Abstract
The superficial peroneal nerve is vulnerable to damage from ankle sprain injuries and fractures as well as surgery to this region. And it is also one of the most commonly involved nerves in complex regional pain syndrome type II in the foot and ankle region. We report two cases of ultrasound-guided pulsed radiofrequency treatment of superficial peroneal nerve for reduction of allodynia in CRPS patients.
Keywords: Allodynia, Complex regional pain syndrome, Neuralgia, Peripheral nerve, Pulsed radiofrequency treatment, Ultrasonography
The superficial peroneal nerve (SPN), a branch of the common peroneal nerve, is vulnerable to damage from ankle sprain injuries, fractures and surgeries to this region. And it is also one of the most commonly involved nerves in complex regional pain syndrome (CRPS) type II in the foot and ankle region [1,2].
Pulsed radiofrequency treatment (PRF) has been used forvarious types of pain treatments and gained popularity in recent years. Although the exact mechanisms of PRF are not yet fully understood until now, PRF is a very attractive procedure because it has several advantages over conventional (thermal) radiofrequency ablation such as simplicity and safety [3]. Introduction of ultrasound technique in various types of peripheral nerve blocks have made these procedures much easier and the same is true with PRF. Thus, its applications for the treatment of the peripheral nerve have been increasing recently. However, the clinical evidenceabout the efficacy of PRF on the peripheral nerve is even more limited, particularly in regards to neuropathic pain.
Current literatures suggested that PRF can reduce mechanical allodynia in animal models [4,5] but there are few reports about clinical experiences regarding the efficacy of PRF on allodynia. Herein, we report a successful use of ultrasound-guided pulsed radiofrequency treatment of the superficial peroneal nerve for the reduction of allodynia in CRPS patients.
CASE REPORT
1. Case 1
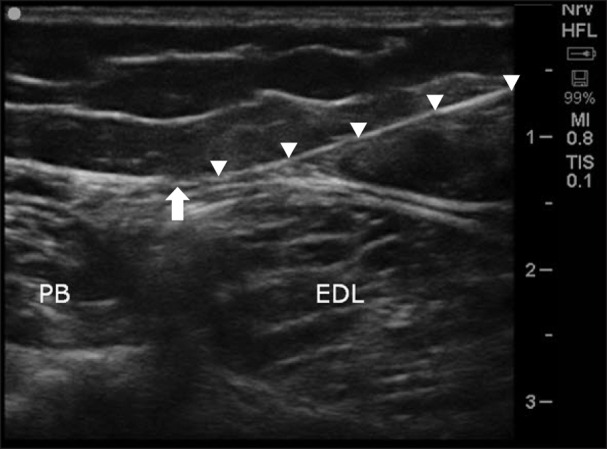
A 51-year-old female patient (BMI 39.3) was referred our clinic, complaining of tactile allodynia, coldness, and electric shock-like pain on her right ankle. Mechanical allodynia was assessed with a brush (visual analogue scale [VAS] score of 7/10) and thermal allodynia was assessed by applying ice and hot water to the affected area (VAS score of 2-3/10). Her pain developed about 1 year earlier after an ankle fracture and subsequent surgery, and gradually worsened. She had already been diagnosed as CRPS type 2, and nerve conduction velocity and electromyographic studies revealed lesions of the SPN and sural nerve. She was treated with medication and physical therapy but it did not relieve her pain at all. A lumbar sympathetic block and subsequent lumbar sympathetic neurolysis was done and the coldness and electric shock-like pain was reduced from a VAS score of 7-8/10 to 2-3/10. However, the allodynia was not diminished (a VAS score of 7/10). As her pain was mainly confined to the anterior ankle and foot dorsum, the SPN block was tried with 3 ml of 1% mepivacaineat in the mid-calf area using an in-plane ultrasound guided approach with a 6-13 MHz linear array transducer (SonoSite S-nerve, SonoSite Inc., Bothell, WA; Linear 6–13 MHz) and it relieved her pain by about 70% (the VAS decreased from 7-8/10 to 2-3/10). However, as the reduction of pain lasted only for a few days, we decided to apply PRF on the SPN. We used a TCU 410 TC-Electrode inside the 22G disposable cannula of 0.9 × 150 mm cannula with an active tip of 5 mm, and the electrode was connected to a radiofrequency lesion generator (Neuro N50, Stryker Leibinger GmbH, Freiburg, Germany). After applying local anesthesia to the skin, the needle was inserted under ultrasound guidance close to the SPN (Fig. 1) where paresthesia was reported at less than 0.3 mA. PRF was applied for 120 seconds at 45 V, with an electrode tip temperature not exceeding 42℃, and it was repeated three times. The treatment resulted in a reduction of the VAS of the allodynia from 7-8/10 to 2-3/10 at the 2-week follow up. Pain relief was maintained during the 4-month follow-up, but was slightly exacerbated to a VAS score of 5-6.
Fig. 1. Ultrasound imaging of applying radiofrequency probe on superficial peroneal nerve. PB: peroneus brevis muscle, EDL: extensor digitorum longus muscle.
2. Case 2
A 20-year-old male patient (BMI 19.8) who presented with complaints of tactile allodynia and coldness in his right foot visited our clinic. He had been diagnosed as CRPS type 1 about two years earlier. He could not undergonerve conduction velocity and electromyographic studies because of severe allodynia in affected area. The assessment of the allodynia was also impossible because of his fear of anyone touching the affected foot. According to the patient, the VAS of the allodynia was 7-8/10. He had a history of treatment with medication, physical therapy, intravenous ketamine therapy, a lumbar sympathetic block, and lumbar sympathetic neurolysis, but their effects had not been satisfactory, especially in the management of the allodynia. We tried superficial peroneal nerve blocks using ultrasound and nerve stimulator guidance because his complaint about allodynia was dominant on the dorsal aspect of the right foot. It provided the reduction of allodynia symptom (the VAS decreasedfrom 7-8/10 to 3-4/10) but it lasted only a few days. PRF on the SPN underultrasound guidance was done in the same manner as described in Case 1. The VAS of the allodynia was decreasedfrom 7-8/10 to 3-4/10 at the 2-week follow up, and he could put on his socks, unlike previous visits with barefoot. The VAS was maintained at 3-4/10 at the 3-month follow-up, but gradually returned to the baseline level of pain after that.
DISCUSSION
CRPS is one of the most disastrous types of neuropathic pain. CRPS occurs mainly in the limbs and is characterized by abnormal pain, motor symptoms, sweating, edema, and autonomic changes of the skin such as hypertrichosis and abnormal sudomotor activity [6]. Several pharmacological and interventional therapies for the treatment of CRPS have been introduced, but there are no definitive treatment modalities established at this point [7].
Mechanical allodynia is pain due to a mechanical stimulus which does not normally provoke pain. It may cause functional impairment of daily activity and result in a declining quality of life. The mechanisms of allodynia are not fully understood but central sensitization is generally the accepted mechanism of allodynia like most neuropathic pain symptoms. Thus, medical treatments such as anticonvulsants, antidepressants, and opioids, or invasive interventional treatments, including sympathetic ganglion neurolysis/radiofrequency denervation, spinal cord stimulation, and the intrathecal pump, are generally accepted as the treatments of choice [8]. However, systemic administration of these medications produce frequent side effects, and interventional treatments are relatively invasive and expensive.
Meanwhile, in addition to peripheral sensitization, the peripheral mechanism has been suggested as a cause for allodynia [5,9]. And there are some reports that peripheral applied treatments can reduce the symptoms of CRPS patients including intradermal/subcutaneous botulinum toxin A, topical ketamine, and lidocaine patches [10,11,12].
PRF treatments are usually performed on the dorsal root ganglion or medial branch of spinal dorsal ramus [3], but PRF treatments on the peripheral nerves for the treatment of chronic pain have gained popularity in recent years [13,14]. There are also some reports about the efficacy of PRF treatment on neuropathic pain symptoms [15,16,17,18]. However, the exact mechanism of PRF in pain relief is still not known, but it is thought that the electromagnetic field may cause a neuromodulatory effect which can interrupt the gene expression of the sensory neurons and molecules involved in sensitization without damaging the surrounding tissue. It can also be applied in the neuropathic pain state [19]. Mechanical allodynia is known to be mediated by altered low-threshold A-β fiber functions such as phenotypic switching, sprouting, and the opening of polysynaptic excitatory synaptic pathways [20]. In our opinion, PRF treatment may affect the A-β input signal, and it can reduce dynamic mechanical allodynia symptoms, although the precise mechanism is not known. Additional controlled trials for proving the efficacy of PRF are needed, especially in neuropathic pain.
In conclusion, we report our experience with 2 patients with CRPS of the foot and ankle treated with PRF on SPN, and to our knowledge, this is the first report of applying PRF treatment on the SPN.
References
- 1.Trevino SG, Panchbhavi VK, Castro-Aragon O, Rowell M, Jo J. The "kick-off" position: a new sign for early diagnosis of complex regional pain syndrome in the leg. Foot Ankle Int. 2007;28:92–95. doi: 10.3113/FAI.2007.0017. [DOI] [PubMed] [Google Scholar]
- 2.Zengerink M, van Dijk CN. Complications in ankle arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2012;20:1420–1431. doi: 10.1007/s00167-012-2063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12:37–41. doi: 10.1007/s11916-008-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: a double-blind placebo-controlled trial of topical ketamine. Pain. 2009;146:18–25. doi: 10.1016/j.pain.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Ozsoylar O, Akçali D, Cizmeci P, Babacan A, Cahana A, Bolay H. Percutaneous pulsed radiofrequency reduces mechanical allodynia in a neuropathic pain model. Anesth Analg. 2008;107:1406–1411. doi: 10.1213/ane.0b013e31818060e1. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S. Complex regional pain syndrome. BMJ. 2015;351:h2730. doi: 10.1136/bmj.h2730. [DOI] [PubMed] [Google Scholar]
- 7.Gierthmühlen J, Binder A, Baron R. Mechanism-based treatment in complex regional pain syndromes. Nat Rev Neurol. 2014;10:518–528. doi: 10.1038/nrneurol.2014.140. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- 9.Coutaux A, Adam F, Willer JC, Le Bars D. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine. 2005;72:359–371. doi: 10.1016/j.jbspin.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Safarpour D, Salardini A, Richardson D, Jabbari B. Botulinum toxin A for treatment of allodynia of complex regional pain syndrome: a pilot study. Pain Med. 2010;11:1411–1414. doi: 10.1111/j.1526-4637.2010.00897.x. [DOI] [PubMed] [Google Scholar]
- 11.Ushida T, Tani T, Kanbara T, Zinchuk VS, Kawasaki M, Yamamoto H. Analgesic effects of ketamine ointment in patients with complex regional pain syndrome type 1. Reg Anesth Pain Med. 2002;27:524–528. doi: 10.1053/rapm.2002.35517. [DOI] [PubMed] [Google Scholar]
- 12.Karmarkar A, Lieberman I. Management of complex regional pain syndrome type II using lidoderm 5% patches. Br J Anaesth. 2007;98:261–262. doi: 10.1093/bja/ael343. [DOI] [PubMed] [Google Scholar]
- 13.Gofeld M, Restrepo-Garces CE, Theodore BR, Faclier G. Pulsed radiofrequency of suprascapular nerve for chronic shoulder pain: a randomized double-blind active placebo-controlled study. Pain Pract. 2013;13:96–103. doi: 10.1111/j.1533-2500.2012.00560.x. [DOI] [PubMed] [Google Scholar]
- 14.Akbas M, Luleci N, Dere K, Luleci E, Ozdemir U, Toman H. Efficacy of pulsed radiofrequency treatment on the saphenous nerve in patients with chronic knee pain. J Back Musculoskelet Rehabil. 2011;24:77–82. doi: 10.3233/BMR-2011-0277. [DOI] [PubMed] [Google Scholar]
- 15.Lim SM, Park HL, Moon HY, Kang KH, Kang H, Baek CH, et al. Ultrasound-guided infraorbital nerve pulsed radiofrequency treatment for intractable postherpetic neuralgia -a case report - Korean J Pain. 2013;26:84–88. doi: 10.3344/kjp.2013.26.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restrepo-Garces CE, Marinov A, McHardy P, Faclier G, Avila A. Pulsed radiofrequency under ultrasound guidance for persistent stump-neuroma pain. Pain Pract. 2011;11:98–102. doi: 10.1111/j.1533-2500.2010.00398.x. [DOI] [PubMed] [Google Scholar]
- 17.Fowler IM, Tucker AA, Mendez RJ. Treatment of meralgia paresthetica with ultrasound-guided pulsed radiofrequency ablation of the lateral femoral cutaneous nerve. Pain Pract. 2012;12:394–398. doi: 10.1111/j.1533-2500.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 18.Todorov L. Pulsed radiofrequency of the sural nerve for the treatment of chronic ankle pain. Pain Physician. 2011;14:301–304. [PubMed] [Google Scholar]
- 19.Randić M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]


