Abstract
Mangroves are unique ecosystems in the coastal tropical and subtropical regions of the Earth. The fluctuation in salinity due to tidal action results in a prolific population of adhered halophilic and halotolerant bacteria in this ecosystem. In this study, a pigment producing adhered bacterial strain Halobacillus trueperi MXM-16 was isolated from mangrove plant litter of Goa. This strain was moderately halophilic, Gram positive rod, catalase positive and capable of utilizing sodium benzoate as a source of carbon. H. trueperi MXM-16, produced a siderophore that was hydroxamate in nature. The non-diffusible yellow pigment was a carotenoid and HPLC studies revealed a peak that was indicative of astaxanthin as one of the component. Further studies on the pigment exhibited its ability to chelate iron from the chrome azurol sulphonate medium behaving as an additional mechanism for iron acquisition.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-016-0591-7) contains supplementary material, which is available to authorized users.
Keywords: Adhered, Siderophores, Carotenoids, Pigment, Astaxanthin, Mangroves, Halophilic
Introduction
In turbid ecosystems like the estuaries and mangroves adhered bacteria are gaining interest as it is now being theorized that these bacteria are responsible for most of the degradation of particulate organic matter in the mangrove ecosystem [1–3]. Mangrove leaf litter is the primary source of particulate organic matter in the mangrove ecosystem.
Mangroves are ecosystems found at the interface region between the freshwater and the saline ecosystems. In this ecosystem salinity plays an important role in determining the type of community structure. Due to the fluctuation in salinity brought about by the influence of tides, the predominant bacteria are envisaged to be halophilic and halotolerant bacteria. An important factor for metabolic activities to take place at optimum level is the availability of essential metals such as iron. Iron forms a part of many important enzymes in bacteria. Owing to the aerobic atmosphere of the planet iron occurs mostly as ferric oxyhydroxide polymers that have low solubility ion. Therefore, to overcome this limitation bacteria have adopted strategies like production of siderophores [4]. These siderophores form complexes with the iron and helps transport in across to the bacterial cell [5, 6]. It is envisaged that in a dynamic ecosystem such as the mangroves, the most effective mineralization would occur if the bacteria are capable of acquiring iron.
In this study, the strain Halobacillus trueperi MXM-16 was isolated from mangrove plant litter. It is the first report on Halobacillus genera producing a hydroxamate siderophore. The siderophore and the pigment produced by the strain was characterized and identified. The pigment was studied further for its ability to chelate iron. The research work also documents for the first time the ability of a carotenoid pigment from Halobacillus genera capable of chelating iron from the environment.
Materials and Methods
Isolation of Adhered H. trueperi MXM-16 from Mangrove Plant Litter
Halobacillus trueperi MXM-16 was isolated from the plant litter using ethylene-diamine-tetra-acetic acid (EDTA) and identified by I6S rRNA sequencing and BLAST analysis and the accession number was obtained as GenBank: KF379752 (Supplementary data) [7].
Studies on Siderophores Produced by H. trueperi MXM-16
The isolate was grown on chrome azurol sulphonate medium (CAS) for 24–48 h at 28 °C. A zone of yellow colouration around the bacterial colony indicated the production of siderophore. Characterization of the siderophore was carried out by growing the isolate in nutrient broth prepared in de-ionized distilled water for 48 h at 28 °C. The broth was centrifuged (Eppendorf 5804R) at 8000 rpm for 20 min at 4 °C and the supernatant decanted and was used for characterization of siderophore. Qualitative analysis of the siderophore was carried out by Neiland’s spectrophotometric assay and tetrazolium salt test for hydroxamate siderophore, Arnow’s assay and Csaky’s spectrophotometric assay for catecholate siderophore and Vogel’s test for carboxylate siderophore [8].
Studies on Pigment Produced by H. trueperi MXM-16
The isolate was grown on ZMB on shaker at 150 rpm for 48 h at 28 °C. The culture suspension was pelleted by centrifuging (Eppendorf 5804R) at 5000 rpm for 10 min at 4 °C. 1 g of the pellet was resuspended in 10 ml of methanol (Merck HPLC grade) and acetone (Merck HPLC grade) each and sonicated (B. Braun Labsonic U) using 0.5 pulses for 2 min. The supernatant containing the pigment was collected by centrifuging (Eppendorf 5804R) at 8000 rpm for 20 min at 4 °C. The pigment was scanned between 190 and 800 nm in a UV–Vis spectrophotometer (Shimadzu UV 2450). The separation of carotenoids was achieved by using the pigment extracted in methanol by HPLC on a C-18 reverse-phase column (Waters Spherisorb ODS, 5 m diameter of 4 mm × 25 mm) in a mobile phase of methanol. Detection of pigment was performed at a wavelength of 342 nm at a flow rate of 1 ml/min and a pressure of 1000 psi. The peaks were monitored over a period of 20 min using an HPLC-Waters equipped with waters 2996 Phase diode array detector.
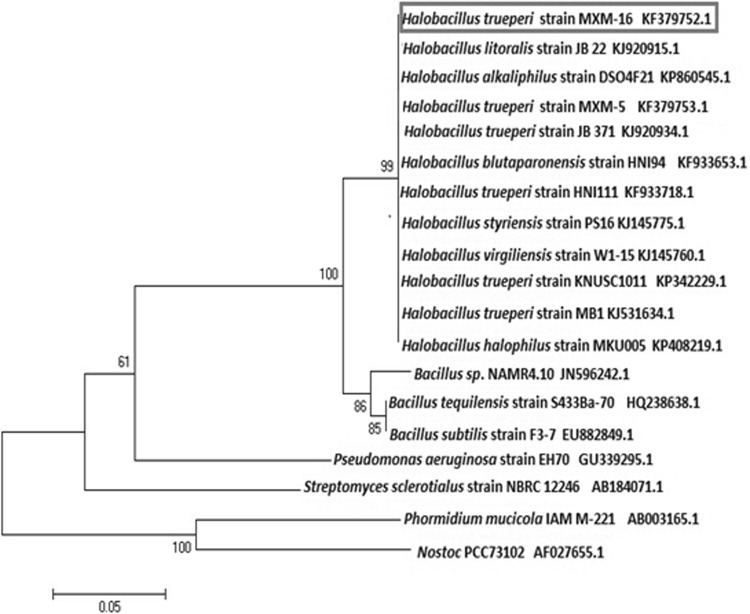
The relationship of this pigment producing strain of Halobacillus trueperi strain MXM-16 with other closely related Halobacillus sp. and also other Bacillaceae bacteria like Exiguobacterium sp. was compared by constructing a phylogenetic tree based on the 16S rRNA sequence by the neighbor joining method.
Analysis of the Pigment to Chelate Iron
CAS solution was prepared in de-ionised distilled water and to 3 ml of this solution 2 ml of the extracted pigment was added and allowed to stand for 15 min at room temperature (28 °C). The control was maintained with methanol or acetone added to CAS solution. The colour change of the CAS solution from blue to yellow indicated the ability of the pigment to chelate iron.
Growth of H. trueperi MXM-16 on Sodium Benzoate
Cells of H. trueperi MXM-16 were grown on ZMB. 5 % of this was inoculated in flasks with minimal salt medium (MSM) supplemented with 0.2 % (w/v) sodium benzoate. The flasks were incubated for 48 h at 28 °C. The ability of the culture to grow on this medium was noted and the cells were subjected to Rothera’s test [9].
Results and Discussion
Isolation and Identification of H. trueperi MXM-16
The bacterial isolate MXM-16 was isolated from mangrove plant litter. It was characterised by morphological, biochemical and molecular identification by 16S rRNA sequencing as H. trueperi MXM-16 (GenBank: KF379752) [7]. It produced a non-diffusible orange pigment and was a moderately halophilic, Gram positive bacillus that was catalase positive and oxidase negative. Other species of Halobacillus are known to produce pigments that range from yellow to orange in colour [10].
Studies on Siderophore Production by H. trueperi MXM-16
The ability of the bacteria to produce siderophores is crucial to the survival of the bacteria as they depend on these chelating molecules to acquire the metal iron from its surrounding environment. H. trueperi MXM-16 was found to produce siderophore (Fig. 1). Characterization of the siderophore indicated that it was a hydroxamate type of siderophore. The Csaky’s test showed a peak between 420 and 450 nm and the tetrazolium assay showed a reddish-pink colouration characteristic of the hydroxamate siderophore. It has been reported that hydroxamate siderophores are highly resistant to environmental degradation associated with the wide range of hydrolytic enzymes that are present in humic soil such as that of mangrove ecosystem [11]. Hydroxamate siderophores produced by Pseudomonas fluorescens [12], Magnetospirillum magneticum [13] and root nodule bacteria such as Rhizobium and Sinorhizobium [14] have been reported. However, production of siderophore by Halobacillus genera is being reported for the first time.
Fig. 1.
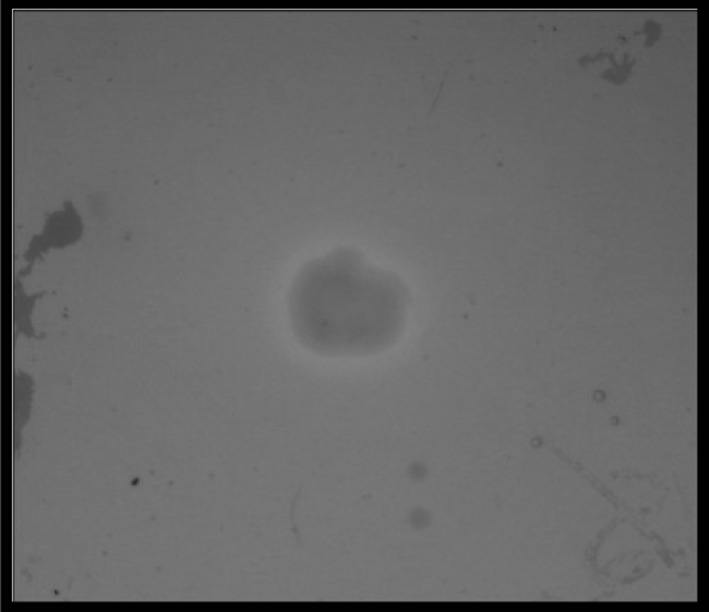
Bacterial strain H. trueperi MXM-16 showing yellowish colouration around the bacterial colony indicative of siderophore production on CAS agar (colour figure online)
Studies on Pigment Produced by H. trueperi MXM-16
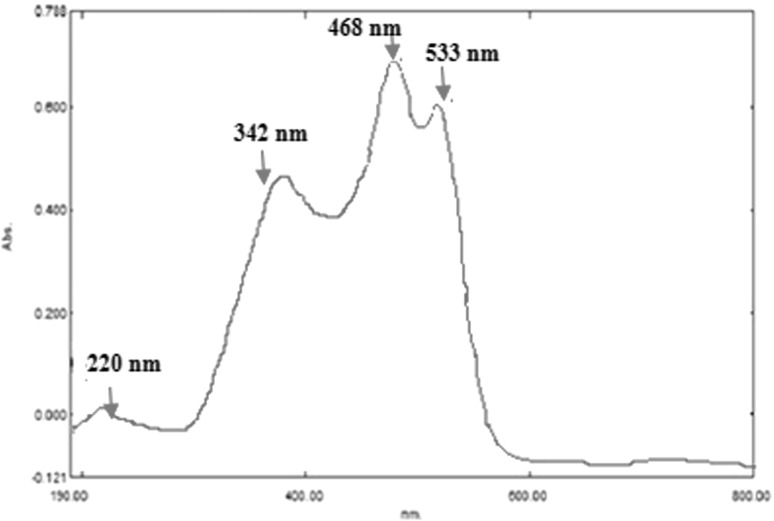
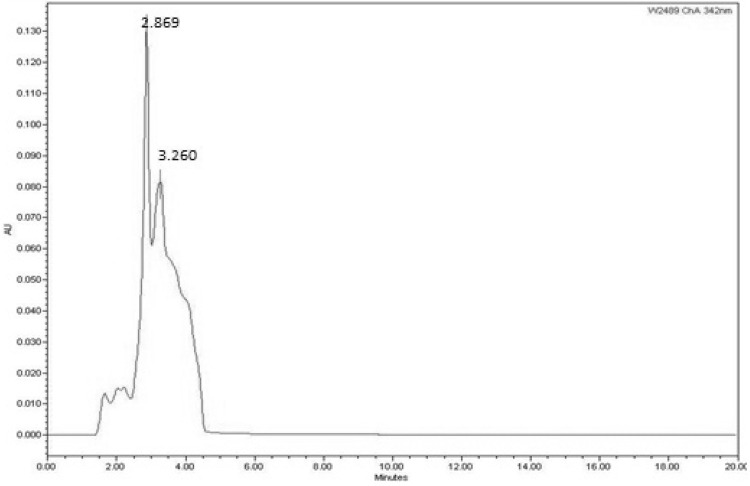
Halobacillus trueperi MXM-16 produced a non-diffusible orange pigment (Fig. 2). The carotenoid compounds in the pigment were identified by the spectral data in the UV–Vis spectrophotometer and HPLC. The UV–Vis scan of the pigment extracted in acetone showed peaks at 553, 468 and 342 nm characteristic of carotenoid pigments owing to their polyene chromophores which absorb the light in the 400–500 nm regions (Fig. 3). This is the basis for the ability of the carotenoids to quench singlet oxygen and appear yellow to orange in colour. These carotenoids are responsible for the cream white, pale yellow or bright orange colouration of the cells in the genus Halobacillus [10]. The HPLC of the pigment revealed two prominent peaks (Fig. 4). The first peak with a retention time of 2.86 min remained unidentified. However, a similar unidentified peak has been reported in HPLC scan in the pigment from Pseudomonas fluorescence. The second peak with the retention of 3.26 min was indicative of a component called astaxanthin. Astaxanthin has previously been reported in Exiguobacterium sp. [15, 16]. Interestingly, Exiguobacterium and Halobacillus both belong to the family Bacillaceae [10] (Fig. 5). Astaxanthin is a keto-carotenoid that belongs to the xanthophyll family of the carotenoids and because of its antioxidant properties and its use as colourant is gaining popularity in the food and drug industry [17].
Fig. 2.
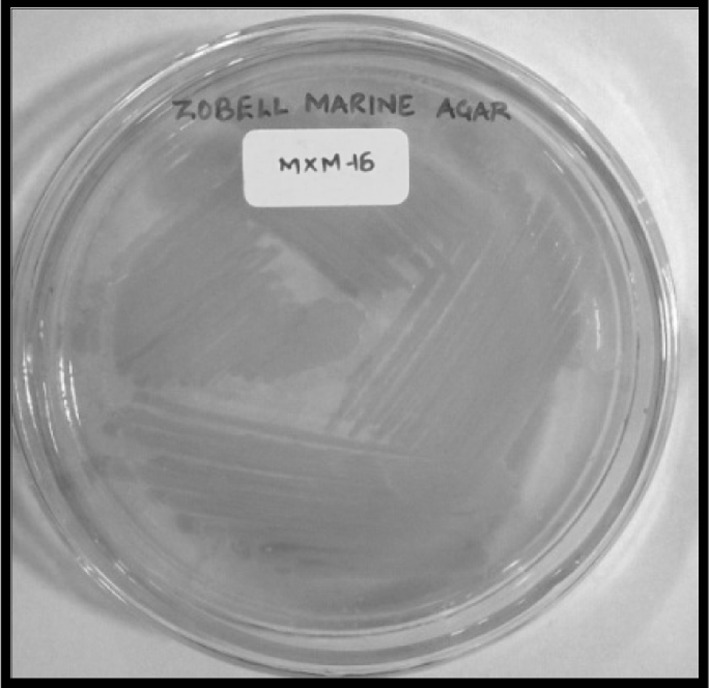
Orange pigmentation by H. trueperi MXM-16 cells grown on ZMA agar
Fig. 3.
UV–Vis scan of the carotenoid pigment produced by H. trueperi MXM-16
Fig. 4.
HPLC profile of the carotenoid pigment produced by H. trueperi MXM-16 at 342 nm
Fig. 5.
Phylogenetic tree depicting evolutionary relationship of H. trueperi MXM-16 (GenBank accession no KF 379752) with other closely related strains of Halobacillus spp.
Carotenoid pigments have been reported from bacteria such as Micrococcus roseus, Staphylococcus aureus and Microbacterium arborescens [18]. These carotenoid pigments are non-diffusible and form an integral part of the bacterial cell membrane and influence the effectiveness of the membrane as a barrier to water molecules, oxygen and other small molecules [19]. Reports also indicate that the bacteria accumulate carotenoids as a response to environmental stress and it thus aids the bacteria to survive in such environments [20].
Ability of the Pigment to Chelate Iron
Significantly, the UV–Vis spectral scan showed a peak at 220 nm in the UV region. Such peaks have been noticed in scans of pyoverdine and azotobactin [21, 22]. Interestingly both pyoverdine and azotobactin are mixed ligand siderophores. Studies have shown that fluorescent pigment pyoverdine from Pseudomonas aeruginosa is one such example that is known to be a powerful scavenger and efficient transporter of Fe(III) [23–25]. In the current investigation, the ability of the pigment to chelate iron from the CAS medium was studied. An interesting observation was that, the CAS solution turned yellow on addition and incubation with the pigment (Fig. 6). This was a significant discovery as it indicated that the pigment aided the bacteria to acquire iron from the CAS medium and the H. trueperi MXM-16 pigment may be associated with a siderophore. The fluorescent pigment pyoverdine, from Pseudomonas aeruginosa is one such example that is known to be a powerful scavenger and efficient transporter of Fe(III) [23, 24]. The ability of the H. trueperi MXM-16 to produce an iron chelating pigment is significant as this genera are characterized by their strict oxidative mechanisms and therefore they display a high iron requirement for satisfying cytochrome and other electron transport system biosynthesis. The discovery and further detailed studies of these carotenoid iron chelating pigments may also pave avenues for their use as taxonomic and phylogenetic markers. Also this is the first report that shows a carotenoid pigment to have the ability to chelate iron. The only other pigment reported so far with this ability is the fluorescent pigment pyoverdine from Pseudomonas [24, 25].
Fig. 6.
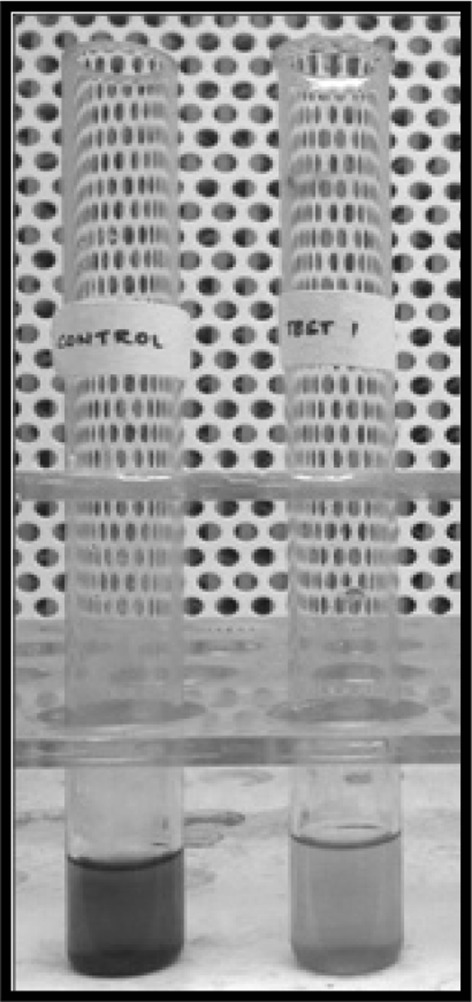
Ability of the pigment to change colour of CAS solution (colour figure online)
Growth of H. trueperi MXM-16 on Sodium Benzoate
Bacteria produce the enzymes oxygenases that have iron as their cofactor. These enzymes are needed to utilize aromatic substrates such as sodium benzoate. The isolate H. trueperi MXM-16 exhibited growth in presence of 0.2 % sodium benzoate. However, the culture did not show any pigmentation. Sand dune bacteria, Pseudomonas aeruginosa TMR2.13 can grow on sodium benzoate up to 2 % and Bacillus sp. can tolerate up to 1 % of sodium benzoate [23, 25, 26]. Earlier studies have shown that utilization of the aromatic compound puts a greater stress on the bacteria for the demand of oxygen as a result of which they downplay the formation of pigment [23]. Research has also shown the ability of Halobacillus salinus and Halobacillus halophilus to degrade a variety of polycyclic aromatic compounds [27]. The Rothera’s test showed a deep purple colour with a ring formation on addition of the sodium nitroprusside solution. The purple colour was a visual indicator of production of β-ketoadipate, a result of the ortho cleavage of the aromatic ring of sodium benzoate. Haloarchaea such as Haloferax sp., Halobacterium piscisalsi, Halobacterium salinarum and Halorubrum ezzemoulense have been reported to degrade aromatic hydrocarbons such as benzene and toluene by the ortho ring cleavage pathway of degradation [28].
Conclusion
Halobacillus trueperi MXM-16 an adhered bacterial strain was isolated from the mangrove plant litter. The research work reported the production of hydroxamate type of siderophore by this strain and also is the first research work that indicates that the genera Halobacillus produce a siderophore. A significant discovery was the ability of the carotenoid pigment of this strain to chelate iron that demonstrated the strategies of this strain to adapt to dynamic and complex ecosystem like the mangroves. The strain could utilize aromatic compounds like sodium benzoate. The results suggests the use of this strain for the bioremediation of soils contaminated with xenobiotics and other aromatic hydrocarbon pollutants in the estuarine and mangrove ecosystems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors wish to thank the financial support provided by the Student Fellowship program for Doctoral studies by Goa University, Prof. Indrani Karunasagar, Dr. Biswajit Maiti. College of Fisheries Mangalore, and Dr. Dnyananda Khanolkar, Goa University for identification studies.
Abbreviations
- ZMB/ZMA
Zobell marine broth/agar
- CAS
Chrome azurol sulphonate
- HPLC
High pressure liquid chromatography
- EDTA
Ethylene-diamine-tetra-acetic acid
- BLAST
Basic local alignment search tool
References
- 1.Crump BC, Armbrust EV, Baross JA. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia river, its estuary, and the adjacent coastal ocean. Appl Environ Microbiol. 1999;65:3192–3204. doi: 10.1128/aem.65.7.3192-3204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith P, Shiah FK, Gloerson K, Ducklow HW, Fletcher M. Activity and distribution of attached bacteria in Chesapeake Bay. Mar Ecol Prog Ser. 1994;108:1–10. doi: 10.3354/meps108001. [DOI] [Google Scholar]
- 3.Gonsalves MJ, Nair S, LokaBharathi PA, Chandramohan D. Abundance and production of particle-associated bacteria and its implication in a mangrove dominated estuary. Aquat Microbial Ecol. 2009;57:151–159. doi: 10.3354/ame01337. [DOI] [Google Scholar]
- 4.Sandy M, Butler A. Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev. 2009;109:4580–4595. doi: 10.1021/cr9002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilands JB. Siderophores: structure, function of microbial iron transport compounds. J Biochem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 6.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 7.Kharangate-Lad A, Bhosle S. Siderophore producing halophilic and halotolerant bacteria adhered to mangrove plant litter. NeBIO. 2014;5:56–60. [Google Scholar]
- 8.Yeole RD, Dave BP, Dube HC. Siderophore production by fluorescent pseudomonads colonizing roots of certain crop plants. Indian J Exp Biol. 2001;39:464–468. [PubMed] [Google Scholar]
- 9.Norris JR, Ribbons DW. Methods in microbiology. London: Academic Press; 1971. [Google Scholar]
- 10.De Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FAS, Karl-Heinz S, Whitman WB. Bergey’s manual of systematic bacteriology: volume 3: the firmicutes. 2. London: Academic Press; 1986. pp. 164–168. [Google Scholar]
- 11.Winkelmann G. Ecology of siderophores with special reference to the fungi. Biometals. 2007;20:379–392. doi: 10.1007/s10534-006-9076-1. [DOI] [PubMed] [Google Scholar]
- 12.Ali SS, Vidhale NN. Bacterial siderophore and their application: a review. Int Curr Microbiol Appl Sci. 2013;2:303–312. [Google Scholar]
- 13.Calugay RJ, Miyashita H, Okamura Y, Matsunaga T. Siderophore production by the magnetic bacterium Magnetospirillum magneticum AMB-1. FEMS Microbiol Lett. 2003;218:371–375. doi: 10.1016/S0378-1097(02)01188-6. [DOI] [PubMed] [Google Scholar]
- 14.Carson C, Meyer JM, Dilworth MJ. Hydroxamate siderophores of root nodule bacteria. Soil Biol Biochem. 2000;32:11–21. doi: 10.1016/S0038-0717(99)00107-8. [DOI] [Google Scholar]
- 15.Sasidharan P, Raja R, Karthik C, Sharma Ranandkumar, Indra Arulselvi P. Isolation and characterization of yellow pigment producing Exiguobacterium sps. J Biochem Technol. 2013;4:632–635. [Google Scholar]
- 16.Nugraheni SA, Khoeri MM, Kusmita L, Widyastuti Y, Radjasa OK. Characterization of carotenoid pigments from bacterial symbionts of sea grass Thalassia hemprichii. J Coast Dev. 2010;14:51–60. [Google Scholar]
- 17.Eldahshan OA, Singab ANB. Carotenoids. J Pharmacogn Phytochem. 2013;2:225–234. [Google Scholar]
- 18.Godinho A, Bhosle S. Carotenes produced by alkaliphilic orange pigmented strain of Microbacterium arborescens AGSB isolated from coastal sand dunes. Ind J Mar Sci. 2008;37:307–312. [Google Scholar]
- 19.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. [PubMed] [Google Scholar]
- 20.Bhosale P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl Microbiol Biotechnol. 2004;63:351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- 21.Tank N, Rajendran N, Patel B, Saraf M. Evaluation and biochemical characterization of a distinctive pyoverdin from a Pseudomonas isolated from chickpea rhizosphere. Braz J Microbiol. 2012;43:639–648. doi: 10.1590/S1517-83822012000200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma M, Gohil NK. Interaction of azotobactin with blocking and mobilizing agents in NTBI assay. Mol BioSyst. 2010;6:1941–1946. doi: 10.1039/c004840b. [DOI] [PubMed] [Google Scholar]
- 23.Gaonkar T, Nayak PK, Garg S, Bhosle S. Siderophore producing bacteria from a sand dune ecosystem and the effect of sodium benzoate on siderophore production by a potential isolate. Sci World J. 2012;2012:1–8. doi: 10.1100/2012/857249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer JM. Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol. 2000;174:135–142. doi: 10.1007/s002030000188. [DOI] [PubMed] [Google Scholar]
- 25.Gaonkar T, Bhosle S. Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere. 2013;93:1835–1843. doi: 10.1016/j.chemosphere.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 26.De Souza T, Bhosle S. Implications of benzoate induced alterations in cell morphology and physiology in Pseudomonas aeruginosa TMR.13 for potential application in bioremediation and monitoring approaches. J Bioremed Biodegrad. 2012 [Google Scholar]
- 27.Marquez MC, Sanchez-Porro C, Ventosa A (2011) Halophilic and haloalkaliphilic, aerobic endospore-forming bacteria in Soil. In: Logan NA, De Vos P (eds) Endospore-forming soil bacteria. Soil biology, vol 27. Springer, Berlin, pp 331. doi:10.1007/978-3-642-19577-8
- 28.Erdoğmuş SF, Mutlu B, Korcan SE, Güven K, Konuk M. Aromatic hydrocarbon degradation by halophilic archaea isolated from Çamalt Saltern Turkey. Water Air Soil Pollut. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.