Abstract
Background
Fatigue is a common symptom in patients with Lyme disease.
Objective
The purpose of this study was to characterize fatigue in untreated adult patients presenting with erythema migrans. Selected variables were assessed to determine if any correlated with the presence or severity of fatigue.
Methods
Fatigue was assessed on the day of the evaluation by a visual analogue scale (VAS), over the past 14 days by the 11-item fatigue severity scale (FSS-11) and over the past 28 days based on a question from the 36-item Short Form General Health Survey version 2.
Results
51 patients with erythema migrans whose mean age was 49.8 years, and 33 (64.7%) of whom were male, were evaluated in this study. The three measures of fatigue were positively correlated with one another (p ≤ 0.01). 26 (51%) had fatigue based on a VAS score above 0. 10 (19.6%) had severe fatigue based on an FSS-11 score of ≥ 4. The strongest correlate for higher fatigue scores was having a greater number of symptoms in addition to fatigue.
Conclusion
Based on the FSS-11 assessment tool, approximately 20% of early Lyme patients have severe fatigue. Having a high total number of symptoms was associated with both the presence and severity of fatigue. Since prior studies have demonstrated the presence of elevated levels of pro-inflammatory cytokines and other molecules in the serum of highly symptomatic patients with erythema migrans, the symptom of fatigue in early Lyme disease may be a component of what has been referred to as the acute sickness response.
Keywords: Lyme disease, Borrelia burgdorferi, Fatigue, Acute sickness response
Lyme disease is the most common tick-borne infection in North America. Recent estimates suggest that approximately 300,000 cases occur annually in the United States [1,2]. At least 70-80% of patients with Lyme disease in the United States present with the objective skin manifestation, erythema migrans [3]. Approximately 65% of patients with erythema migrans in the United States have additional non-specific complaints such as fatigue, musculoskeletal pains, headaches, and others [4]. Fatigue is a common symptom in patients with both early and late manifestations of Lyme disease, as well as in patients with post-treatment Lyme disease symptoms (PTLDS) [4-9]. Studies of patients with PTLDS have often employed both an 11-item Fatigue Severity Scale (FSS-11) and a visual analogue scale (VAS) to quantitate the level of fatigue [6-8]. A score of ≥ 4 is regarded as severe fatigue on the FSS-11 [6]. However, the FSS-11 has not been used in patients with untreated erythema migrans and, thus, it is unknown what proportion of early Lyme disease patients have severe fatigue based on this assessment tool.
The purpose of this study was to evaluate the results of the FSS-11 and other measures of fatigue severity in adult patients with untreated early Lyme disease presenting with erythema migrans and to determine whether certain clinical or demographic factors in such patients correlate with fatigue severity.
Methods
Adult patients with Lyme disease were enrolled between 2011 and 2015 in a prospective study to assess outcomes. Exclusion criteria included: a history of Lyme disease within 12 months, or on-going symptoms from a more remote bout of Lyme disease; pregnancy or being post-partum; an immunocompromising condition; having a diagnosis of fibromyalgia, chronic fatigue syndrome or traumatic brain injury; any prolonged history of undiagnosed or unexplained somatic complaints; and any underlying disease or condition that might interfere with an evaluation of outcome.
The focus of this analysis was specifically on the subset of enrolled subjects with untreated erythema migrans, who had no evidence of a concomitant objective extracutaneous manifestation of Lyme disease. All patients fulfilled the Infectious Diseases Society of America criteria for erythema migrans [10].
Subjects underwent a systematic evaluation for the presence and severity of fatigue prior to antibiotic treatment. The study was conducted at the Lyme Disease Diagnostic Center at New York Medical College and was approved by the institutional review board at New York Medical College.
The level and presence of fatigue was evaluated by three different measures:
The FSS-11, which is an 11-item self-administered questionnaire about the subject's experience with fatigue over the prior two weeks, with each item scored on a scale of 1 to 7 (7 being most severe).
An 8 cm VAS was used to quantitate the level of fatigue that the subject was experiencing on the day of study entry. A score of 0 indicated that fatigue was not present and a score of 8 indicated that the fatigue was extreme/could not be worse. Subjects with positive scores on the VAS were questioned about the duration of fatigue and whether there was another possible explanation for this symptom. One subject with a positive VAS score was not included in this study because fatigue was attributed to a cause other than Lyme disease.
The third measure, arbitrarily called the 28 day fatigue scale (28d-FS), was based on a self-administered question from the 36-item Short Form General Health Survey version 2 (SF-36v2) that inquired about how much of the time during the past four weeks the subject felt tired. A score of 0 indicated “none of the time,” 1 indicated “a little of the time,” 2 indicated “some of the time,” and 3 indicated “most of the time,” and a score of 4 indicated “all of the time.”
Other assessments
At the baseline visit, demographic and clinical data were collected, including data on 11 other symptoms in addition to fatigue, such as headache, joint pain, muscle pain, cognitive complaints and others, as described elsewhere [9]. Serologic testing was performed using the C6 Lyme ELISA kit (Immunetics, Inc., Boston, Massachusetts) according to the manufacturer's recommendations. In addition, patients were evaluated for co-infections with Anaplasma phagocytophilum and Babesia microti by serology (based on a 4-fold rise in titer between acute and convalescent serum samples on testing performed by Focus Diagnostics, Inc., Cypress, California) and in selected cases with highly suggestive clinical features, such as new onset fever while on antibiotic treatment for erythema migrans, by a blood smear or polymerase chain reaction, as described elsewhere [11,12].
Statistical methods
Continuous variables were described using means and standard deviations; categorical variables were described with frequencies and percentages. For univariable comparisons, t-tests assuming unequal variances were used for continuous outcomes. For categorical variables, Fisher's exact test was used. Correlations between symptom and fatigue scales were analyzed using Spearman's correlation (rs). To examine the agreement between symptom and fatigue scales dichotomized at specific cutpoints, a GEE analysis was performed to account for the within-subject correlations among scales. Because of multiple comparisons, a p value of < 0.01 was considered to be significant.
Results
Fifty-one adult patients with untreated erythema migrans were evaluated in this study. The mean age was 49.8 years (range, 20-86 years) and 33 (64.7%) were male (Table 1). Twenty-one (41.2%) had multiple erythema migrans skin lesions. The mean duration ± SD of the erythema migrans skin lesion was 7.88 ± 5.51 days (median 7 days, range 1-22 days) (patients who presented on the same day as the skin lesion was discovered were regarded as having a duration of 1 day in this analysis).
Table 1.
Selected Demographic and Clinical Characteristics of 51 Untreated Patients with Erythema Migrans.
| Characteristic | Findings |
|---|---|
| % male | 64.7 |
| Mean age ± SD in years (median, range) | 49.8 ± 15.6 (50, 20-86) |
| % with multiple erythema migrans skin lesions | 41.2 |
| Mean area ± SD of largest erythema migrans skin lesion in square cm* (median, range) | 179.8 ± 200.8 (99, 11-900) |
| Mean duration of erythema migrans ± SD in days at time of evaluation (median, range) | 7.88 ± 5.51 (7, 1-22) |
| % with symptoms at study entry | 76.5 |
| Mean number of symptoms ± SD (median, range) | 3.27 ± 3.38 (2, 0-12) |
| Mean body mass index ± SD (median, range) | 25.2 ± 3.9 (25.1, 17.2-38.4) |
| % with reactive C6 Lyme serology** | 64.7 |
| Mean C6 ELISA value ± SD | 4.3 ± 3.7 |
| % with Babesia microti co-infection | 7.8 |
| % with Anaplasma phagocytophilum co-infection | 0 |
Area defined as the maximum width times the maximum length
Test result was positive or equivocal
All three measures of fatigue were directly and significantly correlated (Table 2) (28d-FS vs. FSS-11: rs = 0.50, p = 0.009; VAS vs. FSS-11: rs = 0.66, p < 0.001; VAS vs. 28d-FS: rs = 0.48, p = 01.). When the comparison of fatigue measures was restricted to the 26 subjects (51.0%; 95%CI: 36.6%, 65.2%) with a VAS score of > 0, the correlations all remained significant (p ≤ 0.01). However, when the sample was restricted to the 10 subjects with an FSS-11 score of ≥ 4, there was no significant correlation among the three measures of fatigue (28d-FS vs. FSS-11: rs = 0.07, p = 0.85; VAS vs. FSS-11: rs = 0.13, p = 0.71; VAS vs. 28d-FS: rs = 0.15, p = 0.68).
Table 2.
Comparison of Three Measures of Fatigue in 51 Untreated Patients with Erythema Migrans.
| VAS | FSS-11 | 28d-FS | |
|---|---|---|---|
| Mean ± SD score (median, range) | 1.85 ± 2.32 (0.4, 0-8) | 2.57 ± 1.85 (1.73, 1-7) | 1.63 ± 1.04 (2, 0-4) |
| Mean ± SD scores for the 26 patients with a VAS score > 0 (median, range) | 3.63 ± 2.02 ( 3.6, 0.4 – 8) | 3.46 ± 1.89 (3.32, 1-7) | 2.15 ± 0.97 (2, 1-4) |
| Mean ± SD scores for the 10 patients with FSS-11 ≥ 4 (median, range) | 4.6 ± 2.64 (5.2, 0-8) | 5.76 ± 0.99 (5.55, 4-7) | 2.40 ± 0.97 (2.5, 1-4) |
Not all of the FSS-11 scores of ≥ 4 correlated with high VAS scores. Although 6 of the 10 subjects with an FSS-11 of ≥ 4 had a VAS score that also exceeded 4, 4 subjects reported lower scores including 1 subject with a score of 0, a second subject with a score of 0.6 and 2 with scores of 3.8. In contrast, there seemed to be greater consistency between high scores on the VAS and higher FSS-11 scores. Of the 8 subjects whose level of fatigue equaled or exceeded 5 on the VAS (representing 15.7% of the 51 subjects enrolled and 30.8% of the 26 with VAS scores above 0), the FSS-11 score was ≥ 4 for 6 and the other 2 had scores close to 4, of 3.45 and 3.27 respectively. Some discrepancies in scores are not unexpected, since the FSS-11 evaluates fatigue over 14 days, whereas the VAS used in this study inquired about fatigue experienced only on the day of study entry.
Having a higher number of symptoms (inclusive of the symptom of fatigue itself) was associated with significantly greater fatigue scores on all 3 scales (Table 3) (p<0.0001). Other characteristics (Tables 3 and 4), such as younger age, having multiple erythema migrans skin lesions and seroreactivity at enrollment, were associated with at least a trend toward higher levels of fatigue (for seroreactivity and for the C6 ELISA value, this was found only for the FSS-11). Gender, body mass index (BMI), and size and duration of the erythema migrans skin lesion did not correlate with fatigue severity.
Table 3.
Correlation between Fatigue Scales and Selected Clinical and Demographic Characteristics.
| Characteristic | VAS | FSS-11 | 28d-FS |
|---|---|---|---|
| rs value; p value | rs value; p value | rs value; p value | |
| Number of symptoms | +0.78; < 0.0001 | +0.72; < 0.0001 | +0.64; < 0.0001 |
| Age | −0.28; 0.04 | −0.28; 0.05 | −0.40; 0.003 |
| BMI | +0.15; 0.30 | −0.07; 0.63 | −0.01; 0.93 |
| EM area | −0.08; 0.56 | +0.05; 0.75 | −0.07; 0.63 |
| EM duration | −0.08; 0.56 | +0.05; 0.75 | −0.07; 0.63 |
| C6 ELISA value | +0.14; 0.35 | +0.32; 0.02 | +0.20; 0.16 |
BMI = Body mass index
EM = Erythema migrans; EM area = maximum width time maximum length
Table 4.
Fatigue Severity in Relation to Selected Clinical and Demographic Characteristics.
| Characteristic | P value | ||
|---|---|---|---|
| Scales* | Male | Female | |
| VAS | 2.03 ± 2.49 | 1.52 ± 2.01 | 0.43 |
| FSS-11 | 2.51 ± 1.83 | 2.67 ± 1.93 | 0.78 |
| 28d-FS | 1.64 ± 1.11 | 1.61 ± 0.92 | 0.93 |
| Single EM | Multiple EM | ||
| VAS | 1.28 ± 1.97 | 2.67 ± 2.58 | 0.05 |
| FSS-11 | 2.02 ± 1.53 | 3.35 ± 2.01 | 0.02 |
| 28d-FS | 1.40 ± 1.04 | 1.95 ± 0.97 | 0.06 |
| Reactive C6** | Non-reactive C6 | ||
| VAS | 2.16 ± 2.38 | 1.28 ± 2.17 | 0.19 |
| FSS-11 | 2.98 ± 1.89 | 1.81 ± 1.55 | 0.02 |
| 28d-FS | 1.76 ± 1.09 | 1.39 ± 0.92 | 0.21 |
Scores are shown as mean ± SD
Positive or equivocal
Of particular interest are the associations and predisposing factors for severe fatigue. A comparison of the 10 (19.6%) (95% CI = 9.8%, 33.1%) subjects with severe fatigue based on a score of at least 4.0 on the FSS-11 with the 41 other subjects showed no significant differences in age, sex, BMI, presence of multiple erythema migrans skin lesions, duration of erythema migrans, area of the largest skin lesion, frequency of C6 ELISA reactivity, C6 ELISA value, or the presence of a co-infection (all p values > 0.25) (Table 5). Severe fatigue based on an FSS-11 score of ≥ 4 did, however, directly correlate with the number of symptoms the subjects had at the time of the visit (those with an FSS-11 ≥ 4 had a mean ± SD of 7.5 ± 3.06 symptoms versus 2.24 ± 2.58 symptoms for those with an FSS-11 score of < 4, p < 0.001).
Table 5.
Comparison of the 10 subjects with erythema migrans who had an FSS-11 score of ≥ 4 with the other 41 subjects.
| Characteristic | FSS-11 ≥ 4 n=10 | FSS-11 < 4 n=41 | P value |
|---|---|---|---|
| % male | 50 | 68.3 | 0.30 |
| Mean age ± SD in years | 46.3 ± 17.6 | 50.6 ± 15.1 | 0.49 |
| Mean ± SD body mass index | 24.0 ± 13.5 | 25.5 ± 3.9 | 0.25 |
| % with symptoms at study entry | 100 | 70.7 | 0.09 |
| Mean number of symptoms ± SD | 7.5 ± 3.0 | 2.2 ± 1.26 | < 0.001 |
| % with multiple erythema migrans skin lesions | 60 | 36.6 | 0.28 |
| Mean duration ± SD of erythema migrans skin lesion in days | 8.9 ± 7.1 | 7.6 ± 5.2 | 0.60 |
| Mean area ± SD of largest skin lesion in square cm* | 187.7 ± 256.9 | 177.9 ± 188.4 | 0.91 |
| % with reactive C6 Lyme serology** | 80 | 61 | 0.46 |
| Mean ± SD C6 ELISA value | 5.0 ± 3.5 | 4.1 ± 3.8 | 0.50 |
| % with co-infection | 10.0 | 7.3 | 1.0 |
Area defined as the maximum width times the maximum length
Test result was positive or equivocal
A comparison of the 26 subjects with a VAS score above 0 with the 25 whose score was 0 also found no significant differences in any of the parameters listed in Table 5 (data not shown), except for the total number of symptoms (mean ± SD number of symptoms in those subjects with a VAS score of > 0 was 5.38 ± 3.32 versus 1.08 ± 1.58 in those with a VAS score of 0, p < 0.001). There was a trend for subjects to be younger who had VAS scores of > 0 (mean ± age of subjects with a VAS score > 0 was 46.3 ± 13.7 years versus 53.4 ± 16.8 years for those with a VAS score of 0, p = 0.10).
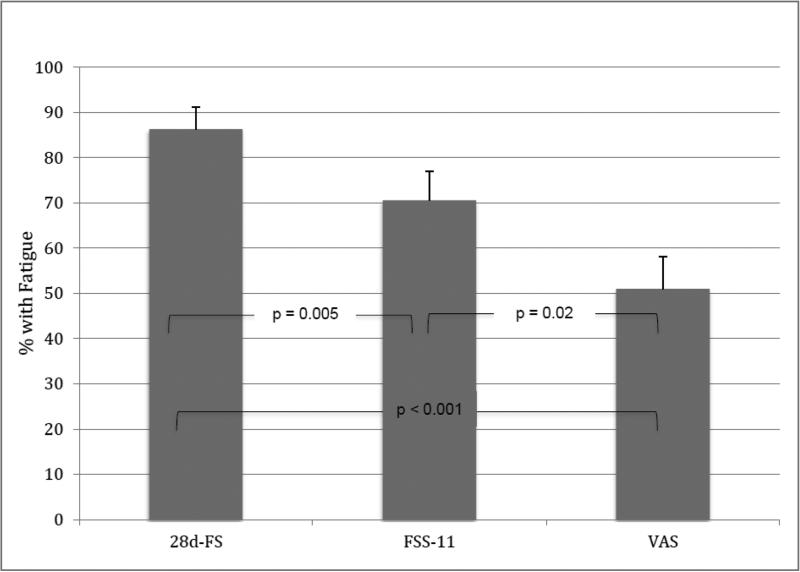
A limitation of the 28d-FS is that it potentially captures any event causing fatigue over a 4 week period and thus would seem to be more likely to generate a larger number of positive responses with some of these unrelated to early Lyme disease. In this study 44 (86.3%) of the 51 subjects had a 28d-FS score of >0 compared with 36 (70.6%) who had an FSS-11 score of >1 and with 26 (51.0%) who had a VAS score of >0 (28d-FS vs. FSS-11: p = 0.02; 28d-FS vs. VAS: p < 0.001; FSS-11 vs. VAS: p = 0.005). The significance of the overall comparison was p < 0.0001 (Figure 1).
Figure 1.
Comparison of the proportion of 51 early Lyme disease patients with a positive score for the presence of fatigue based on three different assessment measures (28d-FS vs FSS-11, p=0.005; 28d-FS vs VAS, p<0.001; FSS-11 vs VAS p=0.02). An overall comparison of the three prevalence figures showed a highly significant difference (p<0.0001).
The duration of fatigue was assessed only for the 26 subjects whose VAS score was > 0; the mean ± duration was 8.08 ± 7.79 days (median = 5.5, range = 1-36) (the mean duration of the erythema migrans skin lesion for this subgroup was very similar at 7.92 ± 5.29 days). There was a trend toward greater fatigue severity in subjects with longer durations of fatigue (rs = 0.46, p = 0.02 for the FSS-11; rs = 0.39, p = 0.053 for the VAS; and rs = 0.50, p = 0.011 for the 28d-FS). However, the small sample size limited more detailed analyses.
Discussion
Fifty-one adult patients with erythema migrans were assessed by three different measures of fatigue. An 8 cm VAS assessed the presence and severity of fatigue on the day of the visit. The FSS-11 assessed the severity of fatigue over the prior 14-days. What we have termed the “28d-FS” determined the frequency that fatigue was experienced over a 28-day time frame. Based on a positive score on the VAS, 26 (50.1%) had fatigue on the day of study entry. Based on the FSS-11, 10 (19.6%) of the enrolled subjects had severe fatigue. Severe fatigue by this criterion was significantly associated with having more total symptoms (p<0.001), but was not associated with age, gender, BMI, having multiple erythema migrans skin lesions, the duration of the erythema migrans skin lesion at the time of evaluation, the area of the largest erythema migrans skin lesion, seropositivity by the C6 ELISA, or the presence of a co-infection.
All of the assessment tools had certain limitations. A limitation of the FSS-11 is the difficulty that subjects may have had in filling out this form if they were not experiencing fatigue at all. For example, one of the questions on the FSS-11 asks to what extent you agree with the statement that “fatigue interferes with carrying out certain duties and responsibilities” which could be interpreted as true even if you do not have fatigue when the questionnaire was administered. This assessment tool was intended to quantitate the level of fatigue in subjects who actually had this symptom. A possible illustrative example of an inaccurate FSS-11 score is that of the seven subjects who had a score of 0 on both the VAS and the 28d-FS, one, nevertheless, had an FSS-11 score of 2.09.
The 28d-FS also appeared to over-estimate the frequency of fatigue due to Lyme disease because it assessed a much longer time period than is typical for early Lyme disease patients prior to consulting with a health care provider. The VAS tool only asked about fatigue on the day of the evaluation and thus some patients with fatigue from Lyme disease on preceding days might have been missed based on this measure of fatigue.
There was a trend toward greater levels of fatigue based on all 3 fatigue scales in patients with disseminated Lyme disease as defined by the presence of multiple erythema migrans skin lesions. However, neither the presence of fatigue by the VAS, nor a high level of fatigue severity based on an FSS-11score of ≥4 was associated with disseminated B. burgdorferi infection. This finding is consistent with a previous study of 104 patients with culture-confirmed erythema migrans that failed to show that fatigue was significantly more common among patients infected with the subgroup of borrelial strains most often associated with hematogenous dissemination, compared with patients who were infected with other strains (based on a ribosomal spacer typing system that differentiates United States strains of B. burgdorferi into 3 distinct subsets) [13].
In the present study, the presence of fatigue by the VAS and severe fatigue based on the FSS-11 were both strongly associated with having a greater number of total symptoms, suggesting that fatigue is a component of what is referred to for other infectious diseases as the “acute sickness response,” which appears to be caused by the presence of pro-inflammatory cytokines and other acute phase proteins [14,15]. Prior studies in the United States of patients with erythema migrans have found evidence for increased serum levels of interferon (IFN)-gamma, interleukin-6 (IL-6), CXCL9 and/or CXCL10, depending on the particular study, in more symptomatic patients [16-18]. Higher serum levels of certain cytokines and chemokines were correlated with the presence of a particular single nucleotide polymorphism (SNP) in the gene encoding Toll-like receptor 1 [17]. Patients with this SNP who were infected with certain borrelial strains had even higher levels of these cytokines and chemokines and were uniformly symptomatic [17]. In patients without this SNP, however, borrelial strain differences did not affect the likelihood of a symptomatic versus asymptomatic infection [17]. These observations suggest that there is a potentially important interaction between the strain of borrelia causing Lyme disease and host factors in contributing to the development of non-specific symptoms such as fatigue.
Fatigue is a common complaint in many other infectious diseases, including other deer tick transmitted infections [12,19-21]. In certain other infections, emerging data suggest that a SNP in the IFN-gamma gene is significantly associated with experiencing severe fatigue per se [15], a topic that should be considered for potential investigation in Lyme disease patients as well.
In conclusion, in this study, as in others [4], fatigue was a common symptom in adult patients with early Lyme disease associated with erythema migrans. The prevalence of this symptom was influenced by the choice of the assessment measure. Based on the FSS-11 assessment tool approximately 20% of such patients may experience severe fatigue. Having more numerous symptoms was significantly associated with reporting the symptom of fatigue and specifically with having severe fatigue as defined by a score of ≥4 on the FSS-11. The symptom of fatigue in early Lyme disease is likely to be a component of what has been referred to as the acute sickness response.
HIGHLIGHTS.
Fatigue was assessed in patients with Lyme disease presenting with erythema migrans (EM).
Over 50% of patients with EM had fatigue, and ~20% had severe fatigue based on the FSS-11 scale.
Having a large number of symptoms was associated with both the presence and severity of fatigue.
This finding suggests that fatigue with EM may be a component of the acute sickness response.
Acknowledgments
The authors thank Denise Cooper, Julia Singer, Sophia Less, Artemio Zavalla and Lisa Giarratano for their assistance.
Funding: RO1 CK 000152 from the Centers for Disease Control and Prevention (CDC) to GPW. This publication was also made possible by support from CTSA Grants Number UL1 TR000142 and KL2 TR000140 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research to EDS. The findings and conclusions of this paper are those of the authors and do not necessarily represent the official position of the CDC or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Wormser reports receiving research grants from Immunetics, Inc., Institute for Systems Biology, Rarecyte, Inc., and Quidel Corporation. He owns equity in Abbott; has been an expert witness in malpractice cases involving Lyme disease; and is an unpaid board member of the American Lyme Disease Foundation. Dr. Shapiro has received royalty payments from UptoDate; has been an expert witness in malpractice cases involving Lyme disease; and is an unpaid board member of the American Lyme Disease Foundation. Other authors have no disclosures.
References
- 1.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:678–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21:1625–31. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: A review. JAMA. 2016;315:1767–1777. doi: 10.1001/jama.2016.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;297:2617–27. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 5.Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 6.Krupp LB, Hyman LG, Grimson R, Coyle PK, Melville P, Ahnn S, et al. Study and Treatment of Post Lyme Disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–30. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- 7.Fallon BA, Keilp JG, Corbera KM, Petkova E, Britton CB, Dwyer E, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- 8.Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Nowakowski J. Long-term assessment of fatigue in patients with culture-confirmed Lyme disease. Am J Med. 2015;128:181–4. doi: 10.1016/j.amjmed.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Weitzner E, McKenna D, Nowakowski J, Scavarda C, Dornbush R, Bittker S, et al. Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis. 2015;61:1800–1806. doi: 10.1093/cid/civ735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Wormser GP, Zhuge J, Villafuerte P, Ip D, Zeren C, et al. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis. 2015;6:376–82. doi: 10.1016/j.ttbdis.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Wormser GP, Aguero-Rosenfeld ME, Cox ME, Nowakowski J, Nadelman RB, Holmgren D, et al. Differences and similarities between culture-confirmed human granulocytic anaplasmosis and early Lyme disease. J Clin Microbiol. 2013;51:954–8. doi: 10.1128/JCM.02929-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, et al. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–5. doi: 10.1086/314922. [DOI] [PubMed] [Google Scholar]
- 14.Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, et al. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behavior in humans. Psychol Med. 2004;34:1289–1297. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- 15.Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun. 2012;26:552–558. doi: 10.1016/j.bbi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strle K, Shin JJ, Glickstein J, Steere AC. A toll-like receptor 1 polymorphism is associated with heightened T-helper 1 inflammatory responses and antibiotic refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar JC, Pope CD, Sellati TJ, Feder HM, Jr., Kiely TG, Dardick KR, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 19.Metlay JP, Fine MJ, Schulz R, Marrie TJ, Coley CM, Kapoor WN, et al. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med. 1997;12:423–430. doi: 10.1046/j.1525-1497.1997.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhalla MC, Wilber ST, Stiffler KA, Ondrejka JE, Gerson LW. Weakness and fatigue in older ED patients in the United States. Am J Emerg Med. 2014;32:1395–1398. doi: 10.1016/j.ajem.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, et al. Disease-specific diagnosis of co-infecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis and Lyme disease. Clin Infect Dis. 2002;34:1184–91. doi: 10.1086/339813. [DOI] [PubMed] [Google Scholar]