Abstract
Background
Current prostate cancer screening guidelines conflict with respect to the age at which to initiate screening.
Objective
To evaluate the effect of prostate-specific antigen (PSA) screening, versus zero screening, starting at age 50-54, on prostate cancer mortality.
Design, Setting, and Participants
This is a population-based cohort study comparing 3,479 men aged 50 through 54 randomized to PSA-screening in the Göteborg population-based prostate cancer screening trial, initiated in 1995, versus 4,060 unscreened men aged 51 to 55 providing cryopreserved blood in the population-based Malmö Preventive Project in the pre-PSA era, during 1982-1985.
Outcome measures and Statistical Analysis
Cumulative incidence and incidence rate ratios of prostate cancer diagnosis, metastasis, and prostate cancer death.
Results and Limitation
At 17 years, regular PSA-screening in Göteborg of men in their early 50s carried a more than 2-fold higher risk of prostate cancer diagnosis compared to the unscreened men in Malmö (IRR 2.56, 95% CI 2.18, 3.02), but resulted in a substantial decrease in risk of metastases (IRR 0.43, 95% CI 0.22, 0.79) and prostate cancer death (IRR 0.29, 95% CI 0.11, 0.67). There were 57 fewer prostate cancer deaths per 10,000 men (95% CI 22, 92) in the screened group. At 17 years, the number needed to invite to PSA-screening and the number needed to diagnose to prevent one prostate cancer death was 176 and 16, respectively. The study is limited by lack of treatment information and the comparison of two different birth cohorts.
Conclusions
PSA screening for prostate cancer can decrease prostate cancer mortality among men aged 50–54, with NNI and NND comparable to those previously reported from the European Randomized Study of Screening for Prostate Cancer for men aged 55-69 years, at similar follow-up. Guideline groups could consider whether guidelines for PSA screening should recommend starting no later than at ages 50-54.
Trial registration
The Göteborg randomized population-based prostate cancer screening trial is registered with the ISRCTN registry (isrctn.com). Identifier: ISRCTN54449243.
Keywords: prostate-specific antigen, prostate cancer, screening
Introduction
Guidelines conflict regarding the age to start prostate specific antigen (PSA) screening[1-6]. The European Association of Urology recommends obtaining a baseline PSA at age 40–45[2] whereas the American Urological Association recommends starting at age 55 for most men.[3]
There is little evidence on the effects of screening men in their early 50’s. The Swedish Göteborg randomized population-based prostate cancer screening trial[7] is unique starting at ages 50-64 and provides a critically important opportunity to determine the impact of starting screening at ages 50-55 on prostate cancer incidence and mortality. While the rate of PSA-testing was low when the trial was initiated[7], a substantial proportion of men in the control arm of the trial has likely been exposed to PSA-testing during recent years[8-9]. Such contamination may dilute the relative difference in prostate cancer incidence and mortality in the conventional intention-to-screen analysis, comparing the randomized arms.
Therefore, an ideal comparison group would be an age-matched pre-PSA era cohort of men with similar background risk and similar follow-up time as the screening group. The Swedish Malmö Preventive Medicine Project (MPP) is one such cohort, in which men gave blood in the early 80’s as part of a cardiovascular risk factor study.[10, 11] Because the PSA-test was not widely disseminated until mid-to-late 1990s in Sweden[12], this cohort has been followed without much PSA-screening at all, as widely accepted and described in previous reports [10,11,13].
Our goal was to evaluate the effect of regular PSA-screening starting before age 55, on prostate cancer incidence, metastasis and prostate cancer mortality, compared to an unscreened population. We hypothesized that starting screening at age 50 would be associated with a larger relative risk reduction than screening starting at 55–69, on the grounds that, for at least some men, cancer would progress from curable to incurable after the age of 50. We also hypothesized that the absolute risk reduction would be lower, because the risk of lethal prostate cancer is lower among younger than older men, given similar follow-up.
METHODS
Subjects, Measurements and Outcomes
The study populations, measurements and outcome ascertainment have been described in detail elsewhere[7, 10-11, 13-16]. The Göteborg trial (ISRCTN54449243) randomized 20,000 men to biennial PSA-screening or to a control group (1:1 ratio) in 1995. Men with a PSA-level above the cut-off (initially 3.0 ng/mL and 2.5 ng/mL since 2005) were recommended further urological work-up including prostate biopsy. The upper age limit for screening was 70 years. [7] In the present study, men in the screened cohort in Göteborg consisted of 3,479 men randomized to screening aged 50 through 54 at their first PSA-invitation date in 1995-1997 and men in the unscreened cohort in Malmö consisted of 4,060 men who provided blood samples at age 51-55 in 1982-1985.
Statistics
Our primary aim was to compare the number of cancers diagnosed, documented distant metastases, and prostate cancer deaths between the screened and unscreened participants. We calculated cumulative incidence for the outcomes of prostate cancer, prostate cancer metastases, overall mortality, and prostate cancer death based on 17 years of follow-up from the time of blood draw. For Göteborg men randomized to screening who never had a PSA test, we used the median date of the blood draw among those attending. Of patients who were not diagnosed with prostate cancer, 81% in Malmö and 62% in Göteborg had 17 years of follow-up. At 13 years, the corresponding proportions were 85% and 88%, respectively.
Incidence rate ratios and incidence rate differences for the effect of screening on prostate cancer diagnosis, metastasis and death were calculated based on 17 years of follow-up. The primary analysis was made on the basis of the intention-to-screen principle. Thus, our approach was biased towards the null, because we compared everyone in Göteborg (whether they attended or not) with only attendees in Malmö. Screening attendees tend to be healthier and more health conscious than the average population invited (‘healthy worker bias’ or ‘healthy screenee bias’[17]). Secondary analyses were performed restricted to attendees only in Göteborg, that is, men randomized to screening who participated and had a PSA test at least once.
Several hypotheses can explain any observed difference in prostate cancer mortality between the two non-contemporaneous cohorts. The first is if more men die of other causes in one cohort, their risk of dying from prostate cancer would be lower. Overall mortality is close to death from other causes and so we calculated the incidence rate difference of overall mortality between Göteborg and Malmö. A second hypothesis would be improvements in treatment over time. If, for instance, an effective drug for prostate cancer was developed between the end of the Malmö cohort and the beginning of the Göteborg cohort, this would lead to a survival advantage for the latter. To assess whether differences in treatment would affect our findings, we investigated the age-standardized prostate cancer mortality in Sweden over the time frame of both cohorts.[18] If prostate cancer mortality rates remained constant, then there would be no evidence that any mortality differences between these two cohorts were related to differences in treatment. The third hypothesis would be that differences in prostate cancer mortality between cohorts were related to an effect of PSA screening.
To quantify the benefits of regular PSA screening, we calculated the number of men needed to invite to screening (NNI) and the number of men needed to be diagnosed (NND) to prevent one man from dying from prostate cancer. We calculated the NNI to screening as the inverse of the absolute risk reduction between the screened and unscreened groups based on 17 years of follow-up. We calculated the NND as the inverse absolute risk reduction multiplied by the excess incidence of prostate cancer diagnosis in the screened group based on 17 years of follow-up. As a sensitivity analysis, we calculated cumulative incidences adjusted for the competing risk of death from other causes.
We believe that prostate cancer metastasis were followed more closely in Göteborg, since these patients were attendees of a screening trial in regular contact with the urology clinic and access to tests such as bone scintigraphy. We therefore performed a sensitivity analysis where we defined Malmö participants without previous documented evidence of distant metastasis but who died of prostate cancer between 17 and 19 years of follow-up as developing distant metastasis at 17 years of follow-up. All analyses were performed using Stata, version 12.0 (StataCorp, College Station, TX).
RESULTS
Over 17 years follow-up, 463 men were diagnosed out of 3,479 men in Göteborg (15.0% cumulative incidence) compared to 225 men out of 4,060 men in Malmö (6.3% cumulative incidence) (Tables 1 and 2). The men randomized to screening in Göteborg who were diagnosed with prostate cancer more frequently exhibited non-palpable clinical stage T1c tumors, whereas the men diagnosed with prostate cancer in Malmö presented with palpable disease and more often presented with distant metastases at diagnosis (p<0.0001). The majority (95%) of prostate cancer patients in Göteborg were diagnosed with biopsy Gleason score ≤ 7 disease. Men in Göteborg were younger (63 vs. 67 years) and had lower PSA-levels at diagnosis (median 4.4 vs.12.8 ng/mL). Of the 463 prostate cancers diagnosed in the Göteborg cohort, 392 were detected through screening (85%). There were 15 men with documented evidence of metastasis during 17 years follow up (cumulative incidence 0.5%) in Göteborg as compared with 42 (cumulative incidence 1.2%) in Malmö, and 7 prostate cancer deaths (cumulative incidence 0.2%) in Göteborg as compared with 29 (cumulative incidence 0.8%) in Malmö (Table 2).
Table 1. Characteristics of participants with a diagnosis of prostate cancer within 17 years of follow-up from blood draw by cohort.
Estimates were given as median (interquartile range) or frequency (percentage).
| Characteristics | Malmö unscreened men (N=225) |
Göteborg men randomized to screening (N=463) |
p-value* |
|---|---|---|---|
| Age at Diagnosis | 67 (64, 69) | 63 (60, 66) | <0.0001 |
| Total PSA Near Diagnosis (ng/mL) (N=639) |
12.8 (7.6, 25.1) | 4.4 (3.5, 6.3) | <0.0001 |
| Unknown | 1 (0.2%) | 48 (21%) | |
| T stage | |||
| T1 unspecified | 16 (7.1%) | 0 (0%) | <0.0001 |
| T1A | 9 (4.0%) | 7 (1.5%) | |
| T1B | 8 (3.6%) | 1 (0.2%) | |
| T1C | 26 (12%) | 350 (76%) | |
| T2 | 74 (33%) | 82 (18%) | |
| T3 | 65 (29%) | 18 (3.9%) | |
| T4 | 11 (4.9%) | 1 (0.2%) | |
| TX | 16 (7.1%) | 4 (0.9%) | |
| Lymph Node Metastases at Diagnosis |
1 (0.4%) | 2 (0.4%) | 1 |
| Distant Metastases at Diagnosis | 18 (8.0%) | 6 (1.3%) | <0.0001 |
| Gleason Biopsy Score | |||
| GS 2-6 | 359 (78%) | ||
| GS 7 | 81 (17%) | ||
| GS 8-10 | 19 (4.1%) | ||
| Unknown | 4 (0.9%) | ||
| WHO Biopsy Grade | |||
| 1 | 79 (35%) | ||
| 2 | 85 (38%) | ||
| 3 | 34 (15%) | ||
| Unknown | 27 (12%) |
p-value calculated using Wilcoxon Rank-Sum test for continuous and Fisher’s exact test for categorical variables
Table 2.
Cumulative incidence during the 17-year follow-up period by group.
| Outcome | Malmö (unscreened) (95% CI) |
Göteborg (screened) (95% CI) |
|---|---|---|
| Prostate Cancer Diagnosis | 6.3% (5.5%, 7.1%) | 15.0% (13.8%, 16.3%) |
| Prostate Cancer Metastasis | 1.2% (0.9%, 1.6%) | 0.5% (0.3%, 0.9%) |
| Prostate Cancer Death | 0.8% (0.6%, 1.2%) | 0.2% (0.1%, 0.5%) |
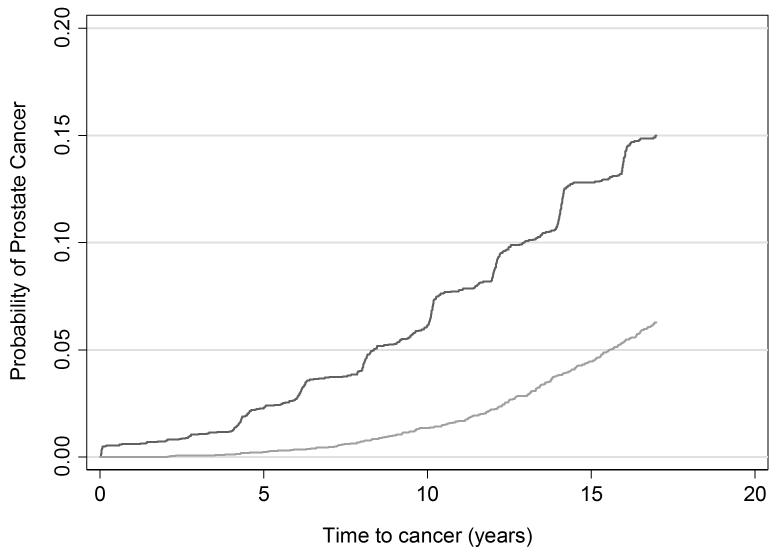
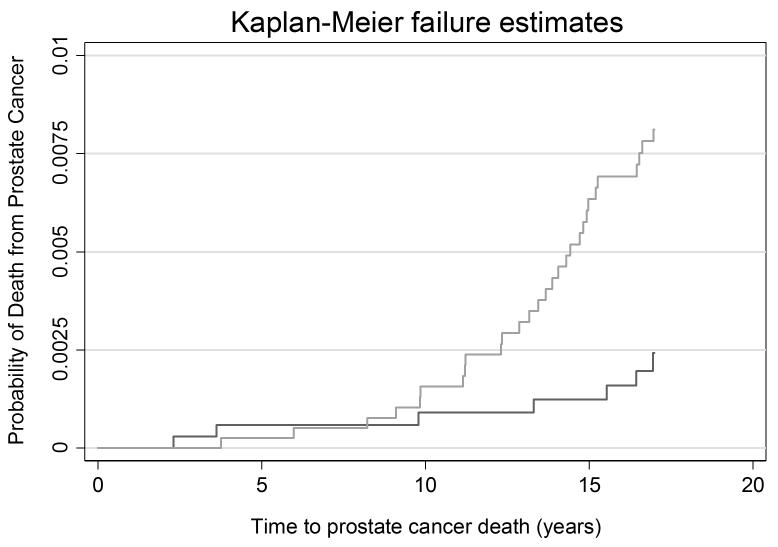
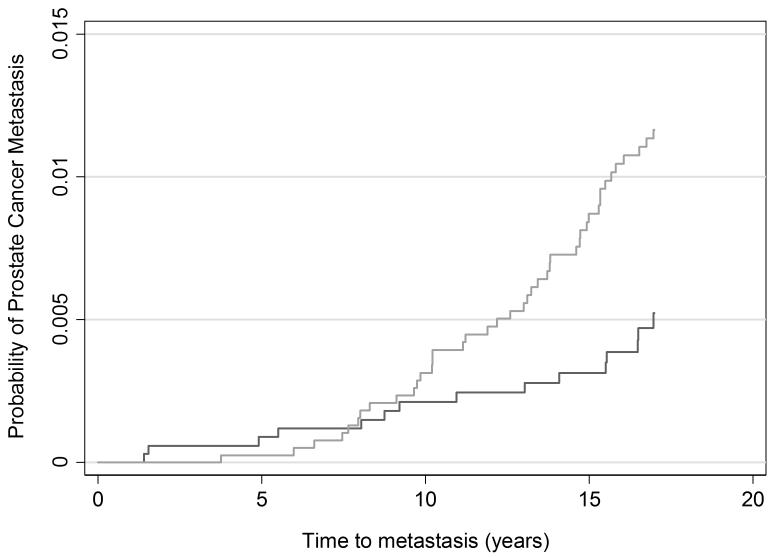
Table 3 displays the 17-year outcome rates per 1,000 person years. Cumulative incidence plots are given in figures 1-3. The rate ratios for incident diagnosis, metastases, and death from prostate cancer were 2.56 (95% CI 2.18, 3.02), 0.43 (95% CI 0.22, 0.79), and 0.29 (95% CI 0.11, 0.67), respectively, at 17 years among screened men compared with the unscreened men based on an intent-to-screen analysis. The rate of prostate cancer death was 0.45 per 1,000 person years (95% CI 0.32, 0.65) among men in Malmö and 0.13 per 1,000 person years (95% CI 0.06, 0.27) among men randomized to screening in Göteborg. Thus, biennial prostate cancer screening starting at age 50–54 carried a substantial risk of being diagnosed with prostate cancer that was more than 2-fold higher in the screened group compared to the unscreened group. Yet, there was a 71% relative reduction in the incidence of prostate cancer death among men invited to screening in Göteborg as compared to Malmö.
Table 3. Incidence rates, rate differences, and rate ratios for 17-year follow-up per 1,000 person years.
Incidence rate difference (IRD) was defined as the incidence in Malmö subtracted from the incidence in Göteborg. Incidence rate ratio (IRR) was defined as the rate in Göteborg over the rate in Malmö.
| Outcome | Incidence rate (95% CI) | IRD (95% CI) | IRR (95% CI) | |
|---|---|---|---|---|
| Malmö (unscreened) |
Göteborg (screened) |
|||
| Prostate Cancer Diagnosis | 3.57 (3.13, 4.07) | 9.14 (8.35, 10.01) | 5.58 (4.62, 6.53) | 2.56 (2.18, 3.02) |
| Prostate Cancer Metastasis | 0.66 (0.49, 0.89) | 0.28 (0.17, 0.47) | −0.38 (−0.62, −0.13) | 0.43 (0.22, 0.79) |
| Prostate Cancer Death | 0.45 (0.32, 0.65) | 0.13 (0.06, 0.27) | −0.32 (−0.51, −0.13) | 0.29 (0.11, 0.67) |
| Overall Mortality | 11.34 (10.54, 12.20) | 11.21 (10.35, 12.15) | −0.13 (−1.35, 1.09) | 0.99 (0.89, 1.10) |
Figure 1. Cumulative risk of prostate cancer diagnosis.
Light grey line: Unscreened men in Malmö
Dark grey line: Men screened in Göteborg
Figure 3. Cumulative risk of prostate cancer death.
Light grey line: Unscreened men in Malmö
Dark grey line: Men screened in Göteborg
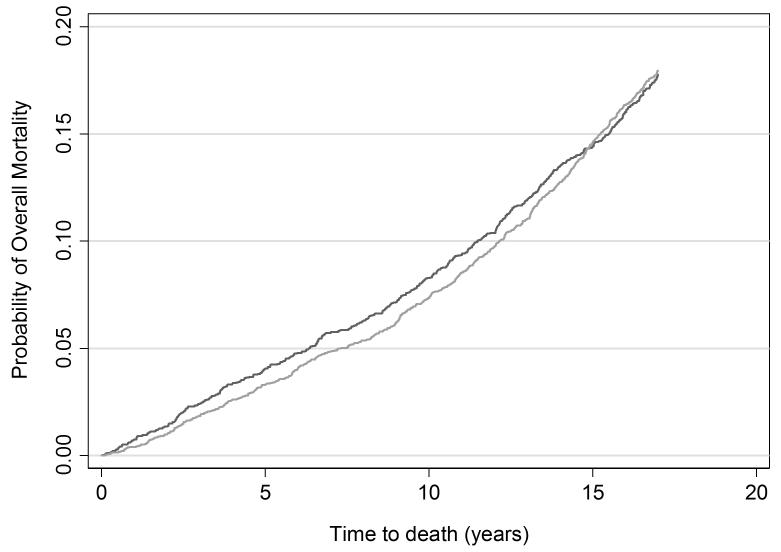
As expected, there was no significant difference in overall mortality between cohorts with an incidence rate difference of −0.1 per 1,000 person years (95% CI −1.3, 1.1) (Table 3 and Figure 4). This suggests that comparisons of prostate cancer mortality are not affected by the competing risk of other cause mortality. With respect to nationwide trends in prostate cancer mortality, between 1987-2012, Swedish age-standardized mortality from prostate cancer remained relatively stable around 60-70 deaths per 100,000 men, with a modest decline seen only in the past few years [18]. It seems implausible that any improvements in the effectiveness of prostate cancer treatment during the study period could explain the large difference in prostate cancer mortality between Göteborg and Malmö.
Figure 4. Cumulative risk of overall mortality.
Light grey line: Unscreened men in Malmö
Dark grey line: Men screened in Göteborg
Table 4 shows the effects of screening on incidence, metastasis, and prostate cancer mortality. The increase in diagnosis incidence associated with screening was 872 (95% CI 723, 1022) per 10,000 men. However, there was a decrease in the risk of death from prostate cancer of 57 (95% CI 22, 92) per 10,000 men at 17 years. Invitation to attend screening starting at age 50-54 significantly reduced prostate cancer mortality with a number needed to be invited to screening of 176 and a number needed to diagnose of 16 to prevent one death from prostate cancer at 17 years.
Table 4.
Difference in risk between Göteborg (screened) and Malmö (unscreened) cohorts per 10,000 men based on 17-years of follow-up.
| Outcome | Risk difference/10,000 men (95% CI) |
|---|---|
| Increase in Prostate Cancer Diagnosis | 872 (723, 1022) |
| Decrease in Prostate Cancer Metastasis | 64 (20, 108) |
| Decrease in Prostate Cancer Death | 57 (22, 92) |
While primary analyses included all men randomized to screening in Göteborg, we also performed a sensitivity analysis restricted to men in the Göteborg cohort who attended at first screening between ages 50–54. The cumulative incidence of prostate cancer diagnosis was 18.3% for attendees in Göteborg, corresponding to a more than 3-fold higher risk of diagnosis as compared to unscreened men in Malmö (IRR 3.20, 95% CI 2.69, 3.80). Excluding non-attendees in Göteborg at age 50-54 revealed a more pronounced prostate cancer-specific mortality rate ratio of 0.21 (95% CI 0.04, 0.69), which is a 79% reduction in prostate cancer mortality that translates to an NNI of 159 and an NND of 20 in order to prevent one prostate cancer death at 17 years. A sensitivity analysis using competing risk methods did not drastically alter the estimates of NNI and NND, which were 222 and 18 for preventing one prostate cancer death. Similarly, the sensitivity analysis classifying men who died from prostate cancer between years 17-19 in Malmö as developing distant metastasis at year 17 did not materially alter our results.
DISCUSSION
In this non-randomized comparison between healthy asymptomatic men invited to the screening arm of a prospective randomized trial of PSA testing in 1995-97 versus a population-based cohort of men providing blood in 1982-85 to determine risk of hypertension, metabolic and cardiovascular factors and who were followed during a period when PSA testing was not widely available, we found that PSA screening at age 50–54 lead to a significant decrease in prostate cancer-specific mortality – 57/10,000 fewer prostate cancer deaths or an NNI of 176 at 17 years. This supports the hypothesis that screening younger men would allow a greater chance of detection before a lethal cancer becomes incurable and is in line with the findings of our previous work on baseline PSA and future risk of metastasis comparing ages 45–49 versus 51–55.[10] However, this benefit came at the expense of importantly increased risk of overdiagnosis – 872/10,000 more prostate cancer diagnoses or an NND of 16 at 17 years. Recalculating NNI and NND at 13 years in the present study gives an NNI of 435 and an NND of 32. Comparing these numbers with the ERSPC trial which screened men ages 55-69 years, the NNI at was considerably higher at 13 years, at 781 while NND was very similar, at 27.[19] We also expect that both NNI and NND will decrease with longer follow-up time as differences in mortality increase more rapidly over time than differences in incidence. We do, however, acknowledge that the approach of basing NND on the excess incidence (incidence rate difference) in the present study and that in the ERSPC as a whole, as well as using a shorter observation time, may include unknown levels of bias.
To mitigate the harms of overdiagnosis by screening, we acknowledge that diagnosis can successfully be uncoupled from immediate treatment through close monitoring of the tumor progression (active surveillance). Use of this regimen is high in the Göteborg trial (over a quarter).[7]. We currently see broader indications and more use of active surveillance across the globe[20-24]. For instance, in Sweden, 59% of men with very low risk and 41% of men with low risk disease choose this strategy.[22] Nonetheless, we acknowledge the possible drawback of earlier screening in extending the length of time that men live with disease. For example, a man destined to be diagnosed with a curable Gleason 7 tumor at age 55 would instead be diagnosed at 50, enduring an additional 5 years of cancer-treatment morbidity and resource implications. Whether starting screening at age 50 is superior to starting at age 55 could not be explicitly addressed in the present study. We also acknowledge that the benefit of screening in absolute terms is small, but finite, and that the perceptions of the value of early detection vis a vis risk of overdiagnosis and overtreatment may vary. Therefore, we propose that a discussion about pros and cons of starting screening at ages 50-54 takes place in the context of shared decision-making to help men make high-quality decisions.
Our study is not devoid of limitations. Our study compares two Swedish cohorts from different time periods; no screening between 1982-5 and 1999-2002 and screening between 1995 and 2012. This raises the obvious question of whether improvements in comparison to a historical cohort reflect changes in treatment patterns or even differential misattribution in cause of death in one situation with a higher disease prevalence due to screening and one with a low disease prevalence in its absence. However, we did not find important differences in prostate mortality rates in Sweden over time, certainly nothing that could explain such a large reduction in prostate cancer mortality between the screened vs. unscreened cohorts (a hazard ratio of 0.29). Given the high accuracy of Swedish death certificates – 96% agreement with the cause of death committee in the Göteborg trial – as well as no support of any change in cause of death determination in a study of men dying from prostate cancer before versus after the introduction of PSA, we find cause of death misattribution unlikely.[25, 26]
ERSPC Göteborg had better results on average than other ERSPC countries[19] and it might be questioned whether the results of ERSPC Göteborg are representative of ERSPC, or whether they can be applied to other settings. We would first note that the overall strategy in Göteborg –screening every two years, median starting age in the mid-fifties[7] – is closer to current US and European guidelines[2-5] than the ERSPC as a whole (median starting age in the early sixties, screening every four years)[19]. As we have argued previously [27], the Göteborg trial would underestimate the mortality benefits of screening due to sub-optimal methods of detection (e.g. sextant biopsy) and treatment (i.e. lower dose radiotherapy) available in the mid 1990’s. Given these considerations, we have no reason to believe that the net benefit of screening men in their early 50’s reported here would be much lower when applied to contemporary settings.
We acknowledge what we do not know whether these results about PSA screening based on the Göteborg trial apply to other screening settings or to other populations..
Conclusion
We found evidence that PSA screening for prostate cancer can decrease prostate cancer mortality among men aged 50–54 and the effect was comparable to that previously reported in the ERSPC for men aged 55–69. Guideline groups could consider whether guidelines for PSA screening should recommend starting no later than at ages 50-54.
Figure 2. Cumulative risk of prostate cancer metastasis.
Light grey line: Unscreened men in Malmö
Dark grey line: Men screened in Göteborg
Acknowledgments
Funding Sources: S.C. was supported by grants from AFA Insurance, the Swedish Cancer Society (Cancerfonden), the Sweden-America Foundation, the Swedish Council for Working Life and Social Research (FAS), the Swedish Society for Medical Research (SSMF) and the Swedish Prostate Cancer Foundation. D.U. was supported by the David H Koch Young Investigator Award through the Prostate Cancer Foundation, the Lowenstein Foundation, the Tegger Foundation, and the Bertha Kamprad Foundation. This work was further supported by: National Cancer Institute [grant No R33 CA 127768-03, R01CA160816, R01 CA175491, P50-CA92629], the NIH/NCI Cancer Center Support Grant P30 CA008748, the Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation, the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program in UK, Swedish Cancer Society project no. 14-0722, and Fundacion Federico SA.
Role of funders in the writing of the manuscript of decision to submit for publication:
None.
Footnotes
Contributors
AV and HL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. HL, AV and JH conceived and designed the study. JH, SC, DU, AG, and HL contributed to the data collection. MA and AV carried out the statistical analyses. SC, MA, and AV drafted the manuscript. All authors interpreted the data and participated in the critical revision of the manuscript for important intellectual content. All authors approved the final submitted version. HL, AV, JH, and SC obtained funding. AV, HL and JH provided administrative, technical, or material support. AV and HL supervised the study.
Declaration of interests
HL holds patents for free PSA, human kallikrein 2, and intact PSA assays, and is named, along with AV, on a patent application for a statistical method to detect prostate cancer. This has been commercialized by Opko Health and AV and HL receive royalties from any sales of the test. AV is on the advisory board of Opko Health and has Opko stock options. AV has a consulting or advisory role in Genome DX and Genomic Health. HL served on an advisory panel for Roche Diagnostics during 2014, and an immediate family member of HL is an employee at Ferring Pharmaceuticals. SC has received travel reimbursement from Sanofi-Aventis. No other author has any conflict of interest to declare. Role of the for-profit health care companies in the writing of the abstract: None.
Presentation at conference: This study was submitted as an abstract and presented as a moderated poster at the annual meeting of the American Urological Association (AUA) in New Orleans, May 18, 2015, Session: Prostate Cancer: Detection and Screening II, abstract #MP60-10.
Patient summary: Guideline recommendations about the age to start prostate-specific antigen (PSA) could be discussed.
Take home message: Guideline recommendations about the age to start prostate-specific antigen (PSA) screening could be discussed.
Twitter summary: Age to start #PSA test to be discussed
References (28 of max 30)
- 1.Moyer VA, U.S. Preventive Services Task Force Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Abrahamsson PA, Artibani W, et al. Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol. 2013;64:347–54. doi: 10.1016/j.eururo.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [Date accessed 2015-10-09];National Comprehensive Cancer Network prostate cancer screening guidelines. 2014 Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf.
- 5.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016 Feb 2; doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 7.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnsrud Godtman R, Holmberg E, Lilja H, Stranne J, Hugosson J. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Göteborg Randomized Population-based Prostate Cancer Screening Trial. Eur Urol. 2015;68:354–60. doi: 10.1016/j.eururo.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson H, Holmström B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2011;129:1881–8. doi: 10.1002/ijc.25846. [DOI] [PubMed] [Google Scholar]
- 10.Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ. 2013;346:f2023. doi: 10.1136/bmj.f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsson S, Assel M, Sjoberg D, Ulmert D, Hugosson J, Lilja H, Vickers A. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: population based cohort study. BMJ. 2014;348:g2296. doi: 10.1136/bmj.g2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stattin P, Carlsson, Holmström B, Vickers A, Hugosson J, Lilja H, Jonsson H. Prostate cancer mortality in areas with high and low prostate cancer incidence. J Natl Cancer Inst. 2014;106:dju007. doi: 10.1093/jnci/dju007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulmert D, Serio AM, O'Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835–41. doi: 10.1200/JCO.2007.13.1490. [DOI] [PubMed] [Google Scholar]
- 15.Ulmert D, Becker C, Nilsson JA, et al. Reproducibility and accuracy of measurements of free and total prostate-specific antigen in serum vs plasma after long-term storage at −20 degrees C. Clin Chem. 2006;52:235–9. doi: 10.1373/clinchem.2005.050641. [DOI] [PubMed] [Google Scholar]
- 16.De Koning HJ, Blom J, Merkelbach JW, et al. Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int. 2003;92(suppl 2):71–78. doi: 10.1111/j.1465-5101.2003.04402.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiss NS, Rossing MA. Healthy screenee bias in epidemiologic studies of cancer incidence. Epidemiology. 1996;7:319–22. [PubMed] [Google Scholar]
- 18.The National Board of Health and Welfare, Sweden [Date accessed 2015-10-09];Causes of death 2012. Available at: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19175/2013-8-6.pdf.
- 19.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21. [Date accessed 2015-10-09];National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology – Prostate Cancer. 2015 Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 22.Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–9. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC, Michigan urological surgery improvement collaborative Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 25.Godtman R, Holmberg E, Stranne J, Hugosson J. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol. 2011;45:226–32. doi: 10.3109/00365599.2011.559950. [DOI] [PubMed] [Google Scholar]
- 26.Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–23. [PubMed] [Google Scholar]
- 27.Vickers AJ, Lilja H. Prostate cancer: estimating the benefits of PSA screening. Nat Rev Urol. 2009;6:301–3. doi: 10.1038/nrurol.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]