Abstract
Inhibition of the mechanistic target of rapamycin (mTOR) has been exploited largely both in solid tumour oncology and solid organ transplantation. More recently mTOR inhibitors such as sirolimus and everolimus have been introduced to the field of allogeneic haematopoietic stem cell transplantation where their unique combination of immunosuppressive purposes offering reduced nephrotoxicity and potential antimalignant effects reflect a unique drug profile that has led to their widespread use in both prophylaxis and therapy of graft‐versus‐host disease (GVHD). On the other hand haematological insufficiency, infectious complications as well as vasculopathies, have been frequently reported as limiting toxicities. Here, we review both the retrospective and prospective experience available to date and stress the need for prospective registration trials to reduce off label use and improve patient safety by optimizing dosing and enhancing pharmacovigilance. Furthermore, we speculate on the future role of mTOR inhibitors in allogeneic haematopoietic stem cell transplantation.
Keywords: allogeneic haematopoietic stem cell transplantation, everolimus, graft‐versus‐host disease, mTOR inhibitors, sirolimus
Introduction
Mechanistic target of rapamycin (mTOR) is a broadly expressed serine/threonine protein kinase downstream of the phosphatidylinositide 3‐kinase (PI3K) and protein kinase B (Akt) pathway (Figure 1) 1, 2. Once activated by upstream signalling, mTOR plays a key role in the regulation of cell metabolism, proliferation, and survival including the structurally distinct mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) 3, 4. Dysregulation of either pathway can be associated with malignant cell transformation and the first‐in‐class mTOR inhibitor sirolimus affecting solely mTORC1 has been proposed as a potent antineoplastic drug. Up to now, mTOR inhibitors are broadly used to treat malignancies such as breast cancer 5, 6, neuroendocrine tumours 7, 8 and renal cell carcinoma 9, 10.
Figure 1.
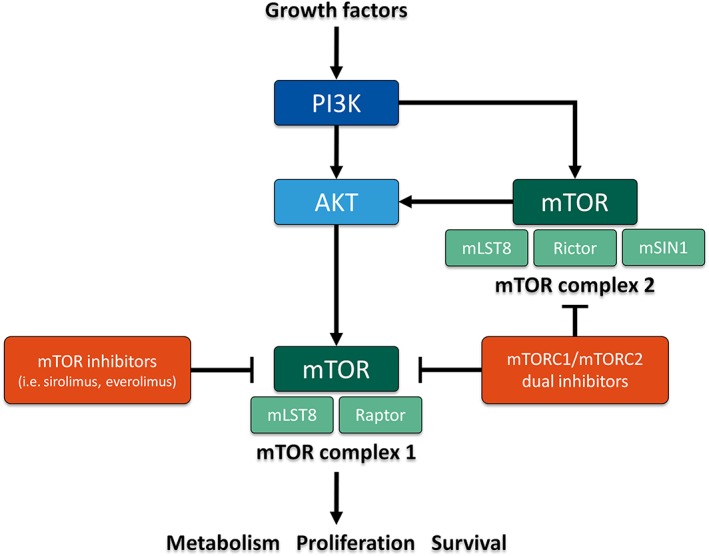
Simplified scheme of the mechanism of action of mTOR inhibitors such as sirolimus and everolimus and novel mTORC1/mTORC2 dual inhibitors. AKT protein kinase B; mLST8 mammalian lethal with SEC13 protein 8; mSIN1 mammalian stress‐activated protein kinase‐interacting protein 1; mTOR mechanistic target of rapamycin; mTORC mTOR complex; PI3K phosphatidylinositide 3‐kinase; Raptor regulatory‐associated protein of mTOR; Rictor rapamycin‐insensitive companion of mammalian target of rapamycin
In addition to these antineoplastic features mTOR inhibitors carry also immunosuppressive properties inhibiting T lymphocytes from proliferation 11, 12. Proven to prevent organ rejection, mTOR inhibitors were registered for immunosuppression in recipients of heart 13, kidney 14 and liver transplantations 15. Inspired by promising results from the field of solid organ transplantation and, as a consequence of medical need, mTOR inhibitors have also been introduced to the field of allogeneic haematopoietic stem cell transplantation (HSCT) to prevent and treat graft‐versus‐host disease (GVHD) 16, 17. Unfortunately and to our best knowledge none of these drugs has been registered yet. With solely limited and largely investigator‐initiated clinical trials currently being open for recruitment 18 a continuing medical need has led to widespread off label use of such drugs despite partly conflicting results from published trials and retrospective analyses. Here, we summarize today's experience with both sirolimus and its 40‐O‐(2‐hydroxyethyl) derivative, everolimus (Figure 2) 19 for prophylaxis and treatment of GVHD.
Figure 2.
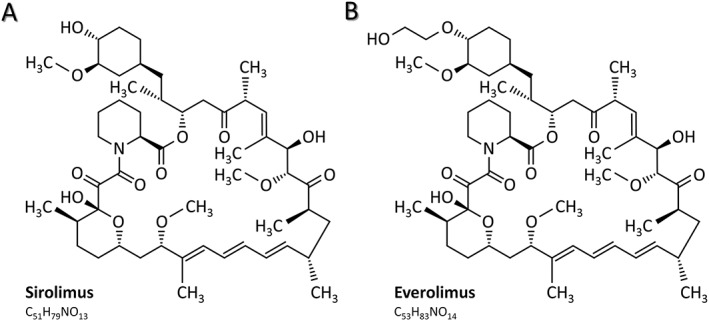
Structural and chemical formulas of sirolimus and everolimus. Everolimus is the 40‐O‐(2‐hydroxyethyl) derivative of sirolimus, a macrolide antimicrobial agent which was initially isolated from Streptomyces hygroscopicus. (A) Structural and chemical formula of sirolimus. (B) Structural and chemical formula of everolimus
Prophylaxis of GVHD
Sirolimus
Following the evidence of efficacy in mice 20 Antin and colleagues reported a phase I/II trial where sirolimus has been employed as GVHD prophylaxis in 41 patients having undergone allogeneic HSCT from unrelated or mismatched related donors 21. Here, sirolimus was added to a well‐established regimen combining the calcineurin inhibitor (CNI) tacrolimus and low dose methotrexate (MTX) resulting in a very low overall grade II to IV acute GVHD (aGVHD) rate of 26%. As a consequence sirolimus was studied more extensively including also randomized controlled trials (RCTs). In these trials combinations of sirolimus with or without CNIs as an immunosuppressive backbone were studied resulting in mixed findings.
Sirolimus in CNI‐based GVHD prophylaxis regimens
The combination of sirolimus with CNIs for GVHD prophylaxis has been studied extensively in both a retrospective and prospective manner. A first RCT was performed by Pidala and colleagues comparing the standard combination of tacrolimus and MTX (n = 37) with tacrolimus and sirolimus (n = 37) in a total of 74 patients 22. Here, the combination of tacrolimus with sirolimus was superior leading to a reduced rate of grade II to IV aGVHD (43% vs. 89%), a reduced rate of moderate to severe chronic GVHD (cGVHD, 24% vs. 64%) and an improved reconstitution of regulatory T lymphocytes. However, no improvement could be shown for overall survival at time of analysis. More recently, a long term follow‐up analysis on all patients of the same study cohort was performed (median follow‐up time of 49 months for the tacrolimus/MTX arm and of 41 months for the tacrolimus/sirolimus arm) 23. While a significantly lower incidence of late aGVHD and moderate to severe cGVHD as well as a significantly lower exposure to steroids and tacrolimus could be shown for the tacrolimus/sirolimus arm, there was no significant difference in complete discontinuation of immunosuppressive medication as well as in overall survival.
Furthermore, Jim and colleagues analyzed changes in quality of life in a subgroup of 71 patients of the above mentioned study cohort (34 patients of the tacrolimus/MTX and all 37 patients of the tacrolimus/sirolimus arm) 24. Using the Functional Assessment of Cancer Therapy‐Bone Marrow Transplant Trial Outcome Index (FACT‐BMT) to assess quality of life prior to HSCT and on days 30, 90, 180, 270 and 360 after HSCT, the tacrolimus/sirolimus group showed significantly less improvement in their quality of life compared with the tacrolimus/MTX group. The authors hypothesized that these differences might be caused by increased nausea and fatigue in patients treated with sirolimus.
A further RCT comparing the combination of tacrolimus and sirolimus with tacrolimus and MTX was performed by Cutler and colleagues 25. In their intention‐to‐treat analysis 151 patients received the sirolimus‐based and 153 patients the sirolimus‐free regimen. While long term outcome was similar in both groups, patients in the tacrolimus/sirolimus arm showed more rapid engraftment and lower incidence of enoral mucositis.
Two further RCTs investigated the addition of sirolimus to a standard regimen of tacrolimus and MTX. Pulsipher and colleagues analyzed 143 paediatric patients receiving allogeneic HSCT for acute lymphoblastic leukaemia 26. Of these, 70 received a prophylactic regimen with tacrolimus and MTX while 73 patients received a triple prophylactic regimen with tacrolimus, MTX and sirolimus. Here, the incidence of grade II to IV aGVHD was significantly lower whereas the incidence of thrombotic microangiopathy (TMA) or sinusoidal obstruction syndrome (SOS) was higher in patients treated with sirolimus. No difference was seen in overall survival. Kornblit and colleagues reported 71 patients treated with a combination of tacrolimus and mycophenolate mofetil (MMF) and 68 patients treated with a triple combination adding sirolimus 27. While toxicity rates and long term outcome were similar in both arms patients treated with triple combination showed a lower incidence of grade II to IV aGVHD and of cytomegalovirus (CMV) reactivation 150 days after allogeneic HSCT. The latter finding was in line with the results from a further trial suggesting protective features against CMV reactivation 28. In this context, sirolimus has been discussed as a salvage treatment option for ganciclovir‐resistant CMV reactivations in kidney graft recipients 29.
Recently, Armand and colleagues performed an RCT comparing the triple regimen of tacrolimus, MTX and sirolimus (n = 66) to a standard regimen either consisting of tacrolimus and MTX or consisting of ciclosporin A (CsA) and MMF (n = 73) in a total of 139 lymphoma patients having received HLA‐matched reduced‐intensity conditioning (RIC) allogeneic HSCT 30. While no significant difference could be shown in terms of cGVHD incidence, relapse, progression‐free survival, non‐relapse mortality and overall survival, incidence of grade II to IV aGVHD was significantly lower in patients treated with tacrolimus/MTX/sirolimus suggesting this triple regimen as a reasonable alternative for GVHD prophylaxis after RIC HSCT.
These results are in line with a retrospective analysis performed by Ceberio and colleagues in 71 lymphoma patients receiving tacrolimus, MTX and sirolimus as GVHD prophylaxis after non‐myeloablative or RIC allogeneic resulting in a low cumulative 1 year incidence of aGVHD (0.28 for grade II to IV and 0.07 for grade III to IV) as well as in a low cumulative 1 year (0.15) and 2 year (0.33) incidence of cGVHD 31.
Besides the above mentioned RCTs, many retrospective analyses have been published reporting on the use of sirolimus as part of GVHD prophylaxis regimens. A special approach was used by Parody and colleagues analyzing 159 patients who received a GVHD prophylaxis regimen containing tacrolimus and sirolimus. Comparing 139 patients with a 8/8 HLA‐matched donor with 20 patients with a 7/8 HLA‐mismatched donor, they could show that this combination could overcome the negative effect of HLA‐mismatch. Although cumulative incidence of grade II to IV aGVHD was significantly higher in the patients with a HLA‐mismatched donor, there was no difference between the two groups regarding 1 year non‐relapse mortality, 3 year event‐free survival and 3 year overall survival 32.
In summary, the addition of sirolimus to CNI‐based GVHD prophylaxis regimens appears to reduce the incidence of grade II to IV aGVHD without affecting overall survival. However, serious side effects such as TMA and SOS may arise from such combinations and thus have to be taken into account.
Sirolimus in CNI‐free GVHD prophylaxis regimens
Schleuning and colleagues evaluated the use of sirolimus in a CNI‐free GVHD prophylaxis regimen retrospectively in 15 patients with leukaemia receiving a combination of sirolimus, MMF and antithymocyte globulin (ATG) 33. Six patients received stem cells from a sibling and nine patients from a HLA‐matched unrelated donor. Rapid engraftment was seen in all patients except one subject who died from invasive aspergillosis early after transplantation. They reported both a favourable grade II to IV aGVHD rate of 21% and a favourable cGVHD rate of 30%. In this retrospective analysis no TMA or SOS were observed.
However, the only prospective trial investigating the combination of sirolimus and MMF for GVHD prophylaxis had to be terminated prematurely. Johnston and colleagues enrolled a total of 11 patients receiving allogeneic HSCT, seven of whom were receiving a busulfane‐based conditioning regimen 34. Grade II to IV aGVHD occurred in six of 11 patients and sirolimus had to be discontinued in four patients due to treatment‐related toxicities including SOS and portal vein thrombosis. Subsequently, the study was terminated. Since all four patients requiring sirolimus discontinuation received a busulfane‐containing preparative regimen, the authors discussed a potential correlation.
The combination of sirolimus with cyclophosphamide for CNI‐free GVHD prophylaxis has been studied in two prospective trials. Solomon and colleagues used post‐transplantation cyclophosphamide and a brief course sirolimus regimen for 26 patients receiving allogeneic HSCT from HLA‐matched related (n = 17) or unrelated (n = 9) donors 35. Rapid and stable engraftment was documented in all patients. Grade II to IV aGVHD occurred in 46% and cGVHD in 31% of all patients. While relapse incidence was estimated to be 32%, no relapses were seen in patients with lymphoid malignancies. Furthermore, only four of 19 patients at risk showed a CMV reactivation. Therefore, GVHD prophylaxis with short course sirolimus and cyclophosphamide seems to be an effective and safe alternative to CNI‐based regimens. In a more recently reported prospective trial, Cieri and colleagues investigated the use of sirolimus and post‐transplantation cyclophosphamide in 40 patients after haploidentical allogeneic HSCT 36. All patients showed a rapid and constant engraftment. While grade II to IV aGVHD was seen in 8% of patients, cumulative incidence of cGVHD was 20% 1 year after HSCT. Ten patients died related to a relapse of their underlying malignancy and non‐relapse mortality occurred in seven patients, mostly due to infections. Due to the low rates of acute and chronic GVHD and the rapid and stable immune reconstitution, this trial also suggests the combination of sirolimus and cyclophosphamide as an attractive option for GVHD prophylaxis.
Currently, MMF and cyclophosphamide are the only agents that have been evaluated as part of sirolimus‐based CNI‐free GVHD prophylaxis regimens. However, bortezomib has also been proposed as a potential combination partner for sirolimus in a preclinical study also showing a maintained graft vs. leukaemia (GVL) effect 37.
Everolimus
Experience on the use of everolimus for primary prophylaxis of GVHD is limited. Platzbecker and colleagues investigated a combination of tacrolimus and everolimus for primary GVHD prophylaxis in a prospective phase II trial in 24 patients with myelodysplastic syndrome or acute myeloid leukaemia 38. Despite a promising overall grade II to IV aGVHD rate of 37%, an unfavourable toxicity profile led to an early termination of the trial. Seven patients had developed TMA leading to acute renal failure in two of them while a further six patients had developed SOS with fatal outcome in two of them. The authors hypothesized that the combination of everolimus with a CNI contributed to the rise of complicating vasculopathies in this trial.
These results are in line with preclinical studies investigating the combination of everolimus with CsA or with MMF in a canine non‐myeloablative HSCT setting 39, 40. Here, the prolonged time to platelet recovery as well as the increased frequency of infectious complications were identified as potential drawbacks to the application of everolimus in primary GVHD prophylaxis.
As a consequence everolimus is currently only employed as part of GVHD prophylaxis in patients developing complications after exposure to conventional immunosuppressive regimens. This includes, for example, the development of posterior reversible encephalopathy syndrome (PRES) under CNIs 41. After discontinuing CNIs and bridging with steroids, everolimus was successfully introduced resulting in an effective prevention of severe GVHD without reoccurrence of PRES 42.
Treatment of acute GVHD
Sirolimus
First line treatment of acute GVHD
To date only one group reported retrospectively on the use of sirolimus as frontline therapy for aGVHD. Pidala and colleagues reported their early promising experience in primary and follow‐up analysis 43, 44. Thirty‐two patients had received a backbone of tacrolimus for primary GVHD prophylaxis in combination with either MTX (n = 29) or MMF (n = 3). After occurrence of aGVHD patients were commenced on sirolimus but continued their primary prophylactic regimen avoiding ‘high’ plasma concentrations of tacrolimus in light of an increased risk for TMA. At time of diagnosis, four patients suffered from grade I, 24 from grade II and four from grade III aGVHD with gastrointestinal tract being the most frequent aGVHD site (66%) followed by skin (53%) and liver (16%). Complete remission of aGVHD was achieved in 16 patients at a median of 14 days after initiation of sirolimus. In the remaining half of patients showing no response to initial sirolimus treatment alone, systemic steroids were added at a median of 9 days after initiation of sirolimus. Among these cases, a further 12 patients achieved a complete remission of aGVHD signs. At the end, only four patients were refractory to sirolimus treatment alone or in combination with steroids resulting in an 88% response rate to this cascading therapeutic approach. TMA was noted in three patients and resolved by reduction of tacrolimus dose in all cases. This to date sole comprehensive assessment of the use of sirolimus for first line aGVHD treatment is limited by many factors including its retrospective nature, the rather limited numbers of patients included, the differences in primary GVHD prophylaxis and the inclusion of patients with grade I to III but not grade IV aGVHD requiring to mark these data as preliminary until being confirmed by larger, more comprehensive or prospective trials. Obviously the authors attempted to avoid the use of steroids by the introduction of sirolimus in the first place. However, the exclusion of systemic steroids from the first line of action has certainly shifted the targeted group of patients to a less severe one explaining why no patients with grade IV were included. Taken together, a clinical trial based on careful risk–benefit assessment for a clearly defined group of patients would have been desirable.
Second line treatment of acute GVHD
In a pilot trial, Benito and colleagues assessed the efficacy and toxicity of sirolimus treatment for steroid‐refactory aGVHD in 21 patients 45. At the time of initiation 10 patients suffered from grade III and 11 patients from grade IV aGVHD. Sirolimus was to be administered for a maximum of 14 days. However, 10 patients were discontinued earlier due to progressive disease (n = 5), myelosuppression (n = 2), seizures (n = 2) and attending physician's preference (n = 1). This resulted in an overall response rate of 57% including five complete and seven partial remissions. Major side effects included hypertriglyceridaemia (n = 8), thrombocytopenia (n = 7), neutropenia (n = 4) and hypercholesterolaemia (n = 3). In light of the limited exposure of several patients to sirolimus these first promising, prospectively generated data have to be interpreted with caution.
A more comprehensive approach was published by Hoda and colleagues 46. In this retrospective study, 34 patients who were refractory or intolerant to steroids received sirolimus for their aGVHD including three cases of grade I, 15 cases of grade II, eight cases of grade III and eight cases of grade IV aGVHD at initiation of sirolimus. The most common aGVHD site was the gastrointestinal tract in 27 patients while skin was affected in 13 patients and liver in four patients. Of note, all patients concurrently received tacrolimus being continued from the aGVHD prophylaxis regimen. Fifteen patients achieved a complete and 11 a partial response resulting in a 76% overall response rate. The most common toxicities possibly associated to sirolimus treatment included cytopenias, hyperlipidaemia and TMA which occurred in seven patients. Regarding the latter, resolution was successfully achieved in all cases by dose reduction or discontinuation of concomitant tacrolimus administration.
In another single centre study, Ghez and colleagues retrospectively identified 22 patients as having received sirolimus as second or further line treatment for their grade II to IV aGVHD 47. Complete remission was achieved in 72% of patients. The toxicity profile included cytopenias with infections consecutively being the main cause of death. TMA occurred in a relatively high proportion of 36% and all of these patients concurrently received CNIs. After discontinuation of the latter and/or sirolimus, TMA frequently resolved. At a median follow‐up of more than 1 year after initiation of sirolimus, nine patients were still alive resulting in a 41% overall survival rate.
Everolimus
To our best knowledge, comprehensive data on the employment of everolimus for the treatment of aGVHD are missing to date.
Treatment of steroid refractory chronic GVHD
By inhibiting fibrogenesis and proliferation of fibroblasts 48, 49, mTOR inhibitors may also improve steroid refractory cGVHD including sclerodermatous cGVHD where skin, subcutaneous tissue and fascia are affected in a complex interplay of chronic inflammation 50. Unfortunately, the number of clinical reports on the administration of mTOR inhibitors in cGVHD is still very much limited to date. Recently, promising results from a mouse model have been reported where the appearance of cGVHD has been successfully suppressed by everolimus 51.
Sirolimus
Johnston and colleagues reported results from a phase II study where 19 patients with treatment refractory cGVHD received a combination of sirolimus, CNIs and steroids 52. In this study a very high primary response rate was noted putting 15 of 16 assessable patients up for clinical improvement. However, severe toxicities including infections and renal impairment limited the overall success of this study markedly and resulted in its premature closure.
A more favourable outcome was reported by Couriel and colleagues from a phase II trial where 35 patients with heavily pre‐treated, steroid refractory cGVHD mainly of the skin received a combination of sirolimus, tacrolimus and steroids 53. Improvement was documented in 22 patients, of whom six showed a complete and 16 a partial response resulting in a 63% overall response rate. Most common adverse events included infections and hyperlipidaemia but also renal insufficiency, cytopenias and TMA. Of note, eight of 11 patients with sclerodermatous cGVHD showed an objective response to treatment suggesting that these patients may have particularly benefitted from this treatment.
Jurado and colleagues retrospectively analyzed 47 patients from eight hospitals having been treated with a sirolimus‐based salvage therapy for refractory or relapsed cGVHD 54. Beside its retrospective nature this study was limited by its diversity with regard to previous treatments and concomitant immunosuppression. While sirolimus had been administered as second line therapy in 12 patients, six patients had received four or more immunosuppressive treatment regimens beforehand. Sirolimus was combined with CNIs in 33 cases, with MMF in nine cases and with steroids in five cases. Response to treatment was seen in 38 patients including 18 patients with complete and 20 with partial remissions. Major adverse events included hyperlipidaemia in 19, renal impairment in 14 and cytopenias in 12 patients. TMA was seen in four patients, all of them receiving a combination of sirolimus and CNI. Thus, this study highlights the therapeutic potential of sirolimus in multiple institutions but provides also confirmatory data on its typical side effects.
Inspired by the suggested efficacy of sirolimus for the treatment of steroid refractory sclerodermatous cGVHD, Jedlickova and colleagues retrospectively analyzed 34 patients with sclerodermatous cGVHD having received treatment with mTOR inhibitors 55. In this report, either sirolimus (n = 13) or everolimus (n = 21) were administered as a true first line therapy largely in combination with steroids or as a single agent therapy in more advanced therapeutic stages. In this study few complete but several partial responses were noted for both sirolimus and everolimus treatment while the most common side effects were limited to infections, coagulopathy and hyperlipidaemia. Of note, TMA was rare and related to high trough concentrations of mTOR inhibitors in the plasma as suggested by a retrospective analysis by Garcia‐Martin and colleagues 56. In this study, a combination of sirolimus and CsA was administered for treatment refractory GVHD in 61 patients including 37 patients with cGVHD. TMA occurred in 13 patients whereby almost half of them showed high trough concentrations of sirolimus. Discontinuation of both CsA and sirolimus solved TMA in half of the patients but continued in four out of six patients with ongoing sirolimus administration. Therefore, a careful monitoring of plasma trough concentrations of sirolimus and early dose adjustments or discontinuation in the case of side effects appear to be critical, particularly in patients with CNI coadministration.
Everolimus
Despite the fact that the use of everolimus as second line treatment for steroid refractory cGVHD has been suggested early on 57, 58, studies employing everolimus for cGVHD treatment are still very limited.
In a first preliminary retrospective assessment, 29 patients with treatment refractory cGVHD had received a CNI free, everolimus‐based treatment regimen. The authors reported a promising response rate of 70% including both complete and partial remissions in conjunction with a bearable toxicity profile 59. As mentioned above, Jedlickova and colleagues retrospectively evaluated 21 patients receiving everolimus as monotherapy or in combination with other immunosuppressants for treatment of severe sclerodermatous cGVHD and reported high response rates 55. At the time of analysis, 17 of these patients had reached complete or partial remissions and steroids could be tapered. However, hyperlipidaemia and impaired wound healing were reported as relevant side events possibly associated with everolimus treatment. As the development of mucosal and skin ulceration is a well‐known side effect of everolimus and has been reported also in context of cGVHD treatment, patients with pre‐existing ulcerations should be treated with caution whilst patients with new ulcerations may have to be discontinued from everolimus treatment 60, 61.
To date the most comprehensive dual centre study on the use of everolimus for the treatment of refractory cGVHD is also limited by its retrospective nature. Mielke and colleagues reported 80 cGVHD patients half of whom achieved complete or partial remissions after initiation of a everolimus‐based salvage therapy 62. Most frequent adverse events included infections and thrombocytopenia and were associated with increased trough concentrations of everolimus. In this study, a single case of relapse of the malignant disease was reported suggesting that both GVL effects in context of cGVHD and anti‐neoplastic properties of everolimus may have contributed to improved disease control 63, 64. Quality of life and cGVHD symptom bother were assessed in a subgroup of 22 patients using standardized questionnaires in a retrospective manner revealing symptom improvement under everolimus treatment as well 65. This is a finding of particular importance since half of the patients are distressed after allogeneic HSCT 66 and compromised quality of life and consecutive mental burden are known to be frequent complications associated with treatment refractory cGVHD 67, 68.
Results from prospective trials are missing to date. Currently, there is one investigator initiated, prospective study open for recruitment where patients with newly diagnosed moderate to severe cGVHD are treated with everolimus and steroids (‘PredEver first’) 69.
Future developments
The widespread use of mTOR inhibitors in allogeneic HSCT was clearly driven by the medical need for immunosuppressants overcoming the limitations in efficacy and toxicity of well‐established drugs for both prophylaxis and treatment of acute and chronic GVHD. Whilst promising response rates particularly for the treatment of cGVHD have been reported, the toxicity profile particularly in combination with CNIs remains limiting. However, very recent developments have put novel agents on the spot such as the JAK1/2 inhibitor ruxolitinib offering almost 90% response rates in steroid refractory acute and chronic GVHD 70. Therefore and with the confirmation of these outstanding results in prospective studies being awaited in a timely manner, less room for mTOR inhibitors in the treatment of GVHD may be left in the nearer future. Thus, future use of mTOR inhibitors may rather favour prophylaxis than treatment of GVHD. Here, combinations without CNIs may offer promising prophylactic regimens with low toxicity rates. The fact that a prophylactic regimen of sirolimus or everolimus and MMF has not yet been comprehensively studied in a prospective manner reflects certainly a missed opportunity allowing to cut off both the nephrotoxicity of CNIs and the microangiopathy likely to be associated with the combination of mTOR inhibitors and CNIs. However, drug interactions and the long half‐life of both sirolimus and everolimus will add to the complexity in clinical practice stressing the need for prospective registration trials to improve patient safety by optimizing dosing and enhancing pharmacovigilance 71, 72, 73. Finally, potential anti‐malignant effects delivered with the use of mTOR inhibitors as well as future mTORC1/mTORC2 dual inhibitors underline a unique drug profile that may protect the role of such in both prophylaxis and treatment of GVHD 74.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that SM received honoraria and travel grants from Novartis, Germany. There is no further support from any organization for the submitted work, there are no further financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and there are no other further relationships or activities that could appear to have influenced the submitted work.
Lutz, M. , and Mielke, S. (2016) New perspectives on the use of mTOR inhibitors in allogeneic haematopoietic stem cell transplantation and graft‐versus‐host disease. Br J Clin Pharmacol, 82: 1171–1179. doi: 10.1111/bcp.13022.
References
- 1. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010; 28: 1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22. [DOI] [PubMed] [Google Scholar]
- 4. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124: 471–84. [DOI] [PubMed] [Google Scholar]
- 5. Wazir U, Wazir A, Khanzada ZS, Jiang WG, Sharma AK, Mokbel K. Current state of mTOR targeting in human breast cancer. Cancer Genomics Proteomics 2014; 11: 167–74. [PubMed] [Google Scholar]
- 6. Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 2015; 12: 342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber HC. Medical treatment of neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes 2013; 20: 27–31. [DOI] [PubMed] [Google Scholar]
- 8. Chan J, Kulke M. Targeting the mTOR signaling pathway in neuroendocrine tumors. Curr Treat Options Oncol 2014; 15: 365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Czarnecka AM, Kornakiewicz A, Lian F, Szczylik C. Future perspectives for mTOR inhibitors in renal cell cancer treatment. Future Oncol 2015; 11: 801–17. [DOI] [PubMed] [Google Scholar]
- 10. Barthelemy P, Hoch B, Chevreau C, Joly F, Laguerre B, Lokiec F, et al. mTOR inhibitors in advanced renal cell carcinomas: from biology to clinical practice. Crit Rev Oncol Hematol 2013; 88: 42–56. [DOI] [PubMed] [Google Scholar]
- 11. Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol 2013; 25: 347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T‐cell differentiation and function. Immunol Rev 2012; 249: 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Radegran G, Gude E, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant 2014; 14: 1828–38. [DOI] [PubMed] [Google Scholar]
- 14. Moes DJ, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today 2015; 20: 1243–9. [DOI] [PubMed] [Google Scholar]
- 15. Klintmalm GB, Nashan B. The role of mTOR inhibitors in liver transplantation: Reviewing the evidence. J Transplant 2014; 2014: 845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ziakas PD, Zervou FN, Zacharioudakis IM, Mylonakis E. Graft‐versus‐host disease prophylaxis after transplantation: a network meta‐analysis. PLoS One 2014; 9: e114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abouelnasr A, Roy J, Cohen S, Kiss T, Lachance S. Defining the role of sirolimus in the management of graft‐versus‐host disease: from prophylaxis to treatment. Biol Blood Marrow Transplant 2013; 19: 12–21. [DOI] [PubMed] [Google Scholar]
- 18. ClinicalTrials.gov . Registered trials as of March 2016. (last accessed 1 March 2016).
- 19. Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman HJ, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo . Transplantation 1997; 64: 36–42. [DOI] [PubMed] [Google Scholar]
- 20. Blazar BR, Taylor PA, Panoskaltsis‐Mortari A, Sehgal S, Vallera DA. In vivo inhibition of cytokine responsiveness and graft‐versus‐host disease mortality by rapamycin leads to a clinical‐pathological syndrome discrete from that observed with cyclosporin A. Blood 1996; 87: 4001–9. [PubMed] [Google Scholar]
- 21. Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low‐dose methotrexate for graft‐versus‐host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 2003; 102: 1601–5. [DOI] [PubMed] [Google Scholar]
- 22. Pidala J, Kim J, Jim H, Kharfan‐Dabaja MA, Nishihori T, Fernandez HF, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica 2012; 97: 1882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pidala J, Kim J, Alsina M, Ayala E, Betts BC, Fernandez HF, et al. Prolonged sirolimus administration after allogeneic hematopoietic cell transplantation is associated with decreased risk for moderate‐severe chronic graft‐versus‐host disease. Haematologica 2015; 100: 970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jim HS, Barata A, Small BJ, Jacobsen PB, Pidala J. Quality of life associated with sirolimus for prevention of graft‐versus‐host disease: results from a randomized trial. Haematologica 2014; 99: 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs. tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014; 124: 1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood 2014; 123: 2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kornblit B, Maloney DG, Storer BE, Maris MB, Vindelov L, Hari P, et al. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after non‐myeloablative unrelated donor transplantation. Haematologica 2014; 99: 1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus‐based graft‐versus‐host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 2007; 110: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozaki KS, Camara NO, Nogueira E, Pereira MG, Granato C, Melaragno C, et al. The use of sirolimus in ganciclovir‐resistant cytomegalovirus infections in renal transplant recipients. Clin Transplant 2007; 21: 675–80. [DOI] [PubMed] [Google Scholar]
- 30. Armand P, Kim HT, Sainvil MM, Lange PB, Giardino AA, Bachanova V, et al. The addition of sirolimus to the graft‐versus‐host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. Br J Haematol 2016; 173: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ceberio I, Devlin SM, Sauter C, Barker JN, Castro‐Malaspina H, Giralt S, et al. Sirolimus, tacrolimus and low‐dose methotrexate based graft‐versus‐host disease prophylaxis after non‐ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant. Leuk Lymphoma 2015; 56: 663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parody R, Lopez‐Corral L, Godino OL, Cadenas IG, Martinez AP, Vazquez L, et al. GVHD prophylaxis with sirolimus‐tacrolimus may overcome the deleterious effect on survival of HLA mismatch after reduced‐intensity conditioning allo‐SCT. Bone Marrow Transplant 2015; 50: 121–6. [DOI] [PubMed] [Google Scholar]
- 33. Schleuning M, Judith D, Jedlickova Z, Stubig T, Heshmat M, Baurmann H, et al. Calcineurin inhibitor‐free GVHD prophylaxis with sirolimus, mycophenolate mofetil and ATG in Allo‐SCT for leukemia patients with high relapse risk: an observational cohort study. Bone Marrow Transplant 2009; 43: 717–23. [DOI] [PubMed] [Google Scholar]
- 34. Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched‐related donor hematopoietic cell transplantation. Bone Marrow Transplant 2012; 47: 581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solomon SR, Sanacore M, Zhang X, Brown S, Holland K, Morris LE, et al. Calcineurin inhibitor–free graft‐versus‐host disease prophylaxis with post‐transplantation cyclophosphamide and brief‐course sirolimus following reduced‐intensity peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 1828–34. [DOI] [PubMed] [Google Scholar]
- 36. Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Post‐transplantation Cyclophosphamide and sirolimus after haploidentical hematopoietic stem cell transplantation using a treosulfan‐based myeloablative conditioning and peripheral blood stem cells. Biol Blood Marrow Transplant 2015; 21: 1506–14. [DOI] [PubMed] [Google Scholar]
- 37. Caballero‐Velazquez T, Sanchez‐Abarca LI, Gutierrez‐Cosio S, Blanco B, Calderon C, Herrero C, et al. The novel combination of sirolimus and bortezomib prevents graft‐versus‐host disease but maintains the graft‐versus‐leukemia effect after allogeneic transplantation. Haematologica 2012; 97: 1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Platzbecker U, von Bonin M, Goekkurt E, Radke J, Binder M, Kiani A, et al. Graft‐versus‐host disease prophylaxis with everolimus and tacrolimus is associated with a high incidence of sinusoidal obstruction syndrome and microangiopathy: results of the EVTAC trial. Biol Blood Marrow Transplant 2009; 15: 101–8. [DOI] [PubMed] [Google Scholar]
- 39. Junghanss C, Rathsack S, Wacke R, Weirich V, Vogel H, Drewelow B, et al. Everolimus in combination with cyclosporin a as pre‐ and post‐transplantation immunosuppressive therapy in nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1061–8. [DOI] [PubMed] [Google Scholar]
- 40. Machka C, Lange S, Werner J, Wacke R, Killian D, Knueppel A, et al. Everolimus in combination with mycophenolate mofetil as pre‐ and post‐transplantation immunosuppression after nonmyeloablative hematopoietic stem cell transplantation in canine littermates. Biol Blood Marrow Transplant 2014; 20: 1301–6. [DOI] [PubMed] [Google Scholar]
- 41. Wu Q, Marescaux C, Wolff V, Jeung MY, Kessler R, Lauer V, et al. Tacrolimus‐associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol 2010; 64: 169–77. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt V, Prell T, Treschl A, Klink A, Hochhaus A, Sayer HG. Clinical Management of Posterior Reversible Encephalopathy Syndrome after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Series and Review of the Literature. Acta Haematol 2016; 135: 1–10. [DOI] [PubMed] [Google Scholar]
- 43. Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft‐versus‐host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15: 881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pidala J, Tomblyn M, Nishihori T, Field T, Ayala E, Perkins J, et al. Sirolimus demonstrates activity in the primary therapy of acute graft‐versus‐host disease without systemic glucocorticoids. Haematologica 2011; 96: 1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K, et al. Sirolimus (rapamycin) for the treatment of steroid‐refractory acute graft‐versus‐host disease. Transplantation 2001; 72: 1924–9. [DOI] [PubMed] [Google Scholar]
- 46. Hoda D, Pidala J, Salgado‐Vila N, Kim J, Perkins J, Bookout R, et al. Sirolimus for treatment of steroid‐refractory acute graft‐versus‐host disease. Bone Marrow Transplant 2010; 45: 1347–51. [DOI] [PubMed] [Google Scholar]
- 47. Ghez D, Rubio MT, Maillard N, Suarez F, Chandesris MO, Delarue R, et al. Rapamycin for refractory acute graft‐versus‐host disease. Transplantation 2009; 88: 1081–7. [DOI] [PubMed] [Google Scholar]
- 48. Salas‐Prato M, Assalian A, Mehdi AZ, Duperre J, Thompson P, Brazeau P. Inhibition by rapamycin of PDGF‐ and bFGF‐induced human tenon fibroblast proliferation in vitro. J Glaucoma 1996; 5: 54–9. [PubMed] [Google Scholar]
- 49. Namba DR, Ma G, Samad I, Ding D, Pandian V, Powell JD, et al. Rapamycin inhibits human laryngotracheal stenosis‐derived fibroblast proliferation, metabolism, and function in vitro. Otolaryngol Head Neck Surg 2015; 152: 881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gratwohl AA, Moutsopoulos HM, Chused TM, Akizuki M, Wolf RO, Sweet JB, et al. Sjogren‐type syndrome after allogeneic bone‐marrow transplantation. Ann Intern Med 1977; 87: 703–6. [DOI] [PubMed] [Google Scholar]
- 51. Shao L, Lie AK, Zhang Y, Wong CH, Kwong YL. Aberrant germinal center formation, follicular T‐helper cells, and germinal center B‐cells were involved in chronic graft‐versus‐host disease. Ann Hematol 2015; 94: 1493–504. [DOI] [PubMed] [Google Scholar]
- 52. Johnston LJ, Brown J, Shizuru JA, Stockerl‐Goldstein KE, Stuart MJ, Blume KG, et al. Rapamycin (sirolimus) for treatment of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant 2005; 11: 47–55. [DOI] [PubMed] [Google Scholar]
- 53. Couriel DR, Saliba R, Escalon MP, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft‐versus‐host disease. Br J Haematol 2005; 130: 409–17. [DOI] [PubMed] [Google Scholar]
- 54. Jurado M, Vallejo C, Perez‐Simon JA, Brunet S, Ferra C, Balsalobre P, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft‐versus‐host disease. Biol Blood Marrow Transplant 2007; 13: 701–6. [DOI] [PubMed] [Google Scholar]
- 55. Jedlickova Z, Burlakova I, Bug G, Baurmann H, Schwerdtfeger R, Schleuning M. Therapy of sclerodermatous chronic graft‐versus‐host disease with mammalian target of rapamycin inhibitors. Biol Blood Marrow Transplant 2011; 17: 657–63. [DOI] [PubMed] [Google Scholar]
- 56. Garcia‐Martin P, Alarcon‐Payer C, Lopez‐Fernandez E, Moratalla L, Romero A, Sainz J, et al. Transplantation‐associated thrombotic microangiopathy in patients treated with sirolimus and cyclosporine as salvage therapy for graft‐versus‐host disease. Ann Pharmacother 2015; 49: 986–94. [DOI] [PubMed] [Google Scholar]
- 57. Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second‐line treatment of chronic graft‐versus‐host disease. Biol Blood Marrow Transplant 2011; 17: 1–17. [DOI] [PubMed] [Google Scholar]
- 58. Schleuning M. Systemic treatment of chronic GVHD. Cell Ther Transplant 2009; 2: e.000050.01. doi:10.3205/ctt-2009‐en‐000050.01. [Google Scholar]
- 59. Klink A, Schilling K, Rapp K, Höffken K, Sayer HG. High overall response rate in calcineurin inhibitor‐free treatment with the mTOR inhibitor everolimus in advanced extensive chronic GvHD after allogeneic stem cell transplantation. Blood (ASH Annual Meeting Abstracts)2008; 112: 2210. [Google Scholar]
- 60. Brown A, Neumayer D, Rafiee‐Tari Z, Krieg T, Eming SA. [Delayed wound healing during therapy of cutaneous graft‐versus‐host disease with everolimus]. Hautarzt 2014; 65: 553–5. [DOI] [PubMed] [Google Scholar]
- 61. Ferte C, Paci A, Zizi M, Gonzales DB, Goubar A, Gomez‐Roca C, et al. Natural history, management and pharmacokinetics of everolimus‐induced‐oral ulcers: insights into compliance issues. Eur J Cancer 2011; 47: 2249–55. [DOI] [PubMed] [Google Scholar]
- 62. Mielke S, Lutz M, Schmidhuber J, Kapp M, Ditz D, Ammer J, et al. Salvage therapy with everolimus reduces the severity of treatment‐refractory chronic GVHD without impairing disease control: a dual center retrospective analysis. Bone Marrow Transplant 2014; 49: 1412–8. [DOI] [PubMed] [Google Scholar]
- 63. Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft‐versus‐host disease prophylaxis after allogeneic hematopoietic stem‐cell transplantation with reduced‐intensity conditioning. J Clin Oncol 2008; 26: 5767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Recher C, Beyne‐Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 2005; 105: 2527–34. [DOI] [PubMed] [Google Scholar]
- 65. Lutz M, Kapp M, Einsele H, Grigoleit GU, Mielke S. Improvement of quality of life in patients with steroid‐refractory chronic graft‐versus‐host disease treated with the mTOR inhibitor everolimus. Clin Transplant 2014; 28: 1410–5. [DOI] [PubMed] [Google Scholar]
- 66. Hefner J, Kapp M, Drebinger K, Dannenmann A, Einsele H, Grigoleit GU, et al. High prevalence of distress in patients after allogeneic hematopoietic SCT: fear of progression is associated with a younger age. Bone Marrow Transplant 2014; 49: 581–4. [DOI] [PubMed] [Google Scholar]
- 67. Dignan FL, Manwani R, Potter MN, Ethell ME, Leonard H, Brennan J, et al. A dedicated GvHD clinic may improve the quality of life for allogeneic stem cell transplant survivors. Clin Transplant 2013; 27: E1–2. [DOI] [PubMed] [Google Scholar]
- 68. Wood WA, Chai X, Weisdorf D, Martin PJ, Cutler C, Inamoto Y, et al. Comorbidity burden in patients with chronic GVHD. Bone Marrow Transplant 2013; 48: 1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. ClincalTrials.gov . Treatment of newly diagnosed moderate or severe chronic graft‐versus‐host disease with prednisone and everolimus (PredEver first). Identifier: NCT01862965. (last accessed 1 March 2016). [DOI] [PMC free article] [PubMed]
- 70. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas‐Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid‐refractory graft‐versus‐host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 2015; 29: 2062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Emoto C, Fukuda T, Venkatasubramanian R, Vinks AA. The impact of CYP3A5*3 polymorphism on sirolimus pharmacokinetics: insights from predictions with a physiologically‐based pharmacokinetic model. Br J Clin Pharmacol 2015; 80: 1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Klawitter J, Nashan B, Christians U. Everolimus and sirolimus in transplantation‐related but different. Expert Opin Drug Saf 2015; 14: 1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grabowsky JA. Drug interactions and the pharmacist: focus on everolimus. Ann Pharmacother 2013; 47: 1055–63. [DOI] [PubMed] [Google Scholar]
- 74. Herrero‐Sanchez MC, Rodriguez‐Serrano C, Almeida J, San‐Segundo L, Inoges S, Santos‐Briz A, et al. Effect of mTORC1/mTORC2 inhibition on T cell function: potential role in graft‐versus‐host disease control. Br J Haematol 2016; 173: 754–68. [DOI] [PubMed] [Google Scholar]


