Abstract
Epidemiological findings support the concept of Developmental Origins of Health and Disease, suggesting that early-life hormonal influences during a sensitive period of development have a fundamental impact on vascular health later in life. The endocrine changes that occur during development are highly conserved across mammalian species and include dramatic increases in circulating IGF-1 levels during adolescence. The present study was designed to characterize the effect of developmental IGF-1 deficiency on the vascular aging phenotype. To achieve that goal, early-onset endocrine IGF-1 deficiency was induced in mice by knockdown of IGF-1 in the liver using Cre-lox technology (Igf1 f/f mice crossed with mice expressing albumin-driven Cre recombinase). This model exhibits low-circulating IGF-1 levels during the peripubertal phase of development, which is critical for the biology of aging. Due to the emergence of miRNAs as important regulators of the vascular aging phenotype, the effect of early-life IGF-1 deficiency on miRNA expression profile in the aorta was examined in animals at 27 months of age. We found that developmental IGF-1 deficiency elicits persisting late-life changes in miRNA expression in the vasculature, which significantly differed from those in mice with adult-onset IGF-1 deficiency (TBG-Cre-AAV8-mediated knockdown of IGF-1 at 5 month of age in Igf1 f/f mice). Using a novel computational approach, we identified miRNA target genes that are co-expressed with IGF-1 and associate with aging and vascular pathophysiology. We found that among the predicted targets, the expression of multiple extracellular matrix-related genes, including collagen-encoding genes, were downregulated in mice with developmental IGF-1 deficiency. Collectively, IGF-1 deficiency during a critical period during early in life results in persistent changes in post-transcriptional miRNA-mediated control of genes critical targets for vascular health, which likely contribute to the deleterious late-life cardiovascular effects known to occur with developmental IGF-1 deficiency.
Keywords: Insulin-like growth factor 1, miRNA, Epigenetics, Post-transcriptional regulation, microRNA
Introduction
Epidemiological findings during the past two decades support the concept of Developmental Origins of Health and Disease, suggesting that early-life events during a sensitive period of development have a fundamental impact on the organism’s later development, tissue structure and function and lifespan (Barker 2004a, b; Gillman 2005). Increasing clinical and experimental evidence, including parabiotic studies, suggest that the endocrine milieu present during development, especially when rapid physical growth occurs, induces cellular programs that affect the pathogenesis of age-related disease (Barker et al. 1989, 2005; Bateson et al. 2004; Eriksson et al. 1999, 2000, 2007; Kajantie et al. 2005; Osmond et al. 2007).
The endocrine changes that occur during development are highly conserved across mammalian species and include dramatic increases in the anabolic hormone IGF-1 during adolescence (due to a significant rise in GH secretion) (Carter et al. 2002; D’Costa et al. 1993; Deak and Sonntag 2012; Sonntag and Csiszar 2012; Sonntag et al. 2000, 1999, 2005b). Levels of circulating IGF-1 can increase several folds during this period compared to pre-adolescent levels. Yet, this increase is highly variable (Edouard et al. 2009; Sorensen et al. 2012). There is increasing experimental and clinical evidence that alterations in IGF-1 levels during development regulate multiple aspects of the aging process and affect the incidence of multiple age-related diseases (Sadagurski et al. 2015; Sonntag and Csiszar 2012). Importantly, developmental IGF-1 deficiency was suggested to extend lifespan in certain murine models of aging, including the Ames dwarf mice and Snell dwarf mice (Panici et al. 2010). The lifespan-extending effects of developmental IGF-1 deficiency have been largely attributed to its anti-cancer effects (Ikeno et al. 2003). On the basis of these observations, hypotheses were put forward proposing that developmental IGF-1 level is an evolutionarily conserved mechanism regulating the aging process (Bartke and Brown-Borg 2004).
The cardiovascular system is an especially important target organ for IGF-1 (Chisalita and Arnqvist 2004; Chisalita et al. 2009; Johansson et al. 2008; Li et al. 2007; Toth et al. 2014, 2015), and there is increasing evidence suggesting that early-life IGF-1 levels may determine cardiovascular health in later life (Sonntag et al. 2005a, 2013). Accordingly, previous studies demonstrate that rodent models with developmental IGF-1 deficiency exhibit a cardiac and/or vascular phenotype in adulthood (Csiszar et al. 2008; Helms et al. 2010; Reddy et al. 2014). For example, adult growth hormone-releasing hormone receptor null dwarf (Little) mice have significantly lower peak and mean aortic velocity and significantly higher aortic impedance than young wild-type mice (Reddy et al. 2014). Adult Ames dwarf mice exhibit cardiac and vascular mitochondrial oxidative stress (Csiszar et al. 2008), whereas adult GH/IGF-1-deficient Lewis dwarf rats exhibit impaired cardiac performance (Cittadini et al. 1997; Longobardi et al. 2000) and impaired vascular stress resistance phenotypes (Bailey-Downs et al. 2012b; Ungvari et al. 2010). However, the mechanistic role of developmental IGF-1 deficiency in regulation of the vascular aging process remains obscure.
MicroRNAs (miRNA) are short, endogenous, non-coding transcripts that regulate the expression of specific messenger RNA (mRNA) targets (Lee et al. 2014; Liu et al. 2015). There is growing evidence that miRNAs control lifespan and the pace of aging in model organisms (Boehm and Slack 2005; Grillari and Grillari-Voglauer n.d.; Ibanez-Ventoso et al. 2006) and that changes in miRNA expression profile also have a role in mammalian aging (Bates et al. n.d.; Inukai et al. 2012; Inukai and Slack 2013; Ito et al. 2010; Maes et al. 2008; Mercken et al. 2013; Smith-Vikos and Slack 2012; Ungvari et al. 2013; Zhang et al. 2012; Zovoilis et al. 2011). Importantly, miRNAs were also reported to regulate several important aspects of endothelial biology and vascular function (Bonauer et al. 2009; Chen et al. 2015; Doebele et al. n.d.; Hergenreider et al. 2012; Kim et al. 2014; Kuehbacher et al. 2007; Leung et al. 2013; Lovren et al. 2012; O’Rourke and Olson 2011; Rotllan et al. 2013; Stellos and Dimmeler 2014; Weber et al. 2014; Zampetaki et al. 2014). Further, age-related changes in miRNA expression were shown to contribute to the development of cardiovascular aging phenotypes (Boon et al. 2013; Csiszar et al. 2014; Ungvari et al. 2013) and the pathogenesis of cardiovascular diseases (Ono et al. 2011). Expression of miRNAs in the cardiovascular system was reported to be regulated by neuroendocrine factors (Hua et al. 2012). Despite these advances, the effects of developmental IGF-1 deficiency on vascular miRNA expression profile have not been elucidated.
The present study was designed to characterize the effect of developmental IGF-1 deficiency on the vascular aging phenotype. To achieve that goal early-onset, isolated endocrine IGF-1 deficiency was induced mice by developmental knockdown of IGF-1 specifically in the liver using Cre-lox technology (Igf1 f/f mice crossed with mice expressing albumin-driven Cre recombinase) (Ashpole et al. 2015). The animals were studied at an age representing ∼75 % of maximal lifespan potential, which corresponds to the biological age of a ∼67-year-old human. To assess vascular health, endothelium-dependent vasorelaxation and vascular ROS production were tested. Due to the emergence of miRNAs as important regulators of vascular aging phenotype (Csiszar et al. 2014; Ungvari et al. 2013), miRNA expression profile in the aorta of mice with developmental IGF-1 deficiency was tested.
Materials and methods
Developmental liver-specific knockdown of Igf1 in mice
To target IGF-1 production early in development, mice homozygous for a floxed exon 4 of the Igf1 gene (Igf1 f/f; in a C57BL/6 background (Toth et al. 2014)) were crossed with mice expressing albumin-driven Cre recombinase, as previously described (Ashpole et al. 2015). The Igf1 f/f mice have the entirety of exon 4 of the Igf1 gene flanked by loxP sites, which allows for genomic excision of this exon when exposed to Cre recombinase. Transcripts of the altered Igf1 gene yield a protein upon translation that fails to bind the IGF receptor. The albumin gene is induced within the liver between post-natal day 10 and 15, thereby decreasing effective IGF-1 production early after birth. Knockdown of IGF-1 was verified by measuring circulating levels of IGF-1 at 2, 12, and at 27 months of age as reported (Ashpole et al. 2015). Mice were used for experimentation at 27 months of age. Wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and utilized as reference controls at 5 and 27 months of age.
As an additional control group, aortas isolated from mice with adult-onset IGF-1 deficiency were also analyzed (Fig.1a). Adult-onset circulating IGF-1 deficiency was induced in Igf1 f/f mice by adeno-associated virus (AAV8)-mediated expression of Cre recombinase in the liver at 5 months of age, as reported (Ashpole et al. 2015). The AAV8 vector was purchased from the University of Pennsylvania Viral Vector Core (Penn Vector Core, Philadelphia, PA, USA; http://www.med.upenn.edu/gtp/vectorcore). Although AAV8 is effective at transducing multiple tissues, the use of thyroxine binding globulin (TBG) promoter allows for the restriction of expression to hepatocytes, as described (Toth et al. 2014). At 5 months of age, Igf1 f/f mice were administered approximately 1.3 × 1010 viral particles of AAV8-TBG-Cre or AAV8-TBG-eGFP via retro-orbital injection, as described (Ashpole et al. 2015; Toth et al. 2014).
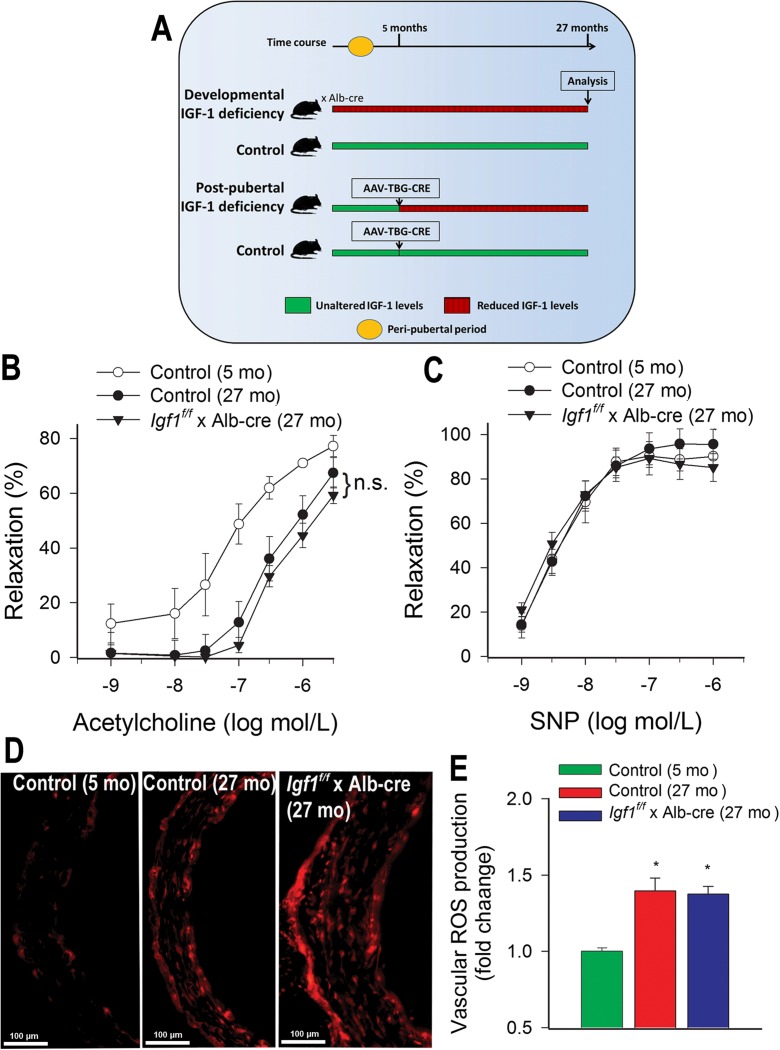
Fig. 1.
Age-related endothelial dysfunction and increased oxidative stress in aortas from mice with developmental IGF-1 deficiency. a Experimental scheme. As a model of developmental IGF-1 deficiency Igf1 f/f mice crossed with mice expressing albumin-driven Cre recombinase (Igf1 f/f x Alb-cre) were used. As a model of adult-onset, post-pubertal IGF-1 deficiency Igf1 f/f mice were injected with TBG-iCre-AAV8 at 5 months of age. Mice were analyzed at 27 month of age. b–c Relaxations in ring preparations of aortas of aged (27 months old) Igf1 f/f x Alb-cre mice and aged (27 months old) and young (5 months old) control mice in response to administration of increasing concentrations of acetylcholine (b) and the NO donor SNP (c). Data are mean ± SEM (n = 6–8). d Representative confocal images showing ethidium fluorescence (representing increased ROS levels) in section of aortas of aged (27 months old) Igf1 f/f x Alb-cre mice and aged (27 months old) and young (5 months old) control mice. Summary data for vascular ROS production are shown in e. Data are mean ± S.E.M. *p < 0.05 vs. young control
Animals were housed in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center, on a 12-h light/12-h dark cycle, and given access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum. All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee of OUHSC in accordance with the ARRIVE guidelines.
Measurement of circulating IGF-1 levels
Venous blood was collected from the submandibular veins of animals from each group (Medipoint, Mineola, NY). Whole blood was centrifuged at 2500×g for 20 min at 4 °C to collect serum, which was then stored at −80 °C. Measurement of serum IGF-1 (Franco et al. 2014; Hill et al. 2015; Rojanathammanee et al. 2014) levels was performed by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol and are reported in ng/mL.
Assessment of vascular endothelial function
Upon euthanasia, aortas were isolated and endothelial function was assessed by measuring relaxation of aortic ring preparations to acetylcholine as previously described (Bailey-Downs et al. 2012a). Endothelial function is an important measure of vascular health (Alonso-Bouzon et al. 2014; Demirci et al. 2014; Gonzalez-Guardia et al. 2014; Grabowska et al. 2015; Heiss et al. 2015; Mourmoura et al. 2014; Walker et al. 2014). In brief, an aorta ring segment 2 mm in length was isolated from each animal and mounted on 40-μm stainless steel wires in myograph chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37 °C; gassed with 95 % air and 5 % CO2). After an equilibration period of 1 h during which an optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), relaxation of pre-contracted (by 10−5 mol/L phenylephrine) vessels to the endothelium-dependent vasodilator acetylcholine (ACh; from 10−9 to 10−6 mol/L) and to an endothelium-independent vasodilator, the NO donor sodium nitroprusside (SNP; from 10−9 to 10−6 mol/L) was obtained.
Measurement of vascular ROS production
The oxidative fluorescent dye dihydroethidium (DHE) was used to assess vascular O2 − production in segments of the aortas as we have previously reported (Csiszar et al. 2007; Pearson et al. 2008; Ungvari et al. 2003, 2010). In brief, freshly isolated aorta segments were incubated with DHE (3 × 10−6 mol/L; for 30 min, at 37 °C, in the dark). The vessels were then washed three times, embedded in OCT medium and cryosectioned. Fluorescent images of the aorta sections were captured using a Leica SP2 confocal laser scanning microscope (Leica Microsystems GmbH, Wetzlar, Germany). Average nuclear DHE fluorescence intensities were assessed using the Metamorph software (Molecular Devices LLC, Sunnyvale, CA) and values for each animal in each group were averaged as reported (Csiszar et al. 2007; Pearson et al. 2008; Ungvari et al. 2003, 2010). Unstained aortas were used for background correction.
Quantitative real-time RT-PCR
A quantitative real time RT-PCR technique was used to analyze miRNA expression profiles in the aorta of mice from each experimental group as reported (Csiszar et al. 2014). In brief, total RNA was isolated with a mirVana™ miRNA Isolation Kit (ThermoFisher Scientific) and was reverse transcribed using TaqMan® MicroRNA Reverse Transcription Kit as described previously (Bailey-Downs et al. 2012a; Csiszar et al. 2014). The expression profile of 641 unique mouse miRNAs in aortas derived from young and aged control mice and aged mice with developmental IGF-1 deficiency was analyzed using the TaqMan Array Rodent MicroRNA A + B Cards Set v3.0 (ThermoFisher Scientific). Expression of miRNAs were normalized to ΔΔCt values using the average of three replicated probes of MammU6, and the resulting expression values were then quantile normalized (Csiszar et al. 2014). Differential expression raw p values were determined using a Student’s t test and corrected using Benjamini-Hochberg multiple hypothesis correction at a q-value (FDR) cutoff of 0.1.
miRNA target prediction and validations
To further understand the consequences of changes in miRNA abundance on regulating vascular aging phenotypes, we used a computational approach to predict targets of differentially expressed miRNAs. After determining miRNAs that were differentially expressed with developmental IGF-1 deficiency and age, we compiled a list of candidate target genes matching the following criteria: (1) Putative targets of miRNAs differentially expressed in aortas of Igf1 f/fxAlb-cre mice, (2) expressed in aorta and show altered expression with age in aorta, (3) co-expressed with IGF-1 across tissues and experimental conditions, and (4) associated with vascular pathophysiology in the published literature. For criterion (1), a list of miRNA-target pairs was obtained from miRBase (Kozomara and Griffiths-Jones 2014), and for each target gene in the database, the number of targeting miRNAs that were significantly up- or downregulated in aging or developmental IGF-1 deficiency was quantified and significance was assessed using the binomial test. The hypothesis tested was that the targeting miRNAs were consistent in their direction of regulation with IGF-1 deficiency or age. For criterion (2), mouse RNA microarray samples were identified in NCBI GEO as deriving from aorta using GEOmetadb (Zhu et al. 2008). Each aorta sample from GEO series accession GSE40156 was annotated with the sample age, and after quantile normalization, a log-linear model was used to quantify the rate of expression change of each gene with time. Additionally, the mean expression of each gene in aorta was approximated by converting each sample’s log-expression vector to a Z-score. Genes with a mean Z-score less than 0 (indicating genes which were expressed at a lower level than the average gene) were excluded from further analysis. For criterion (3), the GAMMA algorithm (Dozmorov et al. 2011; Wren 2009) was used to quantify the correlation of each putative target mRNA with the IGF-1 transcript using the Pearson’s correlation coefficient. For criterion (4), the IRIDESCENT algorithm (Wren and Garner 2004) was used to mine the biomedical literature and quantify the degree of association between each candidate mRNA and terms relating to vascular pathophysiology (e.g., “stroke,” “aneurysm,” “vascular fragility,” “ischemic heart disease,” etc.). IRIDESCENT uses a statistical model to determine whether each gene co-occurs with a term of interest (here, vascular pathophysiology-related terms) more frequently than would be expected by chance, and quantifies this in terms of the mutual information measure. In order to retrieve the most relevant targets, we chose the top-ranked miRNA target genes predicted by these computational approaches. We next validated these predictions with quantitative real-time RT-PCR using TaqMan probes as reported (Csiszar et al. 2013; Toth et al. 2013; Tucsek et al. 2013, 2014).
Statistical analysis
Statistical analysis was carried out by one-way ANOVA followed by Tukey’s post-hoc test or unpaired t test, as appropriate. Dose-response curves for vascular relaxations were analyzed by two-way ANOVA for repeated measures followed by Bonferroni multiple comparison test. A p value less than 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
Developmental liver-specific knockdown of IGF-1
Basic physiological parameters of the experimental cohorts used in the present study were similar to our previous report (Ashpole et al. 2015). Body weight was significantly decreased in the Igf1 f/fxAlb-cre and Igf1 f/f + AAV8-TBG-Cre groups, compared to their age-matched controls (Table 1). Similarly, circulating IGF-1 levels were significantly reduced in the Igf1 f/fxAlb-cre and Igf1 f/f + AAV8-TBG-Cre groups, compared to their respective age-matched controls (Table 1).
Table 1.
Description of experimental animals
| Group | n | Age (days) | Body weight (grams) | IGF-1 levels (ng/mL) |
|---|---|---|---|---|
| 5-month-old control | 7 | n.a. | 25.7 ± 1.5 | 320.9 ± 65.4 |
| 27-month-old control | 7 | 823.4 ± 2.6 | 26.8 ± 3.3 | 314.0 ± 49.9 |
| Igf1 f/f x Alb-Cre | 7 | 823.4 ± 1.9 | 23.2 ± 1.8* | 46.4 ± 12.0* |
| Igf1 f/f x TBG-eGFP-AAV8 | 7 | 821.9 ± 5.6 | 26.4 ± 1.1 | 310.8 ± 68.0 |
| Igf1 f/f x TBG-Cre-AAV8 | 7 | 820.3 ± 5.8 | 23.5 ± 1.3* | 53.2 ± 10.4* |
Average age, body weight, and circulating IGF-1 levels at the time of tissue harvest in each experimental group. The asterisk indicates a significant difference between the treatment group and its respective control group, *p < 0.05, mean ± S.D
n.a. data not available
Endothelial dysfunction and oxidative stress
IGF-1 is known to exert multifaceted vasoprotective effects (Bailey-Downs et al. 2012a, b; Csiszar et al. 2008; Higashi et al. 2010, 2012; Sonntag et al. 2013; Sukhanov et al. 2007; Ungvari and Csiszar 2012; Ungvari et al. 2010) but the role of developmental IGF-1 deficiency in regulating vascular aging has never been investigated. We found that endothelium-dependent aorta relaxation induced by acetylcholine was significantly impaired in aged control mice as compared to young control mice (Fig. 1b). There was no significant difference between acetylcholine-induced responses in aortas of aged Igf1 f/fxAlb-cre mice and aged control mice (Fig.1b). We also investigated the effect of the endothelium-independent relaxing agent SNP, and we found that there was no significant difference among the groups (Fig. 1c).
Analysis of nuclear ethidium fluorescence intensities showed that aging was associated with significant increases in vascular O2 − production in control mice (Fig. 1d–e). There was no significant difference between O2 − production in aortas of aged Igf1 f/fxAlb-cre mice and aged control mice (Fig. 1d–e).
Changes in vascular miRNA expression profile in mice associated with aging and with developmental IGF-1deficiency
We assessed changes in miRNA expression profile in the mouse aorta associated with aging and with developmental IGF-1 deficiency. Principal component analysis and hierarchical clustering of miRNA expression showed a clear separation between the young and aged groups. Aged control mice and aged Igf1 f/fxAlb-cre mice were also separated in the principal component analysis and hierarchical clustering. Figure 2a, b shows changes in miRNA expression in the mouse aorta associated with age and developmental IGF-1 deficiency, respectively. GO terms enriched among miRNAs differentially expressed with age and developmental IGF-1 deficiency are shown in Table 2 and Table 3, respectively.
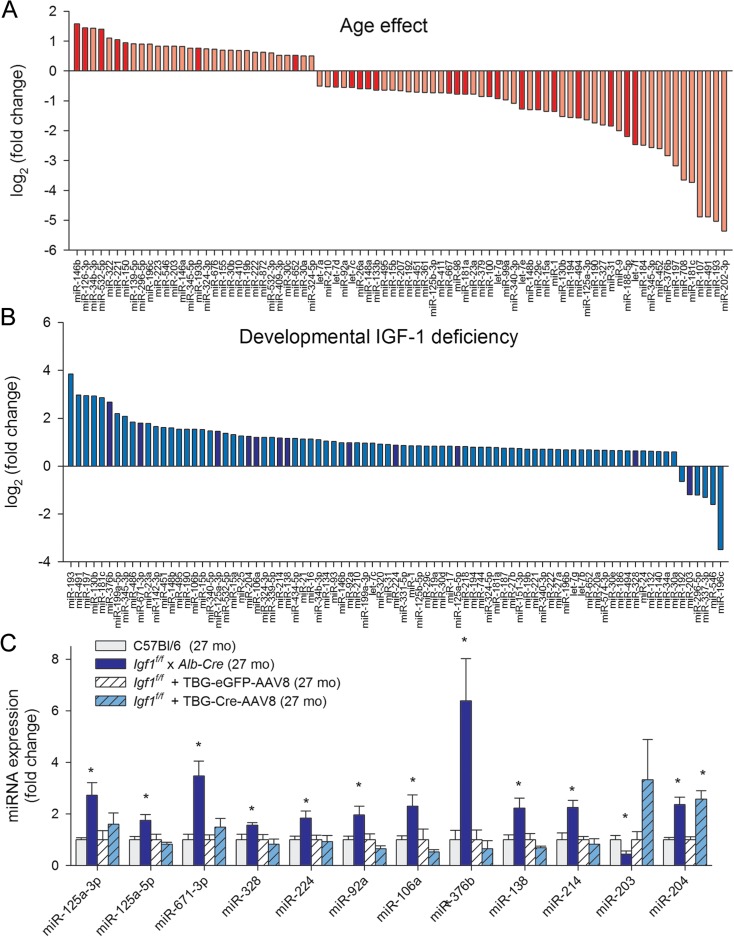
Fig. 2.
Changes in miRNA expression profile in aortas associated with developmental IGF-1 deficiency and aging. a–b Effects of age (a) and developmental IGF-1 deficiency (b) on aortic miRNA expression. The y axis represents the average log2 fold change in miRNA expression levels in aortas derived from aged (27 months old) Igf1 f/f x Alb-cre mice and aged (27 months old) control mice, relative to the corresponding control values. Significant (p < 0.05) changes are highlighted. The x-axis indicates the miRNA rank from the most upregulated to the most downregulated. n = 5–9 for each data point. c Comparison of the effects of developmental IGF-1 deficiency and adult-onset, post-pubertal IGF-1 deficiency. The expression of selected miRNAs significantly dysregulated in aortas of Igf1 f/fxAlb-cre mice was analyzed in aortas of Igf1 f/f + AAV8-TBG-Cre mice by qPCR. Data are normalized to the mean miRNA expression in the aorta of the respective aged control group and are expressed as mean ± SEM (*p < 0.05). The data shows that developmental IGF-1 deficiency and adult-onset IGF-1 deficiency differentially alter miRNA expression in the mouse aorta
Table 2.
GO terms enriched among miRNAs differentially expressed with age in the aorta
| GO terms enriched among miRNAs differentially expressed with age in the aorta |
|---|
| Extracellular matrix |
| Chromatin silencing |
| Rab protein signal transduction |
| Signal transduction |
| Endopeptidase activity |
| Rac GTPase binding |
| Activation of protein kinase activity |
| Negative regulation of cyclin-dependent protein kinase activity |
| Chromatin DNA binding |
| Microtubule |
| Regulation of transcription |
| Cell migration |
| Endosome membrane |
| Intracellular protein transport |
| Negative regulation of cell death |
| Cytokine-mediated signaling pathway |
| Positive regulation of GTPase activity |
| Endosome |
| Stress-activated protein kinase signaling cascade |
| Regulation of mitotic cell cycle |
| Protein tyrosine/serine/threonine phosphatase activity |
| Positive regulation of protein targeting to mitochondrion |
| Cytokine production |
| Negative regulation of extrinsic apoptotic signaling pathway |
| Core promoter proximal region sequence-specific DNA binding |
| Regulation of protein kinase activity |
| Negative regulation of ERK1 and ERK2 cascade |
At least one gene annotated with the GO category listed is targeted by miRNAs that are differentially regulated in the aged mouse aorta. Significance (p < 0.05) was determined by Fisher’s exact test
Table 3.
GO terms enriched among miRNAs differentially expressed with developmental IGF-1 deficiency in the aorta
| GO terms enriched among miRNAs differentially expressed with developmental IGF-1 deficiency |
|---|
| Pattern recognition receptor signaling pathway |
| Production of miRNAs involved in gene silencing by miRNA |
| RISC-loading complex |
| Extracellular matrix |
| Blood vessel remodeling |
| Micro-ribonucleoprotein complex |
| miRNA loading onto RISC involved in gene silencing by miRNA |
| Pre-miRNA binding |
| Negative regulation of translation involved in gene silencing by miRNA |
| RISC complex |
| RNA polymerase II transcription factor binding |
| Negative regulation of cell proliferation |
| Pre-miRNA processing |
| miRNA binding |
| Transcription factor activity, RNA polymerase II core promoter proximal region sequence-specific binding |
| SMAD binding |
| Regulation of transforming growth factor beta receptor signaling pathway |
| Endoplasmic reticulum membrane |
| Positive regulation of protein kinase activity |
| Regulation of transcription from RNA polymerase II promoter |
| Positive regulation of receptor-mediated endocytosis |
| Cytoplasmic mRNA processing body |
| RNA polymerase II transcription coactivator activity |
| Regulation of actin cytoskeleton organization |
| Regulation of protein localization |
| Phospholipid translocation |
| Negative regulation of microtubule depolymerization |
| Adaptive immune response |
| Positive regulation of apoptotic signaling pathway |
| Frizzled binding |
| mRNA polyadenylation |
| Protein tyrosine phosphatase activity |
| Cellular response to cAMP |
| Protein localization to cell surface |
| PDZ domain binding |
| Transcription factor complex |
| Vesicle organization |
| Negative regulation of BMP signaling pathway |
| Negative regulation of extrinsic apoptotic signaling pathway |
| Negative regulation of transforming growth factor beta receptor signaling pathway |
| Poly(A) RNA binding |
| Glycoprotein binding |
| Chromatin DNA binding |
| Peptidyl-tyrosine dephosphorylation |
| Chaperone-mediated protein folding |
| Core promoter proximal region sequence-specific DNA binding |
| Sequence-specific DNA binding |
| Metallopeptidase activity |
| Transcription regulatory region DNA binding |
| Histone deacetylase binding |
| Endocytic vesicle |
| Negative regulation of translation |
| Positive regulation of gene expression |
| Single-stranded RNA binding |
| Endosome membrane |
| mRNA export from nucleus |
| Cytoskeleton |
| Intracellular protein transport |
| Integral component of membrane |
| Regulation of cell proliferation |
| Positive regulation of gene expression |
| Positive regulation of JNK cascade |
| Membrane raft |
| Cell differentiation |
| Vesicle |
| Transcription factor binding |
| Plasma membrane |
| Angiogenesis |
| Sequence-specific DNA binding |
| Negative regulation of transcription |
At least one gene annotated with the GO category listed is targeted by miRNAs that are differentially regulated in the aorta of mice with developmental IGF-1 deficiency. Significance (p < 0.05) was determined by Fisher’s exact test
To differentiate between the effects of IGF-1 deficiency during development and post-pubertal IGF-1 deficiency on vascular phenotype, miRNA expression in aortas of Igf1 f/fxAlb-cre mice and Igf1 f/f + AAV8-TBG-Cre mice was compared. Figure 2c shows that expression of miRNAs that are differentially expressed in the aortas of Igf1 f/fxAlb-cre mice was not altered significantly in aortas of Igf1 f/f + AAV8-TBG-Cre mice, suggesting that developmental IGF-1 status has a critical role in regulation of vascular miRNA expression. The only exception identified was miR-204 whose expression was similarly altered both in Igf1 f/fxAlb-cre mice and Igf1 f/f + AAV8-TBG-Cre mice Igf1 f/fxAlb-cre mice and Igf1 f/f + AAV8-TBG-Cre mice.
Changes in vascular expression of miRNA target genes
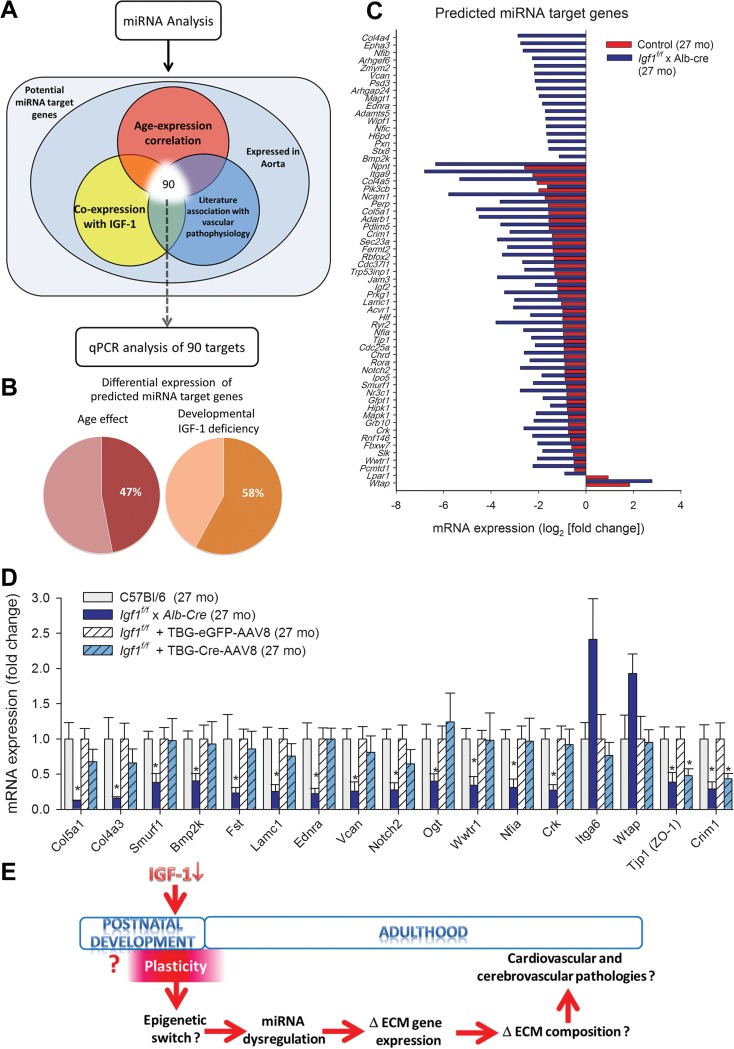
Since the discovery of miRNA regulation of genes, several studies have been focused on predicting the biologically relevant target genes for miRNAs. We have designed a novel selection strategy to predict putative biological targets of differentially expressed miRNAs as shown in Fig. 3a. The top-ranked miRNA target genes predicted by these computational approaches were validated using qPCR. We found that our method successfully predicted miRNA target genes whose aortic expression is significantly impacted by developmental IGF-1 deficiency and age (Fig. 3b). The effects of developmental IGF-1 deficiency and aging on aortic mRNA expression of predicted biological targets of differentially regulated miRNAs are shown in Fig. 3c. Comparison of aortic expression of selected miRNA targets shows that developmental IGF-1 deficiency (Igf1 f/f x Alb-cre) and adult-onset IGF-1 deficiency (Igf1 f/f + TBG-iCre-AAV8) differentially alter expression of a number of targets genes related to extracellular matrix homeostasis and maintenance of vascular structural integrity (Fig. 3d).
Fig. 3.
a Scheme illustrating the selection strategy adopted to predict putative biological targets of differentially regulated miRNAs for qPCR analysis (see Methods). b Percentage of predicted miRNA target genes whose aortic expression is significantly changed with developmental IGF-1 deficiency and age. c qPCR data showing the effect of developmental IGF-1 deficiency and aging on aortic mRNA expression of predicted biological targets of differentially regulated miRNAs. d Comparison of aortic expression of selected miRNA targets (qPCR data) shows that developmental IGF-1 deficiency (Igf1 f/f x Alb-cre) and adult-onset IGF-1 deficiency (Igf1 f/f + TBG-iCre-AAV8) differentially alter expression of genes related to extracellular matrix homeostasis and maintenance of vascular structural integrity. Data are mean ± SEM. *p < 0.05. e Proposed model for epigenetic mechanisms induced by IGF-1 in a critical peripubertal time window impacting vascular health later in life. The scheme depicts preadult periods of adaptive plasticity in the transition between juvenility to adolescence and to adulthood. This transition between developmental stages, which is governed in part by IGF-1, determines cardiovascular health span(Csiszar et al. 2008; Reddy et al. 2014; Sonntag et al. 2005a) and establishes longevity(Panici et al. 2010). We predict that persistent epigenetic mechanisms, including miRNA dysregulation and consequential alterations in extracellular matrix homeostasis contribute to the continued effects of the peripubertal IGF-1 surge later in life
Discussion
The principal new finding of this study is that IGF-1 deficiency through a critical period during early in life determines the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation.
IGF-1 is a critical regulator of development; yet, circulating levels of IGF-1 levels are highly variable during puberty (range: from ∼100 to 800 ng/mL) (Bidlingmaier et al. 2014; Sorensen et al. 2012). In children with short stature, the prevalence of primary IGF-1 deficiency reaches 20 %. The significant variability in peripubertal IGF-1 levels is largely attributed to environmental factors, including socioeconomic status and diet. Protein intake is a key determinant of circulating IGF-1 levels in humans (Fontana et al. 2008), and clinical studies emphasize that nutritional deficiency associated with poverty (which affects over 8 million children in the USA) is a critical factor in the alarming incidence of peripubertal IGF-1 deficiency. Taken together, developmental IGF-1 deficiency and its long-term consequences are significant public health concerns, which affect millions of individuals in addition to those with rare genetic conditions of inherited IGF-1 deficiency.
In invertebrate model organisms, disruption of the insulin/IGF-1 pathway during development was reported to regulate lifespan and/or delay age-related pathophysiological alterations (Kimura et al. 1997). In mammals, the loss of insulin signaling during development is lethal. In recent years, the concept has emerged that alterations in developmental IGF-1 levels in mammals can also regulate aging processes, conferring both anti- and pro-aging effects later in life in an organ system-specific manner (Leiser and Miller 2010; Maynard and Miller 2006; Murakami et al. 2003; Nieves-Martinez et al. 2010; Page et al. 2009; Panici et al. 2010; Ramsey et al. 2002; Sadagurski et al. 2015; Salmon et al. 2005; Sonntag et al. 2005a; Ungvari et al. 2010, 2011; Wang and Miller 2012). Although early studies proposed that developmental IGF-1 deficiency contributes to the extension of lifespan in Ames dwarf mice and Snell dwarf mice (Panici et al. 2010), recent studies demonstrate that mice with isolated developmental endocrine IGF-1deficiency do not exhibit a longevity phenotype (Sonntag and Aspole, 2016, in preparation). Yet, in the same model, developmental endocrine IGF-1deficiency has been linked to marked alterations in healthspan (Ashpole et al. 2015). Interestingly, patients with Laron syndrome (congenital IGF-1 deficiency caused by primary GH insensitivity), who do not exhibit a longevity phenotype, seem to be protected against cancer at old age (Guevara-Aguirre et al. 2011; Steuerman et al. 2011), yet, are also affected by organ-specific symptoms of accelerated aging including osteoporosis, cognitive impairment, and marked obesity (Laron et al. 1999). Additionally, rodent models with developmental IGF-1 deficiency exhibit organ-specific signs of accelerated aging in the central nervous system and the musculoskeletal system (Ekenstedt et al. 2006; Sonntag et al. 2013).
Vascular endothelial and smooth muscle cells abundantly express IGF1R and are more sensitive to IGF-1 than to insulin (Chisalita and Arnqvist 2004; Chisalita et al. 2009; Johansson et al. 2008). Several lines of evidence suggest that normal developmental IGF-1 levels promote vascular health later in life (Ungvari and Csiszar 2012). Epidemiological studies demonstrate that poverty and malnutrition in adolescent children, which is known to be associated with low IGF-1 levels, increases risk for cerebrovascular diseases later in life (Forsdahl 1978; van Abeelen et al. 2012). Further, shorter stature, which is often a consequence of lower-than-normal developmental levels of IGF-1, has been associated with significantly increased risk of coronary heart disease and stroke (Eriksson et al. 2000; Goldbourt and Tanne 2002; Parker et al. 1998). Importantly, in Lewis dwarf rats, restoration of IGF-1 levels in a critical time window of ∼10 weeks around puberty was also shown to increase lifespan by delaying a specific age-related vascular pathology—spontaneous intracerebral hemorrhages (Sonntag et al. 2005a, 2013). In the present study, we found that aged Igf1 f/fxAlb-cre mice exhibited significant endothelial dysfunction and vascular oxidative stress and were not protected from the adverse vascular effects of aging (Fig. 1). Previous studies in human Laron syndrome patients (Guevara-Aguirre et al. 2011), Ames dwarf mice (Csiszar et al. 2008), mice harboring a liver-specific Igf1 deletion (Troncoso et al. 2012) and Lewis dwarf rats (Bailey-Downs et al. 2012b; Cittadini et al. 1997; Longobardi et al. 2000; Ungvari et al. 2010) also show that developmental IGF-1 deficiency compromises cardiovascular health in adulthood. The available data suggest that developmental IGF-1 deficiency also exerts detrimental effects on stress resistance pathways, inflammatory processes and/or changes in structural characteristics of the vasculature later in life (Bailey-Downs et al. 2012b; Csiszar et al. 2008; Reddy et al. 2014; Ungvari et al. 2010). Collectively, our present findings and the aforementioned data from the literature do not support the often-cited hypothesis that developmental GH/IGF-1 deficiency exerts universal anti-aging effects (Panici et al. 2010).
To our knowledge, this is the first study to demonstrate that developmental IGF-1 deficiency elicits persisting late-life changes in miRNA expression profile in the vasculature (Fig. 2). These findings raise the possibility that changes in post-transcriptional control of expression of genes critical targets for vascular health underlie the late-life cardiovascular effects of developmental IGF-1 deficiency. The available evidence supports the concept that a link exists between circulating IGF-1 levels and miRNA expression (Bake et al. 2014; Bates et al. 2010; Fenn et al. 2013; Marino et al. 2010; Victoria et al. 2015). Demonstration of IGF-1-dependent changes in miRNA biology in the vasculature is particularly important (Bonauer et al. 2009; Chen et al. 2015; Doebele et al. n.d.; Hergenreider et al. 2012; Kim et al. 2014; Kuehbacher et al. 2007; Leung et al. 2013; Lovren et al. 2012; O’Rourke and Olson 2011; Rotllan et al. 2013; Stellos and Dimmeler 2014; Weber et al. 2014; Zampetaki et al. 2014) as changes in miRNA expression have been causally linked to the development of cardiovascular aging phenotypes (Boon et al. 2013; Csiszar et al. 2014; Ungvari et al. 2013) and the pathogenesis of cardiovascular diseases (Ono et al. 2011).
The mechanisms by which developmental IGF-1 deficiency alters miRNA expression that persists later in life are presently unknown. Recent studies showed changes in developmental IGF-1 levels during a critical time window in Lewis dwarf rats (Ungvari et al. 2011) and Snell dwarf mice (Pit1dw/dw, which are phenotypically identical to Ames dwarf mice) (Panici et al. 2010) elicits long-lasting changes in cellular phenotypes, which persists in cell culture. These findings are consistent with the concept that changes in developmental IGF-1 levels result in epigenetic modifications to the genome. Recent studies have demonstrated that epigenetic mechanisms, including DNA methylation and histone modification, not only regulate the expression of protein-encoding genes, but also miRNAs, such as miR-203 (Sato et al. 2011). In that regard, it is significant that miR-203 is among the miRNAs selectively regulated by developmental IGF-1 deficiency. Further studies are warranted to test experimentally the role of IGF-1-mediated epigenetic regulation of miRNAs in the vasculature.
Dysregulation of miRNA pathways with developmental IGF-1 deficiency likely have important pathophysiological consequences in the cardiovascular system (Table 2). miRNA-dependent pathways have been shown to regulate multiple aspects of cellular physiology relevant for vascular aging, including angiogenesis (Kuehbacher et al. 2007; Suarez et al. 2007, 2008; Yang et al. 2005), structural integrity of the vessels, replicative senescence (Menghini et al. 2009; Vasa-Nicotera et al. 2011), mechanotransduction (Wu et al. 2011), NO production (Suarez et al. 2007; Wu et al. 2011), endothelial apoptosis (Asada et al. 2008), and inflammation (Suarez et al. 2007). Among the miRNAs whose expression is regulated by developmental IGF-1 deficiency, upregulation of miR-125a-5p has been linked to impaired angiogenesis and endothelial dysfunction (Che et al. 2014), endothelial apoptosis (Svensson et al. 2014), and dysregulation of endothelial tight junctions (Reijerkerk et al. 2013). miR-92a promotes atherosclerosis, endothelial dysfunction (Loyer et al. 2014), and neointima formation (Daniel et al. 2014). miR-126 is a biomarker of clinical atherosclerosis (Kim et al. 2015). miR-376b was reported to inhibit angiogenesis by targeting the VEGFA/Notch1 signaling pathway (Li et al. 2014). A functional link between upregulation of miR-138 and endothelial dysfunction has also been proposed (Sen et al. 2014).
Changes in miRNA expression induced by developmental IGF-1 deficiency likely also play important functional roles in impairing the structural integrity of the vessels, targeting components of the extracellular matrix. Accordingly, miR-328 is a negative regulator of collagen (Col1a1) expression (Rutnam et al. 2013; Rutnam and Yang 2012). miR-21 (Rutnam et al. 2013) and miR-29 (Rutnam et al. 2013) also target collagens, whereas miR-671 downregulates fibronectin (Rutnam and Yang 2012). A link between miR-125a-5p (Rutnam et al. 2013) and impaired synthesis of extracellular matrix has been also documented. Changes in extracellular matrix synthesis and remodeling in the vascular wall during atherosclerosis, development of aneurysms, and the pathogenic processes leading to vascular ruptures (aorta dissection, hemorrhagic stroke, cerebral microhemorrhages) are governed by a wide range of growth factors and cytokines. These autocrine/paracrine mediators and their receptors can also be regulated by miRNAs. Accordingly, miR-224 was reported to modulate extracellular matrix synthesis via regulation of connective tissue growth factor (Chen et al. 2014).
To better understand the pathophysiological relevance of late-life miRNA dysregulation induced by developmental IGF-1 deficiency, we analyzed expression of predicted targets of altered miRNAs known to be involved in maintenance of structural and functional integrity of the vascular system. Using a novel computational approach, we identified miRNA target genes that associate with IGF-1 deficiency, aging, and vascular pathophysiology. Our method accurately predicted genes whose expression was dysregulated in mice with developmental IGF-1 deficiency (Fig. 3). We found that many age-related changes in vascular expression of miRNA target genes were exacerbated in mice with developmental IGF-1 deficiency (Fig. 3b). Further, developmental IGF-1 deficiency and adult-onset IGF-1 deficiency differentially altered expression of the predicted miRNA target genes in the mouse aorta (Fig. 3c). The aforementioned findings provide strong support for the concept that early-life changes in the hormonal milieu have significant impact on cardiovascular health-span later in life, accelerating vascular aging.
Importantly, we confirmed that the expression of multiple extracellular matrix-related genes, including collagen-encoding genes, were preferentially down-regulated in mice with developmental IGF-1 deficiency (Fig. 3c). These results extend previous findings demonstrating that developmental IGF-1 deficiency promotes structural impairment and extracellular matrix remodeling in vessels of aged Lewis dwarf rats, increasing their propensity to spontaneous rupture (Sonntag et al. 2005a). Interestingly, developmental IGF-1 deficiency is also associated with decreased collagen expression in the cardiovascular system of Ames dwarf mice (Helms et al. 2010). Future studies are evidently needed to experimentally dissect the IGF-regulated pathways regulating extracellular matrix homeostasis and vascular remodeling (Bruel and Oxlund 2002; Shai et al. 2010; Ungvari and Csiszar 2012) in the models used.
In addition to collagen encoding genes, we found that other factors controlling vascular integrity are also downregulated in mice with developmental IGF-1 deficiency (Fig. 3d). Bone morphogenetic proteins are important regulators of extracellular matrix homeostasis. Interestingly, our data suggest that developmental IGF-1 deficiency results in dysregulation of BMP signaling pathways in the vascular wall. We found that developmental IGF-1 deficiency results in downregulation of the adapter protein Crk, which is involved in growth regulation, cell migration, and cell adhesion. It is significant that genetic deletion of Crk results in increased vascular fragility (Park et al. 2006). Vascular expression of paxillin was also downregulated in mice with developmental IGF-1 deficiency. Paxillin is expressed at focal adhesions, which adhere the cytoskeleton of smooth muscle cells to the extracellular matrix in the vascular wall and thereby contribute to the tensile strength of the vasculature. We found that developmental IGF-1 deficiency alters the expression of laminin, a major constituent of basement membranes dysregulated in aging (Gavazzi et al. 1995) and α6 integrin, a specific laminin receptor. Both aging and developmental IGF-1 deficiency tend to upregulate Wilms’ tumor 1-associating protein (WTAP), a nuclear protein that interacts with the Wilms’ tumor 1 tumor suppressor gene product (WT1). WTAP is a newly discovered component of the m6 A methyltransferase complex, which plays a critical role in epitranscriptomic regulation of RNA metabolism (Ping et al. 2014). Recent studies show that WTAP inhibits the proliferation of vascular smooth muscle cells and endothelial cells and promotes apoptosis, regulating vascular remodeling (Small et al. 2006, 2007). During development of the vasculature IGF-1 was shown to downregulate WTAP, which is necessary for IGF-1 to confer its antiapoptotic effects, regulating smooth muscle cell fate (Small and Pickering 2009). Another factors affected by developmental IGF-1 deficiency are endothelin receptor A, versican and O-GlcNAc transferase (Ogt), and Wwtr1. Endothelin receptor A is important for vascular development and maintenance of vascular integrity (Donato et al. 2014). Importantly, genome-wide association studies identify EDNRA as a possible factor in the pathogenesis of intracranial aneurysms (Low et al. 2012; Yasuno et al. 2011). The proteoglycan versican has a key role in extracellular matrix assembly and contributes to the pathogenesis of intracranial aneurysms (Sathyan et al. 2014). Changes in the O-linked-N-acetylglucosamine (O-GlcNAc) modification of cytoplasmic and nuclear proteins, catalyzed by O-GlcNAc transferase, regulates a wide range of cellular functions and have been associated with a number of age-related diseases (Fulop et al. 2008). Wwtr1 (TAZ) is a transcriptional coactivator that links mechanosensing of extracellular matrix stiffness to activity of nuclear transcription factors in vascular cells (Dupont et al. 2011). Interestingly, while the aforementioned miRNA target genes were uniquely regulated by developmental IGF-1 deficiency, other targets, such as TJP1 (ZO-1, which plays a role in assembly of tight junctions, regulating endothelial permeability and vascular development) and CRIM1 (which regulates vascular stability and angiogenesis) appear to be affected by post-pubertal IGF-1 status.
Taken together, out of the results of the miRNA profiling experiments and the target validations studies the concept emerges that IGF-1 deficiency during a critical period through development impacts extracellular matrix biology and smooth muscle phenotype later in life via miRNA-regulated pathways, thereby altering the composition and organization of the tissue microenenvironment and contributing to the pathogenesis of age-related vascular diseases. In support of this concept, there is growing evidence that in humans and experimental animals, the origins of pathologies associated with structural weakening of the vascular wall (e.g., intracerebral hemorrhages) occur during puberty, a time of rapid changes in the cerebral circulation and structural brain development (Blakemore et al. 2010; Blanton et al. 2012; Giedd et al. 2006; Goddings et al. 2014; Manz et al. 1979; Peper et al. 2011; Satterthwaite et al. 2014).
Limitations of the study
There are important limitations of our study, including the limited endpoints tested. Further studies are warranted to assess vascular miRNA expression profile and their predicted targets in young Igf1 f/f x Alb-cre mice and in aged Igf1 f/f x Alb-cre mice with peripubertal IGF-1 replacement. Our recent studies suggest that the consequences of a loss of circulating IGF-1 on vertebral bone aging are different in male and female mice due to compensatory changes in IGF-1 signaling (Ashpole et al. 2015). Thus, future studies should determine whether late-life effects of developmental IGF-1 deficiency on vascular health are also sex-specific. There are studies suggesting that IGF-1 deficiency determines intima-media thickness in human patients (Colao et al. 2004); thus, future studies should also determine how experimental IGF-1 deficiency affects neointima formation in our models.
Conclusions
The findings of the present study provide additional experimental evidence in support of the concept that IGF-1 levels in a critical period early in life influence vascular health later in life (Fig. 3e). Among the possible diverse developmental epigenetic processes regulated by IGF-1, our data provide evidence for persistent changes in miRNA-mediated post-transcriptional gene regulation in the vasculature. Importantly, our findings suggest that developmental IGF-1 levels significantly impact post-transcriptional regulation of expression of genes regulating structural integrity of the vasculature, including components of the extracellular matrix. Future studies should fully elucidate the mechanistic effects of developmental IGF-1 levels on the pathogenesis of specific vascular diseases that involve remodeling/degradation of the extracellular matrix (including intracerebral hemorrhages, atherosclerosis, aneurysm), to characterize the peripubertal time window for the late-life effects of developmental IGF-1 on vascular health-span and to study the contribution of individual miRNAs or miRNA clusters regulated by developmental IGF-1 deficiency in controlling gene expression that underlie extracellular matrix remodeling and microvascular aging.
Acknowledgments
This work was supported by grants from the American Heart Association (ST, AC, MNVA, ZT and ZU), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, WES), the Oklahoma IDeA Network for Biomedical Research Excellence (to AC), the NIH (AG031085 to AC; AT006526 to ZU; AG038747 and NS056218 to WES and AC; 5U54GM104938 to JW), the Ellison Medical Foundation (to WES), and the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to JW and ZU; P30 AG028718).
Compliance with ethical standards
All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee of OUHSC in accordance with the ARRIVE guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Stefano Tarantini and Cory B. Giles contributed equally to this work.
References
- Alonso-Bouzon C, Carcaillon L, Garcia-Garcia FJ, Amor-Andres MS, El Assar M, Rodriguez-Manas L. Association between endothelial dysfunction and frailty: the Toledo study for healthy aging. Age (Dordr) 2014;36:495–505. doi: 10.1007/s11357-013-9576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada S, Takahashi T, Isodono K, Adachi A, Imoto H, Ogata T, Ueyama T, Matsubara H, Oh H. Downregulation of dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2512–H2521. doi: 10.1152/ajpheart.00233.2008. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Herron JC, Mitschelen MC, Farley JA, Logan S, Yan H, Ungvari Z, Hodges EL, Csiszar A, Ikeno Y, Humphrey MB, and Sonntag WE (2015) IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res 31(2):443–454 [DOI] [PMC free article] [PubMed]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/S0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell 9:1–18 [DOI] [PMC free article] [PubMed]
- Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010;9:1–18. doi: 10.1111/j.1474-9726.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Korner A, Obermayer-Pietsch B, Hubener C, Dahlgren J, Frystyk J, Pfeiffer AF, Doering A, Bielohuby M, Wallaschofski H, Arafat AM. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99:1712–1721. doi: 10.1210/jc.2013-3059. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Cooney RE, Joormann J, Eugene F, Glover GH, Gotlib IH. Pubertal stage and brain anatomy in girls. Neuroscience. 2012;217:105–112. doi: 10.1016/j.neuroscience.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Muller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- Bruel A, Oxlund H. Growth hormone influences the content and composition of collagen in the aorta from old rats. Mech Ageing Dev. 2002;123:627–635. doi: 10.1016/S0047-6374(01)00409-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Che P, Liu J, Shan Z, Wu R, Yao C, Cui J, Zhu X, Wang J, Burnett MS, Wang S. miR-125a-5p impairs endothelial cell angiogenesis in aging mice via RTEF-1 downregulation. Aging Cell. 2014;13:926–934. doi: 10.1111/acel.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Chuang L, Huang YH, Zhou J, Lim SH, Lee CI, Lin WW, Lin TE, Wang WL, Chen L, Chien S, Chiu JJ. MicroRNA mediation of endothelial inflammatory response to smooth muscle cells and its inhibition by atheroprotective shear stress. Circ Res. 2015;116:1157–1169. doi: 10.1161/CIRCRESAHA.116.305987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- Chisalita SI, Johansson GS, Liefvendahl E, Back K, Arnqvist HJ. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF1 and IGF2. J Mol Endocrinol. 2009;43:231–239. doi: 10.1677/JME-09-0021. [DOI] [PubMed] [Google Scholar]
- Cittadini A, Stromer H, Vatner DE, Grossman JD, Katz SE, Clark R, Morgan JP, Douglas PS. Consequences of growth hormone deficiency on cardiac structure, function, and beta-adrenergic pathway: studies in mutant dwarf rats. Endocrinology. 1997;138:5161–5169. doi: 10.1210/endo.138.12.5591. [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Filippella M, Rota F, Pivonello R, Orio F, Vitale G, Lombardi G. Insulin-like growth factor-1 deficiency determines increased intima-media thickness at common carotid arteries in adult patients with growth hormone deficiency. Clin Endocrinol. 2004;61:360–366. doi: 10.1111/j.1365-2265.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole-rat. Am J Phys. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE and Ungvari ZI (2013) Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and AD. Am J Physiol Heart Circ Physiol [DOI] [PMC free article] [PubMed]
- D’Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil Suppl. 1993;46:87–98. [PubMed] [Google Scholar]
- Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, Bonauer A, Boon RA, Fischer A, Bauersachs J, van Rooij E, Dimmeler S, Sedding DG. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103:564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci B, Demir O, Dost T, Birincioglu M. Antioxidative effect of aspirin on vascular function of aged ovariectomized rats. Age (Dordr) 2014;36:223–229. doi: 10.1007/s11357-013-9569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, and Dimmeler S Members of the microRNA-17-92 cluster exhibit a cell intrinsic anti-angiogenic function in endothelial cells. Blood 115(23):4944–4950 [DOI] [PubMed]
- Donato AJ, Lesniewski LA, Stuart D, Walker AE, Henson G, Sorensen L, Li D, Kohan DE. Smooth muscle specific disruption of the endothelin-a receptor in mice reduces arterial pressure, and vascular reactivity and affects vascular development. Life Sci. 2014;118:238–243. doi: 10.1016/j.lfs.2013.12.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozmorov MG, Giles CB, Wren JD. Predicting gene ontology from a global meta-analysis of 1-color microarray experiments. BMC Bioinformatics. 2011;12(Suppl 10):S14. doi: 10.1186/1471-2105-12-S10-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Edouard T, Grunenwald S, Gennero I, Salles JP, Tauber M. Prevalence of IGF1 deficiency in prepubertal children with isolated short stature. Eur J Endocrinol. 2009;161:43–50. doi: 10.1530/EJE-08-0964. [DOI] [PubMed] [Google Scholar]
- Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum. 2006;54:3850–3858. doi: 10.1002/art.22254. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth, adult income, and risk of stroke. Stroke. 2000;31:869–874. doi: 10.1161/01.STR.31.4.869. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Smith KM, Lovett-Racke AE, Guerau-de-Arellano M, Whitacre CC, Godbout JP. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiol Aging. 2013;34:2748–2758. doi: 10.1016/j.neurobiolaging.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. The cardiovascular survey in Finnmark 1974-75. J Epidemiol Community Health. 1978;32:34–37. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L, Williams FM, Trofimov S, Malkin I, Surdulescu G, Spector T, Livshits G. Assessment of age-related changes in heritability and IGF-1 gene effect on circulating IGF-1 levels. Age (Dordr) 2014;36:9622. doi: 10.1007/s11357-014-9622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop N, Feng W, Xing D, He K, Not LG, Brocks CA, Marchase RB, Miller AP, Chatham JC. Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology. 2008;9:139–151. doi: 10.1007/s10522-007-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Boyle KS, Edgar D, Cowen T. Reduced laminin immunoreactivity in the blood vessel wall of ageing rats correlates with reduced innervation in vivo and following transplantation. Cell Tissue Res. 1995;281:23–32. doi: 10.1007/BF00307955. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbourt U, Tanne D. Body height is associated with decreased long-term stroke but not coronary heart disease mortality? Stroke. 2002;33:743–748. doi: 10.1161/hs0302.103814. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Guardia L, Yubero-Serrano EM, Rangel-Zuniga O, Marin C, Camargo A, Perez-Martinez P, Delgado-Lista J, Gomez-Delgado F, Garcia-Rios A, Tinahones FJ, Roche HM, Perez-Jimenez F, Lopez-Miranda J. Influence of endothelial dysfunction on telomere length in subjects with metabolic syndrome: LIPGENE study. Age (Dordr) 2014;36:9681. doi: 10.1007/s11357-014-9681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska W, Kucharewicz K, Wnuk M, Lewinska A, Suszek M, Przybylska D, Mosieniak G, Sikora E, Bielak-Zmijewska A. Curcumin induces senescence of primary human cells building the vasculature in a DNA damage and ATM-independent manner. Age (Dordr) 2015;37:9744. doi: 10.1007/s11357-014-9744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillari J, Grillari-Voglauer R Novel modulators of senescence, aging, and longevity: small non-coding RNAs enter the stage. Exp Gerontol 45:302–311 [DOI] [PubMed]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez-Mateos A, Kraemer T, Cortese-Krott MM, Kuhnle GG, Spencer JP, Schroeter H, Merx MW, Kelm M. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age (Dordr) 2015;37:9794. doi: 10.1007/s11357-015-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010;6:475–490. doi: 10.7150/ijbs.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab. 2010;21:245–254. doi: 10.1016/j.tem.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Arum O, Boparai RK, Wang F, Fang Y, Sun LY, Masternak MM, Bartke A. Female PAPP-A knockout mice are resistant to metabolic dysfunction induced by high-fat/high-sucrose feeding at middle age. Age (Dordr) 2015;37:9765. doi: 10.1007/s11357-015-9765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Zhang Y, Ren J. IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: role of microRNA-1 and microRNA-133a. J Cell Mol Med. 2012;16:83–95. doi: 10.1111/j.1582-4934.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.B291. [DOI] [PubMed] [Google Scholar]
- Inukai S, de Lencastre A, Turner M, Slack F. Novel microRNAs differentially expressed during aging in the mouse brain. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai S and Slack F (2013) MicroRNAs and the Genetic Network in Aging. J Mol Biol 425(19):3601–3608 [DOI] [PMC free article] [PubMed]
- Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Johansson GS, Chisalita SI, Arnqvist HJ. Human microvascular endothelial cells are sensitive to IGF-I but resistant to insulin at the receptor level. Mol Cell Endocrinol. 2008;296:58–63. doi: 10.1016/j.mce.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Osmond C, Barker DJ, Forsen T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int J Epidemiol. 2005;34:655–663. doi: 10.1093/ije/dyi048. [DOI] [PubMed] [Google Scholar]
- Kim CW, Kumar S, Son DJ, Jang IH, Griendling KK, Jo H. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler Thromb Vasc Biol. 2014;34:1412–1421. doi: 10.1161/ATVBAHA.113.303134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Jung KH, Chu K, Lee ST, Ban J, Moon J, Kim M, Lee SK, Roh JK. Atherosclerosis-related circulating MicroRNAs as a predictor of stroke recurrence. Transl Stroke Res. 2015;6:191–197. doi: 10.1007/s12975-015-0390-1. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- Laron Z, Klinger B, Silbergeld A. Patients with Laron syndrome have osteopenia/osteoporosis. J Bone Miner Res. 1999;14:156–157. doi: 10.1359/jbmr.1999.14.1.156. [DOI] [PubMed] [Google Scholar]
- Lee S, Yu KR, Ryu YS, Oh YS, Hong IS, Kim HS, Lee JY, Kim S, Seo KW, Kang KS. miR-543 and miR-590-3p regulate human mesenchymal stem cell aging via direct targeting of AIMP3/p18. Age (Dordr) 2014;36:9724. doi: 10.1007/s11357-014-9724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Trac C, Jin W, Lanting L, Akbany A, Saetrom P, Schones DE, Natarajan R. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Huang Q, Zhang N, Wang GB, Liu YH. miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol Med Rep. 2014;10:527–535. doi: 10.3892/mmr.2014.2172. [DOI] [PubMed] [Google Scholar]
- Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpcell.00204.2006. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang D, Xu Z, Gao J, Liu M, Liu Y, Jiang M, Zheng D. Dysregulated expression of miR-101b and miR-26b lead to age-associated increase in LPS-induced COX-2 expression in murine macrophage. Age (Dordr) 2015;37:97. doi: 10.1007/s11357-015-9836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi S, Cittadini A, Stromer H, Katz SE, Grossman JD, Clark RG, Morgan JP, Douglas PS. Echocardiographic assessment of cardiac morphology and function in mutant dwarf rats. Growth Hormon IGF Res. 2000;10:242–247. doi: 10.1054/ghir.2000.0160. [DOI] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, Teoh H, Marsden PA, Verma S. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–S90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- Low SK, Takahashi A, Cha PC, Zembutsu H, Kamatani N, Kubo M, Nakamura Y. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet. 2012;21:2102–2110. doi: 10.1093/hmg/dds020. [DOI] [PubMed] [Google Scholar]
- Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–443. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process. Mech Ageing Dev. 2008;129:534–541. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Manz HJ, Vester J, Lavenstein B. Dissecting aneurysm of cerebral arteries in childhood and adolescence. Case report and literature review of 20 cases. Virchows Arch A Pathol Anat Histol. 1979;384:325–335. doi: 10.1007/BF00428233. [DOI] [PubMed] [Google Scholar]
- Marino G, Ugalde AP, Fernandez AF, Osorio FG, Fueyo A, Freije JM, Lopez-Otin C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc Natl Acad Sci U S A. 2010;107:16268–16273. doi: 10.1073/pnas.1002696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SP, Miller RA. Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell. 2006;5:89–96. doi: 10.1111/j.1474-9726.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, Mattison J, Cookson MR, Gorospe M, de Cabo R, Abdelmohsen K. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourmoura E, Couturier K, Hininger-Favier I, Malpuech-Brugere C, Azarnoush K, Richardson M, Demaison L. Functional changes of the coronary microvasculature with aging regarding glucose tolerance, energy metabolism, and oxidative stress. Age (Dordr) 2014;36:9670. doi: 10.1007/s11357-014-9670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, Nicolle MM. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke JR, Olson EN. Modulating the MicroRNArchitecture of an aging aorta. Circ Res. 2011;109:1098–1099. doi: 10.1161/CIRCRESAHA.111.256388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278:1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke. 2007;38:264–270. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- Page MM, Salmon AB, Leiser SF, Robb EL, Brown MF, Miller RA, Stuart JA. Mechanisms of stress resistance in Snell dwarf mouse fibroblasts: enhanced antioxidant and DNA base excision repair capacity, but no differences in mitochondrial metabolism. Free Radic Biol Med. 2009;46:1109–1118. doi: 10.1016/j.freeradbiomed.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:1–7. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Boyd K, Curran T. Cardiovascular and craniofacial defects in Crk-null mice. Mol Cell Biol. 2006;26:6272–6282. doi: 10.1128/MCB.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Lapane KL, Lasater TM, Carleton RA. Short stature and cardiovascular disease among men and women from two southeastern New England communities. Int J Epidemiol. 1998;27:970–975. doi: 10.1093/ije/27.6.970. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36:1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, Zhao X, Li A, Yang Y, Dahal U, Lou XM, Liu X, Huang J, Yuan WP, Zhu XF, Cheng T, Zhao YL, Wang X, Rendtlew Danielsen JM, Liu F, Yang YG. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Hartley CJ, Pham TT, Darlington G, Entman ML, Taffet GE. Young little mice express a premature cardiovascular aging phenotype. J Gerontol A Biol Sci Med Sci. 2014;69:152–159. doi: 10.1093/gerona/glt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TC, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci. 2013;33:6857–6863. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojanathammanee L, Rakoczy S, Kopchick J, Brown-Borg HM. Effects of insulin-like growth factor 1 on glutathione S-transferases and thioredoxin in growth hormone receptor knockout mice. Age (Dordr) 2014;36:9687. doi: 10.1007/s11357-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice—brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013;32:74–85. doi: 10.1016/j.matbio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutnam ZJ, Yang BB. The non-coding 3′ UTR of CD44 induces metastasis by regulating extracellular matrix functions. J Cell Sci. 2012;125:2075–2085. doi: 10.1242/jcs.100818. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, and Miller RA (2015) Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell [DOI] [PMC free article] [PubMed]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Phys Endocrinol Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Sathyan S, Koshy LV, Balan S, Easwer HV, Premkumar S, Nair S, Bhattacharya RN, Alapatt JP, Banerjee M. Association of Versican (VCAN) gene polymorphisms rs251124 and rs2287926 (G428D), with intracranial aneurysm. Meta Gene. 2014;2:651–660. doi: 10.1016/j.mgene.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014;111:8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Most P, Peppel K. Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett. 2014;588:906–914. doi: 10.1016/j.febslet.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol. 2010;30:1916–1924. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small TW, Bolender Z, Bueno C, O’Neil C, Nong Z, Rushlow W, Rajakumar N, Kandel C, Strong J, Madrenas J, Pickering JG. Wilms’ tumor 1-associating protein regulates the proliferation of vascular smooth muscle cells. Circ Res. 2006;99:1338–1346. doi: 10.1161/01.RES.0000252289.79841.d3. [DOI] [PubMed] [Google Scholar]
- Small TW, Penalva LO, Pickering JG. Vascular biology and the sex of flies: regulation of vascular smooth muscle cell proliferation by wilms’ tumor 1-associating protein. Trends Cardiovasc Med. 2007;17:230–234. doi: 10.1016/j.tcm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Small TW, Pickering JG. Nuclear degradation of Wilms tumor 1-associating protein and survivin splice variant switching underlie IGF-1-mediated survival. J Biol Chem. 2009;284:24684–24695. doi: 10.1074/jbc.M109.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Csiszar A. deCabo R, Ferrucci L, and Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1017/S002187829900713X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.B521. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sorensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol. 2012;166:903–910. doi: 10.1530/EJE-12-0106. [DOI] [PubMed] [Google Scholar]
- Stellos K, Dimmeler S. Vascular microRNAs: from disease mechanisms to therapeutic targets. Circ Res. 2014;114:3–4. doi: 10.1161/CIRCRESAHA.113.302762. [DOI] [PubMed] [Google Scholar]
- Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- Svensson D, Gidlof O, Turczynska KM, Erlinge D, Albinsson S, Nilsson BO. Inhibition of microRNA-125a promotes human endothelial cell proliferation and viability through an antiapoptotic mechanism. J Vasc Res. 2014;51:239–245. doi: 10.1159/000365551. [DOI] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A and Ungvari Z (2015) IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14(6):1034–1044 [DOI] [PMC free article] [PubMed]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, and Ungvari Z (2014) IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34(12):1887–1897 [DOI] [PMC free article] [PubMed]
- Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, Del Campo A, Toro B, Battiprolu PK, Aranguiz P, Chiong M, Yakar S, Gillette TG, Hill JA, Abel ED, Leroith D, Lavandero S. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res. 2012;93:320–329. doi: 10.1093/cvr/cvr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z and Csiszar A (2013) Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease J Gerontol A Biol Med Sci 69(10):1212–1226 [DOI] [PMC free article] [PubMed]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending Peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66:501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE, Csiszar A. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:877–891. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Abeelen AF, Elias SG, Bossuyt PM, Grobbee DE, van der Schouw YT, Roseboom TJ, Uiterwaal CS. Cardiovascular consequences of famine in the young. Eur Heart J. 2012;33:538–545. doi: 10.1093/eurheartj/ehr228. [DOI] [PubMed] [Google Scholar]
- Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, Mahe C, Agostini M, Knight RA, Melino G, Federici M. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Victoria B, Dhahbi JM, Nunez Lopez YO, Spinel L, Atamma H, Spindler SR and Masternak MM (2015) Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell 14(6):1055–1066 [DOI] [PMC free article] [PubMed]
- Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr) 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Miller RA. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11:668–674. doi: 10.1111/j.1474-9726.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Kim S, Patterson N, Rooney K, Searles CD. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am J Physiol Heart Circ Physiol. 2014;306:H1192–H1203. doi: 10.1152/ajpheart.00521.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren JD. A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics. 2009;25:1694–1701. doi: 10.1093/bioinformatics/btp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren JD, Garner HR. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics. 2004;20:191–198. doi: 10.1093/bioinformatics/btg390. [DOI] [PubMed] [Google Scholar]
- Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of Kruppel-like factor 2 is mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- Yasuno K, Bakircioglu M, Low SK, Bilguvar K, Gaal E, Ruigrok YM, Niemela M, Hata A, Bijlenga P, Kasuya H, Jaaskelainen JE, Krex D, Auburger G, Simon M, Krischek B, Ozturk AK, Mane S, Rinkel GJ, Steinmetz H, Hernesniemi J, Schaller K, Zembutsu H, Inoue I, Palotie A, Cambien F, Nakamura Y, Lifton RP, Gunel M. Common variant near the endothelin receptor type a (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A. 2011;108:19707–19712. doi: 10.1073/pnas.1117137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Attia R, Mayr U, Gomes RS, Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, Fava M, Barallobre-Barreiro J, Molenaar C, So PW, Abbas A, Jahangiri M, Waltham M, Botnar R, Smith A, Mayr M. Role of miR-195 in aortic aneurysmal disease. Circ Res. 2014;115:857–866. doi: 10.1161/CIRCRESAHA.115.304361. [DOI] [PubMed] [Google Scholar]
- Zhang X, Azhar G, Wei JY. The expression of microRNA and microRNA clusters in the aging heart. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Davis S, Stephens R, Meltzer PS, Chen Y. GEOmetadb: powerful alternative search engine for the gene expression omnibus. Bioinformatics. 2008;24:2798–2800. doi: 10.1093/bioinformatics/btn520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, Bahari-Javan S, Burkhardt S, Sananbenesi F, Fischer A. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30:4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]