Abstract
Aims
Little is known about the impact of inappropriate prescribing (IP) in community‐dwelling adults, aged 80 years and older. The prevalence at baseline (November 2008September 2009) and impact of IP (misuse and underuse) after 18 months on mortality and hospitalization in a cohort of community‐dwelling adults, aged 80 years and older (n = 503) was studied.
Methods
Screening Tool of Older People's Prescriptions (STOPP‐2, misuse) and Screening Tool to Alert to Right Treatment (START‐2, underuse) criteria were cross‐referenced and linked to the medication use (in Anatomical Therapeutic Chemical coding) and clinical problems. Survival analysis until death or first hospitalization was performed at 18 months after inclusion using Kaplan–Meier, with Cox regression to control for covariates.
Results
Mean age was 84.4 (range 80–102) years. Mean number of medications prescribed was 5 (range 0–16). Polypharmacy (≥5 medications, 58%), underuse (67%) and misuse (56%) were high. Underuse and misuse coexisted in 40% and were absent in 17% of the population. A higher number of prescribed medications was correlated with more misused medications (r s = .51, P < 0.001) and underused medications (r s = .26, P < 0.001).
Mortality and hospitalization rate were 8.9%, and 31.0%, respectively. After adjustment for number of medications and misused medications, there was an increased risk of mortality (HR 1.39, 95% CI 1.10, 1.76) and hospitalization (HR 1.26, 95% CI 1.10, 1.45) for every additional underused medication. Associations with misuse were less clear.
Conclusion
IP (polypharmacy, underuse and misuse) was highly prevalent in adults, aged 80 years and older. Surprisingly, underuse and not misuse had strong associations with mortality and hospitalization.
Keywords: aged 80 years and over, hospitalization, inappropriate prescribing, mortality, polypharmacy, primary care
What is Already Known about this Subject
Limited evidence on the clinical outcomes of screening tools for inappropriate prescribing exists.
The effects of polypharmacy and misuse have mainly been studied in cross‐sectional research, but little is known on the effects of underuse.
Few studies of inappropriate prescribing specifically focused on the community‐dwelling oldest old (aged 80 years and over).
What this Study Adds
Polypharmacy, underuse and misuse were correlated and were coexistent in almost half of the oldest old population.
Underuse was associated with increased rates of mortality and hospitalization, even when controlling for polypharmacy and misuse.
For every additional underused medication at baseline, there was a 39% increased risk for mortality and a 26% increased risk for being hospitalized. The electronic application of explicit criteria can aid prescribers in detecting potential hazardous inappropriate prescribing, although further specification of these criteria is needed.
Introduction
Appropriate prescribing of medications is a major challenge in the care for older adults. Older adults are more sensitive to the effects of medications and have a higher prevalence of comorbidities 1. Hence, older adults will have a higher medication intake, potentially putting them at risk for adverse drug events 2, increased morbidity, health care utilization and mortality 3. Yet, polypharmacy cannot be equated with inappropriate prescribing (IP). IP is possible in polypharmacy, yet not every person with polypharmacy will have IP 4.
Prescribing can be potentially inappropriate if the potential benefits are outweighed by the harms, if there is evidence for an equal or more effective, yet lower risk alternative 5, 6 or if omission of potentially beneficial medications is present 7. Tools were developed to identify inappropriate prescribing in older adults, focussing on polypharmacy, underuse and misuse 8. Most of these tools consist of lists of explicit criteria of potentially inappropriate medications, often without the clinical data required. Some criteria address underuse instances, always requiring clinical data 9, 10, 11 and are designed to alert clinicians when to drop or add a medication in individual patients.
The clinical relevance of screening tools for inappropriate prescribing based on these explicit criteria is not yet fully explored. Most studies were cross‐sectional. Gaps in evidence remain, as data from prospective long term cohort studies are scarce 12, 13, 14, 15. Moreover, the oldest old (aged 80 years and over) has been rarely studied as a separate group in primary care settings 16, 17, 18. Finally, polypharmacy, underuse and misuse, although part of the definition of inappropriate prescribing, are seldom concomitantly studied 19.
This study aims to explore the prevalence of inappropriate prescribing (misuse and underuse) in a prospective cohort of community‐dwelling oldest old (aged 80 years and over) and to explore associations with mortality and hospitalization after 18 months.
Methods
The Belfrail‐Med cohort 20, 21 was used (n = 503), consisting of Belgian community‐dwelling patients aged 80 years and over. All subjects were primary care patients, recruited by their own general practitioner (GP). Patients were selected between November 2008 and September 2009. Exclusion criteria were known dementia and in palliative care.
The GPs were responsible for the collection of baseline (demographic, clinical and medication data) and follow‐up data (date and cause of death, date of the first hospitalization). Clinical research assistants were responsible to collect data from the patients, using clinical examinations (e.g. blood pressure, …), and standardized scales (to measure physical activity, activities of daily living…). GPs used their medical records.
Medication handling
The GP recorded all chronic medications at baseline, using the generic name. All chronic medications were codified entered into the Anatomical Therapeutic Chemical classification (WHO ATC/DDD 2013) 22, based on the official register of medications on the Belgian market.
Polypharmacy was defined as the daily intake of five medications or more 23.
Assessing inappropriate prescribing
Inappropriate prescribing was operationalized by the computerized application of criteria for misuse and underuse. For misuse, we applied the clinically oriented Screening Tool for Older Person's Prescriptions (STOPP‐2 criteria). For underuse, we applied the Screening Tool to Alert doctors to Right Treatment (START‐2). These criteria are suitable for use in European countries 24, have been applied and validated in several studies 25, 26, 27 and were recently updated 10.
To assess the prevalence and impact of inappropriate prescribing, the STOPP/START–2 criteria were cross‐referenced and linked to the baseline medications and clinical problems.
This was not possible for all criteria, as only a subset of the STOPP/START–2 criteria could be applied (see box 1). For the START‐2 criteria, 13 out of 34 criteria could be used for our analysis and for the STOPP‐2 criteria, 46 out of 81. Reasons to omit criteria included the absence of data in our database required by the criteria: (1) clinical test results, (2) severity of disease data, (3) short duration of medication and (4) criteria on rank ordering of first choice medications. Other reasons to omit criteria were the unclear definition of clinical problems. Criteria pertaining to diseases excluded in our cohort (e.g. dementia) could also not be applied. Additionally for the STOPP‐2 criteria, we omitted one extra criterion because of possible duplication in scoring. Criterion 32 (benzodiazepines for ≥4 weeks) and 74 (benzodiazepines could increase the risk of fall incidents) were considered too similar. For further analysis, only the former was taken into account. A full overview of the selection process can be found in box 1.
Box 1. Flowchart for the rationale for exclusion of STOPP/START criteria.
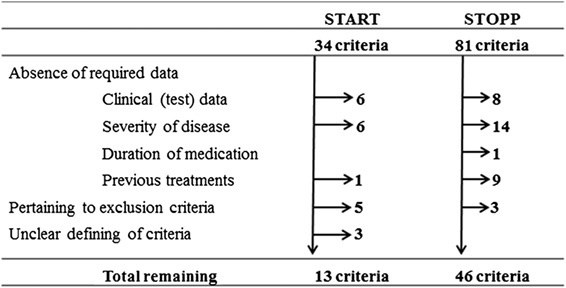
Outcome parameters
Follow‐up data were collected using standardized questionnaires, filled in by the GPs. Data collection on mortality included date and cause of death. Data on hospitalization included the date of the first unplanned hospital stay (longer than 1 day). The full follow‐up period of the Belfrail‐study was 5 years 20, but to observe direct associations with baseline medication use, a shorter follow‐up period was used, setting a cut‐off at 18 months after inclusion in the cohort. All further analyses used the 18 months cut‐off, although we provided in the text data on the 1 year survival rate for future and external comparisons.
Statistical analysis
SPSS 21.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA) was used for analysis.
For all variables, there were less than 5% missing data 20. Normally distributed continuous variables were expressed as means and .d.s. All skewed variables were expressed using the medians and interquartile ranges. Categorical data were expressed using numbers and percentages. Both underuse and misuse were divided into three categories, no (0), low (1–2) and high (3 or more) underuse or misuse of medications. Relationships between skewed data were tested using Spearman rank correlations.
The Kaplan–Meier method was used to estimate the survival rate, with the log‐rank test verifying the differences in survival time between groups. All deceased or hospitalized patients during the 18 months follow‐up period were considered as ‘events’. For hospitalization, additional censoring was done for patients who have died.
Cox proportional hazard models were used to calculate univariate and multivariate hazard ratios for associations with mortality and hospitalization. In univariate analysis, we first tested the associations with inappropriate prescribing, expressed as a continuous variable. Second, we used the above described categories of underuse and misuse (no, low and high), to explore the associations with possible trends in higher mortality and hospitalization rates, for higher categories of underuse or misuse.
Lastly, we tested the interaction between underuse and misuse, by multiplying the number of underused and misused medications of each individual. The statistical significance of each interaction term was evaluated by the likelihood ratio test, comparing nested models with or without inclusion of the interaction term.
A similar exercise was repeated in the multivariate models for both the continuous and categorical variables for underuse and misuse. Now underuse and misuse (continuous and categorical) were corrected for the number of medications taken at baseline. Additionally underuse was corrected for misuse and misuse for underuse.
Ethical approval
The study protocol was approved by the Biomedical Ethics Committee of the Medical School of the Université Catholique de Louvain (UCL), Brussels (B40320084685, on 27/10/2008) and later by the Ethics committee of Ghent University Hospital (B670201421408, on 26/06/2014). All respondents provided informed consent.
Results
The patients in the Belfrail‐Med cohort (n = 503) had a median age of 84.4 (range 80–102) years and 61.2% were female. Hypertension was the most common clinical problem, followed by osteoarthritis and hyperlipidaemia (see Table 1).
Table 1.
Demographics and clinical characteristics of the study population (n = 503)
| Demographic | Total (n = 503) % |
|---|---|
| Mean age in years (range) | 84.4 (80–102) |
| Gender (% female) | 61.2 |
| Living alone | 43.3 |
| Nursing care at home | 36.8 |
| Low education (≤8 years) | 69.2 |
| Clinical a | % |
|---|---|
| Hypertension | 70.4 |
| Osteoarthritis | 57.1 |
| Hyperlipidaemia | 44.1 |
| Heart failure (NYHA a > 0) | 38.4 |
| Obesity (BMI > 30 kg m–2) | 27.9 |
| Osteoporosis | 20.9 |
| Diabetes | 18.9 |
| Post‐myocardial infarction/post‐stroke | 17.7 |
| COPD/asthma | 13.1 |
| Depression | 12.7 |
| Chronic renal failure | 11.1 |
Clinical problems with prevalence above 10% are listed
New York Heart Association (NYHA) functional classification of heart failure
The mean number of medications was 5.4 (range 0–16). Cardiovascular (86.3%), haematological (56.1%) and nervous system drugs (54.5%) were most used.
Prevalence of inappropriate prescribing
Polypharmacy (≥ five medications) was present in 57.7% of the population. Using the START‐2 criteria, underuse was identified in 67.0% of the population (range 0–5) and using the STOPP‐2 criteria, misuse was identified in 56.1% (range 0–6).
In 17.1% of the population, no underuse or misuse was found. Only underuse was present in 26.8% and only misuse in 15.9%. The combination of underuse with misuse was present in 40.2% of the population (of which 31.4% had polypharmacy and 8.7% low medication use).
The most prevalent criterion for underuse was the absence of an angiotensin converter enzyme inhibitor in patients with systolic heart failure (26%) and the absence of antiplatelet therapy in patients with documented coronary, cerebral or peripheral vascular disease (24%). The most prevalent criterion for misuse (35%) was the intake of benzodiazepines for longer than 4 weeks (see box 2 for the prevalence of other criteria).
Box 2. Flowchart for the rationale for exclusion of STOPP/START‐2 criteria.
| Inappropriate prescribing | Most identified | % |
|---|---|---|
| Underuse | Angiotensin converting enzyme (ACE) inhibitor with systolic heart failure and/or documented coronary artery disease | 26.2 |
| Antiplatelet therapy (aspirin or clopidogrel or prasugrel or ticagrelor) with a documented history of coronary, cerebral or peripheral vascular disease | 24.3 | |
| Statin therapy with a documented history of coronary, cerebral or peripheral vascular disease, unless the patient's status is end‐of‐life or age is >85 years | 14.9 | |
| Regular inhaled β2‐adrenoceptor agonist or antimuscarinic bronchodilator (e.g. ipratropium, tiotropium) for mild to moderate asthma or COPD | 10.5 | |
| Vitamin D and calcium supplement in patients with known osteoporosis and/or previous fragility fracture(s) and/or (bone mineral density T‐scores more than −2.5 in multiple sites)* | 9.1 | |
| Misuse | Benzodiazepines for ≥4 weeks | 35.2 |
| Any duplicate drug class prescription e.g. two concurrent NSAIDs, SSRIs, loop diuretics, ACE inhibitors, anticoagulants | 12.5 | |
| Antimuscarinic drugs with dementia, or chronic cognitive impairment or narrow‐angle glaucoma or chronic prostatism** | 10.7 | |
| Use of regular (as distinct from p.r.n.) opioids without concomitant laxative (risk of severe constipation) | 7.8 | |
| Concomitant use of two or more drugs with antimuscarinic/anticholinergic properties | 3.4 |
*Only the clinical indicator osteoporosis could be used. Fragility fractures and bone mineral density scores were not available. **The clinical indicator dementia was an exclusion criteria for this cohort.
Association of inappropriate prescribing with the amount of medications taken
The Spearman rank correlation between the number of medications taken, underuse and misuse is shown in Table 2. The number of medications showed a high positive correlation with misuse (r s 0.51, P < .001), and with underuse (r s 0.26, P < .001). Moreover, there was also a statistically significant correlation between underuse and misuse, in the positive direction (r s 0.19, P < .001).
Table 2.
Description of the medication use and level of inappropriate prescribing
| Description of the medication use | Mean (range) | |
|---|---|---|
| Medication use | 5.4 (0–16) | |
| Underuse | 1.2 (0–5) | |
| Misuse | 0.9 (0–6) | |
| % | ||
| Polypharmacy (≥five drugs daily) | 57.7 | |
| ATC C ‐ Cardiovascular | 86.3 | |
| ATC B ‐ Blood and blood forming | 56.1 | |
| ATC N ‐ Nervous system | 54.5 | |
| ATC A ‐ Alimentary tract and metabolism | 50.1 | |
| ATC M ‐ Musculo‐skeletal system | 23.5 | |
| ATC R ‐ Respiratory system | 15.9 | |
| ATC H ‐ Systemic hormonal preparations | 11.7 | |
| ATC G ‐ Genito‐urinary system and sex hormones | 10.3 | |
| Inappropriate prescribing | Underuse % | Misuse % |
|---|---|---|
| 0 | 33.0 | 43.9 |
| 1–2 | 52.7 | 46.7 |
| 3 or more | 14.3 | 9.3 |
| Combinations | Low medication use (0–4), in % | Polypharmacy (5 or more), in % |
|---|---|---|
| No misuse or underuse | 12.5 | 4.6 |
| Only underuse | 15.3 | 11.5 |
| Only misuse | 5.8 | 10.1 |
| Underuse and misuse | 8.7 | 31.4 |
| Correlations a | r s (P value) | |
|---|---|---|
| Underuse * Misuse | .19 (<0.001) | |
| Underuse * Number of medications | .26 (<0.001) | |
| Misuse * Number of medications | .51 (<0.001) |
All variables are expressed as continuous variables
Survival analysis of inappropriate prescribing on mortality and hospitalization
The mortality rate after18 months was 8.9% (n = 45) and the hospitalization rate 31% (n = 156). Causes of death included cardiovascular and/or cerebrovascular related events (48.9% of deaths), cancer (20.0%),respiratory related events (13.3%) or general deterioration (6.7%).
The survival analysis showed a significant difference between different categories of underuse for both mortality and hospitalization (log rank P < 0.001). The survival rates for mortality after 18 months for those with no, low (1–2) and high underuse (3 or more) were, respectively, 97%, 96% and 88% (see Figure 1). The survival rates for hospitalization after 1 year were, respectively, 85%, 81% and 59% (see Figure 2).
Figure 1.
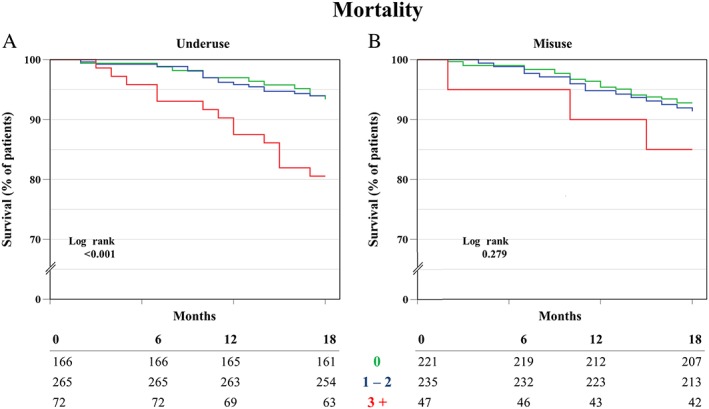
Kaplan‐Meier Survival analysis of time to death for groups of underuse (A), and groups of misuse (B)
Figure 2.
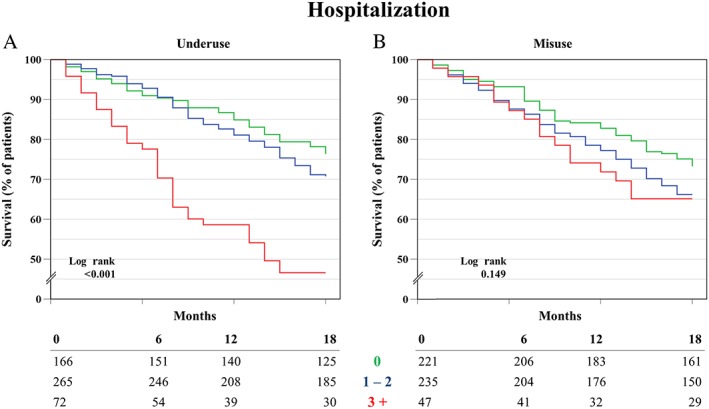
Kaplan‐Meier survival analysis of time to first hospitalization for groups of underuse (A), and groups of misuse (B)
For misuse, no significant difference was found for both outcomes.
Univariate analysis for the impact of inappropriate prescribing
In our previous analysis of polypharmacy, we observed a significant association of the number of medications with mortality and with hospitalization 28. Here, we also looked concomitantly at the additional effects of underuse and misuse (see Table 3). For mortality, underuse expressed as a continuous variable, showed an increased risk (HR 1.43, 95% CI 1.15, 1.78). In categorical analysis, patients with high underuse (three or more) had a 3.3 fold significantly increased risk for mortality compared with those with no underuse. Misuse did not show a significant association with mortality (see Table 3).
Table 3.
Univariate Cox regression analysis of mortality (8.9%) and hospitalization (31.0%) in association with inappropriate prescribing in a cohort of oldest old (n = 503)
| Mortality | Hospitalization | |||
|---|---|---|---|---|
| Continuous | Range | HR (95% CI) | HR (95% CI) | |
| Number of mediations | 0–21 | 1.12 (1.02, 1.22) | 1.14 (1.08, 1.20) | |
| Underuse | 0–5 | 1.43 (1.15, 1.78) | 1.35 (1.19, 1.54) | |
| Misuse | 0–6 | 1.16 (0.92, 1.47) | 1.20 (1.06, 1.36) | |
| Interaction effects | ||||
| Underuse*Misuse | 0–24 | 1.07 (1.00, 1.15) | 1.08 (1.04, 1.12) | |
| Categorical | Cut‐offs | n | MortalityHR (95% CI) | Hospitalization HR (95% CI) |
|---|---|---|---|---|
| Underuse | 0 | 166 | 1 | 1 |
| 1–2 | 265 | .89 (.43, 1.86) | 1.17 (.81, 1.71) | |
| 3 or more | 72 | 3.33 (1.58, 7.04) | 2.79 (1.79, 4.34) | |
| Misuse | 0 | 221 | 1 | 1 |
| 1–2 | 235 | 1.52 (0.80, 2.90) | 1.33 (0.95, 1.86) | |
| 3 or more | 47 | 1.95 (0.7, 5.03) | 1.49 (0.87, 2.55) |
The associations of inappropriate prescribing were first tested, using the continuous variables for underuse and misuse. Using categorical analysis, trends were explored for a higher risk for mortality or hospitalization with a higher degree of underuse or misuse
For hospitalization, underuse expressed as a continuous variable showed an increased risk as well (HR 1.35, 95% CI 1.19, 1.54). In categorical analysis, patients with high underuse (3 or more) had a 2.8 fold significantly increased risk for being hospitalized, compared with those with no underuse. Misuse, yet only when expressed by the continuous variable, showed an increased risk for hospitalization (HR 1.20, 95% CI 1.06, 1.36), but not for mortality.
The interaction effect (multiplying underuse with misuse, range 0–24) was significant as well, for both mortality (HR 1.07, 95% CI 1.00, 1.15, P = 0.044) and hospitalization (HR 1.08, 95% CI 1.04, 1.12).
Multivariate analysis for the impact of inappropriate prescribing
The results of the multivariate analysis are shown in Table 4. After correction for the number of medications and for the number of misused medications, underuse. expressed continuously and categorically, showed significant increased risks for mortality and hospitalization. For every additional underused medication at baseline we observed a 39% increased risk for mortality and a 26% increased risk for hospitalization after 18 months. Compared with those with no underuse, those with high underuse (three or more) showed a 2.9 fold increased risk for mortality and a 2.1 fold risk for hospitalization.
Table 4.
Multivariate Cox regression analysis of mortality (8.9%) and hospitalization (31.0%) in association with inappropriate prescribing in a cohort of oldest old (n = 503)
| Mortality | Hospitalization | |||
|---|---|---|---|---|
| Continuous | Range | HR (95% CI) | HR (95% CI) | |
| Underuse | 0–5 | 1.39 (1.10, 1.76)a | 1.26 (1.10, 1.45)a | |
| Misuse | 0–5 | 0.93 (0.69, 1.24)b | .98 (.84, 1.14)b |
| Categorical | Range | n | Mortality HR (95% CI) | Hospitalization HR (95% CI) |
|---|---|---|---|---|
| Underuse | 0 | 166 | 1 | 1 |
| 1–2 | 265 | .88 (0.41, 1.90) | 1.04 (.71, 1.53) | |
| 3+ | 72 | 2.91 (1.28, 6.61)a | 2.08 (1.29, 3.36)a | |
| Misuse | 0 | 221 | 1 | 1 |
| 1–2 | 235 | 1.16 (0.58, 2.34) | .96 (.67, 1.38) | |
| 3+ | 47 | 1.07 (0.36, 3.17)b | .74 (.41, 1.36)b |
The associations of inappropriate prescribing was first tested using the continuous variables for underuse and misuse. Using categorical analysis, trends were explored for a higher risk for mortality or hospitalization with a higher degree of underuse or misuse
Underuse was corrected for the number of medications and for the number of misused medications
Misuse was corrected for the number of medications and for the number of underused medications
Misuse, after controlling for the number of medications and underuse, did not show significant associations with both mortality and hospitalization.
Discussion
To the best of our knowledge, this study is the first prospective longitudinal cohort study of community‐dwelling older adults, aged 80 years and more, exploring the associations of inappropriate prescribing with mortality and hospitalization, using a computerized version of the STOPP/START–2 criteria.
Main findings
First, we observed a high prevalence of polypharmacy (58%), concurrent with a high prevalence of underuse (67%) and misuse (56%). The combination of polypharmacy, underuse and misuse was present in 31% of the population. Only in 9% of the population, no polypharmacy, no underuse and no misuse were observed.
Second, the Spearman rank correlations suggest that the number of medications were positively correlated with the number of misused medications and also with the number of underused medications.
Lastly, our main finding is that every additional underused medication was associated with a relative increase in mortality rate of 39% and in a hospitalization rate of 26% after 18 months, independent of the number of medications taken and of the number of misused medications.
Limitations of this study
Results of this observational study do not allow causal relations. The relation between inappropriate prescribing and mortality and hospitalization was established out of the proof of a (chronic) inappropriate medication intake throughout the study period. Also, the results cannot be generalized beyond the population of cognitive fit community‐dwelling older persons.
The negative results need to be interpreted with caution, especially the absence of associations with misuse, as the sample size may have resulted in underpowered statistical analysis for this aspect. Additionally, we did not use the full STOPP/START–2 criteria, only those that were applicable in our database and suitable for the computerized evaluation. Also, other authors have made partial use of the STOPP/START criteria for pragmatic reasons 29. However, the criteria applied in this study matched with the most prevalent criteria in other studies 10, 19, 30, 31, 32, 33. Nevertheless, the true prevalence of inappropriate prescribing could have been underestimated in this study. To check this issue for misuse, we repeated the same analysis with the medication only EU(7)‐PIM list 34, also focussing on misuse. Again, only in univariate analysis, we observed a limited association of misuse with hospitalization and not with mortality. All associations of misuse disappeared after entering the number of medications and underuse into the multivariate model.
In our database of prescriptions, over the counter drugs were not included, also possibly underestimating the prevalence of misuse.
Comparison with other findings
In our study, there was a high prevalence of polypharmacy (58%), underuse (67%) and misuse (56%). Interpretation and comparison of the prevalence of inappropriate prescribing must be done with caution, since most studies either used younger aged populations or used the STOPP/START–1 criteria. In other studies, underuse ranged between 23–58% 10, 18, 35, 36, 37 and misuse ranged 21–60% 17, 18, 36, 37, 38. For underuse, our results were over the upper limit of this range and the results for misuse were close to the upper limit of the range.
Cross‐sectional studies focussing on younger age groups and using the Beers 39, 40, 41 or STOPP/START–1 33 criteria have shown higher prevalence of inappropriate prescribing in those who were hospitalized. Associations with mortality have been observed as well, although only in older hip fracture patients 42.
Comparison with the scarce existing longitudinal cohort studies is difficult, as these studies focussed on younger adults (65 years and over), on those in nursing homes, or studied other outcomes such as adverse drug events, economic costs, or geriatric syndromes (falls) 12, 13, 14.
The impact of underuse has also been observed in another cohort, focussing on cardiovascular patients (aged 50–74 years) 15, 43, 44.
Implications for research
This study clearly indicated that higher underuse was associated with higher mortality and with higher hospitalization rate. As this observational study allows no causal inference, we can only formulate hypotheses for further research.
The results of this study suggest that the underuse of medications, next to polypharmacy, was strongly associated with outcomes. An explanation could be the reluctance of GPs to prescribe additional medications in patients with a high multimorbidity and polypharmacy 45, 46 or of a possible aversion of patients for new therapies. The lack of clear evidence of some pharmacotherapies in the oldest age groups may explain reluctance of GPs to adhere to general treatment recommendations in this age group 47. However, most of the START criteria are evidence‐based and should not be overridden.
In addition, deprescribing or not starting medications might be caused by a perception of futility in the face of approaching death in this population. In case this clinical perception is true, this could lead to a higher morbidity in the group of those with underuse, making mortality more the cause rather than the consequence of underuse. However, it should be remembered that this cohort was limited to community‐dwelling active and cognitively fit oldest old, not in palliative care. Another hypothesis could be that substandard prescribing in older adults is a physician trait 48 and an instrumental variable that leads to a combination of polypharmacy, underuse, misuse and higher mortality/hospitalization.
Applicability of the STOPP/START criteria in a particular patient has until now most often been based on the human judgement of a clinical pharmacologist (or similar). Our study indicates that the electronic application of the STOPP/START–2 criteria is feasible, but that further specification of clinical problems and medication groups in the light of computerization is needed 49. Large scale application on big data will need substantial progress in semantic interoperability of clinical data in heterogeneous electronic health records 49, 50, 51.
Implications for practice
The interpretation and transferability of the results to other care settings or other patients must be done with caution. The Belfrail‐med cohort excluded those in nursing homes, those with known dementia and those in palliative care. These community‐dwelling oldest old patients can be considered as the most active and healthy in this age segment.
The findings of our study are in favour of using the STOPP/START–2 criteria in clinical practice or for education purposes of clinicians. They are adapted to European medication markets and can detect underuse.
Using a cut‐off for polypharmacy, with a simple arbitrary point (e.g. ≥ five medications) or as a sole indicator for quality is problematic. Polypharmacy can be a risk for worse outcomes, even when all prescribed medications are justified. In this study, underuse of medications that should have been prescribed for a specific indication may also be hazardous. Our present and previous results indicate that a more patient‐tailored approach is needed to solve this dilemma 28. The discussion on too much medication or too unsafe needs more differentiation and a clear assessment of misuse and underuse using full knowledge on the patient, his/her comorbidities and his/her medications. Computerization of the analysis of medication lists should be considered as a facilitator of the data collection process and the medication chart review, but not as a substitute for assessment of the pharmacological therapy of an individual patient.
In conclusion, inappropriate prescribing (polypharmacy, underuse and misuse) was highly prevalent in community‐dwelling adults, aged 80 years and older. Underuse and misuse were highly correlated and coexisted in almost half of the population. Surprisingly, underuse and not misuse had strong associations with mortality and hospitalization, even when controlling for polypharmacy and misuse. Incentives towards patient‐tailored appropriate prescribing in older adults are needed, taking the number of medications, underuse and misuse into account.
Competing Interests
All authors declare no conflict of interest. All authors have completed the ICMJE uniform disclosure form. There was no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The original BELFRAIL study (B40320084685) was supported by an unconditional grant from the Foundation Louvain. The Foundation Louvain is the support unit of the Université catholique de Louvain and is responsible for developing the education and research projects of the university by soliciting gifts from corporations, foundations and alumni.
Contributors
All authors contributed to this work. B.V., J.D. and O.D. were responsible for conducting the Belfrail study, including data collection. M.A. entered the medication data. M.W. was responsible for analyzing and writing this paper. T.C., R.V.S., M.E. and M.A. contributed in the statistical analysis, interpreting and discussing the results and writing of the paper as well. All authors were responsible for revising this work critically for important intellectual content. All authors are accountable for all aspects of the work.
Supporting information
Addendum S1 STOPP/START–2 criteria that were and were not applied (inclusive rationale for omitting criteria)
Supporting info item
Wauters, M. , Elseviers, M. , Vaes, B. , Degryse, J. , Dalleur, O. , Vander Stichele, R. , Christiaens, T. , and Azermai, M. (2016) Too many, too few, or too unsafe? Impact of inappropriate prescribing on mortality, and hospitalization in a cohort of community‐dwelling oldest old. Br J Clin Pharmacol, 82: 1382–1392. doi: 10.1111/bcp.13055.
Footnotes
Source: https://www.ehealth.fgov.be
References
- 1. Laroche M‐L, Charmes J‐P, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol 2007; 63: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 2010; 107: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Prescribing in elderly people. 1. Appropriate prescribing in elderly people : how well can it be measured and optimised? Lancet 2007; 370: 173–184. [DOI] [PubMed] [Google Scholar]
- 4. Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol 2014; 77: 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med 1991; 151: 1825–1832. [PubMed] [Google Scholar]
- 6. Page RL, Linnebur SA, Bryant LL, Ruscin JM. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clin Interv Aging 2010; 5: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher P, Barry P, O'Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther 2007; 32: 113–121. [DOI] [PubMed] [Google Scholar]
- 8. Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol 2014; 70: 1–11. [DOI] [PubMed] [Google Scholar]
- 9. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel . American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 2: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laroche M‐L, Charmes J‐P, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 2007; 63: 725–731. [DOI] [PubMed] [Google Scholar]
- 12. Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 13. Hamilton H, Gallagher P, Ryan C, Byrne S, O'Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 2011; 171: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 14. García‐Gollarte F, Baleriola‐Júlvez J, Ferrero‐López I, Cuenllas‐Díaz Á, Cruz‐Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc 2014; 15: 885–891. [DOI] [PubMed] [Google Scholar]
- 15. Meid AD, Quinzler R, Freigofas J, Saum K‐U, Schöttker B, Holleczek B, et al. Medication underuse in aging outpatients with cardiovascular disease: prevalence, determinants, and outcomes in a prospective cohort study. PLoS One 2015; 10: e0136339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moriarty F, Bennett K, Fahey T, Kenny RA, Cahir C. Longitudinal prevalence of potentially inappropriate medicines and potential prescribing omissions in a cohort of community‐dwelling older people. Eur J Clin Pharmacol 2015; 71: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahir C, Bennett K, Teljeur C, Fahey T. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol 2014; 77: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalleur O, Boland B, De Groot A, Vaes B, Boeckxstaens P, Azermai M, et al. Detection of potentially inappropriate prescribing in the very old: cross‐sectional analysis of the data from the BELFRAIL observational cohort study. BMC Geriatr 2015; 15: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community‐dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol 2015; 1415–27. [DOI] [PubMed] [Google Scholar]
- 20. Vaes B, Pasquet A, Wallemacq P, Rezzoug N, Mekouar H, Olivier P‐A, et al. The BELFRAIL (BFC80+) study: a population‐based prospective cohort study of the very elderly in Belgium. BMC Geriatr 2010; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wauters M, Elseviers M, Vaes B, Degryse J, Dalleur O, Vander Stichele R, et al. Polypharmacy in a Belgian cohort of community‐dwelling oldest old (80+). Acta Clin Belg 2016; 71: 158–166. [DOI] [PubMed] [Google Scholar]
- 22. WHO . The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Oslo, Norway: World Health Organization. [Google Scholar]
- 23. Veehof LJG, Stewart RE, Haaijer‐Ruskamp FM, Meyboom‐de Jong B. The development of polypharmacy. Fam Pract 2000; 17: 261–267. [DOI] [PubMed] [Google Scholar]
- 24. Levy HB, Marcus E‐L, Christen C. Beyond the Beers criteria: A comparative overview of explicit criteria. Ann Pharmacother 2010; 44: 1968–1975. [DOI] [PubMed] [Google Scholar]
- 25. Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 26. Hill‐Taylor B, Sketris I, Hayden J, Byrne S, O'Sullivan D, Christie R. Application of the STOPP/START criteria: A systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharmacol Ther 2013; 38: 360–372. [DOI] [PubMed] [Google Scholar]
- 27. Ryan C, O'Mahony D, Byrne S. Application of STOPP and START criteria: interrater reliability among pharmacists. Ann Pharmacother 2009; 43: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 28. Wauters M, Elseviers M, Vaes B, Degryse J, Vander Stichele R, Christiaens T, et al. Mortality, hospitalisation, and institutionalisation in a community‐dwelling cohort of oldest old: the impact of medication. Arch Gerontol Geriatr 2016; 65: 9–16. [DOI] [PubMed] [Google Scholar]
- 29. Bjerre LM, Ramsay T, Cahir C, Ryan C, Halil R, Farrell B, et al. Assessing potentially inappropriate prescribing (PIP) and predicting patient outcomes in Ontario's older population: a population‐based cohort study applying subsets of the STOPP/START and Beers' criteria in large health administrative databases. BMJ Open 2015; 5: e010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011; 89: 845–854. [DOI] [PubMed] [Google Scholar]
- 31. San‐José A, Agustí A, Vidal X, Formiga F, Gómez‐Hernández M, García J, et al. Inappropriate prescribing to the oldest old patients admitted to hospital: prevalence, most frequently used medicines, and associated factors. BMC Geriatr 2015; 15: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lang PO, Hasso Y, Dramé M, Vogt‐Ferrier N, Prudent M, Gold G, et al. Potentially inappropriate prescribing including under‐use amongst older patients with cognitive or psychiatric co‐morbidities. Age Ageing 2010; 39: 373–381. [DOI] [PubMed] [Google Scholar]
- 33. Dalleur O, Spinewine A, Henrard S, Losseau C, Speybroeck N, Boland B. Inappropriate prescribing and related hospital admissions in frail older persons according to the STOPP and START criteria. Drugs Aging 2012; 29: 829–837. [DOI] [PubMed] [Google Scholar]
- 34. Renom‐Guiteras A, Meyer G, Thürmann PA. The EU(7)‐PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015; 71: 861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barry PJ, Gallagher P, Ryan C, O'mahony D. START (screening tool to alert doctors to the right treatment)‐‐an evidence‐based screening tool to detect prescribing omissions in elderly patients. Age Ageing 2007; 36: 632–638. [DOI] [PubMed] [Google Scholar]
- 36. Ryan C, O'Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009; 68: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vezmar Kovačević S, Simišić M, Stojkov Rudinski S, Ćulafić M, Vučićević K, Prostran M, et al. Potentially inappropriate prescribing in older primary care patients. Quinn TJ, editor. PLoS One 2014; 9: e95536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wahab MSA, Nyfort‐Hansen K, Kowalski SR. Inappropriate prescribing in hospitalised Australian elderly as determined by the STOPP criteria. Int J Clin Pharmacol 2012; 34: 855–862. [DOI] [PubMed] [Google Scholar]
- 39. Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health 2008; 31: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs Aging 2010; 27: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dedhiya SD, Hancock E, Craig BA, Doebbeling CC, Thomas J. Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother 2010; 8: 562–570. [DOI] [PubMed] [Google Scholar]
- 42. Gosch M, Wörtz M, Nicholas JA, Doshi HK, Kammerlander C, Lechleitner M. Inappropriate prescribing as a predictor for long‐term mortality after hip fracture. Gerontology 2014; 60: 114–122. [DOI] [PubMed] [Google Scholar]
- 43. Meid AD, Lampert A, Burnett A, Seidling HM, Haefeli WE. The impact of pharmaceutical care interventions for medication underuse in older people: a systematic review and meta‐analysis. Br J Clin Pharmacol 2015; 80: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meid AD, Haefeli WE. Age‐Dependent Impact of Medication Underuse and Strategies for Improvement. Gerontology. Karger Publishers; 2016; 62: 491–499. [DOI] [PubMed] [Google Scholar]
- 45. Viktil KK, Blix HS, Reikvam Å. The Janus face of polypharmacy – overuse versus underuse of medication. Norsk Epidemiologi 2008; 147–152. [Google Scholar]
- 46. Ko DT, Mamdani M, Alter DA. Lipid‐lowering therapy with statins in high‐risk elderly patients: the treatment‐risk paradox. JAMA 2004; 291: 1864–1870. [DOI] [PubMed] [Google Scholar]
- 47. Topinková E, Baeyens JP, Michel J‐P, Lang P‐O. Evidence‐based strategies for the optimization of pharmacotherapy in older people. Drugs Aging 2012; 29: 477–494. [DOI] [PubMed] [Google Scholar]
- 48. Huybrechts KF, Gerhard T, Franklin JM, Levin R, Crystal S, Schneeweiss S. Instrumental variable applications using nursing home prescribing preferences in comparative effectiveness research. Pharmacoepidemiol Drug Saf 2014; 23: 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Groot DA, de Vries M, Joling KJ, van Campen JPCM, Hugtenburg JG, van Marum RJ, et al. Specifying ICD9, ICPC and ATC codes for the STOPP/START criteria: a multidisciplinary consensus panel. Age Ageing 2014; 43: 773–778. [DOI] [PubMed] [Google Scholar]
- 50. Dentler K, Numans ME, ten Teije A, Cornet R, de Keizer NF. Formalization and computation of quality measures based on electronic medical records. J Am Med Inform Assoc 21: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moreno‐Conde A, Moner D, da Cruz WD, Santos MR, Maldonado JA, Robles M, et al. Clinical information modeling processes for semantic interoperability of electronic health records: systematic review and inductive analysis. J Am Med Inform Assoc 2015; 22: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Addendum S1 STOPP/START–2 criteria that were and were not applied (inclusive rationale for omitting criteria)
Supporting info item


