Abstract
Purpose
To investigate the epidemiology, clinical manifestations, investigations and management, and prognosis of patients with Henoch-Schonlein purpura (HSP).
Methods
We performed a retrospective review of 212 HSP patients under the age of 18 years who were admitted to Inje University Sanggye Paik Hospital between 2004 and 2015.
Results
The mean age of the HSP patients was 6.93 years, and the ratio of boys to girls was 1.23:1. HSP occurred most frequently in the winter (33.0%) and least frequently in the summer (11.3%). Palpable purpura spots were found in 208 patients (98.1%), and gastrointestinal (GI) and joint symptoms were observed in 159 (75.0%) and 148 (69.8%) patients, respectively. There were 57 patients (26.9%) with renal involvement and 10 patients (4.7%) with nephrotic syndrome. The incidence of renal involvement and nephrotic syndrome was significantly higher in patients with severe GI symptoms and in those over 7 years old. The majority of patients (88.7%) were treated with steroids. There was no significant difference in the incidence of renal involvement or nephrotic syndrome among patients receiving different doses of steroids.
Conclusion
In this study, the epidemiologic features of HSP in children were similar to those described in previous studies, but GI and joint symptoms manifested more frequently. It is essential to carefully monitor renal involvement and progression to chronic renal disease in patients ≥7 years old and in patients affected by severe GI symptoms. It can be assumed that there is no direct association between early doses of steroids and prognosis.
Keywords: Purpura, Schonlein-Henoch, Child, Nephritis, Prognosis, Signs and symptoms, digestive
INTRODUCTION
Henoch-Schonlein purpura (HSP) is the most common type of vasculitis in children [1,2]. Patients with mild clinical symptoms respond well to conservative treatment, but steroids are often required to improve severe abdominal pain and joint symptoms [3,4,5]. Renal involvement is a well-known predictor of poor prognosis, but the rate of progression to chronic renal disease is lower in children than in adults [6,7,8,9]. Hospitalization is often reserved for those cases complicated by severe abdominal or joint pain, and in those patients with proteinuria due to renal involvement. Most cases of HSP can be confidently diagnosed in the presence of the characteristic purpuric spots, but in cases that manifest as severe abdominal pain in the absence of purpura, obtaining a diagnosis can be difficult [10,11]. The use of high dose steroids may be considered in HSP patients presenting with severe gastrointestinal (GI) symptoms (i.e., abdominal pain and hematochezia) or persistent proteinuria. If severe GI symptoms or nephrotic syndrome persist despite steroid treatment, then other treatment modalities such as intravenous immunoglobulin (IVIG), immunosuppressive drugs, or plasmapheresis may be used [12,13,14,15,16,17]. In this study, we investigated the epidemiologic characteristics and clinical courses of children hospitalized with HSP at a tertiary hospital between 2004 and 2015.
MATERIALS AND METHODS
We performed a retrospective review of the medical records of 240 children hospitalized with HSP (diagnosis code: D69.0) at Inje University Sanggye Paik Hospital between January 2004 and February 2015. Three children who were diagnosed at other hospitals before being transferred to Inje University Sanggye Paik Hospital were also included.
A total of 212 patients were included in this study after exclusion of 54 patients due to the passage of more than 1 month from initial diagnosis at another hospital (14 patients), incomplete medical records (13 patients) and 27 due to incorrect coding (27 patients). The study protocol was approved by the Institutional Review Board of the Inje University Sanggye Paik Hospital (SP IRB no. 16-02-005). Diagnosis of HSP was based on American College of Rheumatology Classification Criteria [18]. Recurrence of HSP was defined as the recurrence of symptoms more than 1 month after remission. The data for sex, age, year and month of onset, presence of symptoms (skin, joint, abdominal, renal, and other organs), recurrence of symptoms, results of renal biopsy, dosage and duration of steroid treatment, duration of steroid tapering, IVIG treatment, results of endoscopic exams or radiological exams: abdominal ultrasonography (USG), small bowel series (SBS), computed tomography (CT), and blood tests: white blood cell (WBC) count, platelet count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), were collected by review of medical records. The visual analog scale generally used to evaluate abdominal pain is not applicable for young children with HSP. In this study, abdominal pain was classified into three groups as follows: 1) Severe abdominal pain group: requiring radiological examination to exclude intussusception or appendicitis, confirmation of intussusception, complications requiring surgical or endoscopic intervention, gross hematuria or hematochezia; 2) Mild abdominal pain group: showing GI symptoms not meeting the criteria of the severe group; and 3) No abdominal pain group.
Renal involvement was classified as follows: 1) hematuria-5 red blood cells at high power light microscopy; 2) proteinuria-urine protein:creatinine (UP/UCr) >0.5 in children under 2 years of age, UP/UCr >0.2 in children over 2 years of age; 3) simultaneously meeting the criteria for 1) and 2); and 4) proteinuria over 40 mg/m2/hr or nephrotic range proteinuria (UP/UCr >2.0).
Analysis of steroid treatment was performed in 113 patients who were followed up over a one month period, after the exclusion of 14 patients without steroid treatment and 8 patients with extreme steroid-dosage (<0.5 mg/kg/day or >2.5 mg/kg/day). A total of 113 patients were divided into two groups based on their initial prednisolone dosage: 1) high-dose group (0.5 mg/kg/day-1.5 mg/kg/day), n=94 and 2) low-dose group (1.5 mg/kg/day-2.5 mg/kg/day), n=19. Statistical analysis was performed with SAS ver. 5.1 (SAS Institute, Cary, NC, USA) by chi-square test, Mann-Whitney test, one-way analysis of variances, logistic regression analysis, and Fisher's exact test.
RESULTS
Demographic data
A total of 121 patients were included in the study. HSP was more prevalent in males (male:female= 1.23:1) and the average age of onset was 6.93 (1.75-17.83) years old. Between 2004 and 2007, the annual rate of hospitalization of patients diagnosed with HSP was between 20-25 patients, and this number decreased between 2008 and 2012 to 8-14 patients and subsequently increased to 19-24 patients between 2013 and 2015. The ratio of HSP patients to total hospitalized patients under 18 years old was as follows: 0.25-0.36% (2004-2007), 0.12-0.20% (2008-2012), and 0.31-0.38% (2013-2015). HSP admissions were most common in the winter (33.0%) and least common in the summer (11.3%). The incidence of HSP hospitalization was similar in spring (28.3%) and autumn (27.4%).
Clinical features
1. Clinical manifestation
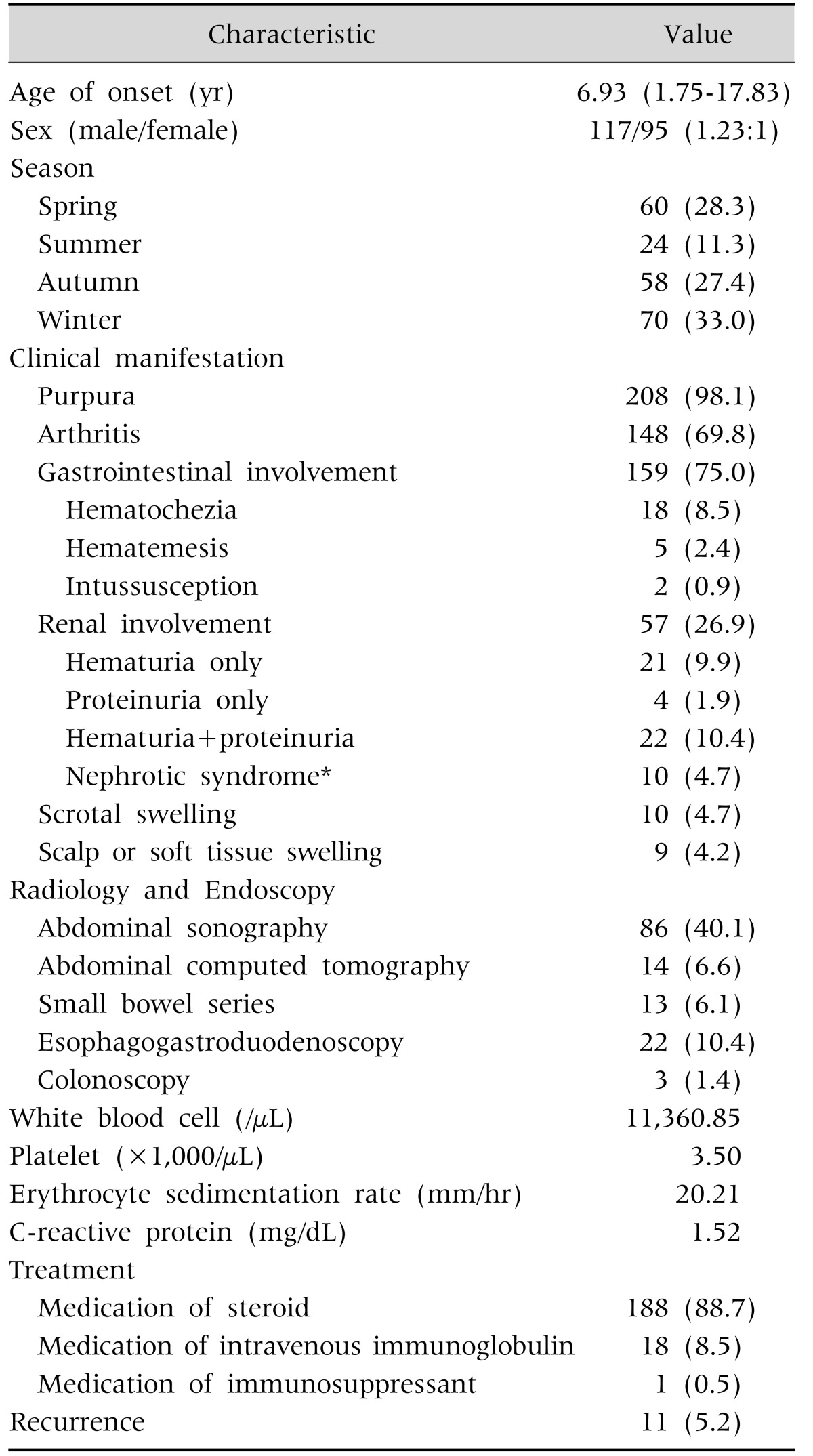
A purpuric rash was observed in 208 patients (98.1%) and joint symptoms were present in 148 patients (69.8%) (Table 1). GI symptoms were present in 159 patients (75.0%), hematemesis in 5 (2.4%), hematochezia in 18 (8.5%), and intussusception in 2 (0.9%). No patients developed complications requiring surgical intervention. Renal involvement was present in 57 patients (26.9%), isolated hematuria in 21 (9.9%), isolated proteinuria not in the nephrotic syndrome range in 5 (2.4%), and co-existence of hematuria and proteinuria in 22 (10.4%). Ten patients (4.7%) experienced proteinuria within the nephrotic range as well as hematuria. HSP nephritis (HSPN) was diagnosed by renal biopsy in 8 of 10 HSP patients associated with nephrotic syndrome (renal biopsy was not performed in 2 HSP patients with nephrotic syndrome as one was transferred to another hospital and the other demonstrated spontaneous recovery of proteinuria and hematuria before renal biopsy). Scalp edema was observed in 9 patients (4.2%) and scrotal edema in 10 patients (4.7%).
Table 1. Clinical Features of Henoch-Schonlein Purpura (n=212).
Values are presented as mean (range), number (%), or number only.
*Up/Ucr (urine protein:creatinine) <2.0 or proteinuria of less than 40 mg/m2/hr.
2. The difference of clinical manifestation according to age
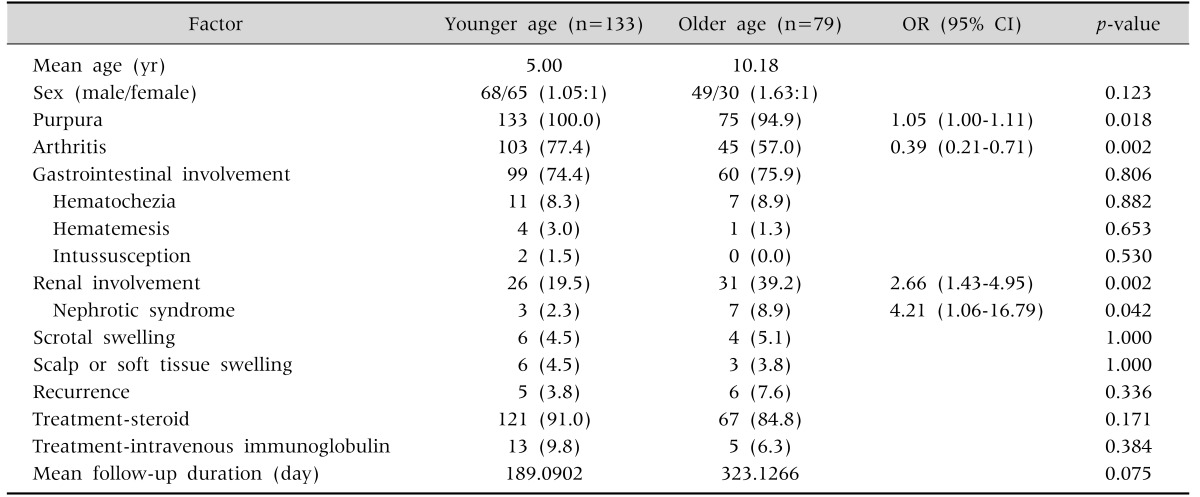
To investigate differences in clinical manifestations, treatment outcomes and prognosis of renal involvement according to age, patients were stratified based on whether they were older or younger than 7 years old (Table 2). A purpuric rash was observed in all HSP patients under 7 years old, but in only 5% of HSP patients over 7 years of age. Joint symptoms were observed frequently in HSP patients under 7 years of age (p-value=0.002; odds ratio [OR], 0.39). Renal involvement and nephrotic syndrome were more frequently observed in HSP patients over 7 years of age. No differences were found in GI symptoms, scrotal involvement, scalp edema, recurrence rate, and proportion of steroid or IVIG treatment between the two groups.
Table 2. Comparison between the Two Age Groups.
Values are presented as number only, number (%), or odds ratio [OR] (95% confidence interval [CI]).
3. The difference according to severity of GI symptoms
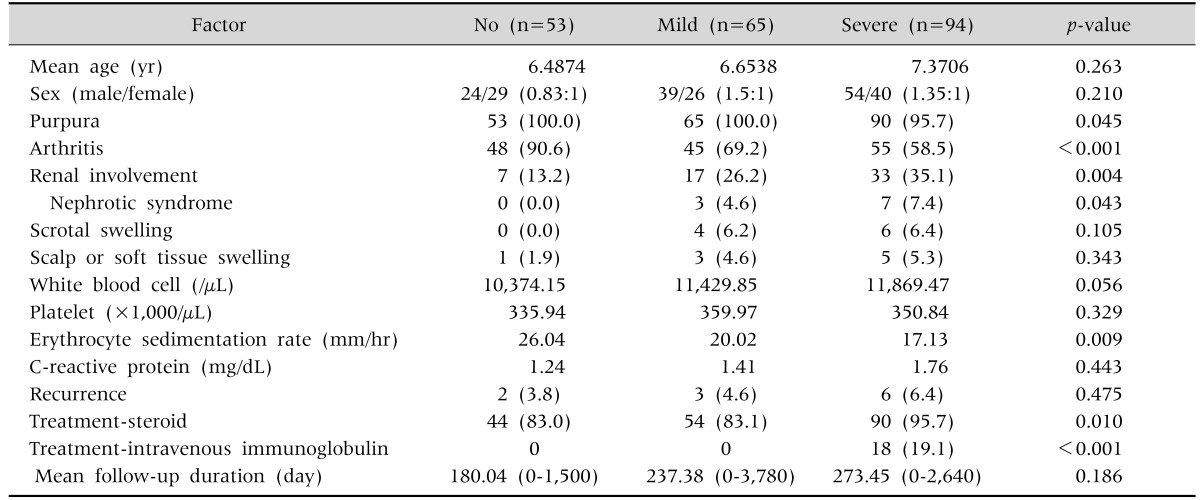
Renal involvement and nephrotic syndrome were more common in patients with severe GI symptoms than in those without GI symptoms, but joint symptoms were less frequently observed in patients with GI symptoms (Table 3). IVIG was only given to patients with severe GI symptoms. The rate of steroid treatment was 95.7% in the severe GI symptoms group, 83.1% in the mild GI symptoms group, and 83.0% in the no GI symptoms group. Scrotal involvement was observed only in patients showing GI symptoms irrespective of symptom grade. There were no significant differences in WBC count, platelet count, and CRP level among the groups. ESR was lower in the group with severe GI symptoms.
Table 3. Comparison between the Three Groups classified by Gastrointestinal Involvement.
Values are presented as number only, number (%), or mean (range).
4. Comparison based on renal involvement
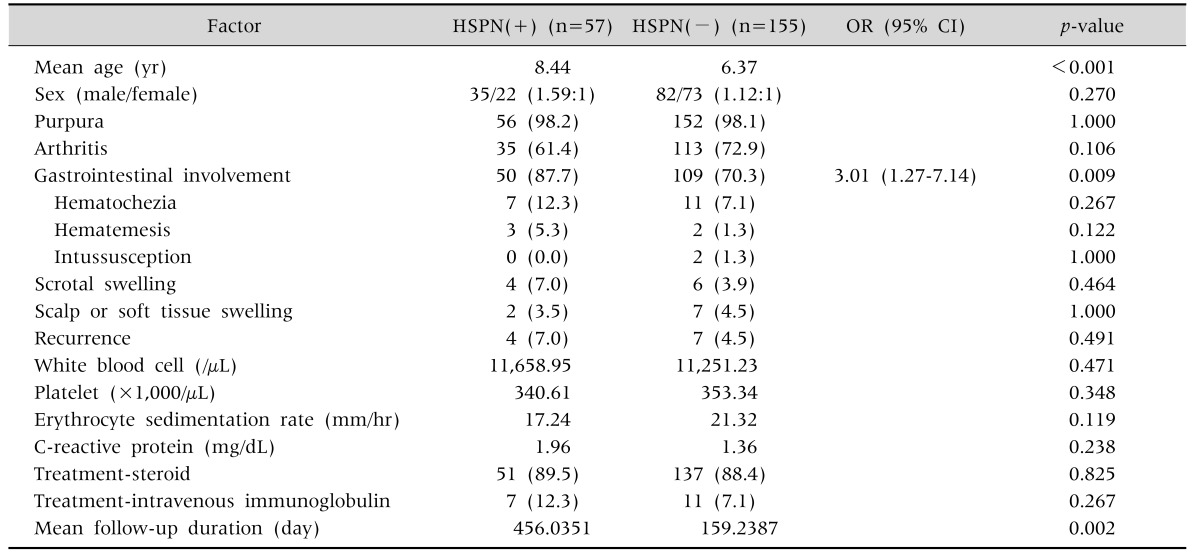
The average age of the renal involvement group was higher than that of the renal non-involvement group (8.44 vs. 6.37 years; p-value<0.001) (Table 4). GI symptoms were more frequently observed in patients with renal involvement than in those without (87.7% vs. 70.3%; OR, 3.01; p-value=0.009). Other factors such as joint symptoms, purpura, sex, recurrence, and laboratory tests showed no significant association with renal involvement.
Table 4. Univariate Analysis of the Risk Factors of Renal Involvement.
Values are presented as number only, number (%), or odds ratio [OR] (95% confidence interval [CI]).
HSPN: Henoch-Schonlein purpura nephritis.
Imaging study & endoscopy
1. Radiological examination
Abdominal USG was performed in 86 patients, SBS in 13 patients, and abdominal CT in 14 patients. Intussusception was diagnosed by USG in 2 patients (Table 1). Most patients had unremarkable findings, although occasionally focal bowel wall thickening was observed. In 9 of 13 HSP patients, segmental wall thickening was noted on the duodenum or small colon in SBS. In two patients with abdominal pain not associated with skin rash, steroid treatment was started on the impression of HSP after SBS. Abdominal CT showed focal bowel wall thickening in 6 patients.
2. Endoscopy
Twenty-two patients were imaged using a gastrofiberscopy and colonoscopy was performed in 3 patients. Twelve patients were found to have erosive gastritis, hemorrhagic gastritis or duodenal ulcers at endoscopy. Endoscopy was performed after diagnosis of HSP in most patients with the exception of two patients who experienced abdominal pain in the absence of a rash. Of the three children examined via colonoscopy, one was diagnosed with inflammatory bowel disease associated with HSP, and the others showed no specific findings.
Treatment and outcome
The majority (88.7%, n=188) of HSP children were treated with steroids. IVIG was used in 8.5% of cases (n=18) and one child received immunosuppressive therapy.
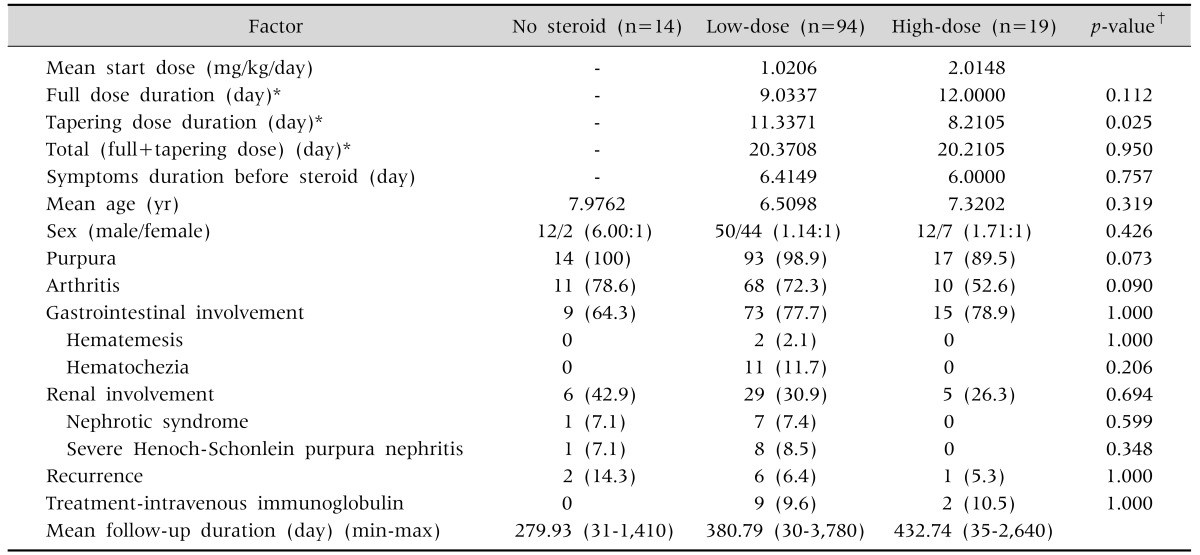
1. Steroid treated group
There was no statistically significant difference in average age, period of initial steroid treatment, presence of joint or GI symptoms, degree of renal involvement, and rate of IVIG treatment between the high and low dose groups (Table 5). The period of steroid treatment with full dose was 12 days in the high-dose group (steroid dose: 1.5-2.5 mg/kg/day) after excluding 5 patients with nephrotic syndrome requiring long-term treatment, and 9.03 days in the low-dose group (steroid dose: 0.5-1.5 mg/kg/day), and this difference was not statistically significant. The period of steroid treatment with tapering dose was significantly shorter in the high-dose group compared to the low-dose group (8.21 vs. 11.3 days, respectively; p=0.025). However, the period of total steroid treatment showed no difference between the high-dose group and low-dose group (20.37 vs. 20.21 days, respectively).
Table 5. Comparison between the Two Steroid-Treated Groups.
Values are presented as number only, number (%), or mean (range).
*Patients with nephrotic syndrome requiring long-term steroid treatment were excluded. †Comparison between high dose group and low dose group.
2. IVIG treated group
IVIG was administered to 18 patients who suffered from persistent abdominal pain despite steroid treatment (total dose=2 g/kg for 1 to 5 days). Complete remission of symptoms was observed in 6 patients within 24 hours after the end of the IVIG infusion. However, variable types of responses were noted after IVIG treatment, including partial improvement (n=5), temporary improvement of symptoms and relapse 2-3 days later (n=5), and no improvement (n=2). Five out of seven patients who experienced temporary or no improvement were transferred to other hospitals at their parent's request. In one patient, GI symptoms and purpuric rash persisted for one month despite treatment with steroids and cyclophosphamide, but completely improved after IVIG treatment. It was difficult to evaluate the long term prognosis of the IVIG treated group due to the high rate of transfer to other hospitals. Of the 18 patients treated with IVIG, only one patient developed nephrotic syndrome and was diagnosed with HSPN by renal biopsy. This patient recovered during the follow-up period.
3. Clinical course and prognosis
After exclusion of 8 transferred patients and 10 lost to follow up, 194 patients were followed up for an average of 261.2 days. Follow-up over one month was possible in 135 patients. Four patients with renal involvement were excluded because they did not visit the outpatient clinic after discharge. The average follow up period of the remaining 53 patients with renal involvement was 490.5 days and 46 patients were followed up for over 1 month. In 10 patients with renal involvement showing nephrotic range proteinuria, 9 cases manifested within one month after diagnosis and one case seven weeks after diagnosis. Persistent elevation of creatinine after discharge was observed in 2 patients. One patient showed aggravation of renal function (Cr 1.12, estimated glomerular filtration rate (eGFR) 85.61 mL/min/1.73 m2) 6 years after onset and was transferred to another hospital. In another patient, renal function (Cr 1.48, eGFR 69.55 mL/min/1.73 m2) was aggravated at 1 month after onset, but improved (Cr 1.01, eGFR >90 mL/min/1.73 m2, mild proteinuria) 2 years later. No patient progressed to end stage renal disease (ESRD) during the study period.
DISCUSSION
HSP is the most common type of vasculitis in children. It is a form of leukocytoclastic vasculitis and is characterized by IgA-mediated injury. It has an incidence of 8-22/100,000 and is more prevalent in males [1,2,19,20]. Although HSP could manifest in all age groups, including adults, it is most frequently diagnosed at 5-7 years of age [4,19,20,21,22,23]. In this study that included children under 18 years of age, the average age at first diagnosis was 6.93 years, and this finding is consistent with previous studies. The frequency of diagnosis of HSP in this study was slightly decreased during 2008-2012 and subsequently increased after 2013, but this was not significant statistically. The peak incidence of HSP was in the winter (33.0%) and lowest in the summer (11.8%), which is in keeping with findings from previous studies [20,23,24].
Rostoker [2] reported that the incidence of skin rash, joint and GI symptoms and renal involvement in one group of patients was 100%, 70%, 66%, and 37%, respectively. In Northern Spain [6], these values were 100%, 63.1%, 64.5%, and 41.2%, respectively, and 100%, 45.3%, 34.4%, and 44.7%, respectively, in Turkey [25]. In this study, the incidence of skin rash, joint and GI symptoms and renal involvement was 98.6%, 69.8%, 75.0%, and 26.9%, respectively, reflecting a higher incidence of GI symptoms but lower incidence of renal involvement compared to those of other countries. In other Korean studies, Choi and Lee [23] reported that skin rash, joint and GI symptoms and renal involvement were 100%, 40.8%, 53.8%, and 18.9%, respectively. In the study by Kang et al. [26], these values were 100%, 55.4%, 56.3%, and 30.4%, respectively, and 93%, 52.4%, 71.4%, and 10.8%, respectively in the study by Hong and Yang [27]. In the present study, the incidence of GI and joint symptoms was relatively high compared to in other Korean studies, which might be due to regional factors and the fact that our study population was recruited from hospitalized patients.
The rate of renal involvement was relatively low in Korea compared to that of other countries. In a Japanese study, Nagamori et al. [28] reported that the renal involvement rate was 15%, which is similar with that of Korea. However, this number was quoted to be as high as 49% in another Japanese study and 31.9% in a recent Chinese study [24,29]. These results suggest that the rate of renal involvement results from an association of multiple factors including ethnic, environmental, and access to medical services.
In the present study, joint symptoms were more frequently observed in younger children. This findings is consistent with the results of a recent Korean study and other studies comparing symptoms of HSP children with those of adults [6,7,9]. In HSP children without abdominal pain, joint pain was a major cause of hospitalization.
In this study, clinical manifestations were assessed by diving patients into two groups based on a reference age of 7 years. In previous studies that compared the clinical manifestations of HSP children with those of HSP adults, the rate of renal involvement was significantly higher in adults [6,7,9,26]. In children, renal involvement and association with nephrotic syndrome was frequently observed in those over 10 years of age [8,9]. Zhao et al. [24] proposed that age greater than 7 is a risk factor for renal involvement by multiple regression analysis. Rostoker [2] suggested that age greater than 5 is a risk factor for renal involvement, while other groups proposed that severe abdominal pain and age over 4 years are risk factors for renal involvement [29]. A recent Korean study stated that age of onset is a poor prognostic factor in HSPN patients [30]. It should be remembered that age of onset of HSP is the most important factor in evaluating prognosis, and thus it is essential to assess whether or not renal involvement is present in older children. In the present study, we found that only GI symptoms (p-value=0.033; OR, 2.701) and age (p-value<0.001) were significant factors associated with renal involvement, the latter of which increased the OR for renal involvement by 1.195 (1.083-1.318) for every 1 year increase in age. In this study, 7 years old (average age of total HSP patients: 6.93 years) was regarded as a reference age for grouping. We confirmed that the rate of renal involvement was significantly higher in older children, and occurred at the same rate in children aged between 6 years and 14 years. These findings suggest that the likelihood of renal involvement increases with age in children with HSP.
In this study, clinical manifestations were assessed by diving patients into two groups based on a reference age of 7 years. In previous studies that compared the clinical manifestations of HSP children with those of HSP adults, the rate of renal involvement was significantly higher in adults [6,7,9,26]. In children, renal involvement and association with nephrotic syndrome was frequently observed in those over 10 years of age [8,9]. Zhao et al. [24] proposed that age greater than 7 is a risk factor for renal involvement by multiple regression analysis. Rostoker [2] suggested that age greater than 5 is a risk factor for renal involvement, while other groups proposed that severe abdominal pain and age over 4 years are risk factors for renal involvement []. A recent Korean study stated that age of onset is a poor prognostic factor in HSPN patients [30]. It should be remembered that age of onset of HSP is the most important factor in evaluating prognosis, and thus it is essential to assess whether or not renal involvement is present in older children. In the present study, we found that only GI symptoms (p-value=0.033; OR, 2.701) and age (p-value<0.001) were significant factors associated with renal involvement, the latter of which increased the OR for renal involvement by 1.195 (1.083-1.318) for every 1 year increase in age. In this study, 7 years old (average age of total HSP patients: 6.93 years) was regarded as a reference age for grouping. We confirmed that the rate of renal involvement was significantly higher in older children, and occurred at the same rate in children aged between 6 years and 14 years. These findings suggest that the likelihood of renal involvement increases with age in children with HSP.
It was initially thought that renal involvement was not usually present at the time of diagnosis. However, several researchers have recently reported that renal involvement is evident in 75-90% of children with HSP within 1 month of diagnosis [1,2,20,31]. These findings were similar to our own, which showed that 90% of cases of renal involvement were manifest within 1 month of HSP onset.
In this study, there was an association between GI symptoms and scrotal involvement, but no significant association was observed between scrotal involvement and renal involvement. Wang et al. [32] reported an association between scrotal involvement, GI symptoms, raised D-dimer, and HSPN. Another recent study in Korea also showed that scrotal involvement was found only in patients with abdominal symptoms [33].
In this study, blood results on admission did not differ among groups. In patients with severe GI symptoms, the ESR was low, but this finding could not be explained rationally. In a previous study, Nagamori et al. [28] suggested that a scoring system comprising 6 laboratory tests (WBC, neutrophil count, albumin, D-dimer, coagulation factor XIII, and sodium) could be used for prognostic purposes. Recently, Hong and Yang [27] suggested a possible association between the grade of acute GI involvement, D-dimer, and fibrin degradation product. Wang et al. [32] reported that the occurrence of HSPN might be increased in patients with joint symptoms who showed an increased D-dimer. More studies are needed to investigate the use of laboratory values as markers of HSP severity and for prognostic purposes.
Characteristic purpura spots are observed in most patients with HSP, but in those cases where skin manifestations are delayed after GI symptoms or absent altogether, accurately diagnosing and thus treating HSP can be difficult [11,33]. In this study, five patients complained of abdominal pain in the absence of purpura at admission (4 patients: no purpura after discharge, 1 patient: purpura 27 days after symptom onset). Focal thickening of the bowel wall in the duodenum and small bowel was observed in two patients by SBS and in one patient by abdominal USG. In one patient, treatment for HSP was started after finding a focal mucosal thickening and hematoma in the duodenum by gastrofiberscopy. These results suggest that non-invasive abdominal USG may be useful as a first line investigation to differentiate HSP from other GI diseases in patients with severe abdominal pain, and that SBS or gastrofiberscopy may be used selectively in patients who do not respond well to conservative treatment [11,21,34,35,36].
Steroid treatment should be considered in HSP patients with severe abdominal pain where possible differential diagnoses include intussusception or GI hemorrhage, and in cases of severe joint pain resulting in immobilization. Masarweh et al. [22] proposed the following HSP admission criteria: 1) scrotal pain and tenderness, 2) moderate abdominal pain, 3) GI hemorrhage, 4) proteinuria, 5) mobilization difficulty, and 6) multiple number of involved joints (>2). In this study, a total of 208 patients out of 212 were hospitalized due to severe GI and joint symptoms. Early steroid treatment could shorten the duration of extra-renal symptoms like severe abdominal pain [3,4,5], and lower the incidence of GI complications [21]. However, steroid treatment in HSP is not yet standardized, which explains the variable range of steroid dosage and treatment periods used in previous studies. Huber et al. [3] and Dudley et al. [37] reported that prednisolone treatment (2 mg/kg/day) for 1 week with subsequent dose reduction for 1 week did not show any significant difference in the incidence of renal and GI complications. In contrast, Ronkainen et al. [4] and Jauhola et al. [20] reported that prednisolone treatment (1 mg/kg/day) for 2 weeks with subsequent dose reduction for 2 weeks did improve renal and extra-renal symptoms in the short-term compared to placebo, but this finding did not significantly affect the patient's subsequent clinical course. In a recent Korean study, dexamethasone was given to HSP patients with severe abdominal pain until symptoms improved, and this was followed by a 2 week course of prednisolone (1 mg/kg/day) [38]. No difference in the rate of recurrence rate and persistence of nephritis was observed between the dexamethasone treated and untreated groups [38]. In another study, there was also no significant difference in treatment results after 4 weeks of follow-up between the hydrocortisone-treated and high dose methylprednisolone-treated groups [39]. According to most previous studies, steroid treatment does not affect the long-term prognosis of HSP [3,20,37,40]. This finding is true even for different types and dosages of steroids [26,38]. In this study, two groups classified according to the initial dose of steroid were compared, and we observed no significant difference in the rate of recurrence of HSP, renal involvement, and progression to nephrotic syndrome. However, this study is limited by the non-standardization of steroid treatment criteria, steroid type, and steroid dosage, as the results were based on a retrospective study performed by multiple clinicians over a long-term period. In this study, ESRD cases were not observed. However, relative underestimation of long-term prognosis in this study should be considered, because some patients transferred to other tertiary hospitals due to persistent elevation of creatinine or refractory severe GI symptoms, and thus could not be included in assessing the prognosis.
At present, there is no retrospective study that has investigated the long-term prognosis of HSP in Korea. There is an ongoing need for a prospective large-scale study to establish a standardized treatment protocol for HSP and accurately assess prognosis.
In children with HSP, the incidence of renal involvement and nephrotic syndrome is higher in those presenting at an older age, and in those patients with severe abdominal pain or GI hemorrhage. These patients should be carefully monitored to detect progression to HSPN. In children with severe abdominal pain, hematemesis, or hematochezia, performing a SBS or gastrofiberscopy could be helpful to differentiate cases of HSP without characteristic purpuric spots. In the future, more prospective and large-scaled studies are needed to set a definite treatment protocol based on long-term prognosis and clinical management.
References
- 1.Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35–40. doi: 10.1097/00002281-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Rostoker G. Schönlein-Henoch purpura in children and adults: diagnosis, pathophysiology and management. BioDrugs. 2001;15:99–138. doi: 10.2165/00063030-200115020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Huber AM, King J, McLaine P, Klassen T, Pothos M. A randomized, placebo-controlled trial of prednisone in early Henoch Schönlein purpura [ISRCTN85109383] BMC Med. 2004;2:7. doi: 10.1186/1741-7015-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronkainen J, Koskimies O, Ala-Houhala M, Antikainen M, Merenmies J, Rajantie J, et al. Early prednisone therapy in henoch-schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241–247. doi: 10.1016/j.jpeds.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Weiss PF, Feinstein JA, Luan X, Burnham JM, Feudtner C. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics. 2007;120:1079–1087. doi: 10.1542/peds.2007-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo-Río V, Loricera J, Mata C, Martín L, Ortiz-Sanjuán F, Alvarez L, et al. Henoch-Schönlein purpura in northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine (Baltimore) 2014;93:106–113. doi: 10.1097/MD.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu S, Liu D, Xiao J, Yuan W, Wang X, Zhang X, et al. Comparison between adults and children with Henoch-Schönlein purpura nephritis. Pediatr Nephrol. 2015;30:791–796. doi: 10.1007/s00467-014-3016-z. [DOI] [PubMed] [Google Scholar]
- 8.Shin JI, Park JM, Shin YH, Hwang DH, Kim JH, Lee JS. Predictive factors for nephritis, relapse, and significant proteinuria in childhood Henoch-Schönlein purpura. Scand J Rheumatol. 2006;35:56–60. doi: 10.1080/03009740510026841. [DOI] [PubMed] [Google Scholar]
- 9.Hong JH, Na HJ, Namgung MG, Choe SO, Han BG, Jeong SH, et al. Different clinical courses of Henoch-Schönlein purpura in children, adolescents and adults. Korean J Pediatr. 2005;48:1244–1251. [Google Scholar]
- 10.Cohen N, Mimouni FB, Friedel N, Amarilyo G. Predictors of hospital length of stay in pediatric Henoch-Schönlein purpura. Rheumatol Int. 2015;35:1561–1564. doi: 10.1007/s00296-015-3257-6. [DOI] [PubMed] [Google Scholar]
- 11.Oh JM, Park JH. Clinical features of Henoch-Schonlein purpura gastroenteropathy without purpura before diagnosis. Korean J Pediatr Gastroenterol Nutr. 2004;7:54–60. [Google Scholar]
- 12.Yang HR, Choi WJ, Ko JS, Seo JK. Intravenous immunoglobulin for severe gastrointestinal manifestation of Henoch-Schönlein purpura refractory to corticosteroid therapy. Korean J Pediatr. 2006;49:784–789. [Google Scholar]
- 13.Jauhola O, Ronkainen J, Autio-Harmainen H, Koskimies O, Ala-Houhala M, Arikoski P, et al. Cyclosporine A vs. methylprednisolone for Henoch-Schönlein nephritis: a randomized trial. Pediatr Nephrol. 2011;26:2159–2166. doi: 10.1007/s00467-011-1919-5. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki Y, Suzuki J, Murai M, Takahashi A, Isome M, Nozawa R, et al. Plasmapheresis therapy for rapidly progressive Henoch-Schönlein nephritis. Pediatr Nephrol. 2004;19:920–923. doi: 10.1007/s00467-004-1514-0. [DOI] [PubMed] [Google Scholar]
- 15.Park JM, Won SC, Shin JI, Yim H, Pai KS. Cyclosporin A therapy for Henoch-Schönlein nephritis with nephrotic-range proteinuria. Pediatr Nephrol. 2011;26:411–417. doi: 10.1007/s00467-010-1723-7. [DOI] [PubMed] [Google Scholar]
- 16.Cherqaoui B, Chausset A, Stephan JL, Merlin E. Intravenous immunoglobulins for severe gastrointestinal involvement in pediatric Henoch-Schönlein purpura: a French retrospective study. Arch Pediatr. 2016;23:584–590. doi: 10.1016/j.arcped.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Başaran Ö, Cakar N, Uncu N, Çelikel BA, Kara A, Cayci FS, et al. Plasma exchange therapy for severe gastrointestinal involvement of Henoch Schönlein purpura in children. Clin Exp Rheumatol. 2015;33:S176–S180. [PubMed] [Google Scholar]
- 18.Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, et al. The american college of rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990;33:1114–1121. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 19.Saulsbury FT. Henoch-Schönlein purpura in children Report of 100 patients and review of the literature. Medicine (Baltimore) 1999;78:395–409. doi: 10.1097/00005792-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Jauhola O, Ronkainen J, Koskimies O, Ala-Houhala M, Arikoski P, Hölttä T, et al. Clinical course of extrarenal symptoms in Henoch-Schönlein purpura: a 6-month prospective study. Arch Dis Child. 2010;95:871–876. doi: 10.1136/adc.2009.167874. [DOI] [PubMed] [Google Scholar]
- 21.Weiss PF, Klink AJ, Localio R, Hall M, Hexem K, Burnham JM, et al. Corticosteroids may improve clinical outcomes during hospitalization for Henoch-Schönlein purpura. Pediatrics. 2010;126:674–681. doi: 10.1542/peds.2009-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masarweh K, Horovitz Y, Avital A, Spiegel R. Establishing hospital admission criteria of pediatric Henoch-Schonlein purpura. Rheumatol Int. 2014;34:1497–1503. doi: 10.1007/s00296-014-2971-9. [DOI] [PubMed] [Google Scholar]
- 23.Choi SM, Lee KY. Clinico-epidemiologic study of Henoch-Schonlein purpura in children, 1987 through 2003. Korean J Pediatr. 2005;48:174–177. [Google Scholar]
- 24.Zhao YL, Liu ZJ, Bai XM, Wang YC, Li GH, Yan XY. Obesity increases the risk of renal involvement in children with Henoch-Schönlein purpura. Eur J Pediatr. 2015;174:1357–1363. doi: 10.1007/s00431-015-2547-z. [DOI] [PubMed] [Google Scholar]
- 25.Anil M, Aksu N, Kara OD, Bal A, Anil AB, Yavaşcan O, et al. Henoch-Schönlein purpura in children from western Turkey: a retrospective analysis of 430 cases. Turk J Pediatr. 2009;51:429–436. [PubMed] [Google Scholar]
- 26.Kang Y, Park JS, Ha YJ, Kang MI, Park HJ, Lee SW, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura. J Korean Med Sci. 2014;29:198–203. doi: 10.3346/jkms.2014.29.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong J, Yang HR. Laboratory markers indicating gastrointestinal involvement of Henoch-Schönlein purpura in children. Pediatr Gastroenterol Hepatol Nutr. 2015;18:39–47. doi: 10.5223/pghn.2015.18.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagamori T, Oka H, Koyano S, Takahashi H, Oki J, Sato Y, et al. Construction of a scoring system for predicting the risk of severe gastrointestinal involvement in Henoch-Schönlein purpura. Springerplus. 2014;3:171. doi: 10.1186/2193-1801-3-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano H, Izumida M, Shimizu H, Ogawa Y. Risk factors of renal involvement and significant proteinuria in Henoch-Schönlein purpura. Eur J Pediatr. 2002;161:196–201. doi: 10.1007/s00431-002-0922-z. [DOI] [PubMed] [Google Scholar]
- 30.Choi HJ, Cho HY, Kim EJ, Lee BS, Kang HG, Ha IS, et al. Prognostic factors in children with Henoch-Schonlein purpura nephritis. J Korean Soc Pediatr Nephrol. 2005;9:183–192. [Google Scholar]
- 31.Tabel Y, Inanc FC, Dogan DG, Elmas AT. Clinical features of children with Henoch-Schonlein purpura: risk factors associated with renal involvement. Iran J Kidney Dis. 2012;6:269–274. [PubMed] [Google Scholar]
- 32.Wang X, Zhu Y, Gao L, Wei S, Zhen Y, Ma Q. Henoch-Schönlein purpura with joint involvement: analysis of 71 cases. Pediatr Rheumatol Online J. 2016;14:20. doi: 10.1186/s12969-016-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Yoon JW, Jeong SJ. Comparison of the clinical manifestations and prognosis of Henoch-Schonlein purpura in children with and without abdominal pain. Korean J Pediatr Gastroenterol Nutr. 2011;14:359–367. [Google Scholar]
- 34.Nam EJ, Kim GW, Kang JW, Im CH, Jeon SW, Cho CM, et al. Gastrointestinal bleeding in adult patients with Henoch-Schönlein purpura. Endoscopy. 2014;46:981–986. doi: 10.1055/s-0034-1377757. [DOI] [PubMed] [Google Scholar]
- 35.Esaki M, Matsumoto T, Nakamura S, Kawasaki M, Iwai K, Hirakawa K, et al. GI involvement in Henoch-Schönlein purpura. Gastrointest Endosc. 2002;56:920–923. doi: 10.1067/mge.2002.129592. [DOI] [PubMed] [Google Scholar]
- 36.Park SH, Nam YN, Park SH, Sim SY, Eun BW, Choi DY, et al. Clinical characteristics of childhood Henoch-Schonlein purpura with duodenal involvement by upper gastrointestinal endoscopy. Korean J Pediatr Gastroenterol Nutr. 2009;12:156–162. [Google Scholar]
- 37.Dudley J, Smith G, Llewelyn-Edwards A, Bayliss K, Pike K, Tizard J. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schonlein purpura (HSP) Arch Dis Child. 2013;98:756–763. doi: 10.1136/archdischild-2013-303642. [DOI] [PubMed] [Google Scholar]
- 38.Shin JI, Lee SJ, Lee JS, Kim KH. Intravenous dexamethasone followed by oral prednisolone versus oral prednisolone in the treatment of childhood Henoch-Schönlein purpura. Rheumatol Int. 2011;31:1429–1432. doi: 10.1007/s00296-010-1507-1. [DOI] [PubMed] [Google Scholar]
- 39.Deng F, Lu L, Zhang Q, Hu B, Wang SJ, Huang N. Henoch-Schönlein purpura in childhood: treatment and prognosis. Analysis of 425 cases over a 5-year period. Clin Rheumatol. 2010;29:369–374. doi: 10.1007/s10067-009-1329-2. [DOI] [PubMed] [Google Scholar]
- 40.Jauhola O, Ronkainen J, Koskimies O, Ala-Houhala M, Arikoski P, Hölttä T, et al. Outcome of Henoch-Schönlein purpura 8 years after treatment with a placebo or prednisone at disease onset. Pediatr Nephrol. 2012;27:933–939. doi: 10.1007/s00467-012-2106-z. [DOI] [PubMed] [Google Scholar]