Abstract
Purpose:
To study the effect of undiagnosed diabetes on the relationship between self-reported diabetes and cognitive impairment.
Methods:
Data were from 1033 participants aged ≥60 from Wave III (2012) of the Mexican Health and Aging Study. Participants were classified as nondiabetic (n = 589), undiagnosed diabetic (n = 201), and self-reported diabetic (n = 243). Multivariate logistic regression models were used to estimate the relationship between self-reported diabetes and severity of cognitive impairment (nonimpaired, moderate impaired, severe impaired).
Results:
Self-reported diabetes was associated with significantly higher odds for severe, but not moderate, cognitive impairment (odds ratio [OR] = 2.70, 95% confidence interval [CI] = 1.39-5.32). The association between self-reported diabetes and severe cognitive impairment decreased by 6.3% when undiagnosed diabetics were included in the nondiabetic category and by 30.4% when undiagnosed diabetics were included in the self-reported diabetes category.
Discussion:
The association between self-reported diabetes and severe cognitive impairment is underestimated when undiagnosed diabetics are not differentiated from self-reported diabetics and nondiabetics.
Keywords: diabetes, cognition, dementia, Mexico, older adults
Introduction
Consistent evidence indicates type 2 diabetes is a risk factor for cognitive impairment among older adults. A Delphi Consensus study identified 19 studies that examined the relationship between diabetes and Alzheimer disease (AD), of which 17 reported a positive association between diabetes and AD. 1 Furthermore, a meta-analysis of 20 longitudinal studies reported that diabetes was associated with a 21% higher risk for mild cognitive impairment, 54% higher risk for AD, and 148% higher risk for vascular dementia. 2 Epidemiological research has focused largely on nonhispanic white older adults, but diabetes has also been identified as a risk factor for cognitive impairment among older Mexican adults. Older Mexican adults who reported being diagnosed with diabetes by a physician have been observed to have 2 times higher odds for dementia compared to nondiabetic older adults. 3 These findings are consistent with those reported by studies of older Mexican Americans. A study of 1617 nondemented Mexican Americans revealed that older adults with treated and untreated diabetes had 2 and 1.5 times higher risk, respectively, for cognitive impairment compared to nondiabetic older Mexican Americans. 4
While type 2 diabetes has been consistently associated with cognitive impairment among older adults, the impact that older adults with elevated blood glucose levels who have not been diagnosed with diabetes (ie, undiagnosed diabetics) may have on this relationship is not known. This is particularly important to study in older Mexican adults because prior research has relied primarily upon self-reported data to determine if a participant has diabetes. 3 A limitation of this approach is cases of undiagnosed diabetes are included in the nondiabetic reference group, which may underestimate the association between self-reported diabetes and cognition. This limitation is concerning, given the high prevalence of undiagnosed diabetes among older Mexican adults. 5,6 Thus, the purpose of this study is to examine the effect that undiagnosed diabetes has on the relationship between self-reported diabetes and severity of cognitive impairment among Mexican adults aged 60 and older. We hypothesize that undiagnosed diabetes will be associated with cognitive impairment and not excluding older adults with undiagnosed diabetes from those who are nondiabetic will result in an underestimation of the relationship between self-reported diabetes and cognitive impairment.
Methods
Data Source and Study Design
This study used a cross-sectional design and used data from a subset of the 2012 Wave of the Mexican Health and Aging Study (MHAS). The MHAS is an ongoing nationally representative longitudinal cohort study of Mexican adults aged 50 and older in 2001. 7 The MHAS was initiated in 2001 and follow-up observations were conducted in 2003 and 2012. A total of 18 465 individuals were interviewed during the 2012 Wave, of which 2086 participants underwent a capillary blood draw for measures of hemoglobin and glycosylated hemoglobin (HbA1c).
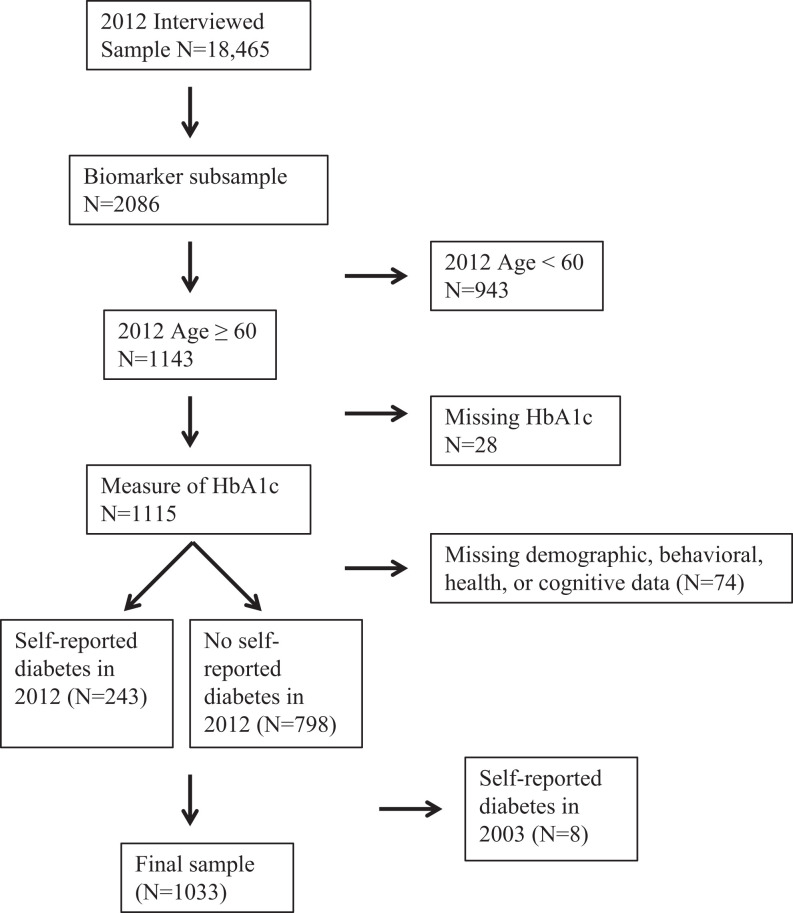
A visual representation for the selection of the final sample is presented in Figure 1. Participants who were selected to provide anthropometric or biomarker data; were aged 60 or older; had data for HbA1c concentration; and had data for demographic, health, and behavioral characteristics were included in the final sample. In order to maximize the final sample size, participants who had completed 2 or more cognitive assessments were included in the final sample. Of the 1041 participants who had data for sociodemographic, health, behavioral, and cognitive data, 8 participants who reported being diagnosed with diabetes during the 2003 observation but reported never being diagnosed with diabetes during the 2012 observation were excluded from the final sample (n = 1033). Participants who were excluded from the final sample were significantly older; were less likely to be married; were more likely to have had a stroke and to be depressed; and had lower scores for orientation, verbal learning, verbal memory, verbal fluency, and attention compared to participants included in the final sample (P < .05).
Figure 1.
Selection of final sample from the Mexican Health and Aging Study.
Assessment of Diabetes Status
Participants who reported having been diagnosed with diabetes by a physician or a medical professional were classified as having self-reported diabetes. Undiagnosed diabetes was determined according to HbA1c concentration. HbA1c provides a reliable measure of circulating glucose in the blood over a 1- to 3-month period. 8 The American Diabetes Association defines diabetes, prediabetes, and normal according to the following HbA1c categories: (1) diabetes: HbA1c ≥6.5%, (2) prediabetes: HbA1c 5.7% to 6.4%, and (3) normal: HbA1c < 5.7%. 9 In the present analysis, participants who reported never being diagnosed with diabetes but had an HbA1c concentration ≥6.5% were classified as having undiagnosed diabetes. Participants classified as prediabetic and normal were defined as nondiabetic.
Defining Moderate and Severe Cognitive Impairment
Cognitive functioning was measured using the Cross-Cultural Cognitive Examination (CCCE). 10 The CCCE includes eight cognitive tasks that each assess a different cognitive domain: (1) verbal memory immediate recall (verbal learning), (2) verbal memory delayed recall (verbal memory), (3) visuospatial (construction), (4) visual memory (visual recall), (5) visual scanning (attention), (6) date naming (orientation), (7) animal naming (verbal fluency), and (8) backward counting (working memory).
The criteria used to classify cognitive impairment were based on the diagnostic criteria for mild cognitive impairment and dementia proposed by the National Institute on Aging-Alzheimer’s Association workgroups. 11,12 Cognitive impairment was defined as performance on 1 or more cognitive assessments that was ≥1.5 SD lower than what would be expected based on the participant’s age and education (0 years, 1-6 years, and 7+ years). The effects of age and education were estimated using a multistep process. First, a sample of MHAS participants who attended the 2012 Wave and were aged 50 to 59 years were identified. This sample was used because it was unlikely to include participants with dementia or other type of cognitive impairment that may bias the relationship between age, education, and cognition. From participants aged 50 to 59 the normative scores for cognition were calculated in the 2001 and 2003 MHAS Waves. 3 Second, the effects of age and education were estimated using a multivariable model that included these characteristics as predictors of each cognitive assessment. Third, the coefficients from these models were used to calculate the expected scores for each participant in the final sample. Finally, participants whose observed score on 1 or more assessments was ≥1.5 SD lower than the expected score were classified as cognitively impaired.
Participants who were defined as cognitively impaired were further classified as moderate or severely impaired based on their ability to perform activities of daily living (ADLs; difficulty walking, bathing or showering, eating, getting out of bed, or using the toilet) and instrumental ADLs (IADLs; difficulty preparing a hot meal, shopping, taking medications, or managing money). Since Mexican culture contributes to gender differences in IADLs (eg, men do not typically shop for groceries or prepare a meal and women do not typically manage money), participants who had difficulty performing 1 or more ADLs and/or 2 or more IADLs were classified as being functionally impaired. 3 Participants who were not cognitively or functionally impaired were classified as having normal cognition. Participants who were cognitively impaired and had no functional impairment were classified as having moderate cognitive impairment. Participants who were cognitively and functionally impaired were classified as having severe cognitive impairment.
Covariates
Covariates were selected based on previous research on the relationship between cognition and diabetes. 4,13,14 Participants were asked to report their age, sex, years of education completed, and marital status (single, married, in a consensual union, divorced, separated from a union, separated from a marriage, widowed from a union, and widowed from a marriage). Education was recoded as 0 years, 1 to 6 years, and 7 or more years and marital status was recoded as married or in a consensual union, not married, and widowed.
Participants were also asked if they engaged in exercise or hard physical work 3 or more times a week and if they had ever experienced a heart attack, stroke, possible stroke, or transient ischemic attack. Depressive symptoms were assessed using 9 questions from the Center for Epidemiological Studies Depression Scale that asked if a participant felt depressed, if everything they did was effort, had restless sleep, felt happy, felt lonely, felt that they enjoyed life, felt sad, felt tired, and felt they had a lot of energy. Participants who had 6 or more symptoms were classified as having high depressive symptoms. 15 Alcohol consumption was defined according to 3 categories: (1) abstainer, (2) former drinker: has consumed alcohol in the past but is not currently drinking alcohol, and (3) current drinker: reported consuming alcohol in the past 3 months. Smoking status was defined as never, former, and current smoker. Body mass index (BMI) was calculated using measured values for height (in meters square) and weight (in kilograms).
Statistical Analysis
Analysis of variance and χ2 tests were used to assess the differences in demographic, behavioral, and health characteristics according to cognitive status. Separate logistic regression models for moderate and severe cognitive impairment were used to assess the association between diabetes and cognitive impairment. This is an appropriate approach when the proportional odds assumption, which is required for ordinal logistic regression, is not valid. 16 Three separate logistic regression models were constructed. Model 1 included 3 categories for diabetes: (1) nondiabetic (reference category), (2) undiagnosed diabetes, and (3) self-reported diabetes. Model 2 used self-report diabetes status as the primary independent variable. In this model, participants with undiagnosed diabetes were included in the same category as participants who did not have diabetes. Model 3 included participants with undiagnosed diabetes in the same category as participants with self-reported diabetes. All models were adjusted for the effects of age, gender, education, marital status, smoking, alcohol consumption, exercise, hypertension, heart attack, stroke, depression, and BMI.
The odds ratio (OR) obtained from model 1 and model 2 were used to calculate the percentage change in the association between self-report diabetes and severity of cognitive impairment when participants with undiagnosed diabetes are included in the same category as those who do not have diabetes. Percentage change was calculated using the following equation: (ORDM1 − ORDM2)/ORDM1, where ORDM1 is the OR of diabetes from model 1 and ORDM2 is the OR of diabetes from model 2. This calculation was also performed using the OR from model 1 and model 3 to calculate the percentage change when participants with undiagnosed diabetes are included in the same category as participants with self-reported diabetes.
Results
Characteristics of Final Sample
Table 1 presents a summary of the demographic, behavioral, and health characteristics of the final sample according to the severity of cognitive impairment. A total of 751 participants had normal cognition, 220 were moderately impaired, and 62 were severely impaired. Participants who were either moderately or severely impaired were older, had lower educational attainment, were more likely to be depressed, had lower BMI, and were less likely to engage in physical exercise compared to participants who were not cognitively impaired (all Ps < .05). Also, participants with self-reported diabetes were more likely to have severe cognitive impairment compared to participants who were nondiabetic (P < .05).
Table 1.
Descriptive Characteristics of 1033 Participants Included in Final Sample According to Severity of Cognitive Impairment.
| Cognitive Status | P- Value | |||
|---|---|---|---|---|
| Characteristic | Normal Cognition (n = 751) | Moderate Impaired (n = 220) | Severe Impaired (n = 62) | |
| Age, mean (SD) | 68.9 (6.9) | 70.0 (8.2) | 73.5 (9.2) | <.01 |
| Sex, n (%) | .25 | |||
| Male | 328 (71.8) | 106 (23.2) | 23 (5.0) | |
| Female | 423 (73.4) | 114 (19.8) | 39 (6.8) | |
| Education, n (%) | <.01 | |||
| 0 years | 137 (18.2) | 63 (28.6) | 26 (41.9) | |
| 1-6 years | 428 (57.0) | 112 (50.9) | 31 (5.4) | |
| 7+ years | 186 (78.8) | 45 (19.1) | 5 (2.1) | |
| Marital status, n (%) | .06 | |||
| Married | 428 (73.4) | 126 (21.6) | 29 (5.0) | |
| Not married | 147 (70.7) | 51 (24.5) | 10 (4.8) | |
| Widowed | 176 (72.7) | 43 (17.8) | 23 (9.5) | |
| Smoking, n (%) | .58 | |||
| Never | 463 (72.7) | 139 (21.8) | 35 (5.5) | |
| Former | 201 (71.3) | 59 (20.9) | 22 (7.8) | |
| Current | 87 (76.3) | 22 (19.3) | 5 (4.4) | |
| Alcohol consumption, n (%) | .16 | |||
| Abstainer | 83 (65.9) | 34 (27.0) | 9 (7.1) | |
| Former | 483 (73.3) | 132 (20.0) | 44 (6.7) | |
| Current | 185 (74.6) | 54 (21.8) | 9 (3.6) | |
| Hypertension, n (%) | .06 | |||
| No | 377 (73.3) | 115 (22.4) | 22 (4.3) | |
| Yes | 374 (72.1) | 105 (20.2) | 40 (7.7) | |
| bStroke, n (%) | .10 | |||
| No | 740 (73.0) | 215 (21.2) | 59 (5.8) | |
| Yes | 11 (57.9) | 5 (26.3) | 3 (15.8) | |
| Heart attack, n (%) | .52 | |||
| No | 721 (72.8) | 212 (21.4) | 58 (5.9) | |
| Yes | 30 (71.4) | 8 (19.0) | 4 (9.5) | |
| Depression, n (%) | <.01 | |||
| No | 575 (75.9) | 160 (21.1) | 23 (3.0) | |
| Yes | 176 (64.0) | 60 (21.8) | 39 (14.2) | |
| Physical exercise, n (%) | <.01 | |||
| No | 327 (53.0) | 160 (25.9) | 130 (21.1) | |
| Yes | 262 (63.0) | 83 (20.0) | 71 (17.1) | |
| HbA1c, mean (SD) | 6.8 (1.7) | 6.8 (1.6) | 7.2 (1.7) | .13 |
| BMI, mean (SD) | 28.9 (5.3) | 27.2 (4.8) | 27.8 (5.3) | <.01 |
| Diabetes status, n (%) | <.01 | |||
| Nondiabetic | 429 (72.8) | 135 (22.9) | 25 (4.2) | |
| Undiagnosed diabetic | 149 (74.1) | 41 (20.4) | 11 (5.5) | |
| Self-report diabetic | 173 (71.2) | 44 (18.1) | 26 (10.7) | |
Note. Bold indicates significance of P < 0.05.
aN = 1033 participants included in final sample. Analysis of variance and χ2 tests used to determine differences in continuous and categorical characteristics, respectively, according to diabetes status. Percentages based on row totals.
bIncludes participants who reported experiencing possible stroke or transient ischemic attack.
Of the 1033 participants included in the final sample, 243 reported being diagnosed with diabetes, 201 were classified as undiagnosed diabetes, and 589 were classified as nondiabetic. Participants with self-reported diabetes were younger, were less likely to exercise, and were more likely to report having been diagnosed with hypertension or a heart attack, to be a former alcohol consumer, and had lower BMI compared to participants with undiagnosed diabetes or nondiabetics (all Ps < .05). Also, participants with self-reported diabetes had higher HbA1c concentration compared to nondiabetics (P < .05) and undiagnosed diabetes (P < .05).
Diabetes Status and Cognitive Impairment
A summary of the results for the association between diabetes and severity of cognitive impairment is provided in Table 2. Significant differences in the odds for severe but not moderate cognitive impairment according to diabetes status were detected in all 3 of the models. In model 1, participants with self-reported diabetes had 2.70 (95% confidence interval [CI] = 1.39-5.32) times higher odds for severe cognitive impairment compared to nondiabetics, whereas participants with undiagnosed diabetes did not have significantly higher odds for severe cognitive impairment compared to nondiabetics (OR = 1.24, 95% CI = 0.53-2.76). The association between self-reported diabetes and severe cognitive impairment decreased when participants with undiagnosed diabetes were included in the nondiabetic category (model 2). Compared to nondiabetics, participants with self-reported diabetes had 2.53 times higher odds (95% CI = 1.37-4.66) when participants with undiagnosed diabetes were included in the nondiabetic category. When participants with undiagnosed or self-reported diabetes were included in the same category (model 3), diabetic participants had 1.88 times higher odds (95% CI = 1.04-3.46) for severe cognitive impairment compared to nondiabetics.
Table 2.
Odds Ratios for Moderate and Severe Cognitive Impairment According to Diabetes Status.a
| Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Model | Non Impaired | Moderate Impairment | Severe Impairment |
| Model 1 | |||
| Nondiabetic (ref) | ∼ | ∼ | ∼ |
| Undiagnosed diabetic | ∼ | 1.07 (0.70-1.61) | 1.24 (0.53-2.76) |
| Self-reported diabetic | ∼ | 0.92 (0.61-1.38) | b2.70 (1.39-5.32) |
| Model 2 | |||
| Nondiabetic or undiagnosed diabetic | ∼ | ∼ | ∼ |
| Self-reported diabetic | ∼ | 0.90 (0.61-1.33) | b2.53 (1.37-4.66) |
| Model 3 | |||
| Nondiabetic | ∼ | ∼ | ∼ |
| Self-reported or undiagnosed diabetic | ∼ | 0.90 (0.65-1.24) | c1.88 (1.04-3.46) |
aAll models adjusted for demographic, health, and behavioral characteristics. Model 1: undiagnosed and self-reported diabetics were included in separate categories. Model 2: participants with undiagnosed diabetes were included in the nondiabetic category. Model 3: undiagnosed diabetes combined with self-reported diabetics. Percentage contribution of undiagnosed diabetes: model 1 versus model 2, 10% decrease; model 1 versus model 3, 48% decrease.
b P < .01.
c P < .05.
Using the OR from model 1 and model 2 indicate that including cases of undiagnosed diabetes in the nondiabetic category contributed to a 6.3% decrease ([2.53-2.70]/2.70) in the association between self-reported diabetes and severe cognitive impairment. The OR from model 1 and model 3 indicate that including participants with undiagnosed diabetes in the same category as those with self-reported diabetes contributed to a 30.4% decrease ([1.88-2.70]/2.70) in the association between diabetes and severe cognitive impairment.
Discussion
This study presents evidence that not separating participants with undiagnosed diabetes from participants with self-reported diabetes or who do not have diabetes underestimates the association between self-reported diabetes and severe cognitive impairment. Self-reported diabetes was associated with 2.70 times higher odds for severe cognitive impairment when cases of undiagnosed diabetes were excluded from the nondiabetic category (model 1). This association decreased but remained statistically significant when participants with undiagnosed diabetes were included in the same category as participants who did not have diabetes (model 2) or had self-reported diabetes (model 3). The 2.70 times higher odds for severe cognitive impairment among older adults with self-reported diabetes is higher compared to other studies that did not account for undiagnosed diabetes. 3,17 -19 While differences in sample population characteristics and study design make it difficult to directly compare our findings to those from prior studies, previous studies that used self-reported data for diabetes status may have underestimated the association between self-reported diabetes and cognitive impairment.
Contrary to previous research, 20,21 we did not observe that undiagnosed diabetes was associated with moderate or severe cognitive impairment. Our inability to detect a statistically significant association may be due to a small sample size. It should also be noted the association between diabetes and severe cognitive impairment decreased by 30.4% when undiagnosed diabetics were combined with self-reported diabetics (model 3). This may be due to older adults with undiagnosed diabetes having lower disease severity than participants who had received a diagnosis of diabetes by a physician. This is supported by the significantly lower HbA1c concentration for participants with undiagnosed diabetes compared to participants with self-reported diabetes. Also, it may be necessary for a person to be exposed to diabetes for a certain period of time before negative effects on cognition can be observed. Participants with undiagnosed diabetes likely have shorter exposure to diabetes compared to participants who reported being diagnosed with diabetes and may not have been exposed to diabetes for a sufficient amount of time for the disease to negatively impact cognition. However, peripheral hyperinsulinemia has been associated with reduced levels of insulin in the brain, which may contribute to altered brain functioning and lead to cognitive impairment. 22
The findings from the present study have important implications. Type 2 diabetes is an important risk factor for dementia, and reducing the prevalence of type 2 diabetes may be an effective strategy in preventing older adults from developing dementia. Previous research indicates a 10% reduction in the global prevalence of type 2 diabetes could potentially prevent approximately 81 000 AD cases and a 25% reduction would prevent approximately 203 000 cases. 23 However, these calculations may underestimate the number of AD cases that can be prevented based on our finding that including cases of undiagnosed diabetes in the nondiabetic or self-reported diabetes categories underestimate the relationship between diabetes and severe cognitive impairment.
This study was able to use self-reported and biomarker data to determine diabetes status and identify undiagnosed cases of diabetes, but important limitations need to be acknowledged. First, this study used a cross-sectional design because data for HbA1c concentration were collected only during the 2012 Wave. A cross-sectional study design cannot account for the relationship between diabetes and mortality. 24 Not accounting for the competing risk of mortality has been found to result in an underestimation of the relationship between diabetes and cognition. 25 Second, HbA1c concentration is the only test of blood glucose included in the MHAS. The American Diabetes Association recommends that a positive test for diabetes be confirmed by repeating the same test. 9 An HbA1c concentration ≥6.5% has been deemed to have acceptable sensitivity and specificity, 26 but a high false negative rate for identifying diabetes cases using HbA1c among patients diagnosed using both fasting plasma glucose and 2-hour postprandial glucose tests has been reported. 27 Third, 282 participants were classified as having cognitive impairment but only 62 participants met the criteria for severe cognitive impairment. The limited number of participants with severe cognitive impairment may be due to participants with low cognitive functioning being unable to complete a direct interview. The MHAS includes a proxy measure to assess cognition among participants who do not receive or are unable to complete a direct interview. However, participants who required a proxy could not be included in the final sample because HbA1c data were collected only for participants who were directly interviewed. Finally, the 2012 subsample is not a random sample of the MHAS population. Therefore, our findings may not be generalizable to all of Mexico or to other populations with different physiological, behavioral, social, and cultural characteristics from Mexico.
In summary, this study presents evidence that the relationship between self-reported diabetes and severe cognitive impairment is underestimated when older adults with undiagnosed diabetes are not differentiated from those with self-reported diabetes or who do not have diabetes. These findings indicate that future studies need to account for undiagnosed diabetes when examining the association between diabetes and cognition. Given the importance of diabetes and cognitive impairment to public health, these findings need to be replicated using data from other sample populations.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the US National Institutes of Health National Institute on Aging [5T32 AG000270-17 to BD, RW; 2P2C HD065702-06; 5R01 AG018016-10 to RW; and P30 AG024832-11 to SAS].
References
- 1. Deckers K, van Boxtel MP, Schiepers OJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234–246. [DOI] [PubMed] [Google Scholar]
- 2. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. [DOI] [PubMed] [Google Scholar]
- 3. Mejia-Arango S, Gutierrez LM. Prevalence and incidence rates of dementia and cognitive impairment no dementia in the Mexican population: data from the Mexican Health and Aging Study. J Aging Health. 2011;23(7):1050–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mayeda ER, Haan MN, Kanaya AM, Yaffe K, Neuhaus J. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013;36(9):2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diaz-Apodaca BA, Ebrahim S, McCormack V, de Cosio FG, Ruiz-Holguin R. Prevalence of type 2 diabetes and impaired fasting glucose: cross-sectional study of multiethnic adult population at the United States-Mexico border. Rev Panam Salud Publica. 2010;28(3):174–181. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Wong R, Ottenbacher K, Al Snih S. Prediabetes, undiagnosed diabetes, and diabetes among mexican adults: findings from the Mexican Health and Aging Study. Ann Epidemiol. 2016;26(3):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong R, Michaels-Obregon A, Palloni A. Cohort profile: the Mexican Health and Aging Study (MHAS). [published January 27, 2015] Int J Epidemiol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy S, Ramsubeik K, Vega KJ, Federico J, Palacio C. Do HbA1C levels correlate with delayed gastric emptying in diabetic patients? J Neurogastroenterol Motil. 2010;16(4):414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(suppl 1):s11–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glosser G, Wolfe N, Albert ML, et al. Cross-cultural cognitive examination: validation of a dementia screening instrument for neuroepidemiological research. J Am Geriatr Soc. 1993;41(9):931–939. [DOI] [PubMed] [Google Scholar]
- 11. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKhann G, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Aging Study. Arch Gen Psychiatry. 2012;69(4):410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Debling D, Amelang M, Hasselbach P, Sturmer T. Diabetes and cognitive function in a population-based study of elderly women and men. J Diabetes Complications. 2006;20(4):238–245. [DOI] [PubMed] [Google Scholar]
- 15. Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11(2):139–148. [DOI] [PubMed] [Google Scholar]
- 16. Bender R, Grouven U. Using binary logistic regression models for ordinal data with non-proportional odds. J Clin Epidemiol. 1998;51(10):809–816. [DOI] [PubMed] [Google Scholar]
- 17. Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39(11):1392–1397. [DOI] [PubMed] [Google Scholar]
- 18. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Emam A, Elhaddad AA, Ramadan E. The risk of clinically diagnosed Alzheimer disease in patients with non insulin dependent diabetes mellitus. Egypt J Neurol Neurosurg Psychiatr. 2010;47(1):419–424. [Google Scholar]
- 20. Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia. 2009;52(6):1031–1039. [DOI] [PubMed] [Google Scholar]
- 21. Gao L, Matthews FE, Sargeant LA, Brayne C, Mrc C. An investigation of the population impact of variation in HbA1c levels in older people in England and Wales: from a population based multi-centre longitudinal study. BMC Public Health. 2008;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayeda ER, Haan MN, Yaffe K, Kanaya AM, Neuhaus J. Does type 2 diabetes increase rate of cognitive decline in older Mexican Americans? Alzheimer Dis Assoc Disord. 2015;29(3):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nathan DM, Balkau B, Bonora E, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo F, Moellering DR, Garvey WT. Use of HbA1c for diagnoses of diabetes and prediabetes: comparison with diagnoses based on fasting and 2-hr glucose values and effects of gender, race, and age. Metab Syndr Relat Disord. 2014;12(5):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]