Abstract
Superoxide dismutase (SOD) and catalase are considered the most important antioxidant enzymes which protect fungus from the oxidant damage of reactive oxygen species. In this study, we collected 44 strains of Trichosporon asahii (T. asahii) from different sources and investigated their SOD and catalase activities. The results showed that the SOD and catalase activities of Clinical group were significantly higher than those of Environment group (p < 0.01). The SOD and catalase activities of T. asahii in Internal passage group went up gradually after passage in mice, and were significantly higher in 5th generation of Internal passage group (p < 0.05). The SOD and catalase activities of Fluconazole-resistant group strains also increased after resistant induction, and the SOD and catalase activities were significantly higher in the 10th generation of Fluconazole-resistant group (p < 0.05). This implied that T. asahii has stronger antioxidant ability. The strains of T. asahii from different sources have different antioxidant abilities, which mainly manifest in the difference of antioxidant enzymatic activities. Clinical group strains have the strongest antioxidant capacity; Internal passage group strains and Fluconazole resistant group strains better; Environmental group strains the lowest. These results also suggested that the antioxidant defensive response of T. asahii might be relevant to its infection mechanism and drug resistance mechanism.
Keywords: Trichosporon asahii, Catalase, Superoxide dismutase, Reactive oxygen species
Introduction
Reactive oxygen species (ROS) mainly include hydrogen peroxide (H2O2), hydroxyl radical (·OH), superoxide anion (O·−2) and so on [1]. Given that they could react with nucleic acids, lipid and proteins, ROS were recognized that they may nearly destroy many cell functions of organisms [1]. Many studies (including bacterium and fungus) have demonstrated that ROS could be produced by phagocytes [1] and antifungal drugs [2] during their killing process. For organisms, they have taken shape a series of ways to escape or repair these oxidant damage to survive from the deleterious ROS [1]. Among them, the antioxidant enzymes [particularly catalase, superoxide dismutase (SOD), peroxidase, glutathione systems] were well studied and related to the virulence or pathogenicity of fungus directly or indirectly [3–9].
Previous studies have shown that SOD and catalase were the most important antioxidant enzymes that work to diminish the oxidant damage of ROS [3–9]. The two antioxidant enzymes have been proved extensively in the studies of common fungus including Candida albicans (C. albicans), Aspergillus fumigatus (A. fumigatus), Cryptococcus neoformans (C. neoformans). Hwang et al. [3] and Martchenko et al. [4] proved that SOD1 and SOD5 were necessary for the virulence of C. albicans when infecting mice, respectively. The catalase of C. albicans may also enhance its pathogenicity to human neutrophils [5]. Similar to C. albicans, the SOD of A. fumigatus may have some role as a virulence factor when defending against neutrophil and phagocyte [6], whereas its mycelial catalase could only protect this fungus from the host for a while [7]. In C. neoformans, its Cu, ZnSOD helped to protect fungal cells from macrophages, but was not a virulence factor as well as its catalase [8, 9].
In recent years, the incidence of disseminated trichosporonosis caused by Trichosporon asahii increases obviously, accounting for 5–10 % of deep infection of fungus in patients [10]. Moreover, T. asahii is becoming more and more resistant to antifungal drugs [10]. Once T. asahii caused disseminated or systemic infection in the body, its mortality could be more than 80 % [11], which making the therapy become more harder. Therefore, it will be of great significance to explore the infection mechanism and pathogenic mechanism of T. asahii from the perspective of antioxidant defense response. In our previous studies, Zong et al. [12] found that three different oxidants (diamide, H2O2 and menadione) induced different levels of oxidative damage to T. asahii. Among them, the killing effect of menadione was the strongest, diamide better, H2O2 the least [12]. In addition, the higher its concentration was, the stronger its killing effect would be [12]. Therefore, we hypothesize that T. asahii also has basic antioxidant defense system. Since T. asahii could invade the host and cause serious infections [10], we infer that there may be a connection between its pathogenicity and their capacity to resist or evade the attack of host immune system. To test this hypothesis, we collected 44 T.asahii strains from different sources, and investigated their SOD and catalase activities, then compared and analyzed the results. This will contribute to further study of antioxidant mechanism and infection mechanism of T. asahii.
Materials and Methods
Strains and Sources
There were 44 strains of T. asahii collected and they were divided into four groups according to their sources. Environmental group: including 3 strains, CBS8904, CBS7137, CBS8520 (CBS-KNAW, The Netherlands). Clinical group: including 9 strains, CBS2479; BZP07001, BZP07002, BZP07003, BZP07004, BZP09001, BZP09002, BZP07005, BZP07005R (General Hospital of Beijing Military Area Command, PLA). Internal passage groups: including 20 strains CBS2479, CBS8904, CBS7137, CBS8520 and their five generations (CBS2479P1-5, CBS8904P1-5, CBS7137P1-5, CBS8520P1-5). Fluconazole-resistant groups: including 12 strains CBS247, CBS8904 and their selected generations (CBS2479 F6, F9, F10, R7, R8, R9; CBS8904F6, F9, F10, R7, R8, R9). The strains above were confirmed to be AS2.2174 (API 20cAUX bioassay and ITS sequence analysis). The Candida parapsilosis ATCC22019 (Peking University First Hospital, China) was used as a control strain.
Induction of Internal Passage Strains
Strains CBS2479, CBS8904, CBS7137, CBS8520 were selected to passage in mice and extended from 1 to 5 generations in turn [13]. Then isolated from kidney tissue and cultured on potato dextrose agar (PDA, Merck KGaA, Germany).
Induction of Fluconazole-Resistant Strains
The strains CBS2479, CBS8904 were cultured on PDA that contained fluconazole with stepwise increasing concentrations to induce highly resistant strains. The resistant strains CBS2479R, CBS8904R [14] were then cultured on fluconazole-free PDA and passaged in turn to obtain the reply strains. We selected the mid and late stage of the induction strains.
Fungal Cells and Enzyme Extracts Preparation
The preparation was employed based on the assay of Linares et al. [15]. The strains were cultured on PDA for 48 h at 35 °C. 1 ml volume of each cell suspension (contained about 1.5 × 108 cfu/ml T. asahii and 0.9 % sterile saline) was transferred to 50 ml Sabouraud Glucose Broth (SDB), and incubated (37 °C, 150 r/min, 48 h). After centrifugation, the cells were washed by sterile water for 3 times, then the cell mat was prepared. They were resuspended by potassium phosphate buffer (PH 7.0, 50 mmol/l), and added 0.5 g glass beads (Sigma, USA). Before the centrifugation, the suspensions above were shaking strongly for 6 times, then the supernatant was collected for the enzymatic assays [15].
Enzymatic Assays
Total SOD activity assay kit, catalase activity assay kit and total protein quantitative assay kit (Biological engineering of Nanjing Jian Cheng, China) were employed to determine SOD and catalase activities following the instructions provided by the manufacture. Repeated three times.
Statistics
SPSS 13.0 statistical software was employed for calculations.
Results
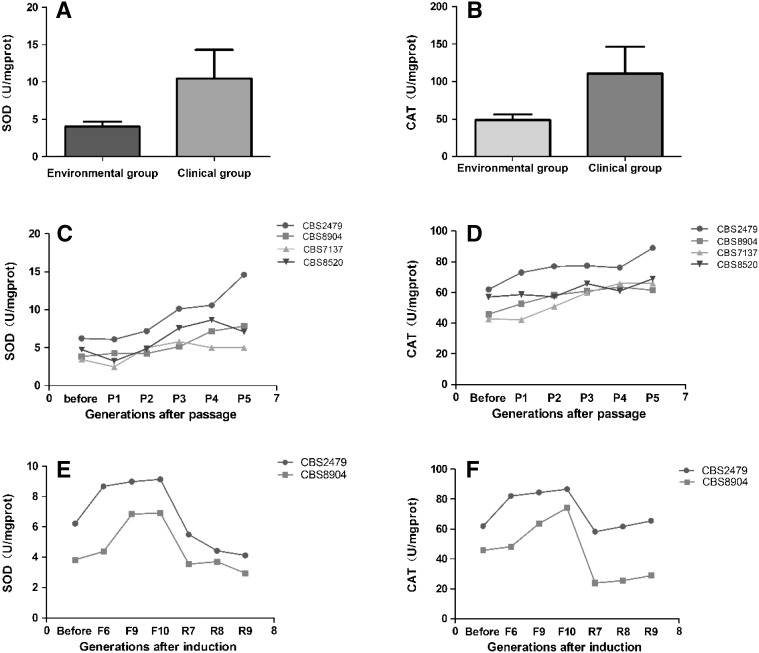
In this study, the SOD activity of the C. parapsilosis ATCC22019 was 41.289 U/mg protein (U/mg), its catalase activity was 4.208 U/mg. Table 1 showed SOD activity of Environmental group of T. asahii CBS8904, CBS7137, CBS8520 were 3.828, 3.46, 4.75 U/mg, the average was 4.01 ± 0.66 U/mg; their catalase activity were 45.833, 42.61, 57.082 U/mg, and the average was 48.51 ± 7.60 U/mg. The SOD activity of Clinical group strains ranged from 6.221 to 16.826 U/mg, the mean value was 10.45 ± 3.87 U/mg, the catalase activity ranged from 61.956 to 164.552 U/mg, the average was 110.56 ± 35.77 U/mg.
Table 1.
Antioxidant enzymatic activities of Environmental group and Clinical group of T. asahii
| Groups | Strains | Sources | SOD (U/mg prot) | Catalase (U/mg prot) |
|---|---|---|---|---|
| Environmental group | CBS 8904 | Corn | 3.828 | 45.833 |
| CBS 7137 | Soil | 3.460 | 42.610 | |
| CBS 8520 | Corruption leaves | 4.750 | 57.082 | |
| Clinical group | CBS 2479 | Nail | 6.221 | 61.956 |
| BZP07001 | Skin | 6.226 | 85.250 | |
| BZP07002 | Sputum | 16.112 | 145.071 | |
| BZP07003 | Sputum | 16.826 | 164.552 | |
| BZP07004 | Urine | 8.804 | 78.330 | |
| BZP07005 | Liver | 9.598 | 81.052 | |
| BZP07005R | Skin | 8.152 | 112.140 | |
| BZP09001 | Sputum | 11.988 | 123.986 | |
| BZP09002 | Sputum | 10.164 | 142.740 |
The data are the mean of triplicate experiments. U: unit of SOD or catalase. The SOD and catalase activities of Clinical group were significantly higher than those of Environment group (p < 0.01)
In Table 2, the two antioxidant enzymatic activities of strains CBS2479, CBS8904, CBS7137, CBS8520 gradually increased after continuous passage in mice for 5 generations. Compared with each parental strain, the SOD activity of descendants of the strain CBS2479 was 0.98-, 1.16-, 1.63-, 1.70-, 2.35-fold higher, and its catalase activity was 1.18-, 1.24-, 1.25-, 1.23-, 1.44-times greater. Until the 5th generation, the SOD activity of other 3 environmental strains CBS8904, CBS7137, CBS8520 were 2.05-, 1.45-, 1.5-fold greater than their parental strain, while their catalase activities were 1.34-, 1.55-, 1.21-times higher.
Table 2.
Antioxidant enzymatic activities of Internal passage group of Trichosporon asahii
| Enzyme (U/mg prot) | Strains | Before passage | Generations | ||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | |||
| SOD | CBS2479 | 6.221 | 6.100 | 7.194 | 10.132 | 10.598 | 14.610 |
| CBS8904 | 3.828 | 4.288 | 4.240 | 5.164 | 7.182 | 7.854 | |
| CBS7137 | 3.460 | 2.490 | 4.970 | 5.833 | 5.023 | 5.016 | |
| CBS8520 | 4.750 | 3.265 | 4.857 | 7.571 | 8.660 | 7.115 | |
| Catalase | CBS2479 | 61.956 | 72.992 | 77.034 | 77.431 | 76.186 | 89.045 |
| CBS8904 | 45.833 | 52.702 | 58.355 | 60.830 | 63.433 | 61.418 | |
| CBS7137 | 42.610 | 42.270 | 50.916 | 59.775 | 65.858 | 66.025 | |
| CBS8520 | 57.082 | 58.664 | 57.196 | 65.749 | 60.964 | 68.964 | |
The data are the mean of triplicate experiments. U: unit of SOD or catalase. The SOD and catalase activities of Internal passage group strains went up gradually after passage in mice, and was significantly higher in 5th generation of Internal passage group (p < 0.05)
In Table 3, the antioxidant enzymatic activity of T. asahii CBS2479, CBS8904 went up with the increasing of generation induced by Fluconazole. Contrast to each parental strain, the SOD activity of CBS2479 that induced the 6th, 9th, 10th generations were 1.40-, 1.44-, 1.47-fold superior than before, their CAT activity were also 1.32-, 1.36-, 1.40-fold higher. However, the SOD activities of strains CBS2479 and CBS8904 gradually returned to normal levels in the late stage of reply induction as well as catalase.
Table 3.
Antioxidant enzymatic activities of Fluconazole-resistant group of T. asahii
| Enzyme (U/mg prot) | Strains | Before induction | Generations | |||||
|---|---|---|---|---|---|---|---|---|
| F6 | F9 | F10 | R7 | R8 | R9 | |||
| SOD | CBS2479 | 6.221 | 8.683 | 8.978 | 9.126 | 5.499 | 4.432 | 4.121 |
| CBS8904 | 3.828 | 4.378 | 6.845 | 6.921 | 3.543 | 3.704 | 2.937 | |
| CAT | CBS2479 | 61.956 | 82.085 | 84.469 | 86.738 | 58.190 | 61.598 | 65.448 |
| CBS8904 | 45.833 | 48.160 | 63.595 | 74.128 | 23.997 | 25.562 | 28.844 | |
The data are the mean of triplicate experiments. U: unit of SOD or catalase. The SOD and catalase activities of Fluconazole-resistant group strains increased after resistant induction, and were significantly higher in the 10th generation of Fluconazole-resistant group (p < 0.05). The antioxidant enzymatic activity of strains after reply induction gradually returned to normal levels
Discussion
In this study, we observed that both of enzymatic activities (SOD and catalase) in Clinical group of T. asahii were significantly higher (p < 0.01) than those of Environment group (Table 1; Fig. 1a, b). The mean SOD activity of the clinical group strains was 10.45 ± 3.87 U/mg, and its mean catalase activity was 110.56 ± 35.77 U/mg. The average SOD activity of Environmental group was 4.01 ± 0.66 U/mg, and its average catalase activity was 48.51 ± 7.60 U/mg. In contrast to Environment group, the SOD activity of Clinical group was about 2.6 times higher, while its catalase activity was about 2.3 times greater. Besides, the antioxidant enzymatic activities of these isolated strains from different clinical sources also manifested certain differences. Given that different strains had different antioxidant capacity, mainly reflected in the activities of antioxidant enzymes, we suggested that the T. asahii clinical group stains may have higher antioxidant capacity than the environment group strains. According to previous studies, we also obtained that the mean SOD activity of Clinical group strains of T. asahii (10.45 ± 3.87 U/mg) was equivalent to that of Cryptococcus (12 ± 0.5 U/mg) [16], and below that of Candida dubliniensis (27.87 ± 20.82 U/mg) [15], significantly lower than that of C. albicans (151.8 ± 73.27 U/mg) [15]. The mean catalase activity of T. asahi Clinical group strains (48.51 ± 7.60 U/mg) was significantly higher than that of A. fumigatus (1.75 ± 0.75 U/mg), Aspergillus terreus (3.5 ± 0.8 U/mg) [17]. Hence, we hypothesized that the antioxidant ability of T. asahii clinical group strains may be close to Cryptococcus, but lower than that of Candida, significantly higher than that of Aspergillus.
Fig. 1.
Antioxidant enzyme SOD (a, c, e) and catalase (b, d, f) activities of different sources of T. asahii. a, b The SOD and catalase activities of Clinical group were significantly higher than those of Environment group (p < 0.01). c, d The SOD and catalase activities of Internal passage group strains went up gradually after passage in mice, and were significantly higher in 5th generation of Internal passage group (p < 0.05). e, f The SOD and catalase activities of Fluconazole-resistant group strains increased after resistant induction, and were significantly higher in the 10th generation of Fluconazole-resistant group (p < 0.05). The antioxidant enzymatic activities of strains after reply induction gradually returned to normal levels
These data also demonstrated that the two enzymatic activities in the Internal passage group of strains (CBS2479, CBS8904, CBS7137, CBS8520 and their generations) were obviously promoted (p < 0.05) after passage (Table 2, Fig. 1c, d). Prior to passage, the mean SOD activity of strains was 4.56 ± 1.23 U/mg, the mean catalase activity was 51.87 ± 9.15 U/mg. To extend the 5th generation, the SOD activity increased to 8.65 ± 4.15 U/mg, and the catalase activity risen to 71.36 ± 12.19 U/mg, increasing 90 and 34 %. In addition, the mean catalase activity of the Internal passage group was higher than that of the Environment group (p < 0.05). These data suggested that once T. asahii invaded the host, its antioxidant defensive mechanism may be stimulated or induced through the interaction with the host immune systems, finally achieved that the antioxidant enzyme activities raised to different extent together with increase of its oxidation resistance [1]. Many studies have demonstrated that SOD and catalase played an important protective role in clearing up or cutting off the production of ROS from macrophages or neutrophils [3–9]. In A. fumigatus, Diamond and Clark [18] found that the catalase could protect hyphae from myeloperoxidase system of neutrophils. So, we speculated that the antioxidant enzymes of T. asahii played a certain role in protecting strains from oxidative damage of host immune systems, even may contribute to rescuing from the attack of host immune systems, further leading to the formation of infection as well.
At the same time, we proved that the two enzyme activities of CBS2479 and CBS8904 went up with the increasing of generation induced by Fluconazole (Table 3; Fig. 1e, f). Before drug-treatment, the mean SOD activity was 5.02 ± 1.69 U/mg, and the mean catalase activity was 53.89 ± 11.40 U/mg. Until the 10th generation of Fluconazole-resistant induction, its SOD activity added to 8.02 ± 1.56 U/mg, and the catalase activity risen to 80.43 ± 8.92 U/mg, which increasing by 60, 49 % (p < 0.05). Furthermore, the two enzymatic activities of the 10th generation strains in Fluconazole-resistant group were higher than those of Environmental group (p < 0.05). For reply induction strains, their antioxidant enzymatic activities gradually returned to normal levels. At the 9th generation of reply induction, the mean SOD activity of strains was 3.53 ± 0.84 U/mg, the mean catalase activity was 47.15 ± 25.88 U/mg. These results implied that fluconazole may simulate or induce the expression of antioxidant enzymatic activity of T. asahii, leaving the antioxidant capacity of these strains further promoted [15]. Because that the activities of SOD and catalase in T. asahii could go down to normal level without fluconazole exposure, we suggested that the induction of antioxidant enzymatic activity may be regulatory [1]. There were other antifungal agents that also could induce antioxidant stress response except fluconazole and amphotericin B [15]. Hoehamer et al. [2] confirmed that the expression of the proteins involved in antioxidant mechanism elevated after the exposure of the azole (ketoconazole) and echinocandin (caspofungin). Blum et al. [17] pointed out that Aspergillus terreus who had inherent resistance to amphotericin B was closely related with its higher catalase activity and had no obvious correlation to its cell wall components and lipid peroxidation level when compared with the A. fumigatus. Consequently, we inferred that fluconazole might also cause a certain degree of oxidative damage to T. asahii during the killing process. Meanwhile, the induced antioxidant enzymes of T. asahii may be one of fluconazole-resistance mechanism, which required to further research.
Moreover, our results also suggested that there was some relationship between the two enzymes SOD and catalase in T. asahii after internal passage and fluconazole resistance induction that they had the same variation tendency, which is in accordance with Linares et al. [15].
In conclusion, this investigation proved that T. asahii has stronger antioxidant defensive mechanism as well as most of other fungus [3–9]. The antioxidant capacity of T. asahii differed according to their sources, mainly reflected in the activities of antioxidant enzymes. Among them, Clinical group strains have the strongest antioxidant capacity; Internal passage group strains and Fluconazole-resistant group strains better; Environmental group strains the lowest. Furthermore, the antioxidant enzymes (SOD and catalase) in T. asahii could be activated or induced by the exposure of host defensive system and fluconazole. The antioxidant defensive response of T. asahii might be relevant to its infection mechanism and drug-resistance mechanism. However, this aspect still needed to further explore.
Acknowledgments
This research was supported by Project No. BWS11J059 of Army “Twelve Five” of China. Special thanks also should go to Xiufeng Han and Zhaoxia Guo who have put considerable time and effort into the supply of strains in the Internal passage group and Fluconazole-resistant group.
Compliance with Ethical Standards
This article does not contain any studies with human participants performed by any of the authors. All animal experiments were performed and supervised with the approval of the local ethical committee and all the experiments were performed according to the National (China) Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
There are no conflicts of interest for this article.
Footnotes
Yangmei Zhang and Haitao Li have contributed equally to this work.
References
- 1.Temple MD, Perrone GG, Dawes IW. Complex cellular responses to reactive oxygen species. Trends Cell Biol. 2005;15:319–326. doi: 10.1016/j.tcb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Hoehamer CF, Cummings ED, Hilliard GM, Rogers PD. Changes in the proteome of Candida albicans in response to azole, polyene, and echinocandin antifungal agents. Antimicrob Agents Chemother. 2010;54:1655–1664. doi: 10.1128/AAC.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology. 2002;148:3705–3713. doi: 10.1099/00221287-148-11-3705. [DOI] [PubMed] [Google Scholar]
- 4.Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15:456–467. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysong DR, Christin L, Sugar AM, Robbins PW, Diamond RD. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect Immun. 1998;66:1953–1961. doi: 10.1128/iai.66.5.1953-1961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holdom MD, Hay RJ, Hamilton AJ. Purification, N-terminal amino acid sequence and partial characterization of a Cu, Zn superoxide dismutase from the pathogenic fungus Aspergillus fumigatus. Free Radic Res. 1995;22:519–531. doi: 10.3109/10715769509150324. [DOI] [PubMed] [Google Scholar]
- 7.Paris S, Wysong D, Debeaupuis JP, Shibuya K, Philippe B, Diamond RD, Latgé JP. Catalases of Aspergillus fumigatus. Infect Immun. 2003;71:3551–3562. doi: 10.1128/IAI.71.6.3551-3562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giles SS, Stajich JE, Nichols C, Gerrald QD, Alspaugh JA, Dietrich F, Perfect JR. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot Cell. 2006;5:1447–1459. doi: 10.1128/EC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushima H, Tokimatsu I, Ishii H, Kawano R, Shirai R, Kishi K, Hiramatsu K, Kadota J. Cloning of the lanosterol 14-α-demethylase (ERG11) gene in Trichosporon asahii: a possible association between G453R amino acid substitution and azole resistance in T. asahii. FEMS Yeast Res. 2012;12:662–667. doi: 10.1111/j.1567-1364.2012.00816.x. [DOI] [PubMed] [Google Scholar]
- 11.Gross JW, Kan VL. Trichosporon asahii infection in an advanced AIDS patient and literature review. AIDS. 2008;22:793–795. doi: 10.1097/QAD.0b013e3282f51ecc. [DOI] [PubMed] [Google Scholar]
- 12.Zong LN, Li HT, Yong RY, Ao JH, Wang WL, Zhu H, Cong L, Wang CM. Experimental study on oxidant sensitivity of Trichosporon asahii. J Pract Dermatol. 2012;5:65–70. [Google Scholar]
- 13.Han XF, Li HT, Yang RY, Zhang YM, Tian YL, ZhouJF YD. The change of morphology and antifungal susceptibility in Trichosporon asahii by in vivo passage and in vivo induction. J Pract Dermatol. 2015;8:6–10. [Google Scholar]
- 14.Guo ZX, Li HT, Yang RY, Zhu H, Wang CM, Liao Y, Xia ZK. In vitro induction and stability evaluation of fluconazole resistance in Trichosporon asahii. Chin J Dermatol. 2013;46:41–44. [Google Scholar]
- 15.Linares CE, Giacomelli SR, Altenhofen D, Alves SH, Morsch VM, Schetinger MR. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev Soc Bras Med Trop. 2013;46:752–758. doi: 10.1590/0037-8682-0190-2013. [DOI] [PubMed] [Google Scholar]
- 16.Dias AL, Brigagão MR, Colepicolo P, Siqueira AM, Silva EG, Paula CR. Superoxide dismutase in Cryptococcus neoformans varieties gattii, grubi, and neoformans. Mem Inst Oswaldo Cruz. 2006;101:107–109. doi: 10.1590/S0074-02762006000100021. [DOI] [PubMed] [Google Scholar]
- 17.Blum G, Perkhofer S, Haas H, Schrettl M, Würzner R, Dierich MP, Lass-Flörl C. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother. 2008;52:1553–1555. doi: 10.1128/AAC.01280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond RD, Clark RA. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982;38:487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]