Abstract
Purpose
To examine antigen-stimulated cytokine production by Behçet disease patients (BD) before and after infliximab infusion.
Methods
PBMCs were obtained before and after infliximab infusion in BD patients with or without recurrent uveitis during at least 1 year of infliximab therapy, and from healthy subjects. PBMCs were cultured with IRBP, and Th-related cytokines in cultures were measured.
Results
Levels of IL-4, IL-6, IL-10 IL-17A, IL-17F, IL-31, IFN-γ, and TNFα were higher in BD before infliximab infusion than in healthy subjects, and these levels were the highest in BD with recurrent uveitis. After infliximab infusion, these cytokine levels were reduced to a greater extent in BD without recurrent uveitis than in BD with recurrence.
Conclusions
Th-related cytokines produced by IRBP-stimulated PBMCs were elevated in BD, and infliximab infusion suppressed these cytokines to a greater extent in BD without recurrent uveitis than in those with recurrence.
Keywords: Behçet disease, infliximab, uveitis
Behçet disease (BD) is a chronic multisystem disease manifesting recurrent ocular, oral, cutaneous, genital, vascular, and neurologic lesions.1,2 Ocular involvement mostly manifests as bilateral panuveitis, and occurs in 60–80% of BD patients.3 Uveitis in BD is characterized by recurrent inflammatory attacks followed by spontaneous remissions. Repeated ocular attacks and progressive retinal vasculitis result in permanent ocular tissue damage, such as extensive retinal atrophy, maculopathy, and optic atrophy.
The etiology of BD remains unknown, but the most credited hypothesis suggests a complex interaction between genetic background and environmental factors. Among these factors, aberrant cellular immunity accelerated by both innate and adaptive immune responses,4–8 such as T-cell-mediated autoimmunity and Th1/Th2 imbalance, may be crucial in the pathogenesis of BD.9–12 In addition, recent studies have proposed that immune responses mediated by Th17 cells, which are characterized by the production of high levels of IL-17, IL-6, and TNF-α but a low level of IFN-γ,13 are upregulated in BD.14,15 In autoimmunity studies, immune responses mediated by retinal autoantigens such as S antigen (S-Ag) and interphotoreceptor retinoid binding protein (IRBP) are involved in the ocular inflammation in BD.16–18 Zhao et al.12 reported that peripheral blood mononuclear cells (PBMCs) from BD patients with active uveitis produced IFN-γ and TNF-α but not IL-2, IL-4, or IL-17 upon S-Ag stimulation. On the other hand, we previously found that PBMCs from BD patients and from healthy controls produced IL-6, IL-10, IL-17, IFN-γ, and TNF-α upon stimulation with IRBP or S-Ag, but IRBP-stimulated IL-6, IFN-γ, and IL-17 production was higher in BD patients than in healthy controls.19 In particular, IRBP-stimulated IFN-γ production was significantly higher in BD patients with active uveitis than in BD patients with remitted uveitis.
Improved understanding of the molecular mechanisms involved in BD has drastically changed the therapy. Prior to the availability of biologic agents, treatment options for severe BD were limited. Corticosteroids and/ or immunosuppressive agents are generally used for the treatment of BD-associated uveitis. Although these therapies occasionally improve the outcome of visual acuity (VA),20–23 they do not always achieve complete remission of ocular inflammation.24 In addition, these regimens are used as long-term treatment,25,26 often causing unacceptable adverse effects. TNF blockade is a new therapeutic approach for uveitis refractory to conventional immunosuppressive therapy. Infliximab is a chimeric IgG1 monoclonal antibody that binds soluble and membrane-bound TNF-α with high affinity, thereby blocking the binding of TNF-α to its receptor. Various studies have shown remarkably beneficial effects of infliximab in the treatment of refractory BD-associated uveitis.27–30 However, these favorable results were based on clinical evaluation. There are few studies exploring the modulation of cellular and molecular mechanisms by infliximab therapy in BD patients. In this study, we isolated PBMCs from BD patients with uveitis before and after infliximab infusion, and examined the quantitative changes of proinflammatory cytokines, and Th1-, Th2-, and Th17-related cytokines produced by these PBMCs when stimulated with retinal self-antigen. Furthermore, we compared cytokine production between BD patients with recurrent uveitis during infliximab treatment and those in whom recurrent uveitis was not observed after initiation of infliximab treatment.
PATIENTS AND METHODS
Patients
Eight BD patients who were treated with infliximab at the Uveitis Clinic of National Defense Medical College Hospital between January and December 2014 were enrolled in this study. BD was diagnosed according to the criteria of the International Study Group for BD.31 The profiles of individual BD patients are shown in Table 1. BD patients were classified into a group with recurrent uveitis (BD-recurrent uveitis group) and a group with remitted uveitis (BD-remitted uveitis group). The recurrence group consisted of BD1, BD3, BD4, and BD8, in which recurrence of uveitis was occasionally observed even after initiation of infliximab treatment. The remission group comprised BD2, BD5, BD6, and BD7, in which uveitis did not recur after initiation of infliximab treatment. Ten healthy subjects were enrolled as controls. The ages (mean ± SD) were 36.8 ± 11.6 years (range: 30–54) in the BD-recurrent uveitis group; 39.8 ± 7.3 years (range: 30–47) in the BD-remitted uveitis group; and 34.6 ± 7.6 years (range: 28–49) in the healthy controls. There were no significant differences in age between the three groups. The mean period of infliximab treatment was 26.9 ± 21.9 months, and all patients were treated for longer than 1 year. This study adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by the institutional review board of the National Defense Medical College. Written informed consent was obtained from all participating patients and control subjects.
TABLE 1.
Profiles of individual BD patients.
| No. | Age (years) |
Sex | Age at uveitis onset (years) |
Other complications | Type of uveitis | Duration of infliximab treatment (months) |
Interval of infliximab infusion (weeks) |
|---|---|---|---|---|---|---|---|
| BD1 | 30 | M | 23 | Oral ulcer Skin lesions | Panuveitis | 14 | 8 |
| BD2 | 39 | M | 37 | Oral ulcer Skin lesions Genital ulcer |
Panuveitis | 18 | 8 |
| BD3 | 54 | M | 51 | Oral ulcer Skin lesions | Panuveitis | 17 | 6 |
| BD4 | 33 | F | 30 | Oral ulcer Skin lesions Genital ulcer Neural lesions |
Panuveitis | 31 | 5 |
| BD5 | 47 | M | 45 | Arthritis | Panuveitis | 26 | 8 |
| BD6 | 30 | M | 18 | Oral ulcer | Panuveitis | 78 | 8 |
| BD7 | 43 | M | 41 | Skin lesions Arthritis | Posterior uveitis | 12 | 8 |
| BD8 | 30 | M | 16 | Oral ulcer Skin lesions | Panuveitis | 23 | 8 |
Preparation of interphotoreceptor retinoid binding protein
Fresh swine retinas were homogenized in 0.03 M disodium monopotassium phosphate buffer (PB; pH 7 6). After centrifugation (48 000 g for 10 min), the supernatant was collected. Saturated ammonium sulfate (pH 7.2) was added to the supernatant until 50% saturation was achieved, and the mixture was left overnight at 4°C. After centrifugation, the precipitate was dissolved in PB and was used as the crude antigen preparation. IRBP was isolated from the crude antigen preparation according to the method of Redmond et al.32 To improve the quality of IRBP, the crude preparation was purified successively by concanavalin A Sepharose affinity chromatography and ion exchange high-performance liquid chromatography.
Isolation of PBMCs
Peripheral venous blood (20 mL) was collected into a heparinized tube before and 1 week after infliximab infusion in BD patients with or without recurrent uveitis during at least 1 year of infliximab therapy, and from healthy controls at any time. PBMCs were isolated immediately by density gradient centrifugation (Ficoll-Hypaque; Pharmacia Biotech, Shanghai, China) and suspended at 2 × 106 cells/mL in RPMI 1640 medium supplemented with 10 mM HEPES, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin (all from BioWhittaker, Walkersville, MD), 1 × 10−5 M 2-ME (Sigma Chemical Co., St Louis, MO), and 10% fetal calf serum (Sigma Chemical Co.).
Cytokine Production Assay
Fresh PBMCs (2 × 105) were added to microwells in triplicate and incubated with IRBP at concentrations of 0, 5, and 10 μg/mL for 48 h. Supernatants were collected, and the concentrations of IL-1β, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFN-γ, soluble CD40 ligand (sCD40L), and TNFα in the supernatants were measured by Bio-Plex kit® (Bio-Rad Laboratories Inc.).
Calculation of Change in Cytokine Production in BD Patients after Infliximab Infusion
The change in cytokine production by IRBP-stimulated PBMCs of BD patients after infliximab infusion was calculated using the following formula (A, level of cytokine before infliximab infusion; B, level of cytokine after infliximab infusion; and C, percentage change.
Statistical Analysis
Cytokine concentrations are presented as mean ± standard deviation for each group. Levels below limits of detection were assigned a numerical value of 0 pg/mL for statistical analysis. Since the levels of cytokines were not normally distributed, statistical comparisons of cytokine levels were performed using Wilcoxon signed-rank test or Steel-Dwass non-parametric multiple comparison test. A value of p<0.05 was considered significant.
RESULTS
Comparison of T Cell Activation Marker and Proinflammatory Cytokines between Behçet Patients and Controls
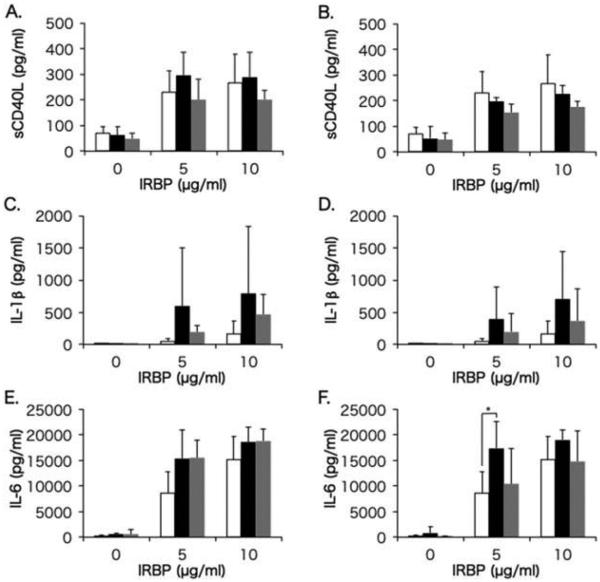
Soluble CD40L is a T cell activation marker, and IL-1β and IL-6 are proinflammatory cytokines produced by various cells such as macrophages and dendritic cells upon interaction with activated T cells. Figure 1 displays levels of sCD40L, IL-1β, and IL-6 produced by IRBP-stimulated PBMCs in the healthy control group, and in BD-recurrent uveitis and BD-remitted uveitis groups before and after infliximab infusion. In BD-recurrent uveitis group, levels of sCD40L, IL-1β, and IL-6 produced by PBMCs before infliximab infusion were higher compared with healthy subjects, and IL-1β, and IL-6 remained higher after infliximab infusion with a significant difference for IL-6 compared with healthy subjects. In the BD-remitted uveitis group, IL-1β and IL-6 produced by PBMCs before infliximab infusion were also higher compared with healthy subjects, but IL-6 was reduced to the level of healthy subjects after infliximab infusion.
FIGURE 1.
Comparisons of sCD40L, IL-1β, and IL-6 between Behçet patients (BD) and healthy controls. Mean levels of sCD40L (A and B), IL-1β (C and D), and IL-6 (E and F) produced by PBMCs in the BD-recurrent uveitis group (black bars) and BD-remitted uveitis group (gray bars) before (A, C, and E) and after (B, D, and F) infliximab infusion are compared with cytokine production in the healthy control group (white bars). PBMCs were stimulated with IRBP at concentrations of 0, 5, and 10 μg/mL. The line above each bar shows standard deviation. *p<0.05 by Steel-Dwass non-parametric multiple comparison test.
Comparison of Th1 and Th17 Cytokines between Behçet Patients and Controls
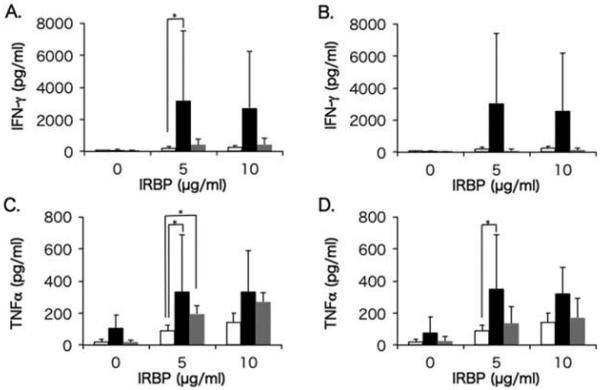
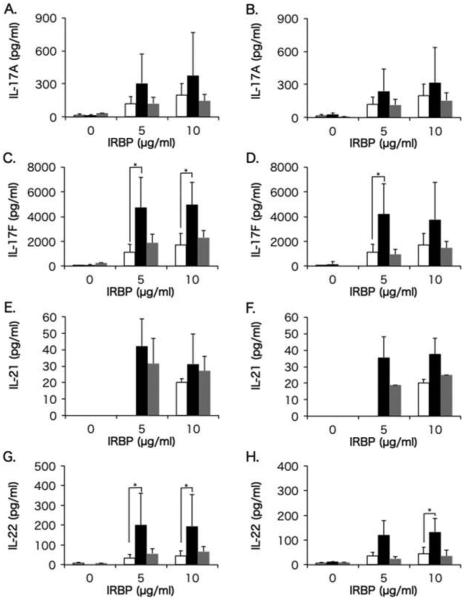
Th1 and Th17 cells have been demonstrated to be involved in the development of uveitis not only in animal uveitis models but also in humans.15,19,33,34 Levels of Th1 cytokines (IFN-γ and TNFα) produced by IRBP-stimulated PBMCs in healthy control group, and in BD-recurrent uveitis and BD-remitted uveitis groups before and after infliximab infusion, are shown in Figure 2, and the data for Th17 cytokines (IL-17A, IL-17F, IL-21, and IL-22) are shown in Figure 3.
FIGURE 2.
Comparisons of IFN-γ and TNFα between Behçet (BD) patients and healthy controls. Mean levels of IFN-γ (A and B) and TNFα (C and D) produced by PBMCs in the BD-recurrent uveitis group (black bars) and BD-remitted uveitis group (gray bars) before (A and C) and after (B and D) infliximab infusion are compared with cytokine production in the healthy control group (white bars). PBMCs were stimulated with IRBP at concentrations of 0, 5, and 10 μg/mL. The line above each bar shows standard deviation. *p<0.05 by Steel-Dwass non-parametric multiple comparison test.
FIGURE 3.
Comparisons of IL-17A, IL-17F, IL-21, and IL-22 between Behçet (BD) patients and healthy controls. Mean levels of IL-17A (A and B), IL-17F (C and D), IL-21 (E and F), and IL-22 (G and H) produced by PBMCs in the BD-recurrent uveitis group (black bars) and BD-remitted uveitis group (gray bars) before (A, C, E and G) and after infliximab infusion (B, D, F and H), compared with cytokine production in the healthy control group (white bars). PBMCs were stimulated with IRBP at concentrations of 0, 5, and 10 μg/mL. The line above each bar shows standard deviation. *p<0.05 by Steel-Dwass non-parametric multiple comparison test.
In BD-recurrent uveitis group, levels of IFN-γ and TNFα produced by IRBP-stimulated PBMCs before infliximab infusion were significantly higher compared with healthy subjects, and TNFα level remained significantly higher after infliximab infusion. In BD-remitted uveitis group, IFN-γ level was significantly higher, while TNFα was not different compared with healthy subjects, and both cytokines were reduced almost to the levels of healthy subjects after infliximab infusion.
In BD-recurrent uveitis group, levels of IL-17A, IL-17F, IL-21, and IL-22 before infliximab infusion were higher, with significant differences for IL-17F and IL-22, compared with healthy subjects, and the levels of IL-17F and IL-22 remained significantly higher after infliximab infusion. In the BD-remitted uveitis group, levels of IL-17F and IL-21 before infliximab infusion were higher compared with healthy subjects, but were reduced to the levels of healthy subjects after infliximab infusion.
Comparison of Th2 Cytokines between Behçet Patients and Controls
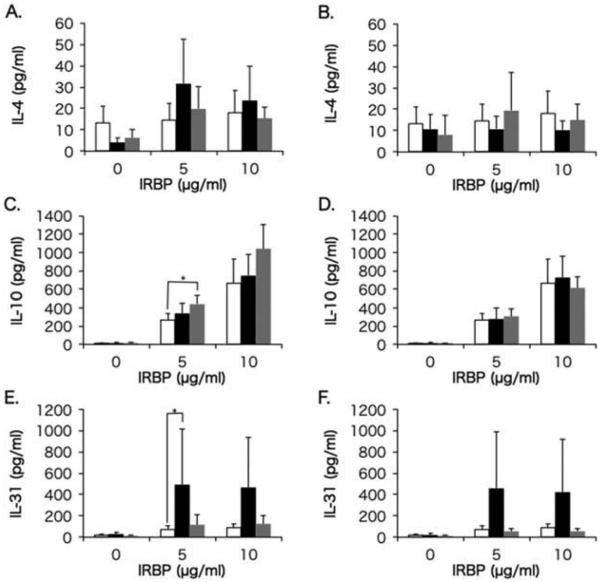
IL-4, IL-10, and IL-31 are Th2 cytokines, and IL-10 is known to be produced also by regulatory T (Treg) cells. Although the effect of IL-31 on uveitis has not been elucidated, both IL-4 and IL-10 play inhibitory roles in the development of uveitis. Levels of IL-4, IL-10, and IL-31 produced by IRBP-stimulated PBMCs in the control group, and in the BD-recurrent uveitis and BD-remitted uveitis groups before and after infliximab infusion, are shown in Figure 4.
FIGURE 4.
Comparisons of IL-4, IL-10, and IL-31 between Behçet (BD) patients and healthy controls. Mean levels of IL-4 (A and B), IL-10 (C and D), and IL-31 (E and F) produced by PBMCs in the BD-recurrent uveitis group (black bars) and BD-remitted uveitis group (gray bars) before (A, C and E) and after infliximab infusion (B, D and F), compared with cytokine production in the healthy control group (white bars). PBMCs were stimulated with IRBP at concentrations of 0, 5, and 10 μg/mL. The line above each bar shows standard deviation. *p<0.05 by Steel-Dwass non-parametric multiple comparison test.
In the BD-recurrent uveitis group, levels of IL-4, IL-10, and IL-31 before infliximab infusion were unexpectedly higher, with a significant difference for IL-31, compared with the healthy controls, and all levels except IL-31 were reduced to almost the same levels of healthy subjects after infliximab infusion.
In the BD-remitted uveitis group, levels of IL-4, IL-10, and IL-31 before infliximab infusion were also higher, with a significant difference for IL-10, compared with healthy controls, and all three cytokines, including IL-31, were reduced to almost the same levels of healthy subjects after infliximab infusion.
Comparison of Proinflammatory and Th1-, Th2-, and Th17-related Cytokines Between BD-recurrent Uveitis and BD-remitted Uveitis Groups Before and After Infliximab Infusion
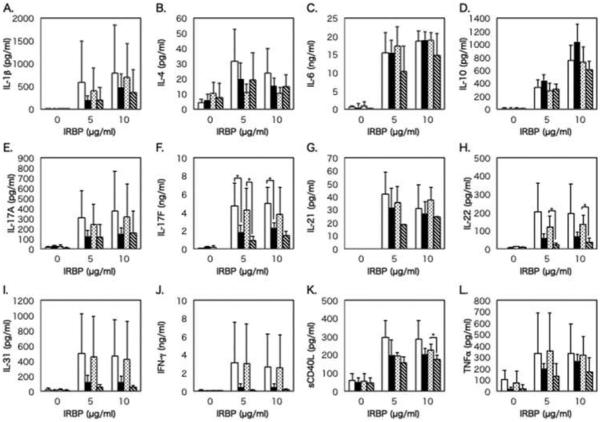
Figure 5 shows the levels of proinflammatory cytokines and Th1-, Th2-, and Th17-related cytokines produced by IRBP-stimulated PBMCs in BD-recurrent uveitis and BD-remitted uveitis groups before and after infliximab infusion. Before infliximab infusion, levels of IL-1β, IL-4, IL-17A, IL-17F, IL-21, IL-22, IL-31, IFN-γ, sCD40L, and TNFα were higher, with a significant difference for IL-17F in BD-recurrent uveitis group than in BD-remitted uveitis group. Only the level of IL-10 was higher in the remission group than in the recurrence group.
FIGURE 5.
Comparison of cytokine production between the Behçet disease (BD)-recurrent uveitis and BD-remitted uveitis groups before and after infliximab infusion. Mean levels of IL-1β (A), IL-4 (B), IL-6 (C), IL-10 (D), IL-17A (E), IL-17F (F), IL-21 (G), IL-22 (H), IL-31 (I), IFN-γ (J), sCD40L (K), and TNFα (L) produced by PBMCs in the BD-recurrent uveitis group before (white bars) and after infliximab infusion (dotted bars) and in the BD-remitted uveitis group before (black bars) and after infliximab infusion (diagonal striped bars). PBMCs were stimulated with IRBP at concentrations of 0, 5, and 10 μg/mL. The line above each bar shows standard deviation. *p<0.05 by Wilcoxon signed-rank test.
After infliximab infusion, levels of cytokines except for IL-4, IL-22, and sCD40L were not apparently reduced in BD-recurrent uveitis group. On the other hand, levels of IL-6, IL-10, IL-17F, IL-22, IL-31, IFN-γ, and TNFααwere lower, with significant differences for IL-17F, IL-22, and sCD40L, in BD-remitted uveitis group.
Correlation of Infliximab Treatment Duration with Change in Cytokine Production after Infliximab Infusion in BD Patients
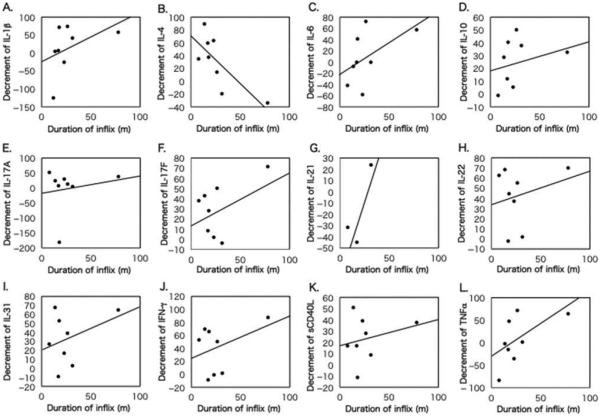
The association between duration of infliximab treatment and change in cytokine production by 5 μg/mL IRBP-stimulated PBMCs after infliximab infusion is shown in Figure 6. A significant negative correlation was observed between infliximab treatment duration and change in IL-4 production. Other cytokines appeared to increase with longer duration of infliximab treatment, although significant differences were not observed.
FIGURE 6.
Association of infliximab treatment duration with change in cytokine production after infliximab treatment in BD patients. Correlation of infliximab treatment duration (months) with change in individual cytokine production by 5 g/mL IRBP-stimulated PBMCs of BD patients was analyzed by Pearson’s correlation coefficient test: (A) IL-1β (y = −22.3 + 1.29x, R2 = 0.18, p = 0.298); (B) IL-4 (y = 71.7−1.49x, R2 = 0.5789, p = 0.028); (C) IL-6 (y = −21.5 + 1.06x, R2 = 0.244, p = 0.213); (D) IL-10 (y = 18.8 + 0.25x, R2 = 0.089, p = 0.472); (E) IL-17A (y = −21.0 + 0.72x, R2 = 0.043, p = 0.623); (F) IL-17F (y = 12.4 + 0.63x, R2 = 0.267, p = 0.190); (G) IL-21 (y = −85.0 + 3.36x, R2 = 0.820, p = 0.279); (H) IL-22 (y = 33.3 + 0.33x, R2 = 0.061, p = 0.554); (I) IL-31 (y = 20.2 + 0.46x, R2 = 0.121, p = 0.399); (J) IFN-γ (y = 22.0 + 0.66x, R2 = 0.146, p = 0.350); (K) sCD40L (y = 17.2 + 0.43x, R2 = 0.061, p = 0.557); and (L) TNFα (y = −31.2 + 1.36x, R2 = 0.297, p = 0.162). Change in cytokine production = level of cytokine before infliximab infusion – level of cytokine after infliximab infusion/level of cytokine before infliximab infusion × 100%.
DISCUSSION
It is widely accepted that Th1 and Th17 immune responses are involved in the pathogenesis of BD. The serum levels of IFN-γ, IL-12, and IL-17 in active BD patients are higher than those in healthy subjects, and Th1and Th17 cells increase in the active phase.35,36 Increases of Th1 and Th17 cells in BD patients are also observed in active uveitis compared with remitted uveitis or healthy controls.14,19,37 The present results that Th1 cytokines and Th17 cytokines produced by PBMCs during infliximab treatment were higher in BD patients with recurrent uveitis than those with remitted uveitis, both before and after infliximab infusion are consistent with these previous reports.
Although the effects of infliximab on immunological events mediated by Th1 and Th17 cells in BD have not yet been elucidated, Sugita et al.37 demonstrated that although ocular fluids of Behçet patients with active uveitis contained IFN-γ, IL-2, TNFα, IL-6, and IL-17, these inflammatory cytokines were undetectable in Behçet patients during infliximab treatment, and that while activated Th cells from Behçet patients produced large amounts of TNF-α and IL-17 upon stimulation by anti-CD3 and anti-CD28 mAbs, such cytokine production was absent in the presence of infliximab. Although our protocol was different from their study and Th2-related cytokines were also inhibited by infliximab, our data did not essentially contradict their results. A study on the effect of infliximab treatment on cytokine response in ankylosing spondylitis (AS) using a similar protocol to ours showed that the numbers of IFNγ-and TNFα-positive CD4+ and CD8+ T cells upon PMA/ionomycin or antigen-specific stimulation decreased significantly after treatment with infliximab.38 However, in vitro TNFα and IL-10 production by LPS-stimulated PBMCs from AS patients did not decrease after treatment with infliximab. In addition, the number of IL-4- or IL-10-positive T cells did not change in AS patients during treatment of infliximab. In the present study, IL-4 produced by PBMCs was apparently reduced after infliximab infusion in BD patients with recurrent uveitis, and IL-10 was reduced in BD patients with remitted uveitis, although the differences were not significant. It is possible that these discrepancies arise due to the different characteristics of the diseases. However, TNFα partially promotes the activation of antigen-presenting cells via innate immune responses.39 Therefore, blockade of TNFα signaling by infliximab possibly impairs antigen-presenting functions of dendritic cells or macrophages, which would interfere with cytokine production by all Th cells including Th2 cells.
In BD patients with remitted uveitis, although levels of most cytokines produced by PBMCs after infliximab infusion were lower than those before infusion, significant differences were not observed. The small number of samples compared with the variability of cytokine production by IRBP-stimulated PBMCs from BD patients would be the major factor for the present results. However, it may also refiect different responses to infliximab in individual BD patients.
Unexpectedly, Th2-related cytokines; IL-4, IL-10, and IL-31, produced by PBMCs from BD patients before infliximab infusion were higher compared with healthy subjects. Consistent with our findings, Raziuddin et al.40 demonstrated that PBMCs from BD patients produced higher levels of IL-4, IL-10, and IL-13, but failed to produce IFN-γ and IL-12 upon stimulation with anti-CD3/anti-CD40 mAbs. In addition, increased serum levels of IL-4, IL-6, IL-10, IL-12, and IFN-γ have also been found in BD patients and these levels were higher in active BD than in remission.35 In BD, uveitis develops as a result of inflammation involving Th1- and Th17-cell mediated immune responses,13–15,19 but the inflammation is self-limiting. Hence, our present study, together with above-mentioned previous reports, seem to argue against an inhibitory role of Th2 cytokines in the development of BD, but may suggest a possibility that Th2 cell-mediated immune responses are passively augmented to compensate for the Th1/Th2 balance by the self-limiting nature of BD uveitis.
On the other hand, IL-4 production in BD patients with remitted uveitis, which was lower than that in BD with recurrent uveitis, was not reduced after infliximab infusion. In addition, a significant negative correlation between the duration of infliximab treatment and change in IL-4 production after infliximab infusion indicates that continuous infliximab treatment ameliorates the inhibition of IL-4 production. These results would suggest the benefit of continuous infliximab treatment for BD-associated uveitis.
Th22 cells, which are characterized by IL-22 and TNFα production, are a relatively new category of Th cells and are distinct from Th1, Th2, and Th17 cells.41 IL-22 gene expression is increased in patients with autoimmune noninfectious uveitis, and has been demonstrated to be involved in the development of uveitis associated with BD.42,43 It is possible that IL-22 and TNFα produced by IRBP-stimulated PBMCs in the present study may be derived from Th22 cells in addition to Th1 and Th17 cells.
The present study has several limitations, including small sample size, variable duration of infliximab therapy, absence of an infliximab-naive group and a disease control group, as well as use of a single antigen for stimulation and gene expression. Since it is not possible to enroll a sufficient number of patients for studies that address all the above issues, a multicenter study has to be planned in the future.
In conclusion, Th1-, Th2-, and Th17-related cytokines, especially IFN-γ, TNFα, IL-10, IL-17F, and IL-22 produced by PBMCs were reduced after infliximab infusion in BD patients with remitted uveitis but not in those with recurrent uveitis. Thus, measurements of these Th1-, Th2-, and Th17-related cytokines produced by IRBP-stimulated PBMCs would provide a quantitative evaluation of the efficacy of infliximab treatment for uveitis in BD patients, although further studies are required to identify specific cytokines that refiect the degree of ocular inflammation and to find a more readily available antigen than IRBP for stimulation and measurements.
Acknowledgments
FUNDING
This work was supported in part by Grant-in-Aid 24592689 for Scientific Research from the Japan Society for the Promotion of Science.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Chajek T, Fainaru M. Behcet’s disease. Report of 41 cases and a review of the literature. Medicine (Baltimore) 1975;54:179–196. doi: 10.1097/00005792-197505000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wong RC, Ellis CN, Diaz LA. Behcet’s disease. Int J Dermatol. 1984;23:25–32. doi: 10.1111/j.1365-4362.1984.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakane T, Takeno M, Suzuki N, et al. Behcet’s disease. N Engl J Med. 1999;341:1284–1291. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 4.Mege JL, Dilsen N, Sanguedolce V, et al. Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behcet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993;20:1544–1549. [PubMed] [Google Scholar]
- 5.de Smet MD, Dayan M. Prospective determination of T-cell responses to S-antigen in Behcet’s disease patients and controls. Invest Ophthalmol Vis Sci. 2000;41:3480–3484. [PubMed] [Google Scholar]
- 6.Meguro A, Ota M, Katsuyama Y, et al. Association of the toll-like receptor 4 gene polymorphisms with Behcet’s disease. Ann Rheum Dis. 2008;67:725–727. doi: 10.1136/ard.2007.079871. [DOI] [PubMed] [Google Scholar]
- 7.Nara K, Kurokawa MS, Chiba S, et al. Involvement of innate immunity in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2008;152:245–251. doi: 10.1111/j.1365-2249.2008.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pay S, Simsek I, Erdem H, et al. Immunopathogenesis of Behcet’s disease with special emphasize on the possible role of antigen presenting cells. Rheumatol Int. 2007;27:417–424. doi: 10.1007/s00296-006-0281-6. [DOI] [PubMed] [Google Scholar]
- 9.Frassanito MA, Dammacco R, Cafforio P, et al. Th1 polarization of the immune response in Behcet’s disease: a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–1974. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Koarada S, Haruta Y, Tada Y, et al. Increased entry of CD4+ T cells into the Th1 cytokine effector pathway during T-cell division following stimulation in Behcet’s disease. Rheumatology (Oxford) 2004;43:843–851. doi: 10.1093/rheumatology/keh195. [DOI] [PubMed] [Google Scholar]
- 11.Ahn JK, Yu HG, Chung H, et al. Intraocular cytokine environment in active Behcet uveitis. Am J Ophthalmol. 2006;142:429–434. doi: 10.1016/j.ajo.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Yang P, He H, et al. S-antigen specific T helper type 1 response is present in Behcet’s disease. Mol Vis. 2008;14:1456–1464. [PMC free article] [PubMed] [Google Scholar]
- 13.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi W, Zhu X, Yang P, et al. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058–3064. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 15.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto JH, Fujino Y, Lin C, et al. S-antigen specific T cell clones from a patient with Behcet’s disease. Br J Ophthalmol. 1994;78:927–932. doi: 10.1136/bjo.78.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobin D, Thillaye B, de Kozak Y, et al. Severe retinochoroidopathy: variations of humoral and cellular immunity to S-antigen in a longitudinal study. Curr Eye Res. 1990;9(Suppl):91–96. doi: 10.3109/02713689008999426. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto JH, Minami M, Inaba G, et al. Cellular autoimmunity to retinal specific antigens in patients with Behcet’s disease. Br J Ophthalmol. 1993;77:584–589. doi: 10.1136/bjo.77.9.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi M, Usui Y, Okunuki Y, et al. Immune responses to interphotoreceptor retinoid-binding protein and S-antigen in Behcet’s patients with uveitis. Invest Ophthalmol Vis Sci. 2010;51:3067–3075. doi: 10.1167/iovs.09-4313. [DOI] [PubMed] [Google Scholar]
- 20.Mamo JG. The rate of visual loss in Behcet’s disease. Arch Ophthalmol. 1970;84:451–452. doi: 10.1001/archopht.1970.00990040453009. [DOI] [PubMed] [Google Scholar]
- 21.Mishima S, Masuda K, Izawa Y, et al. The eighth Frederick H. Verhoeff lecture. Presented by Saiichi Mishima, MD. Behcet’s disease in Japan: ophthalmologic aspects. Trans Am Ophthalmol Soc. 1979;77:225–279. [PMC free article] [PubMed] [Google Scholar]
- 22.Muhaya M, Lightman S, Ikeda E, et al. Behcet’s disease in Japan and in Great Britain: a comparative study. Ocul Immunol Inflamm. 2000;8:141–148. [PubMed] [Google Scholar]
- 23.Hamuryudan V, Ozyazgan Y, Hizli N, et al. Azathioprine in Behcet’s syndrome: effects on long-term prognosis. Arthritis Rheum. 1997;40:769–774. doi: 10.1002/art.1780400425. [DOI] [PubMed] [Google Scholar]
- 24.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, et al. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–380. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Kaklamani VG, Kaklamanis PG. Treatment of Behcet’s disease – an update. Semin Arthritis Rheum. 2001;30:299–312. doi: 10.1053/sarh.2001.19819. [DOI] [PubMed] [Google Scholar]
- 26.Okada AA. Drug therapy in Behcet’s disease. Ocul Immunol Inflamm. 2000;8:85–91. [PubMed] [Google Scholar]
- 27.Sfikakis PP, Theodossiadis PG, Katsiari CG, et al. Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet. 2001;358:295–296. doi: 10.1016/s0140-6736(01)05497-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohno S, Nakamura S, Hori S, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behçet’s disease with refractory uveoretinitis. J Rheumatol. 2004;31:1362–1368. [PubMed] [Google Scholar]
- 29.Goossens PH, Verburg RJ, Breedveld FC. Remission of Behcet’s syndrome with tumour necrosis factor alpha blocking therapy. Ann Rheum Dis. 2001;60:637. doi: 10.1136/ard.60.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-Fernandez S, Hidalgo V, Fernandez-Melon J, et al. Effect of infliximab on threatening panuveitis in Behcet’s disease. Lancet. 2001;358:1644. doi: 10.1016/S0140-6736(01)06677-6. [DOI] [PubMed] [Google Scholar]
- 31.International Study Group for Behçet’s Disease Criteria for diagnosis of Behcet’s disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 32.Redmond TM, Wiggert B, Robey FA, et al. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 33.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura T, Sonoda K-H, Miyazaki Y, et al. Differential roles for IFN-γ and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–214. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 35.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behçet’s disease patients. Scand J Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 36.Hamzaoui K, Bouali E, Ghorbel I, et al. Expression of Th-17 and RORgammat mRNA in Behcet’s Disease. Med Sci Monit. 2011;17:CR227–CR234. doi: 10.12659/MSM.881720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugita S, Kawazoe Y, Imai A, et al. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet’s disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou J, Rudwaleit M, Brandt J, et al. Down-regulation of the nonspecific and antigen-specific T cell cytokine response in ankylosing spondylitis during treatment with infliximab. Arthritis Rheum. 2003;48:780–790. doi: 10.1002/art.10847. [DOI] [PubMed] [Google Scholar]
- 39.Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 40.Raziuddin S, al-Dalaan A, Bahabri S, et al. Divergent cytokine production profile in Behcet’s disease. Altered Th1/ Th2 cell cytokine pattern. J Rheumatol. 1998;25:329–333. [PubMed] [Google Scholar]
- 41.Trifari S, Kaplan CD, Tran EH, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Liu B, Maminishkis A, et al. Gene expression profiling in autoimmune noninfectious uveitis disease. J Immunol. 2008;181:5147–5157. doi: 10.4049/jimmunol.181.7.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugita S, Kawazoe Y, Imai A, et al. Role of IL-22- and TNF-alpha-producing Th22 cells in uveitis patients with Behcet’s disease. J Immunol. 2013;190:5799–5808. doi: 10.4049/jimmunol.1202677. [DOI] [PMC free article] [PubMed] [Google Scholar]