Abstract
Background
Congenital hypothyroidism (CH) is a common endocrine disorder in newborns. The cause of CH is thyroid dysgenesis in 80%–85% of patients. Paired box gene 8 (PAX8) is a thyroid transcription factor that plays an important role in thyroid organogenesis and development. To date 22 different PAX8 gene mutations have been reported.
Methods
Four generations of a Hungarian Jewish family were affected and in the 3 generations studied, 9 males and 4 females were affected and 3 first degree relatives were unaffected. Six were diagnosed at birth (TSH 59–442 mU/L) and 7 were diagnosed at 2–48 years of age (TSH 6–223 mU/L). One affected patient had thyroid hemiagenesis on ultrasound.
Results
Direct sequencing of the PAX8 gene, revealed a novel single nucleotide substitution (c.162 A>T) in exon 2 that resulted in the substitution of the normal serine 54 with a cysteine (S54C), which segregated with elevated serum TSH levels. Other mutations of the same amino acid (S54G and S54R) have been also shown to produce functional impairment.
Conclusion
We report a large family with a novel mutation in the PAX8 gene presenting variable phenotype with high proportion of affected family members.
Keywords: genetic endocrine disorder, hypothyroid, novel mutation, thyroid hemiagenesis, PAX8, thyroid
Introduction
Congenital hypothyroidism (CH, OMIM 218700) is a common endocrine disorder in newborns with an incidence of 1: 4,000 to 1:1,500, depending on the assigned cutoff TSH value [1, 2]. In 80%–85% of cases, CH is secondary to thyroid dysgenesis which presents with a thyroid gland that may be absent (athyreosis), hypoplastic (hypoplasia), or located in an unusual position (ectopy). In the remaining 15–20% of cases, CH results from inborn errors of thyroid hormones biosynthesis, secretion or recycling (Dyshormonogenesis) [3]. Thyroid dysgenesis occurs mostly as a sporadic disease; however, a genetic cause has been demonstrated in about 5% of the reported cases. To date, mutations in genes involved in thyroid organogenesis have been identified in the following genes: thyroid transcription factors 1 and 2 (TTF1 or NKX2.1 and TTF2 or FOXE1), NK2 homeobox 5 (NKX2.5), thyrotropin-receptor (TSHR), transcription factor GLI similar 3 (GLIS3), and the paired box gene 8 (PAX8) [4]. [PAX8 OMIM: 167415]. The latter, a paired domain-containing protein belonging to the Pax family of transcription factors, is expressed in the thyroid gland, kidney, and central nervous system [5]. The PAX8 gene is located on human chromosome 2q12-q14 and consists of 11 exons encoding 128 amino acids protein, which plays an important role in thyroid organogenesis [4]. In the adult thyroid, PAX8 is an essential regulator of thyroid specific genes expression such as thyroid peroxidase (TPO), thyroglobulin (TG) and sodium/iodide symporter (NIS) [6]. PAX8 gene mutations are inherited in an autosomal dominant fashion [4] which contrasts with the recessive inheritance in Pax8 knock out mice [7]. To date 22 different PAX8 gene mutations have been reported in humans [4, 8]. Systematic PAX8 gene mutation screening was performed in 17 cohorts of patients with CH. Mutations occurred with a prevalence of 1.0%, ranging from 0 to 3.4% [8, 9] The clinical phenotype of individuals with identical PAX8 gene mutations can be variable, ranging from overt CH with severe thyroid hypoplasia to subclinical CH with a morphologically normal gland. The majority of PAX8 gene mutations are located within the DNA-binding paired domain and result in a severe reduction in DNA binding affinity [3].
Patient and Methods
Case history
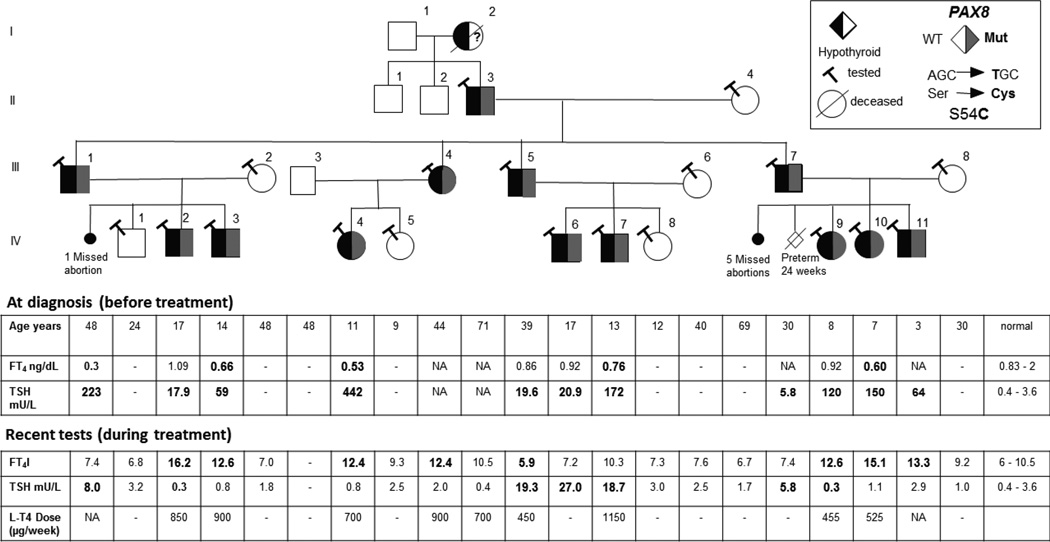
Four generations of a Jewish family of Hungarian origin were affected. Of the three generations studied 9 males and 4 females were affected and 3 were unaffected first degree relatives (Figure 1). Of the 6 miscarriages, 5 occurred in one woman (III-8). Clinical features of all family members are shown in Table 1. Affected individuals had hypothyroidism of variable severity diagnosed at different ages. None of them had autoantibodies to thyroperoxidase or thyroglobulin. Six (patient IV-3, IV-4, IV-6, IV-9, IV-10 and IV-11) were diagnosed at birth with TSH values ranging from 59 to 442 mU/L. The remaining 7 affected individuals were diagnosed at 2–48 years of age with serum TSH values from 6 to 223 mU/L. Note that in Israel the screening program for congenital hypothyroidism is based on measurement of total T4 followed by a confirmatory TSH test on samples with T4 values below the 10th percentile. Thus, it is possible that individuals with T4 within the normal range (for example IV-7) were missed in the neonatal screening. Of note is a striking variability in the initial clinical presentation within the family. For example, in the first nuclear family, patient IV-3 was identified by neonatal screening while his affected brother (IV-2) had a normal TSH value upon neonatal screening and was found to have subclinical hypothyroidism at the age of 6 yr. His father (III-1) was found to have overt hypothyroidism at the age of 48 year when he was admitted to a hospital for severe weakness. He also developed Parkinson’s and chronic kidney disease of unknown cause with normal renal ultrasound. Renal function tests (serum urea and creatinine) were normal in all affected individuals. Ultrasound of the kidney in four other affected family members (IV-6, IV-7, IV-10 and II-3) was normal. In the second nuclear family, patient IV-4 was found to be hypothyroid following neonatal study while her mother was diagnosed at the age of 14 yr. after presenting with delayed puberty. No tests results prior to treatment could be found. In the third nuclear family, patient IV-6 was found to be hypothyroid following neonatal screening while his younger brother (IV-7) was diagnosed at the age of 3 yr. with very high TSH. His father (III-5) was found to have high TSH in adulthood, after presenting with dizziness without hypothyroidism related manifestations. In the fourth nuclear family, patient IV-9 was found to have congenital hypothyroidism with thyroid hemiagenesis, while in her brother (IV-11), hypothyroidism was diagnosed at the age of 3 yr. He had normal thyroid gland imaging and his father (III-7) had asymptomatic subclinical hypothyroidism identified only while testing for the present study. Six affected individuals had neurologic and cognitive abnormalities, developmental delay and/or attention deficit hyperactive disorder.
Figure 1.
Pedigree of the family and results of thyroid function test before and after treatment. Round and square symbols denote females and males, respectively. Each generation corresponds to a roman number. Arabic numbers above each symbol identify the subjects. Laboratory data are aligned below each symbol. Abnormal values are in bold number. Filled and open symbols denote hypothyroid and euthyroid subjects, respectively. Gray symbol denotes presence of heterozygous PAX8 gene mutation. Question mark in the symbol of subject I-2 indicates that while she likely carried the PAX8 gene mutation, this was not confirmed as she was diseased at the time the genetic study was undertaken. Abbreviations: TSH, thyroid-stimulating hormone; FT4; free thyroxine.
Table 1.
Clinical and Genetic Characteristics of Examined Family Members (Abnormal values are in bold number)
| Family Member |
Mutation Carrier |
Gender | Age (years) |
Age at Diagnosis (years) |
TSH at diagnosis (mU/L)* |
FT4 at diagnosis (ng/dl)** |
Thyroid imaging | Current height in cm (SD) |
Current L-T4 Dose (µg/week) |
Comorbidity |
|---|---|---|---|---|---|---|---|---|---|---|
| II-3 | yes | M | 71 | NA | NA | NA | Normal position, texture and size a |
171.5c (−0.8) |
700 | - |
| II-4 | no | F | 69 | - | - | - | - | NA | - | - |
| III-1 | yes | M | 48 | 48 | 223 | <0.3 | NA | 166c (−1.6) |
Unknown dose |
CKD, PD |
| III-2 | no | F | 48 | - | - | - | - | 152c (−1.5) |
- | - |
| III-4 | yes | F | 44 | 14 | NA | NA | NA | 162c (−0.05) |
900 | - |
| III-5 | yes | M | 39 | 30 | 19.6 | 0.86 | NA | 169.8c (−0.8) |
450 | - |
| III-6 | no | F | 40 | - | - | - | - | 167.5c (0.65) |
- | - |
| III-7 | yes | M | 30 | 30 | 5.8 | - | NA | 178c (0.5) |
- | - |
| III-8 | no | F | 30 | - | - | - | - | 152c (−1.5) |
- | - |
| IV-1 | no | M | 24 | - | - | - | - | 171c (−0.7) |
- | - |
| IV-2 | yes | M | 17 | 6 | 17.9 | 1.09 | NA | 167.2 (−1.15) |
850 | - |
| IV-3 | yes | M | 14 | NS | 59 | 0.66 | NA | 147 (−1.96) |
900 | ADHD IGHD |
| IV-4 | yes | F | 11 | NS | 442 | 0.53 | Normal position, normal texture a |
135.3 (−1.31) |
700 | DD |
| IV-5 | no | F | 9 | - | - | - | - | 135 (0.097) |
- | ADHD |
| IV-6 | yes | M | 17 | NS | 20.9 | 0.92 | Hypoechoic, normal position a |
NA | - | LD |
| IV-7 | yes | M | 13 | 3 | 172 | 0.76 | Normal thyroid scan b | 154 (−0.55) |
1150 | ADHD |
| IV-8 | No | F | 12 | - | - | - | - | 153 (0.41) |
- | ADHD |
| IV-9 | yes | F | 8 | NS | 120 | 0.92 | Thyroid hemiagenesis a | 120 (−2.08) |
455 | - |
| IV-10 | yes | F | 7 | NS | 150 | 0.60 | Normal thyroid scan b | 115 (−1.44) |
525 | - |
| IV-11 | yes | M | 3 | NS | 64 | NA | Normal thyroid scan b | 86 (−1.65) |
unknown dose |
DD |
Normal range of TSH: adult 0.4–3.6 mU/L, at birth <20 mU/L
Normal range of FT4: adult 0.83–2 ng/dl, at birth 0.93–1.48 mg/dl
ultrasound thyroid,
technetium scan,
final height
Abbreviations: NA; not available, NS; newborn screening, L-T4; levothyroxine, CKD; chronic kidney disease, IGHD; idiopathic growth hormone deficiency, DD; developmental delay, ADHD: attention deficit hyperactive disorder, LD; learning disorder, SD; standard deviation
Thyroid function tests
Blood was collected locally and shipped for analysis to the Chicago laboratory. TSH, total T4 (TT4) and total T3 (TT3) were measured on the Elecsys Automated System (Roche Molecular Biochemicals GmbH and Hitachi, Ltd., Indianapolis, IN) platform. TrT3, by ZenTech (Liege, Belgium), TG by in house radioimmunoassay, and antibodies to TG and TPO by the Kronus (Star, ID). The free T4 index (FT4I) was calculated from the TT4 and the resin T4 uptake ratio.
DNA analysis
The clinical and genetic studies were approved by the institutional IRBs. After written informed consent was obtained from all participating family members, genomic DNA from peripheral mononuclear blood cells was isolated using QIAamp DNA Mini Kit (QIAGEN) followed by amplification of genomic DNA by polymerase chain reaction and direct sequencing of the PAX8 gene, exons 0 through exon 11. All PCR samples were sequenced using automated fluorescence-based sequencing (373OXL 96 capillary, Applied Biosystem Carlsbad, CA). Primers sequences are available upon request.
Result
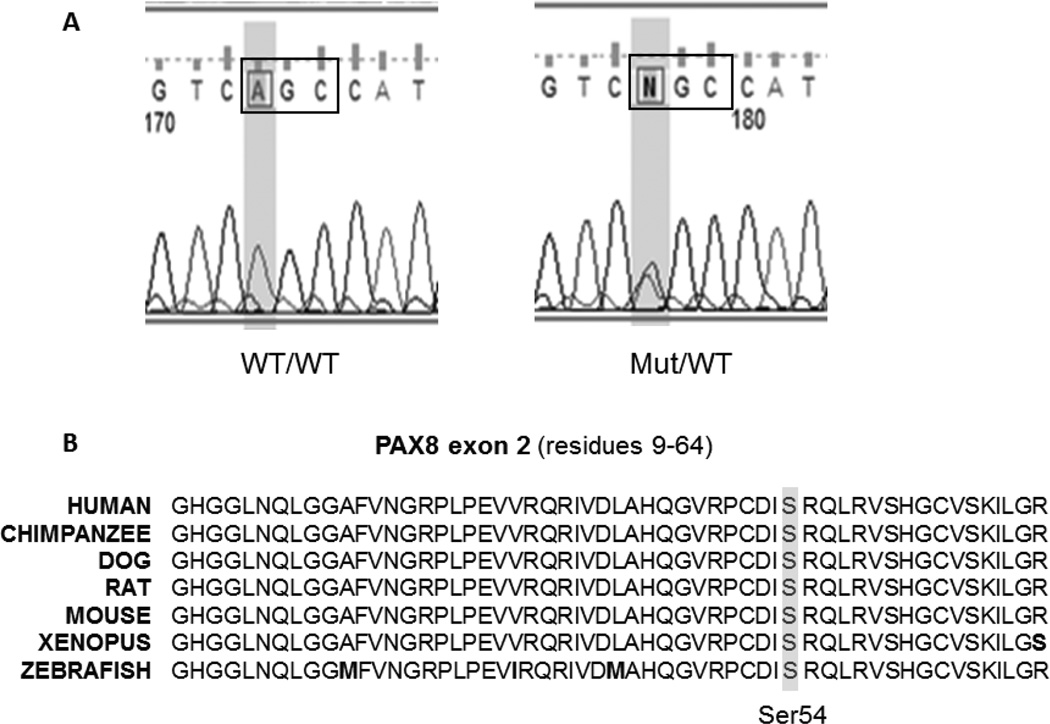
Direct sequencing of exon 0–11 of the PAX8 gene revealed a novel single nucleotide substitution in exon 2 of the PAX8 gene (c.162 A>T) that resulted in the substitution of the normal serine 54 with a cysteine (S54C) (Figure 2A). This S54C mutation cosegregated with a biochemical hypothyroid phenotype in all three-generation tested (Figure 1). Evaluation of the new sequence alterations using ‘PolyPhen-2’ indicated that this variants is “probably damaging”, with a score of 0.997.
Figure 2.
(A) Chromatograms showing sequences for normal (WT/WT) and heterozygote (Mut/WT) covering the region of the mutation. (B) Alignment of the Pax8 amino acid sequence encoded by exon 2 and containing the mutant Ser54 in various species including mammals amphibians and fish. Amino acid differences are in bold letters.
Discussion
We identified a novel mutation in the PAX8 gene, present in all 13 affected individuals of the family. The thyroid phenotype was considerably variable, with respect to i) thyroid function (overt to subclinical hypothyroidism), ii) onset of disease (at birth to late adulthood), iii) thyroid gland anatomy (hemiagenesis to normal). Variable phenotypic expression in PAX8 mutations has been reported in several families. Patients can be euthyroid or severely hypothyroid and the thyroid development can range from athyreosis to normal-sized orthotropic gland [4, 10–16]. Possible explanations for interfamilial variability include a polygenic etiology, epigenetic mechanisms that cause stochastic variations of gene expression at multiple loci, variation in the timing of PAX8 expression in embryonic life, or somatic mutations with a dominant effect in a thyroid development gene [17].
The S54C Pax8 mutation affects a highly conserved amino acid in the paired domain which lies between the second and the third helical region of the N terminal homeodomain-like motif (Figure 2B). Other mutations of the same amino acid (S54G and S54R) have been also shown to exhibit functional impairment. Meeus et al, identified a S54G mutation in a French family with CH. In addition, one of the affected siblings displayed unilateral kidney disease. Functional analysis of the mutant PAX8 demonstrated that it is unable to bind a specific cis-acting element of the promoter and has almost lost the ability to control together with TTF1 TG gene transcription [18]. Hermanns et al, reported a S54R mutation in 2 members of Turkish family. In vitro studies showed that the mutant protein had an impaired binding to the TPO and TG gene promoter-binding sites and exerted a dominant negative effect on the wild type PAX8 [19]. Both reports support a functional impairment of a mutant amino acid at this location.
The PAX8 gene is expressed in the kidney and plays an important role in its development. Urogenital malformations (horseshoe kidney, undescended testes, hydrocele, ureterocele and kidney agenesis) associated with PAX8 gene mutations have been previously reported [18, 20]. One of affected individual of this family had chronic kidney disease but kidney ultrasound showed no abnormal urogenital malformation. Four other affected members of the family were found to have normal kidney ultrasounds. Interestingly, six affected members of the family had neurologic or cognitive abnormalities including Parkinsonism, developmental delay and attention deficit hyperactive disorder (ADHD). This suggests a possible association between the PAX8 gene mutation and neurocognitive impairment, which has not been previously reported. The PAX8 gene is transiently expressed during development in the myencephalon and in the entire length of the neural tube, but no expression is detected in brain at later stages or in adults [5]. Its role in these tissues has not been well demonstrated as in kidney and thyroid and no neurological dysfunction is evidenced in Pax8 knock out mice [7]. Thus, it is currently not possible to determine whether the neurological manifestations could be attributed to the mutation. In this respect, it should be noted that two of the four family members with ADHD did not carry the PAX8 gene mutation (IV-5 and IV-8). Another unusual feature is the high proportion of individuals harboring the mutation (13 of 16) when in autosomal dominant inheritance one would have expected equal number of affected and unaffected.
In conclusion, we report a large family with a novel PAX8 gene mutation (S54C) causing autosomal dominant CH of variable expressivity and high proportion of affected individuals.
Established facts
PAX8 gene mutations are one of the genetic causes of thyroid dysgenesis.
To date 22 different PAX8 gene mutations have been reported in humans.
Novel insights
Identification of a novel single nucleotide substitution (c.162 A>T) in the PAX8 gene that resulted in the replacement of the normal serine 54 with a cysteine (S54C), in a large family with variable magnitude of hypothyroidism but a high proportion of affected individuals.
Acknowledgments
This work is supported in part by grant R37DK15070 from the National Institutes of Health and the Seymour J. Abrams fund for thyroid research (to S.R.). We thank all family members for their participation in this study. We are grateful to the following colleagues for advice in the course of investigation: Dr. Roy E. Weiss, Dr. Alexandra M. Dumitrescu and Dr. Theodora Pappa
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Panudda Srichomkwun, Email: panuddas@uchicago.edu.
Osnat Admoni, Email: Admoni_O@clalit.org.il.
Samuel Refetoff, Email: srefetof@uchicago.edu.
Liat de Vries, Email: liatd@clalit.org.il.
REFERENCES
- 1.Dattani M, Brook CG. Outcomes of neonatal screening for congenital hypothyroidism. Current opinion in pediatrics. 1996;8:389–395. doi: 10.1097/00008480-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Corbetta C, Weber G, Cortinovis F, Calebiro D, Passoni A, Vigone MC, Beck-Peccoz P, Chiumello G, Persani L. A 7-year experience with low blood tsh cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (ch) Clinical endocrinology. 2009;71:739–745. doi: 10.1111/j.1365-2265.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 3.Nettore IC, Cacace V, De Fusco C, Colao A, Macchia PE. The molecular causes of thyroid dysgenesis: A systematic review. J Endocrinol Invest. 2013;36:654–664. doi: 10.3275/8973. [DOI] [PubMed] [Google Scholar]
- 4.Pohlenz J, Van Vliet G, Deladoëy J. Developmental abnormalities of the thyroid. In: Roy E, Weiss SR, editors. Genetic Diagnosis of Endocrine Disorders. Academic Press; 2016. pp. 127–136. [Google Scholar]
- 5.De Felice M, Di Lauro R. Thyroid development and its disorders: Genetics and molecular mechanisms. Endocrine reviews. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- 6.Marotta P, Amendola E, Scarfo M, De Luca P, Zoppoli P, Amoresano A, De Felice M, Di Lauro R. The paired box transcription factor pax8 is essential for function and survival of adult thyroid cells. Mol Cell Endocrinol. 2014;396:26–36. doi: 10.1016/j.mce.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require pax8 gene function. Nature genetics. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 8.Fu C, Chen R, Zhang S, Luo S, Wang J, Chen Y, Zheng H, Su J, Hu X, Fan X, Luo J, Yi S, Lai Y, Li C, Xie B, Shen Y, Gu X, Chen S. Pax8 pathogenic variants in chinese patients with congenital hypothyroidism. Clinica chimica acta; international journal of clinical chemistry. 2015;450:322–326. doi: 10.1016/j.cca.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014;28:133–150. doi: 10.1016/j.beem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 10.de Sanctis L, Corrias A, Romagnolo D, Di Palma T, Biava A, Borgarello G, Gianino P, Silvestro L, Zannini M, Dianzani I. Familial pax8 small deletion (c.989_992delaccc) associated with extreme phenotype variability. J Clin Endocrinol Metab. 2004;89:5669–5674. doi: 10.1210/jc.2004-0398. [DOI] [PubMed] [Google Scholar]
- 11.Hermanns P, Grasberger H, Refetoff S, Pohlenz J. Mutations in the nkx2.5 gene and the pax8 promoter in a girl with thyroid dysgenesis. J Clin Endocrinol Metab. 2011;96:E977–E981. doi: 10.1210/jc.2010-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narumi S, Araki S, Hori N, Muroya K, Yamamoto Y, Asakura Y, Adachi M, Hasegawa T. Functional characterization of four novel pax8 mutations causing congenital hypothyroidism: New evidence for haploinsufficiency as a disease mechanism. Eur J Endocrinol. 2012;167:625–632. doi: 10.1530/EJE-12-0410. [DOI] [PubMed] [Google Scholar]
- 13.Macchia PELP, Krude H, Pirro MT, Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A, Fenzi G, Grüters A, Busslinger M, Di Lauro R. Pax8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nature Genetic. 1998;19:83–86. doi: 10.1038/ng0598-83. [DOI] [PubMed] [Google Scholar]
- 14.Ramos HE, Carre A, Chevrier L, Szinnai G, Tron E, Cerqueira TL, Leger J, Cabrol S, Puel O, Queinnec C, De Roux N, Guillot L, Castanet M, Polak M. Extreme phenotypic variability of thyroid dysgenesis in six new cases of congenital hypothyroidism due to pax8 gene loss-of-function mutations. Eur J Endocrinol. 2014;171:499–507. doi: 10.1530/EJE-13-1006. [DOI] [PubMed] [Google Scholar]
- 15.Bereket ALX, Turoglu T, Aribal E, Refetoff S. Analysis of the pax8 gene in congenital hypothyroidism caused by different forms of thyroid dysgenesis in a father and daughter. J Pediatr Endocrinol Metab. 2004;17:1021–1029. doi: 10.1515/jpem.2004.17.7.1021. [DOI] [PubMed] [Google Scholar]
- 16.Congdon T, Nguyen LQ, Nogueira CR, Habiby RL, Medeiros-Neto G, Kopp P. A novel mutation (q40p) in pax8 associated with congenital hypothyroidism and thyroid hypoplasia: Evidence for phenotypic variability in mother and child. J Clin Endocrinol Metab. 2001;86:3962–3967. doi: 10.1210/jcem.86.8.7765. [DOI] [PubMed] [Google Scholar]
- 17.Fagman H, Nilsson M. Morphogenetics of early thyroid development. Journal of molecular endocrinology. 2011;46:R33–R42. doi: 10.1677/jme-10-0084. [DOI] [PubMed] [Google Scholar]
- 18.Meeus L, Gilbert B, Rydlewski C, Parma J, Roussie AL, Abramowicz M, Vilain C, Christophe D, Costagliola S, Vassart G. Characterization of a novel loss of function mutation of pax8 in a familial case of congenital hypothyroidism with in-place, normal-sized thyroid. J Clin Endocrinol Metab. 2004;89:4285–4291. doi: 10.1210/jc.2004-0166. [DOI] [PubMed] [Google Scholar]
- 19.Hermanns P, Shepherd S, Mansor M, Schulga J, Jones J, Donaldson M, Pohlenz J. A new mutation in the promoter region of the pax8 gene causes true congenital hypothyroidism with thyroid hypoplasia in a girl with down's syndrome. Thyroid. 2014;24:939–944. doi: 10.1089/thy.2013.0248. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho A, Hermanns P, Rodrigues AL, Sousa I, Anselmo J, Bikker H, Cabral R, Pereira-Duarte C, Mota-Vieira L, Pohlenz J. A new pax8 mutation causing congenital hypothyroidism in three generations of a family is associated with abnormalities in the urogenital tract. Thyroid. 2013;23:1074–1078. doi: 10.1089/thy.2012.0649. [DOI] [PubMed] [Google Scholar]