Abstract
Star fruit (Averrhoa carambola) is a nutritious tropical fruit. The aim of this study was to evaluate the production of a star fruit alcoholic fermented beverage utilizing a lyophilized commercial yeast (Saccharomyces cerevisiae). The study was conducted utilizing a 23 central composite design and the best conditions for the production were: initial soluble solids between 23.8 and 25 °Brix (g 100 g−1), initial pH between 4.8 and 5.0 and initial concentration of yeast between 1.6 and 2.5 g L−1. These conditions yielded a fermented drink with an alcohol content of 11.15 °GL (L 100 L−1), pH of 4.13–4.22, final yeast concentration of 89 g L−1 and fermented yield from 82 to 94 %. The fermented drink also presented low levels of total and volatile acidities.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-016-0601-9) contains supplementary material, which is available to authorized users.
Keywords: Star fruit, Fruit wine, Factorial design, Saccharomyces cerevisiae
Introduction
The alcoholic fermentation of fruit can be used for the production of alcoholic drinks and it is commonly realized by yeast such as the Saccharomyces cerevisiae [1, 2]. In such process occurs the production of ethanol and carbon dioxide, which are obtained by the anaerobic conversion of the sugars naturally contained in the fruit or added to it. According to the Brazilian legislation [3], wine is a drink with alcohol content from 4 to 14 °GL (L 100 L−1) at 20 °C, produced from the alcoholic fermentation of healthy, ripe and fresh grapes. The term “fruit wine” is applied to alcoholic fermented drinks produced from fruits other than grapes. Any fruit with reasonable amounts of fermentable sugars can be utilized as must for the production of wine. The usage of different fruits may lead to the obtainment of drinks with different flavors. Therefore, many exotic fruits have been utilized in the production of wine.
Carambola or star fruit (Averrhoa carambola) is a tropical fruit originally from Indonesia and India, being very popular in South-eastern Asia, South Pacific and some regions of Eastern Asia. The carambola tree is grown in other countries out from Asia, such as Colombia, Guiana, Dominican Republic, Brazil and the USA [4]. The star fruit chemical characteristics depend on climatic factors, the type of soil utilized for cultivation, the fruit ripeness level, etc. Almeida et al. [5] characterized ripe star fruit from north-eastern Brazil and obtained average values of soluble solids and pH of 8.0 °Brix and 3.7, respectively. Star fruit is rich in vitamins, oxalic acid, polyphenols, dietary fiber, volatile compounds, etc. Such traits allow innumerable usages for the fruit, as well as providing benefits for the health of the consumers [6, 7].
The aim of this study was to evaluate the production of a star fruit alcoholic fermented drink incorporating the fruit traits in the wine. The effects of the initial concentration of yeast (S. cerevisiae), initial pH and initial sugar concentration were evaluated utilizing a 23 central composite design whereby the fermentation kinetics data and the physico-chemical characteristics of the product were analyzed.
Materials and Methods
Preparation of the Star Fruit Pulp and Must
For the star fruit pulp preparation was selected ripe fruits with good appearance, smell and texture. They were bought at a local supermarket in the region of Campinas-SP, Brazil, in March 2014. The star fruits were cleaned, cut and blitzed in a blender until a pulp consistency was achieved. Thereafter, the pulp was filtered with be means of a cotton cloth, in order to remove the insoluble solids and then pasteurized at 80 °C for 5 min. The pasteurized pulp was transferred to plastic flasks which were closed and left cooling to room temperature and then frozen and stored at −18 °C to the moment they were utilized on the must preparation.
In order to prepare the must for the fermentation, the star fruit pulp was thawed at room temperature (~25 °C) and mixed with saccharose to adjust the concentration of soluble solids indicated on Table 1 (varying between 19 and 25 °Brix). Thereafter, calcium carbonate was added to each test to adjust the pH, also regarding the values indicated on Table 1 (ranging between 4.0 and 5.0).
Table 1.
Kinetic parameters for star fruit alcoholic fermentation at room temperature after 9 days of fermentation
| Runs | SS (°Brix) | pH | Yeast (g L−1) | Alcohol (°GL) | Final pH | Yield (%v/v) | Final yeast (g L−1) |
|---|---|---|---|---|---|---|---|
| 1 | 20.2 (−1) | 4.2 (−1) | 1.6 (−1) | 9.06 | 3.74 | 85.47 | 83.94 |
| 2 | 23.8 (+1) | 4.2 (−1) | 1.6 (−1) | 9.76 | 3.77 | 85.00 | 54.58 |
| 3 | 20.2 (−1) | 4.8 (+1) | 1.6 (−1) | 8.36 | 4.23 | 90.00 | 65.22 |
| 4 | 23.8 (+1) | 4.8 (+1) | 1.6 (−1) | 11.15 | 4.22 | 94.00 | 89.66 |
| 5 | 20.2 (−1) | 4.2 (−1) | 3.4 (+1) | 8.36 | 3.82 | 85.50 | 88.14 |
| 6 | 23.8 (+1) | 4.2 (−1) | 3.4 (+1) | 8.36 | 3.87 | 90.10 | 74.54 |
| 7 | 20.2 (−1) | 4.8 (+1) | 3.4 (+1) | 9.06 | 4.24 | 91.50 | 63.85 |
| 8 | 23.8 (+1) | 4.8 (+1) | 3.4 (+1) | 10.45 | 4.21 | 90.80 | 70.74 |
| 9 | 19.0 (−1.68) | 4.5 (0) | 2.5 (0) | 9.06 | 4.11 | 78.60 | 91.73 |
| 10 | 25.0 (+1.68) | 4.5 (0) | 2.5 (0) | 11.15 | 4.13 | 81.74 | 89.84 |
| 11 | 22.0 (0) | 4.0 (−1.68) | 2.5 (0) | 9.76 | 3.94 | 83.33 | 131.81 |
| 12 | 22.0 (0) | 5.0 (+1.68) | 2.5 (0) | 10.45 | 4.19 | 75.76 | 167.08 |
| 13 | 22.0 (0) | 4.5 (0) | 1.0 (−1.68) | 8.36 | 4.05 | 83.33 | 144.86 |
| 14 | 22.0 (0) | 4.5 (0) | 4.0 (+1.68) | 9.76 | 4.00 | 82.69 | 121.88 |
| 15 | 22.0 (0) | 4.5 (0) | 2.5 (0) | 9.76 | 4.35 | 87.78 | 190.80 |
| 16 | 22.0 (0) | 4.5 (0) | 2.5 (0) | 10.45 | 4.00 | 75.30 | 213.07 |
| 17 | 22.0 (0) | 4.5 (0) | 2.5 (0) | 9.76 | 4.10 | 77.69 | 190.89 |
Central composite rotatable design 23 + 6 star points +3 central points for the star fruit alcoholic fermentation by S. cerevisiae after 9 days at room temperature (~25 °C) without agitation. Independent variables are: the soluble solid concentration (S) (°Brix = g 100 g−1), initial pH and initial yeast concentration (Yeast) (gL−1). Response variables are: the final alcohol content (Alcohol) (°GL = L 100 L−1), final pH, volumetric fermentation yield (Yield) (% v/v) and final yeast concentration (Final Yeast) (g L−1)
Codified values are presented in parenthesis
Alcoholic Fermentation
The must obtained was then utilized for the alcoholic fermentation. A lyophilized commercial yeast S. cerevisiae (Fermentais Lessaffe Group®) was utilized. The lyophilized yeast was rehydrated with a small portion of must and thereafter added to the total must volume (1.0 L). All fermentations were conducted at room temperature (~25 °C), without agitation in glass flasks covered with plastic film, in which small orifices were made to facilitate the elimination of the carbon dioxide produced during the fermentation process. Each fermentation lasted 9 days and the soluble solids consumption of the must, as well as its pH, were measured daily. By the end of the fermentation, the alcoholic content and the yeast concentration were also measured. The total, fixed and volatile acidities were also analyzed for each wine obtained.
Factorial Design
The study of the star fruit alcoholic fermentation by S. cerevisiae was conducted utilizing a central composite rotatable design with 23 factorial points +6 star points +3 central points, totaling 17 tests [8] with independent variables: soluble solids concentration (SS) (19 a 25 °Brix), initial pH (4.0–5.0) and initial yeast concentration (IY) (1.0 a 4.0 g L). The effects of these three variables on the fermentation process responses (alcoholic content (AC), final pH, volumetric fermented yield (VFY) and final yeast concentration (FY)) as well as on the final product (total, fixed and volatile acidities) were evaluated. The results obtained were analyzed by means of the software Statistica 8.0 (Statsoft) and the factorial design matrix is shown on Table 1.
Analytical Methods
The pH was measured directly with a bench pHmeter (Bel Engineering®, W3B model). The soluble solids (S) (°Brix = g 100 g−1) were determined with a portable refractometer (Instrutemp®, model ITREF 25). From the S values, and knowing the stoichiometry ratio of sugar consumption/ethanol production (1: 4 mol of saccharose per mol of ethanol) it was possible to estimate the alcohol content as the function of the fermentation time. At the end of each fermentation a sample was collected to measure the alcohol content (°GL = L 100 L−1) in an Alcolyzer equipment (Anton Paar®). The conversion factors: fermentable sugars into biomass (YX/S = dX/−dS), fermentable sugar into alcohol (YP/S = dP/−dS) and biomass into alcohol (YP/X = dP/dX) were also calculated considering (−dS: g L−1) the fermentable sugar consumption, (dX: g L−1) the cell growth, and (dP: g L−1) the alcohol production. The fixed and total acidities (meq L−1) were measured using titrimetric methods according to Adolfo Lutz Institute [9]; the volatile acidity was calculated by the difference between total and fixed acidities.
Results and Discussion
The responses obtained from the central composite rotatable design applied for the production of star fruit alcoholic fermented by S. cerevisiae can be observed in Table 1. From these results, the analysis of variance (ANOVA) (Online Resource 1) was performed for each response and second order models were evaluated to explain the process.
Considering the ANOVA results for the alcohol content (Online Resource 1), it was possible to obtain a significant and predictive codified model with 94 % confidence (p = 0.06), represented by Eq. 1, where OH is the alcohol content (°GL), S is the initial soluble solids concentration (°Brix), P is the initial pH and Y is the initial yeast concentration (g L−1). The resulting surface responses and contour lines can be observed on the Online Resource 2. The analysis of this figure shows that the best results for the AC, after 9 days of fermentation, were obtained from the highest soluble solids concentrations, the highest pH and the initial yeast concentration levels between −1 (1.6 g L−1) and +1 (3.4 g L−1). These conditions, predicted by the model, can be verified in Table 1, wherein the highest alcohol content were obtained in trials 4, 8, 10 and 12, which resulted in values from 10.45 to 11.15 °GL. The results obtained in this study were better than others cited in the literature, which also used star fruit as a substrate for alcoholic fermentation. Napahde et al. [10] obtained only 0.2 °GL of alcohol after 21 days of fermentation, while Sibounnavong et al. [11] obtained 8.3 °GL (average) after 2 weeks. In other study, Bridgebassie and Badrie obtained a similar alcohol content (from 10.25 to 11.50 °GL) after 4 weeks of fermentation using star fruit must pretreated with different concentrations of pectolase and using different yeast strains [12].
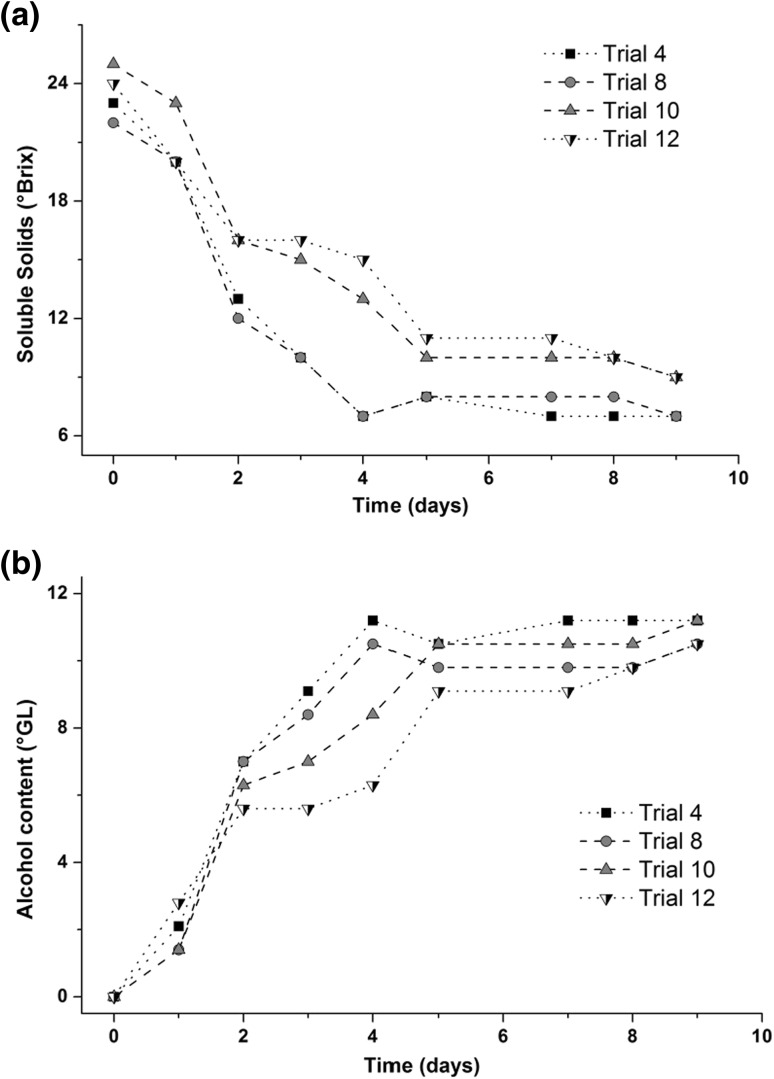
The best conditions (trials 4, 8, 10 and 12) were selected for the analysis of their consumption of soluble solids and the ethanol production during the fermentation time. The obtained kinetic profiles can be seen in Fig. 1. The consumption of soluble solids and the ethanol production showed similar profiles for the selected assays, indicating no significant difference among the chosen conditions. It was also possible to see that after 5 days of fermentation there was a trend for stabilization (Fig. 1), with low sugar consumption and little ethanol production.
| 1 |
Fig. 1.
a Soluble solids (°Brix = g 100 g−1) consumption and b production of alcohol (°GL = L 100 L−1) in star fruit alcoholic fermentation by S. cerevisiae as a function of the fermentation time. The conditions for the concentration of soluble solids (°Brix)/pH/concentration of yeast (g L−1) were: 23.8/4.8/1.6 (trial 4); 23.8/4.8/3.4 (trial 8); 25.0/4.5/2.5 (trial 10); and 22.0/5.0/2.5 (trial 12). The experiments were conducted at room temperature (~25 °C) without agitation, for 9 days. Lines were used to connect the points and guide the eyes
Regarding to the final pH of the star fruit alcoholic fermented, it was not possible to obtain a predictive model (p < 0.10) to represent the process (Online Resource 1). By observing Table 1, however, it was possible to notice that the average final pH value, considering the 17 trials, was 4.05 and the initial value was 4.50, this reduction is a good indicator for the fermentation process because it indicates that most of the substrate was used for ethanol production and cellular growth and there was little formation of acid. Excessive production of acids may indicate microbial contamination by acetic bacteria, excess oxygen in the fermentation medium or excessive fermentation time. Bridgebassie and Badrie [12] obtained a lower final pH of about 3.1 in star fruit wine, but these authors applied a pretreatment in the must using 1 % of citric acid before the fermentation.
Similarly, for the volumetric fermented yield, the correlation with the independent variables was very low (R2 = 0.24) and thus, none of the studied variables had a significant influence on this response (Online Resource 1). Considering the 17 trials (Table 1), the average volumetric fermented yield was 84.6 ± 5.6 % (L 100 L−1). Volume variations in similar processes are common and can occur due to the consumption of must to produce gas (carbon dioxide) during the fermentation.
Regarding to cell growth, i.e., the final yeast concentration, the ANOVA (Online Resource 1) proved that it was possible to obtain a statistically significant and predictive model at 93 % of confidence (p = 0.07). The complete codified model obtained is represented by Eq. 2 where FY is the final yeast concentration (g L−1), S is the soluble solid concentration (°Brix), P is the initial pH, Y is the initial yeast concentration (g L−1). The resulting surface response and the contour curves can be observed on the Online Resource 3, in which it appears that the final concentration of yeast (FY) was the highest in the conditions close to the central points. However, when it comes to alcoholic fermentation process, the best condition for cell growth is not necessarily the best condition for ethanol production. Reddy and Reddy [13] evaluated the effect of different parameters over the S. cerevisiae growth in mango must and it was observed a great influence of temperature; at 25 and at 30 °C they obtained the highest cell populations within 6 and 8 days of fermentation, respectively, with an ethanol production 70 % smaller at 25 °C than at 30 °C (41.3 g L−1 day−1).
| 2 |
The conversion factors were also calculated for each trial (Online Resource 4). The ANOVA was also obtained (data not presented), but it was not possible to obtain second order models to explain each of the conversion factors with a reasonable level of confidence. The results (Online Resource 4) indicate that the highest values of YX/S and YP/S were obtained in trial 12, however, the highest value of YP/X was obtained in trial 2. It confirms the previously mentioned suggestion that a greater microbial growth does not necessarily result in a greater ethanol production. Confronting the three conversion factors and the two models obtained (for OH and FY), the best conditions for the production of star fruit alcoholic fermented (aiming the highest alcohol content) were those in which conditions where the substrate was better used for conversion into product than cell growth. These conditions correspond to trials 4 and 10 which presented the highest initial concentrations of soluble solids (23.8–25.0 °Brix), the highest initial pH (4.8–5.0) and initial concentrations of yeast in the intermediate level (1.6–2.5 g L−1).
Regarding to the acidity levels (Online Resource 4), the obtained values were also evaluated by ANOVA (data not presented) but none of the three responses there were significantly correlations with the independent variables of the process. This means that under all conditions evaluated the resulting acidity values were very similar to each other, a fact that is in agreement with the analysis for the final pH of the fermentation, which had the same behavior. The composition of the acidity of star fruit alcoholic fermented (Online Resource 4) suggests that most of it is related to the fixed acidity (89 %) and a small part (11 %) to the volatile acidity. This is an excellent result, since excessive volatile acidity may indicate microbial contamination or excess of oxygen in both production and storage of fermented drink. Paul and Sahu [14] obtained star fruit alcoholic fermented with a titratable acidity of around 119 meq L−1 and, despite of all the other similar parameters obtained, this value is almost 2.5 times higher than the average value obtained in our study (48.8 meq L−1).
Brazilian law, for instance, specifies some quality standards for grape wines [3], but it does not specify any standard for wines from other fruits. Comparing with the current legislation, star fruit wine obtained showed an average total acidity of 48.8 ± 7.7 meq L−1 similar to the minimum established by Brazilian law (55 meq L−1); however volatile acidity was much lower, 5.4 ± 2.0 meq L−1, than the maximum established (20 meq L−1) indicating low levels of acetic acid, or low oxidation of ethanol. The volatile and total acidities of jabuticaba (Myrciaria jaboticaba) wines determined by da Silvaet al. [15], were higher than those obtained in our study, with values above 185 and 17 meq L−1, respectively.
Other study conducted for the production of watermelon (Citrullus lanatus var. Lanatus) alcoholic fermented [16] led to similar results to those obtained with star fruit fermented for: pH (4.1), final concentration of SS (6.6 °Brix), alcohol content (10 °GL), fermented yield (94 %) and YP/S (0.67). However the final biomass concentration (20 g L−1) and YX/S (0.14) were lower and the fermentation time (48 h) was reduced comparing to those obtained with star fruit wine. These results indicate that the alcoholic fermentation with star fruit was predominantly anaerobic, with good ethanol production. A wine made from jackfruit (Artocarpus heterophyllus) [17] stored for 11 months presented a higher alcohol content (13 °GL) and a higher total acidity (100 meq L−1), but the volatile acidity was very similar to the star fruit wine (6 meq L−1).
Using a similar initial concentration of yeast (1.65 g L−1), Andrade et al. [18]. had a slightly more acidic wine (pH = 3.51) from strawberry (Fragaria ananassa) and similar alcohol content (9.62 °GL) after 30 days of fermentation, with soluble solids consumption varying from 27 to 9 °Brix. For this product, the authors observed that the consumption of soluble solids were more intense in the first 10 days. Tamarind (Tamarindus indica) and soursop (Annona muricata) were also objects of study [19] for the production of alcoholic drinks, resulting in alcohol levels of 8.1 and 6.3 °GL, respectively. Star fruit was applied by Paul and Sahu [14] and it was obtained an alcoholic fermented drink with similar alcohol content of 12.15 °GL, however these authors obtained higher titrable acidity (0.76 % w/w), lower pH (3.94), lower total soluble solid concentration (4.6 °Brix) and they used an inoculum step which added more time to the process.
Conclusion
At the initial conditions of: 23–25 °Brix; pH = 4.8–5.0 and 1.6–2.5 g L−1 of yeast concentration, it was possible to obtain a fermented drink, from star fruit, with: an alcohol content of 11.15 °GL, pH from 4.13 to 4.22, a final yeast concentration of 89 g L−1, volumetric fermentation yield from 82 to 94 % (v/v), total acidity from 42 to 52 meq L−1, a volatile acidity of 5 meq L−1 and fixed acidities of 37–47 meq L−1. The average conversion factors were: YX/S = 0.79; YP/S = 0.67 and YP/X = 1.02. The fermentation kinetics showed that after 5 days of fermentation (~25 °C), the process reached the final stage. The obtained drink had similar characteristics to drinks made from other fruits mentioned in the literature and the differences among their parameters are basically due to the differences in the processes and in the compositions of raw materials used.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Authors are grateful to the Municipal College Professor Franco Montoro (Mogi Guaçu, São Paulo, Brazil) where all the experiments were conducted and also to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for their financial support.
References
- 1.Nyanga LK, Nout MJR, Smid EJ, Boekhout T, Zwietering MH. Fermentation characteristics of yeasts isolated from traditionally fermented masau (Ziziphus mauritiana) fruits. Int J Food Microbiol. 2013;166:426–432. doi: 10.1016/j.ijfoodmicro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Santo DE, Galego L, Gonçalves T, Quintas C. Yeast diversity in the Mediterranean strawberry tree (Arbutus unedo L.) fruits’ fermentations. Food Res Int. 2012;47:45–50. doi: 10.1016/j.foodres.2012.01.009. [DOI] [Google Scholar]
- 3.Brazilian Ministry of Agriculture, Livestock and Supply (2004) Law n° 10970. Ministério da Agricultura, Pecuária e Abastecimento. Lei n. 10970, de 12 de novembro de 2004 (in Portuguese). http://www.agricultura.gov.br. Accessed on 11 Nov 2015
- 4.Warren O, Sargent SA (2011) Carambola (Averrhoa carambola L.). In: Yahia A (ed) Postharvest Biology and Technology of Tropical and Subtropical Fruits: Açai to Citrus. Woodhead Publishing Series in Food Science, Technology and Nutrition, pp 397–414e. doi:10.1533/9780857092762.397
- 5.Almeida MB, Souza WCO, Barros JRA, Barroso PA, Villar FCR. Physical and chemical characterization of star fruits (Averroa carambola L.) produced in Petrolina, PE, Brazil. Rev Semiárido Visu. 2011;1:116–125. [Google Scholar]
- 6.Chau C-F, Chen C-H, Lin C-Y. Insoluble fiber-rich fractions derived from Averrhoa carambola: hypoglycemic effects determined by in vitro methods. LWT Food Sci Technol. 2004;37:331–335. doi: 10.1016/j.lwt.2003.10.001. [DOI] [Google Scholar]
- 7.Wei S-D, Chen H, Yan T, Lin Y-M, Zhou H-C. Identification of antioxidant components and fatty acid profiles of the leaves and fruits from Averrhoa carambola. LWT Food Sci Technol. 2014;55:278–285. doi: 10.1016/j.lwt.2013.08.013. [DOI] [Google Scholar]
- 8.Rodrigues MI, Iemma AF. Experimental design and optimization. Boca Raton: CRC Press; 2015. [Google Scholar]
- 9.Instituto Adolfo Lutz (2008) Analytical Standards of the Adolfo Lutz Institute. Normas Analíticas do Instituto Adolfo Lutz (in Portuguese), São Paulo: IMESP. Accessed on 11 Nov 2015. http://www.ial.sp.gov.br/
- 10.Napahde S, Durve A, Bharati D, Chandra N. Wine Production from Carambola (Averrhoa carambola) juice using Saccharomyces cerevisiae. Asian J Exp Biol Sci. 2010;1:20–23. [Google Scholar]
- 11.Sibounnavong P, Daungpanya S, Sidtiphanthong S, Keoudone C, Sayavong M. Application of Saccharomyces cerevisiae for wine production from star gooseberry and carambola. Int J Agric Technol. 2010;6:99–105. [Google Scholar]
- 12.Bridgebassie V, Badrie N. Effects of different pectolase concentration and yeast strains on carambola wine quality in Trinidad, West Indies. Fruits. 2004;59:131–140. doi: 10.1051/fruits:2004013. [DOI] [Google Scholar]
- 13.Reddy LVA, Reddy OVS. Effect of fermentation conditions on yeast growth and volatile composition of wine produced from mango (Mangifera indica L.) fruit juice. Food Bioprod Process. 2011;8:487–491. doi: 10.1016/j.fbp.2010.11.007. [DOI] [Google Scholar]
- 14.Paul SK, Sahu JK. Process optimization and quality analysis of Carambola (Averrhoa carambola L.) wine. Int J Food Eng. 2014;10:457–465. [Google Scholar]
- 15.Silva PHA, Faria FC, Tonon B, Mota SJD, Pinto VT. Evaluation of the chemical composition of wine produced from jabuticaba (Myrciaria jabuticaba). Avaliação da composição química de fermentados alcoólicos de jabuticaba (Myrciaria jabuticaba) (in Portuguese) Quím Nova. 2008;31:595–600. doi: 10.1590/S0100-40422008000300025. [DOI] [Google Scholar]
- 16.Fontan RDCI, Veríssimo LAA, Silva WS, Bonomo RCF, Veloso CM. Kinetics of the alcoholic fermentation from the preparation of watermelon wine. Cinética da fermentação alcoólica na elaboração de vinho de melancia (in Portuguese) Boletim Centro Pesq Process Aliment. 2011;29:203–210. [Google Scholar]
- 17.Asquieri ER, Rabelo AMDS, Silva AGDM. Fermented jackfruit: study on its physicochemical and sensorial characteristics. Ciên Tecnol Alim. 2008;28:881–887. doi: 10.1590/S0101-20612008000400018. [DOI] [Google Scholar]
- 18.Andrade MB, Perim GA, Santos TRT, Marques RG. Fermentation and characterization of fermented strawberry. Fermentação alcoólica e caracterização de fermentado de morango (in Portuguese) Biochem Biotechnol Rep. 2013;2:265–268. doi: 10.5433/2316-5200.2013v2n3espp265. [DOI] [Google Scholar]
- 19.Mbaeyi-Nwaoha IE, Ajumobi CN. Production and microbial evaluation of table wine from tamarind (Tamarindus indica) and soursop (Annona muricata) J Food Sci Technol. 2015;52:105–116. doi: 10.1007/s13197-013-0972-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.