidation and sumoylation, can induce its ability to template normal tau (for review see Iqbal et al., 2016; Šimić et al., 2016), but whether misfolded tau can catalyse the conformational changes of normal tau to cause the propagation of pathological changes still remains to be elucidated.
At first, it was shown that injection of brain extract from mice that express human mutant Pro301Ser tau into transgenic mice expressing human wild-type tau (ALZ17 model) was sufficient to induce tau pathology not only within, but also adjacent to, the injection site along anatomically connected pathways (Clavaguera et al., 2009). Second, injection of brain extracts from humans who had died with different tauopathies into the hippocampus or cerebral cortex of either ALZ17 or nontransgenic mice was shown to be not only sufficient to drive inclusion formation, but actually effectively reproduced the classic hallmark lesions of the specific tauopathy characteristic of the inoculating brain extract, either AgD, PSP or CBD (Clavaguera et al., 2013). By measuring synaptic levels of total tau using synaptosomes prepared from cryopreserved human postmortem AD and control samples, Sokolow and collaborators demonstrated the abundance of tau, mainly C-terminal truncated tau, in synaptic terminals in aged control and AD samples, whereas tau fragments and dimers/oligomers were found to be a prominent feature of AD synapses (Sokolow et al., 2015). By using quantitative in vitro models, Calafate and collaborators showed that, in parallel to discovered non-synaptic mechanisms, synapses (but not merely the close distance between the cells) enhance the propagation of tau pathology between acceptor hippocampal neurons and tau donor cells (Calafate et al., 2015). Taken together, these studies have provided additional support for the concept that pathologically altered tau species possess a remarkable self-propagating and seeding capacity, and also indicate that seeding-competent tau species are somehow different and distinct across the class of tauopathies (likely depending on a precise biochemical pattern of post-translational modifications that differentially impact conformation and determine aggregate structure), such that the inoculating material acts as an exact template in the new host (Cook et al., 2014).
Tau pathology can indeed be induced and propagated after the injection of tau oligomers (seeds) or aggregates in either wild-type or mutated MAPT transgenic mice (Iba et al., 2013; Peeraer et al., 2015), in a transgenic mouse model overexpressing Pro301Leu mutated MAPT under the control of an inducible neuropsin promoter in the entorhinal cortex (de Calignon et al., 2012; Liu et al., 2012), and tau aggregates can be spread from cell to cell in vitro (Frost et al., 2009; Guo et al., 2013). These new findings suggest that suppressing tau spreading could be an attractive target for the development of disease-modifying therapeutics for AD and other tauopathies, although more in vitro and in vivo studies are needed to determine whether pathologic tau oligomers spread trans-synaptically, by exosomes, or both ways. In the case of soluble monomeric or small oligomeric tau protein, the endocytosis appears to be clathrin-dependent (reviewed in Rubinsztein, 2006). In contrast, larger aggregates of tau could bind heparin in the extracellular matrix and be internalized through macropinocytosis (Holmes et al., 2014). Additionally, it seems that microglia, the primary phagocytes in the brain, may also spread tau via smaller exosome vesicle (40–100 nm in diameter, Asai et al., 2015) or larger ectosome vesicle (50–1000 nm) secretion (Dujardin et al., 2014). Hypotheses on mechanisms by which products of several of the top LOAD risk genes (APOE, BIN1, CLU, ABCA7, CR1 and PICALM), may be involved in spreading tau have been recently formulated (Avila et al., 2015).
1.1.3.4. The role of tau pathology in synaptic damage
Another important aspect of how tau may be involved in neurodegeneration is through its involvement in neurotransmission (for review, see Jadhav et al., 2015). Namely, finding that tau protein can also be phosphorylated on tyrosines, in addition to threonine and serine residues, led to the discovery that human tau Tyr18 in the N-terminal projection domain is phosphorylated in synapses by Fyn tyrosine kinase from the Src family (at least two tyrosine residues - Tyr18 and Tyr29 - are phosphorylated in NFT; Lee et al., 2004). Additionally, as the projection domain of tau in synapses also interacts with postsynaptic density protein 95 (PSD-95) and NMDAR, it is not surprising that tau is essential for NMDA-dependent long-term potentiation (LTP) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA)-dependent long-term depression (LTD), as shown in tau knockout mice (Frandemiche et al., 2014; Jadhav et al., 2015). Thus, pathological tau proteins play an important role in the synaptic impairment of human tauopathies and these changes may occur due to an increase in intracellular Aβ.
Lastly, and importantly, tau acts as a HDAC6 inhibitor. This finding places tau on the map of genotype - environment interactions, because it may mediate environmental stresses via its influence on the regulation of transcriptional activity (Valenzuela-Fernández et al., 2008; Perez et al., 2009). Large protein aggregates such as tau aggregates are excluded from the proteasome and can only be degraded by autophagy in lysosomes. Because selective autophagy of protein aggregates requires ubiquitin-binding receptor proteins such as HDAC6, an excess of tau protein expectedly impairs autophagic clearance by binding to HDAC6 (Leyk et al., 2015). Conversely, decrease in HDAC activity or expression, e.g. by using a novel ubiquitin ligase C-terminus of Hsp70 interacting protein (CHIP) that binds and ubiquitinates HDAC6, could serve to alleviate abnormal tau accumulation (Cook et al., 2012; Cook et al., 2014).
1.1.4 Epigenetic changes in pathogenesis of AD
AD is not an accelerated form of aging (Morrison and Hof, 1997; Šimić et al., 1997) and gene changes alone cannot explain the etiopathogenesis of AD. The moderate concordance of AD among twins (Iacono et al., 2014) suggests other factors, potentially epigenetic and environmental, are related to AD pathogenesis. Epigenetics relate to stable and heritable patterns of gene expression and genomic functions that do not involve changes in DNA sequence, but act at the interface of genetic and environmental factors. Many individual studies have suggested a possible role for epigenetic changes in AD etiology. The most investigated epigenetic mark is DNA methylation, a reversible modification that affects genome function and chromosomal stability through the addition of methyl groups to cytosine located in CpG dinucleotides to form 5-methylcytosine (5mC). In a rare set of monozygotic twins discordant for AD, significantly reduced levels of DNA methylation were observed in the neuronal nuclei of temporal neocortex in the AD twin (Mastroeni et al., 2009). Significantly reduced DNA methylation was also found in entorhinal cortex layer II neurons of 20 AD patients and in particular in the PHF-1/pSer396-immunoreactive NFT-containing neurons (Mastroeni et al., 2010). Together with the repetitive DNA elements Alu and Satellite-α, long interspersed element 1 (LINE-1) is one of the three major contributors of global DNA methylation pattern, which constitute 17%, 4% and 11% of the genome, respectively. Interestingly, a group of AD patients with the best Mini-Mental State Examinaton (MMSE) scores showed a higher level of LINE-1 methylation, than the AD group with the worst MMSE scores (Bollati et al., 2011). However, firm conclusions cannot be drawn yet as, unfortunately, epigenomic studies of AD so far had only limited coverage of DNA methylation sites and microRNAs (miRNAs), whereas other epigenomic markers have not been systematically studied (for review, see Bennett et al., 2015). Of particular interest is the fact that AD patients diplay high homocysteine and low B12 vitamine and folate levels in blood, which represents a physiological response to prevent methionine deficiency is the so-called ‘methyl folate trap’ (Scott and Weir, 1981), and may also occur due to B12 deficiency. This suggests a dysregulation in the S-adenosylmethionine cycle that strictly contributes methyl donors for DNA methylation of the promotors of the genes involved in Aβ processing (Scarpa et al., 2006). A further support for this possibility is the observation of an age-specific epigenetic drifts associated with consistently lower methylation patterns in elderly and LOAD subjects than in young and mid aged people, supporting a strong role for epigenetic effects in the development of AD (Wang et al., 2008).
Environmental toxins, pollutants and metals negatively affect global DNA methylation patterns (LaSalle, 2011). For example, prenatal methylmercury exposure resulted in long-lasting depression-like behavior and hypermethylation of brain-derived neurotropic factor gene (Bdnf) in mouse hippocampus (Onishchenko et al., 2008). Air pollution exposure especially damages the BBB in the brainstem and can trigger an autoimmune response contributing to the neuroinflammatory and AD pathology present in children from very large urban centers (Calderón-Garcidueñas et al., 2015; Brockmeyer and D’Angiulli, 2016). Even though numerous studies connect specific metals and metalloids with Aβ and tau pathology (e.g., Aβ spontaneously self-aggregates in the presence of divalent metals like Fe, Cu, and Zn into amyloid fibrils, Mandel et al., 2007), recently bringing a “metal hypothesis of AD” into focus (Bush and Tanzi, 2008; Bush, 2013; Singh et al., 2014), such data remain rather controversial, warranting further investigations until convincing conclusions might be drawn. Some environmental toxins, such as β-methylamino-l-alanine (BMAA) produced by cyanobacteria cause misfolding and aggregation of various proteins (Dunlop et al., 2013). Chronic dietary exposure to BMAA has been shown to trigger the formation of both NFT and Aβ deposits in the brain of vervet monkeys (Cox et al., 2016).
1.2. Diagnosis of AD
The clinical diagnosis of AD has been generally based on the original 1984 protocol of the National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA; McKhann et al., 1984). It required that the presence of cognitive impairment and a suspected dementia syndrome be confirmed by neuropsychological testing for a diagnosis of possible or probable AD. Similarly to NINCDS-ADRDA, the American Psychiatric Association issued its 4th revised edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV-TR) criteria in 2000. Besides a memory disorder and impairment in at least one additional cognitive domain, the DSM-IV-TR criteria also required both of these impairments to interfere with social functioning or activities of daily living (ADL; American Psychiatric Association, 2000). The advances in newly developed functional neuroimaging techniques, such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET), that had proven their utility to differentiate AD from other possible causes (Okamura et al., 2002; Dougall et al., 2004; Patwardhan et al., 2004), as well as the discovery of distinctive cerebrospinal fluid (CSF) biomarkers (Blennow and Hampel, 2003; Hansson et al., 2006; Blennow et al., 2010; Counts and Mufson, 2010a; Babić et al., 2014), led to a proposed revision of the NINCDS-ADRDA criteria to take into account findings obtained using these methods (Dubois et al., 2007). Unfortunately, multicenter studies showed that usage of enzyme-linked immunosorbent assay (ELISA) kits from different manufacturers significantly affects outcome, making it impossible to use them interchangeably to achieve consensus cut-off values (Babić et al. 2013). In addition to so-called core CSF biomarkers (Aβ1–42, total and tau phosphorylated at Thr181 and Thr231), the usage of new proteomics-based strategies are revealing additional new biomarkers in CSF, some of which have been already validated in clinics (Babić Leko et al., 2016). AD biomarkers are considered of extreme importance due to their use for improving the accuracy of clinical diagnosis, stratification of AD cases, safety monitoring and theragnostics (Blennow et al., 2010).
After this initial effort to incorporate biomarkers into the diagnosis of AD and mild cognitive impairment (MCI; Dubois et al., 2007), the National Institute on Aging and the Alzheimer’s Association (NIA/AA) launched new guidelines for AD in 2011 (Sperling et al., 2011; Albert et al., 2011; McKhann et al., 2011; Hyman et al., 2012; http://www.alz.org/research/diagnostic_criteria/). These guidelines identify three stages of AD: 1) preclinical (presymptomatic) AD (Sperling et al., 2011); 2) MCI, or minor neurocognitive disorder according to the DSM-5 (American Psychatric Association, 2013) due to AD, at which stage it is considered that mild changes in episodic memory and thinking are noticeable and can be measured by neuropsychological testing, but are not severe enough to disrupt a person's daily life (Albert et al., 2011); and 3) dementia (or major neurocognitive disorder, according to DSM-5) due to AD, where impairments in memory, thinking, and behavior decrease a person's ability to function independently in everyday life (McKhann et al., 2011). In addition, the neuropathological criteria for AD have been updated and revised to recognize the preclinical stage of AD (Hyman et al., 2012). In a "preclinical" disease stage, biological changes are under way, but the disease has not yet caused any noticeable (clinical) symptoms. Indeed, it has been shown that in preclinical AD, brain changes caused by the disease may begin even decades before symptoms such as memory deterioration and confusion occur (Braak and Braak, 1997; Braak et al., 2011; Sperling et al., 2011). The guidelines do not include specific diagnostic criteria for this stage; rather, they propose a research agenda to identify biomarkers, such as brain imaging and identification of proteins in CSF, which may signal when presymptomatic brain changes appear (Sperling et al., 2011).
1.3. Neuropathological criteria for AD
The fourth part of the NIA/AA guidelines (Hyman et al., 2012; Montine et al., 2012) updated the 1997 NIA/Reagan Institute neuropathological criteria for AD (Hyman and Trojanowski, 1997). Basically, AD neuropathologic changes are ranked along three parameters to obtain an “ABC score” (A – for amyloid β, Aβ, score: A0: no Aβ or amyloid plaques (AP), A1 Thal phase 1 or 2, A2: Thal phase 3, and A3: Thal phase 4 or 5 (modified from Thal et al., 2002); B – for the Braaks’ six neurofibrillary tangles (NFT) stages (Braak and Braak, 1991; see Fig. 4) that can be reduced to four with improved inter-rater reliability (Nagy et al., 1998): B0: no NFT, B1: Braak stages I/II, with NFT predominantly in entorhinal cortex and closely related areas, B2: stages III/IV, with NFTs more abundant in the hippocampus and amygdala while extending slightly into association cortex, and B3: stages V/VI, with NFT, neuropil threads and dystrophic neurites widely distributed throughout the neocortex and ultimately involving primary motor and sensory areas; and C – for neuritic plaques (composed of a core of Aβ and surrounded by dystrophic neurites made of abnormally hyperphosphorylated tau aggregated into PHF), NP, score: C0: no NP, C1: sparse NP, C2: moderate NP, and C3: frequent NP (modified from the Consortium to Establish a Registry for Alzheimer’s disease, CERAD; Mirra et al., 1991). Recommended brain regions for such tiered evaluation are: the medulla oblongata (including the dorsal motor nucleus of the vagus), pons (including the locus coeruleus, LC), midbrain (including the substantia nigra, SN), cerebellar cortex and dentate nucleus, thalamus and subthalamic nucleus, basal ganglia at the level of anterior commissure with the basal nucleus of Meynert, hippocampus and entorhinal cortex, anterior cingulate cortex, amygdala, mid frontal gyrus, superior and mid temporal gyri, inferior parietal lobule, occipital cortex (Brodmann’s areas 17 and 18), and white matter at the anterior, middle, and posterior cerebral arteries’ watershed areas.
The preferred method for visualization of Aβ plaques is immunohistochemistry for Aβ, and for NFT is immunohistochemistry for tau or phosphorylated tau epitopes (Braak et al., 2006; see Fig. 3). Other acceptable methods for NFT are thioflavin S or sensitive silver histochemical stains (Braak and Braak, 1991). The preferred method for NP is thioflavin S or modified Bielschowsky stain (water solution of silver nitrate, AgNO3; Fig. 2), as recommended by the CERAD protocol (Mirra et al., 1991). Although CAA is not a part of the ABC score, it is suggested to report it using the staging system for CAA of Vonsattel et al. (1991) and association with inheritance of the ε4 allele of apolipoprotein E (APOE) recognized (Thal et al., 2008). Finally, the ABC scores obtained are transformed into one of four levels of AD neuropathologic change: no change, low, intermediate, or high level of AD neuropathologic change, where intermediate or high AD neuropathologic changes are considered as a sufficient explanation for the presence of dementia/major neurocognitive disorder.
1.4. Clinicopathologic correlations
With respect to clinicopathologic correlations, the NIA/AA guidelines also provide a table with the frequency and confidence intervals of cases within each range of ABC scores for CDR sum of boxes score, which represents the sum of scores of clinical impression of symptom severity (ranging from 0 - normal to 3 - marked impairments), in each of six domains of behavioral and cognitive function (Morris, 1993; O’Bryant et al., 2008), to help interpret results from autopsies with incomplete medical records. Although AD is the most common cause of dementia/major neurocognitive disorder and can exist as a pure form in 17–72% of cases irrespective of the clinical symptoms, according to Jellinger and Attems (2015), it commonly coexists with pathologic changes of other diseases that also contribute to cognitive and behavioral impairments.
The most common comorbidities are: 1) Lewy body disease (LBD, a subset of diseases which includes Parkinson’s disease, PD, and dementia with Lewy bodies, DLB, that share the feature of abnormal accumulation of α-synuclein in neurons); 2) cerebrovascular diseases (CVD) that cause vascular brain injury (VBI), including atherosclerosis, arteriolosclerosis (small-vessel disease or lipohyalinosis), and CAA; 3) hippocampal sclerosis (HS); 4) argyrophilic grain disease (AgD); 5) TDP-43 proteinopathy; and 6) cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as well as many other neuropathologic changes.
For a given amount of AD neuropathologic change, cognitive and behavioral symptoms tend to be worse in the presence of these comorbidities (which are frequently missed clinically and may be difficult to identify neuropathologically) that may have an additive or synergistic effect, although their mutual impact often remains unclear (for a review see Nelson et al., 2012, and Jellinger and Attems, 2015). As such, it may be very difficult to judge the extent to which each disease process observed at autopsy may have contributed to a given patient’s cognitive state, particularly when a low level of AD-related neuropathology is observed in the setting of cognitive and behavioral impairment. Additionally, the recommended semiquantitative ABC criteria for routine use does not preclude the possibility that other processes or lesions may critically contribute to the pathophysiology of AD. In this respect it is important to emphasize that soluble forms of both Aβ (Walsh and Selkoe, 2007) and tau protein (Kopeikina et al., 2012) have been implicated in AD pathogenesis, but would not be apparent through using by the conventional techniques used and described in the NIA/AA guidelines.
It needs to be kept in mind that medial temporal lobe NFT may be found in old and very old people in the absence or relative absence of Aβ or NP (Yamada, 2003; Jellinger and Attems, 2007; Nelson et al., 2009). Previously known as “tangle-predominant senile dementia” (TPSD, Jellinger and Attems, 2007) or “tangle-only dementia” (Yamada, 2003), this neuropathological entity has recently been termed primary age-related tauopathy (PART; Crary et al., 2014; Jellinger et al., 2015). Symptoms in persons with PART usually range from normal to amnestic cognitive changes, with only a minority exhibiting profound impairment (Crary et al., 2014). In addition, other diseases that must be considered in the differential diagnosis of dementia/major neurocognitive disorder include tauopathies other than PART, most importantly FTLD (its clinical presentation is usually called frontotemporal dementia, FTD) and its subtypes, such as FTLD-tau, FTLD-TDP (for TDP-43 protein), FTLD-FUS (fused in sarcoma protein), FTLD-UPS (ubiquitin proteasome system), FTLD-ni (no inclusions, also known as dementia lacking distinctive histopathology; Mackenzie et al., 2010), and prion disease. Mutations in several genes have been associated with the majority of genetic FTLD: MAPT (encoding protein tau), GRN (encoding for protein progranulin), TARDBP (encoding transactive response DNA-binding protein 43, TDP-43, which is often associated with amyotrophic lateral sclerosis, ALS, or ALS/FTD, but also with FTD subjects without ALS), VCP (encoding valosin-containing protein, which is also mutated in ALS), C9ORF72 (encoding C9orf72 protein, also mutated in ALS), and TMEM106B (Benussi et al., 2015). Two new loci associated with FTLD have been recently reported: one linked to HLA (human leukocyte antigen) locus on 6p21.3 and the other to 11q14 locus (CHMP2B, whose transcripts are related to lysosomes), suggesting that immune system processes and possibly lysosomal and autophagy pathways, may also potentially be involved in FTLD (Ferrari et al., 2014; Clayton et al., 2015).
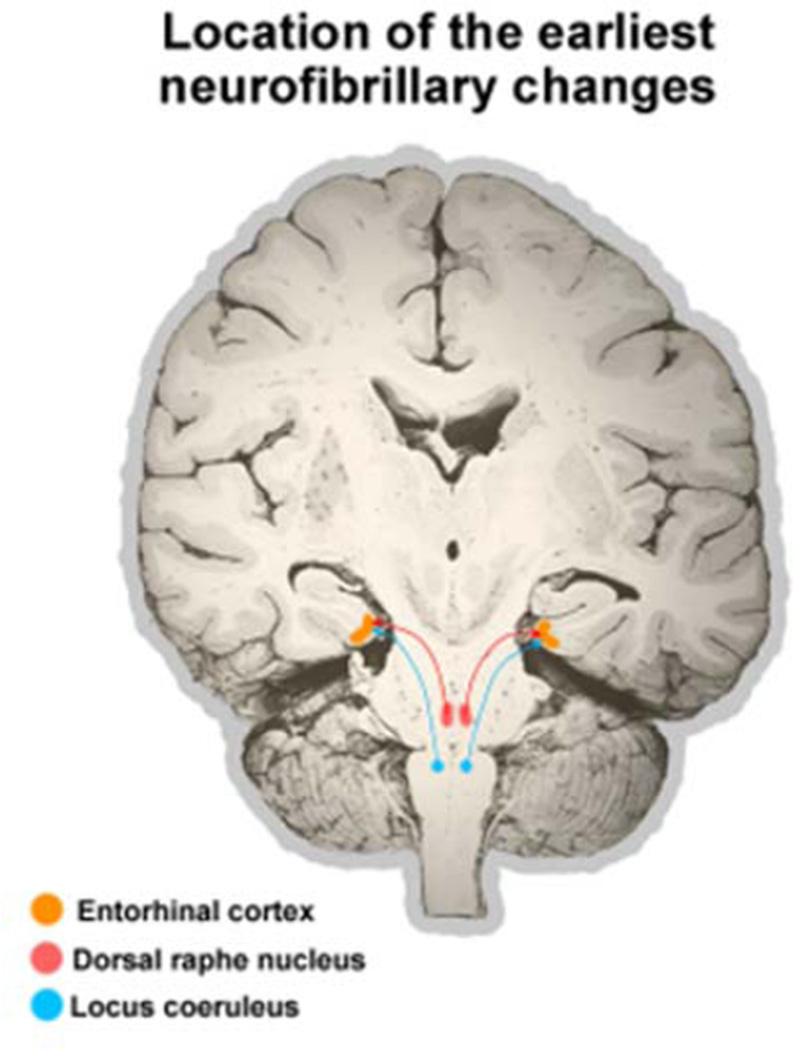
Several recent reports drew attention to the possibility of selective and early involvement of not only the LC (Hyman and Trojanowski, 1997) but also the raphe nuclei, particularly the DRN, in the pathogenesis of AD (Rüb et al., 2000; Grinberg et al., 2009; Michelsen et al., 2008; Šimić et al., 2009). In a clinicopathological series of 118 cases, out of which 38 were categorized as stage B0 (at least four sections at different levels of transentorhinal cortex were free of neurofibrillary changes, based on lack of immunoreactivity for monoclonal antibodies PHF-1 and AT8), and 80 as stage B1 (rare neurofibrillary changes in the transentorhinal cortex), more than 20% of B0 and all of B1 cases had substantial neurofibrillary changes in the DRN. However, because raphe nuclei dysfunction due to neurofibrillary changes is not included even in the new criteria (Hyman et al., 2012; Montine et al., 2012), its possible behavioral consequences are not yet considered as a potential early characteristic clinical feature of AD.
Numerous clinical and neuropathological studies performed from the early 1980s to the present have established compelling links between wide range of structural and functional abnormalities of subcortical monoaminergic systems and the pathophysiology of AD. Here below, we first provide brief general information on involvement of each monoaminergic system in AD, followed by its more detailed chemical neuroanatomy (origins of neurons, distribution of receptors) and functional relevance of their alterations in AD. Finally, we discuss interactions among monoaminergic systems and with the cholinergic system in AD and prospect for future monoamine-based treatments in AD.
2. Chemical neuroanatomy of the monoaminergic systems
2.1. Serotonergic system
The impairment of serotonergic system in AD was shown in humans both in vivo and postmortem. Early non-cognitive behavioral and psychological symptoms of dementia (BPSD), such as disturbances in mood, emotion expression and recognition (Waanders-Oude Elferink et al., 2015), appetite, wake-sleep cycle, confusion, agitation, and depression are probably clinical signs of serotonergic nuclei involvement in AD (Šimić et al., 2009). One of them, sundowning (the increase in one or more abnormal behaviors such as agitation or activity during evening hours), is estimated to occur in about 45% of individuals diagnosed with AD (Scarmeas et al., 2007). Depression as the most pronounced clinical symptom is considered as a risk factor for neurodegeneration (Donovan et al., 2015). AD patients also typically show sleep fragmentation with frequent awakenings during the night, and a propensity to sleep during the daytime (Ancoli-Israel et al., 1994; Lim et al., 2014). Recent animal studies confirmed how early occurence of AD pathology in serotonergic nuclei leads to wake-sleep cycle disturbances (Sterniczuk et al., 2010; Roh et al., 2012).
Many studies found reductions of serotonin (5-hydroxytryptamine, 5-HT), along with its metabolites and receptors in AD postmortem brain tissue (Nazarali and Reynolds, 1992; Garcia-Alloza et al., 2005; Ramirez et al., 2014), as well as reduced serotonergic innervation of the cerebral cortex, amygdala, hippocampus, globus pallidus, lateral nucleus of the thalamus and elsewhere (for review see Trillo et al., 2013).
5-HT is an indoleamine produced from L-tryptophan by the sequential action of tryptophan hydroxylase (TPH, EC 1.14.16.4) and aromatic-L-amino acid decarboxylase (AAAD, EC 4.1.1.28) (Green, 1989). TPH is the rate-limiting enzyme in 5-HT biosynthesis. For many years, only one gene encoding TPH in vertebrates was known. In 2003, a second TPH gene (termed TPH2) was identified on chromosome 12 (Walther et al., 2003). The finding that TPH2 is predominantly expressed in the brain, whereas TPH1 is expressed in peripheral tissues justified the concept of the “central” serotonergic system (Côté et al., 2003; Patel et al., 2004). Unlike other monoamines that are mainly metabolized by monoamine oxidase A (MAO-A, EC 1.4.3.4), serotonergic neurons and astrocytes contain predominantly monoamine oxidase B (MAO-B, EC 1.4.3.4), which metabolizes 5-HT into 5-hydroxyindoleacetic acid (5-HIAA) (Beck et al., 1987; Green, 1989; Fitzgerald et al., 1990). Therefore, the role of MAO-B in serotonergic neurons might be to eliminate intracellular competition of 5-HT with dopamine and other monoamines at low concentrations (as at high concentrations of substrates, MAO isoenzymes loose their selectivity). Besides 5-HT, MAO oxidases catalyze the oxidative deamination of noradrenaline, adrenaline, dopamine, melatonin, tryptamine, histamine, and taurine. In addition to an aldehyde, in MAO catalyzed reaction ammonia and hydrogen peroxide are also formed. Aldehydes are further oxidized by aldehyde dehydrogenase into carboxylic acids, whereas hydrogen peroxide in the presence of transition divalent metals (iron, copper, zinc) may be converted to highly reactive hydroxyl radical (by the Fenton reaction). Thus, along with mitochondrial oxidative phosphorylation, MAO activity is probably the second most significant source of reactive oxygen species (ROS) and oxidative stress in the brain (Edmondson et al., 2014). Another unfavourable consequence of MAO activity, the production of ammonia, puts additional strain on the NH4+-clearing system of monoaminergic neurons making them more vulnerable, because it involves glutamate transporters (Šalković-Petrišić and Riederer, 2010), glutamate dehydrogenase 1 (GLUD1) and glutamine synthetase (also glutamate ammonia ligase, GLUL), which decrease the 2-oxoglutarate (2OG) and glutamate pools. Neurons are very sensitive to the depletion of these pools, especially 2OG, because it decreases adenosine triphosphate (ATP) production in the citric acid cycle. Interestingly, by inhibiting ATP synthase and target of rapamycin (TOR), α-ketoglutarate (α-KG) has been shown to be a key metabolite that mediates longevity by dietary restriction and extends lifespan of Caenorhabditis elegans (Chin et al., 2014). It can be thus concluded that lowered metabolism of monoamine neurotransmitters, as a consequence of therapeutic effect of MAO inhibitors, increases availability of monoamine neurotransmitters, which underlie the antidepressant action, and at the same time decreases oxidative stress, particularly in the case of MAO-B inhibitors. That was the main reason they have been suggested as options for AD treatment (Bortolato et al., 2008; Di Giovanni et al., 2014). MAO inhibitors are therefore an integral part of the concept of the multitarget-directed ligand design strategy based on combination of pharmacophores of diverse compounds to get hybrid drugs. New drugs for AD, for example, may combine acetylcholinesterase inhibitors (AChEI) with compounds acting on metabolism of Aβ, tau, monoamines, iron, transporter activities, channels, etc. (Bolea et al., 2013). Consumption of coffee appears to improve glucose metabolism and reduce the risk of dementia, but more studies are required to identify the active components involved to address this issue (Varghese et al., 2014).
5-HT is produced in serotonergic neuron groups. These groups are mainly embedded in the raphe nuclei. The general organization of the raphe nuclei and the distribution of serotonergic neurons appear to be very similar among mammalian species with the exception of the B4 group in the floor of the fourth ventricle, which is absent in primates, and has been confirmed in humans (Halliday et al., 1988; Halliday and Törk, 1989; Törk, 1990; Hornung 2003, 2004) (see Table 1). The raphe nuclei are located along the midline of the brainstem and span as loosely arranged cells aggregations from the midbrain to the junction of the medulla oblongata with the spinal cord. The continuity of the raphe nuclei is interrupted only by the reticulotegmental nucleus of the pons, which separates the serotonergic neurons into two large groups: one in the rostral pontine and mesencephalic tegmentum (rostral raphe group, B5–B9) and one in the medulla oblongata (caudal raphe group, B1–B3) (Takahashi et al., 1986; Halliday et al., 1988; Baker et al., 1990; Törk, 1990; Törk and Hornung, 1990; Nieuwenhuys et al., 2008).
Table 1.
Overview on the main serotonergic cells groups, their afferent and efferent projections, and functional consequences of their lesion or dysfunction.
| The designation of the serotonergic cell group |
The name of the raphe nucleus where the appropriate serotonergic cell group is located |
Location of the cell group |
Main afferent projections |
Main efferent projections |
Consequences elicited due to a lesion |
|
|---|---|---|---|---|---|---|
| Caudal raphe group | B1 | Nucleus raphe pallidus |
Medulla oblongata |
PAG, reticular formation, parabrachial n. (Kölliker-Fuse), hypothalamus, preoptic area, PFC |
Laminae I, II and V of the dorsal horns of the spinal cord, intermediolateral column of the spinal cord, sensory nuclei of the brain stem, superior colliculus, praetectal nn., intralaminar thalamic nn., preoptic nn., motoneurons of the anterior horn of the spinal cord (via fibers in the lateral and ventral funiculus) including Onuf’s nucleus (Rexed’s lamina IX) |
Loss of pain- controlling input from PAG to the spinal cord; changes in excitability of cranial and spinal motoneurons |
| B2 | Nucleus raphe obscurus |
Loss of regulation of sympathetic system, especially regarding cardiovascular functions; changes in excitability of cranial and spinal motoneurons |
||||
| B3 | Nucleus raphe magnus |
Spinal trigeminal n., gracile n. and cuneate n., PAG, LC (=noradrenergic nucleus A6), n. cuneiformis, gigantocellular reticular n., superior colliculus, inferior colliculus, pretectal nn., hypothalamus, parafascicular thalamic n., hypothalamus, preoptic area,lateral habenular n., central n. of amygdala, zona incerta, BNST, PFC |
Sensory nuclei of the brain stem, cranial motor nuclei, LC (=noradrenergic nucleus A6), superior colliculus, praetectum, intralaminar thamalic nn., preoptic area, septum, hypothalamus (ventromedial, suprachiasmatic), putamen, caudate n., amygdala, mammillary bodies, hippocampal formation, SN, olfactory cortex, lateral geniculate nucleus, habenula, parabrachialis n., noradrenergic nuclei A7 and A5, solitary nucleus, dorsal horn of the spinal cord (laminae I and II) via fibers in the dorsolateral funiculus (Lissauer’s tract), intermediolateral n. |
Lack of endogeneous analgesia (i.e. inhibition of nociceptive transmission in the superficial laminae of the caudal spinal nucleus of the trigeminal nerve and the spinal dorsal horn), diminished motor response to nociceptive stimuli |
||
| Rostral (oral) raphe group | B5 | Pontine raphe nucleus (median and dorsal) |
Pons | Solitary nucleus, PAG, SN, interpeduncular n., hypothalamus, preoptic area, lateral habenular n., vental pallidum, BNST, medial septal n., nucleus of diagonal band (of Broca), PFC |
Cranial motor nuclei, cerebellum, LC, hypothalamus, septum, hippocampus, entorhinal cortex, limbic cortex |
low mood, low self-esteem (hopelessness and pessimism) |
| B6 | ||||||
| B7 | Dorsal raphe nucleus (NRD) |
Mesen- cephalon |
Solitary nucleus, laterodorsal tegmental n. (cholinergic nucleus Ch6), SN, pontine raphe n., LC (= noradrenergic nucleus A6), parabrachialis n. (Kölliker- Fuse), VTA, hypothalamus, tuber cinereum and nuclei tuberis, preoptic area, lateral habenular n., central nucleus of amygdala, ventral pallidum, zona incerta, BNST, nucleus of the diagonal band (of Broca), PFC |
Cerebral cortex, especially transentorhinal and entorhinal cortex (especially from ST, IF, and CC), hippocampal formation (especially from NRC), and PFC (especially from NRL, NRC, NRD CC and CL), olfactory bulb, striatum (including caudate n., putamen, n. accumbens, ventral pallidum, and especially globus pallidus), septum, amygdala (especially central n.), BNST, intralaminar thalamic nn., thalamic relay nn., lateral parabrachial n., retina, pretectum and superior colliculus, SN, n. raphe magnus, n. raphe pallidus, pedunculopontine n., cranial motor nuclei, sensory nuclei of the brain stem, spinal cord |
Mood changes (most often depressive symptoms), anxiety, abnormal conditioned fear response, social withdrawal, confusion, irritability, agitation, restlessness and aggression (first verbal then also physical), disturbances in wake-sleep cycle (“sundowning”), disturbances of NREM sleep, emotion and appetite; oppose the action of dopamine and mediate avoidance of threats; impaired function sensitizes the dopamine system resulting in impulsivity and drug addiction) |
|
| B8 | ||||||
| B9 | ||||||
Legend: BNST, bed nucleus of the stria terminalis; CC, NRD pars caudalis compacta; CL, NRD pars caudalis lamellaris; IF, NRD pars interfascicularis; LC, locus coeruleus; PAG, periaqueductal gray matter; NRD, nucleus raphe dorsalis; NRC, nucleus raphe centralis; NREM, non-rapid eye movement sleep (sleep stages 1–3); NRL, nucleus raphe linearis; SN, substantia nigra; ST, NRD pars supratrochlearis; VTA, ventral tegmental area. Caution: data on the connections of the raphe nuclei (and also other monoaminergic cell groups were mostly collected in rodents and carnivores, while observations in humans and non-human primates are rare). The table is based mainly upon Nieuwenhuys et al., 2008; Michelsen et al., 2008, and Arslan, 2015.
The rostral (oral) raphe group in humans comprises the centromedian part, which consists of the nucleus raphe centralis pars principalis (NRC-P), the nucleus raphe centralis pars annularis (NRC-A), and the nucleus raphe linearis (NRL). The dorsal part is also known as the DRN. There are apparent difficulties when comparisons are made in regard to the DRN subdivisions used in various publications. For example, Ohm and collaborators considered the interfascicular, dorsofascicular and intercalate subnuclei as principal minor subdivisions of DRN (Ohm et al., 1989), whereas Baker and collaborators subdivided the DRN into five subnuclei: interfascicular, ventral, ventrolateral, dorsal, and caudal (Baker et al., 1990). According to the classification made by Braak (1970), which is one of the most commonly used (Michelsen et al., 2008), the DRN is comprised of the pars supratrochlearis (DRN ST), pars interfascicularis (DRN IF), pars caudalis compacta (DRNCC), and the caudal lamellar subnucleus (DRN CL). These nuclei generally correspond to the B5–B9 nuclei originally described in rodents (Dahlström and Fuxe 1964a, 1964b; Fuxe, 1965) (see Fig. 5). The rostral group gives rise to ascending cortical, cerebellar, and local cortical and subcortical projections. The caudal raphe group consists of the nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO), and the nucleus raphe pallidus (NRP), which correspond to B3, B2, and B1, respectively (Fig. 5). The caudal group is reciprocally connected to the brainstem, the cerebellum, and the spinal cord, whereas ascending projections are not as far-reaching as in the case of the rostral group (Table 1).
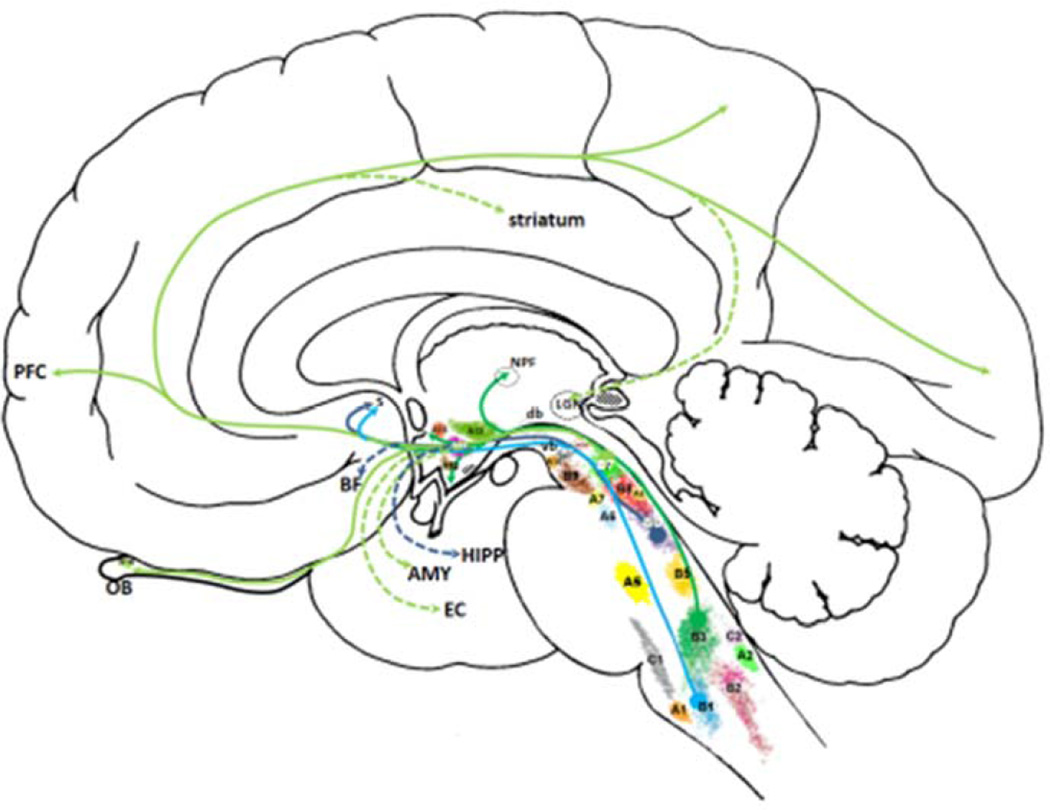
Figure 5.
Schematic drawing of monoaminergic nuclei (except histaminergic and melatonergic cell groups). A1–A7 denote noradrenergic cell groups (A3 is missing in primates), A8–A16 dopaminergic cell groups (A11 is missing in humans, whereas retinal dopaminergic neurons are sometimes denoted as A17 group), B1–B9 serotonergic cell groups (B4 is missing in primates), whereas C1 and C2 denote adrenergic cell groups (C3 group is not present in humans). Histamine neurons in humans are located exclusively in tuberomammilary nucleus (stippled area caudal to A12). Melatonin neurons are located in the pineal gland (stippled area posterior to LGN). Emphasis is given on a rough sketch of two main ascending serotonergic systems: M-fibres with coarse varicosities take their origin from the nucleus raphe pontis (dorsalis, B6, purple lines) and nucleus raphe pallidus (B1, light blue lines) as well as from the nucleus raphe pontis medianus (B5) to a lesser extent (fibers from B5 not drawn) ascending through the tegmental area as the ventral bundle (vb), whereas fibres with small varicosities arise from NRD (light green lines, B7) and nucleus raphe magnus (dark green lines, B3) and collect in a dorsal bundle (db). Large serotonergic axons of the ventral bundle make synaptic contact with their targets, whereas fine axons with small varicosities release serotonin diffusely (volume transmission). AMY, amygdala; BF, basal forebrain; LGN, lateral geniculate nucleus; db, dorsal bundle of serotonergic fibers; EC, entorhinal cortex; HIPP, hippocampus; OB, olfactory bulb; NPF, nucleus parafascicularis; PFC, prefrontal cortex; S, septum; vb, ventral bundle of serotonergic fibers; see Table 1 for the designation of the serotonergic cells groups and their afferent and efferent connections. See text for detailed description.
Quantitative studies of the total number of serotonergic neurons in the raphe nuclei report that the human DRN contains about 235,000 ± 13,000 neurons (Baker et al., 1990), of which approximately 165,000 ± 34,000 neurons (or about 70%) contain serotonin (Baker et al., 1991a). Using a monoclonal antibody raised against TPH (PH8), which recognizes the 5-HT-synthesizing enzyme in formalin-fixed human brain tissue, it was shown that the contingent of serotonergic neurons within the DRN amounts to about 80% of the total neuronal population, while the caudal raphe group contains only about 10–20% of serotonergic neurons (Baker et al., 1991b; Hornung, 2004). The large numbers of scattered 5-HT-synthesizing neurons in the pontine, and particularly in the mesencephalic tegmentum in primates, contrast with their relative paucity in nonprimate species (Baker et al., 1991a).
There are two morphologically distinct classes of serotonergic axons (Kosofsky and Molliver, 1987): beaded axons with large, spherical varicosities (up to 5 µm in diameter), which make synaptic contact with their targets (so-called M-fibers, Törk, 1990), and fine axons with small (smaller than 1 µm in diameter) varicosities (the main fibers, ubiquitous throughout the mammalian cerebral cortex and also called D-fibers, Törk, 1990), which lack membrane junctional complexes (synapse) and release serotonin diffusely through volume transmission (Törk, 1990; Descarries et al., 1991; Descarries and Mechawar, 2000; De-Miguel and Trueta, 2005; Fuxe et al., 2012). The third and least common type was first described in the marmoset, and is thought to be the stem fibers for M-fibers (Hornung et al., 1990). This third type of fibers can be noted throughout the cortex of all mammalian species. The M-fibers are found in the supragranular cortical layers, but can also be occasionally seen in infragranular layers of chimpanzees and humans (Raghanti et al., 2008a). Compared with macaque monkeys, humans and chimpanzees also display a greater density of serotonergic axons in layers V and VI in prefrontal cortical areas 9 and 32 (implicated to mediate working memory and higher cognitive functions), but not in the primary motor cortex (Raghanti et al., 2008). Interestingly, morphological specializations of M-fibers called coils of axons were observed in humans and chimpanzees in all cortical layers, but are absent in macaques and all other primate and non-primate mammalian species (Raghanti et al., 2008a). It has been hypothesized that these morphological features similar to coils described for tyrosine-hydroxylase-immunoreactive axons in humans (Gaspar et al., 1989; Benavides-Piccione and DeFelipe, 2003; Raghanti et al., 2008b) and clusters described for choline acetyltransferase (ChAT)-containing fibers, also in humans, may represent a substrate for a greater capacity for cortical plasticity (hence behavioral flexibility) exclusive to hominoids (anthropoid apes and humans) (Raghanti et al., 2008a).
The described two types of axons originate from different raphe nuclei: M-fibres with coarse varicosities take their origin from the nucleus raphe pontis (medianus and dorsalis; fibers colored in purple in Fig. 5) and nucleus raphe pallidus (light blue fibers in Fig. 5) ascending through the tegmental area as the ventral bundle, whereas D-fibres with small varicosities arise from the DRN (fibers colored in light green in Fig. 5) and nucleus raphe magnus (fibers colored in dark green in Fig. 5) and collect in the dorsal bundle. There is an increase in serotonergic neuron number in the nucleus raphe pontis medianus of cats and primates relative to rodents (Jacobs et al., 1984; Azmitia and Gannon, 1986). The dorsal bundle was identified and described by Forel in 1877; the area containing ascending fibers of this bundle first coincides approximately with the dorsal longitudinal fasciculus (Arslan, 2015) and then with the dorsal trigeminothalamic tract. At the border with the forebrain, the ventral and dorsal bundles join together and continue towards the subthalamus and thalamus and their cortical targets via the medial forebrain bundle that passes through the internal capsule. The dorsal bundle (also known as the dorsal raphe cortical tract) is phylogenetically newer than the ventral bundle, is more prominent in primates (presumably due to an increase in fibers projecting to the cortex through the dorsal pathway, Piñeyro and Blier, 1999) and more vulnerable to neurotoxic amphetamine derivatives methylenedioxyamphetamine and p-chloroamphetamine (Mamounas et al., 1991).
The two systems of serotonergic fibers coexist in most parts of the brain, with the cerebral cortex being the best example of dual contribution to serotonergic innervation. However, although they share many of their targets (Table 1), the main targets of the dorsal bundle (comprised mostly of DRN projections) are the entorhinal cortex, lateral geniculate nucleus, the olfactory bulb, the amygdala, and the striatum, which is almost exclusively innervated by the Dfibers of this system, whereas the pontine dorsal (B6) raphe nucleus mainly projects via the ventral bundle (M-fibers) to the septum, basal forebrain, and especially hippocampus (Steinbusch et al., 1980; Kohler and Steinbusch, 1982; Imai et al., 1986; Morrison and Foote, 1986; Törk, 1990). The pontine median (B5) raphe nucleus connects with the interpeduncular nucleus, substantia nigra, and the mammillary body, but its cortical projection is rather sparse (O’Hearn and Molliver, 1984).
The serotonergic system is one of the oldest neurotransmitter systems and seven distinct serotonergic receptors (5-HT1 – 5HT7), each with several subpopulations of receptors (altogether, at least 14 members in the family are known), mediate both central and peripheral control on numerous physiological functions such as sleep-wake cycle, feeding behavior, thermoregulation, nociception, affective (mood) control and sexual behavior, locomotion and motor control (via interactions with the basal ganglia dopaminergic system), blood coagulation, and cardiovascular homeostasis (Darmon et al., 2015). 5-HT receptors are different in terms of localization and downstream signaling. All 5-HT receptors are G-protein-coupled receptors except 5-HT3, which is a Cys-loop ligand-gated ion channel. 5-HT1 (1A, 1B, 1C, 1D, and 1E) and 5-HT5 receptors (5A and 5B) have an inhibitory effect on adenylyl cyclase through Gαi/o protein. 5-HT2 receptors (2A, 2B, and 2C) activate phospholipase C cascade through the Gαq/11 protein. 5-HT3 receptors (3A, 3B, and recently discovered 3C, 3D and 3E) have a direct influence on the cell’s processes as ion channels. 5-HT4, 5-HT6 and 5-HT7 receptors activate adenylyl cyclase through GαS protein. The majority of 5-HT receptors are postsynaptic, except for 5-HT1B and 5-HT1D receptors, which are mostly presynaptic, and 5-HT1A receptors that are located both on presynaptic and postsynaptic membranes (Seyedabadi et al., 2014 and Darmon et al., 2015).
2.2. Noradrenergic system
The most prominent effect of AD on the noradrenergic system is the loss of up to 70% of locus coeruleus (LC) noradrenergic neurons (Bondareff et al., 1982; Iversen et al., 1983; Zweig et al., 1989). Together with the serotonergic nuclei, the LC is involved in the control of the sleep-wake cycle and, among other symptoms, its impairment can cause early and prominent wake-sleep cycle disturbances in AD (Roh et al., 2012). As noradrenergic projections innervate cerebral vasculature and optimize neurovascular coupling, the diminished ability to couple blood volume to oxygen demand may also contribute to AD pathogenesis (Bekar et al., 2012).
The catecholamine noradrenaline is synthesized from dopamine by the enzyme dopamine-β-hydroxylase (DBH, EC 1.14.17.1) and is metabolized by MAO and catechol-O-methyltransferase (COMT; EC 2.1.1.6). Noradrenaline binds solely to metabotropic α-(α1A, α1B, α1D, α2A, α2B, α2C) and β-adrenoreceptors (β1, β2 and β3) (Ruffolo and Hieble, 1994). The noradrenergic system regulates visceral functions (respiration, cardiovascular function, secretion of hormones), cognitive functions (responsiveness to novel stimuli, vigilance, learning through reinforcement), arousal, attention, sleep-wake cycle, emotion, mood, motor control, and pain control (Harley, 1987; Szabadi, 2013).
The brainstem neurons that produce noradrenaline form noradrenergic nuclei (A1–A7) are divided into dorsal (A2, A4, A6) and ventral (A1, A5, A7) columns. A1, A2, A5 and A7 project to the basal telencephalon, hypothalamus, brainstem, and spinal cord, while A4 and A6 (locus coeruleus, LC) project to the cortex, cerebellum, thalamus, and spinal cord (Pearson et al., 1991). The nucleus locus coeruleus (A6) contains approximately 50% of all noradrenergic neurons and is the only noradrenergic nucleus that innervates the cortex (Samuels and Szabadi, 2008).
While fibers from the LC project to the forebrain, cerebellum and spinal cord, noradrenergic fibers from the lateral brainstem tegmentum project to the ventral forebrain, hypothalamus, amygdala and spinal cord (Heimer, 1995). More precisely, noradrenergic fibres from the LC together with cholinergic and serotonergic fibres form an ascending arousal system (AAS). Noradrenergic and serotonergic fibres form the ventral branch of the AAS, enter the hypothalamus and as a part of the medial forebrain bundle reach the mediobasal telencephalon. On this path, noradrenergic fibers innervate forebrain regions like the basal forebrain, preoptic area, olfactory structures, hypothalamus, thalamus, hippocampus, and neocortex (Foote and Morrison, 1987; Raghanti et al., 2009). Fibers from the LC and A5 and A7 noradrenergic nuclei descend to the spinal cord as a part of tractus pontospinalis and tractus coeruleospinalis, and innervate many regions of the rhombencephalon (Westlund et al., 1982). The cerebellum is also innervated by the noradrenergic fibers from the LC, A5, and A7 noradrenergic nuclei (Voogd et al., 1996). In addition to the neighboring structures, fibers from the A1 and A2 noradrenergic nuclei innervate the thalamus, amygdala, and hypothalamus (Petrov et al., 1993).
The projections from the LC constitute an arousal system, since the noradrenergic neurons are active specifically during waking (Kayama and Koyama, 2003). Broadly speaking, if LC activity is too low, an animal is drowsy and inattentive. If LC activity is too high, the animal is distractible and anxious, but with the intermediate levels of LC activity, the animal is optimally attentive and aroused (España and Scammell, 2011). Mice deficient of noradrenaline exhibit normal sleep and wake states, but fall asleep after exposure to a mild stressor more rapidly than control mice, measured both behaviorally and with electroencephalography (Hunsley and Palmiter, 2003). Noradrenaline tone is also clearly related to cognition as LC neurons in monkeys fire phasically in response to a salient stimulus that signals a reward such as food, but do not respond to a distracting stimulus (Aston-Jones et al., 1994), suggesting that LC activity promote arousal in a way that optimizes attention and task performance (Aston-Jones and Cohen, 2005).
2.3. Dopaminergic system
The reduction of dopamine, dopamine metabolites, and dopamine receptors has been observed in AD (Storga et al., 1996; Trillo et al., 2013). Additionally, polymorphisms in dopaminergic system genes are associated to BPSD in AD (Holmes et al., 2001; Borroni et al., 2004). Enhancement of dopaminergic transmission alleviates cognitive impairment in AD (Martorana et al., 2013; Stefani et al., 2015).
Dopamine is a catecholamine synthesized from tyrosine by the rate-limiting enzyme tyrosine hydroxylase (TH, EC 1.14.16.2) and AAAD, and degraded by the enzymes MAO and COMT. Dopamine binds to five metabotropic receptors (D1–D5) that are divided into two families; D1-like receptors (D1 and D5) and D2-like receptors (D2, D3, and D4) (Wolstencroft et al., 2007; Beaulieu and Gainetdinov, 2011).
Dopamine is produced by dopaminergic neurons that form the A8–A17 nuclei within the mesencephalon and diencephalon. These nuclei are located in the retrorubral field (A8), substantia nigra pars compacta (SNc; A9), ventral tegmental area (VTA; A10), nucleus linearis (A11), preoptic area (A12–A15), olfactory bulb (A16) and retina (A17) (Dahlström and Fuxe, 1964; Frederick et al., 1982). Dopaminergic neurons send their axons through four major dopaminergic tracts: 1) nigrostriatal tract, 2) mesolimbic tract, 3) mesocortical tract and 4) tuberoinfundibular pathway. The nigrostriatal pathway is a dopaminergic projection from the SNc (A9) into the basal ganglia (striatum) and is important for motor control. The mesolimbic and mesocortical tracts are often termed mesocorticolimbic pathway. This pathway originates in the VTA (A10), projects to the nucleus accumbens and further to subcortical structures, hippocampus, and neocortex (Anden et al., 1964; Dahlström and Fuxe, 1964). It is a key component of the reward system, crucial for the development of bonding/attachment and addictive behaviors (Šešo-Šimić et al., 2010). The tuberoinfundibular system projects from hypothalamic arcuate nucleus to the pituitary gland and regulates the secretion of prolactin. In addition to addictive behaviors and attachment disorder, two severe neurologic and psychiatric conditions are the results of the disturbed dopaminergic pathways: Parkinson’s disease (nigrostriatal pathway) and schizophrenia (mesocorticolimbic pathway) (Bogerts et al., 1983; Schultz, 2002; Mladinov et al., 2010).
The dopaminergic system is involved in the regulation of a plethora of physiological functions such as the control of movement, mood, cognitive functions (motivation, attention, working memory, motor planning, thinking and abstract reasoning, temporal analysis, speech and language, learning, maternal/paternal and social behaviors), reward system, emotions, pain control, visceral functions and secretion of hormones (Sawaguchi and Goldman-Rakic, 1991; Jääskeläinen et al., 2001; Schultz, 2002; Iversen and Iversen, 2007; Šešo-Šimić et al., 2010; Yamaguchi et al., 2015). A general theory has been proposed that attributes the evolution of human intelligence and cognitive specializations to an expansion and elaboration of the laminar, sublaminar and regional pattern of dopaminergic cortical innervation (e.g. human-specific coils/clusters of axons and a greater dopamine innervation of infragranular layers in cortical areas involved in high-level processing, such as Brodmann's areas 9 and 32), particularly in the left hemisphere (Hof et al., 1995; Previc, 1999; Raghanti et al., 2008b).
2.4. Histaminergic system
The tuberomammillary nucleus, the major histaminergic nucleus, is affected early by neuropathological changes in AD (Braak and Braak, 1991; Nakamura et al., 1993). A lack of histamine in the AD brain contributes to the disturbances of cognitive functions and sleep. Thus, the enhancers of histamine release in the brain such as histamine H3 receptor antagonists were considered as potential therapeutics in AD (Egan et al., 2012; Grove et al., 2014).
Histamine is synthesized from the essential amino acid histidine by decarboxylation by the histidine decarboxylase (HDC, EC 4.1.1.22). It is mainly metabolized by transmethylation by the N-methyltransferase (HMT, EC 2.1.1.8) to N'-methylhistamine. N'-methylhistamine is further metabolized by MAO-B and aldehyde dehydrogenase. It could by also be metabolized with diamine oxidase (also known as histaminase) to imidazole acetaldehyde (Schayer et al., 1978). Histamine binds to four metabotropic histamine receptors (H1R, H2R, H3R and H4R) and histamine-gated chloride channel (Panula et al., 2015).
Histaminergic neurons in humans are exclusively located in the posterior lateral hypothalamus within the tuberomammillary nucleus into two clusters, one located ventrolaterally, and the other dorsomedially (Panula et al., 1990; Shan et al., 2015). Clusters of histaminergic neurons contain five cell groups: medial, ventral, caudal, lateral, and diffuse. The fact that the lateral hypothalamus contains about 32,000 histaminergic neurons indicates the importance of this system (Airaksinen et al., 1991). Histaminergic neurons give rise to fibers that innervate the whole brain, including the cerebral cortex.
The histaminergic system is involved in the regulation of the level of behavioral arousal, sleep-wake cycle, learning and memory, cognition, attention, control of body temperature, and food uptake (Haas et al., 2003). Injury of the tuberomammillary nucleus causes hypersomnia and blockade of histaminergic neurons with antihistaminic drugs promotes sleep (Brown et al., 2001). Classic antihistamines cause sedation via H1 receptors (Mochizuki et al., 2002).
2.5. Melatonergic system
Secretion of melatonin is decreased in AD (Mishima et al., 1999). Therefore, melatonin supplementation was tested in many clinical trials in the past 20 years as a potential therapy in AD (for review, see Cardinali et al., 2010). While the majority of studies showed ameliorating effects on sundowning, sleep disturbance, and cognitive impairment in AD (for review see Cardinali et al., 2010), some of them failed to confirm these findings (Serfaty et al., 2002; Gehram et al., 2009).
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized in the pineal gland from 5-HT as its precursor (Axelrod, 1974; Namboodiri et al., 1987). More precisely, 5-HT is acetylated by arylalkylamine N-acetyltransferase (AANAT, EC 2.3.1.87) to N-acetylserotonin. By the action of N-acetylserotonin O-methyltransferase (ASMT, EC 2.1.1.4), N-acetylserotonin is turned into melatonin (Wurtman et al., 1968). Melatonin binds to metabotropic MT1 and MT2 melatonin receptors (Dubocovich et al., 2005). Melatonin controls the circadian rhythm of the sleep-wake cycle. Pinealocytes, the main cells of the pineal gland, produce melatonin in high concentration principally during the night (Vaughan et al., 1976).
The suprachiasmatic nucleus (SCN) located in the anterior hypothalamus acts as circadian pacemaker (“Zeitgeber”) and is the main regulator of rhythmic melatonin release. It is activated by light, while light is inhibitory in the pineal gland. Through the direct retinohypothalamic projection, SCN receives information on the luminance of retina (Sadun et al., 1984). SCN projects to hypothalamic paraventricular nucleus. These neurons are connected to the preganglionic sympathetic neurons in the thoracic spinal cord, which project to the superior cervical ganglion (postganglionic sympathetic neurons). These neurons are directly connected with the pineal gland and activate pinealocytes (Nieuwenhuys et al., 2008).
3. Monoaminergic systems in AD
3.1. Alterations of the serotonergic system in AD
Similarly to NIA/AA guidelines, the current clinical criteria for diagnosis of AD of the American Psychiatric Association (DSM-5) are mostly focused on cognitive deficits produced by the dysfunction of hippocampal and association neocortical areas. These changes are neuropsychologically scored as number of points that, in the most commonly used Mini-Mental State Examination (MMSE), ranges from 0 to 30 points, where 30 reflects normal mental status across many cognitive domains (Folstein et al., 1975). Depending on demographic variables such as age, gender, and education, the optimal cutoff point for screening (cognitively normal vs. MCI/minor neurocognitive disorder and dementia/major neurocognitive disorder) can be standardized and validated for any given general population (Boban et al., 2012). However, non-cognitive BPSD have been much less considered, perhaps due to their transient and fluctuating nature and variable severity. The early occurrence of these symptoms, especially mood change (most often depressive mood), anxiety, apathy, social withdrawal/socially intrusive behavior, confusion, irritability, agitation, restlessness, hyperactivity, aggression (first verbal then also physical), psychosis, disinhibition, and disturbances in wake-sleep cycle (“sundowning”), emotion and appetite, and others (hallucinations, delusions, etc.), suggests primarily early involvement of the serotonergic raphe nuclei, particularly the DRN, but also of dopaminergic pathways (Borroni et al., 2010; Martorana et al., 2013; Stefani et al., 2015). An example of early neurofibrillary changes in the DRN of a subject with MCI and BPSD is given in Figure 6. Unfortunately, in spite of the possibility of selective early involvement of the DRN in the pathogenesis of AD (Rüb et al., 2000; for review, see Šimić et al., 2009), among brainstem structures, only the dorsal motor nucleus of the vagus, LC, and SN have been specifically included in the current diagnostic criteria (Hyman et al., 2012; Montine et al., 2012). Additionally, the relationship between depression and AD pathology is of significant interest because diagnosed depression is likely to represents a risk factor of AD (Ownby et al., 2006). In some cases, this can be explained by depression occurring as a preclinical manifestation of neurocognitive disorder/dementia in the context of AD progression. Moreover, chronic or recurrent episodes of depressive symptoms have been found to be associated with hippocampal volume reduction, hypometabolism, and an increased risk for neurocognitive disorder/dementia, suggesting that depression per se is indeed a risk factor for neurodegeneration (Donovan et al., 2015).
Fig. 6.
Bielschowsky silver staining (left panel) and Gallyas silver iodide staining (right panel) of the supratrochlear part of the nucleus raphe dorsalis (NRD) of a 69-year-old woman with mild cognitive impairment (MCI), who had also documented several behavioral and psychological symptoms of dementia (BPSD). Although silver staining seems not to show many changes, on one of the adjacent sections from the same block of tissue more sensitive Gallyas silver iodide reveals a plethora of neurofibrillary changes, including NFT and degenerating neurites. Scale bars = 100 µm.
Extensive serotonergic denervation of the neocortex and hippocampus has been reported in AD (Curcio and Kemper, 1984; Halliday et al., 1992; Chen et al., 2000; for review see Trillo et al., 2013), while reduction of 5-HT as well as its metabolites, primarily 5-HIAA, have been reported in many studies of postmortem AD brains (for instance, Nazarali and Reynolds, 1992; Garcia-Alloza et al., 2005). Both 5-HT and 5-HIAA cortical levels negatively correlate with the number of NFT, suggesting that the impairment of serotonergic system parallels AD progression (Palmer et al., 1987). It has been shown that 5-HT levels are more severely affected in AD brains than the levels of catecholamines (Gottfries et al., 1986). As pointed out by Ramirez et al., similarly to [3H]5-HT binding in AD patients, depletion of 5-HT and its metabolites is more severe in EOAD than LOAD brains, which suggests that serotonergic changes occurring in both aging and AD are degenerative (Ramirez et al., 2014). Depletion of 5-HT also leads to decreased melatonin synthesis, so its concentrations in the CSF samples of AD patients are significantly reduced, particularly in the preclinical stages of the disease (Zhou et al., 2003). As 5-HT is produced from L-tryptophan, reduced dietary intake of tryptophan in AD patients accelerates deterioration of cognitive symptoms (Porter et al., 2000).
Impaired serotonergic functioning has been implicated in many different neurological and psychiatric disorders, among others antisocial personality disorder and depression, both of which have been characterized by the reduced CSF concentrations of 5-HIAA (Deakin, 2003), and higher 5-HT turnover, particularly in female patients (Hou et al., 2006). Animal data suggest that these neurochemical and behavioral changes in adults may have their origins in perinatal exposure, e.g. to 5-HT precursor 5-hydroxytriptophan, 5-HTP, or MAO inhibitors (Blažević and Hranilović, 2013), as well as other pre- and postnatal factors, most likely via epigenetic mechanisms (Pishva et al., 2012). Such changes have also been described as a part of the clinical picture in very early stages of AD (Cummings, 1992; Mychack et al., 2001; Michelsen, 2008). However, as the M-fiber system mainly arises from the median (B5) and dorsal (B6) pontine raphe nuclei it may be of higher relevance for depression and learning and memory, based on its innervation of the hippocampus and the entorhinal cortex (Deakin, 2003; Lei, 2012), while the D-fiber system may be more implicated in personality changes.
The entorhinal cortex is the gate to control the flow of information into and out of the hippocampus and the place from which neurofibrillary degeneration spreads throughout the cerebral cortex (Braak and Braak, 1991). Therefore, it is of great importance to know that the entorhinal cortex receives profuse serotonergic innervations from both the nuclei raphe pontis and DRN and expresses a very high density of serotonergic receptors, including 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT3 and 5-HT6 (Lei, 2012). Mood disorders, depression, and anxiety have been managed with different drugs and diets (Dixon Clarke and Ramsay, 2010), modulating serotonin metabolism (for example, by inhibiting monoamine oxidase A and B), 5-HT transporters and reuptake systems for almost 30 years. Based on preclinical studies, certain 5-HT receptor ligands may the ability to modify or improve memory and cognition, specifically acting at 5-HT1A, 5-HT4 and 5-HT6 receptors (Ramirez et al., 2014).
Together with the noradrenergic LC, the cholinergic pedunculopontine and lateral dorsal tegmental nucleus, with which it is highly interconnected, the serotonergic DRN is also involved in sleep-waking control (España and Scammell, 2011; Monti, 2011). The activity of the DRN is high during waking, low during slow wave sleep and abolished during rapid eye movement (REM) sleep (España and Scammell, 2011). Thus, some drugs, such as modafinil, which modulate 5-HT transmission in the DRN and prefrontal cortex, reduce sleep and promote vigilance (Ferraro et al., 2013), whereas lesions of the raphe nuclei cause insomnia (Monti, 2011). In fact, recent data showed that plaque formation in the brain of the APPswe/PS1ΔE9 mouse model of AD causes the deterioration of the sleep-wake cycle and loss of diurnal fluctuation of Aβ measured in the interstitial fluid (Roh et al., 2012). In addition to the effect that accumulated Aβ causes sleep deprivation, it has been shown that sleep deprivation leads to the inadequate clearance of Aβ (Roh et al., 2012; Ju et al., 2013; Lim et al., 2014; Šimić et al., 2014), contributing to more Aβ accumulation and creating a vicious circle. Besides Aβ release from raphe projection axons (Braak and Del Tredici, 2013), accumulation of Aβ due to sleep deprivation may also add to the pathology of raphe and other brainstem nuclei, particularly in the ‘pre-tangle’ stage.
It has been demonstrated that even cognitively normal individuals with biomarker evidence of preclinical AD have worse quality sleep and sleep efficiency than control individuals (Ju et al., 2013). This in turn can cause a disruption of the default-mode network (DMN) connectivity (for review, see Šimić et al., 2014). Two studies have revealed that the DMN (also known as the resting-state or task-negative network) cortical hubs exhibit high amounts of Aβ deposits in AD (Sheline and Raichle, 2010; Wang et al., 2013) and that clinically normal subjects with high amyloid burden in these regions have significantly reduced functional correlations within the DMN. It is therefore possible that due to DMN neurons’ enhanced (constant) activity dependent processing of APP, these neurons may produce and release more Aβ than occur elsewhere in the neocortex. In a recent cross-sectional cohort study, the decreased CSF values of Aβ1–42 (<500 pg/ml) and increased CSF values of p-tau181 (more than 80 pg/ml) were associated with significant reduction in DMN functional integrity (Wang et al., 2013). The most prominent decreases in functional connectivity were observed between the posterior cingulate and medial temporal regions, and were not attributable to age or structural atrophy, suggesting that both Aβ and tau pathology affect DMN integrity before the clinical onset of AD. Because processing of the APP is activity-dependent, where regional increases in neuronal activity are associated with regional increases in the concentration of Aβ in the interstitial fluid (Cirrito et al., 2005), it can be speculated that due to their constant activity, DMN neurons produce and release more Aβ than occur elsewhere in the neocortex. As DMN connectivity persists during light sleep because self-reflective thoughts do not abruptly cease but rather decrease gradually as a person falls asleep, what particularly matters is the duration of the deepest stages of slow-wave sleep (SWS) during which the activity of DMN is virtually absent and cerebral metabolic rate declines by 43.8% in comparison with wakefulness and REM sleep (Horovitz et al., 2009; Dang-Vu et al., 2010). Out of three models constructed to determine the nature of the interaction among Aβ deposition in the medial prefrontal cortex (mPFC) and its non-REM SW activity, hippocampal activation and memory retention, the model with the best statistical fit was a sleep-dependent one, in which the Aβ impairs memory via its effect on sleep (Mander et al., 2013). As sleep deprivation accelerates Aβ deposition in APP transgenic mice, whereas orexin (hypocretin) deficiency (which increases sleep) decreases it, a bidirectional relationship between sleep and Aβ deposition has been proposed: sleep disruption leads to Aβ deposits and Aβ deposits result in sleep disturbance (Lucey and Holzman, 2015).
Serotonergic receptors are selectively affected in AD, especially 5-HT2 (Ramirez et al., 2014). The loss of 5-HT2A receptors has also been supported by PET imaging (Marner et al., 2012). Reduced 5-HT1A receptor binding in the temporal cortex has been shown to correlate with aggressive behavior in AD patients (Lai et al., 2003), whereas their reduced binding in the hippocampus is better correlated with cognitive decline (Kepe et al., 2006). Also, PET imaging revealed a reduction in 5-HT1A receptor binding in the hippocampus and parahippocampus of patients with mild AD (Truchot et al., 2008). Density of 5-HT1B/1D and 5-HT6 receptors was reduced in the frontal and temporal cortex of AD patients (see Table 2; Garcia Alloza et al., 2004). Studies on polymorphisms in 5-HT receptors support the important role of the serotonergic system in AD too. For instance, T102C polymorphism in the gene for 5-HT2A receptor has been linked to psychosis and psychotic symptoms, including hallucinations and delusions in AD patients (Trillo et al., 2013).
Table 2.
Overview of the involvement of the monoaminergic systems in animal models of Alzheimer’s disease and humans.
| Monoaminergic system |
Component of monoaminergic system in Alzheimer’s disease |
Animal studies | Human subject research | |
|---|---|---|---|---|
| In vivo | Postmortem | |||
| Serotonergic system |
Serotonin innervation (fibers), serotonin, SERT, serotonin metabolites and enzymes |
Progressive degeneration and loss of forebrain afferent 5-HT fibers in APPswe/PS1ΔE9 mouse model of AD (Liu et al., 2008) No 5-HT fiber degeneration and SERT reduction in APPswe/PS1ΔE9 mouse model of AD (Holm et al., 2010) Increased density of 5-HT afferent terminals to hippocampal CA1 field with abnormal 5- HT fiber sprouting in 3×Tg-AD mouse model of AD (Noristani et al., 2011) |
Decreased levels of serotonin, its precursor 5-HTP, and its major metabolite 5-HIAA in CSF in AD (Soininen et al., 1981; Volicer et al., 1985; Zubenko et al., 1986; Bareggi et al., 1982; Blennow et al., 1992; Sjogren et al., 1998) Increased levels of 5-HIAA in CSF in AD (Zubenko et al., 1986; van der Cammen et al., 2006) No change in 5-HIAA levels in CSF in AD (Stuerenburg et al., 2004) |
Significantly decreased concentration of 5-HT in amygdala, caudate nucleus, putamen, and temporal cortex in postmortem AD brains (Nazarali and Reynolds, 1992) Significantly decreased concentration of 5-HIAA in amygdala and caudate nucleus in postmortem AD brains (Nazarali and Reynolds, 1992) Reduced levels of 5-HT and 5-HIAA in postmortem AD brains (Gottfries et al., 1986) Reduced levels of 5-HT and 5-HIAA in postmortem samples of frontal and temporal cortex in AD subjects (Palmer et al., 1987; Garcia-Alloza et al., 2005) ChAT/5-HT ratio in frontal and temporal cortex correlated with MMSE scores in 22 AD patients, whereas AChE/5-HT and ACh/5-HT correlated with MMSE decline in female patients (Garcia-Alloza et al., 2005) Negative correlation of 5-HIAA and tangle formation in temporal and frontal cortex in AD subjects (Palmer et al., 1987) |
| Receptors | 5-HT4 receptor activation decreases quantity of both soluble and insoluble Aβ in the hippocampus of hAPP/PS1 mouse model of AD (Tesseur et al., 2013) Significant decrease of 5-HT2A receptor binding in mPFC of 11-month-old APPswe/PS1ΔE9 mouse model of AD (Holm et al., 2010) |
Reduced 5-HT1A receptor binding in the temporal cortex (Lai et al., 2003), hippocampus (Kepe et al., 2006; Truchot et al., 2008) and parahippocampal gyrus (Truchot et al., 2008) Loss of 5-HT2A receptors exceeds loss of loss of serotonergic projections in early AD (Marner et al., 2012) Thr102Cys polymorphism in the gene for 5-HT2A receptor linked to psychosis and psychotic symptoms in AD patients (Trillo et al., 2013) |
Significantly reduced densities of 5-HT1B/1D and 5-HT6 receptors in frontal and temporal cortex of AD subjects (Garcia Alloza et al., 2004) |
|
| Nuclei | Ectopic cell cycle events that are linked to neurodegenerative process in AD are increased in dorsal raphe of 5 AD mice models (Li et al., 2011) |
Association of early occurrence of BPSD in AD with early pathology of serotonergic raphe nuclei (Borroniet al., 2010; Martorana et al., 2013; Stefani et al., 2015) Greater loss of DRN neurons in depressed AD patients (Zweig et al., 1989) |
As the source of the ascending 5-HT system, the involvement of the oral raphe nuclei, particularly DRN, may be responsible for the early manifestation of the non-cognitive BPSD in AD (Rüb et al., 2000; Grinberg et al., 2009; for review, see Šimić et al., 2009; Braak and Del Tredici, 2013; Šimić et al., 2014) |
|
| Noradrenergic system |
Noradrenaline innervation (fibers), noradrenaline metabolites and enzymes |
Progressive degeneration and loss of forebrain afferent NA fibers in APPswe/PS1ΔE9 mouse model of AD (Liu et al., 2008) Reduced levels of NA within hippocampus, temporoparietal and frontal cortices, and cerebellum in TgCRND8 mouse model of AD, which preceded memory impairment for objects and behavioral despair in tail suspension test (Francis et al., 2012) One-month treatment of 5-month old male 5xFAD transgenic mice with L-DOPS (NA precursor), increases CNS NA levels and improves learning in the Morris water maze task (Kalinin et al., 2012) |
Decreased plasma DBH activity in early AD (Mustapić et al., 2013) Concentrations of NA and its principal metabolite MHPG in CSF increased with the progression of intellectual disability of AD subjects (Tohgi et al., 1992; Elrod et al., 1997) Reduction of NA and MHPG levels in CSF of AD subjects (Martignoni et al., 1991; Sjogren et al., 1998) No change in NA and MHPG levels in CSF of AD subjects (Parnetti et al., 1992; Blennow et al., 1992) |
Decreased concentration of NA in temporal cortex of AD subjects (Nazarali and Reynolds, 1992) Decreased concentration of NA in temporal and frontal cortex of AD subjects (Palmer et al., 1987) Increased concentration of MHPG in frontal cortex of AD subjects (Palmer et al., 1987) Increased ratio of MHPG/NA in temporal cortex of AD subjects (Palmer et al., 1987) |
| Receptors | Decreased density of α1-adrenoreceptors in olfactory bulb, piriform and somatosensory cortex of LRP1 mouse model of AD (von Staden, 2014) Increased density of α2-adrenoreceptors in olfactory bulb, motor and somatosensory cortex, striatum, and hippocampus of LRP1, tg5xFAD and tg5xFAD/LRP1 mice (von Staden, 2014) |
α1-adrenoreceptor agonist prazosin showed positive effects on behavioral symptoms of AD (agitation, aggression) (Wang et al., 2009) β1-adrenergic blockers can cause worsening of delayed memory retrieval in cognitively impaired patients (Gliebus and Lippa, 2007) |
Increased concentration of α2-adrenoreceptors, β1- and β2-adrenoreceptors in cerebellar cortex of aggressive AD patients in comparison to non- aggressive patients (Russo-Neustadt and Cotman, 1997) |
|
| Nuclei | Induced degeneration of LC neurons in APP- transgenic mice resulted in elevated Aβ deposition, increased expression of inflammatory mediators, and impaired microglial Aβ phagocytosis, while supplying the mice with DOPS (precursor of NE) restored microglial functions (Heneka et al., 2010) |
Subjects with AD complicated by depression had significantly fewer LC neurons than non-depressed cases (Zweig et al., 1989) |
LC is affected by neurofibrillary pathology very early during the course of AD (Rüb et al., 2000; Grinberg et al., 2009; for review, see Šimić et al., 2009; Braak and Del Tredici, 2013; Šimić et al., 2014; Pamphlett and Kum Jew, 2015) About 70% of LC neurons lost in the AD brain (Bondareff et al., 1982; Zweig et al., 1989) |
|
| Dopaminergic system |
Dopaminergic innervation (fibers), dopamine metabolites and enzymes |
Elevated level of dopamine in striatum and frontal cortex and reduced levels in the hippocampus (Ambree et al., 2009) and insular cortex (Guzman-Ramos et al., 2012) of a murine and 3xTg-AD mouse models of AD (respectively, compared to wild types) Increased dopamine levels in the brain after treatment with L-DOPA in a murine model of AD (Ambree et al., 2009) |
Decreased levels of dopamine, and dopamine metabolites HVA and DOPAC in CSF in AD (Soininen et al., 1981; Zubenko et al., 1986; Tohgi et al., 1992; Pinessi et al., 1987; Bareggi et al., 1982; Blennow et al., 1992; Sjogren et al., 1998) Increased levels of HVA in CSF in AD (Zubenko et al., 1986; van der Cammen et al., 2006) No change of HVA (Stuerenburg et al., 2004) and dopamine levels (Stefani et al., 2015) in CSF in AD COMT polymorphisms linked with psychosis in AD (Borroni et al., 2004) |
Reduced content of HVA in caudate nucleus of AD subjects (Nazarali and Reynolds, 1992) Increased concentration of HVA in frontal cortex of AD patients (Palmer et al., 1987) Reduced levels of dopamine, dopamine transporter, L- DOPA and DOPAC in AD brains (Storga et al., 1996; Trillo et al., 2013) |
| Receptors | Non-selective dopamine agonist apomorphine reverted perturbed behavioral tasks (such as water maze) caused by 6-OHDA-lesioned rodents (Stefani et al., 2015) |
Polymorphisms in D1 and D3 receptors linked with psychosis and aggression in AD (Holmes et al., 2001) |
Concentrations of L-DOPA, DA, and DOPAC are significantly reduced in post-mortem brains of AD subjects (Storga et al., 1996) Significant reduction in the number of D1 and D2 receptors in the striatum of AD subjects (for review, see Trillo et al., 2013) Density of D3 receptors in striatum was found to be selectively increased in AD subjects with psychosis and associated with Lewy body pathology (Sweet et al., 2001) |
|
| Nuclei | Allopregnanolone reverses the loss of dopaminergic neurons in substantia nigra pars compacta in 3xTgAD mouse model of AD by increasing neurogenesis (Sun et al., 2012) |
Loss of dopaminergic neurons in substantia nigra associated with decreased binding of dopaminergic transporter in striatum in vivo using using123I-N- fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane SPECT (Colloby et al., 2012) |
Substantia nigra affected by tau pathology in later stages of AD (Attems et al., 2012) |
|
| Histaminergic system |
Histamine, histamine metabolites and enzymes |
HDC-knockout mice showed improved contextual fear conditioning and hippocampal CA1 long-term potentiation (Liu et al., 2007) HDC-knockout mice showed improved water-maze performance and impairment in non-reinforced object memory (Dere et al., 2003) |
Reversible AChE inhibitor tacrine given to AD subjects inhibited the activity of HNMT (the histamine deactivating enzyme), resulting in increased levels of histamine (Taraschenko et al., 2005) Slightly decreased t-MeHA levels in CSF of AD subjects (Motawaj et al., 2010) H3R antagonists had modest positive effects on episodic memory in mild to moderate AD subjects, while no improvements were observed on executive functions, working memory, and other cognitive domains (Grove et al., 2014) H3R antagonists had no effect on cognitive functions in AD patients (Haig et al., 2012; Egan et al., 2012) Neurodegenerative pathological changes in tuberomammillary histaminergic system cause disturbances of sleep and thermoregulation in AD patients (for review, see Shan et al., 2013) |
Histamine content was significantly reduced in hypothalamus (42%), hippocampus (43%) and temporal cortex (53% of control value) in postmortem AD brains (Panula et al., 1998) |
| Receptors | Seven H3R antagonists (thioperamide, BF2.649, ABT-239, ABT-288, GSK189254, JNJ-10181457, and PF-03654746) showed wake-promoting effects and improved cognitive efficacy in preclinical models of AD (for review see Brioni et al., 2011) Knockout mice lacking H1R and H2R showed impaired maze performance and object recognition (Dai et al., 2007) |
No change of H3R density in frontal and temporal cortex of AD brains (Medhurst et al., 2009) Higher binding density of H3R antagonist binding in frontal cortex of AD patients with more severe dementia (Medhurst et al., 2009) |
||
| Nuclei | Tuberomammillary hypothalamic area affected relatively early during the course of AD (Braak et al., 1993) |
|||
| Melatonergic system |
Melatonin, melatonin metabolites and enzymes |
Melatonin inhibits Aβ generation and formation of amyloid fibrils, and protects cells from Aβ-mediated toxicity in Tg2576 (Matsubara et al., 2003) and APP 695 (Feng et al., 2004) transgenic mouse model of AD |
Early neuropathological AD changes are accompanied by decreased CSF melatonin levels (Zhou et al., 2003) Melatonin receptors MT1 and MT2 agonist ramelteon suggested as protective against insomnia-induced neuronal damage in AD (Srinivasan et al., 2010) Pineal melatonin levels highly correlate with CSF melatonin levels, which are decreased already in cognitively intact subjects in preclinical AD with the earliest neuropathological changes (Braak stages I–II) (Wu et al., 2013) |
Significantly decreased melatonin levels in CSF of AD patients with ε4/ε4 genotype than in AD patients with ε3/ε4 genotype (Liu et al., 1999) |
| Receptors | Increase of MT2 receptor expression in the hippocampus of adult male Sprague-Dawley rats following chronic treatment with valproic acid (Bahna et al., 2014) |
Overall decrease of MT1 and MT2 melatonin receptors in pineal gland and occipital cortex in AD subjects (Brunner et al., 2006) Overall intensity of MT2 receptor staining distinctly decreased in AD hippocampus (Savaskan et al., 2005) |
||
| Nuclei | Decreased nocturnalmelatonin production and secretion from pineal gland with increasing age (Pandi-Perumal et al., 2005) |
|||
Legend: 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; 5-HTP, 5-hydroxytryptophan; 6-OHDA, 6-hydroxydopamine; Aβ, amyloid β protein; ACh, Acetylcholin; AChE, Acetylcholinesterase; AD, Alzheimer’s disease; APOE, apolipoprotein E; APP, amyloid precursor protein; AT8, antibody specific for phospho-tau epitopes Ser202 and Thr205; BA, Brodmann area; BPSD, behavioral and psychological symptoms of dementia; ChAT, Cholinacetyltransferase; COMT, Catechol-O-methyltransferase; CSF, cerebrospinal fluid; CNS, central nervous system; DBH, dopamin-β-hydroxylase; DOPAC, dihydroxyphenylacetic acid; DRN, dorsal raphe nucleus; HDC, L-histidine decarboxylase; HNMT, histamine-N-methyltransferase; HVA, homovanilic acid; L-DOPA, L-3,4-dihydroxyphenylalanine; L-DOPS, L-threo-dihydroxyphenylserine; LC, locus coeruleus; MHPG, 3-Methoxy-4-hydroxyphenylglycol; MMSE, Mini-Mental State Examination; mPFC, medial prefrontal cortex; NA, noradrenaline; SERT, serotonin transporter; SPECT, single-photon emission computed tomography; t-MeHA, tele-methylhistamine
3.2. Alterations of the serotonergic system in other primary and secondary tauopathies
Although not as prominent as in AD, NFT in the DRN are seen in both PSP and CBD; Ishino et al., 1975; Shiozawa et al., 2000). In PSP, NFT and NT are seen in the nucleus raphe magnus and nucleus raphe obscurus less than one year after initial clinical symptoms and do not correlate in number with the disease duration. It is suspected that tau pathology is present in the preclinical stages of the disease (Rüb et al., 2002). In PSP and postencephalitic parkinsonism (PEP), NFT and NT are found in raphe nuclei, partly contributing to oculomotor abnormalities (Revesz et al., 1996; Yang et al., 2001). Supranuclear gaze palsy was also described in a case with clinical PSP, which was later pathologically confirmed as CBD with NFT and tau-positive inclusions present in the raphe nuclei (Shiozawa et al., 2000). Marked reduction in a number of neurons and NFT pathology to a lesser extent was found in the NRC and DRN in FTD (Yang et al., 2001). Low brain levels of serotonin have been evident in patients with FTD, manifesting aggression, impulsivity, depressive symptoms and alterations in frontal cortex metabolism, leading to clinical improvement after usage of selective 5-HTreceptor inhibitors (Huey et al., 2006; Mendez, 2009). A significant reduction in the number of neurons in the NRC and DRN with NFT in pontine (B5 and B6) and DRN is speculated to contribute to hypersomnia in myotonic dystrophy (Yoshimura et al., 1990; Ono et al., 1995; Ono et al., 1998; Oyamada et al., 2006). In Down’s syndrome, NFT in the raphe nuclei also increase in number with age (Mann et al., 1986). Low 5-HTlevels are seen in the amygdala and cingulate cortex, which receive serotonergic projections from the NRC and DRN, both in AD and Down’s syndrome (Yates et al., 1986). NFT in the raphe nuclei are also seen in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam, Gerstmann-Sträussler-Scheinker syndrome, chronic subacute sclerosing panencephalitis, non-Guamanian motor neuron disease and white matter tauopathy with globular glial inclusions (Yamamoto et al., 1985; Mandybur et al., 1990; Hilton et al., 1995; Yamazaki et al., 1999; Kovacs et al., 2008). Further investigations are needed fully to understand the extent of the raphe nuclei involvement in the clinical expression of these tauopathies.
In an animal model for tau aggregation, THY-Tau22 mouse (Schindowski et al., 2006), which expresses human 4R tau mutated at sites Gly272Val and Pro301Ser under a Thy1.2 promotor, and shows hyperphosphorylation of tau on several AD-relevant epitopes and NFT-like inclusions, in addition to the cognitive impairments, at least some BPSD were seen (Van der Jeugd et al., 2013). Namely, in addition to early tau pathology in hippocampus and basal forebrain (Belarbi et al., 2009), 12-month old THY-Tau22 mice, relative to wild-type littermates displayed increased depression-like and aggressive behavior, co-occurring with disturbances in nocturnal activity (Van der Jeugd et al., 2013). These changes were linked to a decreased hippocampal concentration in 5-HT and 5-HIAA (Van der Jeugd et al., 2013).
3.3. Alterations of the noradrenergic, dopaminergic systems, histaminergic, and melatonergic systems in AD
The LC is the main noradrenergic nucleus affected in AD (Zarow et al., 2003), with up to 70% LC neurons being lost in the brain of AD patients (Bondareff et al., 1982; Zweig et al., 1989). The loss of noradrenergic neurons from the LC correlates with the increase of extracellular Aβ deposition in mice (Heneka et al., 2010), neurofibrillary abnormalities in early stage of AD (Grudzien et al., 2007), onset (Counts and Mufson, 2010b), and duration of dementia (Counts and Mufson, 2010). New studies hypothesize that accumulation of heavy metals such as organic mercury in LC may be an early event in AD, since LC neurons are prone to taking up circulating toxins. Mercury leads to the development of AD pathology within the LC, and pathology is spread further to the neighboring raphe neurons. Actually, dorsal raphe neurons in AD subjects often contain as much hyperphosphorylated tau as those in the LC (Pamphlett and Kum Jew, 2015). To date, clinical and experimental evidence indicate that neurons of the LC modulate several processes that are altered in the brains of AD patients, including synaptic plasticity, neuronal metabolism, and BBB permeability, while enhancement of the brain's noradrenergic neurotransmission reduces both neuroinflammation and cognitive decline (Mravec et al., 2014). Importantly, noradrenergic projections from the LC also regulate neurovascular coupling, so that degeneration of LC neurons diminish the ability to couple blood volume to oxygen demand in AD subjects (Bekar et al., 2012). Apart from its role as a neurotransmitter, noradrenaline may also act as an endogeneous anti-inflammatory agent by inhibition of activation of microglial cells (Feinstein et al., 2002), thereby potentially contributing to the pathogenesis of AD. It seems that the inconsistent findings regarding the role of noradrenaline modulators in improving behavioral symptoms (such as agitation and aggression) in AD patients, such as the use of classical α1-adrenoreceptor agonist prazosin (e.g., Gliebus and Lippa, 2007; and Wang et al., 2009), can only be explained by a strong compensatory mechanism, where the increase in noradrenaline is detected in the absence of metabolite increase consistentwith compensatory activation of surviving noradrenergic projections (Raskind et al., 1999). That the activity and levels of plasma DBH are reduced from early stages of AD suggests that treatment with selective noradrenaline reuptake inhibitors may be helpful in compensating for the loss of noradrenergic activity due to degeneration of neurons in LC in the early stages of AD (Mustapić et al., 2013).
Both the LC and DRN nuclei have been shown to be among the first affected by tau protein abnormalities during the course of AD, causing fluctuating non-cognitive, but also cognitive symptoms of varying degrees of severity (Zweig et al., 1989; for review, see Šimić et al., 2009). In one of the most comprehensive clinicopathological studies that analyzed the earliest AD-related cytoskeletal pathology, 8 of 38 individuals who were categorized as Braak stage 0 and all of the 80 cases characterized as Braak stage 1 or higher than 1 had substantial neurofibrillary changes in the DRN (Grinberg et al., 2009). Braak stages were defined based on the level of neurofibrillary lesions in the transentorhinal cortex. Braak stage 0 had no lesions, stage 1 had rare changes and stages higher than 1 showed more extensive neurofibrillary pathology in the transentorhinal cortex. The finding that all cases of Braak stage 1 and higher, and even a fifth of the cases with Braak stage 0 had neurofibrillary lesions in the brainstem nuclei supports the model in which AD pathology starts in the brainstem and spreads transneuronally to the cortical targets, primarily transentorhinal cortex. This possibility is in agreement with the clinical observations showing that BPSD occur 2–3 years before the onset of cognitive impairment (for review, see Šimić et al., 2009).
Despite the direct harmful effects of hyperphosphorylated soluble tau and progression of intracellular tau protein changes characteristic for AD, as well as their accumulation of heavy metals (Pamphlett and Kum Jew, 2015), many of the tangle-bearing neurons of the LC and DRN are remarkably sturdy and survive for a lifetime (Rüb et al., 2000). Therefore, even when affected by neurofibrillary changes, LC and DRN projecting neurons may release Aβ (Braak and Del Tredici, 2013), as well as soluble monomeric or small oligomeric tau protein, trans-synaptically through M-fibers or, more likely, from the non-synaptic varicosities of their fine D-axons (otherwise dedicated to volume transmission) (Braak and Del Tredici, 2015). As LC and DRN neurons massively project to transentorhinal and entorhinal cortex, it should be also taken into account that they may be contributing to early neurofibrillary changes seen in those regions (Fig. 7), from where tau pathological changes may spread throughout the cerebral cortex. The described presumptive set of events may help explain similarities in size distribution of Aβ deposits in the cerebral cortex of AD subjects and patients with other neurodegenerative disorders (Armstrong, 2012) and the prion-like behavior of misprocessed tau in various tauopathies (de Calignon et al., 2012; Hall et al., 2012; Liu et al., 2012; Avila et al., 2015).
Figure 7.
Schematic drawing of a speculative spreading of tau pathology from LC and DRN to the transentorhinal/entorhinal cortex.
Dopamine, dopamine transporter, L-3,4-dihydroxyphenylalanine (L-DOPA), DOPAC, D1 and D2 receptors are reduced in AD brains (Storga et al., 1996; Trillo et al., 2013). Dopamine is also variably decreased in the CSF of AD subjects (Tohgi et al., 1992; Stefani et al., 2015). D1 and D3 receptor polymorphisms have been linked with psychosis and aggression in AD (Holmes et al., 2001), while certain COMT polymorphisms lead to psychosis in AD (Borroni et al., 2004). Biochemical, genetic, and animal models studies have also documented alterations of the dopaminergic system in AD. Both double mutant APP mice and 3xTg-AD mouse models of AD have reduced dopamine release in the hippocampus and insular cortex (Ambree et al., 2009; Guzman-Ramos et al., 2012). In conclusion, cognitive impairment in AD may, at least in part, be also mediated through deficit in dopaminergic transmission.
Neuropathological changes characteristic for AD are pronounced in tuberomammillary nucleus of the hypothalamus (Braak et al., 1993). These changes may condition disturbances in sleep and thermoregulation in AD patients. The acetylcholinesterase inhibitor tacrine (1,2,3,4-tetrahydro-9-acridinamine monohydrochloride), used as a symptomatic treatment in AD, also increases the levels of histamine by activation of histaminergic hypothalamic neurons and inhibition of histamine-N-methyltransferase (Taraschenko et al., 2005). Histamine H3R antagonists also enhance the release of histamine in the brain. An effect of H3R antagonists on acetylcholine, noradrenaline, and dopamine levels that also modulate cognition has been reported (Medhurst et al., 2007; Galici et al., 2009). As such, H3R antagonists were tested in preclinical and clinical trials for their effect on cognitive processes in AD (see Table 3; for review see Brioni et al., 2011).
Table 3.
Monoamine-based treatments in Alzheimer’s disease.
| Monoaminergic system |
Preclinical trials | Clinical trials | |||
|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | Phase IV | ||
| Serotonergic system | SSP-002392 5-HT4 receptor agonist (Tesseur et al., 2013) |
SR-444 5-HT1A receptor antagonist https://clinicaltrials.gov/show/NCT00499200 |
Lecozotan 5-HT1A receptor antagonist (Sabbagh, 2009) |
Lu AE58054 5-HT6 receptor antagonist https://clinicaltrials.gov/ct2/show/NCT01955161 |
Tandospirone treatment of BPSD (Sato et al., 2007) |
| SB-271046 5-HT6 receptor antagonist (Da Silva Costa-Aze et al., 2012) |
PF-04995274 5-HT4 receptor agonist (Sawant-Basak et al., 2013) |
PRX-3140 5-HT4 receptor agonist (Shen et al., 2011) Nanotherapeutics |
Buspirone treatment of BPSD (Salzman, 2001) |
||
| E-6801 5-HT6 receptor agonist (Kendall et al., 2011) |
RQ-9 5-HT4 receptor agonist (Fujiuchi et al., 20109) |
SB-742457 5-HT6 receptor antagonist (Maher-Edwards et al., 2011) https://clinicaltrials.gov/ct2/show/NCT00710684 |
Escitalopram SSRI https://clinicaltrials.gov/ct2/show/NCT02161458 |
||
| PRX-07034 5-HT6 receptor antagonist EpixPharmaceuticals |
SUVN-502 5-HT6 receptor antagonist https://www.clinicaltrials.gov/ct2/show/NCT02580305 |
||||
| AVN-322 5-HT6 receptor antagonist AvineuroPharmaceuticals |
|||||
| Dimebon antihistamine, affinity for 5-HT6 receptor https://clinicaltrials.gov/ct2/show/NCT00377715 | |||||
| Noradrenergic system | Piperoxane α2-adrenoreceptor antagonist (Zornetzer et al., 1998) |
Atomoxetine noradrenaline reuptake inhibitor https://clinicaltrials.gov/ct2/show/NCT01522404 |
Reboxetine noradrenaline reuptake inhibitor https://clinicaltrials.gov/ct2/show/NCT02374567 |
||
| Fluparoxan α2-adrenoreceptor antagonist (Scullion et al., 2011) | |||||
|
CL316243 β3-adrenoreceptor agonist (Gibbs et al., 2010) | |||||
| L-DOPS Noradrenaline precursor (Heneka et al., 2010; Kalinin et al., 2012) | |||||
| Dopaminergicsystem | Apomorphine dopamine agonist (Brusa et al., 2003; Himeno et al., 2011) |
Bupropion dopamine uptake inhibitor https://clinicaltrials.gov/ct2/show/NCT01047254 |
Levodopa https://clinicaltrials.gov/ct2/show/NCT00306124 |
||
| Risperidone dopamine receptor antagonist https://clinicaltrials.gov/ct2/show/NCT00287742 |
Dextroamphetamine dopamine uptake inhibitor https://clinicaltrials.gov/ct2/show/NCT00254033 |
||||
| Haloperidol D2 receptor antagonist https://clinicaltrials.gov/ct2/show/NCT00009217 and https://clinicaltrials.gov/ct2/show/NCT00000179 |
|||||
| Risperidone dopamine receptor antagonist https://clinicaltrials.gov/ct2/show/NCT00417482 and https://clinicaltrials.gov/ct2/show/NCT00208819 | |||||
| Histaminergic system | Thioperamide H3R antagonist (Prast et al., 1996) |
GSK239512 H3R antagonist https://clinicaltrials.gov/ct2/show/NCT00675090 |
GSK239512 H3R antagonist https://clinicaltrials.gov/ct2/show/NCT01009255 |
||
| BF2.649 H3R antagonist (Ligneau et al., 2007) |
PF-03654746 H3R antagonist https://clinicaltrials.gov/ct2/show/NCT01028911 |
ABT-288 H3R antagonist https://clinicaltrials.gov/ct2/show/NCT01018875 |
|||
| ABT-239 H3R antagonist (Fox et al., 2005 |
|||||
| GSK189254 H3R antagonist (Medhurst et al., 2008) | |||||
| JNJ-10181457 H3R antagonist (Galici et al., 2009) | |||||
| Melatonergic system | Melatonin (Matsubara et al., 2003; Lahiri et al., 2004) |
Melatonin https://clinicaltrials.gov/ct2/show/NCT00544791 |
Melatonin https://clinicaltrials.gov/ct2/show/NCT00000171 |
||
| Circadin https://clinicaltrials.gov/ct2/show/NCT00940589 |
|||||
Legend: BPSD, behavioral and psychological symptoms of dementia; L-DOPS, L-threo-dihydroxyphenylserine; SSRI, serotonin reuptake inhibitor.
The production of melatonin from the pineal gland decreases with the increasing age (Pandi-Perumal et al., 2005). This is relevant as age is the main predisposing factor in AD. The fact that melatonin secretion is also decreased in AD is thus not surprising (Mishima et al., 1999). Decreased melatonin levels in cerebrospinal fluid in preclinical AD could serve as an early biomarker of AD (Zhou et al., 2003). Additionally, patients with the APOE ε4/ε4 genotype have a more prominent melatonin decrease (Liu et al., 1999). Decrease in melatonin secretion results in heightened afternoon agitation seen in so many AD patients (Volicer et al., 2001), sleep disturbance, and circadian rhythm disorganization in patients with AD. In the elderly, melatonin mainly promotes non-rapid eye movement (non-REM) restorative phases of sleep (Monti et al., 1999). In addition to melatonin antioxidant effects, its anti-amyloid properties were also observed. Melatonin inhibits Aβ generation, formation of amyloid fibrils, and protects cells from Aβ-mediated toxicity (Matsubara et al., 2003; Feng et al., 2004). Melatonin receptors MT2 also appear to be important in mechanisms of hippocampal learning and memory in mice (Larson et al., 2006).
3.4. Interactions among monoaminergic systems and with the cholinergic system in AD
Monoaminergic systems are not functioning independently. In fact, their interconnection is obvious at both the anatomical and molecular levels. Often different monoaminergic systems innervate the same brain regions and their fibres together share pathways in the brain, the most obvious example being the medial forebrain bundle. The control of the sleep-wake cycle is mainly regulated by the noradrenergic LC (REM sleep), and the serotonergic DRN and cholinergic pedunculopontine and lateral dorsal tegmental nuclei (non-REM sleep; Arslan, 2015). The serotonergic and melatonergic systems are closely related at the molecular level, 5-HT being the precursor of melatonin (Axelrod, 1974; Namboodiri et al., 1987). Association of the serotonergic and dopaminergic systems also occurs in the activation of 5-HT1B receptors leading to increase of dopamine release in the brain (Gonzalez-Burgos and Feria-Velasco, 2008), while neonatal lesions of raphe nuclei cause compensatory increase in number of dopaminergic fibers in adult rats (Bolte Taylor et al., 1998). Association of histaminergic system with other monoaminergic systems has been shown, for example H3R antagonists stimulate 5-HT, noradrenergic and dopaminergic neurotransmission (Flik et al., 2015). Trillo et al. (2013) elaborated that monoaminergic systems are especially vulnerable in AD due to: 1) the fact that the number of monoaminergic neurons is low in the brain, 2) having long, unmyelinated axons, monoaminergic neurons have increased vulnerability to abnormalities of anterograde or retrograde transport, and 3) monoaminergic fibers innervate many regions affected by amyloid and tau pathology. Besides the possibility that plaques and NFT could damage monoaminergic nerve endings, Aβ and tau could be also retrogradely transported back to their cell bodies (Trillo et al., 2013). Finally, degeneration of the cholinergic neurons is one of the main features of late-stage AD. As the cholinergic system is most important for the generation and modulation of the event related potential component P300 wave (since its initial discovery, it has been shown that it has two components - P3a – related to novelty, and the classic P300, which has been renamed P3b), some parts of the cholinergic magnocellular chain of nuclei that can be of particular clinical significance for non-invasive diagnostics and follow-up of AD subjects could be those rostro-lateral parts of the basal nucleus involved in innervation of the cortical speech areas (Šimić et al., 1999; Boban et al., 2006; Raghanti et al., 2011). Blockade of serotonergic and cholinergic systems leads to loss of learning and memory abilities in rats (Vanderwolf, 1987). Garcia-Alloza et al. (2005) postulated that imbalance of cholinergic and serotonergic system contributes to both cognitive and behavioral symptoms in AD patients. Interdependence of monoaminergic and cholinergic systems can also be seen as a possibility to create potential multifunctional therapeutics for AD treatment in the future. For example, ladostigil, is a reversible AChE and butyrylcholinersterase (BuChE) inhibitor and an irreversible MAO-B inhibitor that combines the mechanisms of action of older drugs rivastigmine (an AChEI) and rasagiline (MAO-B inhibitor) into a single molecule, which also enhances the expression of neurotrophic factors BDNF and glia-derived neurotrophic factor (GDNF), and shows beneficial effects in neurodegenerative rat models (Weinreb et al., 2012). Another example could be the compound ASS234, a multipotent drug that inhibits monoamine oxidase enzymes (MAO A and B), AChE and BuChE, Aβ aggregation, protects cells from Aβ-induced apoptosis, and shows antioxidant properties (Bolea et al., 2013). Therapeutics targeting of cholinergic system only can also show beneficial effects on monoaminergic systems. Treatment with tacrine, an AChEI, increased histamine release in the brain (Taraschenko et al., 2005). Additionally, therapeutics tested for treatment of AD and targeting one monoaminergic system can influence another, such as the supposedly antihistaminic drug latrepirdine, which besides its autophagy-enhancing properties (Steele and Gandy, 2013) has also shown affinity for 5-HT receptors, adrenoreceptors, and dopamine receptors (Okun et al., 2010).
3.5. Monoamine-based treatments in AD
Numerous potential AD therapeutics targeting the serotonergic system have been tested in preclinical and clinical trials (Table 3). 5-HT is seen as having indirect influences on neuronal degeneration and memory deficits (Jia et al., 2014). 5-HT1A, 5-HT4, and 5-HT6 receptors are even considered as novel therapeutic targets in AD (Ramirez et al., 2014). Lecozotan, a 5-HT1A receptor antagonist, was a very promising drug in preclinical and clinical studies, but due to adverse effects failed in phase II of clinical trials (Sabbagh, 2009). Nevertheless, partial agonists of the 5-HT1A receptor, tandospirone and buspirone, are useful in the treatment of BPSD (Salzman, 2001; Sato et al., 2007). Additionally, 5-HT4 receptor agonists increased the release of acetylcholine and reduce Aβ in both preclinical and phase I clinical trials (PF-04995274, PRX-3140, RQ-9; Ramirez et al., 2014). Pharmacological activation of the 5-HT4 receptor by the SSP-002392 compound decreased production and deposition of Aβ in the hAPP/PS1 mouse model of AD, making it another potential AD therapeutic (Tesseur et al., 2013; Pimenova et al., 2014). Interestingly, although they have not found evidence for a direct α-secretase stimulation neither in vivo nor in vitro, De Strooper and collaborators (Tesseur et al., 2013; Pimenova et al., 2014) explained their findings by stating that activation of 5-HT4 receptors had probably stimulated α-secretase activity, which in turn increased non-amyloidogenic proteolysis of APP responsible for the observed decreased soluble and insoluble Aβ concentrations measured (both in cultured human neuroblastoma cells and in the hippocampus of the hAPP/PS1 mouse model of AD). Because Aβ may leak from serotonergic afferents (Braak and Del Tredici, 2013), an alternative and much simpler explanation not considered by the authors would be that it is a decreased activity of the originating serotonergic neurons (due to a pharmacological overstimulation of their target receptors), with consequent decreased Aβ leakage and deposition, responsible for the observed lower concentrations of Aβ (rather than due to a shift in APP processing, as the authors tried to explain). Besides the mentioned ones, several different 5-HT6 receptor antagonists are also currently in clinical trials as drug candidates for AD (Table 3). Because 5-HT6 antagonists were reported to rescue anticholinergic drug-induced amnesia (Hirst et al., 2003), evidence indicates that inhibition of 5-HT6 receptors could facilitate acetylcholine release and, via elevated cholinergic activity, improve memory and learning deficits. Two new agents, PRX-03140 (a 5-HT4 antagonist) and SB-742457 (a 5-HT6 antagonist), have also recently completed phase II trials, whereas LuAE58054, an antagonist of the 5-HT6 receptor, has progressed to a phase III trial with 930 mild to moderate AD patients, in combination with the AChEI donepezil (Jia et al., 2014).
Drugs targeting the noradrenergic system have mainly been used in ameliorating behavioral symptoms in AD, like the α1-adrenoreceptor agonist prazosin (Wang et al., 2009), the β-adrenoreceptor antagonist propranolol (Shankle et al., 1995) for aggression and agitation, and the antidepressant imipramine (Reifler et al., 1989) and venlafaxine (NCT01609348) for depression, as well as β-blockers for slowing cognitive decline (Hajjar et al., 2005; Khachaturian et al., 2006; Rosenberg et al., 2008). Preclinical studies have shown that drugs targeting the noradrenergic system can in fact reduce amyloid burden and neuroinflammation (reviewed in Chalermpalanupap et al., 2013). α2-Adrenoreceptor antagonists improved memory deficits in aged mice (piperoxane; Zornetzer et al., 1998) and APP/PS1 mice (fluparoxan; Scullion et al., 2011), while the β3-adrenoreceptor agonist CL316243 improved learning in chicks impaired by the injection of Aβ42 (Gibbs et al., 2010). The noradrenaline precursor L-thre-odihydroxyphenylserine (L-DOPS) showed very good results in improving memory of APP transgenic mice (Heneka et al., 2010) and DBH−/−, APP/PS1 double-mutant mice (Hammerschmidt et al., 2013) and in reducing Aβ burden in 5xFAD mice (Kalinin et al., 2012). By taking into account the beneficial effects of L-DOPS in animal models of AD, its effects on cognitive functions of demented patients should be further investigated. The efficacy of noradrenaline reuptake inhibitors atomoxetine (used in the treatment of attention-deficit disorder) and reboxetine (used in depression) in the treatment of demented patients is currently in clinical trials. Phase II trials on the effect of atomoxetine on CSF biomarkers of AD in MCI patients is ongoing, whereas the effect of reboxetine in treatment of patients with dementia is investigated along with other potential therapeutics in a large multicenter study currently in phase III of clinical trials (Table 3).
Cognitive impairment in AD can be partially restored by levodopa and dopamine agonists (Martorana et al., 2013). The dopamine agonist rotigotine was shown to be effective in restoring LTP-like cortical plasticity in AD patients (Koch et al., 2014). This finding is not surprising as pharmacological experiments demonstrated the efficacy of the non-selective dopamine agonist apomorphine in reverting perturbed behavioural tasks (such as water-maze) due to oxidopamine-induced parkinsonism in rodents (Brusa et al., 2003), promoting Aβ degradation and protecting of hippocampal neurons from oxidative stress (Himeno et al., 2011). The MAO-B inhibitor selegiline was also considered as a promising therapeutic for AD (Tariot et al., 1987), but it was rejected from further use as it failed to show beneficial effect on cognitive and behavioral functions in AD patients (Burke et al., 1993; Freedman et al., 1998). An effect of many dopaminergic drugs sought in the treatment of AD is to reduce apathy, a behavioral symptom in about 70% of patients (Mitchell et al., 2011). Dopamine uptake inhibitors dextroamphetamine and methylphenidate (Herrmann et al., 2008), used to treat attention deficit hyperactivity disorder and the antidepressant bupropion have been tested for treatment of apathy in AD. Haloperidol, an antipsychotic D2 receptor antagonist has been tested in phase IV clinical trials for treatment of psychosis and agitation in AD. The antipsychotic risperidone, a dopamine receptor antagonist, was tested for the treatment of hallucinations and delusions, and agitation and aggression in AD patients. A currently ongoing phase IV clinical trial is testing the effect of repetitive transcranial magnetic stimulation (rTMS) for treatment of apathy in AD (Table 3).
The research for potential therapeutics targeting histaminergic system in AD focused on histamine H3R antagonists. Seven H3R antagonists showed beneficial effects on cognition in preclinical models: thioperamide, BF2.649, ABT-239, ABT-288, GSK189254, JNJ-10181457 and PF-03654746 (see Table 3; for review, see Brioni et al., 2011). In clinical studies, the H3R antagonist ABT-288 was shown to be safe in healthy elderly subjects and demonstrated efficacy across several cognitive domains (Haig et al., 2014). However, the drug failed in patients with mild to moderate AD (Haig et al., 2012). A pilot randomized controlled trial (RCT) study showed that MK-0249, a histamine H3R inverse agonist, could not improve cognition in mild to moderate AD (Egan et al., 2012). However, the histamine H3R antagonist GSK239512 improved episodic memory in mild to moderate AD patients, but had no effect on executive function and working memory (Grove et al., 2014). This suggests that H3R antagonists can selectively affect cognitive function in AD patients (Grove et al., 2014). The H2R antagonist nizatidine failed to show any benefical effect in AD (Carlson et al., 2002).
Therapeutic effects of melatonin in AD have been tested in 14 trials including AD patients and 8 trials including MCI patients (reviewed in Cardinali et al., 2014). Melatonin supplementation slows the progression of cognitive impairment in AD and MCI patients, ameliorates sundowning and improves sleep (reviewed in Cardinali et al., 2010). A search of ClinicalTrials.gov on 15th February 2016 yielded two studies that tested the effect of melatonin on cognitive functions in MCI patients (phase II) and sleep disturbance associated with AD (phase III). However, some clinical trials in AD patients showed no effects of melatonin on sleep disturbance and agitation (Serfaty et al., 2002; Gehram et al., 2009), suggesting that its efficacy in AD should be further investigated possibly at higher doses (Cardinali et al., 2014). The focus of recent studies is the development of melatonin analogs that could prolong the effect of melatonin. The effect of prolonged-release melatonin on patients with mild to moderate AD is in phase II of clinical trials (Table 3).
4. Conclusions
Pathological changes in monoaminergic nuclei, particularly the noradrenergic LC and serotonergic DRN, but also dopaminergic, histaminergic and melatonergic nuclei and pathways, observed during the early course of AD probably have a profound influence not only on the complex symptoms and pathogenesis in AD, but also in other primary and secondary tauopathies, especially FTD (Boban et al., 2010). The link between depression and AD, depression being a frequent preclinical manifestation of AD, is important in this context. Association of depressive symptoms with hippocampal volume reduction in early stages of AD (Šimić et al., 1997), as well as hypometabolism and an increased risk for neurocognitive disorder or dementia, further strengthen the concept of depression as a risk factor for neurodegeneration in general (Brendel et al., 2015).
One of the problems surrounding depression is that many of its primary symptoms (such as low self-esteem, depressed mood, suicidal ideation, guilt) are by nature difficult to measure in animals. However, the lack of interest in pleasurable experiences may be similar to anhedonia in laboratory animals (Anisman and Matheson, 2005), which can be assessed by a variety of tests, including those based on food consumption and sucrose preference.
Another important connection is disruption of the sleep-wake cycle in AD, which is also most probably caused by the neuropathological changes of the DRN (mostly involved in non-REM sleep) and LC (mostly involved in REM sleep). Both of these brainstem nuclei are among the first to be affected by tau protein abnormalities in the course of LOAD, causing behavioral and cognitive symptoms of variable severity. The possibility that most of the tangle-bearing neurons of the LC and DRN may still release Aβ as well as soluble monomeric or small oligomeric tau protein trans-synaptically by their diffuse projections to the cerebral cortex warrants further investigations of the monoaminergic systems in AD.
In conclusion, evidence from human and animal studies suggest that alterations in serotonergic and noradrenergic transmission may be the link to the early mood, aggression, feeding, and sleep changes observed in AD patients (Šimić et al., 2009). Therefore, expanding our understanding of the brainstem nuclei, particularly the LC and DRN involvement in the early stages of AD to functional concepts beyond neuropathological descriptions, will likely have a significant impact on diagnosis and tracking of AD progression as well as on development of biomarkers, novel therapeutic targets, and preventive strategies. One of the recent breaktroughs in that direction was the successful visualization of the DRN using PET imaging of 5-HT1A receptor binding (Kranz et al., 2012). Other potential pharmacological interventions directed mainly at serotonergic system targets are expected to alleviate symptoms of neurodegeneration, and to expand our understanding of the relationship between monoaminergic systems and the pathogenesis of AD.
A more comprehensive assessment of the genome and improvements in understanding of how the epigenomic influences change the methylome and structure of chromatine through histone protein modifications will enable detection of the early molecular networks that drive AD pathology. Because the human DNA methylomes of different neurodegenerative diseases, including AD, share common epigenomic patterns, at least for the prefrontal cortex (Sanchez-Mut et al., 2016), a key challenge for future studies will be to describe how genetic and environmental interactions shape epigenetic changes of genes related to monoaminergicand other neurotransmitter systems, and their differential vulnerability to AD. Moreover, an evaluation of younger subjects with life experiences such as depression will also be necessary to investigate the role of non-genetic factors to delineate more precisely the cause-effect relationships of molecular events that lead to dementia. Fortunately, the approval of epigenetic drugs for cancer (Adwan and Zawia, 2013) paves the road for the development of such drugs for other disorders, including neurodegenerative diseases such as AD.
Highlights.
Monoaminergic systems are altered in Alzheimer’s disease.
Noradrenergic LC and serotonergic DRN are among the first affected by tau pathology.
Changes in DRN and LC lead to the deterioration of the sleep-wake cycle in AD.
Depression in preclinical AD further indicates monoaminergic alteration in AD.
In view of its early involvement in AD, the serotonergic system could serve as a therapeutic target.
Acknowledgments
This work was supported by The Croatian Science Foundation grant. no. IP-2014-09-9730 ("Tau protein hyperphosphorylation, aggregation, and trans-synaptic transfer in Alzheimer’s disease: cerebrospinal fluid analysis and assessment of protective neuroprotective compounds") and European Cooperation in Science and Technology (COST) Action CM1103 ("Stucture-based drug design for diagnosis and treatment of neurological diseases: dissecting and modulating complex function in the monoaminergic systems of the brain"). PRH is supported in part by NIH grant P50 AG005138.
Abbreviations
- 2OG
2-oxoglutarate
- 5-HT
5-hydroxytryptamine
- 5-HIAA
5-hydroxyindoleacetic acid
- 5mC
5-methylcytosine
- α-KG
α-ketoglutarate
- AAAD
aromatic-L-amino acid decarboxylase
- AANAT
aralkylamine N-acetyltransferase
- AAS
ascending arousal system
- Aβ
amyloid β
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- ADI
Alzheimer’s Disease International
- ADAM10
a disintegrin and metalloprotease 10
- ADL
activities of daily living
- AgD
argyrophilic grain disease
- Alu
Alu (repetitive) element
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- AP
amyloid plaques
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- ASMT
N-Acetylserotonin O-methyltransferase
- ATP
adenosine triphosphate
- BACE
β-site APP cleaving enzyme
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BF
Bayes factor
- BMAA
β-methylamino-l-alanine
- BPSD
behavioral and psychological symptoms of dementia
- BuChE
butyrilcholinesterase
- CAA
cerebral amyloid angiopathy
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CDR
Clinical Dementia Rating
- CERAD
Consortium to Establish a Registry for Alzheimer’s disease
- ChAT
choline acetyltransferase
- CHIP
carboxyl-terminus of Hsp70 interacting protein
- COMT
Catechol-O-methyltransferase
- CSF
cerebrospinal fluid
- CVD
cerebrovascular diseases
- DBH
dopamin-β-hydroxylase
- DLB
dementia with Lewy bodies
- DMN
default-mode network
- DRN
dorsal raphe nucleus
- DRN-CC
dorsal raphe nucleus, pars caudalis compacta
- DRN-CL
dorsal raphe nucleus, caudal lamellar subnucleus
- DRN-IF
dorsal raphe nucleus, pars interfascicularis
- DRN-ST
dorsal raphe nucleus, pars supratroclearis
- DSM
Diagnostic and Statistical Manual for Mental Disorders
- ELISA
Enzyme-Linked Immunosorbent Assay
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degneration
- GWAS
genome-wide association study
- HCHWA-D
hereditary cerebral hemorrhage with amyloidosis – Dutch type
- HDC
histidine decarboxylase
- HLA
human leukocyte antigen
- HMT
histamine N-methyltransferase
- HS
hippocampal sclerosis
- HSV1
herpes simplex virus type 1
- LBD
Lewy body disease
- LC
locus coeruleus
- L-DOPA
L-3,4-dihydroxyphenylalanine
- LINE-1
long interspersed (repetitive) element 1
- LTD
long-term depression
- LTP
long-term potentiation
- MAO-A
monoamine oxidase A
- MAPT
microtubule-associated protein tau
- MCI
mild cognitive impairment
- miRNA
microRNA
- MMSE
Mini-Mental State Examination
- MRI
magnetic resonance imaging
- mtDNA
mitochondrial DNA
- NFT
neurofibrillary tangles
- NIA/AA
National Institute on Aging and the Alzheimer’s Association
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association
- NMDAR
N-methyl-d-aspartate receptors
- NRC-A
nucleus raphe centralis, pars annularis
- NRC-P
nucleus raphe centralis, pars principalis
- NRD
nucleus raphe dorsalis
- NP
neuritic plaques
- NRL
nucleus raphe linearis
- NRM
nucleus raphe magnus
- NRO
nucleus raphe obscures
- NRP
nucleus raphe pallidus
- NT
neuropil threads
- PART
primary age-related tauopathy
- PD
Parkinson’s disease
- PET
positron emission tomography
- PHF
paired helical filaments
- PP2A
protein phosphatase 2A
- PSEN
presenilin
- PSP
progressive supranuclear palsy
- RCT
randomized controlled trial
- REM sleep
rapid eye movement sleep
- rs
reference single nucleotide polymorphism
- rTMS
repetitive transcranial magnetic stimulation
- Sat-α
Satellite-α repetitive element
- SCN
suprachiasmatic nucleus
- SDS
sodium dodecylsulfate
- SF
straight filaments
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SP
senile plaques
- SPECT
single-photon emission computed tomography
- SWS
slow-wave sleep
- TACE
TNF-α converting enzyme
- TH
tyrosine hydroxylase
- TNF-α
tumor necrosis factor alpha
- TPSD
tangle-predominant senile dementia
- TPH
tryptophan hydroxylase
- VBI
vascular brain injury
- VTA
ventral tegmental area
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adwan L, Zawia NH. Epigenetics: a novel therapeutic approach for the treatment of Alzheimer's disease. Pharmacol. Ther. 2013;139:41–50. doi: 10.1016/j.pharmthera.2013.03.010. (PubMed: 23562602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, Suomalainen R, Panula P. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991;44:465–481. doi: 10.1016/0306-4522(91)90070-5. (PubMed: 1719449) [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. (PubMed: 21514249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer´s Association. Alzheimer’s Association Trial Match. [Accessed 17 Apr 2014]; http://www.alz.org/research/clinical_trials/find_clinical_trials_trialmatch.asp. [Google Scholar]
- Ambree O, Richter H, Sachser N, Lewejohann L, Dere E, de Souza Silva MA, Herring A, Keyvani K, Paulus W, Schabitz WR. Levodopa ameliorates learning and memory deficits in a murine model of Alzheimer’s disease. Neurobiol. Aging. 2009;30:1192–1204. doi: 10.1016/j.neurobiolaging.2007.11.010. (PubMed: 18079024) [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders ((IV-TR) 4th. Washington DC: American Psychiatric Publishing; 2000. text revised. [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistic Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing; 2013. (th Ed.) [Google Scholar]
- Anaya F, Lees A, de Silva R. Tau gene promoter rs242557 and allele-specific protein binding. Transl. Neurosci. 2011;2:176–205. [Google Scholar]
- Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. (PubMed: 14187491) [DOI] [PubMed] [Google Scholar]
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, de Silva CG, Guimarães DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite RE, Ferretti-Rebustini RE, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R. Cell nuber changes in Alzheimer's disease relate to dementia, not to plaques and tangles. Brain. 13:3738–3752. doi: 10.1093/brain/awt273. (PubMed: 24136825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. (PubMed: 15925696) [DOI] [PubMed] [Google Scholar]
- Arendt T, Stieler J, Strijkstra AM, Hut RA, Rüdiger J, Van der Zee EA, Harkany T, Holzer M, Härtig W. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 2003;23:6972–6981. doi: 10.1523/JNEUROSCI.23-18-06972.2003. (PubMed: 12904458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA. Size frequency distributions of β-amyloid (Aβ) deposits: a comparative study of four neurodegenerative disorders. Folia Neuropathol. 2012;50:240–249. doi: 10.5114/fn.2012.30524. (PubMed: 23023338) [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. (PubMed: 1549228) [DOI] [PubMed] [Google Scholar]
- Arslan OE. Neuroanatomic basis of clinical neurology. Taylor & Francis, Boca Raton, FL, USA: CRC Press; 2015. [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. (PubMed: 26436904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. (PubMed: 8027789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. (PubMed: 16022602) [DOI] [PubMed] [Google Scholar]
- Attar A, Rahimi F, Bitan G. Modulators of amyloid protein aggregation and toxicity: EGCG and CLR01. Transl. Neurosci. 2013;4:385–409. [Google Scholar]
- Atwood CS, Bowen RL, Smith MA, Perry G. Cerebrovascular requirement for sealant, anti-coagulant and remodeling molecules that allow for the maintenance of vascular integrity and blood supply. Brain Res. Rev. 2003;43:164–178. doi: 10.1016/s0165-0173(03)00206-6. (PubMed: 14499467) [DOI] [PubMed] [Google Scholar]
- Avila J, Gómez-Ramos A, Bolós M. AD genetic risk factors and tau spreading. Frontiers Aging Neurosci. 2015;7:99. doi: 10.3389/fnagi.2015.00099. (PubMed: 26052285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974;184:1341–1348. doi: 10.1126/science.184.4144.1341. (PubMed: 4151465) [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv. Neurol. 1986;43:407–468. (PubMed: 2418648) [PubMed] [Google Scholar]
- Babić M, Vogrinc Ž, Diana A, Klepac N, Borovečki F, Hof PR, Šimić G. Comparison of two commercial enzyme-linked immunosorbent assays for cerebrospinal fluid measurement of amyloid β1–42 and total tau. Transl. Neurosci. 2013;4:234–240. doi: 10.2478/s13380-013-0123-4. (PubMed: 24376914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babić M, Švob Štrac D, Mück-Šeler D, Pivac N, Stanić G, Hof PR, Šimić G. Update on the core and developing cerebrospinal fluid biomarkers for Alzheimer’s disease. Croat. Med. J. 2014;28:347–365. doi: 10.3325/cmj.2014.55.347. (PubMed: 25165049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babić Leko M, Borovečki F, Dejanović N, Hof PR, Šimić G. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J. Alzheimers Dis. 2016;50:765–778. doi: 10.3233/JAD-150705. (PubMed: 26836160) [DOI] [PubMed] [Google Scholar]
- Bahna SG, Sathiyapalan A, Foster JA, Niles LP. Regional upregulation of hippocampal melatonin MT2 receptors by valproic acid: therapeutic implications for Alzheimer's disease. Neurosci. Lett. 2014;576:84–87. doi: 10.1016/j.neulet.2014.05.056. (PubMed: 24909617) [DOI] [PubMed] [Google Scholar]
- Baker KB, Halliday GM, Halasz P, Hornung J-P, Geffen LB, Cotton RGH, Törk I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1990;7:301–320. doi: 10.1002/syn.890070407. (PubMed: 2042112) [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Törk I. Cytoarchitecture of the human dorsal raphe nucleus. J. Comp. Neurol. 1990;301:147–161. doi: 10.1002/cne.903010202. (PubMed: 2262589) [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RGH, Törk I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991a;7:301–320. doi: 10.1002/syn.890070407. (PubMed: 2042112) [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RGH, Törk I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991b;42:757–775. doi: 10.1016/0306-4522(91)90043-n. (PubMed: 1720227) [DOI] [PubMed] [Google Scholar]
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylation tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. (PubMed: 2495152) [DOI] [PubMed] [Google Scholar]
- Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S. Decreased CSF concentrations of homovanillic acid and gamma-aminobutyric acid in Alzheimer's disease. Age- or disease-related modifications? Arch. Neurol. 1982;39:709–712. doi: 10.1001/archneur.1982.00510230035010. (PubMed: 6181768) [DOI] [PubMed] [Google Scholar]
- Basurto-Islas G, Blanchard J, Tung YC, Fernandez JR, Voronkov M, Stock M, Zhang S, Stock JB, Iqbal K. Therapeutic benefits of a component of coffee in a rat model of Alzheimer's disease. Neurobiol. Aging. 2014;35:2701–2712. doi: 10.1016/j.neurobiolaging.2014.06.012. (PubMed: 25034344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. (PubMed: 21715678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. (PubMed: 21303898) [DOI] [PubMed] [Google Scholar]
- Beck O, Lundman A, Jonsson G. 5-Hydroxytryptophol and 5-hydroxyindoleacetic acid levels in rat brain: effects of various drugs affecting serotonergic transmitter mechanisms. J. Neural. Transm. 1987;69:287–298. doi: 10.1007/BF01244349. (PubMed: 2442302) [DOI] [PubMed] [Google Scholar]
- Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J. Cereb. Blood. Flow. Metab. 2012;32:2135–2145. doi: 10.1038/jcbfm.2012.115. (PubMed: 22872230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K, Schindowski K, Burnouf S, Caillierez R, Grosjean ME, Demeyer D, Hamdane M, Sergeant N, Blum D, Buée L. Early tau pathology involving the septo-hippocampal pathway in a tau transgenic model: relevance to Alzheimer's disease. Curr. Alzheimer Res. 2009;6:152–157. doi: 10.2174/156720509787602843. (PubMed: 19355850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb. Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. (PubMed: 12571119) [DOI] [PubMed] [Google Scholar]
- Benitez BA, Karch CM, Cai Y, Jin SC, Cooper B, Carrell D, Bertelsen S, Chibnik L, Schneider JA, Bennett DA, Alzheimer's Disease Neuroimaging Initiative, Genetic and Environmental Risk for Alzheimer's Disease Consortium GERAD. Fagan AM, Holtzman D, Morris JC, Goate AM, Cruchaga C. The PSEN1, p.E318G variant increases the risk of Alzheimer's disease in APOE-ε4 carriers. PLoS Genet. 2013;9:e1003685. doi: 10.1371/journal.pgen.1003685. (PubMed: 23990795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Yu L, Yang J, Srivastava GP, Aubin C, De Jager PL. Epigenomics of Alzheimer's disease. Transl. Res. 2015;165:200–220. doi: 10.1016/j.trsl.2014.05.006. (PubMed: 24905038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Padovani A, Borroni B. Phenotypic heterogeneity of monogenic frontotemporal dementia. Front. Aging Neurosci. 2015;7:171. doi: 10.3389/fnagi.2015.00171. (PubMed: 26388768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia N, Hall G. Untangling the role of tau in Alzheimer’s disease: a unifying hypothesis. Transl. Neurosci. 2013;4:155–133. [Google Scholar]
- Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. (PubMed: 7826280) [DOI] [PubMed] [Google Scholar]
- Blanquet V, Goldgaber D, Turleau C, Créau-Goldberg N, Delabar J, Sinet PM, Roudier M, de Grouchy J. The Aβ protein (AD-AP) cDNA hybridizes in normal and Alzheimer individuals near the interface of 21q21 and q22.1. Ann. Genet. 1987;30:68–69. (PubMed: 3314665) [PubMed] [Google Scholar]
- Blažević S, Hranilović D. Expression of 5-HT related genes after perinatal treatment of 5-HT agonists. Transl. Neurosci. 2013;4:165–171. [Google Scholar]
- Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. (PubMed: 14505582) [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Gottfries CG, Lekman A, Karlsson I, Skoog I, Svennerholm L. Significance of decreased lumbar CSF levels of HVA and 5-HIAA in Alzheimer's disease. Neurobiol. Aging. 1992;13:107–113. doi: 10.1016/0197-4580(92)90017-r. (PubMed: 1371850) [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer's disease. Nat. Rev. Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. (PubMed: 20157306) [DOI] [PubMed] [Google Scholar]
- Boban M, Kostović I, Šimić G. Nucleus subputaminalis: neglected part of the basal nucleus of Meynert. Brain. 2006;129:E42. doi: 10.1093/brain/awl025. (PubMed: 16543395) [DOI] [PubMed] [Google Scholar]
- Boban M, Šarac H, Mimica N, Mladinov M, Süßmair C, Ackl N, Bader B, Huzak M, Danek A, Hof PR, Šimić G. CSF tau proteins in differential diagnosis of dementia. Transl. Neurosci. 2010;1:43–48. [Google Scholar]
- Boban M, Malojčić B, Mimica N, Vuković S, Zrilić I, Hof PR, Šimić G. The reliability and validity of the mini-mental state examination in the elderly Croatian population. Dement. Geriatr. Cogn. Disord. 2012;33:385–392. doi: 10.1159/000339596. (PubMed: 22814030) [DOI] [PubMed] [Google Scholar]
- Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol. Psychiatry. 1983;18:951–969. (PubMed: 6640008) [PubMed] [Google Scholar]
- Bolea I, Gella A, Monjas L, Pérez C, Rodríguez-Franco MI, Marco-Contelles J, Samadi A, Unzeta M. Multipotent, permeable drug ASS234 inhibits Aβ aggregation, possesses antioxidant properties and protects from Aβ-induced apoptosis in vitro. Curr. Alzheimer Res. 2013;10:797–808. doi: 10.2174/15672050113109990151. (PubMed: 23919774) [DOI] [PubMed] [Google Scholar]
- Bollati V, Galimberti D, Pergoli L, Dalla Valle E, Barretta F, Cortini F, Scarpini E, Betazzi PA, Baccarelli A. DNA methylation in repetitive elements and Alzheimer's disease. Brain Behav. Immun. 2011;25:1078–1083. doi: 10.1016/j.bbi.2011.01.017. (PubMed: 21296655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognin S, Zatta P, Drago D, Tognon G, Parnigotto PP, Ricchelli F. Mutual stimulation of Aβ fibrillogenesis by clioquinol and divalent metals. Neuromolecular Med. 10:322–332. doi: 10.1007/s12017-008-8046-x. (PubMed: 18712494) [DOI] [PubMed] [Google Scholar]
- Bolte Taylor J, Cunningham MC, Benes FM. Neonatal raphe lesionsincrease dopamine fibers in prefrontal cortex of adult rats. Neuroreport. 1998;9:1811–1815. doi: 10.1097/00001756-199806010-00026. (PubMed: 9665606) [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/wnl.32.2.164. (PubMed: 7198741) [DOI] [PubMed] [Google Scholar]
- Borroni B, Agosti C, Archetti S, Costanzi C, Bonomi S, Ghianda D, Lenzi GL, Caimi L, Di Luca M, Padovani A. Catechol-O-methyltransferase genepolymorphism is associated with risk of psychosis in Alzheimer Disease. Neurosci. Lett. 2004;370:127–129. doi: 10.1016/j.neulet.2004.08.006. (PubMed: 15488308) [DOI] [PubMed] [Google Scholar]
- Borroni B, Costanzi C, Padovani A. Genetic susceptibility to behavioural and psychological symptoms in Alzheimer disease. Curr. Alzheimer Res. 2010;7:158–164. doi: 10.2174/156720510790691173. (PubMed: 19715553) [DOI] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. (PubMed: 18652859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H. On the nuclei of the human brain stem. II. The raphe nuclei. Z. Zellforsch. Mikrosk. Anat. 1970;107:123–141. (PubMed: 4194341) [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. (PubMed: 16906426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. (PubMed: 1759558) [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. (PubMed: 9330961) [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur. Neurol. 1993;33:403–408. doi: 10.1159/000116984. (PubMed: 8307060) [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J. Neural Transm. 1998;105:801–819. doi: 10.1007/s007020050096. (PubMed: 9869320) [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Mandelkow E-M. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–567. doi: 10.1007/BF00293315. (PubMed: 7522386) [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Amyloid-β may be released from non-junctional varicosities of axons generated from abnormal tau-containing brainstem nuclei in sporadic Alzheimer’s disease: a hypothesis. Acta Neuropathol. 2013;126:303–306. doi: 10.1007/s00401-013-1153-2. (PubMed: 23824268) [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. (PubMed: 26283673) [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. (PubMed: 22002422) [DOI] [PubMed] [Google Scholar]
- Brendel M, Pogarell O, Xiong G, Delker A, Bartenstein P, Rominger A. Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:716–724. doi: 10.1007/s00259-014-2975-4. (PubMed: 25631614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioni JD, Esbenshade TA, Garrison TR, Bitner SR, Cowart MD. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer’s disease. J Pharmacol. Exp. Ther. 2011;336:38–46. doi: 10.1124/jpet.110.166876. (PubMed: 20864505) [DOI] [PubMed] [Google Scholar]
- Brockmeyer A, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl. Neurosci. 2016;7:24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog. Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. (PubMed: 11164999) [DOI] [PubMed] [Google Scholar]
- Brunner P, Sözer-Topcular N, Jockers R, Ravid R, Angeloni D, Fraschini F, Eckert A, Müller-Spahn F, Savaskan E. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer's disease. Eur. J. Histochem. 2006;50:311–316. (PubMed: 17213040) [PubMed] [Google Scholar]
- Brusa L, Bassi A, Stefani A, Pierantozzi M, Peppe A, Caramia MD, Boffa L, Ruggieri S, Stanzione P. Pramipexole in comparison to l-dopa: a neuropsychological study. J. Neural. Transm. 2003;110:373–380. doi: 10.1007/s00702-002-0811-7. (PubMed: 12658365) [DOI] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. (PubMed: 10967355) [DOI] [PubMed] [Google Scholar]
- Burke WJ, Roccaforte WH, Wengel SP, Bayer BL, Ranno AE, Willcockson NK. L-deprenyl in the treatment of mild dementia of the Alzheimer type: results of a 15-month trial. J. Am. Geriatr. Soc. 1993;41:1219–1225. doi: 10.1111/j.1532-5415.1993.tb07306.x. (PubMed: 8227897) [DOI] [PubMed] [Google Scholar]
- Bush AI. The metal theory of Alzheimer's disease. J. Alzheimers Dis. 2013;33(Suppl. 1):S277–S281. doi: 10.3233/JAD-2012-129011. (PubMed: 22635102) [DOI] [PubMed] [Google Scholar]
- Bush AI, Tanzi RE. Therapeutics for Alzheimer's disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. (PubMed: 18625454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R. Pharmacogenetic basis for therapeutic optimization in Alzheimer's disease. Mol. Diagn. Ther. 2007;11:385–405. doi: 10.1007/BF03256262. (PubMed: 18078356) [DOI] [PubMed] [Google Scholar]
- Cacace R, Van den Bossche T, Engelborghs S, Geerts N, Laureys A, Dillen L, Graff C, Thonberg H, Chiang HH, Pastor P, Ortega-Cubero S, Pastor MA, Diehl-Schmid J, Alexopoulos P, Benussi L, Ghidoni R, Binetti G, Nacmias B, Sorbi S, Sanchez-Valle R, Lladó A, Gelpi E, Almeida MR, Santana I, Tsolaki M, Koutroumani M, Clarimon J, Lleó A, Fortea J, de Mendonça A, Martins M, Borroni B, Padovani A, Matej R, Rohan Z, Vandenbulcke M, Vandenberghe R, De Deyn PP, Cras P, van der Zee J, Sleegars K, Van Broeckhoven C Belgium Neurology (BELNEU) Consortium and the European Early-Onset Dementia (EU EOD) Consortium. Rare variant in PLD3 do not affect risk for early-onset Alzheimer’s disease in a European Consortium Cohort. Hum. Mutat. 2015;36:1126–1235. doi: 10.1002/humu.22908. (PubMed: 26411346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafate S, Buist A, Miskiewicz K, Vijayan V, Daneels G, De Strooper B, de Wit J, Verstreken P, Moechars D. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep. 2015;11:1176–1183. doi: 10.1016/j.celrep.2015.04.043. (PubMed: 25981034) [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Park SB, Torres-Jardón R, D’Angiulli A. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimers Dis. 2015;43:1039–1058. doi: 10.3233/JAD-141365. (PubMed: 25147109) [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr. Neuropharmacol. 2010;8:218–227. doi: 10.2174/157015910792246209. (PubMed: 21358972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants. 2014;3:245–277. doi: 10.3390/antiox3020245. (PubMed: 26784870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MC, Tschanz JT, Norton MC, Welsh-Bohmer K, Martin BK, Breitner JC. H2 histamine receptor blockade in the treatment of Alzheimer disease:a randomized, double-blind, placebo-controlled trial of nizatidine. Alzheimer Dis. Assoc. Disord. 2002;16:24–30. doi: 10.1097/00002093-200201000-00004. (PubMed: 11882746) [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain Aβ peptide clearance. Sci. Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. (PubMed: 21715678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Weinshenker D, Levey AI. Targeting norepinephrine in mild cognitive impairment and Alzheimer's disease. Alzheimers Res. Ther. 2013;5:21. doi: 10.1186/alzrt175. (PubMed: 23634965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A, Martinez-Ramirez S, Shoamanesh A, Oliveira-Filho J, Frosch M, Vashkevich A, Ayres A, Rosand J, Gurol ME, Greenberg SM, Viswanathan A. Cerebral amyloid angiopathy with and without hemorrhage: evidence for different disease phenotypes. Neurology. 2015;84:1206–1212. doi: 10.1212/WNL.0000000000001398. (PubMed: 25716356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CPLH, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol. Appl. Neurobiol. 2000;26:347–355. doi: 10.1046/j.1365-2990.2000.00254.x. (PubMed: 10931368) [DOI] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petraschek M, Reue K, Jung ME, Frand AR, Huang J. The metabolite α–ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. (PubMed: 24828042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid Aβ levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. (PubMed: 16364896) [DOI] [PubMed] [Google Scholar]
- Citron M, Teplow DB, Selkoe DJ. Generation of Aβ protein from its precursor is sequence specific. Neuron. 1995;14:661–670. doi: 10.1016/0896-6273(95)90323-2. (PubMed: 7695913) [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue Aβ-protein in both transfected cells and transgenic mice. Nat. Med. 1997;3:67–72. doi: 10.1038/nm0197-67. (PubMed: 8986743) [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramovski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. (PubMed: 19503072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. (PubMed: 23690619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Mizielinska S, Edgar JR, Nielsen TT, Marshall S, Norona FE, Robbins M, Damirji H, Holm IE, Johannsen P, Nielsen JE, Asante EA, Collinge J, FReJA consortium. Isaacs AM. Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology. Acta Neuropathol. 2015;130:511–523. doi: 10.1007/s00401-015-1475-3. (PubMed 26358247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloby SJ, McParland S, O'Brien JT, Attems J. Neuropathological correlates of dopaminergic imaging in Alzheimer's disease and Lewy body dementias. Brain. 2012;135:2798–2808. doi: 10.1093/brain/aws211. (PubMed: 22961551) [DOI] [PubMed] [Google Scholar]
- Cook C, Gendron TF, Scheffel K, Carlomagno Y, Dunmore J, DeTure M, Petrucelli L. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Hum. Mol. Genet. 2012;21:2936–2945. doi: 10.1093/hmg/dds125. (PubMed: 22492994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Carlomagno Y, Gendron TF, Dunmore J, Scheffel K, Stetler C, Davis M, Dickson D, Jarpe M, DeTure M, Petrucelli L. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 2014;23:104–116. doi: 10.1093/hmg/ddt402. (PubMed: 23962722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F, Pham CL, Vink R, Blumbergs PC, Masters CL, van den Heuvel C, Cappai R. The neuroprotective domains of the amyloid precursor protein, in traumatic brain injury, are located in the two growth factor domains. Brain Res. 2011;1378:137–143. doi: 10.1016/j.brainres.2010.12.077. (PubMed: 21215734) [DOI] [PubMed] [Google Scholar]
- Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. (PubMed: 14597720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Putative CSF protein biomarker candidates for amnestic mild cognitive impairment. Transl. Neurosci. 2010a;1:2–8. doi: 10.2478/v10134-010-0004-0. (PubMed: 21311725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J. Neurochem. 2010b;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x. (PubMed: 20132474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016;283:20152397. doi: 10.1098/rspb.2015.2397. (PubMed: 26791617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. (PubMed: 25348064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Karch CM, Jin SC, Benitez BA, Cai Y, Guerreiro R, Harari O, Norton J, Budde J, Bertelsen S, Jeng AT, Cooper B, Skorupa T, Carrell D, Levitch D, Hsu S, Choi J, Ryten M, UK Brain Expression Consortium. Hardy J, Ryten M, Trabzuni D, Weale ME, Ramasamy A, Smith C, Sassi C, Bras J, Gibbs JR, Hernandez DG, Lupton MK, Powell J, Forabosco P, Ridge PG, Corcoran CD, Tschanz JT, Norton MC, Munger RG, Schmutz C, Leary M, Demirci FY, Bamne MN, Wang X, Lopez OL, Ganguli M, Medway C, Turton J, Lord J, Braae A, Barber I, Brown K, Alzheimer’s Research UK Consortium. Passmore P, Craig D, Johnston J, McGuinness B, Todd S, Heun R, Kölsch H, Kehoe PG, Hooper NM, Vardy ER, Mann DM, Pickering-Brown S, Brown K, Kalsheker N, Lowe J, Morgan K, David Smith A, Wilcock G, Warden D, Holmes C, Pastor P, Lorenzo-Betancor O, Brkanac Z, Scott E, Topol E, Morgan K, Rogaeva E, Singleton AB, Hardy J, Kamboh MI, St George-Hyslop P, Cairns N, Morris JC, Kauwe JS, Goate AM. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature. 2014;505:550–554. doi: 10.1038/nature12825. (PubMed: 24336208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Neuropsychiatric aspects of Alzheimer’s disease and other dementing illnesses. In: Stuart C, Yudofsky E, Robert E, Hales E, et al., editors. American Psychiatric Press textbook of neuropsychiatry. 2nd. Washington, DC: American Psychiatric Press; 1992. pp. 605–620. [Google Scholar]
- Curcio CA, Kemper T. Nucleus raphe dorsalis in dementia of the Alzheimer type: neurofibrillary change and neuronal packing density. J. Neuropathol. Exp. Neurol. 1984;43:359–368. doi: 10.1097/00005072-198407000-00001. (PubMed: 6737007) [DOI] [PubMed] [Google Scholar]
- Czech C, Tremp G, Pradier L. Presenilins and Alzheimer's disease: biological functions and pathogenic mechanisms. Prog. Neurobiol. 2000;60:363–384. doi: 10.1016/s0301-0082(99)00033-7. (PubMed: 10670705) [DOI] [PubMed] [Google Scholar]
- Da Silva Costa-Aze V, Dauphin F, Boulouard M. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology. 2012;2221:99–115. doi: 10.1007/s00213-011-2627-3. (PubMed: 22367167) [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the exitstance of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of the brain stem neurons. Acta Physiol. Scand. 1964a;62:1–55. (PubMed: 14229500) [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964b;20:398–399. doi: 10.1007/BF02147990. (PubMed: 5856530) [DOI] [PubMed] [Google Scholar]
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, Sakurai E, Kato M, Okamura N, Kuramasu A, Yanai K. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci. Res. 2007;57:306–313. doi: 10.1016/j.neures.2006.10.020. (PubMed: 17145090) [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–1603. doi: 10.1093/sleep/33.12.1589. (PubMed: 21120121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon M, Al Awabdh S, Emerit M-B, Masson J. Insights into serotonin receptor trafficking: cell membrane targeting and internalization. Prog. Mol. Biol. Transl. Sci. 2015;132:97–126. doi: 10.1016/bs.pmbts.2015.02.009. (PubMed: 26055056) [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. (PubMed: 22365544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and γ-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. (PubMed: 22315713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF. Depression and antisocial personality disorder: two contrasting disorders of 5HT function. J. Neural. Transm. Suppl. 2003;64:79–93. doi: 10.1007/978-3-7091-6020-6_5. (PubMed: 12830930) [DOI] [PubMed] [Google Scholar]
- De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol. Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. (PubMed: 16047543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Topic B, Spieler RE, Haas HL, Huston JP. Histidine-decarboxylase knock out mice show deficient non reinforced episodic object memory, improved negatively reinforced water-maze performance, and increased neo- and ventro-striatal dopamine turnover. Learn. Mem. 2003;10:510–519. doi: 10.1101/lm.67603. (PubMed: 14657262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. (PubMed: 11098652) [DOI] [PubMed] [Google Scholar]
- Descarries L, Seguela E, Watkins KC. Nonjunctional relationships of monoamine axon terminals in the cerebral cortex of adult rat. In: Fuxe K, Agnati LE, editors. Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission. New York: Raven Press; 1991. pp. 53–62. [Google Scholar]
- Diana A, Šimić G, Sinforiani E, Orrù N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to Aβ1–42 peptide. Coll. Antropol. 2008;32(Suppl. 1):51–58. (PubMed: 18405058) [PubMed] [Google Scholar]
- Di Giovanni G, García I, Colangeli R, Pierucci M, Rivadulla ML, Soriano E, Chioua M, Della Corte L, Yáñez M, De Deurwaerdère P, Fall Y, Marco-Contelles J. N-(furan-2-ylmethyl)-N-methylprop-2-yn-1-amine (F2MPA): a potential cognitive enhancer with MAO inhibitor properties. CNS Neurosci. Ther. 2014;20:633–640. doi: 10.1111/cns.12284. (PubMed: 24848125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon Clarke SE, Ramsay RR. Dietary inhibitors of monoamine oxidase. J. Neural. Transm. 2011;118:1031–1041. doi: 10.1007/s00702-010-0537-x. (PubMed: 21190052) [DOI] [PubMed] [Google Scholar]
- Donovan NJ, Hsu DC, Dagley AS, Schultz AP, Amariglio RE, Mormino EC, Okereke OI, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J. Alzheimers Dis. 2015;46:63–73. doi: 10.3233/JAD-142940. (PubMed: 25697700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D Dimebon Investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. (PubMed: 18640457) [DOI] [PubMed] [Google Scholar]
- Dougall NJ, Bruggink S, Ebmeier KP. Systematic review of the diagnostic accuracy of 99mTc-HMPAO-SPECT in dementia. Am. J. Geriatr. Psychiatry. 2004;12:554–570. doi: 10.1176/appi.ajgp.12.6.554. (PubMed: 15545324) [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. (PubMed: 16217123) [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. (PubMed: 17616482) [DOI] [PubMed] [Google Scholar]
- Dunlop RA, Cox PA, Banack SA, Rodgers KJ. The non-protein amino acid BMAA is misincorporated into human proteins in place of l-serine causing protein misfolding and aggregation. PLoS One. 2013;8:e75376. doi: 10.1371/journal.pone.0075376. (PubMed: 24086518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S, Bégard S, Caillierez R, Lachaud C, Delattre L, Carrier S, Loyens A, Galas MC, Bousser L, Melki R, Aurégan G, Hantraye P, Brouillet E, Buée L, Colin M. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One. 2014;9:e100760. doi: 10.1371/journal.pone.0100760. (PubMed: 24971751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson DE. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr. Pharm. Des. 2014;20:155–160. doi: 10.2174/13816128113190990406. (PubMed: 23701542) [DOI] [PubMed] [Google Scholar]
- Egan M, Yaari R, Liu L, Ryan M, Peng Y, Lines C, Michelson D. Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Curr. Alzheimer Res. 2012;9:481–490. doi: 10.2174/156720512800492530. (PubMed: 22272611) [DOI] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer's disease severity on cerebrospinal fluid norepinephrineconcentration. Am. J. Psychiatry. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. (PubMed: 8988954) [DOI] [PubMed] [Google Scholar]
- Endres K, Fahrenholz F. Regulation of α-secretase ADAM10 expression and activity. Exp. Brain Res. 2012;217:343–352. doi: 10.1007/s00221-011-2885-7. (PubMed: 21969210) [DOI] [PubMed] [Google Scholar]
- España RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep. 2011;34:845–858. doi: 10.5665/SLEEP.1112. (PubMed: 21731134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola MF, Lee SP, Nixon RA, Duff K, Helpern JA. Histological co-localization of iron in Aβ plaques of PS/APP transgenic mice. Neurochem. Res. 30:201–205. doi: 10.1007/s11064-004-2442-x. (PubMed: 15895823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Heneka MT, Gavrilyuk V, Dello Russo C, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. (PubMed: 12176079) [DOI] [PubMed] [Google Scholar]
- Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, Zhang JT. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer's disease. J. Pineal Res. 2004;37:129–136. doi: 10.1111/j.1600-079X.2004.00144.x. (PubMed: 15298672) [DOI] [PubMed] [Google Scholar]
- Ferrari R, Hernandez DG, Nalls MA, Rohrer JD, Ramasamy A, Kwok JB, Dobson-Stone C, Brooks WS, Shoefield PR, Halliday GM, Hodges JR, Piquet O, Bartley L, Thompson E, Haan E, Hernández I, Ruiz A, Boada M, Borroni B, Padovani A, Cruchaga C, Cairns NJ, Benussi L, Binetti G, Ghidoni R, Forloni G, Galimberti D, Fenoglio C, Serpente M, Scarpini E, Clarimón J, Lleó A, Blesa R, Waldö ML, Nilsson K, Nilsson C, Mackenzie IR, Hsiung GY, Mann DM, Grafman J, Morris CM, Attems J, Griffiths TD, McKeith IG, Thomas AJ, Pietrini P, Huey ED, Wassermann EM, Baborie A, Jaros E, Tierney MC, Pastor P, Razquin C, Ortega-Cubero S, Alonso E, Perneczky R, Diehl-Schmid J, Alexopoulos P, Kurz A, Rainero I, Rubino E, Pinessi L, Rogaeva E, St George-Hyslop P, Rossi G, Tagliavini F, Giaccone G, Rowe JB, Schlachetzki JC, Uphill J, Collinge J, Mead S, Danek A, Van Deerlin VM, Grossman M, Trojanowski JQ, van der Zee J, Deschamps W, Van Langenhove T, Cruts M, Van Broeckhoven C, Cappa SF, Le Ber I, Hannequin D, Golfier V, Vercelletto M, Brice A, Nacmias B, Sorbi S, Bagnoli S, Piaceri I, Nielsen JE, Hjermind LE, Riemenschneider M, Mayhaus M, Ibach B, Gasparoni G, Pichler S, Gu W, Rossor MN, Fox NC, Warren JD, Spillantini MG, Morris HR, Rizzu P, Heutink P, Snowden JS, Rollinson S, Richardson A, Gerhard A, Bruni AC, Maletta R, Frangipane F, Cupidi C, Bernardi L, Anfossi M, Gallo M, Conidi ME, Smirne N, Rademakers R, Baker M, Dickson DW, Graff-Radford NR, Petersen RC, Knopman D, Josephs KA, Boeve BF, Parisi JE, Seeley WW, Miller BL, Karydas AM, Rosen H, van Swieten JC, Dopper EG, Seelaar H, Pijnenburg YA, Scheltens P, Logroscino G, Cappozzo R, Novelli V, Puca AA, Franceschi M, Postiglione A, Milan G, Sorrentino P, Kristiansen M, Chiang HH, Graff C, Pasquier F, Rollin A, Deramecourt V, Lebert F, Kapogiannis D, Ferrucci L, Pickering-Brown S, Singleton AB, Hardy J, Momeni P. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13:686–699. doi: 10.1016/S1474-4422(14)70065-1. (PubMed: 24943344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, Hernández F, Avila J, Lucas JJ. Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat. Med. 2014;20:881–885. doi: 10.1038/nm.3617. (PubMed: 25038828) [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Beggiato S, Cristina Tomasini M, Fuxe K, Tanganelli S. The vigilance promoting drug modafinil modulates serotonin transmission in the rat prefrontal cortex and dorsal raphe nucleus. Possible relevance for its postulated antidepressant activity. Mini Rev. Med. Chem. 2013;13:478–492. doi: 10.2174/1389557511313040002. (PubMed: 22512571) [DOI] [PubMed] [Google Scholar]
- Filip V, Kolibás E. Selegiline in the treatment of Alzheimer’s disease: a long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer Type Study Group. J. Psychiatry Neurosci. 1999;24:234–243. (PubMed: 10354658) [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Kaplinsky L, Kimelberg HK. Serotonin metabolism by monoamine oxidase in rat primary astrocyte cultures. J. Neurochem. 1990;55:2008–2014. doi: 10.1111/j.1471-4159.1990.tb05789.x. (PubMed: 1700071) [DOI] [PubMed] [Google Scholar]
- Flik G, Folgering JH, Cremers TI, Westerink BH, Dremencov E. Interaction between brain histamine and serotonin, norepinephrine, and dopamine systems: in vivo microdialysis and electrophysiology study. J. Mol. Neurosci. 2015;56:320–328. doi: 10.1007/s12031-015-0536-3. (PubMed: 25820671) [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. (PubMed: 1202204) [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH. Extrathalamic modulation of cortical function. Annu. Rev. Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. (PubMed: 3551766) [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J. Pharmacol. Exp. Ther. 2005;313:176–190. doi: 10.1124/jpet.104.078402. (PubMed: 15608077) [DOI] [PubMed] [Google Scholar]
- Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, McLaurin J, Fahnestock M, Mount HT. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:1934–1944. doi: 10.1038/npp.2012.40. (PubMed: 22491352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandemiche ML, De Serrano S, Rush T, Borel E, Elie A, Arnal I, Lanté F, Buisson A. Activity-dependent tau protein translocation to excitatory synaps is disrupted by exposure to Aβ oligomers. J. Neurosci. 2014;34:6084–6097. doi: 10.1523/JNEUROSCI.4261-13.2014. (PubMed: 24760868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M, Rewilak D, Xerri T, Cohen S, Gordon AS, Shandling M, Logan AG. L-deprenyl in Alzheimer’s disease: cognitive and behavioral effects. Neurology. 1998;50:660–668. doi: 10.1212/wnl.50.3.660. (PubMed: 9521253) [DOI] [PubMed] [Google Scholar]
- Frederick JM, Rayborn ME, Laties AM, Lam DM, Hollyfield JG. Dopaminergic neurons in the human retina. J. Comp. Neurol. 1982;210:65–79. doi: 10.1002/cne.902100108. (PubMed: 6127354) [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. (PubMed: 19282288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiuchi A, Sugiura A, Ohshiro H, Watanabe S, Yamamoto T, Take Y. RQ-00000009, a selective 5-HT4 receptor partial agonist, suppressed brain amyloid-b protein levels and improved memory and cognitive performances in rodents. Alzheimers Dement. 2010;6:S538. [Google Scholar]
- Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G. Vaccination with Aβ peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain. 2003;126:285–291. doi: 10.1093/brain/awg031. (PubMed: 12538398) [DOI] [PubMed] [Google Scholar]
- Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol. Scand. Suppl. 1965;64:37–85. (PubMed: 14319769) [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Díaz-Cabiale Z, Agnati LF. On the role of volume transmission and receptor-receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 2012;1476:119–131. doi: 10.1016/j.brainres.2012.01.062. (PubMed: 22373652) [DOI] [PubMed] [Google Scholar]
- Galici R, Boggs JD, Aluisio L, Fraser IC, Bonaventure P, Lord B, Lovenberg TW. JNJ-10181457, a selective non-imidazole histamine H3 receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology. 2009;56:1131–1137. doi: 10.1016/j.neuropharm.2009.03.011. (PubMed: 19345233) [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ. Cholinergic–serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 2005;43:442–449. doi: 10.1016/j.neuropsychologia.2004.06.007. (PubMed: 15707619) [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Hirst WD, Chen CP, Lasheras B, Francis PT, Ramírez MJ. Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. (PubMed: 14571255) [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-β-hydroxylase. J. Comp. Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. (PubMed: 2563268) [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer’s disease. Am. J. Geriatr. Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. (PubMed: 19155748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Maksel D, Gibbs Z, Hou X, Summers RJ, Small DH. Memory loss caused by Aβ protein is rescued by a β3-adrenoceptor agonist. Neurobiol. Aging. 2010;31:614–624. doi: 10.1016/j.neurobiolaging.2008.05.018. (PubMed: 18632189) [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984b;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. (PubMed 6236805) [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984a;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. (PubMed 6375662) [DOI] [PubMed] [Google Scholar]
- Gliebus G, Lippa CF. The influence of β-blockers on delayed memory function in people with cognitive impairment. Am. J. Alzheimers Dis. Other Demen. 2007;22:57–61. doi: 10.1177/1533317506295889. (PubMed: 17534003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goate AM, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. (PubMed 1671712) [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. (PubMed: 8849730) [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride WO, Saffiotti U, Gajdusek DC. Isolation, characterization, and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer's disease, Down's syndrome and aging. J. Neural Trans. 1987;24:23–28. (PubMed: 2960782) [PubMed] [Google Scholar]
- Golomb G, Wisoff J, Miller DC, Boksay I, Kluger A, Weiner H, Salton J, Graves W. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J. Neurol. Neurosurg. Psychiatry. 2000;68:778–781. doi: 10.1136/jnnp.68.6.778. (PubMed: 10811706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. (PubMed: 9005861) [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer's disease brain. J. Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. (PubMed: 7616230) [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Feria-Velasco A. Serotonin/dopamine interaction in memory formation. Prog. Brain Res. 2008;172:603–623. doi: 10.1016/S0079-6123(08)00928-X. (PubMed: 18772052) [DOI] [PubMed] [Google Scholar]
- Gottfries CG, Bartfai T, Carlsson A, Eckernas S, Svennerholm L. Multiple biochemical deficits in both gray and white matter of Alzheimer brains. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1986;10:405–413. doi: 10.1016/0278-5846(86)90014-x. (PubMed: 3797685) [DOI] [PubMed] [Google Scholar]
- Green JP. Histamine and serotonin. In: Siegel GJ, Agranoff B, Albers RW, Molinoff P, editors. Basic neurochemistry. Fourth. New York, NY, USA: Raven Press; 1987. pp. 253–269. [Google Scholar]
- Grinberg LT, Rüb U, Ferreti REL, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in AD. A precocious onset? Neuropathol. Appl. Neurobiol. 2009;35:406–416. doi: 10.1111/j.1365-2990.2009.00997.x. (PubMed: 19508444) [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Wang X, Wang C, Sohn PD, Theofilas P, Sidhu M, Arevalo JB, Heinsen H, Huang EJ, Rosen H, Miller BL, Gan L, Seeley WW. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 2013;125:581–593. doi: 10.1007/s00401-013-1080-2. (PubMed: 23371364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove RA, Harrington CM, Mahler A, Beresford I, Maruff P, Lowy MT, Nicholls AP, Boardley RL, Berges AC, Nathan PJ, Horrigan JP. A randomized, double-blind, placebo-controlled, 16-week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild-to-moderate Alzheimer’s disease. Curr. Alzheimer Res. 2014;11:47–58. doi: 10.2174/1567205010666131212110148. (PubMed: 24359500) [DOI] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. (PubMed: 16574280) [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. (PubMed: 23150934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI. Pseudophosphorylation of tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J. Neurochem. 2006;97:1005–1014. doi: 10.1111/j.1471-4159.2006.03784.x. (PubMed: 16606369) [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett. 2013;587:717–723. doi: 10.1016/j.febslet.2013.01.051. (PubMed: 23395797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Ramos K, Moreno-Castilla P, Castro-Cruz M, McGaugh JL, Martinez-Coria H, LaFerla FM, Bermudez-Rattoni F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn. Mem. 2012;19:453–460. doi: 10.1101/lm.026070.112. (PubMed: 22984283) [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. (PubMed: 12563283) [DOI] [PubMed] [Google Scholar]
- Haig GM, Bain E, Robieson W, Othman AA, Baker J, Lenz RA. A randomized trial of the efficacy and safety of the H3 antagonist ABT-288 in cognitive impairment associated with schizophrenia. Schizophr. Bull. 2014;40:1433–1442. doi: 10.1093/schbul/sbt240. (PubMed: 24516190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig GM, Meier A, Pritchett Y, Hall C, Gault L, Lenz R. Evaluation of efficacy and safety of the H3 antagonist ABT-288 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2012;8:P601–P602. doi: 10.3233/JAD-140291. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Catoe H, Sixta S, Boland R, Johnson D, Hirth V, Wieland D, Eleazer P. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:67–73. doi: 10.1093/gerona/60.1.67. (PubMed: 15741285) [DOI] [PubMed] [Google Scholar]
- Hall GF, Patuto BA. Is tau ready for admission to the prion club? Prion. 2012;6:223–233. doi: 10.4161/pri.19912. (PubMed: 22561167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Joh TH, Cotton RGH, Howe PRC, Geffen LB, Blessing WW. Distribution of monoamine-synthesizing neurons in the human medulla oblongata. J. Comp. Neurol. 1988;273:301–317. doi: 10.1002/cne.902730303. (PubMed: 2905364) [DOI] [PubMed] [Google Scholar]
- Halliday GM, Törk I. Serotonin-like immunoreactive cells and fibres in the rat ventromedial mesencephalic tegmentum. Brain Res. Bull. 1989;22:725–735. doi: 10.1016/0361-9230(89)90092-0. (PubMed: 2736398) [DOI] [PubMed] [Google Scholar]
- Halliday GM, McCann HL, Pamphlett R, Brooks WS, Creasey H, McCusker E, Cotton RG, Broe GA, Harper CG. Brain stem serotonin-synthesizing neurons in Alzheimer’s disease: a clinicopathological correlation. Acta Neuropathol. 1992;84:638–650. doi: 10.1007/BF00227741. (PubMed: 1281956) [DOI] [PubMed] [Google Scholar]
- Hammerschmidt T, Kummer MP, Terwel D, Martinez A, Gorji A, Pape HC, Rommelfanger KS, Schroeder JP, Stoll M, Schultze J, Weinshenker D, Heneka MT. Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice. Biol. Psychiatry. 2013;73:454–463. doi: 10.1016/j.biopsych.2012.06.013. (PubMed: 22883210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. (PubMed: 16488378) [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Ishizu H, Terada S, Takehisa Y, Tanabe Y, Nishinaka T, Kawai K, Kuroda S, Komoto Y, Namba M. An autopsy case of postencephalitic parkinsonism of von Economo type: some new observations concerning neurofibrillary tangles and astrocytic tangles. Neuropathology. 2000;20:143–148. doi: 10.1046/j.1440-1789.2000.00287.x. (PubMed: 10935451) [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. (PubMed: 1763432) [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. (PubMed: 1566067) [DOI] [PubMed] [Google Scholar]
- Hardy J. Does Aβ 42 have a function related to blood homeostasis? Neurochem. Res. 2007;32:833–835. doi: 10.1007/s11064-006-9221-9. (PubMed: 17186373) [DOI] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. (PubMed: 19457065) [DOI] [PubMed] [Google Scholar]
- Harley CW. A role for norepinephrine in arousal, emotion and learning?: limbic modulation by norepinephrine and the Kety hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1987;11:419–458. doi: 10.1016/0278-5846(87)90015-7. (PubMed: 3321150) [DOI] [PubMed] [Google Scholar]
- Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK, Kummer MP. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. (PubMed: 20231476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, Lanctot KL. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J. Clin. Psychopharmacol. 2008;28:296–301. doi: 10.1097/JCP.0b013e318172b479. (PubMed: 18480686) [DOI] [PubMed] [Google Scholar]
- Hilton DA, Love S, Ferguson I, Newman P. Motor neuron disease with neurofibrillary tangles in a non-Guamanian patient. Acta Neuropathol. 1995;90:101–106. doi: 10.1007/BF00294466. (PubMed: 7572072) [DOI] [PubMed] [Google Scholar]
- Himeno E, Ohyagi Y, Ma L, Nakamura N, Miyoshi K, Sakae N, Motomura K, Soejima N, Yamasaki R, Hashimoto T, Tabira T, LaFerla FM, Kira J. Apomorphine treatment in Alzheimer mice promoting amyloid-b degradation. Ann. Neurol. 2011;69:248–256. doi: 10.1002/ana.22319. (PubMed: 21387370) [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol. Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. (PubMed 14645659) [DOI] [PubMed] [Google Scholar]
- Heimer L. The Human Brain and Spinal Cord. 2nd. New York, Berlin: Springer-Verlag; 1995. [Google Scholar]
- Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK, Kummer MP. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. (PubMed: 20231476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Revishchin AV, Bouras C, Charnay Y, Morgane PJ. Distribution of dopaminergic fibers and neurons in visual and auditory cortices of the harbor porpoise and pilot whale. Brain Res Bull. 1995;36:275–284. doi: 10.1016/0361-9230(94)00202-c. (PubMed: 7697381) [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, Alzheimer's Disease Neuroimaging Initiative. van Duijn CM, Breteler MM, Ikram MA, DeStefano AL, Fitzpatrick AL, Lopez O, Launer LJ, Seshadri S, CHARGE consortium. Berr C, Campion D, Epelbaum J, Dartigues JF, Tzourio C, Alpérovitch A, Lathrop M, EADI1 consortium. Feulner TM, Friedrich P, Riehle C, Krawczak M, Schreiber S, Mayhaus M, Nicolhaus S, Wagenpfeil S, Steinberg S, Stefansson H, Stefansson K, Snaedal J, Björnsson S, Jonsson PV, Chouraki V, Genier-Boley B, Hiltunen M, Soininen H, Combarros O, Zelenika D, Delepine M, Bullido MJ, Pasquier F, Mateo I, Frank-Garcia A, Porcellini E, Hanon O, Coto E, Alvarez V, Bosco P, Siciliano G, Mancuso M, Panza F, Solfrizzi V, Nacmias B, Sorbi S, Bossù P, Piccardi P, Arosio B, Annoni G, Seripa D, Pilotto A, Scarpini E, Galimberti D, Brice A, Hannequin D, Licastro F, Jones L, Holmans PA, Jonsson T, Riemenschneider M, Morgan K, Younkin SG, Owen MJ, O'Donovan M, Amouyel P, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 2011;43:429–435. doi: 10.1038/ng.803. (PubMed: 21460840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm P, Ettrup A, Klein AB, Santini MA, El-Sayed M, Elvang AB, Stensbøl TB, Mikkelsen JD, Knudsen GM, Aznar S. Plaque deposition dependent decrease in 5-HT2A serotonin receptor in AβPPswe/PS1dE9 amyloid over expressing mice. J. Alzheimers Dis. 2010;20:1201–1213. doi: 10.3233/JAD-2010-100117. (PubMed: 20413853) [DOI] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, Belaygorod L, Cairns NJ, Holtzman DM, Diamond MI. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4376–E4385. doi: 10.1073/pnas.1411649111. (PubMed: 25261551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, Lovestone S. Psychosis and aggression in Alzheimer’s disease: the effect of dopaminereceptor gene variation. J. Neurol. Neurosurg. Psychiatry. 2001;71:777–779. doi: 10.1136/jnnp.71.6.777. (PubMed: 11723200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, Geschwind DH, Bird TD, McKeel D, Goate A, Morris JC, Wilhelmsen KC, Schellenberg GD, Trojanowski JQ, Lee VM. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–1917. doi: 10.1126/science.282.5395.1914. (PubMed: 9836646) [DOI] [PubMed] [Google Scholar]
- Hornung JP, Fritschy JM, Törk I. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J. Comp. Neurol. 1990;297:165–181. doi: 10.1002/cne.902970202. (PubMed: 2115053) [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. (PubMed: 14729135) [DOI] [PubMed] [Google Scholar]
- Hornung JP. Raphe nuclei. In: Paxinos G, Mai JK, editors. The human nervous system. Amsterdam: Elsevier; 2004. pp. 425–450. [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain’s default mode network during deep sleep. Proc. Natl. Acad. Sci. USA. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. (PubMed: 19549821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Jia F, Liu Y, Li L. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res. 2006;1095:154–158. doi: 10.1016/j.brainres.2006.04.026. (PubMed: 16713589) [DOI] [PubMed] [Google Scholar]
- Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. (PubMed: 16401839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–526. (PubMed: 12938804) [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. (PubMed: 9641683) [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging - Alzheimer's Association guidelines for the neuropathological assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. (PubMed: 22265587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. (PubMed: 9329452) [DOI] [PubMed] [Google Scholar]
- Iacono D, Volkman I, Nennesmo I, Pedersen NL, Fratiglioni L, Johansson B, Karlsson D, Winblad B, Gatz M. Neuropathologic assessment of dementia markers in identical and fraternal twins. Brain Pathol. 2014;24:317–333. doi: 10.1111/bpa.12127. (PubMed: 24450926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. (PubMed: 23325240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J. Comp. Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. (PubMed: 2419370) [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM, Trojanowski JQ. Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain. 2012;135:807–818. doi: 10.1093/brain/aws013. (PubMed: 22366796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino H, Otsuki S. Distribution of Alzheimer's neurofibrillary tangles in the basal ganglia and brain stem of progressive supranuclear palsy and Alzheimer's disease. Folia Psychiatr. Neurol. Jpn. 1975;29:179–187. doi: 10.1111/j.1440-1819.1975.tb02334.x. (PubMed: 1176072) [DOI] [PubMed] [Google Scholar]
- Iversen LL, Rossor MN, Reynolds GP, Hills R, Roth M, Mountjoy CQ, Foote SL, Morrison JH, Bloom FE. Loss of pigmented dopamine-β-hydrohylase positive cells from locus coeruleus in senile dementia of Alzheimer's type. Neurosci. Lett. 1983;39:95–100. doi: 10.1016/0304-3940(83)90171-4. (PubMed: 6633940) [DOI] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine:50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. (PubMed: 17368565) [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer's disease and other tauopathies. Biochim. Biophys. Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. (PubMed: 15615638) [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. (PubMed: 26635213) [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Gannon PJ, Azmitia EC. Atlas of serotonergic cell bodies in the cat brainstem: an immunocytochemical analysis. Brain Res. Bull. 1984;13:1–31. doi: 10.1016/0361-9230(84)90003-0. (PubMed: 6478260) [DOI] [PubMed] [Google Scholar]
- Jadhav S, Cubinkova V, Zimova I, Brezovakova V, Madari A, Cigankova V, Zilka N. Tau-mediated synaptic damage in Alzheimer's disease. Transl. Neurosci. 2015;6:214–226. doi: 10.1515/tnsci-2015-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain. 2001;90:257–260. doi: 10.1016/S0304-3959(00)00409-7. (PubMed: 11207397) [DOI] [PubMed] [Google Scholar]
- Jazvinšćak Jembrek M, Babić M, Pivac N, Hof PR, Šimić G. Hyperphosphorylation of tau by GSK3β in Alzheimer’s disease: the interaction of Aβ and sphingolipid mediators as a therapeutic target. Transl. Neurosci. 2013;4:466–476. [Google Scholar]
- Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR, Jr, Jicha GA, Knopman DS, Kovacs GG, Mackenzie IR, Masliah E, Montine TJ, Nelson PT, Schmitt F, Schneider JA, Serrano-Pozo A, Thal DR, Toledo JB, Trojanowski JQ, Troncoso JC, Vonsattel JP, Wisniewski T. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015;129:757–762. doi: 10.1007/s00401-015-1407-2. (PubMed: 25778618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, Deng Y, Qing H. Potential therapeutic strategies for Alzheimer’s disease targeting or beyond β-amyloid: insights from clinical trials. Biomed. Res. Int. 2014:837157. doi: 10.1155/2014/837157. (PubMed: 25136630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Atems J. Challenges of multimorbidity of the aging brain: a critical update. J. Neural Transm. 2015;122:505–521. doi: 10.1007/s00702-014-1288-x. (PubMed: 25091618) [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113:107–117. doi: 10.1007/s00401-006-0156-7. (PubMed: 17089134) [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. (PubMed: 22801501) [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer's disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. (PubMed: 23150908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Praticò D. Neuroinflammation and Alzheimer’s disease: lessons learned from 5-lypoxigenase. Transl. Neurosci. 2014;5:197–202. [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. (PubMed: 23479184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA. 2015;112:7501–7506. doi: 10.1073/pnas.1504081112. (PubMed: 26034266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL. The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2012;33:1651–1663. doi: 10.1016/j.neurobiolaging.2011.04.012. (PubMed: 21705113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamah A, Huvent I, Cantrelle FX, Qi H, Lippens G, Landrieu I, Smet-Nocca C. Nuclear magnetic resonance analysis of the acetylation pattern of the neuronal tau protein. Biochemistry. 2014;53:3020–3032. doi: 10.1021/bi500006v. (PubMed: 24708343) [DOI] [PubMed] [Google Scholar]
- Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 77:43–51. doi: 10.1016/j.biopsych.2014.05.006. (PubMed: 24951455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayama Y, Koyama Y. Control of sleep and wakefulness by brainstem monoaminergic and cholinergic neurons. Acta Neurochir. Suppl. 2003;87:3–6. doi: 10.1007/978-3-7091-6081-7_1. [DOI] [PubMed] [Google Scholar]
- Kendall I, Slotten HA, Codony X, Burgueno J, Pauwels PJ, Vela JM, Fone KC. E-6801, a 5-HT6 receptor agonist, improves recognition memory by combined modulation of cholinergic and glutamatergic neurotransmission in the rat. Psychopharmacology. 2011;213:413–430. doi: 10.1007/s00213-010-1854-3. (PubMed: 20405281) [DOI] [PubMed] [Google Scholar]
- Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc. Natl. Acad. Sci. U. S. A. 2006;103:702–707. doi: 10.1073/pnas.0510237103. (PubMed: 16407119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, Moir RD, Becker KD, Tanzi RE. Potential late-onset Alzheimer's disease-associated mutations in the ADAM10 gene attenuate α-secretase activity. Hum. Mol. Genet. 2009;18:3987–3996. doi: 10.1093/hmg/ddp323. (PubMed: 19608551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JC. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch. Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. (PubMed: 16533956) [DOI] [PubMed] [Google Scholar]
- Knezović A, Osmanović-Barilar J, Ćurlin M, Hof PR, Šimić G, Riederer P, Šalković-PetriŠić M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer's disease. J. Neural Transm. 2015;122:577–592. doi: 10.1007/s00702-015-1394-4. (PubMed: 25808906) [DOI] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Bonnì S, Giacobbe V, Bozzali M, Caltagirone C, Martorana A. Dopaminergic modulation of cortical plasticity in Alzheimer's disease patients. Neuropsychopharmacology. 2014;39:2654–2661. doi: 10.1038/npp.2014.119. (PubMed: 24859851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Steinbusch HW. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. (PubMed: 7048127) [DOI] [PubMed] [Google Scholar]
- Kopeikina K, Hyman B, Spires-Jones T. Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 2012;3:223–233. doi: 10.2478/s13380-012-0032-y. (PubMed: 23029602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosofsky BE, Molliver ME. The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. (PubMed: 2463687) [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Majtenyi K, Spina S, Murrell JR, Gelpi E, Hoftberger R, Fraser G, Crowther RA, Goedert M, Budka H, Ghetti B. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J. Neuropathol. Exp. Neurol. 2008;67:963–975. doi: 10.1097/NEN.0b013e318187a80f. (PubMed: 18800011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Savli M, Lanzenberger R. Challenges in the differentiation of midbrain raphe nuclei in neuroimaging research. Proc. Natl. Acad. Sci. USA. 2012;109:E2000. doi: 10.1073/pnas.1206247109. (PubMed: 22711836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. 103:370–376. doi: 10.1007/s00401-001-0477-5. (PubMed: 11904757) [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. (PubMed: 16049337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid β-peptides in the murine cerebral cortex. J. Pineal Res. 2004;36:224–231. doi: 10.1111/j.1600-079X.2004.00121.x. (PubMed: 15066046) [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Keene J, Hope T, Chen CP. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. (PubMed: 12742626) [DOI] [PubMed] [Google Scholar]
- Lambracht-Washington D, Rosenberg RN. Active DNA Ab42 vaccination as immunotherapy for Alzheimer’s disease. Transl. Neurosci. 2012;3:307–313. doi: 10.2478/s13380-012-0037-6. (PubMed: 23741624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP. The epigenetics of aging and neurodegeneration. Prog. Neurobiol. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. (PubMed: 26072273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, Imbesi M, Manev H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci. Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. (PubMed: 16203090) [DOI] [PubMed] [Google Scholar]
- LaSalle JM. A genomic point-of-view on environmental factors influencing the human brain methylome. Epigenetics. 2011;6:862–869. doi: 10.4161/epi.6.7.16353. (PubMed: 21617367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J. Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. (PubMed: 14999081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S. Serotonergic modulation of neural activities in the entorhinal cortex. Int. J. Physiol. Pathophysiol. Pharmacol. 2012;4:201–210. (PubMed: 23320133) [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. (PubMed: 2111584) [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. (PubMed: 7638622) [DOI] [PubMed] [Google Scholar]
- Leyk J, Goldbaum O, Noack M, Richter-Landsberg C. Inhibition of HDAC6 modifies tau inclusion body formation and impairs autophagic clearance. J. Mol. Neurosci. 2015;55:1031–1046. doi: 10.1007/s12031-014-0460-y. (PubMed: 25434725) [DOI] [PubMed] [Google Scholar]
- Li L, Cheung T, Chen J, Herrup K. A comparative study of five mouse models of Alzheimer's disease: cell cycle events reveal new insights into neurons at risk for death. Int. J. Alzheimers Dis. 2011:171464. doi: 10.4061/2011/171464. 2011. (PubMed: 21912750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, Lecomte JM, Parmentier R, Anaclet C, Lin JS, Bertaina-Anglade V, la Rochelle CD, d'Aniello F, Rouleau A, Gbahou F, Arrang JM, Ganellin CR, Stark H, Schunack W, Schwartz JC. BF2.649 [1-{3-[3-(4-chlorophenyl) propoxy] propyl} piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J. Pharmacol. Exp. Ther. 2007;320:365–375. doi: 10.1124/jpet.106.111039. (PubMed: 17005916) [DOI] [PubMed] [Google Scholar]
- Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer's disease: what do we know? Neurodegener. Dis. Manag. 2014;4:351–362. doi: 10.2217/nmt.14.33. (PubMed: 25405649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. (PubMed: 22312444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. (PubMed: 16262633) [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang S, Zhu Y, Fu Q, Zhu Y, Gong Y, Ohtsu H, Luo J, Wei E, Chen Z. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus. 2007;17:634–641. doi: 10.1002/hipo.20305. (PubMed: 17534971) [DOI] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer's disease, and apolipoprotein E-ε4/4 genotype. J. Clin. Endocrinol. Metab. 1999;84:323–327. doi: 10.1210/jcem.84.1.5394. (PubMed: 9920102) [DOI] [PubMed] [Google Scholar]
- Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE, Blue ME, Lee MK. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. (PubMed: 19091971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer E, Vernooij MW, Ikram MA. Cerebral microbleeds: spatial distribution implications. Transl. Neurosci. 2014;5:160–163. [Google Scholar]
- Lowenberg K, Waggoner R. Familial organic psychosis (Alzheimer’s type) Arch. Neurol. 1934;31:737. [Google Scholar]
- Lucey BP, Holtzman DM. How amyloid, sleep and memory connect. Nat. Neurosci. 2015;18:933–934. doi: 10.1038/nn.4048. (PubMed: 26108720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk C, Vandrovcova J, Malzer E, Lees A, de Silva R. Brain tau isoform mRNA and protein correlation in PSP brain. Transl. Neurosci. 2010;1:30–36. [Google Scholar]
- Luna-Muñoz J, Harrington CR, Wischik CM, Flores-Rodríguez P, Avila J, Zamudio SR, De la Cruz F, Mena R, Meraz-Ríos MA, Floran-Garduño B. Phosphorylation of tau protein associated as a protective mechanism in the presence of toxic, C-terminally truncated tau in Alzheimer's disease. In: Zerr I, editor. Understanding Alzheimer's disease. Rijeka, Croatia: InTech; 2013. pp. 89–107. [Google Scholar]
- Luo Y, Ma B, Nussinov R, Wei G. Structural insight into tau protein’s paradox of intrinsically disordered behavior, self-acetylation activity, and aggregation. J. Phys. Chem. Lett. 2014;5:3026–3031. doi: 10.1021/jz501457f. (PubMed: 25206938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QF, Li YM, Du JT, Kanazawa K, Nemoto T, Nakanishi H, Zhao YF. Binding of copper (II) ion to an Alzheimer's tau peptide as revealed by MALDI-TOF MS, CD, and NMR. Biopolymers. 79:74–85. doi: 10.1002/bip.20335. (PubMed: 15986501) [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozenmuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Disckson DW, Trojanowski JQ, Mann DM. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. (PubMed: 19924424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher-Edwards G, Dixon R, Hunter J, Gold M, Hopton G, Jacobs G, Hunter J, Williams P. SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. Int. J. Geriatr. Psychiatry. 2011;26:536–544. doi: 10.1002/gps.2562. (PubMed: 20872778) [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O’Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J. Comp. Neurol. 1991;314:558–586. doi: 10.1002/cne.903140312. (PubMed: 1814975) [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog. Neurobiol. 2007;82:348–360. doi: 10.1016/j.pneurobio.2007.06.001. (PubMed: 17659826) [DOI] [PubMed] [Google Scholar]
- Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer's disease. Brain Pathol. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. (PubMed: 17493042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandybur TI. The distribution of Alzheimer's neurofibrillary tangles and gliosis in chronic subacute sclerosing panencephalitis. Acta Neuropathol. 1990;80:307–310. doi: 10.1007/BF00294649. (PubMed: 1698006) [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO, Marcyniuk B, Ravindra CR. The topography of plaques and tangles in Down's syndrome patients of different ages. Neuropathol. Appl. Neurobiol. 1986;12:447–457. doi: 10.1111/j.1365-2990.1986.tb00053.x. (PubMed: 2946973) [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. (PubMed: 12471243) [DOI] [PubMed] [Google Scholar]
- Marner L, Frokjaer VG, Kalbitzer J, Lehel S, Madsen K, Baaré WF, Knudsen GM, Hasselbalch SG. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer’s disease: a combined [11C]DASB and [18F]altanserin-PET study. Neurobiol. Aging. 2012;33:479–487. doi: 10.1016/j.neurobiolaging.2010.03.023. (PubMed: 20510480) [DOI] [PubMed] [Google Scholar]
- Martignoni E, Bono G, Blandini F, Sinforiani E, Merlo P, Nappi G. Monoamines and related matabolite levels in the cerebrospinal fluid of patients with dementia of Alzheimer type. Influence of treatment with L-deprenyl. J. Neural. Transm. Park. Dis. Dement. Sect. 1991;3:15–25. doi: 10.1007/BF02251133. (PubMed: 1712206) [DOI] [PubMed] [Google Scholar]
- Martorana A, Di Lorenzo F, Esposito Z, Lo Giudice T, Bernardi G, Caltagirone C, Koch G. Dopamine D2-agonist rotigotine effects on cortical excitability and central cholinergic transmission in Alzheimer's disease patients. Neuropharmacology. 2013;64:108–113. doi: 10.1016/j.neuropharm.2012.07.015. (PubMed: 22863599) [DOI] [PubMed] [Google Scholar]
- Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One. 2009;4:e6617. doi: 10.1371/journal.pone.0006617. (PubMed: 19672297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer's disease: decrements in DNA methylation. Neurobiol. Aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. (PubMed: 19117641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara E, Bryant-Thomas T, Pacheco QJ, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J. Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. (PubMed: 12753069) [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS Aβ in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. (PubMed: 21148344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann. NY Acad. Sci. 2000;903:176–179. doi: 10.1111/j.1749-6632.2000.tb06366.x. (PubMed: 10818505) [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. (PubMed: 6610841) [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RS, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging – Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. (PubMed: 21514250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Atkins AR, Beresford IJ, Brackenborough K, Briggs MA, Calver AR, Cilia J, Cluderay JE, Crook B, Davis JB, Davis RK, Davis RP, Dawson LA, Foley AG, Gartlon J, Gonzalez MI, Heslop T, Hirst WD, Jennings C, Jones DN, Lacroix LP, Martyn A, Ociepka S, Ray A, Regan CM, Roberts JC, Schogger J, Southam E, Stean TO, Trail BK, Upton N, Wadsworth G, Wald JA, White T, Witherington J, Woolley ML, Worby A, Wilson DM. GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. J. Pharmacol. Exp. Ther. 2007;321:1032–1045. doi: 10.1124/jpet.107.120311. (PubMed: 17327487) [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Roberts JC, Lee J, Chen CP, Brown SH, Roman S, Lai MK. Characterization of histamine H3 receptors in Alzheimer's Disease brain and amyloid over-expressing TASTPM mice. Br. J. Pharmacol. 2009;157:130–138. doi: 10.1111/j.1476-5381.2008.00075.x. (PubMed: 19222483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF. Frontotemporal dementia: therapeutic interventions. Front. Neurol. Neurosci. 2009;24:168–178. doi: 10.1159/000197896. (PubMed: 19182475) [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog. Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. (PubMed: 18772036) [DOI] [PubMed] [Google Scholar]
- Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, Gan L. Critical roles of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015;21:1154–1162. doi: 10.1038/nm.3951. (PubMed: 26390242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. (PubMed: 2011243) [DOI] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer's type with disturbed sleep-waking. Biol. Psychiatry. 1999;45:417–421. doi: 10.1016/s0006-3223(97)00510-6. (PubMed: 10071710) [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Herrmann N, Lanctot KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci. Ther. 2011;17:411–427. doi: 10.1111/j.1755-5949.2010.00161.x. (PubMed: 20560994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladinov M, Mayer D, Brčić L, Wolstencroft E, Man N, Holt I, Hof PR, Morris GE, Šimić G. Astrocyte expression of D2-like dopamine receptors in the prefrontal cortex. Transl. Neurosci. 2010;1:238–243. [Google Scholar]
- Mochizuki H, Tashiro M, Tagawa M, Kano M, Itoh M, Okamura N, Watanabe T, Yanai K. The effects of a sedative antihistamine, d-chlorpheniramine, on visuomotor spatial discrimination and regional brain activity as measured by positron emission tomography (PET) Hum. Psychopharmacol. 2002;17:413–418. doi: 10.1002/hup.430. (PubMed: 12457377) [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. (PubMed: 25611508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. (PubMed: 21459634) [DOI] [PubMed] [Google Scholar]
- Monti JM, Alvarino F, Cardinali DP, Savio I, Pintos A. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Arch. Gerontol. Geriatr. 1999;28:85–98. doi: 10.1016/s0167-4943(98)00129-0. (PubMed: 15374088) [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frotsch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging - Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. (PubMed: 22101365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. (PubMed: 8232972) [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J. Comp. Neurol. 1986;243:117–138. doi: 10.1002/cne.902430110. (PubMed: 3950077) [DOI] [PubMed] [Google Scholar]
- Motawaj M, Peoc'h K, Callebert J, Arrang JM. CSF levels of the histamine metabolite tele-methylhistamine are only slightly decreased in Alzheimer's disease. J. Alzheimers Dis. 2010;22:861–871. doi: 10.3233/JAD-2010-100381. (PubMed: 20858978) [DOI] [PubMed] [Google Scholar]
- Mravec B, Lejavova K, Cubinkova V. Locus (coeruleus) minoris resistentiae in pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2014;11:992–1001. doi: 10.2174/1567205011666141107130505. (PubMed: 25387337) [DOI] [PubMed] [Google Scholar]
- Murray ME, Kouri N, Lin WL, Jack CR, Jr, Dickson DW, Vemuri P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res. Ther. 2014;6:1. doi: 10.1186/alzrt231. (PubMed: 24382028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapić M, Presečki P, Pivac N, Mimica N, Hof PR, Šimić G, Folnegović-Šmalc V, Mück-Šeler D. Genotype-independent decrease in plasma dopamine β-hydroxylase activity in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;44:94–99. doi: 10.1016/j.pnpbp.2013.02.002. (PubMed: 23416088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychack P, Rosen H, Miller BL. Novel applications of social-personality measures to the study of dementia. Neurocase. 2001;7:131–143. doi: 10.1093/neucas/7.2.131. (PubMed: 11320161) [DOI] [PubMed] [Google Scholar]
- Nagy Z, Yilmazer-Hanke DM, Braak H, Braak E, Schultz C, Hanke J. Assessment of the pathological stages of Alzheimer’s disease in thin paraffin sections: a comparative study. Dement. Geriatr. Cogn. Disord. 1998;9:140–144. doi: 10.1159/000017038. (PubMed: 9622001) [DOI] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants in MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 2011;43:436–441. doi: 10.1038/ng.801. (PubMed: 21460841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Takemura M, Ohnishi K, Suenaga T, Nishimura M, Akiguchi I, Kimura J, Kimura T. Loss of large neurons and occurrence of neurofibrillary tangles in the tuberomammillary nucleus of patients with Alzheimer’s disease. Neurosci. Lett. 1993;151:196–199. doi: 10.1016/0304-3940(93)90019-h. (PubMed: 8506080) [DOI] [PubMed] [Google Scholar]
- Namboodiri MA, Dubbels R, Klein DC. Arylalkylamine N-acetyltransferase from mammalian pineal gland. Methods Enzymol. 1987;142:583–590. doi: 10.1016/s0076-6879(87)42069-7. (PubMed: 3298986) [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Reynolds GP. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: a postmortem study. Cell Mol. Neurobiol. 1992;12:581–587. doi: 10.1007/BF00711237. (PubMed: 1283363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer’s disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. (PubMed: 22487856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, Smith CD, Patel E, Markesbery WR. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009;68:774–784. doi: 10.1097/NEN.0b013e3181aacbe9. (PubMed: 19535994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system. 4th. New York: Springer; 2008. pp. 889–916. [Google Scholar]
- Noristani HN, Meadows RS, Olabarria M, Verkhratsky A, Rodríguez JJ. Increased hippocampal CA1 density of serotonergic terminals in a triple transgenic mouse model of Alzheimer's disease: an ultrastructural study. Cell Death Dis. 2011;2:e210. doi: 10.1038/cddis.2011.79. (PubMed: 21918544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R Texas Alzheimer’s Research Consortium. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Arch. Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. (PubMed: 18695059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res. Bull. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. (PubMed: 6099744) [DOI] [PubMed] [Google Scholar]
- Ohm TG, Heilmann R, Braak H. The human oral raphe system. Architectonics and neuronal types in pigment-Nissl preparations. Anat. Embryol. (Berl.) 1989;180:37–43. doi: 10.1007/BF00321898. (PubMed: 2476947) [DOI] [PubMed] [Google Scholar]
- Okamura N, Arai H, Maruyama M, Higuchi M, Matsui T, Tanji H, Seki T, Hirai H, Chiba H, Itoh M, Sasaki H. Combined analysis of CSF tau levels and [123I] iodoamphetamine SPECT in mild cognitive impairment: implications for a novel predictor of Alzheimer’s disease. Am. J. Psychatry. 2002;159:474–476. doi: 10.1176/appi.ajp.159.3.474. (PubMed: 11870015) [DOI] [PubMed] [Google Scholar]
- Okochi M, Steiner H, Fukumori A, Tanii H, Tomita T, Tanaka T, Iwatsubo T, Kudo T, Takeda M, Haass C. Presenilins mediate a dual intramembranous gamma-secretase cleavage of Notch-1. EMBO J. 2002;21:5408–5416. doi: 10.1093/emboj/cdf541. (PubMed: 12374741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun I, Tkachenko SE, Khvat A, Mitkin O, Kazey V, Ivachtchenko AV. From anti-allergic to anti-Alzheimer's: molecular pharmacology of Dimebon. Curr. Alzheimer Res. 2010;7:97–112. doi: 10.2174/156720510790691100. (PubMed: 19939222) [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castrén E, Ceccatelli S. Long-lasting depression like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. (PubMed: 18485098) [DOI] [PubMed] [Google Scholar]
- Ono S, Kanda F, Takahashi K, Fukuoka Y, Jinnai K, Kurisaki H, Mitake S, Inagaki T, Nagao K. Neuronal cell loss in the dorsal raphe nucleus and the superior central nucleus in myotonic dystrophy: a clinicopathological correlation. Acta Neuropathol. 1995;89:122–125. doi: 10.1007/BF00296355. (PubMed: 7732784) [DOI] [PubMed] [Google Scholar]
- Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, Mitake S, Inagaki T, Yamano T, Nagao K. Loss of serotonin-containing neurons in the raphe of patients with myotonic dystrophy: a quantitative immunohistochemical study and relation to hypersomnia. Neurology. 1998;50:535–538. doi: 10.1212/wnl.50.2.535. (PubMed: 9484393) [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. (PubMed: 16651510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada R, Hayashi M, Katoh Y, Tsuchiya K, Mizutani T, Tominaga I, Kashima H. Neurofibrillary tangles and deposition of oxidative products in the brain in cases of myotonic dystrophy. Neuropathology. 2006;26:107–114. doi: 10.1111/j.1440-1789.2006.00662.x. (PubMed: 16708543) [DOI] [PubMed] [Google Scholar]
- Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res. 1987;401:231–238. doi: 10.1016/0006-8993(87)91408-9. (PubMed: 2434191) [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Kum Jew S. Different populations of human locus ceruleus neurons contain heavy metals or hyperphosphorylated tau: implications for amyloid-β and tau pathology in Alzheimer’s disease. J. Alzheimers Dis. 2015;45:437–447. doi: 10.3233/JAD-142445. (PubMed: 25547633) [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP. Melatonin and sleep in aging population. Exp. Gerontol. 2005;40:911–925. doi: 10.1016/j.exger.2005.08.009. (PubMed: 16183237) [DOI] [PubMed] [Google Scholar]
- Panula P, Airaksinen MS, Pirvola U, Kotilainen E. A histamine-containing neuronal system in human brain. Neuroscience. 1990;34:127–132. doi: 10.1016/0306-4522(90)90307-p. (PubMed: 2325846) [DOI] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WLS, Stark H, Thurmond RL, Haas HL. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. (PubMed: 26084539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, Relja M. Neuronal histamine deficit in Alzheimer's disease. Neuroscience. 1998;82:993–997. doi: 10.1016/s0306-4522(97)00353-9. (PubMed: 9466423) [DOI] [PubMed] [Google Scholar]
- Parnetti L, Gaiti A, Reboldi GP, Santucci C, Mecocci P, Brunetti M, Cadini D, Senin U. CSF monoamine metabolites in old age dementias. Mol. Chem. Neuropathol. 1992;16:143–157. doi: 10.1007/BF03159966. (PubMed: 1381590) [DOI] [PubMed] [Google Scholar]
- Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol. Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. (PubMed: 14960297) [DOI] [PubMed] [Google Scholar]
- Patwardhan MB, McCrory DC, Matchar DB, Samsa GP, Rutschmann OT. Alzheimer’s disease: operating characteristics of PET – a meta-analysis. Radiology. 2004;231:73–80. doi: 10.1148/radiol.2311021620. (PubMed: 15068942) [DOI] [PubMed] [Google Scholar]
- Pearson J, Halliday G, Sakamoto N, Michel P. Catecholaminergic neurons. In: Paxinos G, editor. The Human Nervous System. Academic Press: San Diego; 1990. pp. 1023–1049. [Google Scholar]
- Peeraer E, Bottelbergs A, Van Kolen K, Stancu IC, Vasconcelos B, Mahieu M, Duytschaever H, Ver Donck L, Torremans A, Sluydts E, Van Acker N, Kemp JA, Mercken M, Brunden KR, Trojanowski JQ, Dewachter I, Lee VM, Moechars D. Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of the transgenic mice. Neurobiol. Dis. 2015;73:83–95. doi: 10.1016/j.nbd.2014.08.032. (PubMed: 25220759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Santa-Maria I, Gomez de Barreda E, Zhu X, Cuadros R, Cabrero JR, Sanchez-Madrid F, Dawson HN, Vitek MP, Perry G, Smith MA, Avila J. Tau – an inhibitor of deacetylase HDAC6 function. J. Neurochem. 2009;109:1756–1766. doi: 10.1111/j.1471-4159.2009.06102.x. (PubMed: 19457097) [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res. 1993;609:81–92. doi: 10.1016/0006-8993(93)90858-k. (PubMed: 8099526) [DOI] [PubMed] [Google Scholar]
- Pimenova AA, Thathiah A, De Strooper B, Tesseur I. Regulation of amyloid precursor protein processing by serotonin signaling. PLoS One. 2014;21:e87014. doi: 10.1371/journal.pone.0087014. (PubMed: 2446315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol. Rev. 1999;51:533–591. (PubMed: 10471417) [PubMed] [Google Scholar]
- Pinessi L, Rainero I, De Gennaro T, Gentile S, Portaleone P, Bergamasco B. Biogenic amines in cerebrospinal fluid and plasma of patients with dementia of Alzheimer type. Funct. Neurol. 1987;2:51–58. (PubMed: 3678940) [PubMed] [Google Scholar]
- Pishva E, Kenis G, Lesch KP, Prickaerts J, Steinbusch HMW, van den Hove DLA, van Os J, Rutten BP. Epigenetic epidemiology in psychiatry: a translational neuroscience perspective. Transl. Neurosci. 2012;3:196–212. [Google Scholar]
- Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer’s disease. J. Neurosci. 2004;24:2401–2411. doi: 10.1523/JNEUROSCI.5561-03.2004. (PubMed: 15014115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Lunn BS, Walker LL, Gray JM, Ballard CG, O'Brien JT. Cognitive deficit induced by acute tryptophan depletion in patients with Alzheimer’s disease. Am. J. Psychiatry. 2000;157:638–640. doi: 10.1176/appi.ajp.157.4.638. (PubMed: 10739429) [DOI] [PubMed] [Google Scholar]
- Prast H, Argyriou A, Philippu A. Histaminergic neurons facilitate social memory in rats. Brain Res. 1996;734:316–318. (PubMed: 8896839) [PubMed] [Google Scholar]
- Previc FH. Dopamine and the origins of human intelligence. Brain Cogn. 1999;41:299–350. doi: 10.1006/brcg.1999.1129. (PubMed: 10585240) [DOI] [PubMed] [Google Scholar]
- Qu BX, Gong Y, Moore C, Fu M, German DC, Chang LY, Rosenberg R, Diaz-Arrastia R. Aβ auto-antibodies are reduced in Alzheimer's disease. J. Neuroimmunol. 2014;274:168–173. doi: 10.1016/j.jneuroim.2014.06.017. (PubMed: 25022335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb. Cortex. 2008a;18:584–597. doi: 10.1093/cercor/bhm089. (PubMed: 17586605) [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neuroscience. 2008b;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. (PubMed: 18562124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Spocter MA, Stimpson CD, Erwin JM, Bonar CJ, Allman JM, Hof PR, Sherwood CC. Species-specific distribution of tyrosine hydroxylase-immunoreactive neurons in the prefrontal cortex of anthropoid primates. Neuroscience. 2009;158:1551–1559. doi: 10.1016/j.neuroscience.2008.10.058. (PubMed: 19041377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Šimić G, Watson S, Stimpson CD, Hof PR, Sherwood CC. Comparative analysis of the nucleus basalis of Meynert among primates. Neuroscience. 2011;184:1–15. doi: 10.1016/j.neuroscience.2011.04.008. (PubMed: 21504783) [DOI] [PubMed] [Google Scholar]
- Ramirez MJ, Lai MK, Tordera RM, Francis PT. Serotonergic therapies for cognitive symptoms in Alzheimer’s disease: rationale and current status. Drugs. 2014;74:729–736. doi: 10.1007/s40265-014-0217-5. (PubMed: 24802806) [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer's disease. Biol. Psychiatry. 1999;46:756–765. doi: 10.1016/s0006-3223(99)00008-6. (PubMed: 10494443) [DOI] [PubMed] [Google Scholar]
- Reifler BV, Teri L, Raskind M, Veith R, Barnes R, White E, McLean P. Doubleblind trial of imipramine in Alzheimer’s disease patients with and without depression. Am. J. Psychiatry. 1989;146:45–49. doi: 10.1176/ajp.146.1.45. (PubMed: 2643356) [DOI] [PubMed] [Google Scholar]
- Revesz T, Sangha H, Daniel SE. The nucleus raphe interpositus in the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Brain. 1996;119:1137–1143. doi: 10.1093/brain/119.4.1137. (PubMed: 8813278) [DOI] [PubMed] [Google Scholar]
- Ramakrishna N, Wolfe G, Silverman WP, Brown WT. Chromosome 21q21 sublocalisation of gene encoding Aβ peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987;1:384–385. doi: 10.1016/s0140-6736(87)91754-5. (PubMed: 2880184) [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogeneous tau ameliorates Aβ-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. (PubMed: 17478722) [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci. Transl. Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. (PubMed: 22956200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitne JC, Munger R, Lyketsos CG. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2008;16:883–892. doi: 10.1097/JGP.0b013e318181276a. (PubMed: 18978249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. (PubMed: 17051204) [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Cotman CW. Adrenergic receptors in Alzheimer's disease brain: selective increases in the cerebella of aggressive patients. J. Neurosci. 1997;17:5573–5589. doi: 10.1523/JNEUROSCI.17-14-05573.1997. (PubMed: 9204938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüb U, Del Tredici K, Schultz C, de Vos RA, Jansen Steur EN, Arai K, Braak H. Progressive supranuclear palsy: neuronal and glial cytoskeletal pathology in the higher order processing autonomic nuclei of the lower brainstem. Neuropathol. Appl. Neurobiol. 2002;28:12–22. doi: 10.1046/j.0305-1846.2001.00374.x. (PubMed: 11849559) [DOI] [PubMed] [Google Scholar]
- Rüb U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol. Appl. Neurobiol. 2000;26:553–567. doi: 10.1046/j.0305-1846.2000.00291.x. (PubMed: 11123722) [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Hieble JP. α-Adrenoceptors. Pharmacol. Ther. 1994;61:1–64. doi: 10.1016/0163-7258(94)90058-2. (PubMed: 7938167) [DOI] [PubMed] [Google Scholar]
- Sabbagh MN. Drug development for Alzheimer’s disease: where are we now and where are we headed? Am. J. Geriatr. Pharmacother. 2009;7:167–185. doi: 10.1016/j.amjopharm.2009.06.003. (PubMed: 19616185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun AA, Schaechter JD, Smith LE. A retinohypothalamic pathway in man: light mediation of circadian rhythms. Brain Res. 1984;302:371–377. doi: 10.1016/0006-8993(84)90252-x. (PubMed: 6733517) [DOI] [PubMed] [Google Scholar]
- Salzman C. Treatment of the agitation of late-life psychosis and Alzheimer’s disease. Eur. Psychiatry. Suppl. 2001;1:25s–28s. doi: 10.1016/s0924-9338(00)00525-3. (PubMed: 11520475) [DOI] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part II: Physiological and Pharmacological Manipulations and Pathological Alterations of Locus Coeruleus Activity in Humans. Curr. Neuropharmacol. 2008;6:254–285. doi: 10.2174/157015908785777193. (PubMed: 19506724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mut JV, Heyn H, Vidal E, Moran S, Sayols S, Delgado-Morales M, Schultz D, Ansoleaga B, Garcia-Esparcia P, Pons-Espinal M, de Lagran MM, Dopazo J, Rabano A, Avila J, Dierssen M, Lott I, Ferrer I, Ecker JR, Esteller M. Human DNA methylomes of neurodegenerative diseases show common epigenomic patterns. Transl. Psychiatry. 2016;6:e718. doi: 10.1038/tp.2015.214. (PubMed: 26784972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Mizukami K, Asada T. A preliminary open-label study of 5-HT1A partial agonist tandospirone for behavioural and psychological symptoms associated with dementia. Int. J. Neuropsychopharmacol. 2007;10:281–283. doi: 10.1017/S1461145706007000. (PubMed: 16817981) [DOI] [PubMed] [Google Scholar]
- Saunders A, Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ. Association of apoliprotein E allele ε4 with the late-onset familial and sporadic Alzheimer disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. (PubMed: 8350998) [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Müller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J. Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. (PubMed: 15617532) [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. (PubMed: 1825731) [DOI] [PubMed] [Google Scholar]
- Sawant-Basak A, Coffman KJ, Walker GS, Ryder TF, Tseng E, Miller E, Lee C, Vanase-Frawley MA, Wong JW, Brodney MA, Rapp T, Obach RS. Metabolism of a serotonin-4 receptor partial agonist 4-{4-[4-tetrahydrofuran-3-yloxy)-benzo[d]isoxazol-3-yloxymethyl]- piperidin-1-ylmethyl}-tetrahydropyran-4-ol (TBPT): identification of an unusual pharmacologically active cyclized oxazolidine metabolite in human. J. Pharm. Sci. 2013;102:3277–3293. doi: 10.1002/jps.23542. (PubMed: 23589342) [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, Devanand D, Honig L, Stern Y. Disruptive behavior as a predictor in Alzheimer's disease. Arch. Neurol. 2007;64:1755–1761. doi: 10.1001/archneur.64.12.1755. (PubMed: 18071039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa S, Cavallaro RA, D'Anselmi F, Fuso A. Gene silencing through methylation: an epigenetic intervention on Alzheimer's disease. J. Alzheimers Dis. 2006;9:407–414. doi: 10.3233/jad-2006-9406. (PubMed: 16917149) [DOI] [PubMed] [Google Scholar]
- Schayer RW, Wetterqvist H, Beaven MA, Horakova Z. Metabolism and excretion of histamine. In: Rocha M, editor. Handbook of Experimental Pharmacology. 18/2. Berlin: Springer-Verlag; 1987. pp. 109–173. [Google Scholar]
- Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, White JA, Bonnycastle L, Weber JL, Alonso ME, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258:668–671. doi: 10.1126/science.1411576. (PubMed: 1411576) [DOI] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, Bégard S, Brion JP, Hamdane M, Buée L. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. (PubMed: 16877359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. (PubMed: 12383780) [DOI] [PubMed] [Google Scholar]
- Scott JM, Weir DG. The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet. 1981;2:337–340. doi: 10.1016/s0140-6736(81)90650-4. (PubMed: 6115113) [DOI] [PubMed] [Google Scholar]
- Scullion GA, Kendall DA, Marsden CA, Sunter D, Pardon MC. Chronic treatment with the alpha2-adrenoceptor antagonist fl uparoxan prevents age-related deficits in spatial working memory in APPxPS1 transgenic mice without altering Aβ plaque load or astrocytosis. Neuropharmacology. 2011;60:223–234. doi: 10.1016/j.neuropharm.2010.09.002. (PubMed: 20850464) [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. (PubMed: 1673054) [DOI] [PubMed] [Google Scholar]
- Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int. J. Geriatr. Psychiatry. 2002;17:1120–1127. doi: 10.1002/gps.760. (PubMed: 12461760) [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Fakhfouri G, Ramezani V, Mehr SE, Rahimian R. The role of serotonin in memory: interactions with neurotransmitters and downstream signalling. Exp. Brain Res. 2014;232:723–738. doi: 10.1007/s00221-013-3818-4. (PubMed: 24430027) [DOI] [PubMed] [Google Scholar]
- Shan L, Swaab DF, Bao AM. Neuronal histaminergic system in aging and age-related neurodegenerative disorders. Exp. Gerontol. 2013;48:603–607. doi: 10.1016/j.exger.2012.08.002. (PubMed: 22910064) [DOI] [PubMed] [Google Scholar]
- Shan L, Bao AM, Swaab DF. The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 2015;38:167–177. doi: 10.1016/j.tins.2014.12.008. (PubMed: 25575625) [DOI] [PubMed] [Google Scholar]
- Shankle WR, Nielson KA, Cotman CW. Low-dose pro pranolol reduces aggression and agitation resembling that associated with orbitofrontal dysfunction in elderly demented patients. Alzheimer Dis. Assoc. Disord. 1995;9:233–237. (PubMed: 8749613) [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol. Psychiatry. 2013;74:340–347. doi: 10.1016/j.biopsych.2012.11.028. (PubMed: 23290495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. (PubMed: 9160754) [DOI] [PubMed] [Google Scholar]
- Shen F, Smith JA, Chang R, Bourdet DL, Tsuruda PR, Obedencio GP, Beattie DT. 5-HT4 receptor agonist mediated enhancement of cognitive function in vivo and amyloid precursor protein processing in vitro: a pharmacodynamic and pharmacokinetic assessment. Neuropharmacology. 2011;61:69–79. doi: 10.1016/j.neuropharm.2011.02.026. (PubMed: 21392515) [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. (PubMed: 7596406) [DOI] [PubMed] [Google Scholar]
- Shiozawa M, Fukutani Y, Sasaki K, Isaki K, Hamano T, Hirayama M, Imamura K, Mukai M, Arai N, Cairns NJ. Corticobasal degeneration: an autopsy case clinically diagnosed as progressive supranuclear palsy. Clin. Neuropathol. 2000;19:192–199. (PubMed: 10919351) [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer Aβ protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. (PubMed: 1439760) [DOI] [PubMed] [Google Scholar]
- Singh DB, Kumar Gupta M, Kumar Kesharwani R, Sagar M, Dwivedi S, Misra K. Molecular drug targets and therapies for Alzheimer’s disease. Transl. Neurosci. 2014;5:203–217. [Google Scholar]
- Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD, Deane RJ, Zhong E, Parisi M, Ciszewski J, Kasper RT, Deane R. Low levels of copper disrupt brain Aβ homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA. 2013;110:14771–14776. doi: 10.1073/pnas.1302212110. (PubMed: 23959870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Lopes DH, Du Z, Pang ES, Shanmugam A, Lomakin A, Talbiersky P, Tennstaedt A, McDaniel K, Bakshi R, Kuo PY, Ehrmann M, Benedek GB, Loo JA, Klärner FG, Schrader T, Wang C, Bitan G. Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. J. Am. Chem. Soc. 2011;133:16958–16969. doi: 10.1021/ja206279b. (PubMed: 21916458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren M, Minthon L, Passant U, Blennow K, Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer's disease. Neuobiol. Aging. 1998;19:379–384. doi: 10.1016/s0197-4580(98)00086-4. (PubMed: 9880039) [DOI] [PubMed] [Google Scholar]
- Soininen H, MacDonald E, Rekonen M, Riekkinen PJ. Homovanillic acid and 5-hydroxyindoleacetic acid levels in cerebrospinal fluid of patients with senile dementia of Alzheimer type. Acta Neurol. Scand. 1981;64:101–107. doi: 10.1111/j.1600-0404.1981.tb04392.x. (PubMed: 6172950) [DOI] [PubMed] [Google Scholar]
- Sokolow S, Henkins KM, Bilousova T, Gonzalez B, Vinters HV, Miller CA, Cornwell L, Poon WW, Gylys KH. Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J. Neurochem. 2015;133:368–379. doi: 10.1111/jnc.12991. (PubMed: 25393609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. (PubMed: 21514248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Kaur C, Pandi-Perumal S, Brown GM, Cardinali DP. Melatonin and its agonist ramelteon in Alzheimer's disease: possible therapeutic value. Int. J. Alzheimers Dis. 2010;2011:741974. doi: 10.4061/2011/741974. (PubMed: 21197086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JW, Gandy S. Latrepirdine (Dimebon®), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy. 2013;9:617–618. doi: 10.4161/auto.23487. (PubMed: 23380933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop PH, Haines JL, Rogaev E, Mortilla M, Vaula G, Pericak-Vance M, Foncin JF, Montesi M, Bruni A, Sorbi S, Rainero I, Pinessi L, Pollen D, Polinsky R, Nee L, Kennedy J, Macciardi F, Rogaeva E, Liang Y, Alexandrova N, Lukiw W, Schlumpf K, Tanzi R, Tsuda T, Farrer L, Cantu JM, Duara R, Amaducci L, Bergamini L, Gusella J, Roses A, Crapper McLachlan D. Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nat. Genet. 1992;2:330–334. doi: 10.1038/ng1292-330. (PubMed: 1303289) [DOI] [PubMed] [Google Scholar]
- Stefani A, Olivola E, Liguori C, Hainsworth AH, Saviozzi V, Angileri G, D'Angelo V, Galati S, Pierantozzi M. Catecholamine-based treatment in Alzheimer’s disease patients: expectations and delusions. Front. Aging Neurosci. 2015;7:67. doi: 10.3389/fnagi.2015.00067. (PubMed: 25999852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Dyck RH, Laferla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer's disease: part 1. Circadian changes. Brain Res. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. (PubMed: 20471965) [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ. Medicine. Old drug, new hope for Alzheimer’s disease. Science. 2012;335:1447–1448. doi: 10.1126/science.1220725. (PubMed: 22442467) [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance MA, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to Aβ and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. (PubMed: 8446617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Ou X, Farley JM, Stockmeier C, Bigler S, Brinton RD, Wang JM. Allopregnanolone increases the number of dopaminergic neurons in substantia nigra of a triple transgenic mouse model of Alzheimer's disease. Curr. Alzheimer Res. 2012;9:473–480. doi: 10.2174/156720512800492567. (PubMed: 22272610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW, van der Kooy D, Verhofstad AA, Pellegrino A. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci. Lett. 1980;19:137–142. doi: 10.1016/0304-3940(80)90184-6. (PubMed: 6302595) [DOI] [PubMed] [Google Scholar]
- Storga D, Vrecko K, Birkmayer JG, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 1996;203:29–32. doi: 10.1016/0304-3940(95)12256-7. (PubMed: 8742039) [DOI] [PubMed] [Google Scholar]
- Stuerenburg HJ, Ganzer S, Muller-Thomsen T. 5-Hydroxy indol acetic acid and homovanillic acid concentrations in cerebrospinal fluid in patients with Alzheimer's disease, depression and mild cognitive impairment. Neuro. Endorinol. Lett. 2004;25:435–437. (PubMed: 15665806) [PubMed] [Google Scholar]
- Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, Tanzi RE. ADAM10 missense mutations potentiate Aβ accumulation by impairing prodomain chaperone function. Neuron. 2013;80:385–401. doi: 10.1016/j.neuron.2013.08.035. (PubMed: 24055016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, Lewis DA, DeKosky ST. Alterations of striatal dopamine receptor binding in Alzheimer's disease are associated with Lewy body pathology and antemortem psychosis. Arch. Neurol. 2001;58:466–472. doi: 10.1001/archneur.58.3.466. (PubMed: 11255451) [DOI] [PubMed] [Google Scholar]
- Szabadi E. Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. 2013;27:659–693. doi: 10.1177/0269881113490326. (PubMed: 23761387) [DOI] [PubMed] [Google Scholar]
- Šalković-Petrišić M, Riederer P. Brain glucose transporter protein 2 and sporadic Alzheimer’s disease. Transl. Neurosci. 2010;1:200–206. [Google Scholar]
- Šešo-Šimić Đ, Sedmak G, Hof PR, Šimić G. Recent advances in the neurobiology of attachment behavior. Transl. Neurosci. 2010;1:148–159. [Google Scholar]
- Šimić G, Mrzljak L, Fučić A, Winblad B, Lovrić H, Kostović I. Nucleus subputaminalis (Ayala): the still disregarded magnocellular component of the basal forebrain may be human specific and connected with the cortical speech area. Neuroscience. 1999;89:73–89. doi: 10.1016/s0306-4522(98)00304-2. (PubMed: 10051218) [DOI] [PubMed] [Google Scholar]
- Šimić G, Kostović I, Winblad B, Bogdanović N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J. Comp. Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. (PubMed: 9067838) [DOI] [PubMed] [Google Scholar]
- Šimić G, Gnjidić M, Kostović I. Cytoskeletal changes as an alternative view on pathogenesis of Alzheimer's disease. Period. Biol. 1998a;100:165–173. [Google Scholar]
- Šimić G, Winblad B, Bogdanović N. Relationship between hippocampal neurofibrillary degeneration and neuronal loss in aging and Alzheimer’s disease. Neurobiol. Aging. 1998b;19(Suppl. 4):239. [Google Scholar]
- Šimić G, Lucassen PJ, Krsnik Ž, Krušlin B, Kostović I, Winblad B, Bogdanović N. nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer’s disease. Exp. Neurol. 2000;165:12–26. doi: 10.1006/exnr.2000.7448. (PubMed: 10964481) [DOI] [PubMed] [Google Scholar]
- Šimić G. Pathological tau proteins in argyrophilic grain disease. Lancet Neurol. 2002;1:276. doi: 10.1016/s1474-4422(02)00130-8. (PubMed: 12849422) [DOI] [PubMed] [Google Scholar]
- Šimić G, Bexheti S, Kelović Z, Kos M, Grbić K, Hof PR, Kostović I. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience. 2005;130:911–925. doi: 10.1016/j.neuroscience.2004.09.040. (PubMed: 15652989) [DOI] [PubMed] [Google Scholar]
- Šimić G, Stanić G, Mladinov M, Jovanov Milošević N, Kostović I, Hof PR. Does Alzheimer's disease begin in the brainstem? Neuropathol. Appl. Neurobiol. 2009;35:532–554. doi: 10.1111/j.1365-2990.2009.01038.x. (PubMed: 19682326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimić G, Babić M, Borovečki F, Hof PR. Early failure of the default-mode network and the pathogenesis of Alzheimer's disease. CNS Neurosci. Ther. 2014;20:692–698. doi: 10.1111/cns.12260. (PubMed: 24712393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimić G, Babić Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R, Di Giovanni G, Wischik C, Hof PR. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:E6. doi: 10.3390/biom6010006. (PubMed: 26751493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štefulj J, Panzenboeck U, Hof PR, Šimić G. Pathogenesis, modulation, and therapy of Alzheimer’s disease: a perspective on roles of liver-X receptors. Transl. Neurosci. 2013;4:349–356. [Google Scholar]
- Takahashhi H, Nakashima S, Ohama E, Taked S, Ikuta F. Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain Dev. 1986;8:355–365. doi: 10.1016/s0387-7604(86)80055-9. (PubMed: 3541662) [DOI] [PubMed] [Google Scholar]
- Taraschenko OD, Barnes WG, Herrick-Davis K, Yokoyama Y, Boyd DL, Hough LB. Actions of tacrine and galanthamine on histamine-N-methyltransferase. Methods Find. Exp. Clin. Pharmacol. 2005;27:161–165. doi: 10.1358/mf.2005.27.3.890872. (PubMed: 15834447) [DOI] [PubMed] [Google Scholar]
- Tariot PN, Sunderland T, Weingartner H, Murphy DL, Welkowitz JA, Thompson K, Cohen RM. Cognitive effects of L-deprenyl in Alzheimer’s disease. Psychopharmacology. 1987;91:489–495. doi: 10.1007/BF00216016. (PubMed: 3108930) [DOI] [PubMed] [Google Scholar]
- Terry RD. The pathogenesis of Alzheimer’s disease: an alternative to the amyloid hypothesis. J. Neuropathol. Exp. Neurol. 1996;55:1023–1025. (PubMed: 8857998) [PubMed] [Google Scholar]
- Tesseur I, Pimenova AA, Lo AC, Ciesielska M, Lichtenthaler SF, De Maeyer JH, Schuurkes JA, D'Hooge R, De Strooper B. Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol. Aging. 2013;34:1779–1789. doi: 10.1016/j.neurobiolaging.2013.01.020. (PubMed: 23474291) [DOI] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. (PubMed: 18369648) [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. (PubMed: 12084879) [DOI] [PubMed] [Google Scholar]
- The World Alzheimer Report. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International (ADI); 2015. [Google Scholar]
- Tohgi H, Ueno M, Abe T, Takahashi S, Nozaki Y. Concentrations of monoamines and their metabolites in the cerebrospinal fluid from patients with senile dementia of the Alzheimer type and vascular dementia of the Binswanger type. J. Neural Transm. Park. Dis. Dement. Sect. 1992;4:69–77. doi: 10.1007/BF02257623. (PubMed: 1540305) [DOI] [PubMed] [Google Scholar]
- Törk I. Anatomy of the serotonergic system. Ann. NY Acad. Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion 34–35 (PubMed: 2252340) [DOI] [PubMed] [Google Scholar]
- Törk I, Hornung JP. Raphe nuclei and serotonin containing systems. In: Paxinos G, editor. The Human Nervous System. San Diego, CA, USA: Academic Press; 1990. pp. 1001–1022. [Google Scholar]
- Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C, Luk C, Gibbs JR, Dillman A, Hernandez DG, Arepalli S, Singleton AB, Cookson MR, Pittman AM, de Silva R, Weale ME, Hardy J, Ryten M. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum. Mol. Genet. 2012;21:4094–4103. doi: 10.1093/hmg/dds238. (PubMed 22723018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo L, Das D, Hsieh W, Medina B, Moghadam S, Lin B, Dang V, Sanchez MM, De Miguel Z, Ashford JW, Salehi A. Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013;37:1363–1379. doi: 10.1016/j.neubiorev.2013.05.008. (PubMed: 23707776) [DOI] [PubMed] [Google Scholar]
- Truchot L, Costes N, Zimmer L, Laurent B, Le Bars D, Thomas-Antérion C, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer's disease. Neuroimage. 2008;40:1251–1256. doi: 10.1016/j.neuroimage.2008.01.030. (PubMed: 18313943) [DOI] [PubMed] [Google Scholar]
- Uro-Coste E, Russano de Paiva G, Guilbeau-Frugier C, Sastre N, Ousset PJ, da Silva NA, Lavialle-Guillotreau V, Vellas B, Delisle MB. Cerebral amyloid angiopathy and microhemorrhages after Aβ vaccination: case report and brief review. Clin. Neuropathol. 2010;29:209–216. doi: 10.5414/npp29209. (PubMed: 20569670) [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernández A, Cabrero JR, Serrador JM, Sánchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18:291–297. doi: 10.1016/j.tcb.2008.04.003. (PubMed: 18472263) [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Lee YA, Goto Y. Dopamine in socioecological and evolutionary perspectives: implications for psychiatric disorders. Front. Neurosci. 2015;9:219. doi: 10.3389/fnins.2015.00219. (PubMed: 26136653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C, Haan J, Bakker E, Hardy J, Van Hul W, Webnert A, Vegter-Van der Vlis M, Roos RA. Aβ protein precursor gene and hereditary cerebral hemorrhage with amyloidosis (Dutch) Science. 1990;248:1120–1122. doi: 10.1126/science.1971458. (PubMed: 1971458) [DOI] [PubMed] [Google Scholar]
- van der Cammen TJ, Tiemeier H, Engelhart MJ, Fekkes D. Abnormal neurotransmitter metabolite levels in Alzheimer patients with a delirium. Int. J. Geriatr. Psychiatry. 2006;21:838–843. doi: 10.1002/gps.1569. (PubMed: 16955437) [DOI] [PubMed] [Google Scholar]
- van Groen T. DNA methylation and Alzheimer’s disease. In: Tollefsbol TO, editor. Epigenetics of Aging. Netherlands: Springer; 2010. pp. 315–326. [Google Scholar]
- Van der Jeugd A, Blum D, Raison S, Eddarkaoui S, Buée L, D'Hooge R. Observations in THY-Tau22 mice that resemble behavioral and psychological signs and symptoms of dementia. Behav. Brain Res. 242:34–39. doi: 10.1016/j.bbr.2012.12.008. (PubMed: 23247080) [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Near-total loss of learning and memory as a result of combined cholinergic and serotonergic blockade in the rat. Behav. Brain Res. 1987;23:43–57. doi: 10.1016/0166-4328(87)90241-5. (PubMed: 2950902) [DOI] [PubMed] [Google Scholar]
- Varghese M, Ho L, Wang J, Zhao W, Levine S, Ono K, Mannino S, Pasinetti GM. Green coffee as a novel agent for Alzheimer’s disease prevention by attenuating diabetes. Transl. Neurosci. 2014;5:111–116. [Google Scholar]
- Vaughan GM, Pelham RW, Pang SF, Loughlin LL, Wilson KM, Sandock KL, Vaughan MK, Koslow SH, Reiter RJ. Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J Clin. Endocr. Metab. 1976;42:752–764. doi: 10.1210/jcem-42-4-752. (PubMed: 1262447) [DOI] [PubMed] [Google Scholar]
- Volicer L, Langlais PJ, Matson WR, Mark KA, Gamache PH. Serotoninergic system in dementia of the Alzheimer type. Abnormal forms of 5-hydroxytryptophan and serotonin in cerebrospinal fluid. Arch. Neurol. 1985;42:1158–1161. doi: 10.1001/archneur.1985.04060110040013. (PubMed: 2415092) [DOI] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer's disease. Am. J. Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. (PubMed: 11329390) [DOI] [PubMed] [Google Scholar]
- Von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. (PubMed: 10805776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Staden E. Dissertation, Mathematisch-Naturwissenschaftlichen Fakultät. Rheinischen Friedrich-Wilhelms-Universität Bonn; 2014. Neurotransmitter receptors in mouse models of Alzheimer’s disease. [Google Scholar]
- Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann. Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. (PubMed: 1763890) [DOI] [PubMed] [Google Scholar]
- Voogd J, Jaarsma D, Marani E. The cerebellum. In: Hökfelt T, editor. Chemoarchitecture and Anatomy. Elsevier; Amsterdam: 1996. pp. 1–369. [Google Scholar]
- Waanders-Oude Elferink M, van Tilborg I, Kessels RPC. Perception of emotions in mild cognitive impairment and Alzheimer’s dementia: does intensity matter? Transl. Neurosci. 2015;6:139–149. doi: 10.1515/tnsci-2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. (PubMed: 17286590) [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. (PubMed: 12511643) [DOI] [PubMed] [Google Scholar]
- Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer's disease. PLoS One. 2008;3:e2698. doi: 10.1371/journal.pone.0002698. (PubMed: 18628954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, Raskind MA, Peskind ER. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am. J. Geriatr. Psychiatry. 2009;17:744–751. doi: 10.1097/JGP.0b013e3181ab8c61. (PubMed: 19700947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-Y, Stucky A, Hahn C-G, Wilson R, Bennett D, Arnold S. BDNF-trkB signaling in late life cognitive decline and Alzheimer’s disease. Transl. Neurosci. 2011;2:91–100. [Google Scholar]
- Wang L, Brier MR, Snyder AZ, Thomas JB, Fagan AM, Xiong C, Benzinger TL, Holtzman DM, Morris JC, Ances BM. Cerebrospinal fluid Aβ1–42, phosphorylated tau181, and resting-state functional connectivity. JAMA Neurol. 2013;70:1242–1248. doi: 10.1001/jamaneurol.2013.3253. (PubMed: 23959173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016;17:22–35. doi: 10.1038/nrn.2015.1. (PubMed: 26631930) [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo S-Y, Kirschner MW. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. U. S. A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. (PubMed: 1057175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer's disease treatment. Curr. Drug Targets. 2012;13:483–494. doi: 10.2174/138945012799499794. (PubMed: 22280345) [DOI] [PubMed] [Google Scholar]
- Weller RO. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J. Neuropathol. Exp. Neurol. 1998;57:885–894. doi: 10.1097/00005072-199810000-00001. (PubMed: 9786239) [DOI] [PubMed] [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Descending noradrenergic projections and their spinal terminations. Prog. Brain Res. 1982;57:219–238. doi: 10.1016/S0079-6123(08)64131-X. (PubMed: 7470856) [DOI] [PubMed] [Google Scholar]
- Williams DR. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J. 2006;36:652–660. doi: 10.1111/j.1445-5994.2006.01153.x. (PubMed: 16958643) [DOI] [PubMed] [Google Scholar]
- Wolstencroft EC, Šimić G, thi Man N, Holt I, Lam, le T, Buckland PR, Morris GE. Endosomal location of dopamine receptors in neuronal cell cytoplasm. J. Mol. Histol. 2007;38:333–340. doi: 10.1007/s10735-007-9106-5. (PubMed: 17593530) [DOI] [PubMed] [Google Scholar]
- Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer’s disease are antigentically related. Proc. Natl. Acad. Sci. USA. 1985;82:8729–8732. doi: 10.1073/pnas.82.24.8729. (PubMed 2934737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff G, Young JE, Martinez FJ, Buen F, Gore A, Kinaga J, Li Z, Yuan SH, Zhang K, Goldstein LS. The presenilin-1 ΔE9 mutation results in reduced γ-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Rep. 2013;5:974–985. doi: 10.1016/j.celrep.2013.10.018. (PubMed: 24239350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Feenstra MG, Zhou JN, Liu RY, Toranõ JS, Van Kan HJ, Fischer DF, Ravid R, Swaab DF. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab. 2003;88:5898–5906. doi: 10.1210/jc.2003-030833. (PubMed: 14671188) [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Larin F, Axelrod J, Shein HM, Rosasco K. Formation of melatonin and 5-hydroxyindole acetic acid from 14C-tryptophan by rat pineal glands in organ culture. Nature. 1968;217:953–954. doi: 10.1038/217953a0. (PubMed: 5300432) [DOI] [PubMed] [Google Scholar]
- Yamada M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology. 2003;23:311–317. doi: 10.1046/j.1440-1789.2003.00522.x. (PubMed: 14719548) [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hirano A. Nucleus raphe dorsalis in parkinsonism-dementia complex of Guam. Acta Neuropathol. 1985;67:296–299. doi: 10.1007/BF00687815. (PubMed: 4050345) [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Oyanagi K, Mori O, Kitamura S, Ohyama M, Terashi A, Kitamoto T, Katayama Y. Variant Gerstmann-Sträussler syndrome with the P105L prion gene mutation: an unusual case with nigral degeneration and widespread neurofibrillary tangles. Acta Neuropathol. 1999;98:506–511. doi: 10.1007/s004010051116. (PubMed: 10541874) [DOI] [PubMed] [Google Scholar]
- Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. 2001;101:256–270. doi: 10.1007/s004010000293. (PubMed: 11307626) [DOI] [PubMed] [Google Scholar]
- Yates CM, Simpson J, Gordon A. Regional brain 5-hydroxytryptamine levels are reduced in senile Down's syndrome as in Alzheimer's disease. Neurosci. Lett. 1986;65:189–192. doi: 10.1016/0304-3940(86)90302-2. (PubMed: 2940479) [DOI] [PubMed] [Google Scholar]
- Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013;6:19–33. doi: 10.1177/1756285612461679. (PubMed: 23277790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Otake M, Igarashi K, Matsunaga M, Takebe K, Kudo H. Topography of Alzheimer's neurofibrillary change distribution in myotonic dystrophy. Clin. Neuropathol. 1990;9:234–239. (PubMed: 2272143) [PubMed] [Google Scholar]
- Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal. Res. 2003;35:125–130. doi: 10.1034/j.1600-079x.2003.00065.x. (PubMed: 12887656) [DOI] [PubMed] [Google Scholar]
- Zhou LX, Du JT, Zeng ZY, Wu WH, Zhao YF, Kanazawa K, Ishizuka Y, Nemoto T, Nakanishi H, Li YM. Copper (II) modulates in vitro aggregation of a tau peptide. Peptides. 2007;28:2229–2234. doi: 10.1016/j.peptides.2007.08.022. (PubMed: 17919778) [DOI] [PubMed] [Google Scholar]
- Zornetzer SF. Catecholamine system involvement in age-related memory dysfunction. Ann. NY Acad. Sci. 1985;444:242–254. doi: 10.1111/j.1749-6632.1985.tb37594.x. (PubMed: 2990292) [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Marquis JK, Volicer L, Direnfeld LK, Langlais PJ, Nixon RA. Cerebrospinal fluid levels of angiotensin-converting enzyme, acetylcholinesterase, and dopamine metabolites in dementia associated with Alzheimer's disease and Parkinson's disease: a correlative study. Biol. Psychiatry. 1986;21:1365–1381. doi: 10.1016/0006-3223(86)90328-8. (PubMed: 3024746) [DOI] [PubMed] [Google Scholar]
- Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. Neuropathology of aminergic nuclei in Alzheimer's disease. Prog. Clin. Biol. Res. 1989;317:353–365. (PubMed: 2602423) [PubMed] [Google Scholar]