Abstract
The macrolide rapamycin and its analogues (rapalogs) constitute the first generation of mammalian target of rapamycin (mTOR) inhibitors. Since the introduction of rapamycin as an immunosuppressant, there has been extensive progress in understanding its complex mechanisms of action. New insights into the function of mTOR in different immune cell types, vascular endothelial cells and neoplastic cells have opened new opportunities and challenges regarding mTOR as a pharmacological target. Currently, the two known mTOR complexes, mTOR complex (mTORC) 1 and mTORC2, are the subject of intense investigation, and the introduction of second‐generation dual mTORC kinase inhibitors (TORKinibs) and gene knockout mice is helping to uncover the distinct roles of these complexes in different cell types. While the pharmacological profiling of rapalogs is advanced, much less is known about the properties of TORKinibs. A potential benefit of mTOR inhibition in transplantation is improved protection against transplant‐associated viral infections compared with standard calcineurin inhibitor‐based immunosuppression. Preclinical and clinical data also underscore the potentially favourable antitumour effects of mTOR inhibitors in regard to transplant‐associated malignancies and as a novel treatment option for various other cancers. Many aspects of the mechanisms of action of mTOR inhibitors and their clinical implications remain unknown. In this brief review we discuss new findings and perspectives of mTOR inhibitors in transplantation.
Keywords: immune cells, mammalian target of rapamycin, Raptor, Rictor, transplantation
Introduction
Rapamycin was isolated in 1975 as an antibiotic product of the actinomycete Streptomyces hygroscopicus, obtained from a soil probe collected on Easter Island (Rapa Nui), and was investigated initially for its antifungal properties 1. Since the first description of its immunosuppressive activity in 1977 2, much has been learned about the complex mechanisms of action of this macrolide and its site of action, the mammalian target of rapamycin (mTOR) 3. mTOR is an evolutionarily conserved intracellular serine–threonine kinase that plays a central role in the regulation of cell growth, metabolism and proliferation 4, 5, 6. The catalytic activity of mTOR occurs via at least two distinct complexes − mTOR complex (mTORC) 1 and mTORC2 7. Compared with mTORC1, comparatively little is known about the function of mTORC2. To exert its function, rapamycin forms a complex with the intracellular immunophilin FK506 binding protein 1 A 12 kDa (FKBP12) 8. This complex inhibits the kinase activity of mTOR by directly blocking substrate recruitment and restricting active site access 9. While rapamycin and its analogues, or ‘rapalogs’, almost completely inhibit mTORC1, mTORC2 is affected only after long exposure 10. Specific deletion of genes encoding mTORC1 or mTORC2, and the use of new‐generation dual mTOR kinase inhibitors, known as ‘TORKinibs’ 11, 12, have opened up new possibilities to investigate the discrete functions of each mTOR subunit in immune cells, with implications for their roles in transplantation. Comprehensive review of the role of mTOR in the regulation of immune responses 13, 14, pharmacokinetic and dynamic aspects of rapamycin in transplantation 15, and the advantages and disadvantages of mTOR inhibitors in renal transplantation 16 have been published. In the present brief review, we highlight recent insights that have been gained into the immunobiology and pharmacology of mTOR and its role in transplantation.
Molecular biology of mTORCs
The molecular components of mTORC1 and mTORC2, and the factors and pathways that influence their function, are depicted in Figure 1.
mTORC1
Figure 1.
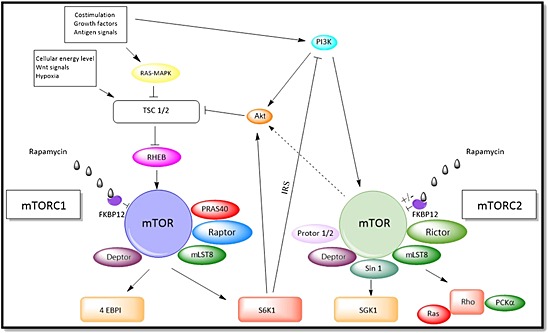
The mammalian target of rapamycin (mTOR) complexes. mTOR complex (mTORC) 1 is formed by mTOR, mammalian lethal with SEC13 protein 8 (mLST8), proline‐rich Akt substrate of 40 kDa (PRAS40), Dep domain‐containing mTOR‐interacting protein (Deptor) and the regulatory associated protein of mTOR (Raptor). Cytokines, growth factors, various nutritional cues and Akt influence the activity of tuberous sclerosis complex (TSC) 1 and TSC2, which control the activity of the GTPase RAS homologue enriched in the brain (RHEB). The interaction with RHEB is followed by phosphorylation of mTOR and leads to mRNA translation by stimulating s6 kinase 1 (S6K1) and phosphorylating eukaryotic translation initiation factor‐binding protein 1 (4E‐BP1), dissociating the inhibitory effect of 4E‐BP1 on eIF4E, a cap‐dependent mRNA translation, in mTORC1 signalling. The activation of S6k1 leads to a negative feedback loop over the phosphatidylinositol 3‐kinase (PI3K)–Akt axis via insulin receptor substrate. mTORC2 is formed by the additional components protein observed with Rictor (Protor) 1/2 and stress‐activated protein kinase‐interacting protein 1 (Sin 1). It is activated by PI3K and is only partially inhibited by rapamycin. Activation of mTORC2 regulates cytoskeletal changes via small GTPase Ras homologue (Rho) and protein kinase Cα (PKCα). Activation of serum‐ and glucocorticoid‐induced protein kinase 1 (SGK1) by mTORC2 regulates the epithelial Na+ channel in kidneys. mTORC2 phosphorylates Akt and can influence the activity of TSC1/2. MAPK, mitogen‐activated protein kinase
mTORC1 is formed by mTOR, mammalian lethal with SEC13 protein 8 (mLST8), proline‐rich Akt substrate of 40 kDa (PRAS40), Dep domain‐containing mTOR‐interacting protein (Deptor) and the regulatory associated protein of mTOR (Raptor). The activity of mTORC1 is controlled by tuberous sclerosis complex (TSC) 1 and TSC2, which act as its main upstream inhibitors. TSC1/2 control the activity of the guanosine triphosphate GTP)‐ase Ras homologue enriched in the brain (RHEB), a protein that interacts directly with mTORC1. Cytokines, growth factors, nutrients 17, costimulatory molecules, as well as cellular energy level and stress influence the activity of TSC1/2. There is evidence that amino acids can directly regulate mTORC1 via Ragulator–Rag GTPases by binding to Raptor and leading it to the surface of lysosomes 18. Recent studies have identified a member of the solute carrier family 38 (SLC38A9) as a key transmembrane protein in this process 19. In dendritic cells (DCs), the late endosomal lysosomal adaptor and mitogen‐activated protein kinase and mTOR 2 (LAMTOR2) complex has been identified as an essential regulator of Langerhans cell homeostasis in vivo 20, suggesting that mTORC1 is important in the immunological regulation of these important antigen (Ag)‐presenting cells (APCs). New studies investigating the role of Rac1 have shown that, by binding directly to mTOR, this member of the Rho family of GTPases is able to activate both mTORC1 and mTORC2, facilitating localization to cellular membranes 21.
mTORC2
In mTORC2, mTOR forms a complex with rapamycin‐insensitive companion of mTOR (Rictor), mLST8, stress‐activated protein kinase‐interacting protein 1 (SIN1) and protein observed with Rictor (Protor) 1 and 2 22. Whereas mTORC1 phosphorylates S6 kinase, mTORC2 phosphorylates Akt, protein kinase Cα (PKCα) and serum‐ and glucocorticoid‐induced protein kinase 1 (SGK‐1), leading to Raptor‐independent rearrangement of the actin cytoskeleton 7 and to the regulation of cell metabolism and survival. While rapamycin is a potent inhibitor of mTORC1, mTORC2 is only partially affected after long‐term exposure 10. A recent study has shown that relative expression of FKBP12 and FKBP51 determines the sensitivity of a cell or tissue to mTORC2 inhibition by rapamycin 23.
Influence of mTOR inhibition on different immune cell populations
Table 1 summarizes the known effects of mTORC1 and mTORC2 inhibition in different immune cell populations.
(DCs)
Table 1.
Effects of mammalian target of rapamycin complex (mTORC) 1 and mTORC2 inhibition on different immune cell types
| Cell type | mTORC 1 inhibition [References] | mTORC 2 inhibition [References] |
|---|---|---|
| Dendritic cells (DCs) | ||
| ‐ Conventional DCs | Suppresses maturation, antigen uptake and micropinocytosis, and induces apoptosis 24, 25, 26; paradoxical augmentation of proinflammatory cytokine production 124 | Augments ability to polarize Th1 and Th17; mTORC2 restrains proinflammatory function of activated DCs 33 |
| ‐ Plasmacytoid DCs | Inhibits activation, modifies cytokine production, enhances Tmem and Treg proliferation 38 | Unknown |
| T cells | Controls Th1 and Th 17 differentiation 38 | Controls Th2 differentiation 125 |
| ‐ Effector T cells | ||
| – CD8 + memory cells | Augments CD8+ Tmem responses in infection 126 | Regulates development of CD8+ cells, altering the quantity and quality of receptors important for cell differentiation 45 |
| – Tregs | Promotes Treg expansion, differentiation and function 50, 78 | Maintains Treg cell stability and coordinates Treg‐mediated control of effector responses 127 |
| NKT cells | Decreases terminal differentiation, reduces peripheral invariant NKT cells, impairs cytokine production 54 | Reduces NKT‐17 cell differentiation, reduces thymic and peripheral NKT cells 55 |
| B cells | Reduces marginal zone formation, decreases antibody (Ab) class switching, alters Ab repertoire 128 | Affects development, survival and function of mature B lineage cells, impairs Ab production 58 |
| MDSCs | Induces T cell suppression by MDSCs, higher expression of iNOS, upregulation of Tregs 42 | Unknown |
| Endothelial cells | Lessens proliferation and cytokine secretion by allogeneic CD4+, upregulates Tregs and reduces infiltration of allogeneic effector T cells into the arterial intima 62 | Antagonizes TNF induction of VCAM‐1 63 |
iNOS, inducible nitric oxide synthase; MDSC, myeloid‐derived suppressor cells; NKT, natural killer T cells; Th, T helper cell; Tmem, T memory cell; TNF, tumour necrosis factor; Tregs, antigen‐specific regulatory T cells; VCAM‐1, vascular cell adhesion molecule‐1.
DCs are important ‘professional’ APCs that play critical roles in the induction and regulation of immunity. They comprise both conventional myeloid DCs (cDCs) and ‘non‐conventional’ plasmacytoid DCs (pDCs), the latter being important sources of type‐1 interferons (IFNs). Multiple studies have explored the influence of rapamycin on DC differentiation and function, and analysed the impact of mTOR inhibition on DC function during transplantation. The stage of DC differentiation, their state of activation, and the duration and timing of their exposure to rapamycin determines the nature of the response. Use of new ATP‐competitive dual mTORC1 and mTORC2 inhibitors is currently enabling new insights into mTOR signalling in DCs and allowing investigators to pose further questions about the roles of these complexes in DCs. In conventional DCs, rapamycin‐induced mTOR inhibition suppresses cell maturation 24, Ag uptake 25, and macro‐ and endocytosis 26 and induces their apoptosis 27. These effects lead to reduced activation and proliferation of alloreactive T cells by DCs 28 via impaired cytokine‐driven activation and differentiation of T helper (Th) 1, Th2 and Th17 cells 29. In contrast to these effects, the stimulation of rapamycin‐preconditioned murine bone marrow (BM)‐derived DCs with the Toll‐like receptor 4 ligand lipopolysaccharide (LPS) results in increased secretion of the proinflammatory cytokine interleukin (IL) 12/p70, although the stimulatory effects of these rapamycin‐conditioned DCs on CD4+T cell activation remain low 30. In a recent study 31 that investigated the extended lifespan of mouse BM‐derived DCs after mTOR inhibition, it was found that mTOR inhibition suppressed the induction of LPS‐mediated inducible nitric oxide synthase (iNOS) in DCs. The reduced transcription of iNOS allowed the cells to continue to use their mitochondria to generate ATP and to use fatty acids or glucose as nutrients to promote metabolism. The same group 32 demonstrated improved outcome after autologous DC vaccination in a murine model using mTOR inhibition, owing to the extended lifespan and prolonged period during which the DCs exhibited an activated phenotype.
In a recent report investigating the role of mTORC2 in DC function, Rictor−/− murine BM‐derived DCs were stimulated with different activating agents. Compared with wild‐type DCs, Rictor−/− DCs displayed an augmented ability to polarize alloreactive Th1 and Th17 cells, both in vitro and in vivo 33, with the implication that mTORC2 activity restrains the proinflammatory function of activated DCs. The Fms‐like tyrosine kinase 3 (Flt3) receptor and its ligand (Flt3L) play an important role in DC development and differentiation 34. Administration of Flt3L, induces marked expansion of all DC subsets, including pDCs and both CD8+ and CD8– cDCs in mouse spleen and bone marrow 35, 36. Flt3L signalling in DCs is only partially understood but is believed to function via the phosphatidylinositol 3‐kinase (PI3K)–Akt–mTOR pathway 37. CD8+ cDCs were particularly responsive to Flt3 signalling, a mechanism that is explained by higher levels of mTOR activity in this DC subset. Recent work has demonstrated a synergistic effect of mTOR inhibition with rapamycin and Flt3L administration on the induction of Ag‐specific regulatory T cells (Tregs) in mice 38. This effect appears to be mediated via the selective expansion of ‘tolerogenic’ pDCs 38. Moreover, a combination of rapamycin and Flt3L promotes organ allograft survival in mice by inducing regulatory DC and allograft autophagy 39.
Myeloid‐derived suppressor cells (MDSCs)
MDSCs differentiate from monocyte and granulocyte precursors under the influence of multiple environmental cues. There is emerging evidence that they play a key role in the regulation of alloimmunity and the induction of experimental organ transplant tolerance 40, 41. Exposure to rapamycin induces T cell suppression by MDSCs and higher expression of iNOS. Moreover, the transcoronary transfer of rapamycin‐conditioned MDSCs can prolong heart allograft survival and upregulate Tregs in mice 42.
Th cells
mTOR inhibitors exert multiple influences on T cell development, homeostasis, activation, differentiation, function and migration. Detailed information about the role of mTOR in T cells can be found in recent reviews 13, 14, 43. The role of mTOR in T cells is evident during the early stages of their maturation in the thymus in response to various environmental cues. The impact of mTOR inhibition is reflected in thymic atrophy in rodents after rapamycin administration 44. mTORC2 appears to be involved in the co/post‐translational processing of membrane‐expressed αβ T cell receptors (TCRs) during thymocyte development. Thus, mTORC2 inhibition affects T cell development via regulation of the quantity and quality of receptors that are important for their functional differentiation 45. In knockout (KO) mice, specific deletion of mTORC1 leads to failure of Th1 and IL‐17‐producing Th (Th17) effector cell differentiation, while mTORC2‐deleted T cells fail to differentiate into Th2 cells 46. Another recent report 47 has revealed a critical role for Akt isoforms and both mTOR complexes in the control of Th17 subset development.
New studies on the role of mTORC1 in Th1 and follicular B helper T (Tfh) cells have demonstrated an important role of the IL‐2‐mTORC1 axis in the signalling, differentiation and metabolism of these cells. Tfh cells display reduced metabolic activity, mitochondrial function and mTOR kinase activity compared with Th1 cells. IL‐2 activation of Akt and mTOR signalling is critical in orchestrating the reciprocal differentiation of Th1 and Tfh cells, and thus IL‐2 conducts two signalling arms that are important for T cell differentiation via signal transducer and activator of transcription 5 (STAT5) and PI3K 48.
Effector memory T cells
Recent studies in mice have defined specific roles for mTORC1 and mTORC2 that link metabolism and CD8+ T effector and memory cell generation, suggesting that these functions could be targeted to promote vaccine efficiency and antitumour immunity 49.
Tregs
Naturally occurring Tregs are CD4+ cells that develop in the thymus. Under normal activating conditions, T cells that lack mTOR differentiate into forkhead box protein (Fox) p3+ Tregs 50, regulate immune responses in vitro and in vivo, and are now the subject of clinical trials for the treatment of graft‐versus‐host disease (GVHD) after BM transplantation and organ allograft rejection. Although multiple studies have reported augmentation of Tregs in response to mTOR inhibition, seemingly paradoxical effects were observed after deletion of Raptor in murine Tregs, resulting in a profound loss of their suppressive activity in vivo and the development of a fatal, early‐onset inflammatory disorder 51. In a nonhuman primate (NHP) model, repeated infusion of Tregs after their ex vivo expansion resulted in longer survival of allogeneic renal transplants when combined with low‐dose rapamycin and antithymocyte globulin 52. Pulsing Tregs from NHPs with rapamycin can enhance their ability to inhibit the proliferation of multiple T cell subpopulations, including CD4+ and CD8+ T cells, as well as Ag‐experienced CD28+CD95+ memory and CD28‐CD95+ effector cell subpopulations 53.
Natural killer T (NKT) cells
NKT cells play a central role in viral and bacterial immune responses, depending on secreted cytokines to induce inflammation or promote immune tolerance. The differentiation and effector function of invariant NKT cells (iNKT), a group of T cells with unique α and β TCR chains, has been shown to be dependent on mTORC1 signalling 54. New studies in murine KO models have identified a crucial role for mTORC2 in NKT cell development, indicating that deficiency in Rictor (and thus the mTORC2 pathway) decreases thymic and peripheral NKT cells and abolishes NKT17 (a NKT effector lineage producing IL‐17) 55. However, deletion of phosphatase and tensin homologue (Pten), which upregulates mTORC2 activity, enhanced NKT17 generation. By contrast, mTORC1 was dispensable for NKT17 generation. Another recent study 56 has investigated the influence of IL‐10 and transforming growth factor β on different rapamycin‐treated iNKT lines and found that the suppressive function of iNKT depended on the nuclear localization of Foxp3. While the expression of Foxp3 was mainly dependent on IL‐10 stimulation, rapamycin was required to promote the nuclear localization of Foxp3.
B cells
mTOR inhibition affects the development and function of B cells. Deletion of TSC‐1 results in a significant reduction in the number of marginal zone (MZ) B cells, an effect that is corrected by administration of rapamycin 57. New studies on the function of mTOR inhibitors in B cells have revealed an important role of mTORC2 in B cell homeostasis. In a KO mouse model, Rictor deletion early in B lymphoid ontogeny had, at most, a modest effect on pro‐ and pre‐B cell progression in the BM. By contrast, striking effects were observed in the development, survival and function of mature B lineage cells, with antibody (Ab) production severely impaired when mature B cells lacked Rictor expression after complete development 58. The blocking of mTORC1 and mTORC2, using the TORKinib AZ8055, resulted in a higher fraction of class‐switching B cells in a dose‐dependent manner 59. Interestingly, vaccine studies have shown that the treatment of mice infected with influenza virus subtype H3N2 (a relatively avirulent subtype of the influenza A virus) with rapamycin results in enhanced protection against lethal infection with the H5N1 virus. This effect was promoted by reduced germinal centre formation and decreased Ab class switching, leading to more cross‐reactive responses owing to an altered Ab repertoire 60.
Influence of mTOR inhibition on endothelial cells (ECs)
Vascular ECs express major histocompatibility complex (MHC) I and II molecules and produce multiple immunostimulatory and inhibitory signals that activate memory CD4+ cells, inducing graft rejection 61. Recent studies of the influence of rapamycin on ECs have shown that, in vitro, rapamycin‐pretreated ECs stimulate less proliferation and cytokine secretion by allogeneic CD4+ cells, owing to the upregulation of programmed death ligand (PD‐L) 1 and PD‐L2. Rapamycin preconditioning of ECs also results in their preferential activation of allogeneic CD4+ CD25hi CD127lo Foxp3+ (Treg) cells and reduced infiltration of allogeneic effector T cells into the arterial intima in vivo 62. Rapamycin‐pretreated ECs also show a reduced ability to capture T cells during venular flow by inhibiting tumour necrosis factor‐induced expression of vascular cell adhesion molecule‐1 (VCAM‐1) on ECs. This effect was shown to be dependent on the inhibition of Rictor, suggesting mTORC2 inhibition as a new therapeutic option to reduce vascular rejection 63. Inhibition of mTORC2 leads to the upregulation of extracellular signal‐regulated kinase 1/2, reducing VCAM‐1 expression by repressing the induction of the transcription factor interferon regulatory factor 1 (IRF‐1), an effect that could be shown in vitro and in vivo after exposure to rapamycin in mice. In an analysis of renal transplant recipients with antiphospholipid syndrome, an autoimmune disease leading to vascular thrombosis and obstetric complications, biopsies from patients treated with rapamycin were compared with those from patients undergoing other immunosuppressive therapy 64. In this study, the formation of intimal hyperplasia by immunoglobulin G Abs was associated with the activation of mTORC1 and mTORC2 in ECs. Patients with antiphospholipid syndrome nephropathy who required transplantation and were treated with rapamycin (sirolimus) had no recurrence of vascular lesions and showed decreased vascular proliferation on biopsy, compared with patients with antiphospholipid Abs who were not receiving rapamycin 64.
Pharmacological aspects of mTOR inhibition
The most commonly used mTOR inhibitors are sirolimus and everolimus. Everolimus is a sister drug of sirolimus that, instead of a hydrogen atom at position 40, has a 2‐hydroxethyl chain substitution, which improves its solubility and bioavailability 65. The main difference between the two agents lies in their pharmacokinetic characteristics and interindividual differences in pharmacokinetics.
Sirolimus
After oral administration of 2.5 mg sirolimus, the drug is absorbed rapidly, reaching a maximal whole blood concentration (Cmax) of 40.5 ± 22.2 μg l−1 (standard deviation) after an average period of 2.7 ± 2.1 h (Tmax), dependent on dose. The exact bioavailability of sirolimus is unknown but has been estimated to be 15 ± 9%, with inter‐ and intraindividual variations depending on intestinal cytochrome P450 (CYP) 3A content 66. The metabolism of sirolimus occurs mainly via CYP3A4, CYP3A5 and CYP2C8 67, with associated interindividual variability due to different expression of these enzymes. An important difference in clearance is found between the Afro‐Caribbean and non‐Afro‐Caribbean populations, with significantly higher metabolism in the former group 68.
Everolimus
Oral administration of 2.5 mg everolimus reaches whole blood Cmax levels of 45 (SD ±21) μg l−1 after an average time of 1.3 (±0.4 h) 69. The total bioavailability is estimated to be about 16%, with interindividual and intraindividual variation 70. Everolimus is metabolized by the same enzymes as sirolimus 71. In contrast to sirolimus, recent findings describe slower metabolism of everolimus in Afro‐Caribbean patients 72.
TORKinibs
Early pharmacokinetic studies have been performed on these dual mTORC1 and mTORC2 inhibitors in cancer patients 73, 74 and in rodents 75, 76. Pharmacokinetic data for the TORKinib AZD2014 show rapid absorption after oral intake, with a median time to peak of 0.5 h and 1 h following a single dose between 50 mg and 100 mg (Cmax: 1664 ng ml−1). Although the elimination half‐life was approximately 3 h, large interpatient variability was seen 77.
Drug combination/conversion strategies in clinical organ transplantation
New immunosuppressive protocols in organ transplantation involve a switch from calcineurin inhibitors (CNIs) to mTOR inhibitors. Recent studies examining changes in T cell subsets in kidney transplant recipients after conversion to rapamycin have shown upregulation of Tregs, accompanied by a reduction in Th17 cells. An increased proportion of CD8+CCR7+ T cells (CD8+ Tregs) that could suppress CD4+ T cell activation in vitro has been found 78. Newer immunosuppressive protocols in renal transplantation have investigated the combination of mTOR inhibition and costimulation (B7‐CD28) blockade (with belatacept) 79. Induction therapy with rabbit antithymocyte globulin in combination with these two agents results in a favourable ratio of Foxp3+ Tregs over the memory T cell compartment 1 year post‐transplant. Despite the presence of alloreactive CD4+ cells in pretransplant donor‐specific memory/effector T cell immune monitoring, no patient showed signs of clinical rejection during the first year. The combination of rapamycin (sirolimus) and belatacept successfully suppressed both donor‐specific and naïve effector T cell responses.
New perspectives of mTOR inhibition in experimental organ transplantation
A new‐generation TORKinib, which blocks both mTORC1 and mTORC2, has recently been shown to prolong allograft survival in a murine organ transplant model 75. In this latter study, simultaneous blocking of both mTOR complexes led to similar immunomodulatory effects as described for rapamycin (upregulation of Foxp3+ Tregs and inhibition of IFNγ production), while the production of cytokines by Th1 and Th17 cells was increased. Recent findings in a rat model showed that chronic rejection could be eliminated through the delivery of a MHC class I molecule into ACI strain recipients of Wistar–Furth hearts at the time of transplantation, together with subtherapeutic cyclosporine. Deregulation of two parallel (RhoA and Rac1) actin pathways played a crucial role in the inhibition of chronic rejection. This implies that both mTORC1 and mTORC2 control cell motility through changes in the organization of the cellular cytoskeleton/RhoA/Rac1 pathway components 80.
New perspectives of mTOR inhibition in clinical organ transplantation
Kidney transplantation
In renal transplantation, special interest in the use of mTOR inhibitors has arisen from their low nephrotoxicity when compared with CNIs 81, and much has been learned since their introduction 82 into clinical transplantation 68. Recent studies report that an early switch to sirolimus in combination with a low‐dose CNI 83 or mTOR monotherapy 84 significantly improves renal graft function and gives rise to a similar risk of acute rejection compared with standard CNI protocols. The use of mTOR inhibitors as monotherapy is still a point of discussion; in a clinical trial comparing CNI‐based immunosuppression with ‘early conversion’ to everolimus‐based CNI‐free therapy, the occurrence of donor‐specific human leucocyte antigen Abs and Ab‐mediated rejection after low‐risk kidney transplantation was increased in the everolimus group 85. A 5‐year follow‐up clinical trial after conversion from a CNI to everolimus at 4.5 months showed significantly improved graft function, along with similar risks of graft loss, mortality, serious adverse events and neoplasms postrandomization 86.
Heart and lung transplantation
In lung transplantation, a large multicentre, randomized, open‐label controlled trial comparing sirolimus with azathioprine in a tacrolimus‐based immunosuppressive regimen including 181 patients showed no significant difference in the overall rate of acute rejection after 1 year 87. In a subgroup analysis by the same investigators, the incidence of cytomegalovirus (CMV) events in transplanted patients given rapamycin (sirolimus) remained significantly lower than in the azathioprine arm, even after adjustment for confounding factors 88. In heart transplantation, a new randomized trial comparing patients undergoing complete CNI withdrawal after 7–11 weeks with standard cyclosporine treatment showed a significant reduction in cardiac allograft vasculopathy after 1 year 89. A long‐term follow‐up comparing the combination of sirolimus and tacrolimus with tacrolimus and mycophenolate mofetil did not show any differences in cardiac allograft vasculopathy progression after 8 years, indicating the need for large randomized clinical trials to compare different drug combinations 90. In addition, in heart transplantation, as in lung and kidney transplantation, treatment with everolimus was found to be associated with a lower incidence of CMV infection compared with azathioprine and mycophenolate mofetil 91.
Infection
Next to improvement in graft function, clinical trials have described a reduced incidence of CMV infection in renal transplant patients under mTOR inhibition 92. In a recent trial conducted in low/moderate immunological risk kidney transplant recipients receiving no CMV prophylaxis, the incidence of CMV infection/disease was significantly lower in those receiving everolimus and reduced‐dose tacrolimus compared with mycophenolate mofetil and standard‐dose tacrolimus 93. One possible explanation for these findings is the selective effect of mTOR inhibitors on T cell differentiation, especially CD8+ T cells 94. In addition, Poglitsch et al. 95 investigated the effect of mTOR inhibition in CMV‐infected macrophages and demonstrated that mTOR is essential for virus replication during the late phases of the viral cycle in myeloid cells. This complex interaction has recently been clarified by in vitro studies in which it could be shown that CMV immediate early proteins can activate PI3K–Akt, resulting in activation of mTORC1 and mTORC2 96. In this study, it was reported that the inhibitory effects of rapamycin on viral growth are due primarily to the presence of Rictor, not Raptor, and that Rictor‐ and Raptor‐containing complexes are modified such that their substrate specificities and rapamycin sensitivities are altered. In another study, where mTOR activity was completely blocked using the dual mTORC1 and mTORC2 inhibitor Torin 1, replication of representative members of the α‐, β‐ and γ‐herpesvirus families was inhibited 97. Recent findings in a murine γ‐herpesvirus infection model, in which treatment with belatacept resulted in increased viral burden, showed that the addition of rapamycin could maintain the number and function of virus‐specific CD8+ T cells 98. The molecular mechanisms underlying these findings remain unclear. Possible explanations are that suppression of Akt and mTOR and augmentation of PD‐1 expression via increased FoxO1 are both a normal and necessary part of the progression of cytotoxic T cell exhaustion that serves not only to prevent excessive immunopathology, but also to sustain virus‐specific cytotoxic T cells during persistent Ag stimulation.
mTOR inhibition and malignant disease
Non‐transplant‐associated malignancies
The mTOR pathway is currently the subject of intense investigation in cancer research, owing to its central role in cell metabolism and proliferation 99. Owing to the complexity of this topic, only a brief overview is presented here. mTOR inhibitors have shown clinical efficacy against a number of malignancies, especially renal cell carcinoma 100, breast cancer 101 and hepatocellular carcinoma (HCC) 102. In a large phase III clinical trial of patients with advanced clear cell renal cell carcinoma (ccRCC), treatment with everolimus prolonged progression‐free survival, with mild or moderate adverse events 103. The largest prospective randomized clinical trial to determine if sirolimus can improve HCC recurrence‐free patient survival (RFS) in liver transplant recipients [Sirolimus in Liver Transplant Recipients with HCC study (SiLVER); 525 patients] has revealed that sirolimus does not improve long‐term RFS beyond 5 years 104. However, a RFS and overall survival benefit was evident in the first 3–5 years, especially in low‐risk patients. Preclinical data support the use of dual mTOR inhibitors for ccRCC, with evidence of increased cell apoptosis in vivo and in vitro 105. A possible explanation for this finding is that negative feedback loops, which regulate PI3K–Akt signalling, are inhibited by rapalogs and may counteract their anticancer efficacy 105. Additionally, the toxicity profiles of new‐generation ATP‐competitive TORKinibs are more attractive compared with those of rapalogs. New phase II trials investigating the use of TORKinibs for advanced vascular endothelium growth factor‐refractory ccRCC have, however, shown AZD2014 to be inferior compared with everolimus regarding progression‐free survival and overall survival 106. The discrepancy between preclinical data and clinical findings is still unclear and raises new questions regarding the role of mTORC1 and mTORC2 in tumour biology.
Transplant‐ associated malignancies
Recent studies investigating the use of rapamycin in liver transplantation in selected patients with HCC report lower tumour recurrence in comparison with conventional immunosuppression 107, 108. Retrospective analysis of the prognosis of patients who developed de novo solid organ tumours after liver transplantation for alcoholic liver disease has shown a significant improvement under everolimus‐based immunosuppression compared with other immunosuppressive protocols 109. In HCC, mTORC1 and mTORC2 pathways, including phosphorylated ribosomal protein S6, p‐Akt, insulin‐like growth factor‐1R and Rictor, are upregulated in 40–50% of the tumours 110. While use of sirolimus did not improve the survival of patients with advanced HCC after the failure of the multikinase inhibitor sorafenib 111, studies using novel dual mTORC1 and mTORC2 inhibitors have shown promising in vitro results. Systematic reviews and meta‐analyses have revealed a reduced risk of malignancies in kidney transplant patients given sirolimus immunosuppression 112, 113. Although overall mortality in one study was increased compared with controls, there may be a subset of patients that benefit from mTOR inhibition. Next to nonskin cancer malignancies, there is also evidence that secondary skin cancer can be prevented by sirolimus treatment in kidney transplant patients 114.
Side effects of mTOR inhibition
Since the introduction of rapamycin and rapalogs for transplantation and other indications such as TSC, various adverse events have been described. Hypertriglyceridaemia is one of the most common metabolic side effects of rapalogs, making dietary and pharmacological treatment (e.g. statins) necessary in many patients 115. Haematological complications described in association with mTOR inhibition include thrombocytopenia, leukopenia, neutropenia, lymphopenia and anaemia 116. These findings were consistent in a systematic review, showing a significant increase in hypercholesterolaemia and anaemia in renal transplant patients after conversion to mTOR inhibitor‐containing immunosuppressive therapy 117. Women treated with mTOR inhibitors for polycystic kidney disease reported menstrual cycle disturbances 3–5 times more commonly than untreated controls; some patients reported amenorrhoea, leading to drug withdrawal in a renal transplant setting 118, 119. Increased risks for wound complications and postoperative lymphocoeles after renal and heart transplantation have been described in recent reviews of the literature. These studies underline the need for meticulous surgical techniques, especially in obese patients with mTOR inhibitory treatment regimens 120, 121. One of the most dreaded complications associated with the use of mTOR inhibitors is noninfectious pneumonitis (NIP), which can result in life‐threatening respiratory distress. In a randomized trial comparing treatment with everolimus vs. placebo in patients with renal cell carcinoma, 16% developed grade 2 (not interfering with daily living) and 3.6% grade 3 (interfering with daily living or oxygen indicated) NIP in the everolimus group 122. Therapy for NIP consists of dose reduction, discontinuation or corticosteroid treatment. Less is known about the side effects of TORKinibs as these new agents have only been used for short periods of time in oncology patients. In a phase I study of patients with advanced solid tumours, those receiving the TORKinib AZD2014 commonly developed nausea, mucositis, diarrhoea and anaemia 77. In a randomized phase II study of everolimus versus AZD2014 in vascular endothelium growth factor‐refractory metastatic ccRCC, grade 3–4 adverse events (most commonly, anaemia, fatigue and nausea) occurred in 35% and 48% of AZD2014 and everolimus patients, respectively 106. It remains unclear whether the perioperative use of TORKinibs would be well tolerated and safe. More detailed elaboration on the safety considerations of mTOR inhibitors can be found in a recent review 123.
Conclusions
In recent years, our knowledge of the complex implications of mTOR inhibition has grown through both basic research and clinical experience. Selective gene KO models and ATP‐competitive mTOR inhibitors have provided new insights into the distinct roles of mTORC1 and mTORC2 in cell growth, metabolism and function. As exclusive mTORC2 inhibitors are not currently available, our understanding of the influence of selective mTORC2 inhibition remains limited to genetic deletion models and RNA silencing. The precise roles of mTORC1 and mTORC2 in many immune cells remain unclear, and interactions between these complexes remain to be studied.
The ability of mTORC1 inhibition to modify the behaviour of APCs, suppress alloreactive effector T cell responses and promote Tregs has bestowed ‘tolerogenic’ properties on rapamycin. However, paradoxical immunostimulatory effects of mTOR inhibition on APCs and T cells, paired with limited immunosuppressive potency, makes further research and refinement of mTOR inhibition strategies essential. Low nephrotoxicity, lower incidences of viral infection and beneficial effects of mTOR inhibition on EC proliferation need to be balanced against known side effects and optimal strategies devised to maximize the therapeutic potential of these important agents.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: MW had support from the DOD for the submitted work; AWT was supported by a National Institutes of Health and a DOD research grant; DF is supported by a Ben J. Lipps Research Fellowship from the American Society of Nephrology Foundation for Kidney Research; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The authors' work was supported by National Institutes of Health (NIH) grant R01 AI67541 and by Department of Defense grant W81XWH. DF is the recipient of an American Society of Nephrology Basic Science Fellowship. MW is supported by the US Department of Defense (DOD) grant MR141044.
Waldner, M. , Fantus, D. , Solari, M. , and Thomson, A. W. (2016) New perspectives on mTOR inhibitors (rapamycin, rapalogs and TORKinibs) in transplantation. Br J Clin Pharmacol, 82: 1158–1170. doi: 10.1111/bcp.12893.
References
- 1. Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY‐22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot 1975; 28: 721–6. [DOI] [PubMed] [Google Scholar]
- 2. Martel RR, Klicius J, Galet S. Inhibition of the immune response by rapamycin, a new antifungal antibiotic. Can J Physiol Pharmacol 1977; 55: 48–51. [DOI] [PubMed] [Google Scholar]
- 3. Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1‐arresting rapamycin–receptor complex. Nature 1994; 369: 756–8. [DOI] [PubMed] [Google Scholar]
- 4. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991; 253: 905–9. [DOI] [PubMed] [Google Scholar]
- 5. Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 2004; 24: 9508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149: 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument‐Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin‐insensitive and raptor‐independent pathway that regulates the cytoskeleton. Curr Biol 2004; 14: 1296–302. [DOI] [PubMed] [Google Scholar]
- 8. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003; 35: 7S–14S. [DOI] [PubMed] [Google Scholar]
- 9. Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature 2013; 497: 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22: 159–68. [DOI] [PubMed] [Google Scholar]
- 11. Feldman ME, Shokat KM. New inhibitors of the PI3K‐Akt–mTOR pathway: insights into mTOR signaling from a new generation of Tor kinase domain inhibitors (TORKinibs). Curr Top Microbiol Immunol 2010; 347: 241–62. [DOI] [PubMed] [Google Scholar]
- 12. Fantus D, Thomson AW. The ups and downs of TORKinibs in transplantation. Transplantation 2015; 99: e117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009; 9: 324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol 2012; 30: 39–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halleck F, Duerr M, Waiser J, Huber L, Matz M, Brakemeier S, Liefeldt L, Neumayer HH, Budde K. An evaluation of sirolimus in renal transplantation. Expert Opin Drug Metab Toxicol 2012; 8: 1337–56. [DOI] [PubMed] [Google Scholar]
- 16. Ponticelli C The pros and the cons of mTOR inhibitors in kidney transplantation. Expert Rev Clin Immunol 2014; 10: 295–305. [DOI] [PubMed] [Google Scholar]
- 17. Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 2013; 14: 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sancak Y, Sabatini DM. Rag proteins regulate amino‐acid‐induced mTORC1 signalling. Biochem Soc Trans 2009; 37: 289–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti‐Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015; 519: 477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sparber F, Scheffler JM, Amberg N, Tripp CH, Heib V, Hermann M, Zahner SP, Clausen BE, Reizis B, Huber LA, Stoitzner P, Romani N. The late endosomal adaptor molecule p14 (LAMTOR2) represents a novel regulator of Langerhans cell homeostasis. Blood 2014; 123: 217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saci A, Cantley LC, Carpenter CL. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell 2011; 42: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor‐1 is required for efficient mTORC2‐mediated activation of SGK1 in the kidney. Biochem J 2011; 436: 169–79. [DOI] [PubMed] [Google Scholar]
- 23. Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin‐mediated mTORC2 inhibition is determined by the relative expression of FK506‐binding proteins. Aging Cell 2015; 14: 265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL‐4‐induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo . Blood 2003; 101: 4457–63. [DOI] [PubMed] [Google Scholar]
- 25. Monti P, Mercalli A, Leone BE, Valerio DC, Allavena P, Piemonti L. Rapamycin impairs antigen uptake of human dendritic cells. Transplantation 2003; 75: 137–45. [DOI] [PubMed] [Google Scholar]
- 26. Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor‐mediated endocytosis by bone marrow‐derived dendritic cells. Blood 2002; 100: 1084–7. [DOI] [PubMed] [Google Scholar]
- 27. Woltman AM, de Fijter JW, Kamerling SW, van Der Kooij SW, Paul LC, Daha MR, van Kooten C. Rapamycin induces apoptosis in monocyte‐ and CD34‐derived dendritic cells but not in monocytes and macrophages. Blood 2001; 98: 174–80. [DOI] [PubMed] [Google Scholar]
- 28. Matsue H, Yang C, Matsue K, Edelbaum D, Mummert M, Takashima A. Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, and dexamethasone) on bidirectional dendritic cell‐T cell interaction during antigen presentation. J Immunol 2002; 169: 3555–64. [DOI] [PubMed] [Google Scholar]
- 29. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha‐dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 2011; 208: 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK‐3 shape the CD4+ T‐cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 2010; 115: 4758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amiel E, Everts B, Fritz D, Beauchamp S, Ge B, Pearce EL, Pearce EJ. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol 2014; 193: 2821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, Pearce EJ. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol 2012; 189: 2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raich‐Regue D, Rosborough BR, Watson AR, McGeachy MJ, Turnquist HR, Thomson AW. mTORC2 deficiency in myeloid dendritic cells enhances their allogeneic Th1 and Th17 stimulatory ability after TLR4 ligation in vitro and in vivo . J Immunol 2015; 194: 4767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waskow C, Liu K, Darrasse‐Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol 2008; 9: 676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev 2010; 234: 32–44. [DOI] [PubMed] [Google Scholar]
- 36. Wu L A Flt3L encounter: mTOR signaling in dendritic cells. Immunity 2010; 33: 580–2. [DOI] [PubMed] [Google Scholar]
- 37. Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity 2010; 33: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biswas M, Sarkar D, Kumar SR, Nayak S, Rogers GL, Markusic DM, Liao G, Terhorst C, Herzog RW. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell‐dependent induction of CD4 + CD25 + FoxP3+ Treg. Blood 2015; 125: 2937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong A, Duan L, Chen J, Fan Z, Zheng F, Tan Z, Gong F, Fang M. Flt3L combined with rapamycin promotes cardiac allograft tolerance by inducing regulatory dendritic cells and allograft autophagy in mice. PLoS One 2012; 7: e46230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, Grisotto M, van Rooijen N, Matesanz R, Tacke F, Ginhoux F, Ding Y, Chen SH, Randolph G, Merad M, Bromberg JS, Ochando JC. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 2010; 120: 2486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boros P, Ochando JC, Chen SH, Bromberg JS. Myeloid‐derived suppressor cells: natural regulators for transplant tolerance. Hum Immunol 2010; 71: 1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura T, Nakao T, Yoshimura N, Ashihara E. Rapamycin prolongs cardiac allograft survival in a mouse model by inducing myeloid‐derived suppressor cells. Am J Transplant 2015; 15: 2364–77. [DOI] [PubMed] [Google Scholar]
- 43. Chapman NM, Chi H. mTOR signaling, Tregs and immune modulation. Immunotherapy 2014; 6: 1295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luo H, Duguid W, Chen H, Maheu M, Wu J. The effect of rapamycin on T cell development in mice. Eur J Immunol 1994; 24: 692–701. [DOI] [PubMed] [Google Scholar]
- 45. Chou PC, Oh WJ, Wu CC, Moloughney J, Ruegg MA, Hall MN, Jacinto E, Werlen G. Mammalian target of rapamycin complex 2 modulates alphabetaTCR processing and surface expression during thymocyte development. J Immunol 2014; 193: 1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 2011; 12: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JS, Sklarz T, Banks LB, Gohil M, Waickman AT, Skuli N, Krock BL, Luo CT, Hu W, Pollizzi KN, Li MO, Rathmell JC, Birnbaum MJ, Powell JD, Jordan MS, Koretzky GA. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol 2013; 14: 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. The interleukin‐2‐mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 2015; 43: 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, Delgoffe GM, Powell JD. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest 2015; 125: 2090–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009; 30: 832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)‐cell function. Nature 2013; 499: 485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma A, Qi S, Song L, Hu Y, Dun H, Massicotte E, Dupuis M, Daloze P, Chen H. Adoptive transfer of CD4 + CD25+ regulatory cells combined with low‐dose sirolimus and anti‐thymocyte globulin delays acute rejection of renal allografts in cynomolgus monkeys. Int Immunopharmacol 2011; 11: 618–29. [DOI] [PubMed] [Google Scholar]
- 53. Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, Kean LS. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant 2012; 12: 1441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Tschumi BO, Corgnac S, Ruegg MA, Hall MN, Mach JP, Romero P, Donda A. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol 2014; 193: 1759–65. [DOI] [PubMed] [Google Scholar]
- 55. Wei J, Yang K, Chi H. Cutting edge: discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J Immunol 2014; 193: 4297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huijts CM, Schneiders FL, Garcia‐Vallejo JJ, Verheul HM, de Gruijl TD, van der Vliet HJ. mTOR inhibition per se induces nuclear localization of FOXP3 and conversion of invariant NKT (iNKT) cells into immunosuppressive regulatory iNKT cells. J Immunol 2015; 195: 2038–45. [DOI] [PubMed] [Google Scholar]
- 57. Benhamron S, Tirosh B. Direct activation of mTOR in B lymphocytes confers impairment in B‐cell maturation and loss of marginal zone B cells. Eur J Immunol 2011; 41: 2390–6. [DOI] [PubMed] [Google Scholar]
- 58. Lee K, Heffington L, Jellusova J, Nam KT, Raybuck A, Cho SH, Thomas JW, Rickert RC, Boothby M. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood 2013; 122: 2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Limon JJ, So L, Jellbauer S, Chiu H, Corado J, Sykes SM, Raffatellu M, Fruman DA. mTOR kinase inhibitors promote antibody class switching via mTORC2 inhibition. Proc Natl Acad Sci U S A 2014; 111: E5076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, Hurwitz J, Chi H, Doherty PC, Thomas PG, McGargill MA. The kinase mTOR modulates the antibody response to provide cross‐protective immunity to lethal infection with influenza virus. Nat Immunol 2013; 14: 1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shiao SL, Kirkiles‐Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo . J Immunol 2007; 179: 4397–404. [DOI] [PubMed] [Google Scholar]
- 62. Wang C, Yi T, Qin L, Maldonado RA, von Andrian UH, Kulkarni S, Tellides G, Pober JS. Rapamycin‐treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest 2013; 123: 1677–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang C, Qin L, Manes TD, Kirkiles‐Smith NC, Tellides G, Pober JS. Rapamycin antagonizes TNF induction of VCAM‐1 on endothelial cells by inhibiting mTORC2. J Exp Med 2014; 211: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Canaud G, Bienaime F, Tabarin F, Bataillon G, Seilhean D, Noel LH, Dragon‐Durey MA, Snanoudj R, Friedlander G, Halbwachs‐Mecarelli L, Legendre C, Terzi F. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 2014; 371: 303–12. [DOI] [PubMed] [Google Scholar]
- 65. Moes DJ, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today 2015; 20: 1243–9. [DOI] [PubMed] [Google Scholar]
- 66. Emoto C, Fukuda T, Cox S, Christians U, Vinks AA. Development of a physiologically‐based pharmacokinetic model for sirolimus: predicting bioavailability based on intestinal CYP3A content. CPT 2013; 2: e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kirchner GI, Winkler M, Mueller L, Vidal C, Jacobsen W, Franzke A, Wagner S, Blick S, Manns MP, Sewing KF. Pharmacokinetics of SDZ RAD and cyclosporin including their metabolites in seven kidney graft patients after the first dose of SDZ RAD. Br J Clin Pharmacol 2000; 50: 449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kahan BD, Knight R, Schoenberg L, Pobielski J, Kerman RH, Mahalati K, Yakupoglu Y, Aki FT, Katz S, Van Buren, CT. Ten years of sirolimus therapy for human renal transplantation: the University of Texas at Houston experience. Transplant Proc 2003; 35: 25S–34S. [DOI] [PubMed] [Google Scholar]
- 69. Neumayer HH, Paradis K, Korn A, Jean C, Fritsche L, Budde K, Winkler M, Kliem V, Pichlmayr R, Hauser IA, Burkhardt K, Lison AE, Barndt I, Appel‐Dingemanse S. Entry‐into‐human study with the novel immunosuppressant SDZ RAD in stable renal transplant recipients. Br J Clin Pharmacol 1999; 48: 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moes DJ, Press RR, den Hartigh J, van der Straaten T, de Fijter JW, Guchelaar HJ. Population pharmacokinetics and pharmacogenetics of everolimus in renal transplant patients. Clin Pharmacokinet 2012; 51: 467–80. [DOI] [PubMed] [Google Scholar]
- 71. Jacobsen W, Serkova N, Hausen B, Morris RE, Benet LZ, Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant Proc 2001; 33: 514–5. [DOI] [PubMed] [Google Scholar]
- 72. Taber DJ, Belk L, Meadows H, Pilch N, Fleming J, Srinivas T, McGillicuddy J, Bratton C, Chavin K, Baliga P. Racial comparisons of everolimus pharmacokinetics and pharmacodynamics in adult kidney transplant recipients. Ther Drug Monit 2013; 35: 753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naing A, Aghajanian C, Raymond E, Olmos D, Schwartz G, Oelmann E, Grinsted L, Burke W, Taylor R, Kaye S, Kurzrock R, Banerji U. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer 2012; 107: 1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huo HZ, Zhou ZY, Wang B, Qin J, Liu WY, Gu Y. Dramatic suppression of colorectal cancer cell growth by the dual mTORC1 and mTORC2 inhibitor AZD‐2014. Biochem Biophys Res Commun 2014; 443: 406–12. [DOI] [PubMed] [Google Scholar]
- 75. Rosborough BR, Raich‐Regue D, Liu Q, Venkataramanan R, Turnquist HR, Thomson AW. Adenosine triphosphate‐competitive mTOR inhibitors: a new class of immunosuppressive agents that inhibit allograft rejection. Am J Transplant 2014; 14: 2173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pike KG, Malagu K, Hummersone MG, Menear KA, Duggan HM, Gomez S, Martin NM, Ruston L, Pass SL, Pass M. Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett 2013; 23: 1212–6. [DOI] [PubMed] [Google Scholar]
- 77. Basu B, Dean E, Puglisi M, Greystoke A, Ong M, Burke W, Cavallin M, Bigley G, Womack C, Harrington EA, Green S, Oelmann E, de Bono JS, Ranson M, Banerji U. First‐in‐human pharmacokinetic and pharmacodynamic study of the dual m‐TORC 1/2 inhibitor AZD2014. Clin Cancer Res 2015; 21: 3412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim KW, Chung BH, Kim BM, Cho ML, Yang CW. The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients. Immunology 2015; 144: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bestard O, Cassis L, Cruzado JM, Torras J, Franquesa M, Gil‐Vernet S, Lucia M, Grinyo JM. Costimulatory blockade with mTor inhibition abrogates effector T‐cell responses allowing regulatory T‐cell survival in renal transplantation. Transpl Int 2011; 24: 451–60. [DOI] [PubMed] [Google Scholar]
- 80. Zhang L, You J, Sidhu J, Tejpal N, Ganachari M, Skelton TS, Kloc M, Li XC, Ghobrial RM. Abrogation of chronic rejection in rat model system involves modulation of the mTORC1 and mTORC2 pathways. Transplantation 2013; 96: 782–90. [DOI] [PubMed] [Google Scholar]
- 81. Fernando M, Peake PW, Endre ZH. Biomarkers of calcineurin inhibitor nephrotoxicity in transplantation. Biomark Med 2014; 8: 1247–62. [DOI] [PubMed] [Google Scholar]
- 82. Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000; 356: 194–202. [DOI] [PubMed] [Google Scholar]
- 83. Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced‐exposure cyclosporine A in de novo kidney recipients. Transplantation 2015; 99: 180–6. [DOI] [PubMed] [Google Scholar]
- 84. Mjornstedt L, Sorensen SS, von zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, Andersson H, Gustafsson B, Solbu D, Holdaas H. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transpl Int 2015; 28: 42–51. [DOI] [PubMed] [Google Scholar]
- 85. Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schonemann C, Zukunft B, Illigens P, Schmidt D, Wu K, Rudolph B, Neumayer HH, Budde K. Donor‐specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 2012; 12: 1192–8. [DOI] [PubMed] [Google Scholar]
- 86. Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Wuthrich RP, Muhlfeld A, Heller K, Porstner M, Veit J, Paulus EM, Witzke O, Investigators ZS. Five‐year outcomes in kidney transplant patients converted from cyclosporine to everolimus: the randomized ZEUS study. Am J Transplant 2015; 15: 119–28. [DOI] [PubMed] [Google Scholar]
- 87. Bhorade S, Ahya VN, Baz MA, Valentine VG, Arcasoy SM, Love RB, Seethamraju H, Alex CG, Bag R, Deoliveira NC, Husain A, Vigneswaran WT, Charbeneau J, Krishnan JA, Durazo‐Arvizu R, Norwick L, Garrity E. Comparison of sirolimus with azathioprine in a tacrolimus‐based immunosuppressive regimen in lung transplantation. Am J Respir Crit Care Med 2011; 183: 379–87. [DOI] [PubMed] [Google Scholar]
- 88. Ghassemieh B, Ahya VN, Baz MA, Valentine VG, Arcasoy SM, Love RB, Seethamraju H, Alex CG, Bag R, DeOliveira NC, Vigneswaran WT, Charbeneau J, Garrity ER, Bhorade SM. Decreased incidence of cytomegalovirus infection with sirolimus in a post hoc randomized, multicenter study in lung transplantation. J Heart Lung Transplant 2013; 32: 701–6. [DOI] [PubMed] [Google Scholar]
- 89. Arora S, Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Botker HE, Radegran G, Gude E, Ioanes D, Solbu D, Sigurdardottir V, Dellgren G, Erikstad I, Solberg OG, Ueland T, Aukrust P, Gullestad L, Investigators S. The effect of everolimus initiation and calcineurin inhibitor elimination on cardiac allograft vasculopathy in de novo recipients: one‐year results of a Scandinavian randomized trial. Am J Transplant 2015; 15: 1967–75. [DOI] [PubMed] [Google Scholar]
- 90. Guethoff S, Stroeh K, Grinninger C, Koenig MA, Kleinert EC, Rieger A, Mayr T, von Ziegler F, Reichart B, Hagl C, Schramm R, Kaczmarek I, Meiser BM. De novo sirolimus with low‐dose tacrolimus versus full‐dose tacrolimus with mycophenolate mofetil after heart transplantation – 8‐year results. J Heart Lung Transplant 2015; 34: 634–42. [DOI] [PubMed] [Google Scholar]
- 91. Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, Wang SS, Dong G, Witte S, Junge G, Potena L. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis 2013; 15: 150–62. [DOI] [PubMed] [Google Scholar]
- 92. Nashan B, Gaston R, Emery V, Saemann MD, Mueller NJ, Couzi L, Dantal J, Shihab F, Mulgaonkar S, Seun Kim Y, Brennan DC. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor‐based immunosuppressive therapy in de novo renal transplant recipients. Transplantation 2012; 93: 1075–85. [DOI] [PubMed] [Google Scholar]
- 93. Tedesco‐Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes‐Freitas T, Aguiar W, Campos E, Gerbase‐DeLima M, Franco M, Medina‐Pestana J. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant 2015; 15: 2655–64. [DOI] [PubMed] [Google Scholar]
- 94. Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: rapamycin augments pathogen‐specific but not graft‐reactive CD8+ T cell responses. J Immunol 2010; 185: 2004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, Krmpotic A, Jonjic S, Horl WH, Zlabinger GJ, Puchhammer E, Saemann MD. CMV late phase‐induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant 2012; 12: 1458–68. [DOI] [PubMed] [Google Scholar]
- 96. Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor‐ and rictor‐containing complexes. Proc Natl Acad Sci U S A 2006; 103: 14182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moorman NJ, Shenk T. Rapamycin‐resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol 2010; 84: 5260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pinelli DF, Wakeman BS, Wagener ME, Speck SH, Ford ML. Rapamycin ameliorates the CTLA4–Ig‐mediated defect in CD8(+) T cell immunity during gammaherpesvirus infection. Am J Transplant 2015; 15: 2576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liao H, Huang Y, Guo B, Liang B, Liu X, Ou H, Jiang C, Li X, Yang D. Dramatic antitumor effects of the dual mTORC1 and mTORC2 inhibitor AZD2014 in hepatocellular carcinoma. Am J Cancer Res 2015; 5: 125–39. [PMC free article] [PubMed] [Google Scholar]
- 100. Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt‐Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ, Global AT. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. N Engl J Med 2007; 356: 2271–81. [DOI] [PubMed] [Google Scholar]
- 101. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012; 366: 520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cholongitas E, Mamou C, Rodriguez‐Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int 2014; 27: 1039–49. [DOI] [PubMed] [Google Scholar]
- 103. Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, Group R‐S. Efficacy of everolimus in advanced renal cell carcinoma: a double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008; 372: 449–56. [DOI] [PubMed] [Google Scholar]
- 104. Geissler EK, Schnitzbauer AA, Zulke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrion VS, Jamieson N, Scholz T, Colledan M, Fandrich F, Becker T, Soderdahl G, Chazouilleres O, Makisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Konigsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open‐label phase 3 trial. Transplantation 2016; 100: 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zheng B, Mao JH, Qian L, Zhu H, Gu DH, Pan XD, Yi F, Ji DM. Pre‐clinical evaluation of AZD‐2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett 2015; 357: 468–75. [DOI] [PubMed] [Google Scholar]
- 106. Powles T, Wheater M, Din O, Geldart T, Boleti E, Stockdale A, Sundar S, Robinson A, Ahmed I, Wimalasingham A, Burke W, Sarker SJ, Hussain S, Ralph C. A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGF‐refractory metastatic clear cell renal cancer. Eur Urol 2015; 69: 450–6. [DOI] [PubMed] [Google Scholar]
- 107. Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus‐based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010; 51: 1237–43. [DOI] [PubMed] [Google Scholar]
- 108. Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus‐based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 2008; 14: 633–8. [DOI] [PubMed] [Google Scholar]
- 109. Thimonier E, Guillaud O, Walter T, Decullier E, Vallin M, Boillot O, Dumortier J. Conversion to everolimus dramatically improves the prognosis of de novo malignancies after liver transplantation for alcoholic liver disease. Clin Transplant 2014; 28: 1339–48. [DOI] [PubMed] [Google Scholar]
- 110. Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van Laarhoven, S, Fiel MI, Di Feo A, Hoshida Y, Yea S, Toffanin S, Ramos A, Martignetti JA, Mazzaferro V, Bruix J, Waxman S, Schwartz M, Meyerson M, Friedman SL, Llovet JM. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008; 135: 1972–83, 83 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL, Dorval E, Peck‐Radosavljevic M, Santoro A, Daniele B, Furuse J, Jappe A, Perraud K, Anak O, Sellami DB, Chen LT. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE‐1 randomized clinical trial. JAMA 2014; 312: 57–67. [DOI] [PubMed] [Google Scholar]
- 112. Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, Barsoum R, Bernasconi C, Blydt‐Hansen TD, Ekberg H, Felipe CR, Firth J, Gallon L, Gelens M, Glotz D, Gossmann J, Guba M, Morsy AA, Salgo R, Scheuermann EH, Tedesco‐Silva H, Vitko S, Watson C, Fergusson DA. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta‐analysis of individual patient data. BMJ 2014; 349: g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yanik EL, Gustafson SK, Kasiske BL, Israni AK, Snyder JJ, Hess GP, Engels EA, Segev DL. Sirolimus use and cancer incidence among US kidney transplant recipients. Am J Transplant 2015; 15: 129–36. [DOI] [PubMed] [Google Scholar]
- 114. Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra AL, Hofbauer GF, Pouteil‐Noble C, Campistol JM, Kanitakis J, Roux AS, Decullier E, Dantal J, Group TS. Sirolimus and secondary skin‐cancer prevention in kidney transplantation. N Engl J Med 2012; 367: 329–39. [DOI] [PubMed] [Google Scholar]
- 115. Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier‐Kriesche HU, Weir MR, Wilkinson A. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant 2008; 8: 1384–92. [DOI] [PubMed] [Google Scholar]
- 116. Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R, Camposano S, Paolini J, Fennessy F, Lee N, Woodrum C, Manola J, Garber J, Thiele EA. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF‐ D levels decrease. PLoS One 2011; 6: e23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Murakami N, Riella LV, Funakoshi T. Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta‐analysis. Am J Transplant 2014; 14: 2317–27. [DOI] [PubMed] [Google Scholar]
- 118. Monaco AP. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation 2009; 87: 157–63. [DOI] [PubMed] [Google Scholar]
- 119. Braun M, Young J, Reiner CS, Poster D, Wuthrich RP, Serra AL. Ovarian toxicity from sirolimus. N Engl J Med 2012; 366: 1062–4. [DOI] [PubMed] [Google Scholar]
- 120. Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation 2012; 94: 547–61. [DOI] [PubMed] [Google Scholar]
- 121. Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int 2011; 24: 1216–30. [DOI] [PubMed] [Google Scholar]
- 122. White DA, Camus P, Endo M, Escudier B, Calvo E, Akaza H, Uemura H, Kpamegan E, Kay A, Robson M, Ravaud A, Motzer RJ. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med 2010; 182: 396–403. [DOI] [PubMed] [Google Scholar]
- 123. Somers MJ, Paul E. Safety considerations of mammalian target of rapamycin inhibitors in tuberous sclerosis complex and renal transplantation. J Clin Pharmacol 2015; 55: 368–76. [DOI] [PubMed] [Google Scholar]
- 124. Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol 2010; 185: 3919–31. [DOI] [PubMed] [Google Scholar]
- 125. Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, Illei P, Sharma A, Naray‐Fejes‐Toth A, Fejes‐Toth G, Misra‐Sen J, Horton MR, Powell JD. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol 2014; 15: 457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T‐cell differentiation. Nature 2009; 460: 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 2015; 16: 178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Boor PP, Metselaar HJ, Mancham S, van der Laan LJ, Kwekkeboom J. Rapamycin has suppressive and stimulatory effects on human plasmacytoid dendritic cell functions. Clin Exp Immunol 2013; 174: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]


