Abstract
Strong epidemiological and experimental evidence indicate that both age and hypertension lead to significant functional and structural impairment of the cerebral microcirculation, predisposing to the development of vascular cognitive impairment (VCI) and Alzheimer’s disease. Preclinical studies establish a causal link between cognitive decline and microvascular rarefaction in the hippocampus, an area of brain important for learning and memory. Age-related decline in circulating IGF-1 levels results in functional impairment of the cerebral microvessels; however, the mechanistic role of IGF-1 deficiency in impaired hippocampal microvascularization remains elusive. The present study was designed to characterize the additive/synergistic effects of IGF-1 deficiency and hypertension on microvascular density and expression of genes involved in angiogenesis and microvascular regression in the hippocampus. To achieve that goal, we induced hypertension in control and IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8) by chronic infusion of angiotensin II. We found that circulating IGF-1 deficiency is associated with decreased microvascular density and exacerbates hypertension-induced microvascular rarefaction both in the hippocampus and the neocortex. The anti-angiogenic hippocampal gene expression signature observed in hypertensive IGF-1 deficient mice in the present study provides important clues for subsequent studies to elucidate mechanisms by which hypertension may contribute to the pathogenesis and clinical manifestation of VCI. In conclusion, adult-onset, isolated endocrine IGF-1 deficiency exerts deleterious effects on the cerebral microcirculation, leading to a significant decline in cortical and hippocampal capillarity and exacerbating hypertension-induced cerebromicrovascular rarefaction. The morphological impairment of the cerebral microvasculature induced by IGF-1 deficiency and hypertension reported here, in combination with neurovascular uncoupling, increased blood-brain barrier disruption and neuroinflammation reported in previous studies likely contribute to the pathogenesis of vascular cognitive impairment in elderly hypertensive humans.
Keywords: Cerebral blood flow, Mild cognitive impairment, Ischemia, Brain aging, Vascular aging, Dementia, Alzheimer’s disease
Introduction
There is growing evidence that alterations of the cerebral microcirculation play a key role in age-related decline in higher brain function (Toth et al. 2013a; Tucsek et al. 2003; Tucsek et al. 2014; Zlokovic 2011). Normal brain function is critically dependent on a continuous, tightly-controlled supply of oxygen and nutrients through adequate cerebral blood flow. The human brain receives almost 15 % of the cardiac output through a network of over 600 km of capillaries. In the brain, the number of endothelial cells is very similar to that of neurons (Garcia-Amado and Prensa 2012) and nearly every neuron is supplied by its own capillary, with an average distance of 8–20 μm between the neuron and the microvessels. Importantly, there is strong evidence that aging is associated with a decline in cerebral capillary density (“microvascular rarefaction”) and that decreases in cerebromicrovascular density contribute to the age-related decline in regional cerebral blood flow (Mitschelen et al. 2009; Riddle et al. 2003; Khan et al. 2001; Lynch et al. 1999; Sonntag et al. 1997; Martin et al. 1991; Moeller et al. 1996; Farkas and Luiten 2001; Kawamura et al. 1993; Krejza et al. 1999; Schultz et al. 1999; Bentourkia et al. 2000; Hagstadius and Risberg 1989; Pagani et al. 2002). The resulting mismatch between energy supply and demand has been causally linked to significant cognitive impairment (reviewed in (Khan et al. 2001; Sonntag et al. 1997; Ingraham et al. 2008; Sonntag et al. 2000; Warrington et al. 2011; Warrington et al. 2012). Recent research substantiates that endocrine mechanisms play a critical role in age-related cerebromicrovascular pathology (Sonntag et al. 2013; Ungvari and Csiszar 2012). Principally, the age-related decline in circulating levels of insulin-like growth factor-1 (IGF-1) has been linked to microvascular aging and cognitive decline (reviewed recently in (Sonntag et al. 2013). IGF-1, which is secreted mainly by the liver and mediates the actions of growth hormone, is a pleiotropic growth factor which confers multifaceted pro-angiogenic effects, regulating microvascular remodeling (Lopez-Lopez et al. 2004) and protection against endothelial injury (Bailey-Downs et al. 2012a; Bailey-Downs et al. 2012b). We have recently demonstrated that mice with isolated decreases of circulating IGF-1 levels exhibit aging-like microvascular phenotypes, including impaired neurovascular coupling and cerebromicrovascular autoregulatory dysfunction (Lopez-Lopez et al. 2004; Toth et al. 2015; Toth et al. 2014a). Despite these advances, the role of circulating IGF-1 deficiency in age-related cerebromicrovascular rarefaction remains elusive.
Hypertension substantially contributes to cerebromicrovascular damage and promotes the development of vascular cognitive impairment. Hypertension-induced microvascular rarefaction has been observed both in humans (Wolf et al. 1994) and in laboratory rodents (Sokolova et al. 1985). Recent evidence shows that old age exacerbates hypertension-induced cerebromicrovascular rarefaction (Toth et al. 2013a). Further, low circulating IGF-1 levels increase the risk for hypertension-induced microvascular brain damage in elderly patients (Angelini et al. 2009), findings which have been also replicated in laboratory animals (Toth et al. 2014a). Despite these advances, the synergistic/additive effects of circulating IGF-1 deficiency and hypertension on cerebral microvascular density have not been explored.
The present study was designed to test the hypotheses that isolated circulating IGF-1 deficiency promotes microvascular rarefaction in the hippocampus and the neocortex and leads to structural maladaptation of the cerebral microcirculation to hypertension, mimicking the aging phenotype. To test our hypotheses, we used a novel mouse model of adult-onset, isolated endocrine IGF-1 deficiency induced by adeno-associated viral knockdown of IGF-1 specifically in the liver of post-pubertal mice using Cre-lox technology (Igf1 f/f + TBG-Cre-AAV8) (Bailey-Downs et al. 2012a; Toth et al. 2015). Hypertension was induced in control and IGF-1 deficient mice by chronic infusion of angiotensin II and changes in microvascular density and expression of genes involved in regulation of angiogenesis and microvascular regression in the hippocampus were assessed.
Materials and methods
Post-developmental liver-specific knockdown of Igf1 in mice
Male mice homozygous for a floxed exon 4 of the Igf1 gene (Igf1 f/f) (Liu et al. 1998) in a C57BL/6 background were purchased from Jackson Laboratories. These mice have the entirety of exon 4 of the Igf1 gene flanked by loxP sites, which allows for genomic excision of this exon when exposed to Cre recombinase. Transcripts of the altered Igf1 gene yield a protein upon translation that fails to bind the IGF receptor. Animals were housed in the Rodent Barrier Facility at OUHSC, on a 12 h light/12 h dark cycle, with access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum.
To target hepatocytes, adeno-associated viruses (AAVs) were purchased from the University of Pennsylvania Vector Core (Philadelphia, PA). At 4 months of age, approximately 1.3 × 1010 viral particles (as assayed by genome content at the University of Pennsylvania) of AAV8.TBG.PI.Cre.rBG or AAV8.TBG.PI.eGFP.WPRE.bGH were administered to Igf1 f/f mice to knockdown IGF-1 or as a control, respectively. Mice were anesthetized with ketamine/xylazine (100 and 15 mg/kg, respectively), and given retroorbital injections of virus diluted to the appropriate concentration in 100 μl 0.9 % saline. While AAV8 is effective at transducing multiple tissues after i.v. delivery, including liver, the thyroxine binding globulin (TBG) promoter restricts expression solely to hepatocytes (Toth et al. 2015; Toth et al. 2014a). Dosages were determined empirically in preliminary studies. All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee of OUHSC in accordance with the ARRIVE guidelines.
Measurement of serum IGF-1 levels
Submandibular venous blood was collected into microcentrifuge tubes using a sterile lancet (Medipoint, Mineola, NY) according to the manufacturer’s instructions. Whole blood was centrifuged at 2500×g for 20 min at 4 °C to collect serum, which was then stored at −80 °C. IGF-1 concentration in the serum samples was measured by ELISA (R&D Systems, Minneapolis, MN) as reported (Toth et al. 2015; Toth et al. 2014a). An IGF-1 control sample, with aliquots stored at −80 °C, was included on each plate. Serum IGF-1 levels are reported in nanogram/milliliter.
Infusion of angiotensin II
To induce hypertension, Alzet mini-osmotic pumps (Model 2006, 0.15 μl/h, 42 days; Durect Co, Cupertino, CA) were implanted into Igf1 f/f + TBG-Cre-AAV8 and control mice (2-month post-AAV injection), as previously described (Toth et al. 2013a). Pumps were filled either with saline vehicle or solutions of angiotensin II (Sigma Chemical Co., St. Louis, MO, USA) that delivered (subcutaneously) 1000 ng/min/kg of angiotensin II for 28 days (Toth et al. 2013a). Pumps were placed into the subcutaneous space of ketamine/xylazine anesthetized mice through a small incision in the back of the neck that was closed with surgical sutures. All incision sites healed rapidly without the need for additional medication.
Blood pressure measurements
Systolic blood pressure of mice in each experimental group was measured by the tail cuff method (CODA Non-Invasive Blood Pressure System, Kent Scientific Co., Torrington, CT) before and 2 weeks (to check for pump failure) and 4 weeks (before the terminal experiments) after the minipump implantation (Toth et al. 2013a).
Analysis of microvascular density: immunofluorescent labeling and confocal microscopy
Mice were transcardially perfused with PBS; the brains were removed and hemisected. From the right hemispheres, the hippocampi were isolated and frozen for subsequent analysis. The left hemispheres were fixed overnight in 4 % paraformaldehyde, then they were cryoprotected in a series of graded sucrose solutions (10, 20, and 30 % overnight), and frozen in Cryo-Gel (Electron Microscopy Sciences, Hatfield, PA) as described (Toth et al. 2014a). Coronal sections of 70 μm were cut through the hippocampus and stored free-floating in cryopreservative solution (25 % glycerol, 25 % ethylene glycol, 25 % 0.2 M phosphate buffer, 25 % distilled water) at −20 °C. Selected sections were ∼1.6 mm caudal to Bregma, representing the more rostral hippocampus. After washing (3 × 5 min with TBS plus 3 × 5 min with 1× TBS + 0.25 % TritonX-100), sections were treated with 1 % of sodium-borohydride solution for 5 min. After a second washing step (3 × 5 min with distilled water plus 3 × 5 min with 1× TBS) and blocking in 5 % BSA/TBS (with 0.5 % Triton X-100, 0.3 M glycin and 1 % fish gelatin; for 3 h), sections were immunostained using a mouse anti-CD31 (1: 50; Cat N: 550274, BD Pharmingen, San Jose, CA) primary antibody (for two nights at 4 °C) to label endothelial cells. Sections were washed for 3 × 5 min with TBS plus 3 × 5 min with 1× TBS + 0.25 % TritonX-100. For nuclear counterstaining, Hoechst 33342 was used. Then, the sections were transferred to slides and coverslipped. Confocal images were captured using a Leica SP2 MP confocal laser scanning microscope.
Immunofluorescent labeling for CD31 was used to identify microvessels in the brain as we previously described (Toth et al. 2013a; Tucsek et al. 2014; Warrington et al. 2011). Capillary density in the CA1 region of the hippocampus was quantified, using stereological methods, as the length of blood vessels <10 μm in diameter per volume of tissue using Neurolucida with AutoNeuron (MicroBrightField, Williston, VT). Brain regions were identified based on reference (Hof et al. 2000). The total length of capillaries (mm) was divided by the volume of brain tissue scanned (mm3) to obtain capillary density (length per tissue volume) within the CA1 region for each animal. The experimenter was blinded to the groups and treatments of the animals throughout the period of blood vessel staining and analysis.
Targeted qPCRarray to analyze mRNA expression of pro- and anti-angiogenic factors
A quantitative real time RT-PCR technique was used to analyze mRNA expression of genes known to be involved in regulation of angiogenesis and microvascular regression in hippocampi of mice from each experimental group as reported (Toth et al. 2013a; Tucsek et al. 2003; Tucsek et al. 2014; Csiszar et al. 2013). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (Bailey-Downs et al. 2012a). Hippocampal mRNA expression of pro- and anti-angiogenic genes was analyzed using validated TaqMan probes (Applied Biosystems) and a Strategen MX3000 platform, as previously reported (Tucsek et al. 2014). Quantification was performed using the efficiency-corrected ΔΔCt method. Using the Bioconductor HTqPCR package (Dvinge and Bertone 2009), arrays were first rescaled to ΔCt values using Ywhaz, B2m, and Hprt as a reference, and then quantile normalized. “Undetermined” Ct values were replaced with the global maximum Ct value. Differential expression significance was determined using the Bioconductor limma package, and the directionality and magnitude of the angiogenic signature was assessed using both the t statistic and Gene Set Enrichment Analysis (Subramanian et al. 2005) as implemented in the Bioconductor GSA package.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests. All statistical comparisons were performed using Prism 5.0 for Windows (Graphpad Software, La Jolla, CA), and were considered significant at p < 0.05. Data are expressed as mean ± S.E.M.
Results
Circulating IGF-1 levels and blood pressure measurements
Although the factors responsible for the deleterious effects of aging on behavior and neuronal function (Baciu et al. 2016; Berghuis et al. 2015; Campbell et al. 2014; Doi et al. 2015; Haider et al. 2014; Hofmann et al. 2014; Kumar and Thakur 2015; Lopez et al. 2014; Manich et al. 2014; Salminen et al. 2014; Samaras et al. 2014; Sarubbo et al. 2015; Loprinzi 2016; Wallis et al. 2016) are not completely understood, there is strong evidence that in elderly humans a decline in circulating IGF-1 levels (Franco et al. 2014) plays a critical pathophysiological role. To understand the effects of IGF-1 deficiency on the cerebral microcirculation in the present study, we used a novel mouse model of adult-onset isolated endocrine IGF-1 deficiency, which phenotypically better mimics age-related IGF-1 deficiency observed in humans that most other available rodent models of GH/IGF-1 deficiency (Arum et al. 2014a; Hill et al. 2015; Rojanathammanee et al. 2014; Wiesenborn et al. 2014; Arum et al. 2014b; Schneider et al. 2014). Serum IGF-1 levels and physiological parameters obtained in the same experimental cohorts of animals used for the present study have been recently reported (Toth et al. 2014a). Accordingly, mice receiving TBG-Cre-AAV8 had significantly decreased (by ∼75 %) serum IGF-1 levels compared with control mice receiving TBG-eGFP-AAV8. Both groups had similar serum IGF-1 levels prior to administration of liver-targeted viruses. Previously, we reported that knockdown of IGF-1 in this model does not lead to change in body weight, serum insulin, IGFBP-1 and −2, adiponectin and glucose levels (Toth et al. 2015). To induce hypertension, we used angiotensin II, which has special relevance for aging research (Salminen et al. 2014; Mellor et al. 2014; Schuch et al. 2014; Simon et al. 2015). We found that blood pressure was significantly increased (by ∼50 %) in both control and IGF-1 deficient mice receiving Ang II infusion (Toth et al. 2014a). Although previous studies reported that in mice a∼80 % reduction in serum IGF-1 levels may be associated with a∼5 mmHg increase in blood pressure (Tivesten et al. 2002), and in our studies, no significant interaction between IGF-1 levels and blood pressure was noted.
IGF-1 deficiency exacerbates hypertension-induced cerebromicrovascular rarefaction
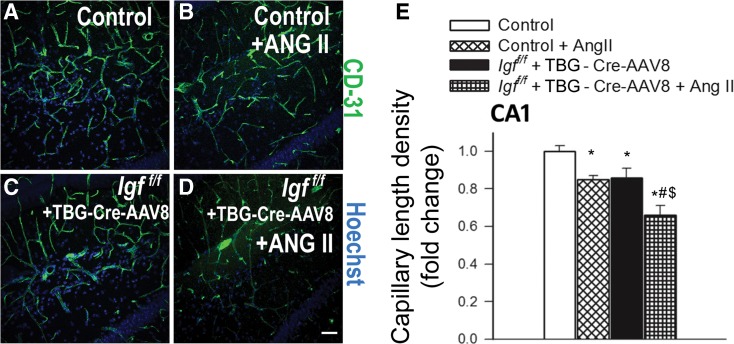
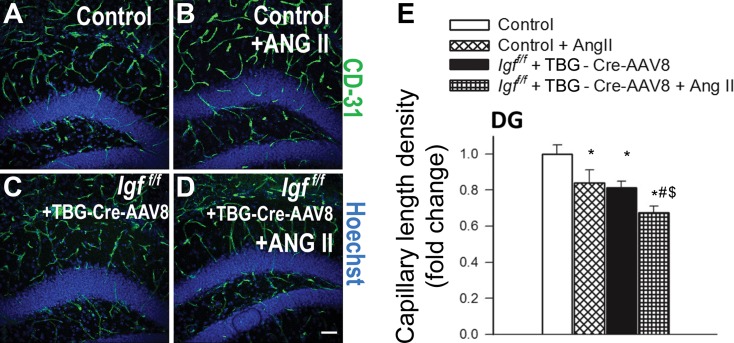
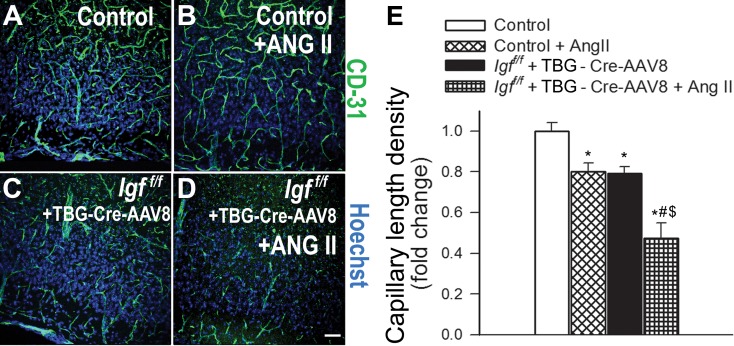
We quantified the effects of IGF-1 deficiency and hypertension on brain capillary density in the hippocampus of mice. Capillaries were identified by their expression of CD31 (Fig. 1a–e), using a lumen diameter of 10 μm or less as a standard identifier. We found that IGF-1 deficiency was associated with significant decreases in capillary density in the CA1 region (Fig. 1) and the dentate gyrus (Fig. 2) of the hippocampus, indicating significant cerebromicrovascular rarefaction. To determine whether the microvascular rarefaction could be observed in brain regions outside of the hippocampus, we next measured capillary density in the retrosplenial cortex of the control and IGF-1 deficient mice. We found that within this region IGF-1 deficiency also reduced capillary density by ∼20 % (Fig. 3).
Fig. 1.
IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the CA1 region of the mouse hippocampus. a–d Representative confocal images showing CD31-positive capillary endothelial cells (green) in the CA1 region of the hippocampi of in normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Hoechst 33342 was used for nuclear counterstaining (scale bar: 100 μm). e Summary data for hypertension-induced changes of capillary length density in the CA1 region of the hippocampus in control and IGF-1 deficient mice. Data are mean ± S.E.M. *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
Fig. 2.
IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the dentate gyrus of the mouse hippocampus. a–d Representative confocal images showing CD31-positive capillary endothelial cells (green) in the dentate gyrus (DG) of the hippocampi of in normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Hoechst 33342 was used for nuclear counterstaining (scale bar: 100 μm). e Summary data for hypertension-induced changes of capillary length density in the dentate gyrus of the hippocampi in control and IGF-1 deficient mice. Data are mean ± S.E.M. *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
Fig. 3.
IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the retrosplenial cortex of mice. a–d Representative confocal images showing CD31-positive capillary endothelial cells (green) in the retrosplenial cortex of normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Hoechst 33342 was used for nuclear counterstaining (scale bar: 100 μm). e Summary data for hypertension-induced changes of capillary length density in the retrosplenial cortex in control and IGF-1 deficient mice. Data are mean ± S.E.M. *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
Previous studies demonstrate that IGF-1 deficiency impairs functional adaptation of the cerebral circulation to hypertension (Toth et al. 2014a). Here we demonstrate that IGF-1 deficiency exacerbates hypertension-induced cerebromicrovascular rarefaction, as indicated by the substantial decline in CD31-positive capillaries both in the hippocampus and the retrosplenial cortex of hypertensive Igf1 f/f + TBG-iCre-AAV8 mice (Fig. 1, Fig. 2, and Fig. 3, respectively).
Effects of circulating IGF-1deficiency and hypertension on hippocampal angiogenic gene expression signature
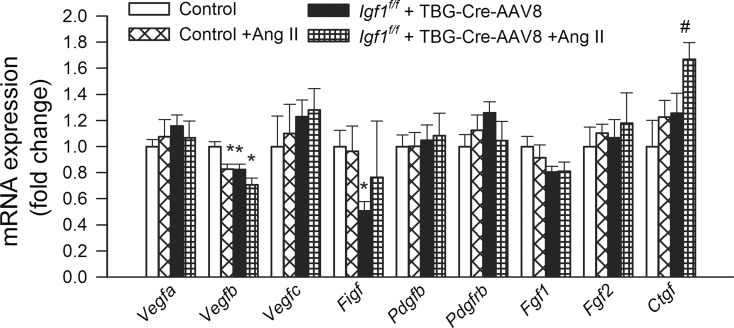
To elucidate the mechanisms contributing to cerebromicrovascular rarefaction, we detected and analyzed the mRNA expression of 90 angiogenesis- and rarefaction-associated genes in the mouse hippocampi (Tucsek et al. 2014). The effects of IGF-1 deficiency and hypertension on the expression of cytokines regulating angiogenesis (Vegfa, Vegfb, Vegfc, Figf, Pdgfb, Fgf1, Fgf2, and Ctgf) are shown in Fig. 4. Expression of vascular endothelial growth factor A (Vegfa), a potent inducer of angiogenesis, which prevents microvascular rarefaction (Steeghs et al. 2010), was unaltered. Importantly, both hypertension and IGF-1 deficiency were associated with down-regulation of Vegfb. VEGF-B is a pro-angiogenic growth factor, which is constitutively expressed in cerebral microvessels and plays a role only in the maintenance of the microcirculatory network (Nag et al. 2002) and adaptation to ischemic challenges (Bellomo et al. 2000). Among other members of the VEGF, family expression of Vegfc (Witzenbichler et al. 1998) was unchanged, whereas expression of Vegfd (C-fos-induced growth factor/FIGF) (Debinski et al. 2001) was down-regulated by IGF-1 deficiency. Expression of platelet-derived growth factor (Pdgfβ) (He et al. 2015) and fibroblast growth factors-1 and −2 (Fgf-1, Fgf-2) was unaltered by either IGF-1 deficiency and hypertension, whereas expression of connective tissue growth factor (Ctgf) was up-regulated in hypertensive IGF-1 deficient mice.
Fig. 4.
Effects of IGF-1 deficiency and hypertension on the hippocampal expression of cytokines regulating angiogenesis. QRT-PCR data showing mRNA expression of Vegfa, Vegfb, Vegfc, Figf, Pdgfb, Pdgfrb, Fgf1, Fgf2, and Ctgf in hippocampi of normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Data are mean ± S.E.M. (n = 5–8 in each group). *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
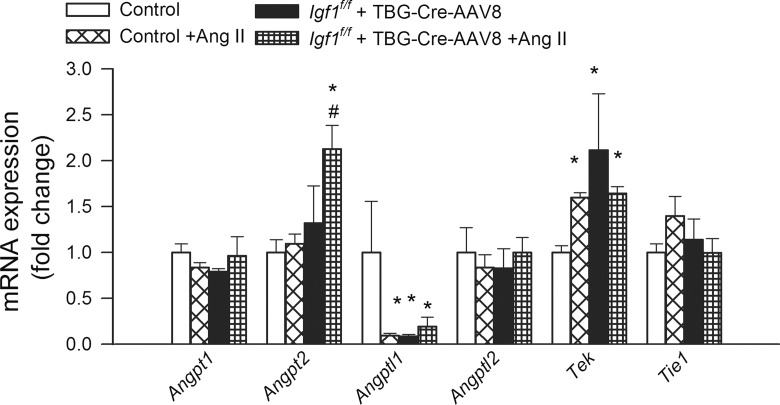
The effects of IGF-1 deficiency and hypertension on the expression of the pro-angiogenic factor Angpt1, the closely related factors Angptl1 and Angptl2, the anti-angiogenic factor Angpt2, and the angiopoietin receptors Tek and Tie1 are shown in Fig. 5. The up-regulation of Angpt2 (an antagonist of the pro-angiogenic angiopoetin-1) in hypertensive IGF-1 deficient mice is significant, as angiopoetin 2 expression has been causally linked to capillary rarefaction (Lobov et al. 2002; Cao et al. 2007).
Fig. 5.
Effects of IGF-1 deficiency and hypertension on the hippocampal expression of angiopoietins and related factors. QRT-PCR data showing mRNA expression of the pro-angiogenic factor Angpt1, the closely related factors Angptl1 and Angptl2, the anti-angiogenic factor Angpt2 and the angiopoietin receptors Tek and Tie1 in hippocampi of normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Data are mean ± S.E.M. (n = 5–8 in each group). *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
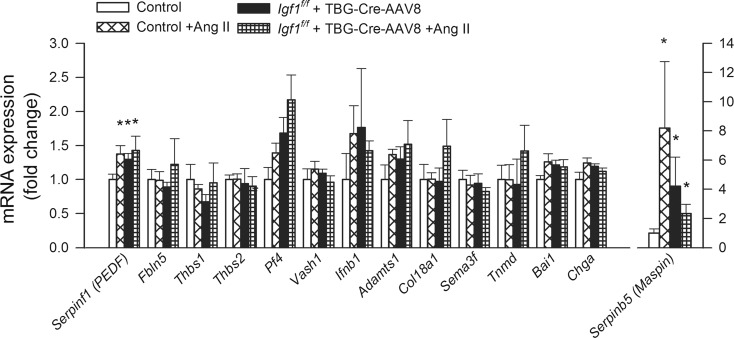
Figure 6 shows the effects of IGF-1 deficiency and hypertension on the hippocampal expression of the angiogenesis inhibitors Serpinf1 (PEDF), fibulin-5 (Fbln5) (Sullivan et al. 2007), thromhospondin-1 (Thbs1) (Lawler 2002), Thbs2 (Volpert et al. 1995 ), the potent anti-angiogenic chemokine platelet factor 4 (Pf4) (Bikfalvi 2004); vasohibin-1 (Vash1), which is a newly recognized negative regulator of angiogenesis produced by endothelial cells (Sato 2013); interferon-β (Ifnb1) (Takano et al. 2014), Adamts1 (“a disintegrin and metalloproteinase with thrombospondin motifs 1”), which inhibits angiogenesis (Lee et al. 2006) by suppressing endothelial cell proliferation; Col18a1, whose expression level impacts endostatin signaling and endothelial angiogenic capacity (Li and Olsen 2004) (endostatin, a potent inhibitor of angiogenesis, is a 20-kDa C-terminal fragment derived from type XVIII collagen); semaphorin-3F (Sema3f) (Ungvari et al. 2011b ; Frisbee et al. 2007 ); tenomodulin (Tnmd) (Oshima et al. 2003); brain-specific angiogenesis inhibitor 1 (Bai1; also known as adhesion G protein-coupled receptor B1 [ADGRB1]) (Nishimori et al. 1997); chromogranin A (Chga), which encodes the precursor to several angiogenesis inhibitor peptides including vasostatin-1 and vasostatin-2 (Helle and Corti 2015) and maspin (“mammary serine protease inhibitor”; encoded by the Serpinb5 gene (Qin and Zhang 2010).
Fig. 6.
Effects of IGF-1 deficiency and hypertension on the hippocampal expression of angiogenesis inhibitors. QRT-PCR data showing mRNA expression of Serpinf1 (PEDF), Fbln5, Thbs1, Thbs2, Pf4, Vash1, Ifnb1, Adamts1, Col18a1, Sema3f, Tnmd, Bai1, Chga, and Serpinb5, in hippocampi of normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Data are mean ± S.E.M. (n = 5–8 in each group). *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
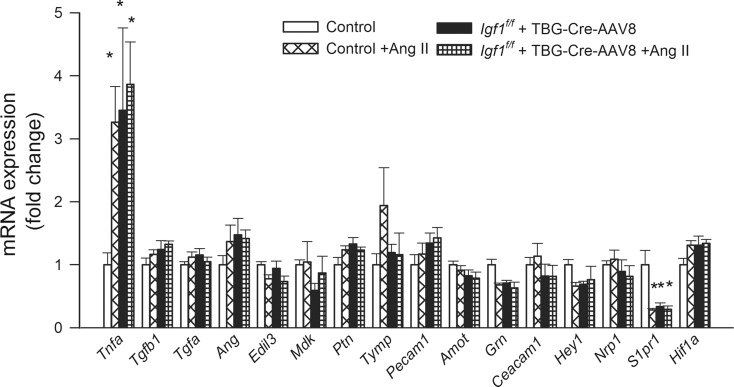
Figure 7 shows the expression of Tnfa, whose overproduction has been causally linked to microvascular rarefaction (Frisbee et al. 2014); Tgfb1, which regulates multiple aspects of the angiogenic process and contributes to hypertension-induced microvascular rarefaction in the heart (Koitabashi et al. 2011); Tgfa; angiogenin (Ang, also known as ribonuclease 5), which is a potent stimulator of angiogenesis and an inhibitor of endothelial apoptosis; Edil3 (EGF-like repeats and discoidin I-like domains 3), which encodes a glycoprotein secreted by endothelial cells that regulates apoptosis, cell migration (Zhong et al. 2003) and induces cerebral angiogenesis in mice (Fan et al. 2008); midkine (Mdk, also known as neurite growth-promoting factor 2 or NEGF2), which is a pleiotropic growth factor regulating cell proliferation, cell migration and promoting angiogenesis (Mashour et al. 2001); pleiotrophin (Ptn; also known as heparin-binding brain mitogen [HBBM], heparin-binding growth factor 8 [HBGF-8], neurite growth-promoting factor 1 [NEGF1], heparin affinity regulatory peptide [HARP] or heparin-binding growth associated molecule [HB-GAM]), which is a pro-angiogenic growth factor that is structurally related to midkine and whose expression in the adult brain is induced by ischemia; Tymp (thymidine phosphorylase, also known as platelet-derived endothelial cell growth factor [ECGF1], which stimulates endothelial cell proliferation and induces angiogenesis in the brain (Hayashi et al. 2007); platelet endothelial cell adhesion molecule (Pecam1; also known as CD31), which confers pro-angiogenic effects (Park et al. 2015); angiomotin (Amot), which regulates endothelial cell migration and angiogenic capacity; the growth factor granulin (Grn), which plays a role in regulation of blood vessel formation; carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1; also known as CD66a), which confers pro-angiogenic effects (Wagener and Ergun 2000); the basic helix-loop-helix-type transcriptional repressor Hey1, which is a primary target gene of the Notch signaling pathway and is thought to confer pro-angiogenic effects (Wang et al. 2014); neuropilin-1 (Nrp1), which modulates VEGF- and semaphorin signaling, regulating multiple aspects of angiogenesis (Fantin et al. 2014); sphingosine-1-phosphate receptor 1 (S1pr1, also known as endothelial differentiation gene 1 [EDG1]) (Fujii et al. 2012); and Hif1a, which also regulate processes underlying angiogenesis and microvascular rarefaction.
Fig. 7.
Effects of IGF-1 deficiency and hypertension on the hippocampal expression of factors regulating processes underlying angiogenesis and/or microvascular rarefaction. QRT-PCR data showing mRNA expression of Tnfa, Tgfb1, Tgfa, Ang, Edil3, Mdk, Ptn, Tymp, Pecam1, Amot, Grn, Ceacam1, Hey1, Nrp1, S1pr1, and Hif1a in hippocampi of normotensive control (Igf1 f/f + TBG-eGFP-AAV8), hypertensive control (Igf1 f/f + TBG-eGFP-AAV8 + Ang II), normotensive IGF-1 deficient (Igf1 f/f + TBG-Cre-AAV8), and hypertensive IGF-1 deficient mice (Igf1 f/f + TBG-Cre-AAV8 + AngII). Data are mean ± S.E.M. (n = 5–8 in each group). *p < 0.05 vs. normotensive control, # p < 0.05 vs. normotensive IGF-1 deficient; $ p < 0.05 vs. hypertensive control mice
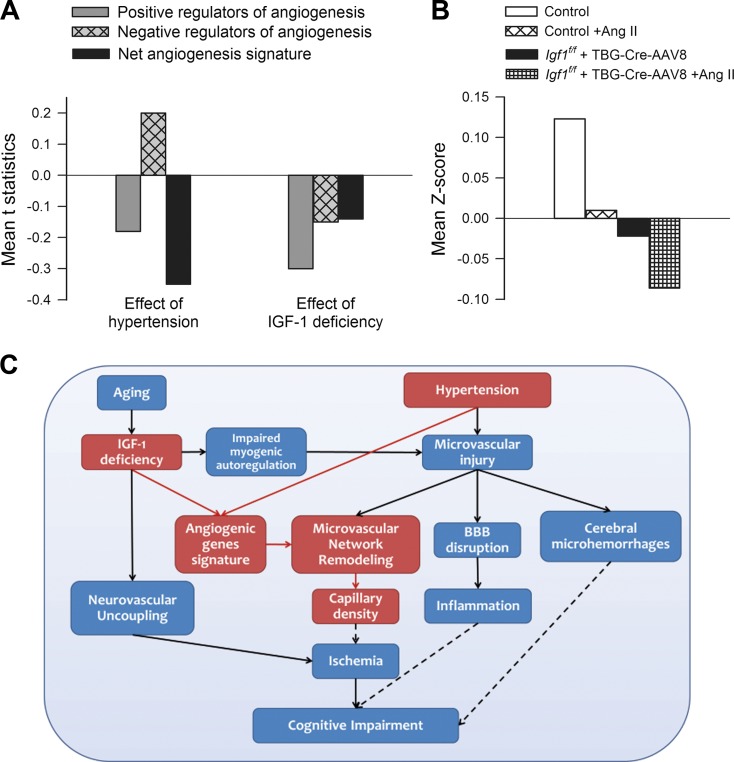
To determine whether the array data suggests a positive or negative angiogenesis signature for various conditions, genes annotated with the Gene Ontology terms for “positive regulation of angiogenesis” (GO:0045766; example: Vegfa) and “negative regulation of angiogenesis” (GO:0016525; example: Thbs) were obtained and intersected with the genes on the array. Genes annotated with both terms were removed. To assess the directionality of the angiogenesis signature, t statistic values were calculated. Figure 8a depicts mean t statistic for positive and negative angiogenesis regulators, and the signed aggregate of both groups. To interpret the gene expression data, Gene Set Enrichment Analysis (GSEA) scores were also calculated for positive regulators of angiogenesis (Fig. 8b). Both hypertension and IGF-1 deficiency appear to decrease the angiogenesis signature. The interaction of IGF-1 deficiency and hypertension appear to have significant negative effect, implying that IGF-1 deficiency and hypertension act in a strongly synergistic manner toward altering the angiogenic gene expression signature.
Fig. 8.
Hypertension in IGF-1 deficiency is associated with a gene expression signature in the mouse hippocampus that favors microvascular rarefaction. a Mean t statistic for positive and negative angiogenesis regulators, and the signed aggregate of both groups. “effect of hypertension” refers to hypertensive vs. normotensive groups, “effect of IGF-1 deficiency” refers to IGF-1 deficient vs control groups. b Gene set enrichment analysis (GSEA) scores for positive regulators of angiogenesis. Both hypertension and IGF-1 deficiency decreases the angiogenesis signature. Exacerbation of the effects of hypertension by IGF-1 deficiency is evident. c Proposed scheme depicting the mechanisms by which IGF-1 deficiency may exacerbate hypertension-induced microvascular damage and promote the pathogenesis of vascular cognitive impairment. The model predicts that IGF-1 deficiency impairs both functional and structural adaptation of the cerebral microcirculation to hypertension. Solid lines represent links supported by existing experimental evidence (see Discussion for details). Dashed lines represent proposed mechanistic links among microvascular injury, regional ischemia, microhemorrhages, neuroinflammation, and cognitive impairment, which should be tested experimentally by future studies
Discussion
The results of this study suggest that adult-onset, isolated endocrine IGF-1 deficiency is associated with a significant decline in microvascular density both in the hippocampus and the neocortex, mimicking the aging phenotype (Khan et al. 2001; Sonntag et al. 1997; Amenta et al. 1995; Bell and Ball 1981; Hicks et al. 1983). Our findings, taken together with results of previous studies obtained in mice with developmental liver-specific knockdown of IGF-1 (Lopez-Lopez et al. 2004), suggest that circulating level of IGF-1 is a critical regulator of brain capillarity. These findings are clinically relevant as circulating IGF-1 levels significantly decrease with age in humans (Franco et al. 2014; O’Connor et al. 1998), and restoration of circulating IGF-1 in older laboratory animals was shown to significantly increase cerebromicrovascular density (Sonntag et al. 1997).
It is generally considered that cerebromicrovascular rarefaction contributes to a decline in cerebral blood flow (CBF) that reduces metabolic support for neural signaling, thereby promoting neuronal dysfunction (Riddle et al. 2003; Sonntag et al. 1997; Khan et al. 2002; Troen et al. 2008). The hippocampus is a key brain region involved in learning and memory, one of the main regions affected in Alzheimer’s disease and it is known to be particularly vulnerable to ischemia. The retrosplenial cortex is also implicated in a wide range of cognitive functions including episodic memory and navigation. Importantly, previous studies demonstrate that rarefaction of the hippocampal microvasculature and/or the retrosplenial cortex in various pathophysiological conditions predicts cognitive dysfunction and impaired performance on spatial memory tests in the absence of or preceding neurodegeneration (Tucsek et al. 2014; Warrington et al. 2012; Troen et al. 2008).
Previous studies showed that hypertension promotes microvascular rarefaction in the brain and this effect is significantly exacerbated in old age (Toth et al. 2013a). This is the first study to demonstrate that circulating IGF-1 deficiency exacerbates the deleterious effects of hypertension on hippocampal microvascular density (Fig. 1–3), mimicking the aging phenotype. Importantly, the available human evidence suggest that hypertension in IGF-1 deficient patients also significantly compromise higher brain function (Angelini et al. 2009). Early studies on decreased capillary density in the peripheral circulation of hypertensive experimental animals and human patients proposed that rarefaction can be either structural (capillary attrition) or functional, associated with impaired recruitment of nonperfused capillaries (Chen et al. 1981; Hashimoto et al. 1987; Ono et al. 1989; Prewitt et al. 1986; Prewitt et al. 1982; Prewitt et al. 1984; Stacy and Prewitt 1989; Sullivan et al. 1983). Our studies provide additional evidence that interaction of IGF-1 deficiency and hypertension promote structural rarefaction in the mouse brain, extending previous findings obtained in hypertensive patients (Wolf et al. 1994; Bell and Ball 1981; Bell and Ball 1990; Abernethy et al. 1993; Mann et al. 1986) and animal models of aging (Sonntag et al. 1997; Wiesenborn et al. 2014), hypertension (Toth et al. 2013a) and IGF-1 deficiency (Lopez-Lopez et al. 2004), but further studies are needed to understand their synergistic effects on the cerebral microvasculature.
The mechanisms underlying IGF-1 deficiency-related structural microvascular rarefaction are likely multifaceted. There is substantial evidence that the cerebral microcirculation is subject to continuous dynamic structural adaptation, a concept that implies a high plasticity of the cerebral microvascular network (Riddle et al. 2003). Accordingly, there is a dynamic balance between capillary regression and growth, which is regulated by various paracrine factors produced by brain cells. It is generally accepted that impaired angiogenesis, due to dysregulated production of autocrine/paracrine regulators of angiogenic processes, is a critical mechanism involved in cerebromicrovascular rarefaction (Tucsek et al. 2014; Ungvari et al. 2010). Here, we demonstrate that in the mouse hippocampus IGF-1 deficiency decrease the angiogenesis gene expression signature (e.g., decreasing the expression of pro-angiogenic genes and up-regulating several anti-angiogenic factors). In accordance with our findings, there is strong in vitro evidence that IGF-1 regulates multiple aspects of the angiogenic process, including induction of endothelial cell proliferation, migration, and tube formation (Viana et al. 2015). Furthermore, previous studies demonstrate that treatment of mice with IGF-1 results in formation of new capillaries in the mouse brain (Lopez-Lopez et al. 2004). Our data suggest that hypertension also results in dysregulated expression of pro- and anti-angiogenic genes, and these effects tend to be exacerbated by IGF-1 deficiency. The IGF-1 dependent mechanisms responsible for impairment of endothelial angiogenic capacity likely also include dysregulation of Nrf2, a newly discovered regulator of endothelial angiogenic processes (Valcarcel-Ares et al. 2012). This concept is supported by the findings that aging is associated with Nrf2 dysfunction in endothelial cells (Ungvari et al. 2011a; Ungvari et al. 2011b) and that IGF-1 deficiency exacerbates Nrf2 dysfunction (Bailey-Downs et al. 2012a), mimicking the aging phenotype.
Previous studies have established a causal link among decreased bioavailability of NO, impaired angiogenesis and microvascular rarefaction (Frisbee et al. 2007). Endothelium-derived NO is both a downstream mediator of VEGF signaling and a critical regulator of microvascular endothelial cell viability. Previous studies have shown that IGF-1 deficiency may impair endothelial NO bioavailability by increasing breakdown of NO due to elevated cellular production of ROS (Toth et al. 2015) and/or by down-regulating eNOS (Csiszar et al. 2008). Thus, it is possible that impaired microvascular NO mediation contributes to microvascular rarefaction associated with circulating IGF-1 deficiency. Collectively, on the basis of our recent and previous findings, we propose that a decline in pro-angiogenic stimuli and up-regulation of anti-angiogenic factors is an important cause for both cerebromicrovascular rarefaction associated with IGF-1 deficiency and the exacerbation of hypertension-induced decreases in capillary density in IGF-1 deficient animals.
It is also likely that a significant part of IGF-1 deficiency-related and hypertension-induced microvascular rarefaction can be ascribed to endothelial injury and apoptosis. IGF-1 is known to exert diverse anti-apoptotic effects (Higashi et al. 2012), including regulation of Nrf2 activity (Bailey-Downs et al. 2012a; Bailey-Downs et al. 2012b) in endothelial cells. Importantly, our previous studies provide evidence that circulating IGF-1 deficiency impairs cellular stress resistance pathways in the vasculature, exacerbating oxidative stress-mediated endothelial apoptosis (Bailey-Downs et al. 2012a; Bailey-Downs et al. 2012b). Further studies are warranted to determine the role for increased microvascular apoptosis in hypertension-induced microvascular rarefaction in IGF-1 deficient mice.
Several additional mechanisms may also be considered to contribute to structural microvascular rarefaction in IGF-1 deficiency, including pericyte damage (Toth et al. 2013a), increased precapillary arteriolar constriction and cessation of capillary blood flow, increased susceptibility to microemboli, platelet adhesion and macrophage activation, and formation of string vessels (Brown 2010). Further, the mechanisms underlying the exacerbation of hypertension-induced microvascular injury in IGF-1 deficiency are also likely to include hemodynamic factors (Fig. 8c). There is strong evidence that in healthy young animals pressure-induced myogenic constriction of proximal arterial branches of the cerebrovascular tree acts as a critical homeostatic mechanism that assures that increased systemic arterial pressure cannot penetrate the distal portion of the cerebral microcirculation and cause damage to the thin-walled arteriolar and capillary microvessels (Toth et al. 2013a; Toth et al. 2014a; Kontos et al. 1978; Harper and Bohlen 1984). In cerebral resistance arteries isolated from hypertensive young control animals (Toth et al. 2013a; Toth et al. 2013b), the myogenic constriction at high pressures is augmented, suggesting that the pressure range for autoregulatory cerebrovascular protection is extended. The aforementioned functional adaptation of cerebral arteries to higher systemic blood pressure is believed to protect the cerebral microcirculation from pressure-induced injury (Toth et al. 2013a; Toth et al. 2014a). Our recent studies demonstrate that cerebral arteries of IGF-1 deficient mice do not exhibit a hypertension-induced adaptive increase in myogenic tone observed in mice with normal IGF-1 levels (Toth et al. 2014a), which mimics the aging phenotype (Toth et al. 2013a; Toth et al. 2013c; Springo et al. 2015). Pathological loss of autoregulatory protection in IGF-1 deficiency likely allows high blood pressure to penetrate the distal, injury-prone portion of the cerebral microcirculation, leading to significant downstream damage.
In addition to microvascular rarefaction, the impairment of microvascular dilator mechanisms is also likely to contribute to dysregulation of CBF and cognitive decline in IGF-1 deficiency (Toth et al. 2015) (Fig. 8c). Our recent studies demonstrate that IGF-1 deficiency leads to profound neurovascular dysregulation, characterized by impaired CBF responses induced by synaptic activity (Toth et al. 2015), which recapitulates cerebromicrovascular alterations present in aged mice (Toth et al. 2014b). Circulating IGF-1 deficiency also appears to compromise the barrier function of the cerebromicrovascular endothelial cells (Toth et al. 2014a) and exacerbate hypertension-induced disruption of the blood-brain barrier, promoting low-grade neuroinflammation (Toth et al. 2014a), mimicking the aging phenotype (Toth et al. 2013a). This was an important finding, as there is growing evidence causally linking blood-brain barrier disruption and neuroinflammation to age-related cognitive decline (Montagne et al. 2015; Carnevale et al. 2012).
In conclusion, adult-onset, isolated endocrine IGF-1 deficiency exerts multifaceted deleterious effects of the cerebral microcirculation, leading to a significant decline in cortical and hippocampal capillarity and exacerbating hypertension-induced cerebromicrovascular rarefaction. The morphological impairment of the cerebral microvasculature induced by IGF-1 deficiency and hypertension, in association with neurovascular uncoupling, increased blood-brain barrier disruption and neuroinflammation reported in previous studies (Toth et al. 2015; Toth et al. 2014a) likely contribute to the pathogenesis of vascular cognitive impairment in the elderly.
Acknowledgments
This work was supported by grants from the American Heart Association (to ST, MNVA, PT, AC, ZT, and ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Institute on Aging R01-AG047879 to AC; R01-AG038747 to WES, R01-NS056218 to AC and WES; 5U54GM104938 to JYW, the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center (to JYW and ZU; P30 AG028718), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, WES), the Oklahoma IDeA Network for Biomedical Research Excellence (to AC), the Oklahoma Nathan Shock Center of Excellence in the Biology of Aging (P30 AG050911 to JDW and WES), and the Reynolds Foundation (to WES, ZU and AC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Stefano Tarantini, Zsuzsanna Tucsek, and M. Noa Valcarcel-Ares contributed equally to this manuscript.
References
- Abernethy WB, Bell MA, Morris M, Moody DM. Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp Neurol. 1993;121:270–274. doi: 10.1006/exnr.1993.1095. [DOI] [PubMed] [Google Scholar]
- Amenta F, Ferrante F, Mancini M, Sabbatini M, Vega JA, Zaccheo D. Effect of long-term treatment with the dihydropyridine-type calcium channel blocker darodipine (py 108-068) on the cerebral capillary network in aged rats. Mech Ageing Dev. 1995;78:27–37. doi: 10.1016/0047-6374(94)01513-L. [DOI] [PubMed] [Google Scholar]
- Angelini A, Bendini C, Neviani F, Bergamini L, Manni B, Trenti T, Rovati R, Neri M. Insulin-like growth factor-1 (igf-1): relation with cognitive functioning and neuroimaging marker of brain damage in a sample of hypertensive elderly subjects. Arch Gerontol Geriatr. 2009;49(Suppl 1):5–12. doi: 10.1016/j.archger.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Arum O, Rickman DJ, Kopchick JJ, Bartke A. The slow-aging growth hormone receptor/binding protein gene-disrupted (ghr-ko) mouse is protected from aging-resultant neuromusculoskeletal frailty. Age (Dordr) 2014;36:117–127. doi: 10.1007/s11357-013-9551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Saleh JK, Boparai RK, Kopchick JJ, Khardori RK, Bartke A. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age (Dordr) 2014;36:9651. doi: 10.1007/s11357-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M, Boudiaf N, Cousin E, Perrone-Bertolotti M, Pichat C, Fournet N, Chainay H, Lamalle L, Krainik A. Functional mri evidence for the decline of word retrieval and generation during normal aging. Age (Dordr) 2016;38:3. doi: 10.1007/s11357-015-9857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the nrf 2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Growth hormone and igf-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564. doi: 10.1093/gerona/glr197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53:299–318. doi: 10.1007/BF00690372. [DOI] [PubMed] [Google Scholar]
- Bell MA, Ball MJ. Neuritic plaques and vessels of visual cortex in aging and Alzheimer’s dementia. Neurobiol Aging. 1990;11:359–370. doi: 10.1016/0197-4580(90)90001-G. [DOI] [PubMed] [Google Scholar]
- Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, Townson S, Wells C, Little M, Cummings MC, Hayward NK, Kay GF. Mice lacking the vascular endothelial growth factor-b gene (vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.RES.86.2.e29. [DOI] [PubMed] [Google Scholar]
- Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci. 2000;181:19–28. doi: 10.1016/S0022-510X(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Berghuis KM, Veldman MP, Solnik S, Koch G, Zijdewind I, Hortobagyi T. Neuronal mechanisms of motor learning and motor memory consolidation in healthy old adults. Age (Dordr) 2015;37:9779. doi: 10.1007/s11357-015-9779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin Thromb Hemost. 2004;30:379–385. doi: 10.1055/s-2004-831051. [DOI] [PubMed] [Google Scholar]
- Brown WR. A review of string vessels or collapsed, empty basement membrane tubes. J Alzheimers Dis. 2010;21:725–739. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Sharman E, Bondy SC. Age-related differences in the response of the brain to dietary melatonin. Age (Dordr) 2014;36:49–55. doi: 10.1007/s11357-013-9542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J, Clary BM, Li CY, Kontos CD, Dewhirst MW. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:3835–3844. doi: 10.1158/0008-5472.CAN-06-4056. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen II, Prewitt RL, Dowell RF. Microvascular rarefaction in spontaneously hypertensive rat cremaster muscle. Am J Phys. 1981;241:H306–H310. doi: 10.1152/ajpheart.1981.241.3.H306. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived gh/igf-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, Deak F, Sonntag WE, Ungvari ZI (2013) Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and ad. Am J Physiol Heart Circ Physiol [DOI] [PMC free article] [PubMed]
- Debinski W, Slagle-Webb B, Achen MG, Stacker SA, Tulchinsky E, Gillespie GY, Gibo DM. Vegf-d is an x-linked/ap-1 regulated putative onco-angiogen in human glioblastoma multiforme. Mol Med. 2001;7:598–608. [PMC free article] [PubMed] [Google Scholar]
- Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T. Effects of white matter lesions on trunk stability during dual-task walking among older adults with mild cognitive impairment. Age (Dordr) 2015;37:120. doi: 10.1007/s11357-015-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvinge H, Bertone P. Htqpcr: high-throughput analysis and visualization of quantitative real-time pcr data in r. Bioinformatics. 2009;25:3325–3326. doi: 10.1093/bioinformatics/btp578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhu W, Yang M, Zhu Y, Shen F, Hao Q, Young WL, Yang GY, Chen Y. Del-1 gene transfer induces cerebral angiogenesis in mice. Brain Res. 2008;1219:1–7. doi: 10.1016/j.brainres.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Herzog B, Mahmoud M, Yamaji M, Plein A, Denti L, Ruhrberg C, Zachary I. Neuropilin 1 (nrp1) hypomorphism combined with defective vegf-a binding reveals novel roles for nrp1 in developmental and pathological angiogenesis. Development. 2014;141:556–562. doi: 10.1242/dev.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/S0301-0082(00)00068-X. [DOI] [PubMed] [Google Scholar]
- Franco L, Williams FM, Trofimov S, Malkin I, Surdulescu G, Spector T, Livshits G. Assessment of age-related changes in heritability and igf-1 gene effect on circulating igf-1 levels. Age (Dordr) 2014;36:9622. doi: 10.1007/s11357-014-9622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Goodwill AG, Frisbee SJ, Butcher JT, Brock RW, Olfert IM, DeVallance ER, Chantler PD. Distinct temporal phases of microvascular rarefaction in skeletal muscle of obese zucker rats. Am J Physiol Heart Circ Physiol. 2014;307:H1714–H1728. doi: 10.1152/ajpheart.00605.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Samora JB, Basile DP. Angiostatin does not contribute to skeletal muscle microvascular rarefaction with low nitric oxide bioavailability. Microcirculation. 2007;14:145–153. doi: 10.1080/10739680601131242. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Ueda Y, Ohtake H, Ono N, Takayama T, Nakazawa K, Igarashi Y, Goitsuka R. Blocking s1p interaction with s1p(1) receptor by a novel competitive s1p(1)-selective antagonist inhibits angiogenesis. Biochem Biophys Res Commun. 2012;419:754–760. doi: 10.1016/j.bbrc.2012.02.096. [DOI] [PubMed] [Google Scholar]
- Garcia-Amado M, Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstadius S, Risberg J. Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn. 1989;10:28–43. doi: 10.1016/0278-2626(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S. Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordr) 2014;36:9653. doi: 10.1007/s11357-014-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419. doi: 10.1161/01.HYP.6.3.408. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Prewitt RL, Efaw CW. Alterations in the microvasculature of one-kidney, one-clip hypertensive rats. Am J Phys. 1987;253:H933–H940. doi: 10.1152/ajpheart.1987.253.4.H933. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Wang XQ, Zhang HZ, Deguchi K, Nagotani S, Sehara Y, Tsuchiya A, Nagai M, Shoji M, Abe K. Induction of platelet derived-endothelial cell growth factor in the brain after ischemia. Neurol Res. 2007;29:463–468. doi: 10.1179/016164107X164139. [DOI] [PubMed] [Google Scholar]
- He X, Cheng R, Benyajati S, Ma JX. Pedf and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 2015;128:805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle KB, Corti A. Chromogranin a: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci. 2015;72:339–348. doi: 10.1007/s00018-014-1750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks P, Rolsten C, Brizzee D, Samorajski T. Age-related changes in rat brain capillaries. Neurobiol Aging. 1983;4:69–75. doi: 10.1016/0197-4580(83)90057-X. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and igf-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Arum O, Boparai RK, Wang F, Fang Y, Sun LY, Masternak MM, Bartke A. Female papp-a knockout mice are resistant to metabolic dysfunction induced by high-fat/high-sucrose feeding at middle age. Age (Dordr) 2015;37:9765. doi: 10.1007/s11357-015-9765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Young WC, Bloom FE, Belichenko PV, Celio MR. Comparative cytoachitectonic atlas of the c57bl/6 and 129/sv mouse brains. New York, NY: Elsevier; 2000. [Google Scholar]
- Hofmann JW, McBryan T, Adams PD, Sedivy JM. The effects of aging on the expression of wnt pathway genes in mouse tissues. Age (Dordr) 2014;36:9618. doi: 10.1007/s11357-014-9618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63:12–20. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- Kawamura J, Terayama Y, Takashima S, Obara K, Pavol MA, Meyer JS, Mortel KF, Weathers S. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res. 1993;19:225–240. doi: 10.1080/03610739308253935. [DOI] [PubMed] [Google Scholar]
- Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci. 2001;56:B364–B371. doi: 10.1093/gerona/56.8.B364. [DOI] [PubMed] [Google Scholar]
- Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/S0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Phys. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol. 1999;172:213–218. doi: 10.2214/ajr.172.1.9888770. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thakur MK. Age-related expression of neurexin 1 and neuroligin 3 is correlated with presynaptic density in the cerebral cortex and hippocampus of male mice. Age (Dordr) 2015;37:17. doi: 10.1007/s11357-015-9752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. Adamts1 mediates the release of antiangiogenic polypeptides from tsp1 and 2. EMBO J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Olsen BR. Increased angiogenic response in aortic explants of collagen xviii/endostatin-null mice. Am J Pathol. 2004;165:415–424. doi: 10.1016/S0002-9440(10)63307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Grinberg A, Westphal H, Sauer B, Accili D, Karas M, LeRoith D. Insulin-like growth factor-i affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the cre/loxp system in transgenic mice. Mol Endocrinol. 1998;12:1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays vegf-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez ME, Cuesta P, Garces P, Castellanos PN, Aurtenetxe S, Bajo R, Marcos A, Delgado ML, Montejo P, Lopez-Pantoja JL, Maestu F, Fernandez A. Meg spectral analysis in subtypes of mild cognitive impairment. Age (Dordr) 2014;36:9624. doi: 10.1007/s11357-014-9624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi PD. Multimorbidity, cognitive function, and physical activity. Age (Dordr) 2016;38:8. doi: 10.1007/s11357-016-9874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200. doi: 10.1016/S0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Manich G, del Valle J, Cabezon I, Camins A, Pallas M, Pelegri C, Vilaplana J. Presence of a neo-epitope and absence of amyloid beta and tau protein in degenerative hippocampal granules of aged mice. Age (Dordr) 2014;36:151–165. doi: 10.1007/s11357-013-9560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Eaves NR, Marcyniuk B, Yates PO. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol Aging. 1986;7:321–330. doi: 10.1016/0197-4580(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Ratner N, Khan GA, Wang HL, Martuza RL, Kurtz A. The angiogenic factor midkine is aberrantly expressed in nf1-deficient schwann cells and is a mitogen for neurofibroma-derived cells. Oncogene. 2001;20:97–105. doi: 10.1038/sj.onc.1204026. [DOI] [PubMed] [Google Scholar]
- Mellor KM, Curl CL, Chandramouli C, Pedrazzini T, Wendt IR, Delbridge LM. Ageing-related cardiomyocyte functional decline is sex and angiotensin ii dependent. Age (Dordr) 2014;36:9630. doi: 10.1007/s11357-014-9630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschelen M, Garteiser P, Carnes BA, Farley JA, Doblas S, Demoe JH, Warrington JP, Yan H, Nicolle MM, Towner R, Sonntag WE. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neuroscience. 2009;164:918–928. doi: 10.1016/j.neuroscience.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Eskandarian MR, Davis J, Eubanks JH. Differential expression of vascular endothelial growth factor-a (vegf-a) and vegf-b after brain injury. J Neuropathol Exp Neurol. 2002;61:778–788. doi: 10.1093/jnen/61.9.778. [DOI] [PubMed] [Google Scholar]
- Nishimori H, Shiratsuchi T, Urano T, Kimura Y, Kiyono K, Tatsumi K, Yoshida S, Ono M, Kuwano M, Nakamura Y, Tokino T. A novel brain-specific p53-target gene, bai1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene. 1997;15:2145–2150. doi: 10.1038/sj.onc.1201542. [DOI] [PubMed] [Google Scholar]
- O’Connor KG, Tobin JD, Harman SM, Plato CC, Roy TA, Sherman SS, Blackman MR. Serum levels of insulin-like growth factor-i are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci. 1998;53:M176–M182. doi: 10.1093/gerona/53A.3.M176. [DOI] [PubMed] [Google Scholar]
- Ono Z, Prewitt RL, Stacy DL. Arteriolar changes in developing and chronic stages of two-kidney, one clip hypertension. Hypertension. 1989;14:36–43. doi: 10.1161/01.HYP.14.1.36. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Shukunami C, Honda J, Nishida K, Tashiro F, Miyazaki J, Hiraki Y, Tano Y. Expression and localization of tenomodulin, a transmembrane type chondromodulin-i-related angiogenesis inhibitor, in mouse eyes. Invest Ophthalmol Vis Sci. 2003;44:1814–1823. doi: 10.1167/iovs.02-0664. [DOI] [PubMed] [Google Scholar]
- Pagani M, Salmaso D, Jonsson C, Hatherly R, Jacobsson H, Larsson SA, Wagner A. Regional cerebral blood flow as assessed by principal component analysis and (99 m) tc-hmpao spet in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging. 2002;29:67–75. doi: 10.1007/s00259-001-0676-2. [DOI] [PubMed] [Google Scholar]
- Park S, Sorenson CM, Sheibani N. Pecam-1 isoforms, enos and endoglin axis in regulation of angiogenesis. Clin Sci (Lond) 2015;129:217–234. doi: 10.1042/CS20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt RL, Cardoso SS, Wood WB. Prevention of arteriolar rarefaction in the spontaneously hypertensive rat by exposure to simulated high altitude. J Hypertens. 1986;4:735–740. doi: 10.1097/00004872-198612000-00008. [DOI] [PubMed] [Google Scholar]
- Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Phys. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- Prewitt RL, Chen II, Dowell RF. Microvascular alterations in the one-kidney, one-clip renal hypertensive rat. Am J Phys. 1984;246:H728–H732. doi: 10.1152/ajpheart.1984.246.5.H728. [DOI] [PubMed] [Google Scholar]
- Qin L, Zhang M. Maspin regulates endothelial cell adhesion and migration through an integrin signaling pathway. J Biol Chem. 2010;285:32360–32369. doi: 10.1074/jbc.M110.131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/S1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Rojanathammanee L, Rakoczy S, Kopchick J, Brown-Borg HM. Effects of insulin-like growth factor 1 on glutathione s-transferases and thioredoxin in growth hormone receptor knockout mice. Age (Dordr) 2014;36:9687. doi: 10.1007/s11357-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen LE, Schofield PR, Pierce KD, Conturo TE, Tate DF, Lane EM, Heaps JM, Bolzenius JD, Baker LM, Akbudak E, Paul RH. Impact of the agtr 1 a1166c polymorphism on subcortical hyperintensities and cognition in healthy older adults. Age (Dordr) 2014;36:9664. doi: 10.1007/s11357-014-9664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, Lutgers HL, Kochan NA, Crawford JD, Campbell LV, Wen W, Slavin MJ, Baune BT, Lipnicki DM, Brodaty H, Trollor JN, Sachdev PS. The impact of glucose disorders on cognition and brain volumes in the elderly: the Sydney memory and ageing study. Age (Dordr) 2014;36:977–993. doi: 10.1007/s11357-013-9613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo F, Ramis MR, Aparicio S, Ruiz L, Esteban S, Miralles A, Moranta D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr) 2015;37:9777. doi: 10.1007/s11357-015-9777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y. The vasohibin family: a novel family for angiogenesis regulation. J Biochem. 2013;153:5–11. doi: 10.1093/jb/mvs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Bartke A, Kopchick JJ, Masternak MM. Effect of growth hormone receptor gene disruption and pma treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age (Dordr) 2014;36:9701. doi: 10.1007/s11357-014-9701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch JB, Constantin PC, da Silva VK, Korb C, Bamberg DP, da Rocha TJ, Fiegenbaum M, de Oliveira A, Jr, Tisser LA, de Andrade FM. Ace polymorphism and use of ace inhibitors: effects on memory performance. Age (Dordr) 2014;36:9646. doi: 10.1007/s11357-014-9646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, O’Leary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport. 1999;10:2493–2496. doi: 10.1097/00001756-199908200-00011. [DOI] [PubMed] [Google Scholar]
- Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW. The renin-angiotensin system and prevention of age-related functional decline: where are we now? Age (Dordr) 2015;37:9753. doi: 10.1007/s11357-015-9753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova IA, Manukhina EB, Blinkov SM, Koshelev VB, Pinelis VG, Rodionov IM. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc Res. 1985;30:1–9. doi: 10.1016/0026-2862(85)90032-9. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in cns and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and igf-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1017/S002187829900713X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–530. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy DL, Prewitt RL. Attenuated microvascular alterations in coarctation hypertension. Am J Phys. 1989;256:H213–H221. doi: 10.1152/ajpheart.1989.256.1.H213. [DOI] [PubMed] [Google Scholar]
- Steeghs N, Rabelink TJ, op’t Roodt J, Batman E, Cluitmans FH, Weijl NI, de Koning E, Gelderblom H. Reversibility of capillary density after discontinuation of bevacizumab treatment. Ann Oncol. 2010;21:1100–1105. doi: 10.1093/annonc/mdp417. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab Investig. 2007;87:818–827. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Prewitt RL, Josephs JA. Attenuation of the microcirculation in young patients with high-output borderline hypertension. Hypertension. 1983;5:844–851. doi: 10.1161/01.HYP.5.6.844. [DOI] [PubMed] [Google Scholar]
- Takano S, Ishikawa E, Matsuda M, Yamamoto T, Matsumura A. Interferon-beta inhibits glioma angiogenesis through downregulation of vascular endothelial growth factor and upregulation of interferon inducible protein 10. Int J Oncol. 2014;45:1837–1846. doi: 10.3892/ijo.2014.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivesten A, Bollano E, Andersson I, Fitzgerald S, Caidahl K, Sjogren K, Skott O, Liu JL, Mobini R, Isaksson OG, Jansson JO, Ohlsson C, Bergstrom G, Isgaard J. Liver-derived insulin-like growth factor-i is involved in the regulation of blood pressure in mice. Endocrinology. 2002;143:4235–4242. doi: 10.1210/en.2002-220524. [DOI] [PubMed] [Google Scholar]
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Laniado Schwartzman M, Sonntag WE, Ungvari ZI (2013a) Role of 20-hete, trp channels & bkca in dysregulation of pressure-induced ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol [DOI] [PMC free article] [PubMed]
- Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-hete, trpc channels, and bkca in dysregulation of pressure-induced ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z (2015) Igf-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. in press [DOI] [PMC free article] [PubMed]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of nadph oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin ii-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z (2014a) Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. in press [DOI] [PMC free article] [PubMed]
- Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008;105:12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A (2003) Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and alzheimer’s disease. J Gerontol Biol Med Sci. in press [DOI] [PMC free article] [PubMed]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A. The emerging role of igf-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. doi: 10.1093/gerona/gls072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, nrf2 dysfunction and nf-kb activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana IM, de Almeida ME, Lins MP, dos Santos Reis MD, de Araujo Vieira LF, Smaniotto S. Combined effect of insulin-like growth factor-1 and cc chemokine ligand 2 on angiogenic events in endothelial cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- Wagener C, Ergun S. Angiogenic properties of the carcinoembryonic antigen-related cell adhesion molecule 1. Exp Cell Res. 2000;261:19–24. doi: 10.1006/excr.2000.5038. [DOI] [PubMed] [Google Scholar]
- Wallis LJ, Viranyi Z, Muller CA, Serisier S, Huber L, Range F. Aging effects on discrimination learning, logical reasoning and memory in pet dogs. Age (Dordr) 2016;38:6. doi: 10.1007/s11357-015-9866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He Z, Xia T, Li X, Liang D, Lin X, Wen H, Lan K. Latency-associated nuclear antigen of kaposi sarcoma-associated herpesvirus promotes angiogenesis through targeting notch signaling effector hey1. Cancer Res. 2014;74:2026–2037. doi: 10.1158/0008-5472.CAN-13-1467. [DOI] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300:H736–H744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Arend O, Schulte K, Ittel TH, Reim M. Quantification of retinal capillary density and flow velocity in patients with essential hypertension. Hypertension. 1994;23:464–467. doi: 10.1161/01.HYP.23.4.464. [DOI] [PubMed] [Google Scholar]
- Wiesenborn DS, Ayala JE, King E, Masternak MM. Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr) 2014;36:9709. doi: 10.1007/s11357-014-9709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu JS, Isner JM. Vascular endothelial growth factor-c (vegf-c/vegf-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol. 1998;153:381–394. doi: 10.1016/S0002-9440(10)65582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]