Abstract
In order to advance the assisted reproductive technologies used in animals and human beings, it is important to accumulate basic informations about underlying molecular mechanisms that shape the biological processes of reproduction. From within seminal plasma, proteins perform a wide variety of distinct functions that regulate major reproductive events such as fertilization. The ability of such proteins to bind and interact with different antagonistic ions and biomolecules such as polysaccharides, lipids, and other proteins present in the male and female reproductive tract define these capabilities. Over the last two decades, extensive work has been undertaken in an attempt to define the role of seminal plasma proteins, of which, Gelatin binding proteins (GBPs) represent a large family. GBPs comprise of known group of Bovine seminal plasma (BSP) protein family, matrix metallo proteinases (MMP 2 and MMP 9) and fibronectin, which have been widely studied. The presence of a type II repeat is a characteristic feature of GBPs, which is similar in structure to the fibronectin type II domain (fn2), which has ability to bind multiple ligands including gelatin, glycosaminoglycans, choline phospholipids, and lipoproteins. Two fn2 domains are present within the BSP protein family, while, three fn2 domains are found in gelatinases (MMP-2 and MMP9), and ELSPBP1 (Epididymosomes Transfer Epididymal Sperm Binding Protein 1) contains four long fn2 domains. For the most part BSP proteins are exclusively expressed in seminal vesicles although mBSPH1, mBSPH2 and hBSPH1 are all expressed in the epididymis. The expression of gelatinases has been demonstrated in several organs and tissues such as the prostate, testis, epididymis, ovary, human placenta, cervix and endometrial wall. This review intends to bring current updates on the role of GBPs in reproductive physiology to light, which may act as basis for future studies on GBPs.
Keywords: Gelatin binding proteins (GBPs), Bovine seminal plasma (BSP) proteins, MMP-2, MMP-9, Fibronectin type II (fn2) domain, Reproductive physiology
Introduction
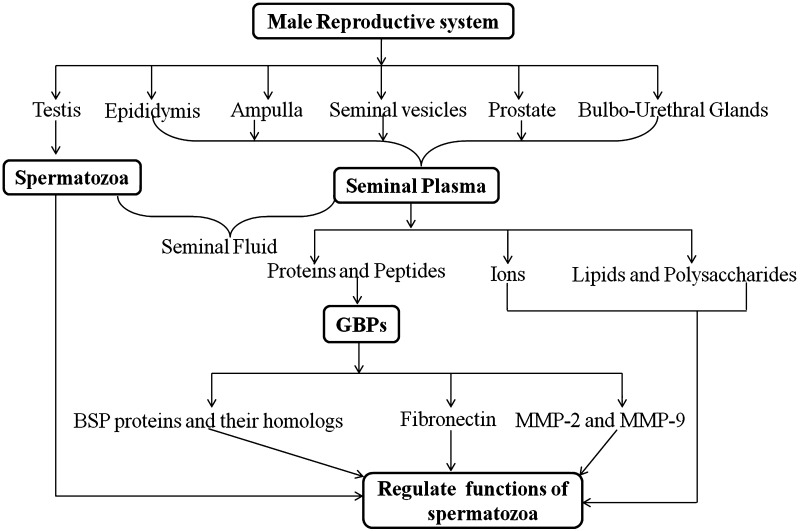
Reproduction is the foundation of life and is essential for maintaining the generation of species on earth. It is known that mammalian reproduction is achieved sexually by internal fertilization involving both male and female gametes. Prior to this event the maturation of sperm and oocyte must be achieved within the respective male and female reproductive systems and involves several established steps and external factors. Spermatozoa develop in the testes, mature in the epididymis and are ejaculated along with seminal fluids, which provide nutrition and protection for sperm deposited in the female reproductive tract. This fluid contains a liquid fraction known as seminal plasma, which is secreted from various accessory sex glands such as the epididymis, seminal vesicles, ampullae, prostate and the bulbourethral glands [1]. Apart from a plethora of minerals, metal ions, sugars, peptides and basic amines, seminal plasma contains more than one thousand proteins, which regulate the different functions of spermatozoa and allows them to achieve fertilization potential [1]. Bicarbonate ions were reported to modulate motility of spermatozoa and destabilize the plasma membrane during capacitation [2], and zinc ions plays role in chromatin stability of sperms [3]. The proteomic study of seminal plasma has detailed a large array of extracellular proteins, proteases and other proteins secreted from the accessory sex glands [4]. However, the structure and functional association of seminal plasma proteins at the molecular level are creating a great deal of medical as well as physiological interest. The sources and components of SP and their specific functions are compiled in the Fig. 1.
Fig. 1.
Schematic representation of male reproductive system and their constituents secretion. The model highlights gelatin binding proteins (GBPs) as an important constituent of seminal plasma, which regulate the sperm functions
Amongst the proteins found in seminal plasma, gelatin binding proteins (GBPs) are a highly conserved group and have been thoroughly explored in various mammal species over the past few decades [5]. In the present review we detail the current available literatures on GBPs in relation to their biological functions, particularly with respect to reproductive physiology.
Gelatin
Gelatin has been characterized as a ligand for proteins present in the seminal fluids of many mammals, which are called GBPs [5]. Although, no known biological function of gelatin and GBPs interactions has been reported so far, gelatin has been used to purify GBPs from different sources such as seminal plasma, seminal vesicles and sperm in different mammals [5]. In best of my knowledge, it is also not clear that whether gelatin is naturally present in seminal plasma or is liberated from collagen. In future, more work is needed to elucidate the exact role of gelatin, and the significance of gelatin and GBP interactions in seminal plasma.
Gelatin is a soluble protein molecule, derived from the denaturation of the collagen type I molecule either by heating or acidic and alkaline treatments. Successive treatment of collagen with heat, breaks hydrogen bonds, destabilizes its triple helix structure, and finally converts it to a coiled structural form of gelatin [6]. Depending upon the pH treatment of collagen, two kinds of gelatin may be produced commercially. Gelatin type A is produced with acidic treatment, having an isoelectric point (pI) of 8.9, while gelatin type B is formed under alkaline conditions and shows a pI of 4.5. The overall commercial value of gelatin is related to its gel strength and thermostabilty, which is mainly dependent on two key points: (1) its species specific composition of amino acids, (2) its molecular weight distribution, established during the processing of collagen [6]. Gelatin is comprised of many glycine amino acids (occurring once every three residues) as well as proline and hydroxyl proline residues. The chemical structure is represented by -Ala-Gly-Pro-Arg-Gly-Glu-4Hyp-Gly-Pro- [6].
Gelatin Binding Proteins (GBPs)
BSP Proteins and their Homologs
Various studies have revealed that BSP proteins can be purified and characterized using gelatin agarose column chromatography from a number of sources such as seminal plasma and seminal vesicles in different mammals including the bull, stallion, goat, buffalo, boar, ram and bison [5, 7]. BSP homologue genes (BSPH4, BSPH5 and BSPH6) have been recognized in the bovine model [7]. In addition, BSP homologous DNA sequences were also characterized in the genomes of other mammals including humans, mice, rabbits, dogs and chimpanzees [8]. In the mouse and in humans, BSP homologs were characterized as mouse BSP homolog 1–3 (mBSPH1, mBSPH2 and mBSPH3) and human BSP homolog 1 (hBSPH1) [7, 9]. The significant difference between the BSP proteins found in other species and those in mice and humans is the site of their expression in accessory sex glands and their concentration found in seminal plasma. BSP homologs are expressed in the epididymis in mice and humans, whilst the site of expression is commonly the seminal vesicles in other mammals [9]. Approximately 60 % of seminal plasma proteins are made up of BSP proteins in most mammalian species which is in stark contrast to the 0.01 % found in the mouse and human [8, 9]. This variation in the site of expression amongst species may be because of differences in species specificity in terms of the maturation of spermatozoa, in contrast to the bull, the semen of mice and humans coagulates just after ejaculation and the coating of spermatozoa by seminal plasma proteins may not occur extensively. Mammalian species producing BSP homologs in the epididymis do not appear to require the coating of sperm with seminal plasma proteins, in contrast to those producing BSP homologs in the seminal vesicles [8, 10]. Structurally, all epididymal BSP proteins identified have one common feature distinct from the other BSPs: the presence of the C-terminal tail. In the proteins expressed by seminal vesicle glands, the C-terminus always ends with cysteine, whereas the BSP proteins of epididymal origin contain an additional C-terminal tail composed of 1–5 amino acids after the last cysteine [11]. The sources and type of GBPs are listed in Table 1.
Table 1.
Types of gelatin binding proteins (GBPs) identified from different sources in male reproductive tracts in mammals
| Organism | Sources | Types of GBPs | References |
|---|---|---|---|
| Bovine | Seminal plasma | BSP A1, BSP A2, BSP A3, BSP-30 kDa, PDC-109 | [5, 11, 41] |
| Goat | Seminal plasma | GSP-14 kDa, GSP-15 kDa, GSP-20 kDa and GSP-22 kDa | [5, 11, 41] |
| Bison | Seminal vesicle | BiSV-16 kDa, BiSV-17 kDa, BiSV-18 kDa, and BiSV-28 kDa | [5, 11] |
| Ram | Seminal plasma | RSP-15 kDa, RSP-16, RSP-22 kDa and RSP-24 kDa | [5, 11] |
| Stallion (horse) | Seminal plasma | HSP1, HSP2, EQ-12 | [41] |
| Human, bovine, canine, ram | Seminal plasma | MMP-2 and MMP-9 | [12, 14, 16, 41] |
| Bovine, human, dog, horse and pig | Epididymis | Epididymal sperm-binding protein 1 (ELSPBP1) | [8, 23, 25] |
| Human | Epididymis | hBSPH1 | [7, 9, 11] |
| Mouse | Epididymis | m BSPH1 and mBSPH2 | [7, 9, 11] |
| Porcine | Seminal plasma | pB1 | [7] |
| Buffalo | Seminal plasma | BSP homologs | [11, 41] |
Other GBPs
MMP 2 and MMP 9
MMP 2 and MMP 9 have been characterized as GBPs in bovine seminal plasma using gelatin zymography [12]. Furthermore, MMP 2 and MMP 9 have been shown to be expressed in multiple genital organs including the testicular, epididymal fluids and seminal plasma amongst mammals such as ram, boar, stallion, buffaloes and canine [13–16]. With regards to reproduction, MMP2 and MMP9 have been associated with ovulation [17] and implantation of the embryo [18]. These two proteins exhibit three gelatin binding fn2 domains at the catalytic site as compared two fn2 domain in BSP proteins and their homologs [19]. These domains are mainly essential for binding with substrates of MMP-2 and MMP-9, such as gelatins, collagens and laminine [20]. Matrix metalloproteinases (MMPs) cleave the extracellular matrix components (ECM) of tissues and fluids and guide the secretion of various cytokines and growth factors [20]. MMPs are categorized into four groups of (1) gelatinases, cleaving collagen substrates, (2) stromolysins acting on non collagenous components, (3) Collagenases which targets the fibril forms of collagens and (4) MT-MMps which are transmembrane enzymes, which do not act on ECM components but rather are involved in the activation of other MMPs [20].
MMPs are initially released in an inactive form such as proMMPs and converted into the active form by the removal of some inhibitory peptides. The molecular weight of proMMPs is usually 10 kDa less than the active form. Human seminal plasma possesses latent as well as active types of MMP 2 and MMP 9 with latent MMPs more predominant than the active form. In addition, the expression of proMMP 2 and MMP 9 increases sharply up to maximum levels 2 h after capacitation, which implies a role in triggering the hyperactivation of spermatozoa [21]. In addition, the inactive form of MMP 9 has been associated with semen parameters in humans and its concentration has been found to be higher in patients showing low sperm counts [21]. Hence, these proteins can be correlated with infertility in human.
Epididymal Sperm-Binding Protein 1 (ELSPBP1)
ELSPBP1 was characterized as a sperm binding protein, expressed in the epididymis and containing the highest number of the four fn2 domains in comparison to other GBPs [22, 23]. ELSPBP1 has been also described as HE12 in humans and CE12 in dogs [23, 24]. Furthermore ELSPBP1 orthologs have been recognized in equine, porcine and bovine systems [25] while no DNA homologs of ELSPBP1 were observed in either the rat or mouse [25]. The role of ELSPBP1 in male reproductive physiology is yet not clear but common sequence identity with BSP proteins, suggests some common characteristics. The sequence identity of fn2 domains in both BSP proteins and ELSPBP1 suggests that an identical two dimensional structure and similar amino acid residues participate in lipid binding [25]. This indicates an identical binding pattern for both proteins, but different expression patterns in the reproductive tract pointing different functions in spermatozoa. The study of ELSPBP1 expression in the bull epididymis utilizing a specific antibody has revealed an association with its role in maintaining spermatozoa cell volume during sperm maturation [26]. More recently, ELSPBP1 has been observed to be more prominent in extracts of spermatozoa in sub fertile bulls and has been exclusively identified in dead spermatozoa [23, 27]. Furthermore, it was investigated that the epididymis secretes ELSPBP1 complexed with an epididymosome, which transports ELSPBP1 to dead spermatozoa in the presence of zinc [24, 27]. However, the roles of ELSPBP1 in dead spermatozoa are matter of investigations in future.
Gelatin Binding Domain
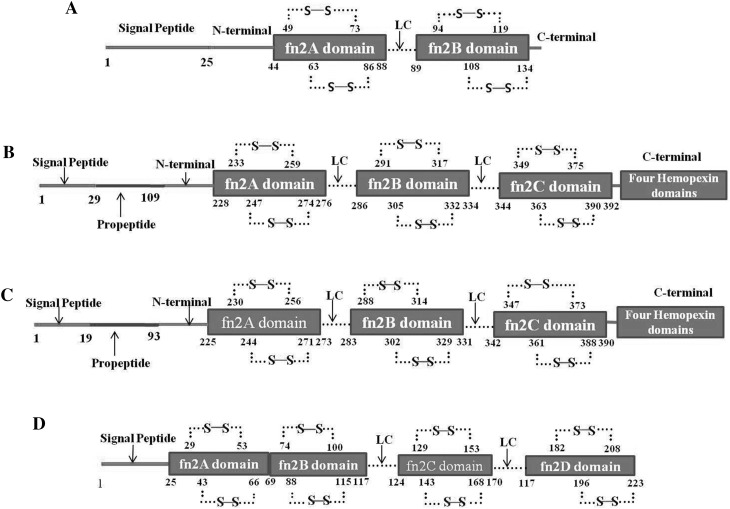
GBPs are characterized by the presence of a fibronectin type II (fn2) domain, also called the gelatin binding domain which works as a functional unit for GBPs proteins. The BSP super family proteins possess common conserved two fn2 domains with variable acidic N-terminal extension. These two domains are arranged in tandem repeat of fn2A and fn2B, and separated by linker chain (LC) of 7–9 amino acids followed by acidic N-terminal extension. Some of BSP proteins (especially epididymal in origin) contain also small variable carboxyl terminal end. Typically, in BSP proteins each fn2 domain contains eight cysteine residues, which make four disulphide bonds, two in each domain [5, 7, 11, 28] (Fig. 2a). Based on data available in uniprot, there are three fn2 domains that exist in MMP-2 and MMP-9 and a total of 12 cysteine residues, four in each domain, which are covalently linked with six disulphide bonds (two in each domain) (Fig. 2b, c). Similarly uniprot based data shows that in ELSPBP1, four fn2 domains are arranged in repeats of fn2A, fn2B, fn2C and fn2D, each domain possessing four cysteine amino acids, and forming two disulphide bonds per domain. There is no LC present between fn2A and fn2B (Fig. 2d). Thus, each fn2 domain of all known GBPs appears to possess two disulphide bonds. The characteristics of the fn2 domain in all GBPs were prepared from data available in uniprot and are summarized in Table 2.
Fig. 2.
Schematic representation of gelatin binding proteins (GBPs) structure based on the sequences and data available in uniprot site (http://www.uniprot.org/). a BSP Protein (PDC-109) contains two gelatin binding domains fn2A and fn2B, which are connected with linker chain (LC) of 7–9 amino acids. Two disulphide bonds (⋯S–S⋯) are present in each domain. b MMP-2 and c MMP-9 carry three gelatin binding domains at N terminal, which are tandemly arranged as fn2A, fn2B and fn2C and they are joined with each other by LC. Their C-terminal contains four hemopexin domains also d ELSPBP1 is a long fn2 domain containing protein. Each all four domains have two disulphide bonds. fn2A domain of GBPs is followed by N terminal and signal peptides. There is no LC between fn2A and fn2B domain in ELSPBP1. The uniprot identification numbers of these proteins are UniProtKB-P02784 for BSP protein (PDC-109), UniProtKB-P08253 for MMP-2, UniProtKB-P14780 for MMP-9 and UniProtKB-Q7YR83 for ELSPBP1
Table 2.
Features of gelatin binding (fn2) domain in different proteins
| Characteristcs of fn2 domains | Gelatin binding proteins(GBPs) | ||
|---|---|---|---|
| BSP protein family and their homologs | MMP-2 and MMP-9 | ELSPBP1 | |
| Number | 2 | 3 | 4 |
| Glycosylation | + (except BSP A3 and hBSPH1) |
+ | + |
| Number of (-S–S-) disulfide bonds | 4 | 6 | 8 |
| Site of expression | Seminal vesicles (except mBSPH1, mBSPH2, and hBSPH1 from epididymis) | Testis, epididymis, ovary, endometrium, prostate, cervix | Epididymis |
Functions of GBPs
Capacitation
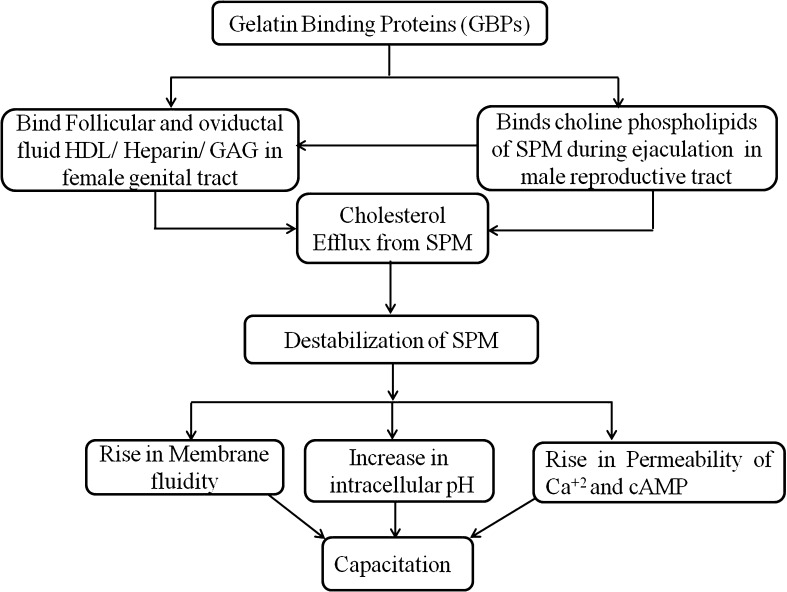
Capacitation is an important phenomenon in fertilization, which occurs in the female reproductive tract after the ejaculation of spermatozoa and is an essential step in sperm gaining the ability to fuse with an oocyte and achieve successful fertilization. It has been speculated that spermatozoa unite with SP containing BSP proteins during ejaculation. Such proteins bind with choline phospholipids present in sperm plasma membranes and stimulate cholesterol discharge, this is called the first cholesterol efflux [11, 29]. Within the female genital tract, BSP proteins have been shown to interact with high density lipids (HDL) present in oviductal and follicular fluids, affecting further cholesterol discharge from sperm plasma membranes, this is known as the second cholesterol efflux [11, 29]. This cholesterol efflux causes the destabilization of the spermatozoa plasma membrane, which in turn rise in calcium ions permeability, alteration in intracellular pH, elevation in membrane fluidity, which finally leads to capacitation [11, 29]. Further, HDL and glycosaminoglycans (GAGs) such as heparin have been displayed to induce capacitation in the female reproductive tract and GAGs interact with BSP proteins perhaps regulating capacitation [11, 29], which is essential for fertilization.
Recombinant human BSPH1 has been shown to induce capacitation in humans in a similar way to the murine rec-BSPH1 in murine species [9, 29]. The functions of murine BSPH2 in capacitation is not known, although a high sequence similarity with murine BSPH1 has been observed. Therefore, murine BSPH2 may also have a specific role in the capacitation of spermatozoa though further study is needed to fully elucidate any proposed mechanism of sperm capacitation by murine BSPH2. It has been suggested that during the maturation of sperm in the epididymis, murine BSPH1 proteins bind to and destabilize the membranes of spermatozoa, then, following ejaculation in the female genital tract, the sperm BSPH1 complex may easily bind with high density lipids (HDL) present in oviductal fluid, inducing capacitation [29]. This possible role of BSP proteins in the induction of capacitation could be used to understand the braoder involvement of GBPs family in the future. The complete pathway for regulation of capacitation by GBPs is shown in Fig. 3.
Fig. 3.
The proposed mechanisms of capacitation induced by gelatin binding proteins (GBPs) were framed according to information discussed in this article. Abreviation: SPM–Sperm plasma membrane, HDL–High density lipid and GAG–Glycosaminoglycans
Menstruation Cycle
Menstruation is the regular monthly discharge of blood and mucosal tissue from the inner lining of the uterus through the vagina. Previously, menstruation was purely described as an event of ischemic necrosis of endometrial epithelial linings, producing vasoconstriction of arterioles, finally leading to bleeding. Presently, it is understood that the endometrium produces plenty of MMPs during menstruation and consequently MMPs have been regarded as effectors molecules in endometrial disruption during this process in women. MMP-2 and MMP-9 contain an fn2 domain near catalytic sites, and as such cleave ECM components at neutral pH and are involved in various physiological processes including the remodeling and disruption of tissues during normal estrous and menstrual cycles and pregnancy [30, 31]. In addition, MMP-2 mRNA has been shown to be expressed in the bovine endometrium and placenta throughout the whole gestation period, which suggests that these genes are one of the main endometrial remodeling factors for implantation and pre-partum in cattle [32]. As reviewed, stromal cells produces MMP-2, which acts on ECM components including epithelial cells and the vascular basement membrane, degrading the integrity of the epithelial lining and blood vessels of the endometrium, ultimately causing bleeding [33]. Progesterone and tissues inhibitors of metalloproteinases (TIMPs) have been described as key molecules regulate the expression of MMPs, which is essential in maintaining the time and space relation between expressions of MMPs and disruption of epithelial linings [34, 35].
Establishment of Sperm Reservoir in Oviduct
Following male ejaculation inside female reproductive tract, after ejaculation, millions of spermatozoa are deposited; however, only a few thousands of them ever manage to approach the oviductal isthmus after crossing the uterotubal junction to form a reservoir for spermatozoa [36]. The ampulla–isthmus has been identified as a reservoir for sperm in many mammals such as cattle, horse, pigs, mice, hamsters, guinea pigs and camelids [5, 36–38]. The occurrence of a sperm reservoir performs significant for three main reasons; (1) It reduces polyspermy by permitting fewer spermatozoa to reach the site of fertilization in the ampulla, (2) It allows for the selection of highly viable sperm (3) and lastly it helps to regulate capacitation and the induction of hyper activation of spermatozoa [38, 39]. The mechanisms involved in formation of this reservoir are not fully understood, but it is believed that spermatozoa bind with the epithelial linings of the oviduct, which produce a hub during passage in female genital canal. This binding has been thought to be vital in maintaining the viability and motility of sperm in vitro [28, 40]. Furthermore, sperm binding to oviductal epithelia is facilitated by a Lewis like trisaccheride molecule present in oviductal membranes which contains a fucose molecule, and PDC-109 present in the sperm plasma membrane has a demonstrable affinity for fucose [5, 28, 40]. Therefore, PDC-109 might have potential role in oviductal binding of spermatozoa.
Another hypothesis for sperm reservoir formation in the female genital tract has been forwarded involving the coating of spermatozoa with BSP proteins in the epididymis during maturation by the binding of phospholipids to the plasma membrane. Upon reaching oviduct, these BSP coated spermatozoa are coordinated towards the oviductal reservoir by binding to the epithelia of the oviduct [28, 41]. This binding inhibits premature capacitation and enhances the viability of spermatozoa [41, 42]. Further to this, BSP1 has been shown to remain present on spermatozoa even after capacitation, suggesting a function in the binding of spermatozoa to the epithelium of the ampulla [43]. More work is required in order to know mechanisms underlying the actions performed by GBPs during sperm reservoir formation.
Cryopreservation
Cryopreservation is a procedure used in order to preserve semen in assisted reproductive technology, and involves sequential steps of cooling, freezing and finally the thawing of spermatozoa. This process negatively affects spermatoza in several ways such as ultrastructural damage sustained to plasma membranes and cytoskeleton, rises in intracellular Ca+2 and K+1 ions levels, reduced tolerance to stress, and reduction in cAMP levels. Overall, these alterations reduce the life span and fertilizing capacity of spermatozoa [44, 45]. The addition of seminal plasma after semen thawing has been used to enhance the resistance of spermatozoa to cold shock and overcome the deleterious consequences of cryopreservation in the ram [10, 46]. In addition, SP incorporating to semen prior to cyropreservsation has led to the recovery of lost motility, acrosomal integrity, mitochondrial respiration and viability of spermatozoa during semen preservation in liquid nitrogen [47, 48]. These cryoprotective effects of SP have been largely attributed to some proteins available within it. GBPs such as, RSVP14 and RSVP20 have been exhibited to have antioxidant properties in ram seminal plasma and protect ram sperm from oxidative stresses and premature capacitation (cryocapacitation) during cryopreservation [8, 49]. Recently, it has been described that two SP proteins RSVP20 and lactotransferin of ram SP are conserved in three breeds of ram and bind to spermatozoa and maintain their fertilizing ability during cryopreservation of semen in this species [49]. The exact molecular mechanism by which the reversal of these deleterious effects during cryopreservation is achieved is not fully understood. In several studies, it has been reported that SP proteins are adsorbed onto the surfaces of boar spermatozoa and that this may allow damaged spermatozoa surfaces to retain their original shape during cryopreservation [43, 50].
Cryocapicitation caused by cryopreservation reduces the fertilizing ability of spermatozoa, negatively impacting the efficiency of artificial reproduction techniques which may often rely on frozen semen such as artificial insemination. A reduced impact of cryocapacitation has been observed when SP proteins have been added to semen extender during the cryopreservation of boar semen, [51]. The addition of SP to spermatozoa in cryopreservation reduces the cryocapacitation of boar sperm and enhances the fertilizing ability of both cryopreserved ram and boar spermatozoa [49, 51]. It is believed that cryocapacitation is induced partially by reactive oxygen species and those GBPs such as RSVP14 and RSVP20 in ram SP protect sperm from these cryoinjuries via antioxidant action against ROS species [8, 49, 51]. Hence, such proteins may be used to formulate more optimal semen extenders for the cryopreservation of semen.
Sperm Maturation
Functionally immature sperm are synthesized in the testis, and then pass to the epididymis, where their maturation is completed. Throughout this process spermatozoa experience many biochemical and morphological modifications such as changes in pH, membrane remodeling and alterations in plasma membranes. Maturation of spermatozoa is partly controlled by addition of epididymal fluid proteins, which alter the sperm plasma membrane. This alteration is caused by the proteolysis of some proteins that leads to the disappearance or redistribution of protein domains within plasma membranes [52]. The proteolysis of different proteins varies amongst animal species, such as ADAMs (disintegrin and metalloproteinases) in rodents and monkeys [53]. MMPs such as MMP-2 and MMP-9 and as well as other gelatinolytic proteases have been observed in the fluids of the testis and the epididymis of canines [54]. In mammals, MMP-2 has been shown to be secreted mostly in the epididymis and is associated with membrane remodeling functions during sperm maturation [55, 56]. However, spermatozoa only gain their complete maturation and fertilizing capacity during capacitation inside the female reproductive tract, a process highly regulated by BSP proteins [28, 29, 31, 56].
Sperm Motility
Motility is key factor defining the fertility of sperm as only sufficiently active sperms will reach the fertilization site once deposited in the female reproductive tract. SP is crucial modulator of sperm motility in mammals and several SP proteins have been linked to the motility of spermatozoa [57]. Most proteins associated with motility are usually located within the mid piece of spermatozoa and BSP proteins have been detected on the mid piece of spermatozoa in several species, which hints at a biological function in the motility of spermatozoa [reviewed in [5]. The motility of spermatozoa is known to be regulated by intracellular calcium signaling molecules, indicating a regulatory function of Ca+2-ATPase in spermatozoa motility through this signaling pathway [58]. Further, it was reported that PDC-109 interacts with Ca+2-ATPase and enhances the motility of spermatozoa in the bovine model by increasing the pumping efficiency of Ca+2-ATPase in an irreversible and cooperative way across the plasma membrane [reviewed in [28]. It has been shown that buffalo spermatozoa treated with heparin binding proteins show increased motility, and the fn2 domains of GBPs also share a similar heparin binding property [28], suggesting a role in sperm motility for this molecule.
Oocyte Maturation and Ovulation
Ovulation is triggered by a preovulatory wave of luteinizing hormone initiated from the pituitary gland, which ultimately results in the release of mature oocytes from ovarian follicles. This process requires extensive remodeling of granulose cell membranes and the ECM breakdown of the follicular wall, prior to the liberation of oocytes [59]. Extracellular MMPs have been correlated with ovarian tissue remodeling in various studies, and are seen as important for the follicular microenvironment and ovulation [59–61]. In addition, MMP-2 and MMP-9 (gelatinases) are expressed in mammalian ovaries [62]. During follicular development in humans, the size of a follicle is thought to expand around 400 times its original size before ovulation, requiring tremendous remodeling of the ECM both outside and inside follicles and gelatinases plays key role in this process. Furthermore, human MMP-2 expression has been shown to increase as follicles grow over time and its expression has also been found to be higher in the corpus luteum [63]. MMP-2 and MMP-9 activity was demonstrated in inside follicles and higher rates of activity were observed in women suffering from polycystic ovary syndrome as compared to women experiencing normal ovulation [64]. TIMP regulate the function of gelatinases, which are essential for the controlled remodeling of ECM during normal follicular development and ovulation [64]. In addition, both MMPs and their TIMPs have also been implicated during sperm oocyte interactions [65]. Recently, MMP2 levels and activity has been significantly correlated with oocyte maturation and fertilization rates, suggesting that MMP-2 activity can be used as a prognostic marker in follicular fluids of IVF/ICSI patients [66]. These observations infer that these proteins play a crucial role in the maturation of oocytes and ovulation, which are important for successful fertilization.
Chaperon like Activity
Molecular chaperones are a functionally related group of proteins, which are essential for maintaining cellular physiology under both normal and unfavorable environments. Chaperones enable the proper folding of normal and misfolded proteins. However, chaperons proteins and their behavior in semen have not been well documented to date. For the first time, Seminal PDC-109 was identified as a molecular chaperon in SP and the functional similarities of PDC-109 with well characterized chaperon proteins R-crystallin and spectrin has been studied under in vitro conditions [67]. Some proteins like G6PD are very prone to aggregate in SP even in their native state, it was hypothesized therefore that PDC-109 interacts with this protein and make facilitates their active confirmation [67]. The most common feature of PDC-109, which forms an oligomeric protein structure of 150 kDa, is its polydisperty. The oligomerization into smaller components results in the loss of the chaperon like activities of PDC-109. This conformational flexibility of PDC-109 helps in its chaperon like activities, where, it binds with targeted proteins and protects them from different kinds of stress conditions [67].
Amylodogenesis is the abnormal deposition of denatured proteins occurring during the maturation of spermatozoa in the epididymis [67]. Some proteins in SP have been detected very prone to amyloid formation and amyloid synthesis in the testis and epididymis, and they have been correlated with male infertility [68]. Bovine PDC 109 reported as molecular chaperone, which may stops amyloid formation by binding denatured or unfolded proteins [67, 68]. More recently, chaperon like activity has also been demonstrated in equine SP protein HSP1/2, a gelatin binding protein, homologous to PDC-109. HSP1/2 exhibits chaperon activity and inhibits the aggregation (induced by heat) of targeted proteins such as insulin [69]. The behavior of GBPs as molecular chaperones in vivo has yet to be fully understood and is a possible area of future study.
Conclusion
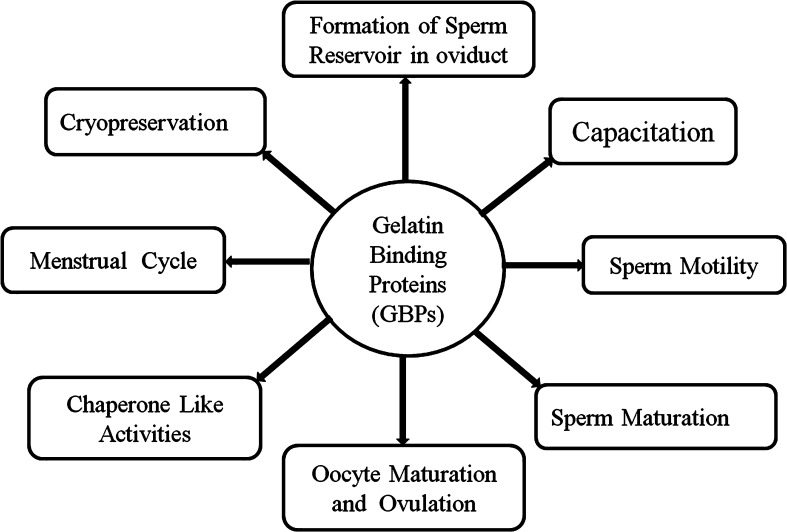
GBPs have been extensively studied as a ubiquitous protein family in many mammalian species, however, the origin and function of GBPs is still largely a mystery and is a focused area of continuous research. As an important protein family, much of the current information on GBPs has been discussed in this review. GBPs play an important role in many aspect of reproduction with many functions attributed to GBPs such as physiological roles in sperm motility, sperm viability, cell volume regulation, sperm capacitaion, the acrosome reaction, formation of the oviductal sperm reservoir, the menstruation cycle and ovulation in females (Fig. 4). The cryopreservation of sperm provides a means of maintaining genetic stocks and has broad usages in artificial insemination and other reproductive technologies. The protective role of GBPs during cryopreservation may be beneficial when designing chemically defined semen extenders, (free of products such as egg yolk), which will help in improving semen cryopreservation techniques.
Fig. 4.
Divergent role of gelatin binding proteins (GBPs) in different steps of reproduction, such as capacitation, formation of sperm reservoir in oviduct, cryopreservation, menstrual cycle, sperm motility, sperm maturation, maturation of oocytes and ovulation and chaperone like activities
Although, plenty of work has been done on defining the role GBPs with respect to reproductive physiology, more study is required to know how GBPs binds to gelatin in seminal plasma and regulate important physiological processes. In addition, mostly studies to date have focused on a few proteins within GBP family such as the BSP proteins; PDC-109, fibronectin, MMP2 and MMP9. Further investigations are required to explore the functions of other proteins within this family to understand their relevance to the physiology of reproduction.
Acknowledgments
The authors wish to thank Department of President’s Affairs (DOPA), Abu Dhabi, United Arab Emirates (UAE) for financial support and resources for this study.
Contributor Information
Sanjay Kumar, Email: sanjay.syadavbiotech@gmail.com.
Shreesh Ojha, Email: shreeshojha@uaeu.ac.ae.
References
- 1.Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66:11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Wang DK, Chen LM. The physiology of bicarbonate transporters in mammalian reproduction. Biol Reprod. 2012;5:86–99. doi: 10.1095/biolreprod.111.096826. [DOI] [PubMed] [Google Scholar]
- 3.Björndahl L, Kvist U. Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod. 2010;16:23–29. doi: 10.1093/molehr/gap099. [DOI] [PubMed] [Google Scholar]
- 4.Batruch I, Smith CR, Mullen BJ, Grober E, Lo KC, Diamandis EP, Jarvi KA. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J Proteom Res. 2012;11:1503–1511. doi: 10.1021/pr200812p. [DOI] [PubMed] [Google Scholar]
- 5.Plante G, Prud’homme B, Fan J, Lafleur M, Manjunath P. Evolution and function of mammalian binder of sperm proteins. Cell Tissue Res. 2016;363:105–127. doi: 10.1007/s00441-015-2289-2. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- 7.Manjunath P, Lefebvre J, Jois PS, Fan J, Wright MW. New nomenclature for mammalian BSP genes. Biol Reprod. 2009;80:394–397. doi: 10.1095/biolreprod.108.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano E, Martínez AB, Arruga D, Pérez-Pé R, Sánchez-Ferrer Á, Muiño-Blanco T, Cebrián-Pérez JA. New Insights into the phylogeny and gene context analysis of binder of sperm proteins (BSPs) PLoS ONE. 2010;9:e0137008. doi: 10.1371/journal.pone.0137008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plante G, Fan J, Manjunath P. Murine binder of Sperm homolog 2 (BSPH2): the black sheep of the BSP superfamily. Biol Reprod. 2014;90:1–12. doi: 10.1095/biolreprod.113.114272. [DOI] [PubMed] [Google Scholar]
- 10.Manjunath P. new insights into the understanding of the mechanism of sperm protection by extender components. Anim Reprod. 2012;9:809–815. [Google Scholar]
- 11.Plante G, Manjunath P. Epididymal binder of sperm genes and proteins: what do we know a decade later? Andrology. 2015;3:817–824. doi: 10.1111/andr.12089. [DOI] [PubMed] [Google Scholar]
- 12.Pipan MZ, Kosec M, Mrkun J, Zrimsek P. Gelatinases in boar seminal plasma and their relation to semen indicators. Acta vet brno. 2010;79:491–496. doi: 10.2754/avb201079030491. [DOI] [Google Scholar]
- 13.Gurupriya VS, Roy SC, Dhama K, Gopinath D, Rekha V, Aswathi PB, John JK, Gopalakrishnan A. Proteases and proteases inhibitors of semen–a review. Adv Anim Vet Sci. 2014;2:447–456. doi: 10.14737/journal.aavs/2014/2.8.447.456. [DOI] [Google Scholar]
- 14.Saengsoi W, Shia WY, Shyu CL, Wu JT, Warinrak C, Lee WM, Cheng FP. Detection of matrix metalloproteinase (MMP)-2 and MMP-9 in canine seminal plasma. Anim Reprod Sci. 2011;127:114–119. doi: 10.1016/j.anireprosci.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Gurupriya VS, Divyashree BC, Roy SC. Cryogenic changes in proteases and antiprotease activities of buffalo (Bubalus bubalis) and cattle (Bos taurus) semen. Theriogenology. 2014;81:396–402. doi: 10.1016/j.theriogenology.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Souza CE, Rego JP, Lobo CH, Oliveira JT, Nogueira FC, Domont GB, Fioramonte M, Gozzo FC, Moreno FB, Monteiro-Moreira AC, Figueiredo JR, Moura AA. Proteomic analysis of the reproductive tract fluids from tropically-adapted Santa Ines rams. J Proteom. 2012;75:4436–4456. doi: 10.1016/j.jprot.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Portela VM, Veiga A, Price CA. Regulation of MMP2 and MMP9 metalloproteinases by FSH and growth factors in bovine granulosa cells. Genet Mol Biol. 2009;32:516–520. doi: 10.1590/S1415-47572009005000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orief YI, Alabd MM, Alkasar YS, Koritam AG, Deghedy A. The role of matrix metalloproteinase-2 in the culture media in embryo implantation rate in normogonadotrophic cases undergoing ICSI. Middle East Fertil Soc J. 2013;18:278–283. doi: 10.1016/j.mefs.2012.11.001. [DOI] [Google Scholar]
- 19.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur Respir J. 2012;39:197–209. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 20.Shiomi T, Lemaître V, D’Armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60:477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dua JW, Xub KY, Fangc LY, Qid XL. Detection and analysis of MMP-2 and MMP-9 in seminal plasma. J Men Health. 2012;9:216–219. doi: 10.1016/j.jomh.2012.07.002. [DOI] [Google Scholar]
- 22.Frenette G, Girouard J, D’Amours O, Allard N, Tessier L, Sullivan R. Characterization of two distinct populations of epididymosomes collected in the intraluminal compartment of the bovine cauda epididymis1. Biol Reprod. 2012;87:1–11. doi: 10.1095/biolreprod.112.101691. [DOI] [PubMed] [Google Scholar]
- 23.D’Amours O, Frenette G, Fortier M, Leclerc P, Sullivan R. Proteomic comparison of detergent-extracted sperm proteins from bulls with different fertility indexes. Reproduction. 2010;139:545–556. doi: 10.1530/REP-09-0375. [DOI] [PubMed] [Google Scholar]
- 24.D’Amours O, Frenette G, Bordeleau LJ, Allard N, Leclerc P, Blondin P, Sullivan R. Epididymosomes transfer epididymal sperm binding protein 1 (ELSPBP1) to dead spermatozoa during epididymal transit in bovine. Biol Reprod. 2012;18:87–94. doi: 10.1095/biolreprod.112.100990. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan R. Epididymosomes: a heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J Androl. 2015;7:726–729. doi: 10.4103/1008-682X.155255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahin E, Petrunkina AM, Ekhlasi-Hundrieser M, Hettel C, Waberski D, Harrison RA, Topfer-Petersen E. Fibronectin type II-module proteins in the bovine genital tract and their putative role in cell volume control during sperm maturation. Reprod Fertil Dev. 2009;21:479–488. doi: 10.1071/RD08209. [DOI] [PubMed] [Google Scholar]
- 27.D’Amours O, Bordeleau LJ, Frenette G, Blondin P, Leclerc P, Sullivan R. Binder of sperm 1 and epididymal sperm binding protein 1 are associated with different bull sperm subpopulations. Reproduction. 2012;143:759–771. doi: 10.1530/REP-11-0392. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava N, Jerome A, Srivastava SK, Ghosh SK, Kumar A. Bovine seminal PDC-109 protein: an overview of biochemical and functional properties. Anim Reprod Sci. 2013;138:1–13. doi: 10.1016/j.anireprosci.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Plante G, Therien I, Manjunath P. Characterization of recombinant murine binder of sperm protein homolog 1 and its role in capacitation. Biol Reprod. 2012;87:1–11. doi: 10.1095/biolreprod.111.096644. [DOI] [PubMed] [Google Scholar]
- 30.Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab. 2012;303:55–70. doi: 10.1152/ajpendo.00553.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy SC, Ghosh J. Dynamic in vivo changes in the activities of gelatinases, matrix metalloproteinases (MMPs), and tissue inhibitor of metalloproteinases (TIMPs) in buffalo (Bubalus bubalis) uterine luminal fluid during estrous cycle and early pregnancy. Mol Reprod Dev. 2010;77:944–953. doi: 10.1002/mrd.21240. [DOI] [PubMed] [Google Scholar]
- 32.Ulbrich SE, Meyer SU, Zitta K, Hiendleder S, Sinowatz F, Bauersachs S, Büttner M, Fröhlich T, Arnold GJ, Reichenbach HD, Wolf E, Meyer HH. Bovine endometrial metallopeptidases MMP14 and MMP2 and the metallopeptidase inhibitor TIMP2 participate in maternal preparation of pregnancy. Mol Cell Endocrinol. 2011;332:48–57. doi: 10.1016/j.mce.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Henriet P, Gaide Chevronnay HP, Marbaix E. The endocrine and paracrine control of menstruation. Mol Cell Endocrinol. 2012;358:197–207. doi: 10.1016/j.mce.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Diaz PS, Solar PA, Juica NE, Orihuela PA, Cardenas H, Christodoulides M, Vargas R, Velasquez LA. Differential expression of extracellular matrix components in the fallopian tubes throughout the menstrual cycle. Reprod Biol Endocrinol. 2012;16:10–56. doi: 10.1186/1477-7827-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Xu X, He B, Li Y, Chen X, Wang J. A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Hum Reprod. 2013;28:1670–1678. doi: 10.1093/humrep/det052. [DOI] [PubMed] [Google Scholar]
- 36.Apichela S, Valz-Gianinet JN, Schuster S, Jimenez-Dıaz MA, Roldan-Olarte M, Miceli DC. Lectin binding patterns and carbohydrate mediation of sperm binding to llama oviductal cells in vitro. Anim Reprod Sci. 2010;118:344–353. doi: 10.1016/j.anireprosci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Defaus S, Aviles M, Andreu D, Gutierrez-Gallego R. Identification of bovine sperm surface proteins involved in carbohydrate-mediated fertilization interactions. Mol Cell Proteom. 2016 doi: 10.1074/mcp.M115.057703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tienthai P. The porcine sperm reservoir in relation to the function of hyaluronan. J Reprod Dev. 2015;61:245–250. doi: 10.1262/jrd.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coy P, García-Vázquez FA, Visconti PE, Avilés M. Roles of the oviduct in mammalian fertilization. Reproduction. 2012;144:649–660. doi: 10.1530/REP-12-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tecle E, Gagneux P. Sugar-coated sperm: unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev. 2015;82:635–650. doi: 10.1002/mrd.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafleur M, Courtemanche L, Karlsson G, Edwards K, Schwartz JL, Manjunath P. Bovine binder-of-sperm protein BSP1 promotes protrusion and nanotube formation from liposomes. Biochem Biophys Res Commun. 2010;399:406–411. doi: 10.1016/j.bbrc.2010.07.088. [DOI] [PubMed] [Google Scholar]
- 42.Ardon F, Suarez SS. Cryopreservation increases coating of bull sperm by seminal plasma binder of sperm proteins BSP1, BSP3, and BSP5. Reproduction. 2013;146:111–117. doi: 10.1530/REP-12-0468. [DOI] [PubMed] [Google Scholar]
- 43.Hung PH, Suarez SS. Alterations to the bull sperm surface proteins that bind sperm to oviductal epithelium. Biol Reprod. 2012;18:80–88. doi: 10.1095/biolreprod.112.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pons-Rejraji H, Bailey JL, Leclerc P. Cryopreservation affects bovine sperm intracellular parameters associated with capacitation and acrosome exocytosis. Reprod Fertil Dev. 2009;21:525–537. doi: 10.1071/RD07170. [DOI] [PubMed] [Google Scholar]
- 45.Blässe AK, Oldenhof H, Ekhlasi-Hundrieser M, Wolkers WF, Sieme H, Bollwein H. Osmotic tolerance and intracellular ion concentrations of bovine sperm are affected by cryopreservation. Theriogenology. 2012;78:1312–1320. doi: 10.1016/j.theriogenology.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Juyena NS, Stelletta C. Seminal plasma: an essential attribute to spermatozoa. J Androl. 2012;33:536–551. doi: 10.2164/jandrol.110.012583. [DOI] [PubMed] [Google Scholar]
- 47.Rovegno M, Feitosa WB, Rocha AM, Mendes CM, Visintin JA, D’Avila Assumpção ME. Assessment of post-thawed ram sperm viability after incubation with seminal plasma. Cell Tissue Bank. 2013;14:333–339. doi: 10.1007/s10561-012-9317-1. [DOI] [PubMed] [Google Scholar]
- 48.de Andrade AF, Zaffalon FG, Celeghini EC, Nascimento J, Bressan FF, Martins SM, de Arruda RP. Post-thaw addition of seminal plasma reduces tyrosine phosphorylation on the surface of cryopreserved equine sperm, but does not reduce lipid peroxidation. Theriogenology. 2012;77:1866–1872. doi: 10.1016/j.theriogenology.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Bernardini A, Hozbor F, Sanchez E, Fornés MW, Alberio RH, Cesari A. Conserved ram seminal plasma proteins bind to the sperm membrane and repair cryopreservation damage. Theriogenology. 2011;76:436–447. doi: 10.1016/j.theriogenology.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 50.Mendoza N, Casao A, Pérez-Pé R, Cebrián-Pérez JA, Muiño-Blanco T. New insights into the mechanisms of ram sperm protection by seminal plasma proteins. Biol Reprod. 2013;88:1–15. doi: 10.1095/biolreprod.112.105650. [DOI] [PubMed] [Google Scholar]
- 51.Vadnais ML, Roberts KP. Effects of seminal plasma on cooling-induced capacitative changes in boar sperm. J Androl. 2010;28:416–422. doi: 10.2164/jandrol.106.001826. [DOI] [PubMed] [Google Scholar]
- 52.Van Tilburg MF, Rodrigues MA, Moreira RA, Moreno FB, Monteiro-Moreira AC, Cândido MJ, Moura AA. Membrane-associated proteins of ejaculated sperm from morada nova rams. Theriogenology. 2013;79:1247–1261. doi: 10.1016/j.theriogenology.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Cho C. Testicular and epididymal ADAMs: expression and function during fertilization. Nat Rev Urol. 2012;9:550–560. doi: 10.1038/nrurol.2012.167. [DOI] [PubMed] [Google Scholar]
- 54.Warinrak C, Wu JT, Hsu WL, Liao JW, Chang SC, Cheng FP. Expression of matrix metalloproteinases (MMP-2, MMP-9) and their inhibitors (TIMP-1, TIMP-2) in canine testis, epididymis and semen. Reprod Domest Anim. 2015;50:48–57. doi: 10.1111/rda.12448. [DOI] [PubMed] [Google Scholar]
- 55.Moura AA, Souza CE, Stanley BA, Chapman DA, Killian GJ. Proteomics of cauda epididymal fluid from mature Holstein bulls. J Proteom. 2010;73:2006–2020. doi: 10.1016/j.jprot.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Caballero J, Frenette G, Sullivan R. Post testicular sperm maturational changes in the bull: important role of the epididymosomes and prostasomes. Vet Med Int. 2010;2011:1–13. doi: 10.4061/2011/757194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodrigues MAM, Souzaa CEA, Martinsa JAM, Regoa JPA, Oliveirab JTA, Domontc G, Nogueirac FCS, Mouraa AA. Seminal plasma proteins and their relationship with sperm motility in Santa Ines rams. Small Rumin Res. 2013;109:94–100. doi: 10.1016/j.smallrumres.2012.07.032. [DOI] [Google Scholar]
- 58.Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2 + -ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS ONE. 2013;8:e80181. doi: 10.1371/journal.pone.0080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu YX, Liu XM, Nin LF, Shi L, Chen SR. Serine protease and ovarian paracrine factors in regulation of ovulation. Front Biosci (Landmark Ed) 2013;18:650–664. doi: 10.2741/4128. [DOI] [PubMed] [Google Scholar]
- 60.Kim SH, Kang CW, Min KS, Yoon JT. Matrix metalloproteinases are important for follicular development in normal and miniature pigs. Biotechnol Lett. 2014;36:1187–1196. doi: 10.1007/s10529-014-1474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 2015;19:1–15. doi: 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basini G, Bussolati S, Baioni L, Grasselli F. Gelatinases (MMP2 and MMP9) in swine antral follicle. BioFactors. 2011;37:117–120. doi: 10.1002/biof.153. [DOI] [PubMed] [Google Scholar]
- 63.Vos MC, van der Wurff AA, Last JT, de Boed EA, Smeenk JM, van Kuppevelt TH, Massuger LF. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod Biol Endocrinol. 2014;31:1–8. doi: 10.1186/1477-7827-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baka S, Zourla K, Kouskouni E, Makrakis E, Demeridou S, Tzanakaki D, Hassiakos D, Creatsas G. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilization. In Vivo. 2010;24:293–296. [PubMed] [Google Scholar]
- 65.Laflamme BA, Wolfner MF. Identification and function of proteolysis regulators in seminal fluid. Mol Reprod Dev. 2013;80:80–101. doi: 10.1002/mrd.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang WJ, Liu FC, Hsieh JS, Chen CH, Hsiao SY, Lin CS. Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles. Reprod Biol Endocrinol. 2015;13:102. doi: 10.1186/s12958-015-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sankhala RS, Swamy MJ. The major protein of bovine seminal plasma, PDC-109, is a molecular chaperone. Biochemistry. 2010;49:3908–3918. doi: 10.1021/bi100051d. [DOI] [PubMed] [Google Scholar]
- 68.Silva JV, Yoon S, Domingues S, Guimarães S, Goltsev AV, da Cruz E, Silva EF, Mendes JF, da Cruz E, Silva OA, Fardilha M. Amyloid precursor protein interaction network in human testis: sentinel proteins for male reproduction. BMC Bioinform. 2015;16:1–12. doi: 10.1186/s12859-014-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sankhala RS, Kumar CS, Singh BP, Arangasamy A, Swamy MJ. HSP-1/2, a major protein of equine seminal plasma, exhibits chaperone-like activity. Biochem Biophys Res Commun. 2012;427:18–23. doi: 10.1016/j.bbrc.2012.08.120. [DOI] [PubMed] [Google Scholar]