Abstract
Aims
The risk of hypoglycaemia may differ among sulphonylureas (SUs), but evidence from head‐to‐head comparisons is sparse. Performing a network meta‐analysis to use indirect evidence from randomized controlled trials (RCTs), we compared the relative risk of hypoglycaemia with newer generation SUs when added to metformin.
Methods
A systematic review identified RCTs lasting 12–52 weeks and evaluating SUs added to inadequate metformin monotherapy (≥1000 mg/day) in type 2 diabetes. Adding RCTs investigating the active comparators from the identified SU trials, we established a coherent network. Hypoglycaemia of any severity was the primary end point.
Results
Thirteen trials of SUs and 14 of oral non‐SU antihyperglycaemic agents (16 260 patients) were included. All reported hypoglycaemia only as adverse events. Producing comparable reductions in HbA1C of −0.66 to −0.84% (−7 to −9 mmol/mol), the risk of hypoglycaemia was lowest with gliclazide compared to glipizide (OR 0.22, CrI: 0.05 to 0.96), glimepiride (OR 0.40, CrI: 0.13 to 1.27), and glibenclamide (OR 0.21, CrI: 0.03 to 1.48). A major limitation is varying definitions of hypoglycaemia across studies.
Conclusions
When added to metformin, gliclazide was associated with the lowest risk of hypoglycaemia between the newer generation SUs. Clinicians should consider the risk of hypoglycaemia agent‐specific when selecting an SU agent.
Keywords: hypoglycaemia, network meta‐analysis, oral antiglycaemic agents, relative safety, sulphonylurea, type 2 diabetes
What is Already Known about this Subject
Adding a sulphonylurea derivate (SU) to metformin remains a commonly used second‐line strategy for management of type 2 diabetes.
The individual SU agents differ pharmacologically and may confer different risk of hypoglycaemia but there are still few robust designed RCTs that compare the SU agents head to head.
Gliclazide and glimepiride have been associated with lower risk of mortality compared with glibenclamide.
What this Study Adds
When added to metformin, gliclazide confers the lowest risk of hypoglycaemia between the newer generation SU agents.
Introduction
For more than 50 years, oral sulphonylurea derivates (SUs) have been fundamental in the medical treatment of type 2 diabetes (T2DM) and despite an expanding number of treatment options, adding an SU is still one of the second‐line choices recommended after initial metformin monotherapy 1. Effective in lowering glucose levels, the SUs increase the secretion of insulin through the blocking of ATP‐sensitive potassium channels in pancreatic beta cells 2. An important side effect is hypoglycaemia that one in five patients with T2DM experience in some form each year 3. Hypoglycaemia has a negative impact on the patients' quality of life 4, 5, 6 and even mild hypoglycaemia can be a psychological burden to patients, and fear of hypoglycaemia may inhibit adherence to treatment 7. More severe episodes may interfere with level of consciousness, balance, coordination and vision and precipitate falls and injury or even coma, seizures and strokes 8.
Including the SUs as a homogeneous drug class, several systematic reviews and meta‐analyses have examined the effect of noninsulin antidiabetic drugs 4, 5, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17. There is, however, a vast amount of evidence indicating that the SU agents differ in terms of tissue selectivity 18, insulin secretory profiles 19, stimulation of insulin secretion during hypoglycaemia 20, risk of hypoglycaemia 12, 21, 22, 23, 24 and effect on counter‐regulatory defence during hypoglycaemia 25. Hypoglycaemia seems associated with cardiac ischaemia 26 and a recent network meta‐analysis revealed that gliclazide and glimepiride were associated with a lower risk of cardiovascular and all‐cause mortality compared with glibenclamide 27.
An expanding number of oral anti‐glycaemic agents are available, but when sufficient glycaemic control cannot be maintained with lifestyle management and metformin, adding an SU is cost‐effective 28 and SUs remain the most commonly used second‐line oral antidiabetic drugs 29. Newer generation SUs, gliclazide, glipizide, glibenclamide (glyburide) and glimepiride have taken the place of the first generation agents tolbutamide, chlorpropamide, acetohexamide and tolazamide 2. However, robust designed RCTs comparing the currently available agents are still few 22, 24 and because the patents of all SUs have expired, it is unlikely that further RCTs will be performed to assess whether differences in pharmacological properties among the individual SUs translate into differences in risk of adverse events.
In the absence of robust comparative trials, a network meta‐analysis is a powerful alternative approach to compare efficacy and safety of several treatment options. In contrast to conventional pairwise meta‐analysis, a network meta‐analysis allows the combination of evidence from studies that have one or more treatments in common and provides estimates of the relative efficacy between all interventions whether or not they have been trialled against each other 30, 31. Comparing the relative effectiveness with this approach, the effect of randomization from the individual trials is preserved 31.
To provide information needed to assist prescribers in selecting a specific SU for clinical use, we conducted this systematic review and network meta‐analysis of available direct and indirect evidence to compare the relative risk of hypoglycaemia among SUs when used in patients with T2DM not controlled by metformin monotherapy.
Materials and methods
Data sources and searches
A systematic literature review of PubMed, EMBASE, and Cochrane Library electronic databases was performed to identify clinical papers (Appendix S1). First, RCTs comparing glimepiride, gliclazide, glibenclamide (glyburide) and glipizide to placebo or an oral non‐SU agent were identified. In order to connect the network and produce reliable estimates of the relative safety and efficacy, we applied the same inclusion criteria and searched the databases a second time for RCTs comparing the non‐SU comparators in the identified SU trials with each other or with placebo. We did not contact authors to identify additional studies or confirm data.
Study selection
The two authors independently conducted the literature search and study selection. Searching the databases from inception until 8 January 2016, each record was included according to the following criteria: RCT with a duration of 12–52 weeks of SU in patients with T2DM aged 18 or older, who had received metformin monotherapy ≥1000 mg for at least 4 weeks and required add‐on therapy with another oral antihyperglycaemic agent due to inadequate control (HbA1C > 6.5% (47.5 mmol/mol)). Only studies reporting outcomes of hypoglycaemia were included. Potential relevant papers and abstracts were obtained and the full text editions were reviewed for inclusion. Furthermore, we supplemented the electronic database search by examining reference lists of the included papers. Extension studies and dose‐finding studies together with studies using SU combinations were excluded. No language exclusion criterion was applied.
Data extraction and quality assessment
Data abstraction and risk of bias assessment using the Jadad scale 32 was performed by one author and checked by the other. Only trials rated 3 out of 5 or greater were included in the final analysis. We extracted the following information from each trial: (i) first author's name, (ii) year of publication, (iii) number of participants, (iv) duration of follow‐up, (v) base‐line characteristics (age, gender, body mass index (BMI), HbA1C, duration of diabetes, metformin dosage and duration), (vi) drug name, drug class, dose and dosing (fixed or flexible), (vii) end points (overall hypoglycaemia of any severity, severe hypoglycaemia, mean change in HbA1C and change in body weight).
Data synthesis and analysis
We constructed the network meta‐analyses by combining direct and indirect evidence. The outcomes were hypoglycaemia of any severity (the primary outcome), HbA1C and body weight. Hypoglycaemia was analysed as a dichotomous end point and reported as odds ratio (OR) with 95% credibility interval (CrI), while changes in HbA1C and body weight were analysed as continuous variables and reported as absolute differences with 95% CrI 31. CrI is the Bayesian analogue to the confidence interval in frequentist statistics. Trial arms in which the agents were administered at doses below clinical recommended doses were excluded from the meta‐analyses (Appendix S2). All analyses were conducted as random effects models based on the assumption of heterogeneity across the RCTs.
In addition to the assumptions required for a standard pairwise meta‐analysis, network meta‐analyses are based on the assumptions that covariates acting as treatment modifiers are similar across trials and that the indirect evidence is consistent with the direct evidence 33, 34. We assessed potential incoherence in the closed loops of the evidence network in terms of discrepant results from direct and indirect evidence by running both inconsistency analyses and node split models 35. Publication bias were analysed using comparison‐adjusted funnel plots 36.
Finally, we did a sensitivity analysis of hypoglycaemia excluding the GUIDE study 22 that used the gliclazide modified release formulation (MR) and provided no information on baseline metformin doses.
The network meta‐analyses were performed using a Bayesian Markov‐chain Monte Carlo method and fitted in the free open source software, ADDIS version 1.16.3 (Research Institute SOM, Faculty of Economics & Business, University of Groningen, The Netherlands; http://drugis.org/addis).
Results
Characterization of trials included in the analyses
The search identified 541 records, of which 26 fulfilled our inclusion criteria (Figure 1) 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62. Though no data on the baseline metformin dosage were provided, we decided not to exclude the only head‐to‐head comparison of SUs in a relevant population, the GUIDE study 22. None of the 27 included studies reported hypoglycaemia or safety as the primary outcome. The analyses included 16 260 patients, 1878 of which had been randomized to placebo and 5572 to SU (glibenclamide: 261; gliclazide: 847; glimepiride: 2981; and glipizide: 1483). In total, 1637 patients (10.1%) had experienced one or more episodes of hypoglycaemia, including 1249 (22.4%) of the SU‐treated patients, and 42 (2.2%) of those receiving metformin monotherapy (placebo). Appendix S2 shows the study characteristics.
Figure 1.
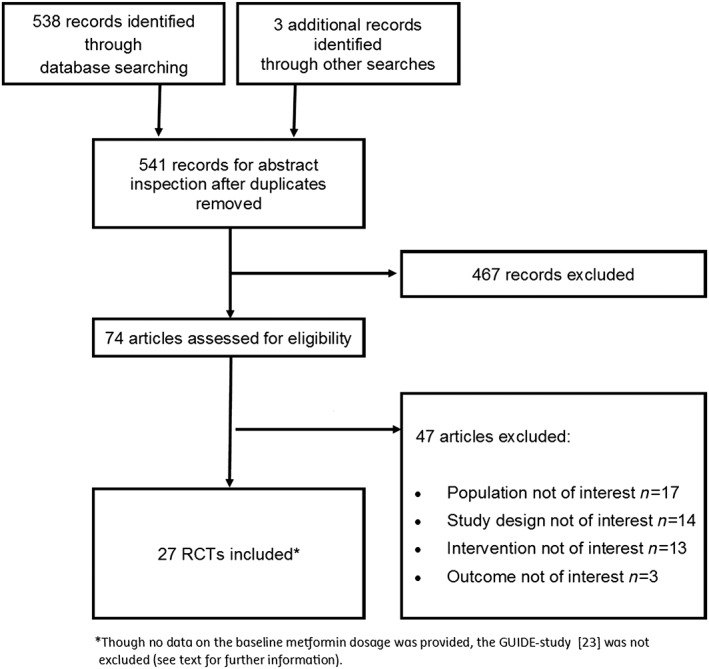
PRISMA flow chart of study selection results
The evidence network comprised 57 study arms from the 27 trials (Figure 2) lasting 16–52 weeks. The baseline HbA1c differed across the trials with the lower inclusion threshold between 6.5 and 8.0% (47.5 and 63.9 mmol/mol) and the higher threshold between 8.5 and 16.7% (63.9 and 159 mmol/mol) (Appendix S2). The metformin dosage and duration at baseline also varied across the trials, although the patients used ≥1500 mg for ≥2 or 3 months in most studies. While six trials reported no criteria for hypoglycaemia 42, 44, 47, 54, 58, 62, the remainder used various criteria ranging from symptoms suggestive of hypoglycaemia to symptomatic hypoglycaemia confirmed by a glucose measurement. Hypoglycaemic episodes requiring third party or medical assistance were typically classified as severe. Yet, no consistent criteria were applied for minor hypoglycaemia.
Figure 2.
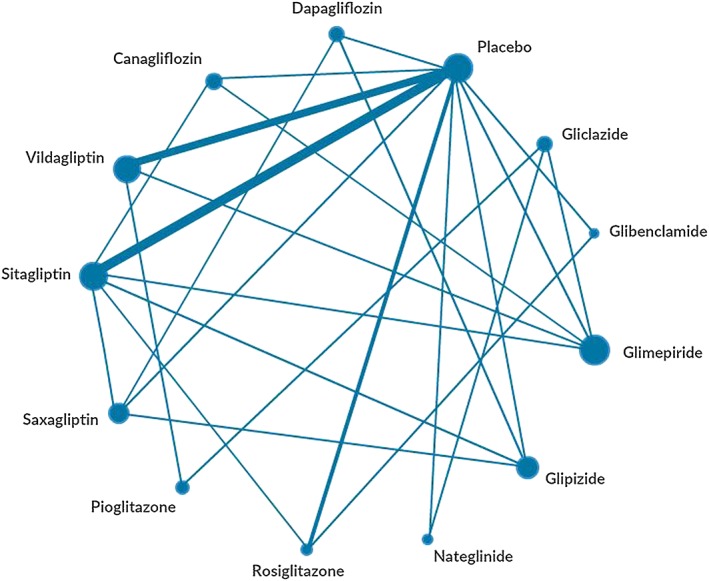
The evidence network of eligible comparisons for hypoglycaemia. The width of the lines is proportional to the number of trials comparing each pair of treatments, and the area of each node is proportional to the number of randomized participants (sample size)
Reflecting variable methodological quality, the mean Jadad score was 4 out of 5, most often due to missing information about the randomization (15 trails) or blinding (12 trials). No definition of hypoglycaemia was mentioned in 1 out of 11 trials rated 5 out of 5 and in 2 out of 5 trials rated 4 out of 5 in Jadad (Appendix S2). Nine trials provided no definition of severe hypoglycaemia, one of which rated 5 out of 5 and two of which rated 4 out of 5 in Jadad. Visual inspection of comparison‐adjusted funnel plots 36 (Appendices S3 and S4) did not suggest any small study effects or publication bias.
Table 1 shows baseline demographic and metabolic characteristics from the 27 included trials. The number of patients randomized ranged from 122 to 2789. The mean age was similar across the study arms (53–62 years) and the gender distribution equal, although two studies had a relative high proportion of females (59%) 45, 58. Mean diabetes duration ranged from 4.6 to 8.4 years, baseline HbA1c from 6.4 to 9.3% (46.4 to 78.1 mmol/mol), fasting plasma glucose from 8.0 to 12.2 mmol/l, and BMI from 25.5 to 32.9 kg/m2.
Table 1.
Baseline demographic and metabolic characteristics of the randomized patients by study
| Study | Group a | No. | Mean age (y) | Female (%) | Known duration of diabetes (y) | HbA 1c , (%) | HbA 1c (mmol/mol) | Fasting plasma glycose, ( mmol l ‐1 ) | Body weight (kg) | Body mass index ( kg m ‐2 ) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fonseca et al. 47 | Rosiglitazone | l | 58.3 (8.8) | 32 | 8.3 (6.3) | 8.9 (1.3) | 73.8 | 12.2 (3.1) | NR | 29.8 (3.9) |
| Placebo | 116 | 58.8 (9.2) | 26 | 7.3 (5.7) | 8.6 (1.3) | 70.5 | 11.9 (2.9) | NR | 30.3 (4.4) | |
| Charpentier et al. 43 | Glimepiride | 147 | Median 56.8 | 42 | Median 5.6 | 6.4 (1.1) | 46.4 | 10.4 (1.8) | 81.2 (NR) | 29.5 (NR) |
| Placebo | 75 | Median 56.7 | 40 | Median 7.0 | 7.8 (1.2) | 61.7 | 10.6 (1.8) | 82.2 (NR) | 29.2 (NR) | |
| Marre et al. 52 | Glibenclamide | 101 | 58.0 (13.0) | 51 | 5.9 (5.4) | 7.9 (1.6) | 62.8 | 10.7 (3.0) | 84.7 (15.1) | 30.1 (4.6) |
| Placebo | 104 | 57.5 (11.5) | 40 | 5.4 (4.9) | 8.1 (1.8) | 65.0 | 11.0 (3.2) | 84.9 (17.6) | 29.9 (4.7) | |
| Marre et al. 53 | Nateglinide | 160 | 57.3 (10.5) | 39 | 6.8 (5.5) | 8.2 (NR) | 66.1 | 9.9 (2.5)b | 85.2 (13.9)b | 29.3 (3.5) |
| Placebo | 152 | 56.4 (10.3) | 45 | 6.5 (6.5) | 8.3 (NR) | 67.2 | 10.1 (2.5)b | 84.9 (14.7)b | 29.6 (3.9) | |
| Schernthaner et al. 22 | Gliclazide | 405 | 60.5 (9.9) | 49 | 5.6 (5.9) | 8.4 (1.1) | 68.3 | 10.2 (2.6) | 83.1 (14.3) | 30.5 (4.8) |
| Glimepiride | 440 | 60.6 (10.5) | 48 | 5.8 (5.8) | 8.2 (1.0) | 66.1 | 10.1 (2.6) | 83.8 (16.0) | 30.6 (4.9) | |
| Feinglos et al. 45 | Glipizide | 61 | 57.7 (10.7) | 54 | 6.5 (NR) | 7.5 (NR) | 58.5 | 8.6 (1.5)b | 90.0 (18.7) | 31.7 (4.4) |
| Placebo | 61 | 58.8 (10.0) | 59 | 4.6 (NR) | 7.6 (NR) | 59.6 | 8.7 (1.5)b | 90.8 (18.4) | 32.1 (4.9) | |
| Matthews et al. 54 | Pioglitazone | 317 | 56 (9.2) | 49 | 5.8 (5.1) | 8.7 (1.1) | 49.7 | 11.8 (3.1) | 91.8 (16.2) | 32.6 (5.0) |
| Gliclazide | 313 | 57 (9.0) | 51 | 5.5 (5.1) | 8.5 (0.9) | 69.4 | 11.3 (2.6) | 92.7 (17.4) | 32.6 (5.8) | |
| Charbonnel et al. 42 | Sitagliptin | 464 | 54.4 (10.4) | 44 | 6.0 (5.0) | 8.0 (0.8) | 63.9 | 9.4 (2.3) | 86.7 (17.8) | 30.9 (5.3) |
| Placebo | 237 | 54.7 (9.7) | 41 | 6.6 (5.5) | 8.0 (0.8) | 63.9 | 9.7 (2.3) | 89.6 (17.5) | 31.5 (4.9) | |
| Garber et al. 48 | Glibenclamide | 160 | 56 (NR) | 44 | 5 (4) | 8.5 (1.2) | 69.4 | 10.6 (2.9) | 93 (17) | 32 (5) |
| Rosiglitazone | 158 | 56 (NR) | 35 | 6 (5) | 8.4 (1.1) | 68.3 | 10.4 (2.7) | 94 (18) | 32 (5) | |
| Ristic et al. 59 | Nateglinide | 133 | 62.0 (11.0) | 46 | 7.2 (6.3) | 7.7 (0.6) | 60.7 | 9.0 (1.5) | NR | 28.5 (3.5) |
| Gliclazide | 129 | 61.6 (10.1) | 50 | 6.7 (5.6) | 7.6 (0.6) | 59.6 | 8.7 (1.5) | NR | 29.5 (3.6) | |
| Nauck et al. 55 | Sitagliptin | 588 | 56.8 (9.3) | 43 | 6.5 (6.1) | 7.7 (0.9) | 60.7 | 8.8 (1.9) | 89.5 (17.6) | 31.2 (5.0) |
| Glipizide | 584 | 56.6 (9.8) | 39 | 6.2 (5.4) | 7.6 (0.9) | 59.6 | 8.8 (2.1) | 89.7 (17.5) | 31.3 (5.2) | |
| Bolli et al. 39 | Vildagliptin | 295 | 56.3 (9.3) | 38 | 6.4 (4.9) | 8.4 (1.0) | 68.3 | 10.9 (2.6) | 91.8 (18.5) | 32.2 (5.6) |
| Pioglitazone | 281 | 57.0 (9.7) | 36 | 6.4 (5.2) | 8.4 (0.9) | 68.3 | 11.0 (2.7) | 91.2 (16.9) | 32.1 (5.1) | |
| Bosi et al. 40 | Vildagliptin | 185 | 53.9 (9.5) | 39 | 5.8 (4.7) | 8.4 (1.0) | 68.3 | 9.9 (2.6) | 95.3 (20.3)b | 32.9 (5.0) |
| Placebo | 182 | 54.5 (10.3) | 47 | 6.2 (5.3) | 8.3 (0.9) | 67.2 | 10.1 (2.4) | 94.8 (24.2)b | 33.2 (6.1) | |
| Raz et al. 58 | Sitagliptin | 96 | 53.6 (9.5) | 49 | 8.4 (6.5) | 9.3 (0.9) | 78.1 | 11.2 (2.6) | 81.5 (16.8) | 30.1 (4.4) |
| Placebo | 94 | 56.1 (9.5) | 59 | 7.3 (5.3) | 9.1 (0.8) | 76.0 | 11.0 (2.4) | 81.2 (19.4) | 30.4 (5.3) | |
| Scott et al. 63 | Sitagliptin | 94 | 55.2 (9.8) | 45 | 4.9 (3.5) | 7.8 (1.0) | 61.7 | 8.7 (1.7) | 83.1 (17.1) | 30.3 (4.7) |
| Rosiglitazone | 87 | 54.8 (10.5) | 37 | 4.6 (4.0) | 7.7 (0.8) | 60.7 | 8.7 (1.8) | 84.9 (18.5) | 30.4 (5.5) | |
| Placebo | 92 | 55.3 (9.3) | 41 | 5.4 (3.7) | 7.7 (0.9) | 60.7 | 8.9 (2.1) | 84.6 (16.5) | 30.0 (4.5) | |
| DeFronzo et al. 44 | Saxagliptin | 191 | 54.7 (9.6) | 46 | 6.4 (4.7) | 8.1 (0.8) | 65.0 | 10.0 (2.6) | 87.3 (17.0) | 31.2 (4.7) |
| Placebo | 179 | 54.8 (10.2) | 46 | 6.7 (5.6) | 8.1 (1.0) | 65.0 | 9.7 (2.4) | 87.1 (17.8) | 31.6 (4.8) | |
| Ferrannini et al. 46 | Vildagliptin | 1396 | 57.5 (9.1) | 47 | 5.7 (5.2) | 7.3 (0.6) | 56.3 | 9.2 (2.3) | 89.0 (NR) | 31.8 (5.3 |
| Glimepiride | 1393 | 57.5 (9.3) | 46 | 5.8 (5.0) | 7.3 (0.7) | 56.3 | 9.2 (2.2) | 88.6 (NR) | 31.7 (5.3) | |
| Goodman et al. 50 | Vildagliptin | 248 | 54.9 (10.8) | 47 | NR | 8.5 (1.0) | 69.4 | 10.8 (2.8) | NR | 31.4 (4.7) |
| Placebo | 122 | 54.5 (9.7) | 33 | NR | 8.7 (1.0) | 71.6 | 11.1 (2.8) | NR | 31.7 (4.3) | |
| Bailey et al. 38 | Dapagliflozin | 135 | 52.7 (9.9) | 43 | 6.1 (5.4) | 7.9 (0.8) | 62.8 | 8.7 (2.2) | 86.3 (17.5) | 31.2 (5.1) |
| Placebo | 137 | 53.7 (10.3) | 45 | 5.8 (5.1) | 8.1 (1.0) | 65.0 | 9.2 (2.6) | 87.7 (19.2) | 31.8 (5.3) | |
| Göke et al. 49 | Saxagliptin | 428 | 57.5 (10.3) | 51 | 5.5 (4.5) | 7.7 (0.9) | 60.7 | 9.0 (2.3) | 88.7 (18.6) | 31.5 (5.7) |
| Glipizide | 430 | 57.6 (10.4) | 46 | 5.4 (4.7) | 7.7 (0.9) | 60.7 | 8.9 (2.2) | 88.6 (19.6) | 31.3 (6.2) | |
| Scheen et al. 61 | Saxagliptin | 403 | 58.8 (10.1) | 53 | 6.3 (5.0) | 7.7 (1.0) | 60.7 | 8.9 (2.5) | NR | 31.1 (5.3) |
| Sitagliptin | 398 | 58.1 (10.5) | 49 | 6.3 (4.7) | 7.7 (0.9) | 60.7 | 8.9 (2.4) | NR | 30.9 (5.5) | |
| Arechavaleta et al. 37 | Sitagliptin | 516 | 56.3 (9.7) | 45 | 6.8 (4.6) | 7.5 (0.7) | 58.5 | 8.0 (1.8) | 80.6 (15.2) | 29.7 (4.5) |
| Glimepiride | 519 | 56.2 (10.1) | 46 | 6.7 (4.8) | 7.5 (0.8) | 58.5 | 8.1 (1.9) | 82.0 (16.7) | 30.2 (4.4) | |
| Nauck et al. 56 | Dapagliflozin | 406 | 58 (9) | 45 | 6 (5) | 7.7 (0.9) | 60.7 | 9.0 (2.1) | NR | 31.7 (5.1) |
| Glipizide | 408 | 59 (10) | 45 | 7 (6) | 7.7 (0.9) | 60.7 | 9.1 (2.3) | NR | 31.2 (5.1) | |
| Pan et al. 57 | Vildagliptin | 145 | 54.2 (9.6) | 50 | 4.9 (4.8) | 8.1 (0.9) | 65.0 | 8.8 (2.0) | 71.6 (11.9) | 26.1 (3.3) |
| Placebo | 144 | 54.5 (9.7) | 54 | 5.2 (4.6) | 8.0 (0.8) | 63.9 | 8.8 (2.1) | 69.8 (11.2) | 25.5 (3.1) | |
| Cefalu et al. 41 | Glimepiride | 482 | 56.3 (9.0) | 45 | 6.6 (5.0) | 7.8 (0.8) | 61.7 | 9.2 (2.1) | 86.5 (19.8) | 30.9 (5.5) |
| Canagliflozin | 485 | 55.8 (9.2) | 50 | 6.7 (5.5) | 7.8 (0.8) | 61.7 | 9.1 (2.0) | 86.6 (19.5) | 31.0 (5.4) | |
| Lavalle‐González et al. 51 | Placebo | 183 | 55.3 (9.8) | 49 | 6.8 (5.3) | 8.0 (0.9) | 63.9 | 9.1 (2.1) | 86.6 (22.4) | 31.1 (6.1) |
| Canagliflozin | 367 | 55.3 (9.2) | 55 | 7.1 (5.4) | 7.9 (0.9) | 62.8 | 9.6 (2.5) | 85.4 (20.9) | 31.4 (6.3) | |
| Sitagliptin | 366 | 55.5 (9.6) | 53 | 6.8 (5.2) | 7.9 (0.9) | 62.8 | 9.4 (2.3) | 87.7 (21.6) | 32.0 (6.1) | |
| Rosenstock et al. 60 | Saxagliptin | 176 | 55 (10) | 47 | 8.2 (5.5) | 9.0 (1.1) | 74.9 | 10.7 (2.5) | 88.0 (18.7) | 31.8 (5.1) |
| Dapagliflozin | 179 | 54 (10) | 50 | 7.4 (5.4) | 8.9 (1.2) | 73.8 | 10.3 (2.7) | 86.3 (18.6) | 31.5 (5.3) |
Data are presented as mean with standard deviation (SD) unless stated otherwise. NR, not reported; HbA1c, glycosylated haemoglobin
Only study arms included in the meta‐analysis are shown
SD calculated from standard error of mean (SE)
Meta‐analyses
Our primary network meta‐analysis for hypoglycaemia was based on data from 27 studies. The inconsistency and note‐split models revealed no statistically significant inconsistency between direct and indirect evidence. Table 2 shows the comparative risk of hypoglycaemia of any severity with the four SUs + metformin. Among the SUs, the risk of hypoglycaemia was lowest with gliclazide. Only the risk associated with glipizide, however, was statistically higher than the risk with gliclazide (OR 4.60, CrI: 1.04, 19.48). Figure 3 shows the estimated probability that, given the priors and the data, each of the SU agents has the lowest rate of hypoglycaemia (rank 1), the second lowest (rank 2), etc. Gliclazide ranked best among the SUs.
Table 2.
Comparative risk of hypoglycaemia of any severity. Results are the odds‐ratios (ORs) with 95% credibility intervals (CI). ORs less than 1 favour the column defining treatment in terms of lower risk of hypoglycaemia. Results are statistically significant where the CIs do not cross 1.
| Gliclazide | |||
| 0.40 (0.13, 1.27) | Glimepiride | ||
| 0.21 (0.03, 1.48) | 0.51 (0.09, 2.83) | Glibenclamide | |
| 0.22 (0.05, 0.96) | 0.54 (0.18, 1.64) | 1.04 (0.18, 6.85) | Glipizide |
Figure 3.
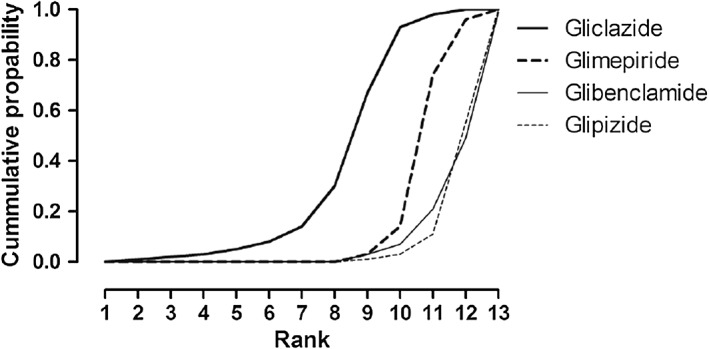
The cumulative rankogram of the estimated probability that, given the priors and the data, each SU agent has the lowest rate of hypoglycaemia (rank 1), the second lowest (rank 2), etc., among the compared agents
Figure 4 shows the comparative risk of hypoglycaemia when comparing all the individual oral anti‐glycaemic agents using placebo as reference. A significantly higher risk of hypoglycaemia than with placebo was noted for nateglinide, glimepiride, glipizide, and glibenclamide, but not for gliclazide (OR 2.91, CrI: 0.87–9.93). Moreover, the risk with gliclazide was not statistically different from that of the other non‐SU agents included in the present analysis with the exception of pioglitazone (OR 9.75, CrI: 2.40–42.38) (Appendix S5).
Figure 4.
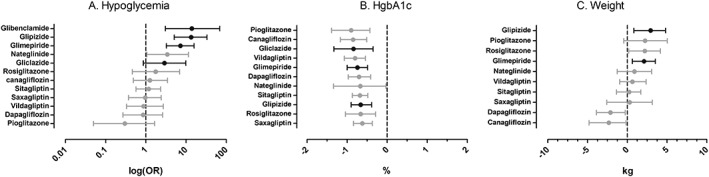
Comparative risk of hypoglycaemia (A) and comparative effect on HgbA1c (B) and body weight (C) when comparing the individual oral anti‐glycaemic agents + metformin using metformin + placebo as reference. Results with 95% credibility intervals (CI) are: (A) odds ratios (ORs); (B) absolute difference (%); and (C) absolute difference (kg)
Excluding the GUIDE study from the analysis led to only minor changes in the ORs for hypoglycaemia and did not affect the overall rank of the four SU agents (Appendix S6). Thus, OR for hypoglycaemia with gliclazide vs. placebo increased from 2.91 (CrI: 0.87–9.93) to 4.65 (CrI: 0.79–28.91) while OR with glimepiride vs. placebo decreased from 7.25 (CrI: 3.25–15.93) to 6.43 (CrI: 2.77–15.26).
A total of 22 studies reported data on severe hypoglycaemia 37, 39, 40, 41, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 60, 61 and this outcome was rare for all drug classes. Most trials reported zero events. Severe hypoglycaemia affected none of the patients enrolled for glibenclamide or gliclazide compared to 0–2.1% of the patients enrolled for glimepiride and 0–2.6% of the patients enrolled for glipizide.
The secondary network meta‐analysis for efficacy on HbA1C was based on 21 RCTs. Six RCTs reported no measure of variance and were not included in the analysis of HbA1C 47, 48, 50, 52, 53, 54. Because this also counted for both RCTs on glibenclamide 48, 52, the relative efficacy of glibenclamide could not be estimated. Overall, the SU and non‐SU agents produced statistically similar mean change from baseline in HbA1C that ranged from −0.60 to −0.90% (−6.1 to −9.9 mmol/mol) compared to placebo (Figure 4). The efficacy was similar across all included comparators (Appendix S7).
Most RCTs reported data on weight change from baseline, but only 13 reported measures of variance and were included in our secondary network meta‐analysis 37, 38, 39, 40, 41, 43, 46, 51, 53, 55, 56, 60, 63. Compared with metformin monotherapy, glimepiride and glipizide produced a body weight gain of 2.11 kg (CrI: 0.64–3.53 kg) and 2.94 (CrI: 0.84–4.83 kg), respectively (Figure 4 and Appendix S8). The weight gain associated with glibenclamide and gliclazide could not be estimated.
Discussion
In this systematic review, we examined the risk of hypoglycaemia associated with four SUs and eight non‐SU antihyperglycaemic drugs by compiling direct and indirect evidence from 27 RCTs in patients with T2DM inadequately controlled by metformin monotherapy. This analysis is the first to synthesize the available safety data to compare relative risk of hypoglycaemia among the different SU agents. Indicating that the risk of hypoglycaemia may differ among the SUs with gliclazide having the lowest of the four, our results are consistent with direct evidence from the only sufficiently powered head‐to‐head trial, the GUIDE study 22, which demonstrated fewer hypoglycaemic episodes with gliclazide than with glimepiride. Although our analysis suggests a relevant difference between the SU agents, the credibility intervals are wide, reflecting considerable clinical uncertainty.
Interestingly, a recent systematic review compared the relative risk of mortality and adverse cardiovascular events with the SUs 27. Including both RCTs and controlled observational studies, Simpson et al. also undertook a network meta‐analysis and reported that gliclazide use was associated with the lowest risk of mortality followed by glimepiride, glipizide and glibenclamide. Relating this to our results may lend support to the notion of a causal relation between hypoglycaemia and adverse cardiac outcomes 64.
Although severe hypoglycaemia was rare, our results support the general view that SUs entail a significant risk of hypoglycaemia 65. The mechanistic basis for this might be that all SUs bind to the SU receptor subunit of ATP‐sensitive potassium channels and trigger exocytosis of insulin from storage granules in pancreatic β‐cells even at low plasma glucose levels 19, where the insulin secretion in humans normally is almost non‐existent. Moreover, some of the SUs seem to impair the neuroendocrine and metabolic counter‐regulatory response to hypoglycaemia 25, 66.
There are considerable differences in pharmacological properties among the individual SUs in terms of intrinsic activity, potency and in onset and duration of action 67, but the extent to which these differences translate into differences in risk of hypoglycaemia is not fully understood. In contrast to the more physiological biphasic response induced by gliclazide 19, glibenclamide enhances basal insulin secretion and fasting insulin levels more than glipizide 68, and induces a delayed monophasic insulin response. Glibenclamide also has two metabolites with hypoglycaemic activity and a relatively long mean elimination half‐life of 15 hours 69. Another important difference is the actions during recovery from hypoglycaemia, where glibenclamide but not glimepiride stimulates insulin secretion at low glucose levels 20. Another difference relates to the pancreatic specificity. While gliclazide and glipizide seem to bind selectively to SUR1 receptors on the insulin secreting pancreatic β‐cells, glimepiride and glibenclamide seem less selective 18. In this context, it might be important that ATP‐sensitive potassium channels, although with varying SU receptor subunits, can be found both in glucagon secreting pancreatic α‐cells and in the brain, heart, vessels and other tissues 67. In addition to specificity for the pancreatic β‐cell KATP channel, gliclazide differs from the other SUs and does not activate the cAMP sensor Epac2 to Rap1 signalling, which promotes insulin granule exocytosis 70.
Two formulations of gliclazide are available and we included two studies that used the immediate release formulation (IR) 54, 59 and one that used the modified release formulation (MR) 22. Including only studies of gliclazide IR in a sensitivity analysis of hypoglycaemia, we found no effect on the overall rank of the SU agents (Appendix S6). Still, the OR for hypoglycaemia with gliclazide increased from 2.91 to 4.65. This indicates that the MR formulation might confer a lower risk of hypoglycaemia and is in keeping with a head‐to‐head comparison of gliclazide IR and MR that reports a lower (but statistically insignificant) risk of hypoglycaemia with gliclazide MR 71.
Despite variable safety profiles, our results indicate a comparable overall effect of the SUs on glycaemic control. This is in line with a series of systematic reviews of the efficacy of oral anti‐hyperglycaemic drugs 4, 6, 9, 10, 14. Thus, applying a similar network meta‐analysis approach, McIntosh et al. reported that SUs as a group resulted in a decrease from baseline in HbA1c of 0.79% relative to metformin monotherapy and increased the risk of hypoglycaemia significantly (OR 8.2) 6.
Strength and weaknesses
The strength of our analysis is the use of network meta‐analysis incorporating both direct and indirect evidence in a single analysis to get an integrated and coherent picture of the relative risk. We included relatively large studies of fairly good quality assessing exposure to the SU for 16–52 weeks and found a reasonably low inter‐trial heterogeneity adding to the robustness of our study. We did not include trials of longer duration in order to limit heterogeneity in our main outcome, the cumulative risk of hypoglycaemia. Moreover, to avoid attrition bias, the extension arms of primary studies were not included. Although our focus was on the SUs, the enrichment of the network with non‐SU trials might also have strengthened the analyses since the exclusion of treatments can affect substantially the estimated treatment effect and occasionally the ranking of treatments 72.
Major limitations of our study relate to those of any meta‐analysis in the absence of sufficiently powered head‐to‐head RCTs. Thus, the included studies comprise patients with different baseline characteristics. Moreover, network meta‐analyses rely on the assumption that data from the different trials are interchangeable, and differences in study design, methodology and patient populations may invalidate this assumption and result in biased estimates from the indirect comparisons. Other potent effect modifiers and causes of heterogeneity and inconsistency in the evidence network such as sex, age, disease duration, and differences in non‐pharmacological care or co‐medication were not considered. For example, it was not possible to take into account concomitant use of SU‐potentiating or ‐antagonizing drugs or risk factors for hypoglycaemia such as alcohol intake, long diabetes duration or presence of diabetes complications. Furthermore, the lack of hypoglycaemia definition in some trials is a limitation that was not fully accounted for in our trial quality assessment.
In addition to similarity between studies, consistency is an important assumption that should hold for network meta‐analyses where indirect estimates are derived 33. Use of inconsistency models and note splitting did not reveal any significant network inconsistency in the present study.
There are other important limitations to consider when interpreting our findings. Thus, we included only trials described as double‐blinded RCTs; however, most of the included trials did not report randomization or blinding procedures. In most cases, forced titration of metformin was applied, and the patients were not truly inadequately controlled with metformin monotherapy according to recent guidelines advocating individualized treatment goals 73, 74. Many trials used flexible dosing; the actual doses used may have varied across studies. We included only published RCTs, but RCTs may underestimate the true risk of hypoglycaemia as patients in RCTs are treated according to a rigorous protocol and patients with hypoglycaemia problems are excluded by design.
None of the included trials reported hypoglycaemia and weight gain as primary outcomes, but only as adverse events and in no cases did an independent committee perform adjudication of hypoglycaemia outcomes. The risk of hypoglycaemia of any severity was our primary outcome and the definition of hypoglycaemia varied across trials. Severe episodes of hypoglycaemia were rare and are generally far less common in clinical trials compared to clinical observational studies. Although severe episodes are much more relevant from a safety, quality of life, and health economic point of view, the occurrence of non‐severe episodes may also be important to patients. Hence, Schloot et al. analysed real life data from SU‐treated patients and found that the reported rate of non‐severe hypoglycaemia was an independent risk factor for severe hypoglycaemia 75.
Constructing a network with newer generation SU studies and comparing the risk of hypoglycaemia, several second‐line oral anti‐hyperglycaemic agents and several relevant outcomes were intentionally left out of the analyses. We did not consider the risk and efficacy of SUs when used alone or in combination with other agents than metformin and we included no specific safety outcomes other than hypoglycaemia and weight gain. The study duration was limited to 52 weeks and did not allow inference on the long‐term risk of SU treatment. Finally, patients included in clinical trials are often younger, healthier and more compliant than the general diabetic patient is, so we would expect a lower incidence of medication‐related adverse events than in real‐life settings.
Faced with only sparse direct evidence from head‐to‐head comparisons, our analyses based on data from well‐conducted RCTs may represent important data that can assist the clinicians and other decision makers when choosing between the newer generation SUs. The selection of an antidiabetic drug should always be individualized and based on, for example, contraindications, co‐morbidity and drug interactions, and ideally agents that are less prone to precipitate hypoglycaemia should be preferred. When metformin monotherapy fails, adding agents such as incretin‐based therapy and/or sodium‐glucose linked transporter‐2 inhibitors might be rational from a clinical point of view as these agents carry limited risk of hypoglycaemia 76. However, as these agents are still quite expensive, adding an SU might be a more affordable alternative for many patients.
Conclusion
The risk of hypoglycaemia does not seem to pertain to SUs as a drug class as such. We conclude that when added to metformin, gliclazide confers the lowest risk of hypoglycaemia between the newer generation SU agents.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Contributors
S.E.A. performed the study concept and design, data acquisition, statistical analysis and interpretation of data, drafting the manuscript, and critical revision of the manuscript. M.C. performed the study design, data acquisition, interpretation of data, drafting the manuscript, and critical revision of the manuscript.
Supporting information
Appendix S1 Search strategy
Appendix S2 Study characteristics
Appendix S3 Comparison adjusted funnel‐plot of placebo‐controlled trials
Appendix S4 Comparison adjusted funnel‐plot of trials comparing a sulphonylurea agent (SU) with a non‐SU agent
Appendix S5 Comparative risk of hypoglycaemia of any severity. Results are the odds‐ratios (ORs) with 95% credibility intervals (CI). ORs less than 1 favour the column defining treatment in terms of lower risk of hypoglycaemia. Results are statistically significant where the CIs do not cross 1
Appendix S6 Sensitivity‐analysis with exclusion of the GUIDE‐study (Schernthaner et al. Eur. J. Clin. Invest 2004;34: 535‐42)
Appendix S7 Glycaemic control. Comparative efficacy on change in HbA1c from baseline. Differences less than 0 favour the column defining treatment. Results are statistically significant where the 95% credibility intervals do not cross 0
Appendix S8 Comparative efficacy on change in body weight from baseline. Differences less than 0 favour the column defining treatment. Results are statistically significant where the 95% credibility intervals do not cross 0
Supporting info item
Andersen, S. E. , and Christensen, M. (2016) Hypoglycaemia when adding sulphonylurea to metformin: a systematic review and network meta‐analysis. Br J Clin Pharmacol, 82: 1291–1302. doi: 10.1111/bcp.13059.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 2. Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 2004; 64: 1339–1358. [DOI] [PubMed] [Google Scholar]
- 3. Simon D, de Pablos‐Velasco P, Parhofer KG, Gonder‐Frederick L, Duprat Lomon I, Vandenberghe H, et al. Hypoglycaemic episodes in patients with type 2 diabetes – risk factors and associations with patient‐reported outcomes: the PANORAMA study. Diabetes Metab 2015; 41: 470–479. [DOI] [PubMed] [Google Scholar]
- 4. Hirst JA, Farmer AJ, Dyar A, Lung TW, Stevens RJ. Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta‐analysis. Diabetologia 2013; 56: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta‐analysis. Diabetes Obes Metab 2012; 14: 810–820. [DOI] [PubMed] [Google Scholar]
- 6. McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, et al. Second‐line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed‐treatment comparison meta‐analysis. Open Med 2011; 5: e35–e48. [PMC free article] [PubMed] [Google Scholar]
- 7. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med 2008; 25: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014; 10: 711–722. [DOI] [PubMed] [Google Scholar]
- 9. Belsey J, Krishnarajah G. Glycaemic control and adverse events in patients with type 2 diabetes treated with metformin + sulphonylurea: a meta‐analysis. Diabetes Obes Metab 2008; 10: 1–7. [DOI] [PubMed] [Google Scholar]
- 10. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos SS, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147: 386–399. [DOI] [PubMed] [Google Scholar]
- 12. Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta‐analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30: 389–394. [DOI] [PubMed] [Google Scholar]
- 13. Gross JL, Kramer CK, Leitao CB, Hawkins N, Viana LV, Schaan BD, et al. Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta‐analysis. Ann Intern Med 2011; 154: 672–679. [DOI] [PubMed] [Google Scholar]
- 14. Monami M, Lamanna C, Marchionni N, Mannucci E. Comparison of different drugs as add‐on treatments to metformin in type 2 diabetes: a meta‐analysis. Diabetes Res Clin Pract 2008; 79: 196–203. [DOI] [PubMed] [Google Scholar]
- 15. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010; 303: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 16. Salas M, Caro JJ. Are hypoglycaemia and other adverse effects similar among sulphonylureas? Adverse Drug React Toxicol Rev 2002; 21: 205–217. [DOI] [PubMed] [Google Scholar]
- 17. Zintzaras E, Miligkos M, Ziakas P, Balk EM, Mademtzoglou D, Doxani C, et al. Assessment of the relative effectiveness and tolerability of treatments of type 2 diabetes mellitus: a network meta‐analysis. Clin Ther 2014; 36: 1443–1453. [DOI] [PubMed] [Google Scholar]
- 18. Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: a systematic review. Diabetes Obes Metab 2012; 14: 130–138. [DOI] [PubMed] [Google Scholar]
- 19. Gregorio F, Ambrosi F, Cristallini S, Pedetti M, Filipponi P, Santeusanio F. Therapeutical concentrations of tolbutamide, glibenclamide, gliclazide and gliquidone at different glucose levels: in vitro effects on pancreatic A‐ and B‐cell function. Diabetes Res Clin Pract 1992; 18: 197–206. [DOI] [PubMed] [Google Scholar]
- 20. Szoke E, Gosmanov NR, Sinkin JC, Nihalani A, Fender AB, Cryer PE, et al. Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism 2006; 55: 78–83. [DOI] [PubMed] [Google Scholar]
- 21. Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev 2001; 17: 467–473. [DOI] [PubMed] [Google Scholar]
- 22. Schernthaner G, Grimaldi A, Di MU, Drzewoski J, Kempler P, Kvapil M, et al. GUIDE study: double‐blind comparison of once‐daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest 2004; 34: 535–542. [DOI] [PubMed] [Google Scholar]
- 23. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc 1996; 44: 751–755. [DOI] [PubMed] [Google Scholar]
- 24. Tessier D, Dawson K, Tetrault JP, Bravo G, Meneilly GS. Glibenclamide vs gliclazide in type 2 diabetes of the elderly. Diabet Med 1994; 11: 974–980. [DOI] [PubMed] [Google Scholar]
- 25. Joy NG, Tate DB, Davis SN. Counterregulatory responses to hypoglycemia differ between glimepiride and glyburide in non diabetic individuals. Metabolism 2015; 64: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003; 26: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 27. Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 43–51. [DOI] [PubMed] [Google Scholar]
- 28. Klarenbach S, Cameron C, Singh S, Ur E. Cost‐effectiveness of second‐line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. CMAJ 2011; 183: E1213–E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdelmoneim AS, Eurich DT, Gamble JM, Simpson SH. Use patterns of antidiabetic regimens by patients with type 2 diabetes. Can J Diabetes 2013; 37: 394–400. [DOI] [PubMed] [Google Scholar]
- 30. Lumley T. Network meta‐analysis for indirect treatment comparisons. Stat Med 2002; 21: 2313–2324. [DOI] [PubMed] [Google Scholar]
- 31. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011; 14: 417–428. [DOI] [PubMed] [Google Scholar]
- 32. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 33. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013; 159: 130–137. [DOI] [PubMed] [Google Scholar]
- 34. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC, et al. Conducting indirect‐treatment‐comparison and network‐meta‐analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011; 14: 429–437. [DOI] [PubMed] [Google Scholar]
- 35. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 36. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arechavaleta R, Seck T, Chen Y, Krobot KJ, O'Neill EA, Duran L, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double‐blind, non‐inferiority trial. Diabetes Obes Metab 2011; 13: 160–168. [DOI] [PubMed] [Google Scholar]
- 38. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 39. Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24‐week, randomized, double‐blind study. Diabetes Obes Metab 2008; 10: 82–90. [DOI] [PubMed] [Google Scholar]
- 40. Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007; 30: 890–895. [DOI] [PubMed] [Google Scholar]
- 41. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 42. Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643. [DOI] [PubMed] [Google Scholar]
- 43. Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med 2001; 18: 828–834. [DOI] [PubMed] [Google Scholar]
- 44. Defronzo RA, Hissa MN, Garber AJ, Arias P, Niskanen L, Xie J, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009; 32: 1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feinglos M, Dailey G, Cefalu W, Osei K, Tayek J, Canovatchel W, et al. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabetes Res Clin Pract 2005; 68: 167–175. [DOI] [PubMed] [Google Scholar]
- 46. Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahren B, Byiers S, et al. Fifty‐two‐week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2009; 11: 157–166. [DOI] [PubMed] [Google Scholar]
- 47. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA 2000; 283: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 48. Garber A, Klein E, Bruce S, Sankoh S, Mohideen P. Metformin‐glibenclamide versus metformin plus rosiglitazone in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2006; 8: 156–163. [DOI] [PubMed] [Google Scholar]
- 49. Göke B, Gallwitz B, Eriksson J, Hellqvist A, Gause‐Nilsson I. Saxagliptin is non‐inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52‐week randomised controlled trial. Int J Clin Pract 2010; 64: 1619–1631. [DOI] [PubMed] [Google Scholar]
- 50. Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res 2009; 41: 368–373. [DOI] [PubMed] [Google Scholar]
- 51. Lavalle‐González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marre M, Howlett H, Lehert P, Allavoine T. Improved glycaemic control with metformin‐glibenclamide combined tablet therapy (Glucovance) in type 2 diabetic patients inadequately controlled on metformin. Diabet Med 2002; 19: 673–680. [DOI] [PubMed] [Google Scholar]
- 53. Marre M, Van GL, Usadel KH, Ball M, Whatmough I, Guitard C. Nateglinide improves glycaemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diabetes Obes Metab 2002; 4: 177–186. [DOI] [PubMed] [Google Scholar]
- 54. Matthews DR, Charbonnel BH, Hanefeld M, Brunetti P, Schernthaner G. Long‐term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: a randomized, comparative study. Diabetes Metab Res Rev 2005; 21: 167–174. [DOI] [PubMed] [Google Scholar]
- 55. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double‐blind, non‐inferiority trial. Diabetes Obes Metab 2007; 9: 194–205. [DOI] [PubMed] [Google Scholar]
- 56. Nauck MA, Del PS, Meier JJ, Duran‐Garcia S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care 2011; 34: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, et al. Efficacy and tolerability of vildagliptin as add‐on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2012; 14: 737–744. [DOI] [PubMed] [Google Scholar]
- 58. Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24: 537–550. [DOI] [PubMed] [Google Scholar]
- 59. Ristic S, Collober‐Maugeais C, Pecher E, Cressier F. Comparison of nateglinide and gliclazide in combination with metformin, for treatment of patients with type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone. Diabet Med 2006; 23: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015; 38: 376–383. [DOI] [PubMed] [Google Scholar]
- 61. Scheen AJ, Charpentier G, Ostgren CJ, Hellqvist A, Gause‐Nilsson I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2010; 26: 540–549. [DOI] [PubMed] [Google Scholar]
- 62. Scott DA, Boye KS, Timlin L, Clark JF, Best JH. A network meta‐analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab 2013; 15: 213–223. [DOI] [PubMed] [Google Scholar]
- 63. Scott R, Loeys T, Davies MJ, Engel SS. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10: 959–969. [DOI] [PubMed] [Google Scholar]
- 64. Sanon VP, Sanon S, Kanakia R, Yu H, Araj F, Oliveros J, et al. Hypoglycemia from a cardiologist's perspective. Clin Cardiol 2014; 37: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hemmingsen B, Schroll JB, Lund SS, Wetterslev J, Gluud C, Vaag A, et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 2013; 4: CD009008. [DOI] [PubMed] [Google Scholar]
- 66. Landstedt‐Hallin L, Adamson U, Lins PE. Oral glibenclamide suppresses glucagon secretion during insulin‐induced hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab 1999; 84: 3140–3145. [DOI] [PubMed] [Google Scholar]
- 67. Melander A. Kinetics‐effect relations of insulin‐releasing drugs in patients with type 2 diabetes: brief overview. Diabetes 2004; 53: S151–S155. [DOI] [PubMed] [Google Scholar]
- 68. Cozma LS, Luzio SD, Dunseath GJ, Langendorg KW, Pieber T, Owens DR. Comparison of the effects of three insulinotropic drugs on plasma insulin levels after a standard meal. Diabetes Care 2002; 25: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 69. Jonsson A, Rydberg T, Ekberg G, Hallengren B, Melander A. Slow elimination of glyburide in NIDDM subjects. Diabetes Care 1994; 17: 142–145. [DOI] [PubMed] [Google Scholar]
- 70. Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab 2012; 14: 9–13. [DOI] [PubMed] [Google Scholar]
- 71. Drouin P. Diamicron MR once daily is effective and well tolerated in type 2 diabetes: a double‐blind, randomized, multinational study. J Diabetes Complications 2000; 14: 185–191. [DOI] [PubMed] [Google Scholar]
- 72. Mills EJ, Kanters S, Thorlund K, Chaimani A, Veroniki AA, Ioannidis JP. The effects of excluding treatments from network meta‐analyses: survey. BMJ 2013; 347: f5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014; 104: 1–52. [DOI] [PubMed] [Google Scholar]
- 74. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauk M, et al. Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577–1596. [DOI] [PubMed] [Google Scholar]
- 75. Schloot NC, Haupt A, Schutt M, Badenhoop K, Laimer M, Nicolay C, et al. Risk of severe hypoglycemia in sulfonylurea‐treated patients from diabetes centers in Germany/Austria: How big is the problem? Which patients are at risk? Diabetes Metab Res Rev 2016; 32: 316–324. [DOI] [PubMed] [Google Scholar]
- 76. Heller S, Amiel SA, Khunti K. Hypoglycaemia, a global cause for concern. Diabetes Res Clin Pract 2015; 110: 229–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy
Appendix S2 Study characteristics
Appendix S3 Comparison adjusted funnel‐plot of placebo‐controlled trials
Appendix S4 Comparison adjusted funnel‐plot of trials comparing a sulphonylurea agent (SU) with a non‐SU agent
Appendix S5 Comparative risk of hypoglycaemia of any severity. Results are the odds‐ratios (ORs) with 95% credibility intervals (CI). ORs less than 1 favour the column defining treatment in terms of lower risk of hypoglycaemia. Results are statistically significant where the CIs do not cross 1
Appendix S6 Sensitivity‐analysis with exclusion of the GUIDE‐study (Schernthaner et al. Eur. J. Clin. Invest 2004;34: 535‐42)
Appendix S7 Glycaemic control. Comparative efficacy on change in HbA1c from baseline. Differences less than 0 favour the column defining treatment. Results are statistically significant where the 95% credibility intervals do not cross 0
Appendix S8 Comparative efficacy on change in body weight from baseline. Differences less than 0 favour the column defining treatment. Results are statistically significant where the 95% credibility intervals do not cross 0
Supporting info item


