Abstract
Rapamycin and modified rapamycins (rapalogs) have been used to prevent allograft rejection after organ transplant for over 15 years. The mechanistic target of rapamycin (mTOR) has been determined to be a key component of the mTORC1 complex which consists of the serine/threonine kinase TOR and at least five other proteins which are involved in regulating its activity. Some of the best characterized substrates of mTORC1 are proteins which are key kinases involved in the regulation of cell growth (e.g., p70S6K) and protein translation (e.g., 4E‐BP1). These proteins may in some cases serve as indicators to sensitivity to rapamycin‐related therapies. Dysregulation of mTORC1 activity frequently occurs due to mutations at, or amplifications of, upstream growth factor receptors (e.g., human epidermal growth factor receptor‐2, HER2) as well as kinases (e.g., PI3K) and phosphatases (e.g., PTEN) critical in the regulation of cell growth. More recently, it has been shown that certain rapalogs may enhance the effectiveness of hormonal‐based therapies for breast cancer patients who have become resistant to endocrine therapy. The combined treatment of certain rapalogs (e.g., everolimus) and aromatase inhibitors (e.g., exemestane) has been approved by the United States Food and Drug Administration (US FDA) and other drug regulatory agencies to treat estrogen receptor positive (ER+) breast cancer patients who have become resistant to hormonal‐based therapies and have progressed. This review will summarize recent basic and clinical research in the area and evaluate potential novel therapeutic approaches.
Keywords: endocrine resistance, everolimus, exemestane, drug resistance, metastasis, rapamycin
Introduction
It has been shown that the PI3K/PTEN/Akt/mTORC1 kinase cascade is frequently expressed aberrantly in breast and other cancers, which can result in abnormal proliferation. One clinical method to suppress the activity of this cascade is to inhibit the mTOR kinase, a key component present in the mTORC1 complex in this pathway. One approved drug family which suppresses mTOR is the rapamycin family (as known as rapalogs). Rapamycin was originally observed and prescribed to prevent allograft rejection in organ transplant patients. Now rapamycin and certain rapalogs are being used to treat various cancer patients, including breast cancer patients.
Breast cancer is one of the most frequent malignancies in women as approximately one in eight women will develop breast cancer in their lifetime. In the United States, this corresponds to approximately 231 840 new cases of invasive breast cancer each year, which will result in approximately 40 290 deaths 1.
One of the recent major advances in breast cancer therapy is the discovery that certain breast cancers which remain hormone receptor positive (HR+) but have become resistant to endocrine therapy can be treated by the modified rapamycin, everolimus. Everolimus (AFINITOR®), in combination with the aromatase inhibitor exemestane (AROMASIN®), was approved by the US FDA in 2012 to treat HR+, HER2− breast cancer. Everolimus targets the mTORC1 complex, which is often dysregulated in breast cancers that have become resistant to hormone‐based therapies. Suppression of mTORC1 results in inhibition of p70S6K and other proteins. The expression of these proteins is dysregulated in breast cancer patient samples as compared to normal tissues. p70S6K expression is associated with a poor prognosis in breast cancer 2.
Rapamycin and rapalogs used in treatment of human diseases
Rapalogs are synthetic drugs which are analogues of rapamycin. Rapamycin was originally purified from the bacterium Steptomyces hygroscopicus. The rapalogs have better pharmacokinetic kinetic properties than the natural rapamycin. mTORC1 blockers (rapalogs) have been evaluated in clinical trials with breast and other cancer patients 3, 4, 5, 6, 7. Interestingly, different rapalogs have displayed different effects. For example, temsirolimus (TORISEL®) did not show any benefits when compared with endocrine therapy alone. Sirolimus (RAPAMUNE®, rapamycin) has shown promising results in phase II clinical trials 7.
Rapamycin (sirolimus, RAPAMUNE®) was approved by the US FDA to prevent rejection in kidney transplant patients. Temsirolimus (TORISEL®) was approved by the US FDA and European Medicines Agency (EMA) for the treatment of renal cell carcinoma in 2007. Everolimus (AFINITOR®) was approved for the treatment of advanced kidney cancer in 2009 and for prevention of organ transplantation rejection after renal transplant in 2010. In addition, it was approved for treatment of subependymal giant cell astrocytoma (SEGA) that is associated with tuberous sclerosis (TS) in 2010 in those patients who cannot have surgical intervention. It was also approved in 2011 for treatment of patients containing inoperable or metastatic neuroendocrine tumours of pancreatic origin. Everolimus has been approved by the USA FDA for the prevention of organ rejection in liver transplant patients since 2013. Since 2015, sirolimus has been approved by the US FDA for the treatment of lymphangioleiomyomatosis (LAM). LAM is a rare disease affecting lungs, kidneys and the lymphatic system and is progressive.
The rapalogs temsirolimus (TORISEL®) and everolimus (AFINITOR®) are being used to treat breast and renal cancer patients 7, 8, 9. The identification of markers which could predict response to therapy is important. Furthermore, the effects of a combination of these rapalogs with other anti‐cancer drugs are being evaluated.
Endocrine therapy‐aromatase inhibitors (AIs)
Commonly used non‐steroidal AIs include letrozole (FEMARA®) and arimidex (ANASTROZOLE®). These non‐steroidal AIs inhibit estrogen synthesis by a reversible competition for aromatase.
Exemestane (AROMASIN®) is an oral steroidal AI. It binds irreversibly to aromatase and inhibits its activity. It prevents the conversion of cholesterol into pregnenolone thereby suppressing the conversion of androgenic precursors into estrogens. AI therapy has been used in the treatment of all stages (0 to IV) of breast cancer. AI therapy has been used to prevent metastasis of estrogen receptor positive (ER+) breast cancers. AI therapies have been associated with musculoskeletal problems and hot flashes 9. A diagram illustrating the site of action of certain estrogen‐receptor antagonists and AIs and their effects on ERα‐mediated gene expression is presented in Figure 1.
Figure 1.
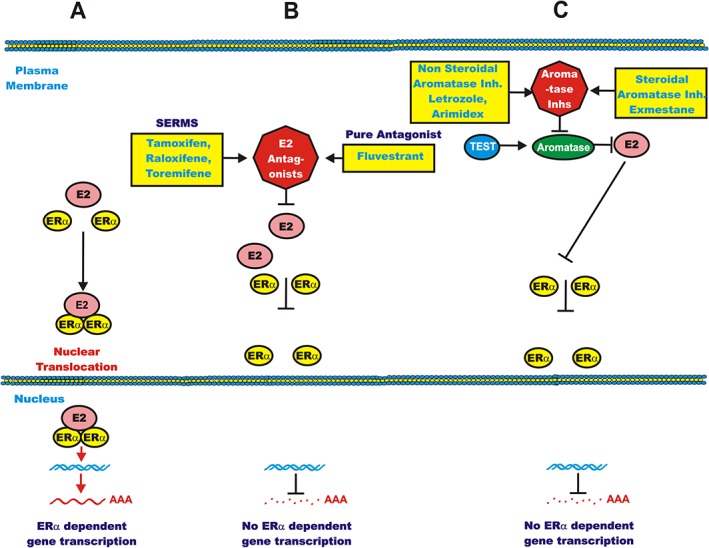
Effects of estrogen, estrogen receptor antagonists and aromatase inhibitors on ERα‐mediated signalling: (A) normal ERα‐mediated signalling; (B) estrogen receptor antagonists block ERα signalling; (C) lack of conversion of testosterone (TEST) to estrogen by aromatase inhibitors preventing conversion. SERMs = selective estrogen‐receptor modulators
Endocrine therapy estrogen‐receptor antagonists
4‐hydroxyl‐tamoxifen (4HT, NOLVADEX®) binds to the ER and competes with estrogen. 4HT belongs to the group of compounds which are called selective estrogen‐receptor modulators (SERMS) (Figure 1). Other SERMS include raloxifene and toremifene. SERMS accelerate the proteasomal degradation of ERα. Fulvestrant (FASLODEX®) is a pure estrogen receptor antagonist. Exemestane treatment is superior to tamoxifen treatment as documented in a phase III clinical trial 10. The superior effects of AIs in comparison to tamoxifen, especially in combination with rapalogs such as everolimus, have been summarized recently 7, 8, 9, 10.
Markers of sensitivity to therapy
The expression and localization of p70S6K1, p70S6K2 and phosphorylated (activated) p70S6K (T389) were examined in two large breast cancer studies. p70S6K1 was associated with high proliferation, HER2 expression and cytoplasmic Akt expression. p70S6K2 was associated with ER+, low proliferation and nuclear Akt (S473) expression. The expression of phosphorylated p70S6K (T389) was correlated with a low benefit to treatment with tamoxifen. The cytoplasmic vs. nuclear localization of p70S6K was determined to be related to benefit with tamoxifen as nuclear p70S6K was associated with reduced benefit to tamoxifen. In contrast, cytoplasmic p70S6K was associated with a significant benefit to tamoxifen. High p70S6K1 protein expression was associated with a poor prognosis whether it was located in the nucleus or cytoplasm. Thus, p70S6K expression and localization could be a marker to predict those patients which may benefit from tamoxifen 11.
Combining estrogen‐receptor antagonists and rapalogs in breast cancer therapy
Approximately 75% of breast cancer patients are HR+ and may be initially sensitive to hormonal‐based therapies which target the HR. The development of resistance to hormonal‐based therapies is a significant problem in breast cancer treatment. The PI3K/PTEN/Akt/mTORC1 pathway is frequently hyper‐activated in endocrine‐resistant breast cancer.
A link between ERα and mTORC1 has recently been discovered. mTORC1 can phosphorylate ERα on S167 via p70S6K which results in its activation. mTORC1 can phosphorylate ERα on S104/S106 which leads to the transcription of ERα‐target genes 12.
The results of a randomized phase II trial of the combination of tamoxifen and everolimus in patients with HER2−, metastatic breast cancer that had previously been treated with AI in the TAMRAD study were presented. The TAMRAD study was a randomized, noncomparative phase II study. The TAMRAD study attempted to determine the efficacy and safety of tamoxifen in combination with everolimus in AI‐resistant metastatic breast cancer patients. The TAMRAD study revealed that the combination of tamoxifen and everolimus increased the clinical benefit rate (CBR) from 42% with tamoxifen alone to 61% with everolimus and tamoxifen. The time to progression (TTP) increased from 4.5 months with tamoxifen alone to 8.6 months in those patients treated with tamoxifen and everolimus. The risk of death was reduced by 55% upon treatment with tamoxifen and everolimus vs. tamoxifen alone 13. A letter to the editor commented on the appropriateness of the study design in this clinical trial 14. The TAMRAD trial showed benefits to those patients who initially responded to AIs but subsequently became resistant. However, the combination did not appear to be beneficial for patients with de novo, previously untreated metastatic HR+ breast cancer 15.
Clinical samples were examined from 52 patients in the TAMRAD trial. Subgroups were identified based on TTP and examined for various markers. The tamoxifen/everolimus‐treated samples with the most improvement in TTP compared with the tamoxifen‐only treated subgroup patients were determined to express high levels of p4EBP1, low levels of 4EBP1, LKB1, pAkt and PI3K. In 45 patient samples examined for mutation at PIK3CA and KRAS, 20% had mutations at PIK3CA and one patient (2%) had a mutation at KRAS. In this study, a positive correlation between downstream targets of mTORC1 and PI3K/PTEN/Akt/mTORC1 activation and everolimus efficiency was observed. The authors pointed out that a larger number of patient samples should be examined 15.
The BRE‐43 study examined the effects of a combination of everolimus and the complete ER antagonist fulvestrant (FASLODEX®) in ER+ patients with metastatic breast cancer. The phase II trial examined this drug combination with postmenopausal woman that were previously treated with an AI who exhibited disease progression or relapse. It was determined that everolimus plus fulvestrant was effective in AI‐pretreated ER+ breast cancer patients whose cancer had metastasized. However, not all patients benefited from this therapeutic approach. The discovery of additional biomarkers may allow the selection of patients who will benefit 16.
Combining aromatase inhibitors and rapalogs in breast cancer therapy
The effects of everolimus has been examined in combination with the nonsteroidal AI letrozole (FEMARA®) in metastatic ER+ breast cancer patients in a phase II randomized study. The addition of everolimus to letrozole increased the response rate from 59.1 to 68.1%. Downregulation of phosphoribosomal protein S6 (rpS6) was detected in the everolimus‐treated patients 17.
The effects of combining letrozole with temsirolimus have been examined in a phase III clinical trial as a first line endocrine therapy in postmenopausal women who had locally advanced or metastatic breast cancer 18. This study revealed that adding temsirolimus to letrozole did not appear to improve the progression‐free survival (PFS) in this group of AI‐naïve breast cancer patients.
Everolimus in combination with exemestane for HR+, HER2− metastatic breast cancer patients
The Breast Cancer Trials of Oral Everolimus‐2 (BOLERO‐2) study evaluated the effects of combining everolimus and exemestane for endocrine‐resistant HR+, HER2− breast cancer 19. The FDA approved the combination of everolimus and exemestane to treat HR+, HER2− breast cancer patients who progressed on treatment with letrozole or anastrazole (Arimidex®, a nonsteroidal AI) AI treatments. The everolimus and exemestane combined treatment resulted in better therapeutic outcomes 19. The primary end point in the BOLERO‐2 clinical trial was PFS and indicated that the combination of everolimus and exemestane improved by more than two‐fold PFS in HR+, HER2− breast cancer patients that had progressed after treatment with nonsteroidal AIs. In contrast, analysis of the secondary end point, which was overall survival (OS), did not result in a statistically significant difference (Clinical Trial #NCT00863655) 20.
The BOLERO‐2 clinical trial also revealed that treatment with everolimus and exemestane had positive effects on bone marrow turnover and progressive disease in bone 21.
Inflammation of the mucous membrane of the mouth (stomatitis) is the most frequent side effect associated with everolimus treatment. If stomatitis is severe, it may result in either discontinuation of everolimus treatment or methods to relieve the stomatitis (steroid‐based mouth rinses) 22.
A summary of a meta‐analysis of randomized breast cancer clinical trials with patients treated with AIs with or without everolimus has been published 23. The analysis was obtained from six studies which included 3693 women who were treated with everolimus plus exemestane or placebo plus exemestane. Everolimus plus exemestane increased the overall response rate (ORR), relative risk, PFS and clinical benefit compared to placebo control as well as the adverse side effects compared to the placebo control (stomatitis, rash, hyperglycaemia, diarrhoea, anorexia and pneumonitis).
Everolimus in combination with letrozole as frontline therapy for HR+, HER2− metastatic breast cancer patients
The Breast Cancer Trials of Oral Everolimus‐4 (BOLERO‐4) study is a phase II clinical trial determining the effects of everolimus plus letrozole as a first‐line therapy for HR+, HER2− metastatic breast cancer patients 24. This trial will evaluate whether there is a role for continuing everolimus and AI treatment after progression. This trial will also examine whether the combination of everolimus plus letrozole can be used after progression in those breast cancer patients treated with everolimus plus exemestane 25.
Activation of the PI3K/PTEN/AKT/MTOR pathway in endocrine‐ resistant breast cancer
The PI3K/PTEN/AKT/mTORC pathway is frequently dysregulated in many different cancer types including: breast 26, 27, pancreatic 28, 29, prostate 30, brain 31, 32, leukaemia 33, 34 and others 35, 36, 37, 38. This pathway is regulated at many steps by tumour suppressor and oncogenes 35, 36. Mutations can occur at multiple steps in this pathway 37, 38. In some cases dysregulation of this signalling pathway can result in drug and therapeutic resistance 39. The mutation rates of components of this and other pathways in breast and other cancers can be found at The Cancer Atlas Data Base: https://tcga‐data.nci.nih.gov/tcga/. There are many different mechanisms which can result in alteration of the activity of the PI3K pathway. Activating mutations at many different genes can occur (e.g., PIK3CA), while mutations which silence tumour suppressor genes can occur (e.g., PTEN). In addition, epigenetic mechanisms as well as miRNAs and lncRNAs can alter the activity of this pathway 40, 41. A diagram illustrating where some of the mutations that occur in signalling pathways is presented in Figure 2.
Figure 2.
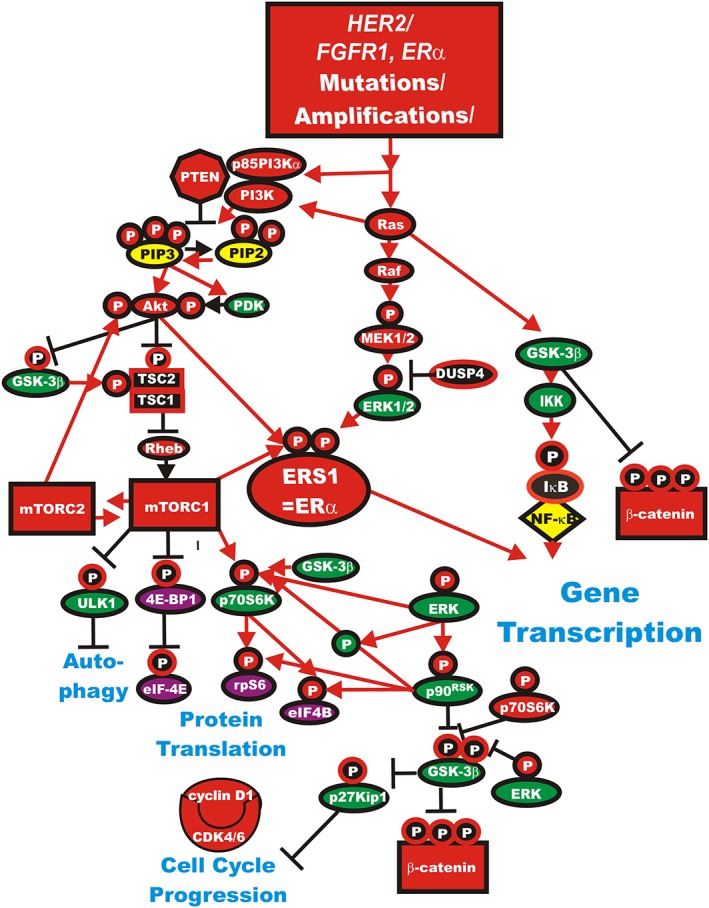
Genetic mutations which result in activation of the Ras/PI3K/PTEN/Akt/mTORC1 and Ras/Raf/MEK/ERK signalling pathways and contribute to malignant transformation and therapy resistance. Sometimes dysregulated expression of growth factor receptors occurs by genetic mutations, translocations or genomic amplifications which can lead to activation of the Ras/Raf/MEK/ERK, Ras/PI3K/PTEN/Akt/mTORC1 and other signalling pathways. Genes in the Ras/PI3K/PTEN/Akt/mTORC1 and Ras/Raf/MEK/ERK pathways that have activating mutations detected in human cancer and proliferative diseases are indicated in red ovals and squares. Other key genes are indicated in green ovals. Red arrows indicate activating events in pathways. Blocked black arrows indicating inactivating events in pathways
Targeting the PI3K/PTEN/Akt/mTORC1 pathway is a very active area of basic and clinical research as well as patient therapy 42, 43. Various components of this pathway have been targeted including PI3K 44, PTEN 45, 46, Akt 47, mTORC1 44 as well as critical downstream components including GSK‐3 37, 38 and NF‐κB 48. A diagram illustrating the PI3K/PTEN/Akt/mTORC1 pathway and some sites where inhibitors target key components is presented in Figure 3.
Figure 3.
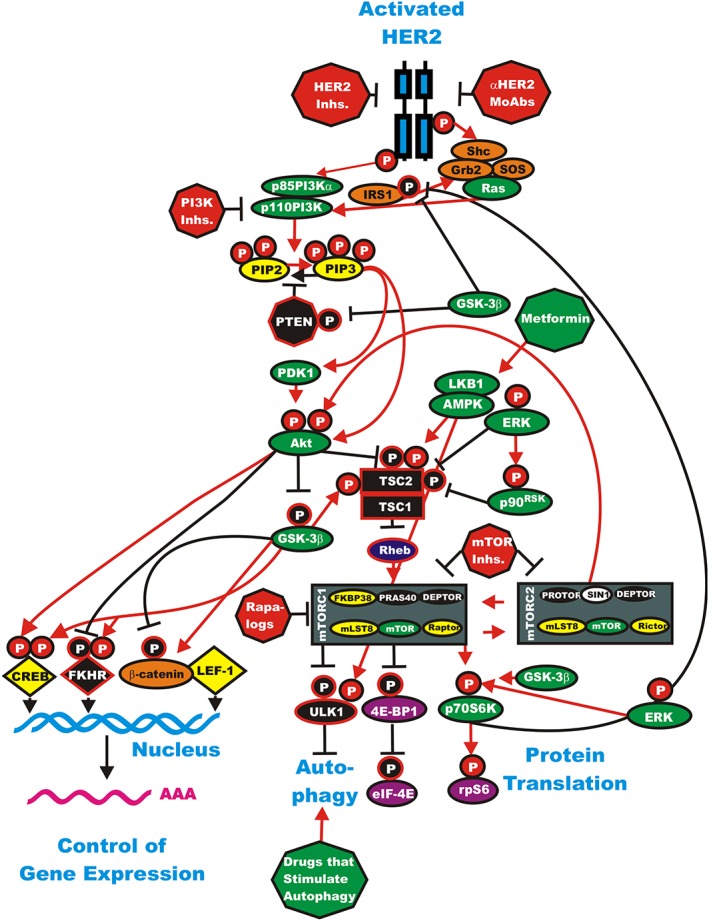
Overview of the HER2/PI3K/PTEN/Akt/mTORC pathway and potential sites for intervention with small molecule membrane‐permeable inhibitors and monoclonal antibodies (MoAbs). The HER2 receptor is indicated in blue. In this figure it is depicted as a homodimer, although it can also heterodimerize with other EGFR family members. The downstream PI3K/PTEN/Akt/mTORC1 pathway is regulated by Ras (indicated in green ovals), PTEN indicated in a black octagon, insulin regulated substrate 1 (IRS1) Shc, Grb2, Sos and β‐catenin are indicated in orange ovals. Kinases are indicated in green ovals. The p85 regulatory subunit of PI3K (p85PI3Kα) is indicated in a green oval. The phosphatases which inhibit steps in this pathway are indicated in black octagons. TSC1 and TSC2 are indicated in black squares. PIP2 and PIP3 are indicated in yellow ovals. The mTORC1 blockers (rapalogs), PI3K and mTOR inhibitors are indicated in red octagons. The AMPK activator metformin is indicated in a green octagon. mTOR interacting proteins, which positively regulate mTOR activity, are indicated in yellow ovals. mTOR interacting proteins which negatively regulate mTOR activity are indicated in black ovals. Transcription factors activated by either ERK or Akt phosphorylation are indicated in yellow diamonds. The FKHR transcription factor that is inactivated by Akt phosphorylation is indicated by a black diamond and a white P in a black circle. FKHR is also activated by GSK‐3β phosphorylation which is indicated by a white P in a red circle. mRNA initiation factors and proteins associated with the ribosome are indicated in magenta ovals. mTORC1 phosphorylates the unc‐51‐like kinase 1 (ULK1) which results in the suppression of autophagy. ULK1 is indicated in a black oval. In contrast, AMPK activates both ULK1 and autophagy as well as TSC activity. Proteins involved in the regulation of translation are indicated in purple ovals. Red arrows indicate activating events in pathways. Black arrows indicate inactivating events in pathways. Activating phosphorylation events are depicted in red circles with Ps with a black outlined circle. Inactivating phosphorylation events are depicted in black circles with Ps with a red outlined circle
Tamoxifen will inhibit the growth of bulk adherent breast cancer cells (BC) but not the breast cancer stem cells (CSCs). Tamoxifen‐treated CSCs were determined to express proteins involved in the PI3K/PTEN/Akt/mTORC1 pathway including proteins associated with ribosome biogenesis and mRNA translation including p70S6K1, rpS6 and eukaryotic translation initiation factor 4E‐binding protein 1 (4E‐BP1). Combined treatment of the breast CSCs with tamoxifen and rapamycin or the rapalog everolimus or the dual PI3K/mTOR inhibitor PF‐04691502 suppressed mammosphere formation. These studies indicate the importance of the mTORC1 pathway in mammosphere formation and endocrine resistance 49.
Everolimus in combination with other drugs to treat herceptin‐resistant breast cancer patients
Activation of the PI3K/PTEN/Akt/mTORC1 pathway has been shown to be involved in herceptin resistance in HER2+ breast cancers. A phase II safety and clinical activity trial with oral sirolimus, in combination with herceptin (trastuzumab), was performed with patients with metastatic breast cancer who exhibited disease progression after prior herceptin therapy. The combination of sirolimus and herceptin appeared to be well tolerated in this study. There was also some disease activity. Thus, in some HER‐resistant tumours, mTOR inhibition may overcome herceptin resistance 50.
It was known that rapalogs would suppress herceptin resistance in PTEN‐deficient tumours and make the normally herceptin‐resistant tumours sensitive to herceptin. The BOLERO‐1 clinical trial (ClinicalTrials.gov, number NCT00876395) examined the safety of combining everolimus and herceptin and paclitaxel in the treatment of advanced HER2+ breast cancer patients. The advanced breast cancer patients enrolled in this study had not been treated with either herceptin or chemotherapy within 12 months of randomization. This study revealed that the PFS was not significantly different between the full analysis population (everolimus and herceptin and paclitaxel vs. placebo and herceptin and paclitaxel). Although this study did not meet its prespecified criteria for significance, the authors reported that the HR‐/HER2+ population treated with everolimus needs further evaluation as in this subgroup, a 7.2‐month prolongation was observed in the arm that received everolimus and herceptin and paclitaxel in comparison to placebo and herceptin and paclitaxel 51.
The BOLERO‐3 phase III clinical trial examined the effects of everolimus in combination with other drugs (vinorelbine, NAVELBINE®) in HER2+, herceptin‐resistant breast cancer patients. Addition of everolimus to the combination of herceptin plus vinorelbine prolonged PFS in advanced herceptin‐resistant breast cancer patients who had previously been treated with taxanes (ClinicalTrials.gov number NCT01007942) 52. While the BOLERO‐3 trial was statistically significant, the clinical usefulness may be limited and did not lead to an indication in this breast cancer subtype.
Use of rapalogs to overcome herceptin resistance in HER2+ breast cancers
In a RNA interference study of 8000 genes, only PTEN suppression was determined to be responsible for herceptin resistance in cells which overexpressed HER2 53. Moreover, this study determined that PTEN‐deficient tumours had lower responses to herceptin than PTEN‐positive tumours. In subsequent studies, it was demonstrated that activation of the PI3K/PTEN/Akt/mTOR pathway was associated with shorter PFS in herceptin‐treated patients 54. When everolimus was combined with chemotherapy, sensitivity to herceptin was observed in breast cancer models which overexpressed HER2 55.
Combining everolimus and chemotherapeutic drugs to treat triple negative breast cancer (TNBC)
The effects of combining everolimus and chemotherapeutic drugs (e.g., carboplatin) have been examined in a clinical trial with metastatic TNBC. The trial with everolimus and carboplatin demonstrated efficacy in PFS for 3 months. In this study, clinical benefit was defined as (complete remission (CR) + partial remission (PR) + stable disease (SD) lasting ≥ 6 months). This trial demonstrated a clinical benefit of 36% (ClinicalTrials.gov NCT01127763) 56.
The effects of combining everolimus with neoadjuvant chemotherapy with paclitaxel for 12 weeks followed by 5‐flurouracil, epirubicin and cyclophosphamide every three weeks for four cycles was evaluated in patients with TNBC. In this study, no significant differences were observed between the everolimus‐treated and the nontreated groups 57.
Markers of sensitivity to rapamycin/rapalogs
A total of 302 tumour specimens from HR+, HER2− patient samples from participants of the BOLERO‐2 clinical trial were examined by next‐generation sequencing for genetic mutations. Interestingly, it was determined that the benefit from everolimus on PFS was present regardless of genetic alterations in PIK3CA, FGFR1 and CCND1 or dysregulation of the signalling pathways of which they are components. However, differences in everolimus benefit were observed in PI3KCA exon specific mutations (exon 20 vs. exon 9) as well as the degree of chromosomal instability 58.
Detection of single nucleotide polymorphism (SNP) signatures may be an approach to find patients which show activation of mTOR. Such signatures may define patients who are sensitive to rapalog treatment 59.
Combining everolimus with metformin
The effects of combining everolimus with the adenosine 5′‐monophosphate‐activated protein kinase (AMPK) activator metformin have been evaluated. The site of action of metformin is shown in Figure 3. This combination was determined to inhibit the growth and mammosphere formation better than either drug by itself. The combination of everolimus and metformin also suppressed the phosphorylation of downstream targets including rpS6 and 4E‐BP1 in HCC1429 cells. The combination of everolimus and metformin was also determined to suppress the development of xenografts better than treatment with either drug by itself 60. There is a clinical trial examining the effects of everolimus and metformin in breast cancer therapy (NCT01627067).
Combining everolimus with PI3K and TOR inhibitors
Recently, it has become apparent that blocking signalling pathways at multiple levels may be more effective than blocking a pathway at a single position. This may result from multiple mechanisms including intricate negative and positive feedback pathways as well as mutations at different components. TOR inhibitors actually block the kinase activity of TOR, specifically the TOR serine/threonine kinase that is present in both mTORC1 and mTORC2. Rapalogs block the activity of mTORC1 but not normally mTORC2. There are also dual PI3K and TOR inhibitors which block both PI3K and TOR. The effects of combining the mTORC1 blocker everolimus with PI3K and TOR kinase inhibitors has been examined in a panel of 30 breast cancer cell lines. A correlation between everolimus IC50 values and p70S6K phosphorylation was observed in these studies. In contrast, a correlation between everolimus IC50 values and Akt or ERK phosphorylation was not observed. The effects of combining everolimus and the kinase inhibitors were also examined on four everolimus‐resistant cell lines and inhibition of proliferation was observed. Thus, it may be possible to enhance the effects of everolimus with mTOR and PI3K inhibitors on certain breast cancers 61.
Resistance to rapamycin
In some breast cancer models, activated Akt signalling is associated with rapamycin resistance. This may be due to phosphorylation of the transcriptional repressor 4E‐BP1. While rapamycin or the Akt inhibitor MK2206 solo treatment did not have significant effects on suppressing 4E‐BP1 phosphorylation, growth or mobility of tumour cells, the combined treatment did have benefits both in vitro and in vivo. The combined treatment suppressed the phosphorylation of the proline‐rich Akt substrate of 40 kDa (PRAS40) on S183 and T246 which are normally mediated by mTORC1 and Akt respectively. This resulted in enhanced binding of dephosphorylated PRAS40 to Raptor/mTOR which repressed mTORC1‐mediated 4E‐BP1 phosphorylation and protein translation. These studies provide a mechanism of how rapamycin and Akt inhibitors may enhance suppression of tumour growth 62.
The epithelial mesenchymal transition (EMT) has been proposed to confer rapamycin resistance. The epithelial protein E‐cadherin was observed to be expressed at higher levels in rapamycin‐sensitive cells. In contrast, E‐cadherin was expressed at lower levels in mesenchymal breast cancer cells that were less sensitive to rapamycin. MCF‐7 breast cancer cells transfected with the constitutively active Snail transcription factor were resistant to rapamycin. Inhibition of ZEB1 transcription factor by transfection of ZEB siRNA in mesenchymal breast cancer cells promoted mesenchymal to epithelial transition (MET), E‐cadherin expression and sensitivity to rapamycin. The effects of rapamycin on cell growth could be enhanced by treatment of the mesenchymal cells with the MEK inhibitor trametinib 63.
Resveratrol is a stilbenoid (natural phenol) found in the skin of red grapes, blueberries, raspberries, mulberries and other plants. Resveratrol is induced when the plant is attacked by bacteria or fungi. The effects of rapamycin and resveratrol on the induction of apoptosis and autophagy have been examined in breast cancer cells. Breast cancer cells may often become resistant to rapamycin treatment by upregulation of the Ras/PI3K/PTEN/Akt/mTORC1 pathway due to the induction of autophagy which results in the prevention of apoptosis. Addition of rapamycin and resveratrol to ER+ breast cancer cells was shown to induce apoptosis while inhibiting Akt activation 64.
Lipins are multifunctional proteins involved in lipid metabolism. Lipins can act as enzymes by dephosphorylating phosphatidic acid to diacylglycerol. Lipins can also function as co‐transcriptional regulators. The role of lipin‐1 in rapamycin sensitivity has recently been investigated. Lipins have been observed to be upregulated in certain tumours including breast cancers. Lipins stimulate the proliferation of breast tumour lines as knock‐down of lipin‐1 reduced proliferation of breast cancer cells but not of normal cells. Knock‐down of lipin‐1 resulted in activation of RhoA which suppressed cell migration. Autophagy was also induced when lipin‐1 was suppressed. Propranolol is a sympatholytic nonselective beta blocker which will inhibit lipins. Suppression of lipins with propranolol increased sensitivity of the cells to rapamycin 65.
p27Kip1 has recently been shown to be a predictive biomarker for the response to rapamycin in certain patient‐derived xenograft (PDX) models. Breast cancer cells which expressed high levels of p27Kip1 were observed to be sensitive to rapamycin. Rapamycin treatment was determined to decrease phosphorylation of p70S6K and 4E‐BP1 66.
PDX breast cancer models are being developed by many investigators and there are some models commercially available 67, 68. All the human intrinsic breast cancer subtypes are represented in various breast PDX models. Many small molecule inhibitors (e.g., Bcl‐2 inhibitors), drugs such as rapalogs and hormonal‐based therapies and others either have been or will be evaluated in PDX models of breast cancer 69, 70.
p70S6K and 4E‐BP1 are two key downstream substrates of mTORC1. Knock‐down of both p70S6K and 4E‐BP1 resulted in a transforming growth factor‐β (TGF‐β) dependent G1 arrest in the TNBC MDA‐MB‐231 cell line. Nanomolar concentrations of rapamycin led to inhibition of p70S6K phosphorylation in MDA‐MB‐231 cells; however, much higher doses (micromolar) were required to inhibit 4E‐BP1 phosphorylation, which resulted in the liberation of eIF4E to promote protein translation. Micromolar concentrations of rapamycin were required to induce G1 arrest indicating the importance of 4E‐BP1. G1 arrest was determined to be increased by TGF‐β signalling and downregulation of Rb phosphorylation by p70S6K and 4E‐BP1 respectively 71.
Phorbol esters (PMA) can induce either proliferation or cell cycle arrest depending on the cell type and culture conditions. PMA will hyperactivate the Raf/MEK/ERK pathway in SKBr3 breast cancer cells which in turn induces p21Cip‐1, cell cycle arrest, and cellular senescence (geroconversion). mTOR and p70S6K were involved in geroconversion. PMA has been shown to induce cell cycle arrest while constitutively active mTOR mediated geroconversion. Rapamycin suppressed geroconversion and maintained quiescence. PMA elicited its effects via phosphorylation of p70S6K on T389 and S6 on S240/244, which was inhibited by rapamycin treatment. In contrast, in the presence of PMA, phosphorylation of p70S6K on T421/S424 and S6 on S235/236 were rapamycin‐insensitive. These studies indicated that rapamycin can decrease geroconversion induced by PMA without preventing PMA‐induced cell cycle arrest 72.
Rapamycin and autophagy
The effects of rapamycin and the anti‐malarial drug hydroxychloroquine on autophagy in cancer patients have been examined. Rapamycin and chemotherapy are known to enhance autophagy while hydroxychloroquine may inhibit it. Analysis of a limited patient cohort suggested that rapamycin and hydroxychloroquine treatment in combination with chemotherapy resulted in the classification of autophagy as an oncotarget 73.
Other approaches to targtet mTORC1 activity – mTOR inhibitors
AZD2014 is a novel mTOR kinase inhibitor which blocks the activity of both mTORC1 and mTORC2. It has proved to be more effective in inducing growth inhibition in several breast cancer cell lines than everolimus. AZD2014 has been examined in combination with fulvestrant. This combination resulted in tumour growth inhibition or regression in ER+ breast cancer models. The effects of AZD2014 have also been examined in the CTC174 explant model which has a mutated ER. AZD2014 is currently in phase II clinical study development 74.
AZD2014 is being examined in at least six clinical trials with breast cancer patients. Some are in combination with: fulvestrant (NCT01597388 and NCT02216786), the cyclin‐dependent kinase4/6 inhibitor, palbociclib (IBRANCE®) and fulvestrant (NCT02599714), the MEK inhibitor Selumetinib (NCT02583542), the oral Akt inhibitor AZD5363 (NCT02208375) or with various other drugs to determine the efficacy of high throughput genome analysis as a therapeutic decision tool (NCT0229999).
A phase I dose‐escalation study has been performed with the mTORC1/mTORC2 kinase inhibitor CC‐223 in cancer patients with either advanced solid tumours or multiple myeloma. Suppression of downstream targets of mTORC1/mTORC2 was observed in patient biopsies. This phase I study concluded that treatment with CC‐223 was tolerable, with manageable toxicities and antitumour activity including regression 75.
Rapalogs and bone cancer pain
The PI3K/PTEN/Akt/mTORC1 pathway has also been shown to be involved in bone cancer pain. Rapamycin was shown to prevent protein kinase C epsilon (PKC‐epsilon) and protein kinase A (PKA) normally induced by morphine treatment. Suppression of mTORC1 may be an approach to block bone pain in certain breast cancer patients who exhibit activation of PI3K/PTEN/Akt/mTORC, PKC‐epsilon and PKA 76.
Combining PI3K and dual PI3K/MTORC1 inhibitors
The effects of combining the PI3K inhibitor pilaralisib or the PI3K/mTOR dual inhibitor voxtalisib with letrozole has been examined in phase I/II clinical trials in HR+, HER2− breast cancers that were refractory to nonsteroidal AI therapy. Interestingly, in these studies, no association between PI3K pathway mutations and efficacy was observed. These studies indicated that AI and PI3K or PI3K/mTOR inhibitors may be combined to potentially treat endocrine therapy‐resistant HR+, HER‐ metastatic breast cancer patients 77.
The effects of the dual PI3K/mTOR inhibitor NVP‐BEZ235 and either an autophagy inhibitor or autophagy gene knockdown have been examined in MCF‐7 cells. Combining NVP‐BEZ235 and autophagy inhibitors were shown to result in increased growth inhibition 78.
Summary
HR+ breast cancer patients are often treated with endocrine therapies. However, a large proportion of the patients will develop resistance to endocrine therapy and disease progression occurs. Increased PI3K/PTEN/Akt/mTORC1 pathway activity is observed in patients who underwent either endocrine or HER2−targeted therapies. This review has focused on the recent studies on targeting the PI3K/PTEN/Akt/mTORC1 pathway in HR+ breast cancer patients which have become resistant to AIs. Over the past decade, it has become apparent that certain ER+ breast cancer patients may become resistant to nonsteroidal AIs. Part of their resistance may be due to activation of the PI3K/PTEN/Akt/mTORC1 pathway, which is a key pathway involved in proliferation and the prevention of apoptosis. Rapalogs which inhibit mTORC1 were determined to block the AI resistance documenting the importance of this pathway in AI resistance in HR+ breast cancers. Various rapalogs have been examined in clinical trials and one of the most promising is everolimus. This review has also summarized the effects of rapalogs on other breast cancer types including HR‐/HER2+ breast cancer. In addition, more novel inhibitors which target the kinase activity of mTOR and suppress both mTORC1 and mTORC2 may be more effective in suppressing AI resistance in combination with AIs. Other drugs and natural products which when combined with rapalogs may eventually be useful in breast cancer therapy (e.g., metformin and resveratrol) have been discussed.
In summary, there are a large number of clinical trials which will determine the effects of everolimus on breast cancer therapy. There are numerous clinical trials listed on ClinicalTrials.gov for the everolimus and breast cancer therapy https://clinicaltrials.gov/ct2/results?term=%22everolimus%22+AND+%22breast+cancer%22&Search=Search. In Table 1, clinical trials with breast cancer patients which examine the effects of rapalogs combined with agents that target HER2 and/or epidermal growth factor receptor (EGFR) or insulin‐like growth factor receptor (IGF‐1R) and hormonal‐based therapy are presented. In Table 2, clinical trials with breast cancer patients which examine the effects of rapalogs combined with chemotherapeutic drugs, PI3K/mTOR kinase inhibitors and metformin are presented. The diverse array of drugs being combined with rapalogs documents the intensity of developing effective approaches to treat breast cancer. These numerous clinical trials and basic research studies point to the significant roles that specific targeted therapy has had on breast cancer treatment which may be extended to other tumours, especially hormonal‐responsive cancer.
Table 1.
Breast cancer clinical trials with rapalogs alone or in combination with EGFR/HER2/IGFR inhibitors or hormonal‐based agents (to April 2016)
| Official trial name | Clinical trial # | Phase of trial | Type of cancer patient in trial | Status of trial | Intervention | Publications |
|---|---|---|---|---|---|---|
| Rapalogs * | ||||||
| A randomized study of mTOR inhibition by RAD001 (everolimus) in invasive breast cancer patients after pre‐operative use of anthracycline and/or taxane‐based chemotherapy | NCT01088893 | II | BC | Unknown because information has not been verified recently | Everolimus | Not provided |
| A phase II trial of short‐term everolimus (RAD001) to predict response in women with operable breast cancer | NCT00855114 | II | BC | Withdrawn | Everolimus (mTORC1 blocker) | Not provided |
| Phase II trial of CCI‐779 (temsirolimus) in patients with locally advanced or metastatic breast cancer | NCT00376688 | II | Male BC, recurrent BC, stage IIIA, stage IIIB, stage IIIC, stage IV BC | Ongoing, but not recruiting | Temsirolimus (mTORC1 blocker) | Not provided |
| A phase II trial of RAD001 in triple negative metastatic breast cancer | NCT00827567 | II | Breast cancer | Terminated | RAD001 (everolimus) | 79, 80, 81 |
| Randomized, double blind, multicentre phase III trial evaluating the safety and benefit of adding everolimus to adjuvant hormone therapy in women with poor prognosis, ER+ and HER2− primary breast cancer who remain free of disease after receiving 3 years of adjuvant hormone therapy | NCT01805271 | III | ER+, HER2− BC | Recruiting | Everolimus | Not provided |
| RADAR: a randomized discontinuation phase II study to determine the efficacy of RAD001 in breast cancer patients with bone metastases | NCT00466102 | II | BC | Ongoing, but not recruiting | RAD001 | Not provided |
| A randomized phase II study of two different schedules of RAD001C in patients with recurrent/metastatic breast cancer | NCT00255788 | II | BC | Completed | Everolimus | Not provided |
| Rapalogs plus herceptin † | ||||||
| A brief dose escalation followed by a phase II study of RAD001 in combination with trastuzumab in HER2‐positive metastatic breast cancer | NCT00458237 | I/II | BC | Ongoing, but not recruiting | Everolimus, trastuzumab | 82 |
| A Phase Ib/II Study Investigating the Combination of Everolimus With Trastuzumab and Paclitaxel in Patients With HER2‐overexpressing Metastatic Breast Cancer | NCT00426556 | I | Metastatic BC | Completed | Everolimus, trastuzumab, paclitaxel | Not provided |
| A phase Ib study investigating the combination of everolimus with trastuzumab and vinorelbine in patients with HER2‐overexpressing metastatic breast cancer | NCT00426530 | I | Breast neoplasms, neoplasm metastasis | Completed | Everolimus, trastuzumab, vinorelbine | 83 |
| A phase II study of rapamycin (rapamune, sirolimus) and trastuzumab (herceptin) for patients with HER‐2 receptor positive metastatic breast cancer | NCT00411788 | II | BC | Recruitment is unknown | Rapamycin, trastuzumab | Not provided |
| A phase II study evaluating the efficacy and tolerability of everolimus (RAD001) In combination with trastuzumab and vinorelbine in the treatment of progressive HER2‐positive breast cancer brain metastases | NCT01305941 | II | HER2+ BC | Recruiting | Everolimus, vinorelbine, trastuzumab | Not provided |
| A randomized phase III, double‐blind, placebo‐controlled multicenter trial of everolimus in combination with trastuzumab and paclitaxel, as first line therapy in women with HER2‐positive locally advanced or metastatic breast cancer | NCT00876395 | III | BC | Ongoing, not recruiting | Everolimus, trastuzumab,paclitaxel | 51 |
| A phase II neoadjuvant study of RAD001 (everolimus) in combination with paclitaxel and trastuzumab for operable HER2‐positive breast cancer | NCT01163929 | II | BC | Withdrawn prior to enrolment | Paclitaxel | Not provided |
| A phase II study evaluating the efficacy and tolerability of everolimus (RAD001) in combination with trastuzumab and vinorelbine in the treatment of progressive HER2‐positive breast cancer brain metastases | NCT01305941 | II | HER2+ BC | Recruiting | Everolimus, vinorelbine, trastuzumab | Not provided |
| Phase I‐II study of trastuzumab in combination with RAD001 in patients with HER‐2 overexpressing, PTEN‐deficient metastatic breast cancer progressing on trastuzumab‐based therapy | NCT00317720 | I/II | BC metastatic | Completed | Trastuzumab, RAD001 | 82 |
| A phase II, randomized, multicentre study, assessing value of adding everolimus (RAD001) to trastuzumab as preoperative therapy of HER‐2 positive primary breast cancer amenable to surgery | NCT00674414 | II | BC | Terminated due to accrual issue (82 points accrued/120 expected) | Trastuzumab, everolimus | Not provided |
| Randomized phase II trial of trastuzumab or everolimus in hormone‐refractory metastatic breast cancer | NCT00912340 | II | BC | Ongoing, not recruiting | Trastuzumab (herceptin), everolimus | Not provided |
| A randomized phase III, double‐blind, placebo‐controlled multicentre trial of daily everolimus in combination with trastuzumab and vinorelbine, in pretreated women with HER2/Neu overexpressing locally advanced or metastatic breast cancer | NCT01007942 | III | HER2/Neu overexpressing locally advanced BC, metastatic BC | Completed | Everolimus, vinorelbine, trastuzumab | 84 |
| A phase II trial of oral deforolimus (AP23573; MK‐8669), an mTOR inhibitor, in combination with trastuzumab for patients with HER2‐positive trastuzumab‐refractory metastatic breast cancer | NCT00736970 | II | BC | Completed | Ridaforolimus, trastuzumab | 85 |
| Rapalogs plus EGFR/HER2 or IGF‐1R inhibitors ‡ | ||||||
| Phase II trial of lapatinib and RAD001 for HER2‐positive metastatic breast cancer | NCT01283789 | II | Metastatic BC | Ongoing not recruiting | Lapatinib, RAD‐001 | Not provided |
| Phase I study of combined temosirolimus, erlotinib and cisplatin in advanced solid tumours | NCT00998036 | I | TNBC | Completed | Temsirolimus, cisplatin, erlotinib | Not provided |
| *A phase III trials programme exploring the integration of bevacizumab, everolimus (RAD001), and lapatinib into current neoadjuvant chemotherapy regimes for primary breast cancer | NCT00567554 | III | BC | Completed | Epirubicin, cyclophosphamide, docetaxel, bevacizumab, paclitaxel, everolimus, trastuzumab, lapatinib | 86, 87, 88 |
| Phase I/II trial of an oral mTOR protein kinase inhibitor (everolimus, RAD001) in combination with an oral EGFR tyrosine kinase inhibitor (erlotinib, Tarceva™) in patients with metastatic breast cancer | NCT00574366 | I | BC | Completed | Erlotinib (EGFR1 inhibitor), everolimus (RAD001) | Not provided |
| *Phase Ib/II single‐arm trial evaluating the combination of lapatinib, everolimus and capecitabine for the treatment of patients with HER2‐positive metastatic breast cancer with CNS progression after trastuzumab | NCT01783756 | I/II | Central nervous system metastases HER2+ BC, male BC, recurrent BC, stage IV BC | Currently recruiting | Lapatinib ditosylate, everolimus, capecitabine | Not provided |
| Phase I/II trial of IMC‐A12 in combination with temsirolimus in patients with metastatic breast cancer | NCT00699491 | I/II | Male BC, recurrent BC, stage IV BC | Ongoing, but not recruiting | Cixutumumab (an IGF‐1R inhibitor), temsirolimus | Not provided |
| Phase II trial of lapatinib in combination with everolimus in triple negative metastatic or locally advanced breast cancer | NCT01272141 | II | BC | Terminated | Lapatinib, everolimus | Not provided |
| GCC 0901 – a phase II study of letrozole in combination with lapatinib followed by an addition of everolimus in postmenopausal women with advanced endocrine resistant breast cancer | NCT01499160 | II | Breast neoplasms, endocrine breast diseases, neoplasm metastasis | Ongoing, not recruiting | Letrozole, lapatinib, everolimus | Not provided |
| Rapalogs plus hormonal‐based therapies § | ||||||
| A multicentre randomized, double blind, placebo‐controlled, phase II study to compare endocrine treatment alone vs. endocrine treatment with everolimus in patients with HR+/HER2− metastatic breast cancer and progression after previous treatment with exemestane and everolimus | NCT01773460 | III | Metastatic BC | Terminated | Everolimus | Not provided |
| *Phase II study of everolimus in combination with exemestane vs. everolimus alone vs. capecitabine in the treatment of postmenopausal women with ER + locally advanced, recurrent, or metastatic breast cancer after recurrence or progression on prior letrozole or anastrozole | NCT01783444 | II | BC | Ongoing, not recruiting | Capecitabine (a pro‐drug, which is converted to 5‐flurouracil (5‐FU) which inhibits thymidylate synthase, exemestane, everolimus | Not provided |
| A phase Ib/II trial of LEE011 in combination with everolimus (RAD001) and exemestane in the treatment of postmenopausal women with ER+, HER2− locally advanced or metastatic breast cancer | NCT01857193 | I/II | BC | Currently recruiting | LEE011, a CDK4/6 inhibitor, exemestane, everolimus | Not provided |
| A phase IV multicentre, open label study of postmenopausal women with ER+ locally advanced or metastatic breast cancer treated with everolimus (RAD001) in combination with exemestane, with exploratory epigenetic marker analysis | NCT01743560 | IV | ER+ advanced BC | Ongoing, but not recruiting | RAD001, exemestane | Not provided |
| An open‐label, phase II, single‐arm study of everolimus in combination with letrozole in the treatment of postmenopausal women with ER+ HER2 negative metastatic or locally advanced breast cancer | NCT01698918 | II | ER+ BC | Ongoing, not recruiting | Everolimus, letrozole, exemestane | Not provided |
| *Phase II study of everolimus in combination with exemestane vs. everolimus alone vs. capecitabine in the treatment of postmenopausal women with ER+ locally advanced, recurrent, or metastatic breast cancer after recurrence or progression on prior letrozole or anastrozole | NCT01783444 | II | BC | Ongoing, not recruiting | Capecitabine, exemestane, everolimus | Not provided |
| A phase IIIB, multicentre, open label study for postmenopausal women with ER+ locally advanced or metastatic breast cancer treated with everolimus (RAD001) in combination with exemestane: 4EVER – efficacy, safety, health economics, translational research | NCT01626222 | III | Metastatic BC | Completed | Exemestane, everolimus | Not provided |
| A phase II study of combined fulvestrant (faslodex) and RAD001 (everolimus) in advanced/metastatic breast cancer after aromatase inhibitor failure | NCT00570921 | II | BC | Completed | RAD001, fulvestrant | 16 |
| Circulating FGF21 levels and efficacy of exemestane, everolimus and metformin in postmenopausal women with HR+ metastatic breast cancer and BMI ≥ 25 | NCT01627067 | II | BC | Ongoing, not recruiting | Everolimus, exemestane metformin | Not provided |
| A randomized double‐blind, placebo‐controlled study of everolimus in combination with exemestane in the treatment of postmenopausal women with ER+ locally advanced or metastatic breast cancer who are refractory to letrozole or anastrozole | NCT00863655 | III | BC | Completed | Everolimus, exemestane | 19, 89, 90, 91, 92, 93 |
| Phase II open label study of everolimus (RAD001) in combination with letrozole in the treatment of postmenopausal women with locally advanced or metastatic breast cancer women with ER+ after failure of tamoxifen and or anestrozole or examestane | NCT01231659 | IV | Postmenopausal women, locally advanced, or metastatic BC | Ongoing, but not recruiting | Everolimus, letrozole | Not provided |
| Randomized, double‐blind, placebo‐controlled phase II trial of fulvestrant (faslodex) plus everolimus in postmenopausal patients with HR+ metastatic breast cancer resistant to aromatase inhibitor therapy | NCT01797120 | II | Metastatic BC | Ongoing, not recruiting | Fulvestrant, everolimus | No results posted yet |
| Phase III randomized, placebo‐controlled clinical trial evaluating the use of adjuvant endocrine therapy ± one year of everolimus in patients with high‐risk, HR+ and HER2/Neu negative breast cancer | NCT01674140 | III | BC | Recruiting | Anastrozole, everolimus, exemestane, goserelin acetate, letrozole, leuprolide acetate, tamoxifen citrate | Not provided |
| A phase II, double‐blind, randomized, placebo‐controlled, multicentre study assessing the value of adding everolimus to letrozole as preoperative therapy of primary breast cancer in postmenopausal women | NCT00107016 | II | BC | Completed | RAD001, letrozole | 17 |
| A phase II randomized open‐label study of letrozole in combination with two dose levels and schedules of oral temsirolimus (CCI‐779), or letrozole alone, in postmenopausal women with locally advanced or metastatic breast cancer | NCT00062751 | II | BC | Completed | Letrozole, temsirolimus (CCI‐779) | Not provided |
| Phase II study assessing the tolerance and efficacy of tamoxifen alone vs. the association tamoxifen‐RAD001 (everolimus) in patients with anti‐aromatase resistant breast metastatic cancer | NCT01298713 | II | BC mTOR proteins | Ongoing, not recruiting | Tamoxifen, everolimus | 15 |
| A phase II randomized trial of the combination of ridaforolimus and exemestane, compared to ridaforolimus, dalotuzumab and exemestane in high proliferation, ER+ breast cancer patients | NCT01605396 | II | BC | Ongoing, but not recruiting | Ridaforolimus, dalotuzumab, exemestane | Not provided |
| GCC 0901 – a phase II study of letrozole in combination with lapatinib followed by an addition of everolimus in postmenopausal women with advanced endocrine resistant breast cancer | NCT01499160 | II | Breast neoplasms, endocrine breast diseases, neoplasm metastasis | Ongoing, not recruiting | Letrozole, lapatinib, everolimus | Not provided |
| A phase II randomized open‐label study of letrozole in combination with two dose levels and schedules of oral temsirolimus (CCI‐779), or letrozole alone, in postmenopausal women with locally advanced or metastatic breast cancer | NCT00061971 | II | BC | Completed | Letrozole, temsirolimus (CCI‐779) | Not provided |
| A phase III randomized, placebo‐controlled, double‐blind study of oral CCI‐779 administered in combination with letrozole vs. letrozole alone as first line hormonal therapy in postmenopausal women with locally advanced or metastatic breast cancer | NCT00083993 | III | Breast neoplasms, neoplasm metastasis | Terminated | Temsirolimus (CCI‐779) for 34 months, letrozole for 34 months | 18 |
| A two‐part adaptive, randomized trial of ridaforolimus in combination with dalotuzumab compared to exemestane or compared to ridaforolimus or dalotuzumab monotherapy in ER+ breast cancer patients | NCT01234857 | II | BC | Completed | Ridaforolimus, dalotuzumab, exemestane | Not provided |
Everolimus (RAD001, AFINITOR®), Temsirolimus (CCI‐779, TORISEL®).
Everolimus (RAD001), Temsirolimus (CC1‐779), Deforolimus (Ridaforolimus, AP23573, MK‐8669) and Trastuzumab (Herceptin).
Everolimus (RAD001),Temsirolimus (CCI‐779) and EGFR/HER2 Inhibitor (Lapatinib [TYKERB®], EGFR inhibitor Erlotinib [TARCEVA®]) and IGF‐R1 Inhibitors (IMC‐A12, Cixutumumab).
Everolimus (RAD001), Ridaforolimus (Deforolimus), Temsirolimus (CCI‐779), and Hormonal Therapy (Exemestane [AROMASIN®], Fulvestrant [FASLODEX®], Letrozole [FEMARA®].
Table 2.
Breast cancer clinical trials with rapalogs and chemotherapy or small molecule kinase inhibitors or metformin (up to April 2016)
| Official trial name | Clinical trial # | Phase of trial | Type of cancer patient in trial | Status of trial | Intervention | Publications |
|---|---|---|---|---|---|---|
| Rapalogs and chemotherapy* | ||||||
| Phase II trial of RAD001 plus carboplatin in patients with triple‐negative metastatic breast cancer | NCT01127763 | II | BC | Completed | RAD001, carboplatin | 56 |
| A randomized study of mTOR inhibition by RAD001 (everolimus) in invasive breast cancer patients after pre‐operative use of anthracycline and/or taxane‐based chemotherapy | NCT01088893 | II | BC | Recruitment unknown because the information has not been verified recently | Everolimus | Not provided |
| Phase I, open label, dose escalation study of the safety, tolerability, and pharmacokinetics of the combination RAD001 plus docetaxel in patients with metastatic breast cancer | NCT00253318 | I | BC | Terminated | Docetaxel, RAD001, dexamethasone, a glucocorticoid/ steroidal drugs that has anti‐inflammatory, immunosuppressant properties | Not provided |
| Phase II, open label, dose escalation study of the safety, tolerability, and pharmacokinetics of the combination RAD001 plus docetaxel in patients with metastatic breast cancer | NCT01825265 | II | BC | Withdrawn prior to enrolment | Docetaxel, RAD001, dexamethasone | Not provided |
| Phase I/II study of weekly abraxane and RAD001 in women with locally advanced or metastatic breast cancer. A study of the Cancer Institute of New Jersey Oncology Group (CINJOG) | NCT00934895 | I/II | BC | Ongoing, not recruiting | Everolimus, abraxane. Abraxane is paclitaxel bonded to albumin as a delivery vehicle | Not provided |
| A phase Ib/II study of cisplatin, paclitaxel, and RAD001 in patients with metastatic breast cancer | NCT01031446 | I/II | BC | Completed | Cisplatin, everolimus, paclitaxel | Not provided |
| Everolimus (RAD001) in combination with intravenous carboplatin in taxane‐ and anthracycline‐pretreated patients with progressive metastatic breast cancer | NCT00930475 | I/II | BC | Unknown | RAD001 in combination with carboplatin. Carboplatin is related to cisplatin, but is modified | Not provided |
| Open label randomized clinical trial of standard neoadjuvant chemotherapy (paclitaxel followed by FEC) vs. the combination of paclitaxel and RAD001 followed by FEC in women with triple receptor‐negative breast cancer (CRAD001C24101) | NCT00499603 | II | BC | Completed | Paclitaxel, 5‐FU, epirubicin, cyclophosphamide, RAD001 | Not provided |
| A phase I study of cisplatin, paclitaxel, and RAD001 patients with metastatic breast cancer | NCT00680758 | I | BC | Completed | Cisplatin, everolimus, paclitaxel | Not provided |
| A phase II neo‐adjuvant study of cisplatin, paclitaxel with or without RAD001 in patients with triple‐negative locally advanced breast cancer | NCT00930930 | II | BC | Completed | Cisplatin, everolimus, paclitaxel | Not provided |
| A phase I pilot study of the oral mTOR inhibitor RAD001 in combination with capecitabine for metastatic breast cancer | NCT00473005 | I | BC | Terminated due to principal investigator leaving sponsor | Capecitabine, RAD001 | Not provided |
| Phase I study of combined temosirolimus, erlotinib and cisplatin in advanced solid tumours | NCT00998036 | I | TNBC | Completed | Temsirolimus, cisplatin, erlotinib | Not provided |
| A phase Ib study of combination of temsirolimus (Torisel®) and pegylated liposomal doxorubicin (PLD, Doxil®/Caelyx®) in advanced or recurrent breast, endometrial and ovarian cancer | NCT00982631 | I | Advanced/recurrent BC, endometrial cancer, ovarian cancer | Recruitment is unknown because the information has not been verified recently | Temsirolimus/PLD | Not provided |
| A phase I study of the mTOR inhibitor rapamycin (rapamune, sirolimus) in combination with abraxane (paclitaxel protein‐bound particles) in advanced solid cancers | NCT00337376 | I | Advanced solid cancers | Terminated | Rapamune, abraxane | Not provided |
| Phase I study of docetaxel and temsirolimus in resistant solid malignancies | NCT00703625 | I | Resistant solid malignancies | Completed | Temsirolimus, docetaxel | Not provided |
| Randomized phase II study to compare vinorelbine in combination with the mTOR inhibitor everolimus vs. vinorelbin monotherapy for second‐line treatment in advanced breast cancer | NCT01520103 | II | HER2− metastatic BC, HER2− locally advanced BC | Recruiting | Vinorebine, everolimus | Not provided |
| Phase I clinical trial of temsirolimus and vinorelbine in advanced solid tumours | NCT01155258 | I | Male BC, | Ongoing, but not recruiting | Temsirolimus, vinorelbine ditartrate | Not provided |
| Neoadjuvant phase II study of everolimus plus cisplatin in triple negative breast cancer patients with residual disease after standard chemotherapy | NCT01931163 | II | BC, TNBC | Recruiting | Everolimus | Not provided |
| Everolimus and PI3K or PI3K/mTOR inhibitors† | ||||||
| A phase I study of BKM120 and everolimus in advanced solid malignancies | NCT01470209 | I | Solid tumours | Recruiting participants | BKM120, everolimus | Not provided |
| An open‐label, multicenter phase I dose‐finding study of RAD001 (everolimus, Afinitor®) in combination with BEZ235 in patients with advanced solid tumours | NCT01482156 | I | Advanced solid tumours, metastatic BC, metastatic renal cell carcinoma | Completed | RAD001 + BEZ235, a dual inhibitor of PI3K and mTOR | Not provided |
| Rapalogs and Akt inhibitors‡ | ||||||
| Phase I parallel protocol of MK‐8669 (ridaforolimus) + MK‐2206 and MK‐8669 (ridaforolimus) + MK‐0752 doublets (MK‐MK) in patients with advanced cancer | NCT01295632 | I | Advanced cancer | Completed | Ridaforolimus, MK‐0752 (gamma secretase inhibitor, a notch signalling pathway inhibitor). MK‐2206 | 94, 95 |
| Rapalogs and metformin§ | ||||||
| A phase I study of temsirolimus in combination with metformin in advanced solid tumours | NCT00659568 | I | BC, endometrial cancer, kidney cancer lung cancer, lymphoma, unspecified adult solid tumour, protocol specific | Completed | Metformin hydrochloride, temsirolimus | Not provided |
| Circulating FGF21 levels and efficacy of exemestane, everolimus and metformin in postmenopausal women with HR+ metastatic breast cancer and BMI ≥ 25 | NCT01627067 | II | BC | Ongoing, but not recruiting participants | Everloimus, exemestane, metformin | Not provided |
Everolimus (RAD001, Afinitor®), Temsirolimus (CCI‐779, Torisel®), Sirolimus (Rapamycin, Rapamune Sirolimus), Ridaforolimus (Deforolimus, AP23573, MK‐8669) and Chemotherapy (Abraxane, Anthracycline, Capecitabine, Carboplatin, Cisplatin, Cyclophosphamide, Docetaxel, Epirubicin, 5‐Flurouracil, Paclitaxel, Taxane, Vinorelbin).
PI3K (BKM120, Buparlisib), PI3K/mTOR (BEZ235, Dactolisib).
Ridaforolimus and Akt Inhibitors (MK‐2206).
Everolimus, Temsirolimus and Metformin.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years.
JAM and SLA were supported in part from an East Carolina University School of Medicine Internal Seed Grant Program. AMM was supported in part by an Italian MIUR FIRB grant RBAP11ZJFA_001.
Steelman, L. S. , Martelli, A. M. , Cocco, L. , Libra, M. , Nicoletti, F. , Abrams, S. L. , and McCubrey, J. A. (2016) The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol, 82: 1189–1212. doi: 10.1111/bcp.12958.
References
- 1. American Cancer Society , http://www.cancer.org/acs/groups/content/@research/documents/document/acspc‐046381.pdf (last accessed 15 December 2015).
- 2. Bostner J, Karlsson E, Eding CB, Perez‐Tenorio G, Franzén H, Konstantinell A, et al. S6 kinase signaling: tamoxifen response and prognostic indication in two breast cancer cohorts. Endocr Relat Cancer 2015; 22: 331–43. [DOI] [PubMed] [Google Scholar]
- 3. Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI‐779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005; 23: 5314–22. [DOI] [PubMed] [Google Scholar]
- 4. Dancey JE, Curiel R, Purvis J. Evaluating temsirolimus activity in multiple tumors: a review of clinical trials. Semin Oncol 2009; 36 (Suppl 3): S46–58. [DOI] [PubMed] [Google Scholar]
- 5. Buckner JC, Forouzesh B, Erlichman C, Hidalgo M, Boni JP, Dukart G, et al. Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs 2010; 28: 334–42. [DOI] [PubMed] [Google Scholar]
- 6. Fleming GF, Ma CX, Huo D, Sattar H, Tretiakova M, Lin L, et al. Phase II trial of temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat 2012; 136: 355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arena F. Clinical implications of recent studies using mTOR inhibitors to treat advanced hormone receptor‐positive breast cancer. Cancer Manag Res 2014; 6: 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royce ME, Osman D. Everolimus in the treatment of metastatic breast cancer. Breast Cancer (Auckl) 2015; 9: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumachi F, Santeufemia DA, Basso SM. Current medical treatment of estrogen receptor‐positive breast cancer. World J Biol Chem 2015; 6: 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zucchini G, Geuna E, Milani A, Aversa C, Martinello R, Montemurro F. Clinical utility of exemestane in the treatment of breast cancer. Int J Womens Health 2015; 7: 551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bostner J, Karlsson E, Eding CB, Perez‐Tenorio G, Franzén H, Konstantinell A, et al. S6 kinase signaling: tamoxifen response and prognostic indication in two breast cancer cohorts. Endocr Relat Cancer 2015; 22: 331–43. [DOI] [PubMed] [Google Scholar]
- 12. Alayev A, Salamon RS, Berger SM, Schwartz NS, Cuesta R, Snyder RB, et al. mTORC1 directly phosphorylates and activates ERα upon estrogen stimulation. Oncogene 2015. doi:10.1038/onc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bachelot T, Bourgier C, Cropet C, Ray‐Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 2012; 30: 2718–24. [DOI] [PubMed] [Google Scholar]
- 14. Choi JH, Jung SH. Appropriate design of prospective studies. J Clin Oncol 2013; 31: 510–1. [DOI] [PubMed] [Google Scholar]
- 15. Treilleux I, Arnedos M, Cropet C, Wang Q, Ferrero JM, Abadie‐Lacourtoisie S, et al. Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol 2015; 26: 120–5. [DOI] [PubMed] [Google Scholar]
- 16. Massarweh S, Romond E, Black EP, Van Meter E, Shelton B, Kadamyan‐Melkumian V, et al. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)‐positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res Treat 2014; 143: 325–32. [DOI] [PubMed] [Google Scholar]
- 17. Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor‐positive breast cancer. J Clin Oncol 2009; 27: 2630–7. [DOI] [PubMed] [Google Scholar]
- 18. Wolff AC, Lazar AA, Bondarenko I, Garin AM, Brincat S, Chow L, et al. Randomized phase III placebo‐controlled trial of letrozole plus oral temsirolimus as first‐line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol 2013; 31: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hortobagyi GN. Everolimus plus exemestane for the treatment of advanced breast cancer: a review of subanalyses from BOLERO‐2. Neoplasia 2015; 17: 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone‐receptor‐positive, human epidermal growth factor receptor‐2‐negative advanced breast cancer: overall survival results from BOLERO‐2. Ann Oncol 2014; 25: 2357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gnant M, Baselga J, Rugo HS, Noguchi S, Burris HA, Piccart M, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO‐2. J Natl Cancer Inst 2013; 105: 654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Divers J, O'Shaughnessy J. Stomatitis associated with use of mTOR inhibitors: implications for patients with invasive breast cancer. Clin J Oncol Nurs 2015; 19: 468–74. [DOI] [PubMed] [Google Scholar]
- 23. Qiao L, Liang Y, Mira RR, Lu Y, Gu J, Zheng Q. Mammalian target of rapamycin (mTOR) inhibitors and combined chemotherapy in breast cancer: a meta‐analysis of randomized controlled trials. Int J Clin Exp Med 2014; 7: 3333–43. [PMC free article] [PubMed] [Google Scholar]
- 24. Southwest Oncology Group . S1207 hormone therapy with or without everolimus in treating patients with breast cancer (e3). Bethesda, MD: National Library of Medicine (US), 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT01674140?term=S1207+Hormone+Therapy+With+or+ Without+Everolimus+in+Treating+Patients+With+Breast+Cancer&rank=1 (last accessed 15 December 2015). [Google Scholar]
- 25. Novartis Pharmaceuticals . Open‐label, phase II, study of everolimus plus letrozole in postmenopausal women with ER+, HER2‐ metastatic or locally advanced breast cancer (Bolero‐4). Bethesda, MD: National Library of Medicine (US), 2000. .Available at: https://clinicaltrials.gov/ct2/show/ NCT01698918 (last accessed 15 December 2015). [Google Scholar]
- 26. Gonzalez‐Angulo AM, Ferrer‐Lozano J, Stemke‐Hale K, Sahin A, Liu S, Barrera JA, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 2011; 10: 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Libra M, et al. Targeting breast cancer initiating cells: advances in breast cancer research and therapy. Adv Biol Regul 2014; 56: 81–107. [DOI] [PubMed] [Google Scholar]
- 28. Fitzgerald TL, Lertpiriyapong K, Cocco L, Martelli AM, Libra M, Candido S, et al. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Reg 2015; 59: 65–81. [DOI] [PubMed] [Google Scholar]
- 29. Baer R, Cintas C, Therville N, Guillermet‐Guibert J. Implication of PI3K/Akt pathway in pancreatic cancer: when PI3K isoforms matter? Adv Biol Regul 2015; 59: 19–35. [DOI] [PubMed] [Google Scholar]
- 30. Bertrand FE, McCubrey JA, Angus CW, Nutter JM, Sigounas G. NOTCH and PTEN in prostate cancer. Adv Biol Regul 2014; 56: 51–65. [DOI] [PubMed] [Google Scholar]
- 31. Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. NGF and ProNGF: regulation of neuronal and neoplastic responses through receptor signaling. Adv Biol Regul 2015; 58: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jhanwar‐Uniyal M, Gillick JL, Neil J, Tobias M, Thwing ZE, Murali R. Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes. Adv Biol Regul 2015; 57: 64–74. [DOI] [PubMed] [Google Scholar]
- 33. Fragoso R, Barata JT. PTEN and leukemia stem cells. Adv Biol Regul 2014; 56: 22–9. [DOI] [PubMed] [Google Scholar]
- 34. Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv Biol Regul 2015; 57: 10–6. [DOI] [PubMed] [Google Scholar]
- 35. Laurent PA, Severin S, Gratacap MP, Payrastre B. Class I PI 3‐kinases signaling in platelet activation and thrombosis: PDK1/Akt/GSK3 axis and impact of PTEN and SHIP1. Adv Biol Regul 2014; 54: 162–74. [DOI] [PubMed] [Google Scholar]
- 36. Maertens O, Cichowski K. An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv Biol Regul 2014; 55: 1–14. [DOI] [PubMed] [Google Scholar]
- 37. McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Basecke J, et al. Diverse roles of GSK‐3: Tumor promoter‐tumor suppressor, target in cancer therapy. Adv Biol Regul 2014; 54: 176–96. [DOI] [PubMed] [Google Scholar]
- 38. McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. GSK‐3 as potential target for therapeutic intervention in cancer. Oncotarget 2014; 5: 2881–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCubrey JA, Abrams SL, Fitzgerald TL, Cocco L, Martelli AM, Montalto G, et al. Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv Biol Regul 2015; 57: 75–101. [DOI] [PubMed] [Google Scholar]
- 40. Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget 2014; 5: 4603–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chappell WH, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, et al. Novel roles of androgen receptor, epidermal growth factor receptor, TP53, regulatory RNAs, NF‐kappa‐B, chromosomal translocations, neutrophil associated gelatinase, and matrix metalloproteinase‐9 in prostate cancer and prostate cancer stem cells. Adv Biol Regul 2016; 60: 64–87. [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto‐Ibusuki M, Arnedos M, André F. Targeted therapies for ER+/HER2‐ metastatic breast cancer. BMC Med 2015; 13: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma CX. PI3K pathway as a therapeutic target in breast cancer. Am J Hematol Oncol 2015; 11: 23–9. [Google Scholar]
- 44. Martelli AM, Lonetti A, Buontempo F, Ricci F, Tazzari PL, Evangelisti C, et al. Targeting signaling pathways in T‐cell acute lymphoblastic leukemia initiating cells. Adv Biol Regul 2014; 56: 6–21. [DOI] [PubMed] [Google Scholar]
- 45. Ciuffreda L, Falcone I, Incani UC, Del Curatolo A, Conciatori F, Matteoni S, et al. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul 2014; 56: 66–80. [DOI] [PubMed] [Google Scholar]
- 46. Spinelli L, Lindsay YE, Leslie NR. PTEN inhibitors: an evaluation of current compounds. Adv Biol Regul 2015; 57: 102–11. [DOI] [PubMed] [Google Scholar]
- 47. Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul 2014; 55: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ukaji T, Umezawa K. Novel approaches to target NF‐κB and other signaling pathways in cancer stem cells. Adv Biol Regul 2014; 56: 108–15. [DOI] [PubMed] [Google Scholar]
- 49. Karthik GM, Ma R, Lövrot J, Kis LL, Lindh C, Blomquist L, et al. mTOR inhibitors counteract tamoxifen‐induced activation of breast cancer stem cells. Cancer Lett 2015; 367: 76–87. [DOI] [PubMed] [Google Scholar]
- 50. Acevedo‐Gadea C, Hatzis C, Chung G, Fishbach N, Lezon‐Geyda K, Zelterman D, et al. Sirolimus and trastuzumab combination therapy for HER2‐positive metastatic breast cancer after progression on prior trastuzumab therapy. Breast Cancer Res Treat 2015; 150: 157–67. [DOI] [PubMed] [Google Scholar]
- 51. Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first‐line treatment for patients with HER2‐positive advanced breast cancer (BOLERO‐1): a phase 3, randomised, double‐blind, multicentre trial. Lancet Oncol 2015; 16: 816–29. [DOI] [PubMed] [Google Scholar]
- 52. André F, O'Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab‐resistant, HER2‐positive, advanced breast cancer (BOLERO‐3): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet Oncol 2014; 15: 580–91. [DOI] [PubMed] [Google Scholar]
- 53. Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6: 117–27. [DOI] [PubMed] [Google Scholar]
- 54. Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007; 12: 395–402. [DOI] [PubMed] [Google Scholar]
- 55. Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2‐overexpressing cancer cells. Clin Cancer Res 2009; 15: 7266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singh J, Novik Y, Stein S, Volm M, Meyers M, Smith J, et al. Phase 2 trial of everolimus and carboplatin combination in patients with triple negative metastatic breast cancer. Breast Cancer Res 2014; 16: R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gonzalez‐Angulo AM, Akcakanat A, Liu S, Green MC, Murray JL, Chen H, et al. Open‐label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor‐negative breast cancer. Ann Oncol 2014; 25: 1122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA 3rd, Pritchard KI, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer: results from BOLERO‐2. J Clin Oncol 2016; 34: 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deluche E, Onesti E, Andre F. Precision medicine for metastatic breast cancer. Am Soc Clin Oncol Educ Book 2015; 35: e2–7. [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Wei J, Li L, Fan C, Sun Y. Combined use of metformin and everolimus is synergistic in the treatment of breast cancer cells. Oncol Res 2015; 22: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leung EY, Askarian‐Amiri M, Finlay GJ, Rewcastle GW, Baguley BC. Potentiation of growth inhibitory responses of the mTOR inhibitor everolimus by dual mTORC1/2 inhibitors in cultured breast cancer cell lines. PLoS One 2015; 10: e0131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mi W, Ye Q, Liu S, She QB. AKT inhibition overcomes rapamycin resistance by enhancing the repressive function of PRAS40 on mTORC1/4E‐BP1 axis. Oncotarget 2015; 6: 13962–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Holder AM, Akcakanat A, Adkins F, Evans K, Chen H, Wei C, et al. Epithelial to mesenchymal transition is associated with rapamycin resistance. Oncotarget 2015; 6: 19500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem 2015; 116: 450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brohée L, Demine S, Willems J, Arnould T, Colige AC, Deroanne CF. Lipin‐1 regulates cancer cell phenotype and is a potential target to potentiate rapamycin treatment. Oncotarget 2015; 6: 11264–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ding XF, Yin DQ, Chen Q, Zhang HY, Zhou J, Chen G. Validation of p27KIP1 expression levels as a candidate predictive biomarker of response to rapalogs in patient‐derived breast tumor xenografts. Tumour Biol 2015; 36: 1463–9. [DOI] [PubMed] [Google Scholar]
- 67. Kabos P, Finlay‐Schultz J, Li C, Kline E, Finlayson C, Wisell J, et al. Patient‐derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen‐dependent gene signatures. Breast Cancer Res Treat 2012; 135: 415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Landis MD, Lehmann BD, Pietenpol JA, Chang JC. Patient‐derived breast tumor xenografts facilitating personalized cancer therapy. Breast Cancer Res 2013; 15: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL‐2 with the BH3 mimetic ABT‐199 in estrogen receptor‐positive breast cancer. Cancer Cell 2013; 24: 120–9. [DOI] [PubMed] [Google Scholar]
- 70. Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient‐derived xenograft models of breast cancer and their predictive power. Breast Cancer Res 2015; 17: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chatterjee A, Mukhopadhyay S, Tung K, Patel D, Foster DA. Rapamycin‐induced G1 cell cycle arrest employs both TGF‐β and Rb pathways. Cancer Lett 2015; 360: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leontieva OV, Blagosklonny MV. Tumor promoter‐induced cellular senescence: cell cycle arrest followed by geroconversion. Oncotarget 2014; 5: 12715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chi KH, Ko HL, Yang KL, Lee CY, Chi MS, Kao SJ. Addition of rapamycin and hydroxychloroquine to metronomic chemotherapy as a second line treatment results in high salvage rates for refractory metastatic solid tumors: a pilot safety and effectiveness analysis in a small patient cohort. Oncotarget 2015; 6: 16735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guichard SM, Curwen J, Bihani T, D'Cruz CM, Yates JW, Grondine M, et al. AZD2014, an inhibitor of mTORC1 and mTORC2, is highly effective in ER+ breast cancer when administered using intermittent or continuous schedules. Mol Cancer Ther 2015; 14: 2508–18. [DOI] [PubMed] [Google Scholar]
- 75. Bendell JC, Kelley RK, Shih KC, Grabowsky JA, Bergsland E, Jones S, et al. A phase I dose‐escalation study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual mTORC1/mTORC2 kinase inhibitor CC‐223 in patients with advanced solid tumors or multiple myeloma. Cancer 2015; 121: 3481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jiang Z, Wu S, Wu X, Zhong J, Lv A, Jiao J, et al. Blocking mammalian target of rapamycin alleviates bone cancer pain and morphine tolerance via μ‐opioid receptor. Int J Cancer 2016; 138: 2013–20. [DOI] [PubMed] [Google Scholar]
- 77. Blackwell K, Burris H, Gomez P, Lynn Henry N, Isakoff S, Campana F, et al. Phase I/II dose‐escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone‐receptor‐positive and HER2‐negative metastatic breast cancer refractory to a non‐steroidal aromatase inhibitor. Breast Cancer Res Treat 2015; 154: 287–97. [DOI] [PubMed] [Google Scholar]
- 78. Ji Y, Di W, Yang Q, Lu Z, Cai W, Wu J. Inhibition of autophagy increases proliferation inhibition and apoptosis induced by the PI3K/mTOR inhibitor NVP‐BEZ235 in breast cancer cells. Clin Lab 2015; 61: 1043–51. [DOI] [PubMed] [Google Scholar]
- 79. Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Improved clinical and cell cycle response with an mTOR inhibitor, daily oral RAD001 (everolimus) plus letrozole versus placebo plus letrozole in a randomized phase II neoadjuvant trial in ER+ breast cancer. J Clin Oncol 2008; 26 (suppl) abstr 530. [Google Scholar]
- 80. Jerusalem GH, Dieras V, Cardoso F, Bergh J, Fasolo A, Rorive A, et al. Multicenter phase I clinical trial of daily and weekly RAD001 in combination with vinorelbine and trastuzumab in patients with HER2‐overexpressing metastatic breast cancer with prior resistance to trastuzumab. J Clin Oncol 2008; 26 (suppl) abstr 1057. [Google Scholar]
- 81. Tanaka C, O'Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol 2008; 26: 1596–602. [DOI] [PubMed] [Google Scholar]
- 82. Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2‐overexpressing metastatic breast cancer who progressed on trastuzumab‐based therapy. J Clin Oncol 2011; 29: 3126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jerusalem G, Fasolo A, Dieras V, Cardoso F, Bergh J, Vittori L, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre‐treated patients with HER2‐overexpressing metastatic breast cancer. Breast Cancer Res Treat 2011; 125: 447–55. [DOI] [PubMed] [Google Scholar]
- 84. Andre F, O'Regan R, Ozgurogulu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab‐resistant, HER‐2‐positive, advanced breast cancer (BOLERO‐3): a randomized, double‐blind, placebo‐combined phase 3 trial. Lancet Oncol 2014; 15: 580–91. [DOI] [PubMed] [Google Scholar]
- 85. Seller M, Ray‐Coguard I, Melichar B, Yardley DA, Wang RX, Dodion PF, et al. Oral ridaforolimus plus trastuzumab for patients with HER2+ trastuzumab‐refractory metastatic breast cancer. Clin Breast Cancer 2015; 15: 60–5. [DOI] [PubMed] [Google Scholar]
- 86. von Minckwitz G, Loibl S, Untch M, Eidtmann H, Rezai M, Fasching PA, et al. GBG/AGO‐B study groups .Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2‐negative primary breast cancer (GBG44‐GeparQuinto). Ann Oncol 2014; 25: 2363–72. [DOI] [PubMed] [Google Scholar]
- 87. von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Müller BM, Denkert C, Loibl S, Nekljudova V, Untch M; German Breast Group ; Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Groups . Neoadjuvant chemotherapy and bevacizumab for HER2‐negative breast cancer. N Engl J Med 2012; 366: 299–309. [DOI] [PubMed] [Google Scholar]
- 88. Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. German Breast Group ; Arbeitsgemeinschaft Gynäkologische Onkologie–Breast Study Group .Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline‐taxane‐based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 2012; 13: 135–44. [DOI] [PubMed] [Google Scholar]
- 89. Rugo HS, Pritchard KI, Gnant M, Noguchi S, Piccart M, Hortobagyi G, et al. Incidence and time course of everolimus‐related adverse events in postmenopausal women with hormone receptor‐positive advanced breast cancer: insights from BOLERO‐2. Ann Oncol 2014; 25: 808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO‐2 final progression‐free survival analysis. Adv Ther 2013; 30: 370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Campone M, Beck JT, Gnant M, Neven P, Pritchard KI, Bachelot T, et al. Health‐related quality of life and disease symptoms in postmenopausal women with HR(+), HER(‐) advanced breast cancer treated with everolimus plus exemestane versus exemestane monotherapy. Curr Med Res Opin 2013; 29: 1463–73. [DOI] [PubMed] [Google Scholar]
- 92. Noguchi S, Masuda N, Iwata H, Mukai H, Horiguchi J, Puttawibul P, et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2‐negative, hormone‐receptor‐positive breast cancer in BOLERO‐2. Breast Cancer 2014; 21: 703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012; 366: 520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Piha‐Paul SA, Munster PN, Hollebecque A, Argiles G, Dajani O, Cheng JD, et al. Results of a phase 1 trial combining ridaforolimus and MK‐0752 in patients with advanced solid tumors. Eur J Cancer 2015; 51: 1865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gupta S, Argilies G, Munster PN, Hollebecque A, Dajani O, Cheng JD, et al. A Phase I trial combined ridaforolimus and MK‐2206 in patients with advanced malignancies. Clin Cancer Res 2015; 21: 5235–44. [DOI] [PubMed] [Google Scholar]


