Abstract
Aims
Roux‐en‐Y gastric bypass (RYGB) alters the anatomical structure of the gastrointestinal tract, which can result in alterations in drug disposition. The aim of the present study was to evaluate the oral disposition of two compounds belonging to the Biopharmaceutical Classification System Class II – fenofibrate (bile salt‐dependent solubility) and posaconazole (gastric pH‐dependent dissolution) – before and after RYGB in the same individuals.
Methods
A single‐dose pharmacokinetic study with two model compounds – namely, 67 mg fenofibrate (Lipanthyl®) and 400 mg posaconazole (Noxafil®) – was performed in 12 volunteers pre‐ and post‐RYGB. After oral administration, blood samples were collected at different time points up to 48 h after administration. Plasma concentrations were determined by high‐performance liquid chromatography in order to calculate the area under the concentration–time curve up to 48 h (AUC0–48 h), the peak plasma concentration (Cmax) and the time to reach peak concentration (Tmax).
Results
After administration of fenofibrate, no relevant differences in AUC0–48 h, Cmax and Tmax between the pre‐ and postoperative setting were observed. The geometric mean of the ratio of AUC0–48 h post/pre‐RYGB for fenofibrate was 1.10 [95% confidence interval (CI) 0.87, 1.40; P = 0.40]. For posaconazole, an important decrease in AUC0–48 h and Cmax following RYGB was shown; the geometric mean of the AUC0–48 h post/pre‐RYGB ratio was 0.68 (95% CI 0.48, 0.96; P = 0.03) and the geometric mean of the Cmax pre/post‐RYGB ratio was 0.60 (95% CI 0.39, 0.94; P = 0.03). The decreased exposure of posaconazole could be explained by the increased gastric pH and accelerated gastric emptying of fluids post‐RYGB. No difference for Tmax was observed.
Conclusions
The disposition of fenofibrate was not altered after RYGB, whereas the oral disposition of posaconazole was significantly decreased following RYGB.
Keywords: absorption, bariatric surgery, fenofibrate, pharmacokinetics, posaconazole, Roux‐en‐Y gastric bypass
What is Already Known about this Subject
Roux‐en‐Y gastric bypass (RYGB) has an effect on various factors influencing drug absorption, including an increased gastric pH, delayed inlet of bile acids and reduced surface area.
Only a few studies have investigated changes in drug disposition after RYGB.
What this Study Adds
The oral disposition of fenofibrate was not altered after RYGB.
The oral disposition of posaconazole was significantly decreased following RYGB.
Caution is needed when prescribing basic drugs, which are highly dependent on the acidic environment in the stomach for their solubility/dissolution behaviour.
Introduction
Over recent decades, the prevalence of obesity has increased dramatically. Obesity is associated with several physiological changes which alter drug disposition. The increased prevalence of obesity has led to an increased demand for bariatric surgery, especially Roux‐en‐Y gastric bypass (RYGB), which offers the most significant and sustainable weight reduction in morbidly obese patients [body mass index (BMI) ≥ 40 kg m−2 or ≥ 35 kg m−2 with obesity‐related diseases) based on currently available data 1, 2. Besides weight loss and subsequently reduced BMI and percentage body fat, RYGB results in anatomical and physiological changes in the gastrointestinal (GI) tract by reducing the gastric capacity and bypassing the duodenum and proximal jejunum 3, 4. These changes may alter the pharmacokinetics of any given drug. However, the extent to which the absorption of a specific drug or class of drugs is altered remains unknown. The available data show no clear prediction of changes in oral drug exposure before and after RYGB. Oral exposure can be reduced following RYGB (as reported for azithromycin 5); it can remain unaltered (as reported for levothyroxine 6); or can be increased (as reported for metformin 7). A systematic approach to studying the influence of RYGB on oral drug exposure is lacking because of variations in study design and a lack of standardization of surgical procedures, making the results difficult to compare 8. We have previously performed a pharmacokinetic study of metoprolol in patients pre‐ and post‐RYGB, and the same design was used in the current pharmacokinetic study, allowing direct comparison of the results 9.
As mentioned above, several anatomical and physiological changes are associated with a RYGB. In patients with RYGB, the gastric remnant and bypassed biliary limb are reconnected to the intestine, 75–150 cm distal to the anastomosis between the gastric pouch and the distal part of the jejunum 10. This alteration in GI anatomy results in a delayed action of bile acids, as bile salts and the drug/food do not come into contact until they reach the common channel in the mid‐jejunum. Furthermore, RYGB leads to the formation of a small gastric pouch; this, in addition to the widespread use of antacid medication following surgery, results in an increased gastric pH. The increased gastric pH affects drug dissolution and the solubility of ionizable compounds, and subsequently their absorption 11, 12. With these changes in mind, we chose our study drugs on the basis of their absorption characteristics. In the present study, we investigated the influence of RYGB on the disposition of fenofibrate and posaconazole. Both compounds belong to the Biopharmaceutical Classification System (BCS) class II (high permeability, low solubility) 13. Fenofibrate (neutral) was selected as a test compound because it has been demonstrated that its solubility is highly dependent on bile salt concentrations 14. In light of a delayed contact with bile salts after surgery, a decrease in absorption was expected. Fenofibrate is a lipid‐lowering agent and is available as a micronized formulation (Lipanthyl® capsule) and a nanonized formulation (Lipanthylnano® tablet). In the present study, we chose the micronized tablet, as our focus was to analyse the drug substance and not the formulation characteristics. Posaconazole (a weak base) was selected as a model compound in view of the fact that a relationship has been demonstrated between residence time in the acidic environment of the stomach and systemic exposure. As a result of a reduction in acid production and a shorter residence time in the stomach after RYGB, a lower systemic exposure was expected.
Posaconazole is a broad‐spectrum anti‐fungal drug for the treatment of invasive fungal infections. In the present study, we used the suspension (Noxafil®) 15.
Subjects and methods
Selection of patients
Obese patients with a planned RYGB surgery at the University Hospitals Leuven, Belgium, were recruited. Patients who had previously undergone bariatric surgery or who had renal and hepatic impairment were not included in the study. Pregnant and breastfeeding women were also not included. RYGB surgery was performed in all recruited patients by the same surgeon and according to the same procedure. In brief, the jejunum was divided 30 cm from the ligament of Treitz and anastomosed to a 30 ml proximal gastric pouch. The jejunum was reanastomosed 120 cm distally to the gastrojejunostomy. All mesenteric defects were closed. The study was approved by the medical ethics committee of the University Hospitals Leuven (ML8433) and was performed in accordance with the 1964 Helsinki Declaration and its later amendments. The study is listed in the European Clinical Trials database (EudraCT), with reference number 2012–001 244‐22. All patients gave written informed consent.
Study design and procedure
In one group (12 patients; 11 Caucasian and one of African descent), a single‐dose pharmacokinetic study with 67 mg fenofibrate (Lipanthyl®) was performed before and 6–9 months after RYGB {average 6.9 [standard deviation (SD) 1.0] months; further referred to as 6 months after RYGB}. In another group of 12 patients (10 Caucasian, and one of African and one of South American descent), a single‐dose pharmacokinetic study with 400 mg posaconazole (10 ml Noxafil®, an oral suspension containing 40 mg ml−1) was performed before and 6–9 months after RYGB [average 6.7 (SD 0.7) months; further referred to as 6 months after RYGB]. As lung cancer was diagnosed in a patient post‐RYGB, there was dropout of one participant in the posaconazole study. One of the patients in the posaconazole study had previously undergone a cholecystectomy.
The extent of absorption, distribution, metabolism, and excretion of disposition of fenofibrate and posaconazole was estimated by determining the area under the curve (AUC0–48 h), peak plasma concentration (Cmax) and time to reach peak concentration (Tmax) of fenofibric acid and posaconazole, respectively.
Following an overnight fast of at least 10 h, subjects came to the clinical pharmacology unit of the University Hospitals Leuven. Weight and height were measured using calibrated equipment. Weight was measured to the nearest 0.1 kg, with the subjects having an empty bladder and wearing indoor clothing with empty pockets and without shoes. The BMI (kg m−2) was calculated by dividing the weight (kg) by the square of the height (m2). The percentage weight loss from baseline was calculated to express the change in weight.
Dual‐energy X‐ray absorptiometry was performed (Hologic Discovery) to measure the amount of body fat mass 16.
After the insertion of an intravenous catheter, one group of subjects ingested 67 mg fenofibrate with 150 ml water, and the other group ingested 10 ml posaconazole 40 mg ml−1 with 150 ml water. After oral administration, blood samples were collected into heparinized tubes at 15 min, 30 min, 60 min, 90 min, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 24 h and 48 h. The blood samples were centrifuged immediately after collection (1800 g, 10 min, 4°C); plasma samples were stored at −20°C until analysis.
A standardized meal and standardized snack were administered 4 h and 8 h after drug administration, respectively. Participants had to consume the entire meal. The use of water was allowed ad libitum, except for 1 h before and 4 h after drug administration. During the first 4 h after administration of the drugs, the patients had to remain semi‐supine in bed. After the 10‐h blood sample, the subjects were discharged and had to return on the two subsequent mornings for the 24‐ and 48‐h blood sampling. As proton pump inhibitors (PPIs), H2‐receptor antagonists and antacids can influence drug absorption, the recruited patients were asked to stop taking these drugs during the week preceding the study. Other prescription drugs were checked to verify that they had no pharmacokinetic interactions with the study drug. On the first morning of the study, the patients were not allowed to take any of their medication.
High‐performance liquid chromatography (HPLC) analysis
Fenofibrate
After absorption of fenofibrate, it is quantitatively converted to its active metabolite, fenofibric acid 17. Fenofibric acid was determined in plasma to be indicative of the oral exposure from fenofibrate. Before HPLC–ultraviolet (UV) analysis, fenofibric acid was extracted from plasma samples. 100 μl of a stock solution of the internal standard carbamazepine solution (20 μM in 1 M HCl) was added to 500 μl plasma. Subsequently, 400 μl HCl (1 M) was added and vortexed (±10 s) in order to precipitate plasma proteins. To extract fenofibric acid and carbamazepine, 6 ml dichloromethane was added and samples were shaken for 1 min. After centrifugation (2880 g, 15 min, 4°C), the water layer was discarded and the organic layer was evaporated under a stream of air until dryness. The residue was dissolved in 1 ml methanol. Following evaporation, 200 μl of mobile phase was added to the residue and injected into the HPLC system (Waters, Milford, MA, USA). Carbamazepine and fenofibric acid were detected at a wavelength of 287 nm (Waters 2487 UV Detector). Retention times of 4.5 min and 8 min were generated with a flow rate of 1 ml min−1 for carbamazepine and fenofibric acid, respectively. Running conditions started with acetonitrile: 25 mM acetic acid buffer pH 3.5 (50:50 v/v). Acetonitrile concentrations increased up to 60% over 3 min. Following elution of fenofibric acid, the column was rinsed for 2 min with acetonitrile:water (90:10 v/v), followed by 1 min with water: 25 mM acetic acid buffer pH 3.5 (75:25 v/v) and subsequently re‐equilibrated under the starting conditions for 2 min.
The calibration curve was based on a stock solution of fenofibric acid in acetonitrile. Blank plasma samples were spiked and treated the same way as the samples. Linearity was observed between 158 μM and 0.31 μM. Method validation resulted in accuracy and precision errors of less than 5% and 8%, respectively, for a concentration of 9.8 μM. Quality control samples (9.8 μM) were included on the days of analysis and resulted in a relative SD of less than 5%.
Posaconazole
Analysis was performed by extracting posaconazole from plasma samples as described by Walravens et al. 27. Concentrations of posaconazole were determined using HPLC/fluorescence analysis. Briefly, 100 μl of internal standard solution (2.5 μM itraconazole in 0.2 N HCl) was added to 1000 μl of plasma. Subsequently, the sample was alkalized with 500 μl 2 N NaOH. After addition of 4 ml diethylether, samples were vortexed for 30 s and directly centrifuged (2880 g, 5 min, 4°C). Finally, the organic layer was transferred to a clean glass tube and evaporated to dryness under a gentle stream of air. A volume of 300 μl of the mobile phase [methanol:20 mM acetic acid buffer pH 3.3 (76:24 v/v)] was added to the remaining residue. After centrifugation (2880 g, 5 min, 4°C), 50 μl of the supernatant was injected into the Hitachi Elite LaChrom HPLC system and analysed by the Hitachi Elite LaChrom L‐2480 fluorescence detector (excitation wavelength 240 nm, emission wavelength 385 nm). A gradient run of 19 min was performed in order to obtain a retention time of 7.9 min and 12.1 min on the Novapak C‐18 column for posaconazole and itraconazole, respectively. Gradient elution at a constant flow rate of 1 ml min−1 was performed as follows: methanol:20 mM acetic acid buffer pH 3.3 (76:24) for 2 min followed by methanol:20 mM acetic acid buffer pH 3.3 (81:19) for 7 min; followed by a rinsing step with 100% methanol for 3 min; then re‐equilibration for 5 min with methanol:20 mM acetic acid buffer pH 3.3 (76:24) before the next injection. A calibration curve was made based on stock solutions of posaconazole and itraconazole in dimethyl sulfoxide. Linearity was observed between 2000 nM and 7.8 nM. Quality control samples of 500 nM and 50 nM, which were analysed together with the plasma samples, resulted in an accuracy and precision error of less than 10%.
Data analysis
The AUC0–48 h of the concentration–time curves was determined using the linear trapezoidal rule. All results are presented as mean (95% CI). To compare the characteristics of the patients, paired t‐tests were performed. To estimate the magnitude of the effect of RYGB on the disposition of fenofibrate/posaconazole, a ratio paired t‐test was performed, determining the geometric mean of the ratios (post/pre) with 95% CI for AUC0–48 h and Cmax, or the mean difference (pre‐post) with 95% CI for Tmax. For that purpose, the values of AUC0–48 h and Cmax were log‐transformed, the difference between the transformed variables was estimated with 95% CI, and the mean and confidence limits were back‐transformed to the original scale. The preoperative data for the patient who dropped out of the posaconazole study were omitted from the analysis. Data were analysed using SPSS Statistics 22 (IBM Corp., Armonk, New York, USA). Multiple linear regression analysis was performed to investigate if there was a correlation between the difference in AUC0–48 h and gender, age, difference in BMI, fat percentage and percentage weight loss. Statistical significance was set at P < 0.05.
Results
Fenofibrate
For the fenofibrate study, 12 patients (seven female) with a mean age of 43.3 (95% CI 34.0, 52.6) years were included and completed follow‐up. Weight, BMI, percentage weight loss, and total and abdominal fat mass percentage were significantly decreased post‐RYGB (see Table 1).
Table 1.
Characteristics of the participants, shown as mean [95% confidence interval (CI)]
| Before RYGB | After RYGB | P value | |
|---|---|---|---|
| Fenofibrate (n = 12) | |||
| Weight (kg) | 118 (105, 131) | 83.7 (74.2, 93.2) | <0.001 |
| BMI (kg m −2 ) | 40.4 (38.2, 42.6) | 28.8 (26.6, 30.9) | <0.001 |
| Percentage weight loss | ‐ | 28.8 (25.7, 31.8) | <0.001 |
| Total fat percentage | 42.5 (38.2, 46.8) | 31.0 (25.0, 37.0) | <0.001 |
| Abdominal fat percentage | 44.0 (41.2, 46.7) | 29.1 (23.8, 34.4) | <0.001 |
| Posaconazole (n = 11) | |||
| Weight (kg) | 123 (110, 135) | 87.2 (77.7, 96.7) | <0.001 |
| BMI (kg m −2 ) | 40.8 (37.0, 44.6) | 29.0 (26.2, 31.8) | <0.001 |
| Percentage weight loss | ‐ | 28.8 (24.4, 33.3) | <0.001 |
| Total fat percentage | 41.9 (36.8, 47.0) | 31.6 (25.9, 37.4) | <0.001 |
| Abdominal fat percentage | 41.9 (36.8, 47.1) | 28.3 (23.0, 33.5) | <0.001 |
BMI, body mass index; RYGB, Roux‐en‐Y gastric bypass.
The observed concentration–time profiles are shown in Figure 1. The mean AUC0–48 h, AUC from 0 to infinity (AUC0–∞) and Cmax were comparable before and after RYGB, as shown in Table 2. The geometric mean of the ratio of AUC0–48 h post/pre‐RYGB for fenofibrate was 1.10 (95% CI 0.87, 1.40; P = 0.40) and the geometric mean of the ratio of Cmax post/pre‐RYGB was 1.12 (95% CI 0.79, 1.59; P = 0.49). The mean difference in Tmax was −0.58 h (95% CI −4.72, 3.55).
Figure 1.
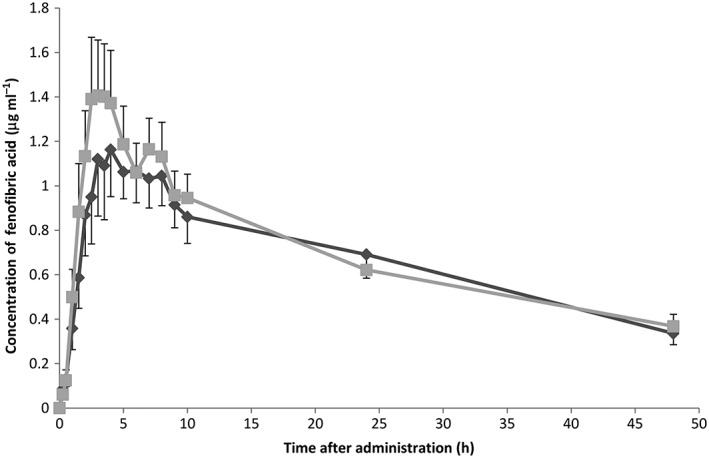
Observed plasma concentration–time profiles of fenofibrate over 48 h after oral administration, shown as mean concentration ± standard error of the mean.  Preoperative,
Preoperative,  Postoperative
Postoperative
Table 2.
Pharmacokinetic parameters, shown as mean [95% confidence interval (CI)]
| Before RYGB | After RYGB | |
|---|---|---|
| Fenofibrate (n = 12) | ||
| AUC 0–48 h (μg ml −1 * h) | 31.3 (21.8, 40.8) | 33.3 (25.0, 41.5) |
| AUC 0–∞ (μg ml −1 * h) | 46.9 (29.6, 64.2) | 51.7 (32.4, 71.1) |
| C max (μg ml −1 ) | 1.37 (0.87, 1.87) | 1.57 (1.01, 2.12) |
| T max (h) | 4.54 (3.38, 5.70) | 5.12 (1.23, 9.02) |
| Posaconazole (n = 11) | ||
| AUC 0–48 h (μg ml −1 * h) | 3.11 (1.38, 4.85) | 1.81 (1.37, 2.26) |
| AUC 0–∞ (μg ml −1 * h) | 9.49 (5.26, 13.7) | 4.37 (2.91, 5.84) |
| C max (μg ml −1 ) | 0.12 (0.04, 0.21) | 0.06 (0.04, 0.07) |
| T max (h) | 7.68 (−1.52, 16.9) | 6.46 (2.43, 10.5) |
AUC0–48 h, area under the concentration–time curve up to 48 h; AUC0–∞, area under the concentration–time curve from 0 to infinity; Cmax, peak plasma concentration; RYGB, Roux‐en‐Y gastric bypass; Tmax, time to reach peak concentration.
When comparing the individual AUC0–48 h postoperatively with preoperatively, two patients had a >25% decrease in AUC0–48 h and four had a >25% increase in AUC0–48 h. For the others, the AUC0–48 h was comparable before and after surgery. No correlation with other variables was identified.
Posaconazole
For the posaconazole study, 12 patients were included, 11 of whom (seven female), with a mean age of 37.4 (95% CI 30.0, 44.7) years, completed the study. Weight, BMI, percentage weight loss, and total and abdominal fat mass percentage were significantly decreased post‐RYGB (see Table 1).
The observed concentration–time profiles are shown in Figure 2. The RYGB had an important impact on the AUC0–48 h and AUC0–∞ after oral administration of posaconazole, as shown in Table 2. The geometric mean of the ratio of AUC0–48 h post/pre‐RYGB was 0.68 (95% CI 0.48, 0.96; P = 0.03). Cmax was also decreased after surgery; the geometric mean of the ratio for Cmax post/pre‐RYGB was 0.60 (95% CI 0.39, 0.94; P = 0.03). The difference in Tmax was 1.23 h (95% CI −8.86, 11.31). No correlation with other variables was identified.
Figure 2.
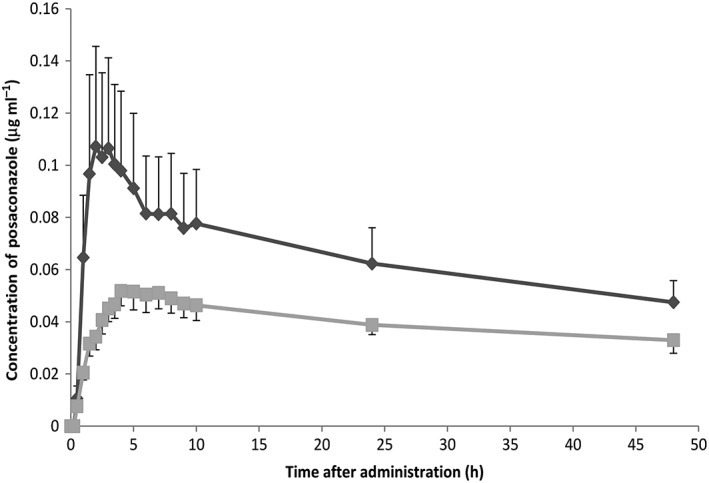
Observed plasma concentration–time profiles of posaconazole over 48 h after oral administration, shown as mean concentration ± standard error of the mean.  Preoperative,
Preoperative,  Postoperative
Postoperative
Discussion
We investigated the pharmacokinetic parameters of a neutral compound (fenofibrate) and a weak base (posaconazole) in patients before and after RYGB. We showed that the mean pharmacokinetic parameters of the neutral compound (fenofibrate) were unaltered after RYGB, whereas for the weak base (posaconazole), the oral exposure was decreased after RYGB.
Both fenofibrate and posaconazole are lipophilic drugs belonging to BCS class II (low solubility, high permeability). The solubility of fenofibrate is highly dependent on bile acid/phospholipid concentrations 14. These ingredients are responsible for the formation of micelles, which increase the solubility of lipophilic drugs. RYGB changes the anatomical structure of the GI tract, resulting in a delayed inlet of bile acids 10. This could explain the delayed exposure of fenofibrate to bile acids, which can result in a reduced exposure after oral administration. However, the mean oral exposure of fenofibrate was comparable before and after RYGB. This could be explained by a compensation mechanism after RYGB. Patti et al. 19 showed that the fasting total serum bile acid concentration is twice as high in patients 2–4 years after RYGB, compared with overweight or obese individuals without bariatric surgery. This was confirmed in patients 4 days and 1 year post‐RYGB 20. This increase might be associated with an increased secretion of bile acids in the small intestine, compensating for the delayed inlet of bile acids post‐RYGB and resulting in no relevant impact of RYGB on the pharmacokinetic parameters of oral fenofibrate disposition after this intervention. However, no information about the intestinal concentration of bile acids post‐RYGB is available. Interestingly, no increase in Tmax was observed for fenofibrate. It is possible to hypothesize that the interval between administration of the drug and its subsequent contact with bile acids is comparable before and after surgery, despite the delayed inlet of bile acids. An explanation could be that the delayed inlet of bile acids might be compensated by the accelerated gastric emptying of fluids after RYGB and thus be similar; accelerated gastric emptying has already been demonstrated in previous studies 21, 22, 23, 24, 25. Furthermore, the changes in body composition can influence fenofibrate disposition, although no information about the impact of obesity on fenofibrate disposition is available. It is worth mentioning that large interindividual differences were observed: some patients had a decrease in the oral exposure of fenofibrate, and others an increase; for most patients, the oral exposure pre‐ and postoperatively was the same. This might reflect large inter‐ and intraindividual differences in the amount of bile acid secretion, which have also been shown in healthy volunteers 26.
Posaconazole is a lipophilic, weak base and we have demonstrated an important decrease in AUC0–48 h and Cmax post‐RYGB. Dissolved posaconazole might be absorbed quickly as it has a high permeability 27. However, it has a low solubility, and the intraluminal pH and the residence time in the stomach have an important role in the intestinal absorption of this agent; a previous study showed that an increase in gastric pH is associated with a reduction in the absorption of posaconazole, while a longer residence time in the stomach increased absorption 18. These findings were confirmed by Krishna et al., who showed that the oral exposure and Cmax of posaconazole were decreased if the gastric pH was increased by the intake of a PPI (esomeprazole), and were increased when posaconazole was administered with an acidic beverage 28. In patients undergoing RYGB, a small gastric pouch is created, in which gastric acid secretion is negligible as the majority of the parietal cells (i.e. acid‐producing cells) are bypassed 11, 12. This results in an elevated gastric pH, which affects the solubility of drugs, including posaconazole as a weak base. In the present study, the mean AUC0–48 h and Cmax were decreased after RYGB by 42% and 50%, respectively, compared with 37% and 42%, respectively, in patients using esomeprazole 18. This suggests that the increase in gastric pH is not the only contributor to the reduction in oral exposure postoperatively. After RYGB, the residence time in the stomach is also changed. It has also been shown that gastric emptying for liquids is accelerated after RYGB 21, 22, 23, 24, 25. Faster gastric emptying is associated with a shorter gastric residence time, and subsequently a shorter dissolving period for posaconazole, eventually resulting in a reduced AUC0–48 h and Cmax, and especially a reduction in the rate and extent of absorption. Furthermore, the reduction in body weight post‐RYGB can have an impact on the disposition of posaconazole. However, posaconazole dosages should not be changed for increased body weight, although follow‐up studies are needed to investigate the impact of obesity on the disposition of posaconazole 29.
These observations may have significant clinical implications. The results from the present study might explain why posaconazole was found to be ineffective in a patient who had undergone RYGB 30. The patient was treated with posaconazole for 10 days and the levels were well below the minimal inhibitory concentration, despite strict adherence and no coadministration of a medication that could interfere with the absorption of posaconazole. A switch to oral isavuconazole was necessary in this case 30. Isavuconazole is a BCS class I compound (high solubility and high permeability) 31; this might explain why its disposition is less influenced by RYGB, as in a previous pharmacokinetic study of metoprolol, another BCS class I compound, we showed that RYGB had no significant impact on its oral exposure 9. However, it should be noted that the results for isavuconazole were from a case report and we do not know the generalizability of this finding. This case report highlights the importance of pharmacokinetic studies in the RYGB patient population in order to avoid underdosing. Therapeutic drug monitoring for posaconazole after RYGB should be considered in order to ensure that minimal inhibitory concentrations are reached.
Furthermore, we need to take into account that obesity is associated with many changes in body composition, which can have an influence on the volume of distribution (VD), especially for lipophilic compounds. In general, lipophilic compounds will have an increase in VD in obese vs. normal‐weight individuals, although there are exceptions 31, 32. Both fenofibrate and posaconazole are lipophilic drugs and may be associated with an increase in VD before RYGB as obesity is associated with an excessive fat mass accumulation. Posaconazole, in particular, has a remarkable distribution in the body; one study found a 40‐fold higher concentration in the tissue than in the serum 33. We have shown that the fat mass percentage after surgery was significantly decreased, which might have resulted in a decreased VD post‐RYGB. These changes might be reflected in lower plasma concentrations before surgery.
To increase the solubility of posaconazole, coadministration of an acidic carbonated beverage, such as Coca‐Cola®, can help 18. However, in patients who have undergone RYGB, the intake of such drinks is not recommended as it can result in gastric problems and dumping syndrome 34. Furthermore, the intake of fenofibrate and posaconazole with high‐fat food could increase intraluminal solubility by increasing bile acid flow 33. However, in the present study we preferred to administer the drugs in a fasted state, for two reasons. First, the results on the gastric emptying of solids after RYGB are contradictory 23, 25. This implies that if the drug was administered during a fed state, more interindividual differences would be observed. Furthermore, we wanted to use the same design as in our previously performed pharmacokinetic study of metoprolol, in order to enable a comparison to be made between the different drugs analysed 9.
Recently, a delayed‐release tablet formulation containing 100 mg posaconazole was developed using the hot‐melt extrusion technique, in which posaconazole is dispersed in hypromellose acetate succinate, which is pH sensitive 33. The tablet has the advantage that it results in a higher exposure to the drug, and this may be accompanied by fewer interindividual differences. Moreover, this exposure is not affected by changes in motility or gastric pH 33. It is possible that the increased gastric pH post‐RYGB has less influence on posaconazole exposure from the tablet formulation than from the oral suspension. In a future study, it would be interesting to test the tablet formulation of posaconazole in this population group, in order to have a better idea of the influence of RYGB on different formulations of the same drug, as we have already done for metoprolol (immediate‐ and controlled‐release formulation). Based on the results of the pharmacokinetic studies performed with metoprolol, fenofibrate and posaconazole in patients before and after RYGB, we can state that caution is especially needed when prescribing basic drugs which are highly dependent on the acidic environment in the stomach for dissolution/solubility. Overall, the strength of the present pharmacokinetic study was the design as it was performed in the same patient group both before and after the operation, ensuring that there were no interindividual differences between the groups. Furthermore, the patients underwent the same type of surgery, performed by the same surgeon.
In conclusion, fenofibrate and posaconazole are both BCS class II compounds with high permeability and low solubility, but fenofibrate is a neutral compound and posaconazole a weak base. The pharmacokinetic parameters for the disposition of fenofibrate were found to be comparable before and after RYGB. This was in contrast with the important decrease in the oral exposure of posaconazole after RYGB, which could be explained by the increase in gastric pH and accelerated gastric emptying of fluids post‐RYGB. Caution is needed when prescribing other basic drugs which are highly dependent on the acidic environment in the stomach for dissolution/solubility. The disposition of these drugs might also be decreased, resulting in underdosing.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
We would like to thank all study participants. IG received a PhD scholarship from the Agency for Innovation by Science and Technology, Flanders, IWT‐111 328. BVdS is the recipient of a ‘Fundamenteel Klinisch Navorserschap FWO Vlaanderen’.
Contributors
IG, BH, BVDS, RM, JdH, ML, CM, VF and PA wrote the manuscript. IG, BVDS, JdH, ML, CM, VF and PA designed the research. IG, BH and RM performed the research. IG, BH, BVDS, CM, VF and PA analysed the data.
Gesquiere, I. , Hens, B. , Van der Schueren, B. , Mols, R. , de Hoon, J. , Lannoo, M. , Matthys, C. , Foulon, V. , and Augustijns, P. (2016) Drug disposition before and after gastric bypass: fenofibrate and posaconazole. Br J Clin Pharmacol, 82: 1325–1332. doi: 10.1111/bcp.13054.
References
- 1. WHO – Obesity and overweight 2015. Fact sheet N°311.
- 2. Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost‐effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 215–357. [DOI] [PubMed] [Google Scholar]
- 3. De Smet J, Van Boxclaer J, Boussery K. The influence of bypass procedures and other anatomical changes in the gastrointestinal tract on the oral bioavailability of drugs. J Clin Pharmacol 2013; 53: 361–76. [DOI] [PubMed] [Google Scholar]
- 4. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev 2010; 11: 41–50. [DOI] [PubMed] [Google Scholar]
- 5. Padwal RS, Ben‐Eltriki M, Wang X, Langkaas LA, Sharma AM, Birch DW, et al. Effect of gastric bypass surgery on azithromycin oral bioavailability. J Antimicrob Chemother 2012; 67: 2203–6. [DOI] [PubMed] [Google Scholar]
- 6. Gkotsina M, Michalaki M, Mamali I, Markantes G, Sakellaropoulos GC, Kalfarentzos F, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid 2013; 23: 414–9. [DOI] [PubMed] [Google Scholar]
- 7. Padwal RS, Gabr RQ, Sharma AM, Langkaas LA, Birch DW, Karmali S, et al. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care 2011; 34: 1295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenblatt HK, Greenblatt DJ. Altered drug disposition following bariatric surgery: a research challenge. Clin Pharmacokinet 2015; 54: 573–9. [DOI] [PubMed] [Google Scholar]
- 9. Gesquiere I, Darwich AS, Van der Schueren B, de Hoon J, Lannoo M, Matthys C, et al. Drug disposition and modelling before and after gastric bypass: immediate and controlled release metoprolol formulations. Br J Clin Pharmacol 2015; 80: 1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neff KJ, Olbers T, le Roux CW. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med 2013; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux‐en‐Y gastric bypass for morbid obesity. Ann Surg 1993; 218: 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci 1994; 39: 315–20. [DOI] [PubMed] [Google Scholar]
- 13. Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 2005; 22: 11–23. [DOI] [PubMed] [Google Scholar]
- 14. Mohsin K. Design of lipid‐based formulations for oral administration of poorly water‐soluble drug fenofibrate: effects of digestion. AAPS PharmSciTech 2012; 13: 637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schiller DS, Fung HB. Posaconazole: an extended‐spectrum triazole antifungal agent. Clin Ther 2007; 29: 1862–86. [DOI] [PubMed] [Google Scholar]
- 16. Bazzocchi A, Ponti F, Cariani S, Diano D, Leuratti L, Albisinni U, et al. Visceral fat and body composition changes in a female population after RYGBP: a two‐year follow‐up by DXA. Obes Surg 2015; 25: 443–51. [DOI] [PubMed] [Google Scholar]
- 17. Guivarc'h PH, Vachon MG, Fordyce D. A new fenofibrate formulation: results of six single‐dose, clinical studies of bioavailability under fed and fasting conditions. Clin Ther 2004; 26: 1456–69. [DOI] [PubMed] [Google Scholar]
- 18. Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole: monitoring of intraluminal and plasma drug concentrations. Clin Pharmacokinet 2011; 50: 725–34. [DOI] [PubMed] [Google Scholar]
- 19. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009; 17: 1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonen M, Dali‐Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux‐en‐Y gastric bypass. Obes Surg 2012; 22: 1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang G, Agenor K, Pizot J, Kotler DP, Harel Y, Van Der Schueren BJ, et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP). Obes Surg 2012; 22: 1263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naslund I, Beckman KW. Gastric emptying rate after gastric bypass and gastroplasty. Scand J Gastroenterol 1987; 22: 193–201. [DOI] [PubMed] [Google Scholar]
- 23. Horowitz M, Cook DJ, Collins PJ, Harding PE, Hooper MJ, Walsh JF, et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg 1982; 69: 655–7. [DOI] [PubMed] [Google Scholar]
- 24. Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon‐like peptide‐1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006; 91: 1735–40. [DOI] [PubMed] [Google Scholar]
- 25. Dirksen C, Damgaard M, Bojsen‐Moller KN, Jorgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux‐en‐Y gastric bypass. Neurogastroenterol Motil 2013; 25: 346–e255. [DOI] [PubMed] [Google Scholar]
- 26. Riethorst D, Mols R, Duchateau G, Tack J, Brouwers J, Augustijns P. Characterization of human duodenal fluids in fasted and fed state conditions. J Pharm Sci 2015; 105: 673–81. [DOI] [PubMed] [Google Scholar]
- 27. Gubbins PO, Krishna G, Sansone‐Parsons A, Penzak SR, Dong L, Martinho M, et al. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Chemother 2006; 50: 1993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 2009; 53: 958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Payne KD, Hall RG. Dosing of antifungal agents in obese people. Expert Rev Anti Infect Ther 2016; 14: 257–67. [DOI] [PubMed] [Google Scholar]
- 30. Knoll BM. Pharmacokinetics of oral isavuconazole in a patient after Roux‐en‐Y gastric bypass surgery. J Antimicrob Chemother 2014; 69: 3441–3. [DOI] [PubMed] [Google Scholar]
- 31. Schmitt‐Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, et al. Multiple‐dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 2006; 50: 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin JH, Saleem M, Looke D. Therapeutic drug monitoring to adjust dosing in morbid obesity – a new use for an old methodology. Br J Clin Pharmacol 2012; 73: 685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Percival KM, Bergman SJ. Update on posaconazole pharmacokinetics: comparison of old and new formulations. Curr Fungal Infect Rep 2014; 8: 139–45. [Google Scholar]
- 34. Tack J, Deloose E. Complications of bariatric surgery: dumping syndrome, reflux and vitamin deficiencies. Best Pract Res Clin Gastroenterol 2014; 28: 741–9. [DOI] [PubMed] [Google Scholar]


