Abstract
We aimed to investigate whether aging increases the susceptibility of hepatic and renal inflammation or fibrosis in response to high-fat diet (HFD) and explore the underlying genetic alterations. Middle (10 months old) and old (20 months old) aged, male C57BL/6N mice were fed either a low-fat diet (4 % fat) or HFD (60 % fat) for 4 months. Young (3 months old) aged mice were included as control group. HFD-induced liver and kidney injuries were analyzed by serum and urine assay, histologic staining, immunohistochemistry, and reverse-transcription real-time quantitative polymerase chain reaction. Total RNA sequencing with next-generation technology was done with RNA extracted from liver tissues. With HFD feeding, aged was associated with higher serum alanine aminotransferase levels, marked infiltration of hepatic macrophages, and increased expression of inflammatory cytokines (MCP1, TNF-α, IL-1β, IL-6, IL-12, IL-17A). Importantly, aged mice showed more advanced hepatic fibrosis and increased expression of fibrogenic markers (Col-I-α1, αSMA, TGF-β1, TGF-β2, TGFβRII, PDGF, PDGFRβII, TIMP1) in response to HFD. Aged mice fed on HFD also showed increased oxidative stress and TLR4 expression. In the total RNA seq and gene ontology analysis of liver, old-aged HFD group showed significant up-regulation of genes linked to innate immune response, immune response, defense response, inflammatory response compared to middle-aged HFD group. Meanwhile, aging and HFD feeding showed significant increase in glomerular size and mesangial area, higher urine albumin/creatinine ratio, and advanced renal inflammation or fibrosis. However, the difference of HFD-induced renal injury between old-aged group and middle-aged group was not significant. The susceptibility of hepatic fibrosis as well as hepatic inflammation in response to HFD was significantly increased with aging. In addition, aging was associated with glomerular alterations and increased renal inflammation or fibrosis, while the differential effect of aging on HFD-induced renal injury was not remarkable as shown in the liver.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9938-6) contains supplementary material, which is available to authorized users.
Keywords: Aging, High-fat diet, Liver, Fibrosis
Introduction
The remarkable improvement in human lifespan over past century has led to a dramatic increase in the elderly population in the world. According to the report by World Health Organization, the number of people aged 65 or older is projected to grow from an estimated 524 million in 2010 to nearly 1.5 billion in 2050 (Organization WH 2011). A person gradually loses the ability to maintain homeostasis with aging and subsequently becomes vulnerable to external stress or damage (López-Otín et al. 2013). Indeed, aging has been considered as a major risk factor for most chronic diseases. Currently, non-communicable diseases including ischemic heart disease, cerebrovascular disease, chronic obstructive disability, cancer, and diabetes commonly affect the elderly people and impose great burden on global health (Organization WH 2011).
Aging predisposes to structural and functional impairment of liver as well as various changes in liver cells which is associated with increased risk of hepatic injury (Schmucker 2005; Hohn & Grune 2013). Aging is also associated with increased lipid accumulation in non-adipose tissues, including the liver. Previous studies reported that aging increases the prevalence rates of the metabolic syndrome and non-alcoholic fatty liver disease (NAFLD) in human (Park et al. 2006; Amarapurkar et al. 2007). Generally, the prevalence rate of the NAFLD among adults is estimated at 15–30 %, whereas overall prevalence rate of NAFLD among elderly people aged more than 65 years was 35.1 % (Amarapurkar et al. 2007; Chalasani et al. 2012; Koehler et al. 2012). Also, as the population ages, the prevalence rate of non-alcoholic steatohepatitis or fibrosis also tends to increase (Frith et al. 2009; Noureddin et al. 2013). However, there was limited data about the differential effect of high-fat diet (HFD) feeding on the progression of NAFLD according to aging and underlying biological or genetic mechanisms are not well understood. Aging is also associated with progressive decrease of renal function as well as glomerular, vascular, and parenchymal changes (Choudhury & Levi 2011; Bolignano et al. 2014). In addition, HFD feeding in mice has been known to induce characteristics of metabolic syndrome including obesity, hyperglycemia, hyperinsulinemia, dyslipidemia, and hypertension. These systemic changes combined with local alterations in the kidney with aging are considered to further increase the risk of development or progression of chronic kidney disease (Deji et al. 2009). However, the effect of aging on HFD-induced renal injury has not been determined.
In this study, we investigated whether aging increases the susceptibility of hepatic and renal inflammation or fibrosis in response to HFD feeding and explored the underlying hepatic alterations of gene expression using next-generation total RNA sequencing technology.
Materials and methods
Animals and HFD-induced liver and kidney injury model
Male C57BL/6N mice, young (3 months old, n = 9) mice purchased from Charles River Laboratories (Wilmington, MA) and middle-aged (10 months old, n = 14) mice and old-aged (20 months old, n = 14) mice from the US National Institute on Aging stock located in Charles River Laboratories were used in this experiment. Mice were maintained under 12-h light/dark cycles with unlimited access to food and water. Middle-aged and old-aged mice were fed either a low-fat diet (LFD; 4 % of total calories supplied by fat, Teklad 4 % fat mouse diet, Harlan Laboratories Inc., Madison, WI) or a HFD (60 % fat, Teklad research diet TD. 06414, Harlan Laboratories Inc., Madison, WI). Young mice were included as a control group without HFD feeding (Supplemental Fig. 1). Therefore, mice were divided into five groups as follows: young control group (Ycon), middle-aged LFD group (Mlfd), old-aged LFD group (Olfd), middle-aged HFD group (Mhfd), and old-aged HFD group (Ohfd). Mice were sacrificed after feeding LFD or HFD for 4 months and blood and urine samples were collected. Serum levels of alanine aminotransferase (ALT) were measured using a commercial kit (Thermo Fisher Scientific Inc., Middletown, VA). The urine albumin and creatinine were measured with a mouse Albuwell ELISA kit and a Creatinine Companion kit (Exocell, Philadelphia, PA) according to the manufacturer’s protocol and expressed as urinary albumin creatinine ratio (UACR). All animal experiments were in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication No. 86-23, revised 1985) and approved by the University of California, San Diego Animal Care Committee.
Histologic analysis and immunohistochemistry
Liver and kidney were harvested, fixed with 10 % buffered formalin, embedded in paraffin, and cut into 5-μm-thick sections. For frozen sections, tissues were fixed with 4 % buffered formalin and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek Inc., Torrence, CA). All liver specimens were stained with hematoxylin and eosin (H&E) and examined in a blinded fashion by a single pathologist. The hepatic steatosis on H&E stain was calculated in 5 random low-power views on each slide using Image J Software (National Institutes of Health, Bethesda, MD). Routine Sirius red staining was performed to visualize collagen deposition in liver and kidney tissues. Hepatic and renal fibrosis was assessed by way of morphometric analysis of the Sirius red-positive staining using Image J software. For kidney tissues, periodic acid Schiff (PAS) staining was performed to evaluate structural changes of kidney. To evaluate the glomerular size and mesangial matrix area, 15 randomly selected glomeruli were analyzed using i-solution software (Advanced Imaging Concepts, Princeton, NJ). Immunohistochemistry was performed on paraffin sections with anti-alpha smooth muscle actin (anti-αSMA) (Abcam, San Francisco, CA), anti-desmin (Thermo Fisher Scientific Inc., Waltham, MA), anti-F4/80 (eBioscience, San Diego, CA), and anti-Ly6G (eBioscience, San Diego, CA) antibodies using MOM kit (Vector Laboratories, Burlingame, CA) following by 3,3′-diaminobenzidine tetrahydrochloride staining (Vector Laboratories, Burlingame, CA) and counterstaining with hematoxylin. Immunofluorescent staining was performed on frozen sections with anti-4-hydroxy-2-nonenal (anti-4-HNE) (Alpha Diagnostic Intl Inc., San Antonio, TX) antibody.
RNA extraction and real-time quantitative polymerase chain reaction
Liver and kidney tissues were collected into cryo vials which were immediately put into liquid nitrogen and then stored at −80 °C for later RNA extraction. Tissues were homogenized using a homogenizer, and total RNA was isolated from homogenized tissue with RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the protocol provided by manufacturer. Reverse transcription was performed with 1 μg of RNA in an Eppendorf Mastercycler (Hamburg, Germany) using a high-capacity complementary DNA reverse-transcription kit (ABI, Foster City, CA). Annealing of primers was performed at 25 °C for 10 min, followed by elongation at 37 °C for 2 h and inactivation of the enzyme at 85 °C for 5 min. Polymerase chain reaction (PCR) for F4/80, tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein (MCP)-1, interleukin (IL)-1β, IL-6, IL-10, IL-12, IL-13, IL-17A, IL-17A receptor (IL-17AR), IL-22, IL-25, IL-27, CD3, CD19, collagen type I alpha1 (Col-I-α1), collagen type IV (Col-IV), desmin, alpha smooth muscle actin (αSMA), transforming growth factor (TGF)-β1, TGF-β2, TGFβ receptor-II (TGFβRII), platelet-derived growth factor (PDGF), PDGF receptor β-II (PDGFRβII), tissue inhibitor metalloproteinase (TIMP) 1, matrix metalloproteinase (MMP)-2, MMP-9, nicotinamide adenine dinucleotide phosphate oxidases (NOX) 1, NOX2, NOX4, P22Phox, P67Phox, toll-like receptor (TLR) 4, bambi, p53, p21, p16 was performed in duplicate in a 7500 Fast Real-Time PCR System (Applied Biosystems). The sequences for primers were mainly from PrimerBank (http://pga.mgh.harvard.edu/primerbank) and appear in Supplement Table 1. Primers to these genes were purchased from Integrated DNA Technologies (San Diego, CA). Real-time quantitative PCR was performed using Fast SYBR® Green (Applied Biosystems). The cycling parameters were denaturation at 95 °C for 30 s and extension at 60 °C for 1 min (for 40 cycles). All samples were normalized to hypoxanthine-guanine phosphoribosyl transferase (HPRT), which served as an endogenous control. Values were expressed as fold induction in comparison with young age controls.
RNA-Seq analysis
Strand-specific mRNA-sequencing libraries (polyA+) were generated and barcoded using Illumina’s TruSeq stranded mRNA library prep kits. Each RNA-Seq library was sequenced on an Illumina HiSeq2500 to a depth of approximately 25 million reads. Sequencing reads were aligned to the mouse genome (NCBI MGSCv37, mm9) using “Spliced Transcript Alignment to a Reference” (STAR), using only uniquely alignable reads for downstream analysis (Dobin et al. 2013). Gene expression levels of reference sequence transcripts and normalized genome browser visualization files were created using HOMER (Heinz et al. 2010). Differentially expressed genes, including false discovery rate calculations, were performed using software package empirical analysis of digital gene expression in R (edgeR) (Robinson et al. 2010). Gene ontology analysis and clustering were performed using HOMER and Cluster3.0/Java Tree View, respectively.
Statistical analysis
Results were expressed as mean ± standard error of the mean from at least 3 independent experiments. Data between groups were analyzed by a 2-tailed t test using Excel. A P value of less than .05 was considered statistically significant.
Results
Hepatic steatosis and inflammation with aging and HFD feeding
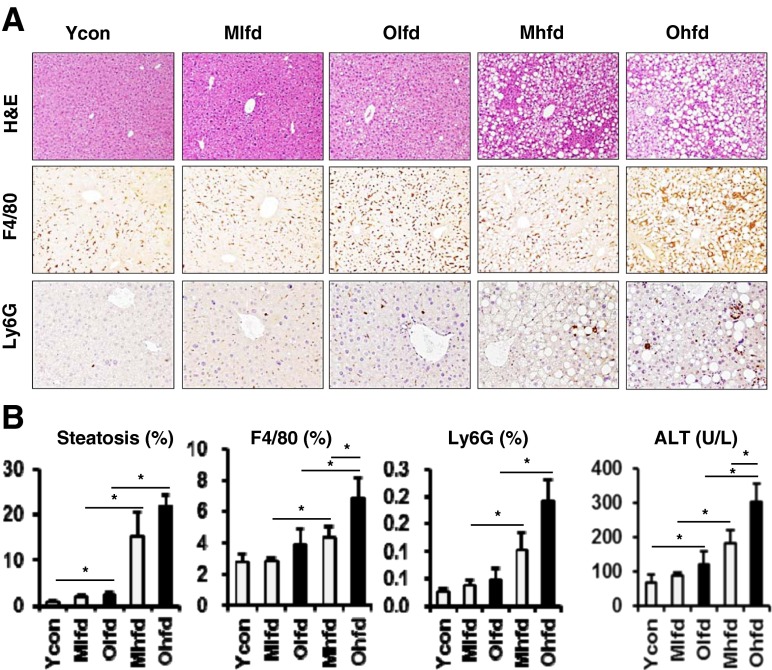
Old-aged mice had much more of hepatic lipid droplets compared to young age control mice in the H&E stain of liver sections (Fig. 1a, b). Compared to LFD groups, both middle-aged and old-aged HFD groups showed markedly increased hepatic steatosis, but the difference between two aged groups was not significant (Fig. 1a, b). We analyzed hepatic infiltration of inflammatory cells by immunohistochemical staining with anti-F4/80 (marker for macrophage) and anti-Ly6G (marker for neutrophil) antibodies. Both F4/80 and Ly6G positive staining cells were significantly increased in HFD groupscompared to LFD groups (Fig. 1a, b). Furthermore, compared to middle-aged HFD group, old-aged HFD group showed significantly increased F4/80 positive cells, which means more increased infiltration of liver macrophages (Fig. 1a, b). We also assessed the effect of aging on hepatic necroinflammation by serum ALT level. Serum ALT level was higher in HFD groups compared to LFD groups and further significantly higher in old-aged HFD group compared to middle-aged HFD group (Fig. 1c).
Fig. 1.
Hepatic steatosis and inflammation with aging and HFD feeding. a Representative images of H&E stain (original magnification ×100) and immunohistochemical staining for anti-F4/80 and anti-Ly6G antibodies (×200). b Image analysis for hepatic steatosis, anti-F4/80- and anti-Ly6G-positive cells. c Serum ALT levels. Ycon, young control; Mlfd, middle-aged low-fat diet; Olfd, old-aged low-fat diet; Mhfd, middle-aged high-fat diet; Ohfd, old-aged high-fat diet. *P < 0.05
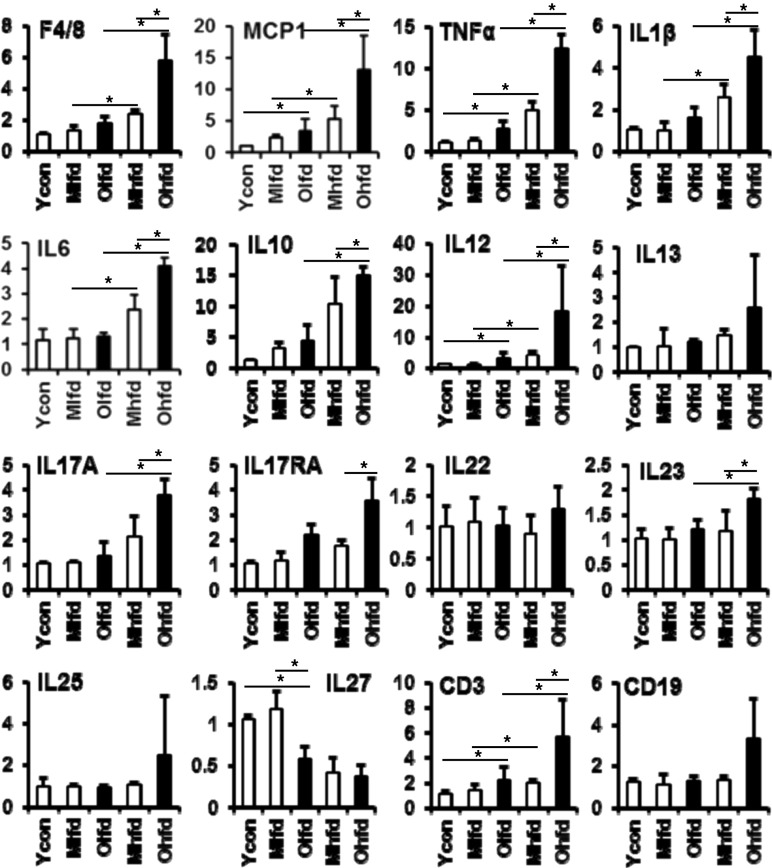
We analyzed the expressions of mRNA levels for inflammatory markers by real-time quantitative PCR (Fig. 2). Both HFD groups showed more increased expressions of F4/80 mRNA levels than LFD groups, which was still more significantly increased in old-aged HFD group compared to middle-aged HFD group. Similarly, the expressions of hepatic pro-inflammatory cytokines including MCP1, TNF-α, IL-1β, IL-6, IL-12, IL-17A, IL-17AR, IL-23 were increased, but the expression of anti-inflammatory cytokine such as IL-27 was decreased with aging or HFD feeding. The expression of IL-10 was increased in the HFD groups compared to LFD groups, further significantly increased in the old-aged HFD group. In addition, the expression of CD3, a marker for lymphocytes, was also significantly increased in both HFD groups with further increase in the old-aged HFD group.
Fig. 2.
Changes of mRNA expressions of hepatic inflammatory cytokines. Expressions of all genes were analyzed by real-time PCR. *P < 0.05
Advanced hepatic fibrosis with aging and HFD feeding
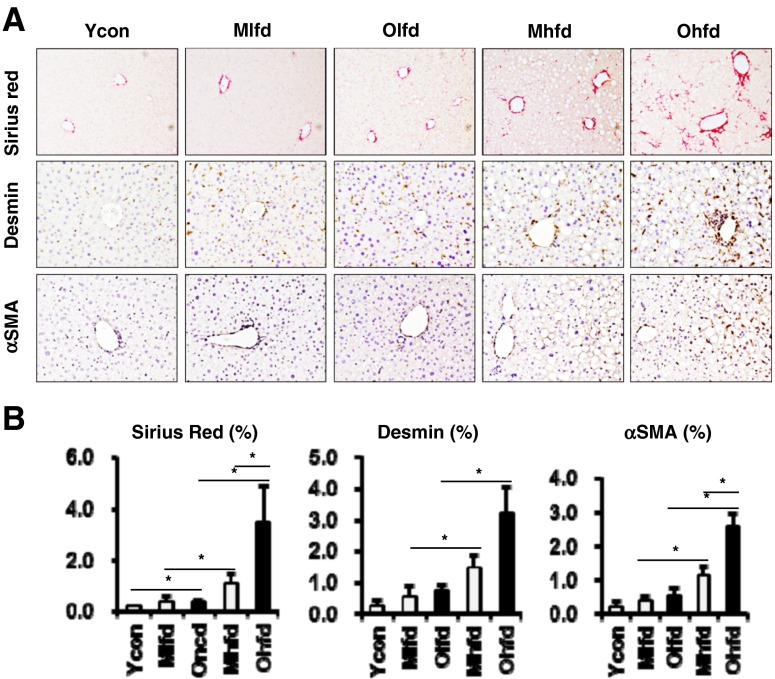
Sirius red staining of liver sections showed that old-aged mice had significant hepatic fibrosis compared to young control mice (Fig. 3a, b). Furthermore, compared to LFD groups, both HFD groups showed significantly increased hepatic fibrosis which was more advanced in old-aged HFD group compared to middle-aged HFD group (Fig. 3a, b). We analyzed the infiltration of hepatic stellate cells (HSCs) by immunohistochemical staining with anti-desmin and anti-αSMA antibodies. Both desmin- and αSMA-positive staining cells were markedly increased in HFD groups compared to LFD groups. Interestingly, compared to middle-aged HFD group, old-aged HFD group showed more significantly increased αSMA-positive cells, which means more increased levels of activated HSCs (Fig. 3a, b).
Fig. 3.
Hepatic fibrosis with aging and HFD feeding. a Representative images of Sirius red stain (original magnification ×100) and immunohistochemical staining for anti-desmin and anti-αSMA antibodies (×200). b Sirius red positive area, anti-desmin and anti-αSMA-positive cells were analyzed by image J. *P < 0.05
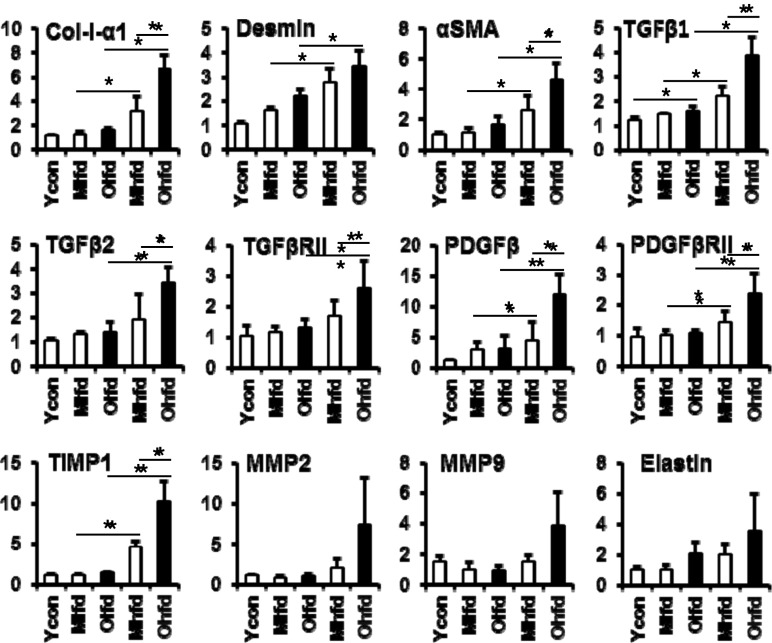
We analyzed the expressions of mRNA levels for hepatic fibrogenic markers by real-time quantitative PCR (Fig. 4). Compared to LFD groups, HFD groups showed increased expressions of Col-I-α1 mRNA which was significantly higher in the old-aged HFD group than middle-aged HFD group. The expressions of other hepatic fibrogenic markers such as αSMA, TGF-β1, TGF-β2, TGFβRII, PDGF, PDGFRβII, TIMP1 were increased in the HFD groups compared to LFD groups and further significantly higher in the old-aged HFD group than middle-aged HFD group. The expression of MMP2, MMP9, and elastin mRNAs was also increased in the old-aged HFD group, but statistically not significant.
Fig. 4.
Changes of mRNA expressions of hepatic fibrogenic markers. Expressions of all genes were analyzed by real-time PCR. *P < 0.05
Oxidative stress and mRNA expressions for ROS production and TLR4 in liver
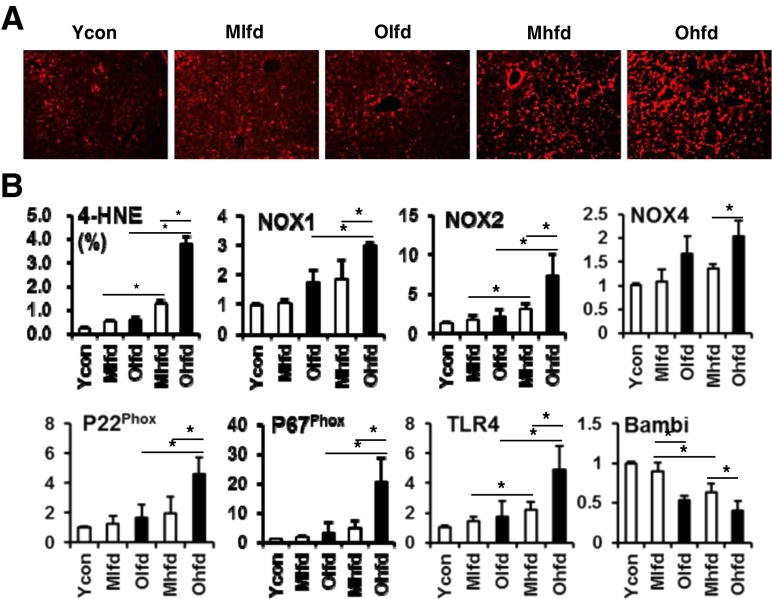
We further analyzed oxidative stress and the expression of ROS markers and TLR4 which were known to be involved in the progression of hepatic fibrosis of NAFLD. Immunofluorescent staining for 4-HNE showed significantly increased oxidative stress in the HFD groups compared to LFD groups and which were more marked in the old-aged HFD group than middle-aged HFD group (Fig. 5a). The hepatic expressions of ROS production-related genes such as NOX1, NOX2, NOX4, P22phox, P67phox were up-regulated in the old-aged HFD group compared to middle-aged HFD group (Fig. 5b). Furthermore, old-aged HFD group showed significantly increased expression of TLR4 with down-regulation of TGF-β pseudoreceptor, Bambi compared to middle-aged HFD group.
Fig. 5.
Hepatic oxidative stress and expression of ROS production related genes, TLR4, and Bambi. a Representative images of immunofluorescent staining for anti-4-HNE antibody (original magnification ×100). b Image analysis for anti-4-HNE-positive area and real-time PCR analysis for expression of ROS production related genes, TLR4, and Bambi. *P < 0.05
Genetic changes in liver with aging and HFD
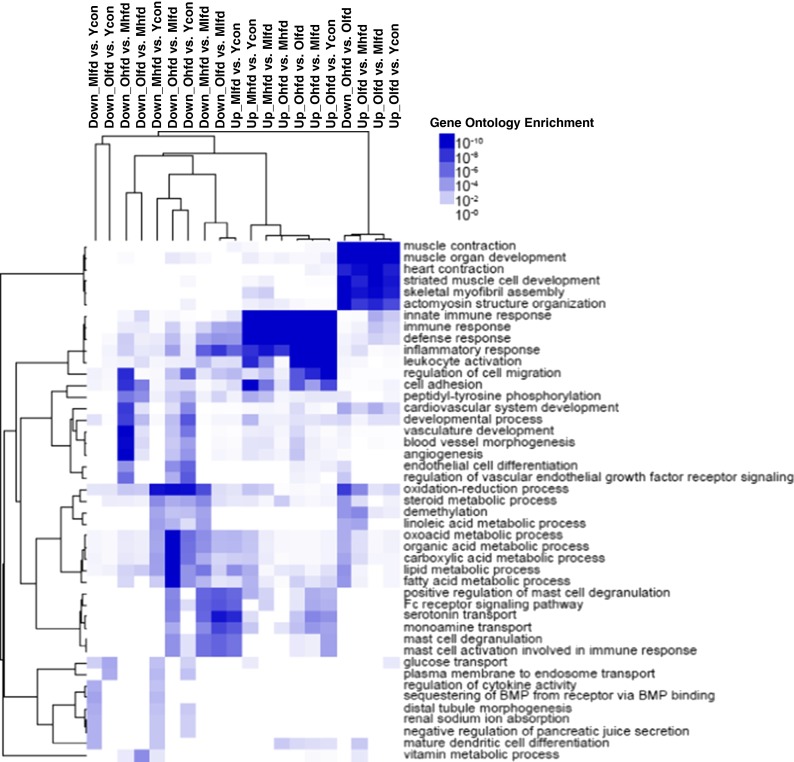
We explored underlying changes of hepatic gene expression related to aging and HFD with total RNA-seq analysis. When we analyzed total 23987 genes, compared to middle-aged HFD group, old-aged HFD groups showed 129 differentially expressed genes of which 72 genes were up-regulated and 57 genes were down-regulated. This set of genes was enriched in GO biological process annotations linked to innate immune response, immune response, defense response, inflammatory response, cell migration, cell adhesion, cardiovascular system development, developmental process, vascular development, blood vessel morphogenesis, angiogenesis, and endothelial cell differentiation (Fig. 6). Table 1 summarizes major canonical pathways and upstream regulators which were strongly induced in old-aged HFD group compared to middle-aged HFD group.
Fig. 6.
Gene ontology analysis. Gene ontology analysis and clustering were performed using HOMER and Cluster3.0/Java Tree View, respectively
Table 1.
Differentially expressed hepatic genes from RNA-seq analysis between Ohfd vs. Mhfd groups
| Top canonical pathways | ||
|---|---|---|
| Name | P value | Overlap |
| Agranulocyte adhesion and diapedesis | 1.64E-06 | 7.4 % (14/189) |
| Hepatic fibrosis/hepatic stellate cell activation | 1.44E-04 | 6.0 % (11/183) |
| Granulocyte adhesion and diapedesis | 4.69E-04 | 5.6 %(10/177) |
| Calcium signaling | 4.90E-04 | 5.6 % (10/178) |
| Mitotic roles of polo-like kinase | 5.60E-04 | 9.1 % (6/66) |
| Top upstream regulators | ||
| Upstream regulator | P value of overlap | Predicted activation |
| Lipopolysaccharide | 4.18E-21 | Activated |
| TNF | 1.05E-18 | Activated |
| Dextran sulfate | 9.11E-17 | Activated |
| PTGER2 | 1.02E-13 | Activated |
| IL-6 | 1.83E-13 | Activated |
TNF tumor necrosis factor, PTGER2 prostaglandin E receptor 2, IL-6 interleukin 6
Renal injury with aging and HFD feeding
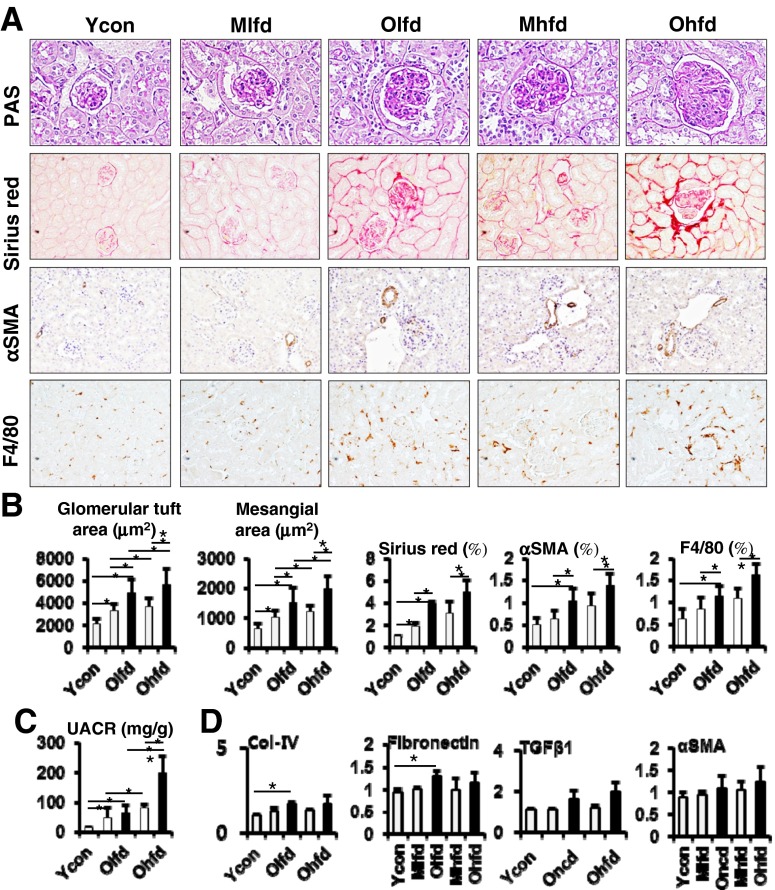
We also investigated the effect of aging on HFD-induced renal injury. On PAS staining, the glomerular size and mesangial matrix area were significantly greater in old-aged mice than young or middle-aged mice and also greater in HFD groups than LFD groups (Fig. 7a, b). Furthermore, it was significantly greater in old-aged HFD group compared to middle-aged HFD group. Consistent to the findings of glomerular changes, UACR were significantly increased with aging and further increased on HFD feeding (Fig. 7c). Sirius red staining showed significant increase of renal fibrosis with aging (Fig. 7a, b). However, there was no significant further increase of renal fibrosis with HFD feeding among same aging groups. Similarly, in the immunohistochemical staining for anti-αSMA and anti-F4/80 antibodies, αSMA- and F4/80-positive cells were markedly increased with aging, but no significant further increase with HFD feeding among same aging groups (Fig. 7a, b). The expression fibrogenic genes such as Col-IV and fibronectin were up-regulated with aging, but no significant change for TGF-β1, αSMA (Fig. 7d).
Fig. 7.
Glomerular changes and renal fibrosis with aging and HFD feeding. a Representative images of PAS stain (original magnification ×400), Sirius red stain (×100), and immunohistochemical staining for anti-αSMA and anti-F4/80 antibodies (×200). b Image analysis for glomerular tuft area, mesangial area, Sirius red-positive area, anti-αSMA- and anti-F4/80-positive cells. c Urinary albumin creatinine ratio (UACR). d Expression of renal fibrogenic genes analyzed by real-time PCR. *P < 0.05
Discussion
Aging is often accompanied by abdominal obesity and excessive visceral fat which causes insulin resistance and metabolic syndromes (Barzilai et al. 2012). Insulin is associated with increased secretion of free fatty acids from lipolysis in fatty tissues, while the synthesis of neutral fat is boosted in the liver by an increased intake of free fatty acids and subsequently results in accumulation of excessive fat in the liver. In addition, the cellular and molecular hallmarks such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication are generally considered to contribute to the aging process (López-Otín et al. 2013). Thus, these aging-related changes may affect the phenotype of liver and kidney injury in response to HFD. In this study, we showed that the susceptibility of hepatic fibrosis as well as hepatic inflammation in response to HFD was significantly increased with aging. In addition, aging also contributed to glomerular alterations and increased renal inflammation or fibrosis, while the effect of aging on HFD-induced renal injury was not remarkable.
Although there has been limited data available regarding the differential effect of HFD feeding on the progression of NAFLD and/or renal injury, a few papers have been published on these topics recently (Sheedfar et al. 2014; van der Heijden et al. 2015a). A recent study reported that increased hepatic CD36 expression with age is associated with enhanced hepatic fat uptake and increased susceptibility to NAFLD (Sheedfar et al. 2014). Meanwhile, in another study, HFD-induced obesity primes inflammation in adipose tissue prior to development of hepatic inflammation (van der Heijden et al. 2015a). Aging is associated with increased visceral adiposity linked to adipose tissue inflammation and systemic increase in pro-inflammatory cytokines. Thus, we thought that this inflamm–aging may contribute to enhanced hepatic inflammation in response to HFD feeding. Our study demonstrated that aging is associated with more marked hepatic infiltration of Kupffer cells and increased expression of inflammatory cytokines in response to HFD. Previous study reported that normal aging increases intestinal permeability through remodeling of tight junction proteins (Tran & Greenwood-Van Meerveld 2013). Old animals showed up-regulation of the inflammatory cytokines IFN-γ, IL-6, and IL-1β in colonic biopsies which activate both canonical and non-canonical NF-kB pathways in intestinal epithelial cells. These changes, in turn, lead to decreased expression of the tight junction proteins such as zonula occludens-1, occludin, and junctional adhesion molecule-A, but increased expression of claudin-2 (Tran & Greenwood-Van Meerveld 2013). Therefore, the increased intestinal permeability with aging may enhance the activation of Kupffer cells which contribute to increased expression of inflammatory cytokines with HFD feeding in aged mice. Indeed, our results showed that the expressions of hepatic inflammatory cytokines such as TNF-α, IL-1β, IL-6, and MCP1 were significantly increased in old-aged HFD group compared to middle-aged HFD group. In addition, the expression of IL-17A was up-regulated with aging and HFD feeding. IL-17A, mainly produced by CD4+ Th17 cells, was recently known to stimulate Kupffer cells and promote the progression of NAFLD from steatosis to steatohepatitis (Kisseleva 2014).
It has been suggested that aging is associated with an acceleration of the progression of hepatic fibrosis induced by various etiologies, such as hepatitis C virus or alcohol (Floreani 2007). Several studies investigated the association of age-dependent liver injury and fibrosis using a mouse model of chronic injury. Collins et al. reported that old (18 months) mice had a significantly greater fibrogenic response to carbon tetrachloride injury than middle-aged (9 months) mice (Collins et al. 2013). Another study also demonstrated higher susceptibility to fibrosis with increasing age using carbon tetrachloride injection model, in which macrophages expressing Th2 cytokines is mainly involved (Mahrouf-Yorgov et al. 2011). Recently, Fontana et al. implicated that aging promotes the development of murine steatohepatitis in response to HFD feeding which was underlined by increased pro-inflammatory reaction with M1 macrophage polarization (Fontana et al. 2013). Based on these findings, we supposed the increased susceptibility of hepatic fibrosis with aging in the HFD-induced liver injury. Indeed, our study demonstrated that the susceptibilities of hepatic fibrosis as well as hepatic inflammation in response to HFD were significantly increased with aging. In the previous studies, known molecular mechanisms for the accumulation of excessive fat in the liver and damage to hepatic cells due to aging include increased ROS formation, DNA damage, activation of p300-C/EBP-dependent neutral fat synthesis, telomere shortening, decreased autophagy, increased M1 macrophage inflammatory responses, activation of NF-kB pathways, and hepatocyte senescence (Sheedfar et al. 2013; Amir & Czaja 2011; Aravinthan et al. 2013; Franceschi et al. 2000; Jin et al. 2013). In this study, old-aged mice showed significantly increased expression of TLR4 in response to HFD injury compared to middle-aged mice. The intracellular signaling of the TLR4 decreased the expression of the TGF-β pseudoreceptor Bambi, which in turn will upregulate TGF-β signaling, again leading to increased fibrosis (Brenner et al. 2011). In addition, in this study, old-aged mice showed significantly increased expression of NOX genes compared to middle-aged mice. NOX was known as a multicomponent enzyme complex that generates ROS in response to injury, which leads to hepatic fibrosis directly or stimulate further inflammation (Paik & Brenner 2011). In support, RNA-Seq analysis of the whole liver tissues revealed that canonical pathways of agranulocyte adhesion and diapedesis, hepatic fibrosis/hepatic stellate cell activation, granulocyte adhesion and diapedesis, calcium signaling, and mitotic roles of polo-like kinases were significantly changed in the old-aged HFD group compared to middle-aged HFD group.
In the previous reports, renal aging is accompanied with glomerular, vascular, and parenchymal changes leading to progressive decline in renal function (Choudhury & Levi 2011; Martin & Sheaff 2007; Wiggins 2012). A range of factors has been proposed that could have a role in renal aging. Clinical factors, including hypertension, diabetes mellitus, obesity, abnormal lipid levels and vitamin deficiency, as well as tissue factors such as increased activity of the renin-angiotensin-aldosterone system, increased levels of advanced glycation end products, oxidative stress, decreased nitric oxide, TGF-β, have been associated with increasing renal aging (Choudhury & Levi 2011). In addition, HFD-induced obese mice showed pathophysiological changes including albuminuria, increase in glomerular tuft area, mesangial expansion, renal lipid accumulation, increased collagen in glomeruli, increased macrophage infiltration in medulla, and impaired sodium handling (Deji et al. 2009). Therefore, we could suppose that aging combined with HFD feeding are considered to further increase the risk of development or progression of renal injury. Indeed, our study showed significant increase in glomerular size, mesangial expansion, higher urine albumin/creatinine ratio, and more advanced renal inflammation or fibrosis with aging and HFD. However, the differential effect of aging on HFD-induced renal injury was not remarkable as shown in the liver. This might be explained with higher susceptibility of liver to inflammatory stimuli from increased intestinal permeability with aging. Other factors such as genetic background of mice and duration of HFD feeding also could be considered. Further studies are required to explore any potential impact of aging on HFD-induced renal injury. Consistent to our results, a recent study also demonstrated a gradual age-dependent occurrence of mild albuminuria, tubulo-interstitial and glomerular sclerotic changes, and increased expression of inflammatory mediators (van der Heijden et al. 2015b). Furthermore, compared to LFD mice, HFD mice developed severely aggravated structural and morphological changes that showed features typical of amyloidosis (van der Heijden et al. 2015b).
In conclusion, the susceptibility of hepatic fibrosis as well as hepatic inflammation in response to HFD was significantly increased with aging. In addition, aging also contributed to glomerular alterations and increased renal inflammation or fibrosis, while the differential effect of aging on HFD-induced renal injury was not remarkable in this study. Proper lifestyle modifications including LFD with high content in antioxidants might represent the most plausible approach to maintain healthy liver aging.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 75 kb)
(DOC 551 kb)
Compliance with ethical standards
Conflict of interest
None.
Financial support and sponsorship
This research was supported by The GlaxoSmithKline Research Fund of the Korean Association for the study of the Liver (In Hee Kim).
Support: NIH R01MH094151-01 and MH019934, and the Sam and Rose Stein Institute for Research on Aging, University of California, San Diego.
References
- Organization WH . Global health and aging: World Health Organization. 2011. [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker DL. Age-related changes in liver structure and function: implications for disease ? Exp Gerontol. 2005;40(8-9):650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Hohn A, Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1(1):140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, et al. Prevalence of nonalcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6(3):161–163. [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Koehler EM, Schouten JN, Hansen BE, van Rooij FJ, Hofman A, Stricker BH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol. 2012;57(6):1305–1311. doi: 10.1016/j.jhep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55(6):607–613. doi: 10.1159/000235677. [DOI] [PubMed] [Google Scholar]
- Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58(5):1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury D, Levi M. Kidney aging—inevitable or preventable? Nat Rev Nephrol. 2011;7(12):706–717. doi: 10.1038/nrneph.2011.104. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:65–80. doi: 10.1016/j.arr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296(1):F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedfar F, Sung MM, Aparicio-Vergara M, Kloosterhuis NJ, Miquilena-Colina ME, Vargas-Castrillon J, et al. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging (Albany NY) 2014;6(4):281–295. doi: 10.18632/aging.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden RA, Sheedfar F, Morrison MC, Hommelberg PP, Kor D, Kloosterhuis NJ, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging (Albany NY) 2015;7(4):256–268. doi: 10.18632/aging.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68(9):1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T. Does interleukin-17 play the villain in nonalcoholic steatohepatitis? Hepatology. 2014;59(5):1671–1672. doi: 10.1002/hep.26955. [DOI] [PubMed] [Google Scholar]
- Floreani A. Liver diseases in the elderly: an update. Dig Dis. 2007;25(2):138–143. doi: 10.1159/000099478. [DOI] [PubMed] [Google Scholar]
- Collins BH, Holzknecht ZE, Lynn KA, Sempowski GD, Smith CC, Liu S, et al. Association of age-dependent liver injury and fibrosis with immune cell populations. Liver Int. 2013;33(8):1175–1186. doi: 10.1111/liv.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrouf-Yorgov M, de L’hortet AC, Cosson C, Slama A, Abdoun E, Guidotti JE, et al. Increased susceptibility to liver fibrosis with age is correlated with an altered inflammatory response. Rejuvenation Res. 2011;14(4):353–363. doi: 10.1089/rej.2010.1146. [DOI] [PubMed] [Google Scholar]
- Fontana L, Zhao E, Amir M, Dong H, Tanaka K, Czaja MJ. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57(3):995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12(6):950–954. doi: 10.1111/acel.12128. [DOI] [PubMed] [Google Scholar]
- Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5(2):159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravinthan A, Scarpini C, Tachtatzis P, Verma S, Penrhyn-Lowe S, Harvey R, et al. Hepatocyte senescence predicts progression in non-alcohol-related fatty liver disease. J Hepatol. 2013;58(3):549–556. doi: 10.1016/j.jhep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Jin J, Iakova P, Breaux M, Sullivan E, Jawanmardi N, Chen D, et al. Increased expression of enzymes of triglyceride synthesis is essential for the development of hepatic steatosis. Cell Rep. 2013;3(3):831–843. doi: 10.1016/j.celrep.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DA, Seki E, Taura K, Kisseleva T, Deminicis S, Iwaisako K, et al. Non-alcoholic steatohepatitis-induced fibrosis: Toll-like receptors, reactive oxygen species and Jun N-terminal kinase. Hepatol Res. 2011;41(7):683–686. doi: 10.1111/j.1872-034X.2011.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik YH, Brenner DA. NADPH oxidase mediated oxidative stress in hepatic fibrogenesis. Korean J Hepatol. 2011;17(4):251–257. doi: 10.3350/kjhep.2011.17.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007;211(2):198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- Wiggins JE. Aging in the glomerulus. J Gerontol A Biol Sci Med Sci. 2012;67(12):1358–1364. doi: 10.1093/gerona/gls157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden RA, Bijzet J, Meijers WC, Yakala GK, Kleemann R, Nguyen TQ, et al. Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci Rep. 2015;5:16474. doi: 10.1038/srep16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 75 kb)
(DOC 551 kb)