Abstract
Introduction
Mice with a complete absence of tissue factor (TF) die during embryonic development whereas mice with low levels of TF (Low-TF mice) survive to adulthood. Low-TF mice exhibit spontaneous hemorrhage in various organs, including the lung. In contrast, mice can survive without protease-activated receptor (PAR)-4, which is the major thrombin receptor on mouse platelets. We determined the effect of combining a deficiency PAR-4 (primary hemostasis) with a deficiency in TF (secondary hemostasis) on embryonic development and survival of adult mice.
Materials and Methods
Low-TF mice (mTF−/−, hTF+/+) were crossed with PAR-4−/− mice to generate heterozygous mice (mTF+/−, hTF+/−, PAR-4+/−). These mice were intercrossed to generate Low-TF mice lacking PAR-4. Mice surviving to wean were genotyped and survival was monitored for 6 months.
Results
We observed the expected number of Low-TF,PAR-4−/− mice at wean indicating survival in utero and after birth. However, an absence of PAR-4 was associated with premature death of all Low-TF,PAR-4−/− mice in the 6 month observational period. This compares with 40% death of the Low-TF,PAR-4+/+ mice (p=0.003). Low-TF,PAR-4+/− mice had an intermediate phenotype with 55% of the mice dying within 6 months. The primary cause of mortality of Low-TF,PAR-4−/− mice was pulmonary hemorrhage.
Conclusions
Low-TF,PAR-4−/− mice survive into adulthood, but combining a deficiency of primary hemostasis (PAR-4 deficiency) with secondary hemostasis (low levels of TF) leads to premature death primarily due to pulmonary hemorrhage.
Keywords: Hemostasis, platelet, transgenic mice, pulmonary hemorrhage
INTRODUCTION
Tissue factor (TF) is the major physiological activator of coagulation. After vessel injury, thrombin generated by the coagulation cascade cleaves fibrinogen to fibrin that stabilizes the platelet clot. Previous studies have shown that embryos with a complete deficiency of TF die at embryonic day ~E9.5 due to a defect in yolk sac vascular development1–3. Similarly, a complete absence of other components of the clotting cascade, namely FVII, FV, FX, and prothrombin, leads to death due to either mid-gestational yolk sac vascular defects in embryos or hemorrhage in neonates after birth4–8. A deficiency of fibrinogen is not associated with any mid-gestational death, although there is a variable degree of death (10–50%) due to abdominal bleeds after birth depending on genetic background9,10.
In mice, thrombin also activates platelets via protease-activated receptor (PAR)-411. PAR-4 deficient mice survive to adulthood but have increased bleeding after tail transection11. However, a combined deficiency of both fibrinogen and PAR-4 results in fatal neonatal bleeding similar to the absence of prothrombin12. Similar results were observed when a deficiency of fibrinogen was combined with thrombocytopenia caused by an absence of the transcription factor NF-E213. These results indicate that thrombin has two major functions: cleavage of fibrinogen to fibrin and activation of platelets12–14.
Various mice have been generated with reduced levels of procoagulant proteins. For instance, Low-TF mice express low levels (~1% of wild-type levels) of human TF from a minigene on a mouse TF null background15. The majority of Low-TF mice (~80%) survive to wean but exhibit spontaneous bleeding in the lung, heart, testis and brain later in life, and also have hemostatic defects in the placenta and uterus15–19. Mice have been generated with 5–10% of wild-type prothrombin and live to adulthood without excess bleeding except after tail transection or in traumatic situations, such as fighting20. A transgene expressing FV from an albumin promoter at less than 0.1% of wild-type FV activity was able to support the survival of adult mice but was unable to rescue FV null embryos21. In addition to the generation of mice with defects in hemostasis, other mice have been generated that are prothrombotic, such as mice lacking the anticoagulant tissue factor pathway inhibitor (TFPI), as well as mice with mutations in FV (FV Leiden) and thrombomodulin (TMpro)22–24.
Mice with pro-hemorrhagic or pro-coagulant phenotypes have been intercrossed to either rebalance thrombin generation or exacerbate a mild phenotype8,25. For example, combining FV Leiden mice with TFPI+/− mice is lethal26. A deficiency of TFPI leads to intrauterine death due to a consumptive coagulopathy22; however, the embryonic lethality of TFPI deficient mice can be rescued by reducing levels of TF using Low-TF mice27. The chronic lung bleeding and fatal pulmonary hemorrhages that are observed in adult Low-TF mice were also markedly reduced when TFPI was absent. Similarly, other mutations that lead to intrauterine death, such as endothelial cell protein C receptor, thrombomodulin, and antithrombin deficiency can be rebalanced by decreasing levels of TF28,29. Recently, it was shown that a deficiency in PAR-4 rescued ~40% of TFPI null embryos30. These mice lived to adulthood but were more prothrombotic with fibrin deposition in the liver and higher susceptibility to TF-induced pulmonary embolism.
In this study we analyzed the effect of combining a defect in primary hemostasis (PAR-4 deficiency) with a defect in secondary hemostasis (low levels of TF) on development during embryogenesis and survival in adulthood.
MATERIAL AND METHODS
Mice
All studies were performed in accordance with the guidelines of the animal care and use committees of UNC-Chapel Hill and comply with National Institutes of Health guidelines. Low-TF mice (mTF−/−,hTF+/+) were generated as previously described 15 and were backcrossed 6 generation to a C57BL/6J genetic background. We observed ~80% of the expected number of Low-TF mice at wean on this genetic background27. Genotyping for the wild-type mTF allele and the hTF transgene was performed by polymerase chain reaction (PCR) as described previously15. The mutant TF allele was identified using a primer in the promoter (mTF-1097) and a primer in PGK-NEO. We use the notation +/− to describe the status of the mTF alleles in Low-TF mice. All Low-TF mice have at least one copy of the hTF transgene. PAR4−/− breeding pairs on a C57BL/6J background were a gift from Dr. Shaun Coughlin, University of California, San Francisco11. Low-TF mice (mTF+/−,hTF+/+) mice were crossed with PAR4+/− mice and mice with the genotype mTF+/−,hTF+/−,PAR-4+/− were intercrossed.
Postmortem analysis of mice
All dead mice that were not decomposed were analyzed for bleeding. Fatal bleeding was mostly observed in the lungs or brain. Lungs were fixed and sectioned for histological analysis.
Data Analysis
Statistical analysis of the distribution of genotypes was analyzed by Chi-square test assuming the null hypothesis. Log-rank analysis was used to determine statistical significance of the survival of the different mice. Differences were determined to be statistically significant at a P value of <0.05.
RESULTS
Generation of Low-TF,PAR-4−/− mice
Previously, we have observed ~80% survival of Low-TF mice at wean on this C57BL/6J genetic background (backcrossed 7 generations)27. We examined the frequency of Low-TF,PAR-4−/− mice in two different breeding strategies. First, we intercrossed mTF+/−,hTF+/−,PAR-4+/− mice. We generated 69 mice and found that at wean the number of Low-TF,PAR-4−/− mice did not differ significantly from the expected number (p = 0.90) (Table 1). As expected from our earlier study27, all three Low-TF groups had slightly lower numbers than expected due to ~20% embryonic lethality. In a second breeding we crossed mTF−/−,hTF+/−, PAR-4+/− mice with mTF+/−,hTF+/−,PAR-4+/− mice. We generated 39 mice in this breeding. As expected from the first breeding, the number of Low-TF,PAR-4−/− mice observed at wean did not differ from the expected number (p=0.62) (Table 1). These results indicate that an absence of PAR-4 does not affect the survival of Low-TF mice during embryonic development and early adulthood.
Table 1.
Expected and observed numbers of mice surviving to wean. Low-TF status is associated with a ~20% lethality on this C57BL/6J background27, which is also observed in our study when comparing the observed/expected survival ratio of all mTF−/− mice to all mTF+/+ mice. Chi-square test did not show any additional effect of PAR-4 status on survival in any of the groups. hTF+ indicates that at least one allele of human TF is present (hTF+/− or hTF+/+).
| Breeding pair | Genotype | Expected % | Observed % | Observed # | Obs/E % | ||
|---|---|---|---|---|---|---|---|
| mTF | hTF | PAR-4 | |||||
| mTF+/−,hTF+/−, PAR-4+/− X mTF+/−,hTF+/−, PAR-4+/− (breeding 1) | −/− | + | −/− | 6 | 4 | 3 | 70 |
|
| |||||||
| −/− | + | +/− | 13 | 12 | 8 | 93 | |
|
| |||||||
| −/− | + | +/+ | 6 | 3 | 2 | 46 | |
|
| |||||||
| +/− | + | −/− | 13 | 12 | 8 | 93 | |
|
| |||||||
| +/− | + | +/− | 25 | 38 | 26 | 151 | |
|
| |||||||
| +/− | + | +/+ | 13 | 9 | 6 | 70 | |
|
| |||||||
| +/+ | + | −/− | 6 | 4 | 3 | 70 | |
|
| |||||||
| +/+ | + | +/− | 13 | 13 | 9 | 104 | |
|
| |||||||
| +/+ | + | +/+ | 6 | 6 | 4 | 93 | |
|
| |||||||
| mTF−/−,hTF+/−, PAR-4+/− X mTF+/−,hTF+/−, PAR-4+/− (breeding 2) | −/− | + | −/− | 13 | 10 | 4 | 82 |
|
| |||||||
| −/− | + | +/− | 25 | 28 | 11 | 113 | |
|
| |||||||
| −/− | + | +/+ | 13 | 3 | 1 | 21 | |
|
| |||||||
| +/− | + | −/− | 13 | 21 | 8 | 164 | |
|
| |||||||
| +/− | + | +/− | 25 | 26 | 10 | 103 | |
|
| |||||||
| +/− | + | +/+ | 13 | 13 | 5 | 103 | |
Survival of Low-TF mice with different levels of PAR-4
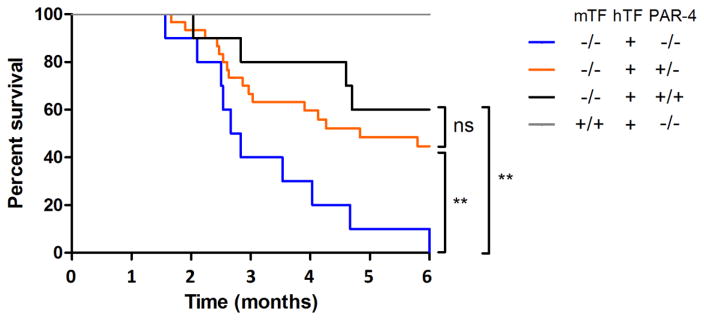
In a previous study27 we observed a relatively high rate of mortality of Low-TF mice on the same C57BL/6J background as used in this study (backcrossed 7 generations) within 6 months. We monitored mice surviving to wean over a 6-month period to determine if reducing levels of PAR-4 affect the survival of Low-TF mice. We used mTF+/+,hTF+/−,PAR-4−/− mice as controls because these mice exhibit normal survival11. We observed that 100% of the Low-TF,PAR-4−/− mice died during the observation period of 6 months (Figure 1). The median survival time of these mice was 83 days. In contrast, only 40% of the Low-TF,PAR-4+/+ mice died during the observational period (p=0.003), and these mice had a median survival time of >180 days. Low-TF−/−,PAR-4+/− mice had an intermediate phenotype with 55% of the mice dying within 6 months and a median survival time of 145 days. All experimental groups had a significantly reduced survival compared to control PAR-4 deficient mice owing to the hemostatic defect caused by Low-TF. These results indicate that PAR-4 is required to maintain hemostasis in Low-TF mice.
Figure 1. Survival of Low-TF mice with different levels of PAR-4.
The survival of all experimental groups is significantly reduced compared to the control group (TF+/+,PAR-4+/+; n=7). Low-TF,PAR-4−/− (n=10) show significantly lower survival compared to Low-TF,PAR-4+/+ (n=10) and Low-TF,PAR-4+/− (n=30) mice (** p<0.01). mTF: mouse tissue factor. hTF+ indicates that at least one allele of human TF is present (hTF+/− or hTF+/+).
Pulmonary hemorrhage is increased in mice with low levels of tissue factor and PAR-4 deficiency
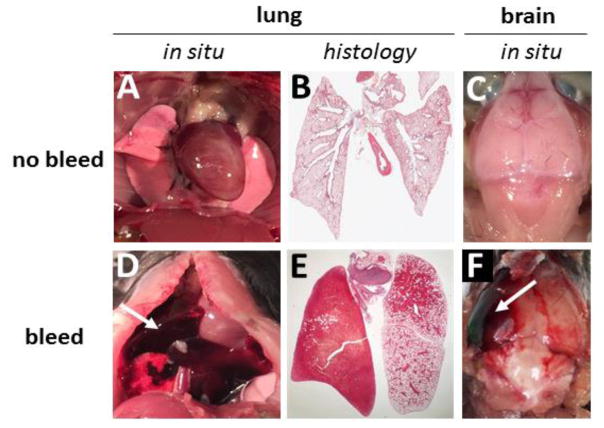
In a previous study using Low-TF mice on the same C57BL/6J background we observed a high rate of death due to pulmonary hemorrhage within a 6 month period27. In the current study we two Low-TF,PAR-4+/+ mice died of pulmonary hemorrhage and one died of an intracranial hemorrhage (Table 2). All Low-TF, PAR-4−/− mice that were analyzed (n=8) died of pulmonary hemorrhage (Table 2) (Figure 2). Similarly, the majority of Low-TF,PAR-1+/− mice that were analyzed (n=14) died from pulmonary hemorrhage (Table 2). Two testicular bleeds (1 Low-TF,PAR-4−/−, 1 Low-TF,PAR-4+/−) and one gastrointestinal hemorrhage (Low-TF,PAR-4+/−) were observed in mice that had died from pulmonary hemorrhage. These results indicate that combining defects in primary and secondary hemostasis leads to fatal pulmonary hemorrhage in mice.
Table 2.
Cause of death. Cause of death during the 6-month observation period was analyzed by necropsy. hTF+ indicates that at least one allele of human TF is present (hTF+/− or hTF+/+).
| mTF−/−, hTF+, PAR-4−/− | mTF−/−, hTF+, PAR-4+/− | mTF−/−, hTF+, PAR-4+/+ | mTF+/+, hTF+, PAR-4−/− | |
|---|---|---|---|---|
|
| ||||
|
pulmonary hemorrhage intracranial hemorrhage not analyzed |
8 | 13 | 2 | 0 |
|
| ||||
| 0 | 1 | 1 | 0 | |
|
| ||||
| 2 | 5 | 0 | 0 | |
Figure 2. Lung and brain bleeds in Low-TF mice lacking PAR-4.
Gross pictures of lungs and brains and histology pictures of the lungs of control mice (A–C), Low-TF,PAR-4−/− mice (D–E), and a Low-TF,PAR-4+/− mouse (F).
DISCUSSION
Our results show that a deficiency of PAR-4 does not affect the survival of Low-TF mice during embryonic development or early adulthood. Previous studies showed that combining fibrinogen deficiency with either a PAR-4 deficiency or thrombocytopenia led to death of all mice shortly after birth12,13. The key difference of our study is that due to low levels of TF small amounts of thrombin are still present, which we propose prevents embryonic lethality and developmental defects seen in other studies.
Our results demonstrate that PAR-4 deficiency led to a dose-dependent increase in mortality when combined with low levels of TF. While only 40% of the Low-TF mice with wild-type PAR-4 died during the 6-month observation, all of the Low-TF mice with PAR-4 deficiency died. No mice died during the first one and a half months. This observation may be explained by accumulation of small bleeds that have not reached a critical threshold this early in life to cause death. The cause of death was mainly pulmonary hemorrhage. This result indicates that in the setting of reduced coagulation with minimal TF the activation of PAR-4 is important for hemostasis, particularly in the lung. Our results suggest that other primary activators of platelets, such as collagen, do not substitute for the lack of activation by thrombin through PAR-4.
Although an absence of PAR-4 on platelets is the most likely explanation for our observations, it is possible that PAR-4 on other cell types may also contribute to maintain hemostasis. Recently, we examined the importance of platelets in maintaining hemostasis in Low-TF mice31. We found that Low-TF mice treated with clopidogrel showed significantly more bleeding due to instability of the hemostatic plug compared to wild-type controls that were treated with clopidogrel31.
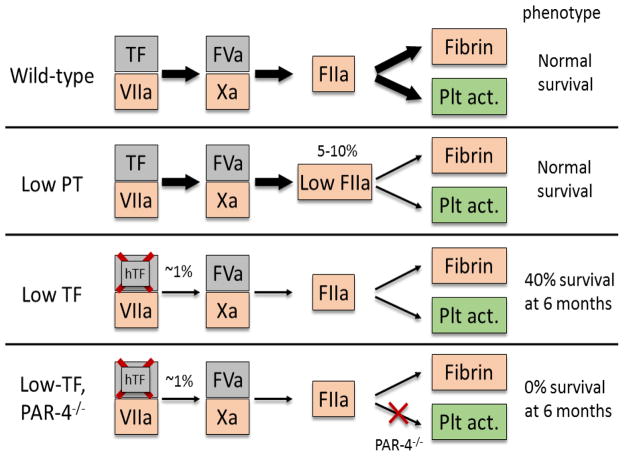
Compared to mice expressing low levels of prothrombin that survived to adulthood without major spontaneous bleeds, the phenotype of Low-TF,PAR-4−/− mice studied here is more severe due to both a lower level of thrombin activity and an absence of thrombin activation of platelets (Figure 3). Low prothrombin mice have 5–10% of wild-type thrombin levels and are therefore able to activate platelets through PAR-4 in the event of a bleed20. This is not possible in the Low-TF,PAR4−/− mice studied here due to a lack of PAR-4.
Figure 3. Summary of the effect of decreasing levels of tissue factor, prothrombin or PAR-4 in mice.
Genetic defects in tissue factor (TF), prothrombin(PT) and/or PAR-4 are shown with the effect on fibrin formation and platelet activation (Plt act.).
The coagulation system is regulated in a tissue-specific manner32. Thrombomodulin, for example, is an important anticoagulant in the lung, and heart, but not in the liver32. The fibrinolytic pathway is important in all three organs, while none of these mechanisms are essential in the brain32. A possible explanation for the high rate of pulmonary hemorrhage in the current study may be vessel damage induced by continuous mechanical stress in conjunction with a limited capacity to contain bleeds into the empty spaces of the alveoli once the hemostatic barrier is compromised. Mechanical stress is also experienced by the heart, which leads to small bleeds into the myocardium that eventually cause cardiac fibrosis in Low-TF mice16. Indeed, Low-TF,PAR-4−/− mice exhibited similar bleeds (data not shown). However, bleeds in the myocardium are likely to be contained by the tight organization of the cardiac myocytes. Intracranial hemorrhages were observed in only two mice. A previous study examined the effect of genetically eliminating prothrombin in adult mice and found that these mice died within 7 days of either cardiac or intracranial hemorrhage but did not show any signs of pulmonary hemorrhage33. This discrepancy may be because high levels of TF are expressed in the heart and brain to provide additional hemostatic protection34 whereas the current study uses mice with low levels of TF.
In summary, our results indicate that combining genetic defects in primary and secondary hemostasis results in fatal hemorrhage in adult mice. Interestingly, both antiplatelet and anticoagulant drugs are used in some patients, such as those with both cardiovascular disease and atrial fibrillation. In the ATLAS ACS-TIMI 51 trial addition of the FXa inhibitor rivaroxaban to antiplatelet drugs led to a dose-dependent increase in major bleeding but not fatal bleeding35. Our study suggests that combing antiplatelet and anticoagulant drugs may increase susceptibility to pulmonary hemorrhage. Understanding organ-specific hemostasis may allow us to predict or prevent bleeding events in patients taking multiple anti-thrombotic drugs.
Highlights.
Mice with a deficiency of TF and PAR-4 survive to wean
Combining low levels of TF with a deficiency of PAR-4 leads to premature death
The primary cause of death of Low-TF,PAR-4−/− mice is pulmonary hemorrhage
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank Ying Zhang for technical support and Dr. Silvio Antoniak, Dr. Saravanan Subramaniam, Wyeth Alexander, and Dr. Weeranun Bode for helpful comments.
Footnotes
Competing Interests Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael F Bode, Email: michael_bode@med.unc.edu.
Nigel Mackman, Email: nigel_mackman@med.unc.edu.
References
- 1.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Müller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383(6595):73–5. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 2.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmbäck K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93(13):6258–63. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88(5):1583–7. [PubMed] [Google Scholar]
- 4.Cui J, O’Shea KS, Purkayastha A, Saunders TL, Ginsburg D. Fatal haemorrhage and incomplete block to embryogenesis in mice lacking coagulation factor V. Nature. 1996;384(6604):66–8. doi: 10.1038/384066a0. [DOI] [PubMed] [Google Scholar]
- 5.Dewerchin M, Liang Z, Moons L, Carmeliet P, Castellino FJ, Collen D, Rosen ED. Blood coagulation factor X deficiency causes partial embryonic lethality and fatal neonatal bleeding in mice. Thromb Haemost. 2000;83(2):185–90. [PubMed] [Google Scholar]
- 6.Sun WY, Witte DP, Degen JL, Colbert MC, Burkart MC, Holmbäck K, Xiao Q, Bugge TH, Degen SJ. Prothrombin deficiency results in embryonic and neonatal lethality in mice. Proc Natl Acad Sci U S A. 1998;95(13):7597–602. doi: 10.1073/pnas.95.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue J, Wu Q, Westfield La, Tuley Ea, Lu D, Zhang Q, Shim K, Zheng X, Sadler JE. Incomplete embryonic lethality and fatal neonatal hemorrhage caused by prothrombin deficiency in mice. Proc Natl Acad Sci U S A. 1998;95(13):7603–7. doi: 10.1073/pnas.95.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan KA, Weiler H, Lord ST. Mouse models in coagulation. Thromb Haemost. 2002;87(4):563–74. [PubMed] [Google Scholar]
- 9.Suh TT, Holmbäck K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9(16):2020–33. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 10.Iwaki T, Sandoval-Cooper MJ, Paiva M, Kobayashi T, Ploplis VA, Castellino FJ. Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse. Am J Pathol. 2002;160(3):1021–34. doi: 10.1016/S0002-9440(10)64923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413(6851):74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 12.Camerer E, Duong DN, Hamilton JR, Coughlin SR. Combined deficiency of protease-activated receptor-4 and fibrinogen recapitulates the hemostatic defect but not the embryonic lethality of prothrombin deficiency. Blood. 2004;103(1):152–4. doi: 10.1182/blood-2003-08-2707. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo JS, Zogg M, Talmage KE, Degen JL, Weiler H, Isermann BH. Role of fibrinogen- and platelet-mediated hemostasis in mouse embryogenesis and reproduction. J Thromb Haemost. 2004;2(8):1368–79. doi: 10.1111/j.1538-7836.2004.00788.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton JR, Cornelissen I, Coughlin SR. Impaired hemostasis and protection against thrombosis in protease-activated receptor 4-deficient mice is due to lack of thrombin signaling in platelets. J Thromb Haemost. 2004;2(8):1429–35. doi: 10.1111/j.1538-7836.2004.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101(3):560–9. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlinski R, Fernandes A, Kehrle B, Pedersen B, Parry G, Erlich J, Pyo R, Gutstein D, Zhang J, Castellino F, Melis E, Carmeliet P, Baretton G, Luther T, Taubman M, Rosen E, Mackman N. Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction. Proc Natl Acad Sci U S A. 2002;99(24):15333–8. doi: 10.1073/pnas.242501899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlich J, Parry GC, Fearns C, Muller M, Carmeliet P, Luther T, Mackman N. Tissue factor is required for uterine hemostasis and maintenance of the placental labyrinth during gestation. Proc Natl Acad Sci U S A. 1999;96(14):8138–43. doi: 10.1073/pnas.96.14.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlinski R, Pedersen B, Erlich J, Mackman N. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb Haemost. 2004:444–450. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- 19.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5(8):1693–700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun WY, Coleman MJ, Witte DP, Degen SJF. Rescue of prothrombin-deficiency by transgene expression in mice. Thromb Haemost. 2002;88(6):984–91. [PubMed] [Google Scholar]
- 21.Yang TL, Cui J, Taylor JM, Yang A, Gruber SB, Ginsburg D. Rescue of fatal neonatal hemorrhage in factor V deficient mice by low level transgene expression. Thromb Haemost. 2000;83(1):70–7. [PubMed] [Google Scholar]
- 22.Huang ZF, Higuchi D, Lasky N, Broze GJ. Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90(3):944–51. [PubMed] [Google Scholar]
- 23.Cui J, Eitzman DT, Westrick RJ, Christie PD, Xu ZJ, Yang AY, Purkayastha AA, Yang TL, Metz AL, Gallagher KP, Tyson JA, Rosenberg RD, Ginsburg D. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96(13):4222–6. [PubMed] [Google Scholar]
- 24.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101(9):1983–91. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25(11):2273–81. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 26.Eitzman DT, Westrick RJ, Bi X, Manning SL, Wilkinson JE, Broze GJ, Ginsburg D. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor V Leiden. Circulation. 2002;105(18):2139–42. doi: 10.1161/01.cir.0000017361.39256.82. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen B, Holscher T, Sato Y, Pawlinski R, Mackman N. A balance between tissue factor and tissue factor pathway inhibitor is required for embryonic development and hemostasis in adult mice. Blood. 2005;105(7):2777–82. doi: 10.1182/blood-2004-09-3724. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Zheng X, Gu J-M, Ferrell GL, Brady M, Esmon NL, Esmon CT. Extraembryonic expression of EPCR is essential for embryonic viability. Blood. 2005;106(8):2716–22. doi: 10.1182/blood-2005-01-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, Mackman N, Weiler H. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9(3):331–7. doi: 10.1038/nm825. [DOI] [PubMed] [Google Scholar]
- 30.Ellery PER, Maroney SA, Cooley BC, Luyendyk JP, Zogg M, Weiler H, Mast AE. A balance between TFPI and thrombin-mediated platelet activation is required for murine embryonic development. Blood. 2015;125(26):4078–4085. doi: 10.1182/blood-2015-03-633958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getz TM, Piatt R, Petrich BG, Monroe D, Mackman N, Bergmeier W. Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition. J Thromb Haemost. 2015;13(3):417–25. doi: 10.1111/jth.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg RD, Aird WC. Vascular-bed--specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340(20):1555–64. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- 33.Mullins ES, Kombrinck KW, Talmage KE, Shaw Ma, Witte DP, Ullman JM, Degen SJ, Sun W, Flick MJ, Degen JL. Genetic elimination of prothrombin in adult mice is not compatible with survival and results in spontaneous hemorrhagic events in both heart and brain. Blood. 2009;113(3):696–704. doi: 10.1182/blood-2008-07-169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackman N. Bleeding hearts. Blood. 2009;113(3):500–1. doi: 10.1182/blood-2008-11-188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]