Abstract
Objective
The present study investigated whether the glycoprotein (GP)IIb/IIIa receptor blocker abciximab might be a successful bridging strategy to achieve adequate levels of platelet inhibition rapidly in cases where prasugrel is used in morphine‐pretreated ST‐elevation myocardial infarction (STEMI) patients.
Methods
In a prospective observational cohort study, 32 patients presenting with STEMI were given prasugrel at a loading dose of 60 mg. Patients were stratified into four groups, according to morphine and/or abciximab use. Adenosine diphosphate (ADP)‐induced platelet aggregation was measured at four time points: at baseline, and at 2 h, 1 day and 2 days after prasugrel loading.
Results
Morphine use was associated with a three‐fold higher level of ADP‐induced platelet aggregation 2 h after prasugrel loading compared with no morphine/no abciximab (P = 0.019). However, when abciximab was infused in the catheterization laboratory, the effect of morphine on ADP‐induced platelet aggregation disappeared (P = 0.884). This interaction was also seen in the presence of high on‐treatment platelet reactivity (HTPR) at 2 h; while HTPR was seen in 88% of morphine users/no abciximab users, it was found in only 17–20% in the three other groups (P = 0.003). The effect of morphine disappeared by day 1 – 2.
Conclusion
The infusion of the GPIIb/IIIa receptor blocker abciximab allows immediate and efficient platelet inhibition in STEMI patients concomitantly receiving the oral ADP receptor blocker prasugrel and morphine.
Keywords: abciximab, morphine, platelet, prasugrel
What is Already Known about this Subject
A drug–drug interaction between morphine and the three P2Y12 receptor inhibitors clopidogrel, prasugrel and ticagrelor has been reported.
GPIIb/IIIa receptor blockers are rapidly acting drugs, with a potent inhibitory effect on the final common pathway of platelet aggregation, and also on signalling events downstream of ADP‐induced platelet aggregation.
What this Study Adds
A single dose of intravenous morphine was associated with a delay in the onset of prasugrel action.
The morphine–prasugrel interaction was apparent at an early stage (at 2 h) after prasugrel administration, and did not persist for longer than 1 day after a single morphine administration.
Abciximab reduced platelet aggregation during a primary percutaneous coronary intervention when morphine was administered concomitantly with prasugrel, and might be a useful bridging strategy to improve platelet inhibition in such patients.
Introduction
The concomitant administration of multiple drugs is common in patients presenting with ST‐elevation myocardial infarction (STEMI), which could lead to drug–drug interactions. Some of these interactions may occur for P2Y12 receptor inhibitors, with the possible consequence of insufficient inhibition of platelet function in a setting where maximum inhibition would be desirable 1, 2.
Morphine, recommended for pain relief, is frequently used in STEMI patients. Recently, an interaction between morphine and the P2Y12 receptor inhibitors clopidogrel, prasugrel and ticagrelor has been reported 3, 4, 5, 6. This is probably due to the morphine‐induced inhibition of gastric emptying, which might delay the absorption of other oral drugs, prolonging the time to reach peak concentration (Tmax) and therefore slowing their onset of action 7.
Intravenous glycoprotein (GP) IIb/IIIa receptor blockers are also commonly used in patients with STEMI. GPIIb/IIIa receptor blockers are rapidly acting drugs, with a potent inhibitory effect on the final common pathway of platelet aggregation, and also on signalling events downstream of ADP‐induced platelet aggregation 8. Therefore, the administration of intravenous GPIIb/IIIa receptor blockers may overcome a delayed onset of action of orally administered drugs, including P2Y12 receptor blockers, in the setting of morphine coadministration. Against this background, we aimed to investigate whether the concomitant use of prasugrel and abciximab might be a successful bridging strategy to achieve sufficient inhibition of platelet aggregation rapidly in cases where prasugrel is used in morphine‐pretreated STEMI patients undergoing primary percutaneous coronary intervention (PCI).
Methods
Study design
This prospective observational cohort study was performed at the Medical University of Vienna. The ethics committee of the Medical University of Vienna approved the study protocol in accordance with the Declaration of Helsinki. Participants were recruited into the study between May 2013 and June 2015. Inclusion criteria comprised the provision of written informed consent before study entry, treatment with an in‐hospital loading dose of prasugrel for an acute STEMI, receipt of primary PCI and being aged >18 years. The exclusion criterion was participation in interventional trials. Thirty‐two consecutive patients fulfilling the inclusion and exclusion criteria were enrolled. All patients received a loading dose of prasugrel (60 mg) in the emergency department or in the catheter laboratory followed by a once‐daily dose of 10 mg prasugrel. Intravenous morphine was administered in the ambulance before reaching the hospital or in the emergency department, and its use was at the discretion of the treating physician. An abciximab bolus was administered in the catheterization laboratory according to the current recommendations, at the discretion of an interventional cardiologist, and maintained for 12 h as a continuous infusion according to the label. Intravenous aspirin (250 mg) was administered to all patients during the prehospital phase. Blood samples were obtained from patients at baseline (before the loading dose of prasugrel), and then after 2 h, 1 day and 2 days at 8 a.m. (Figure 1A).
Figure 1.
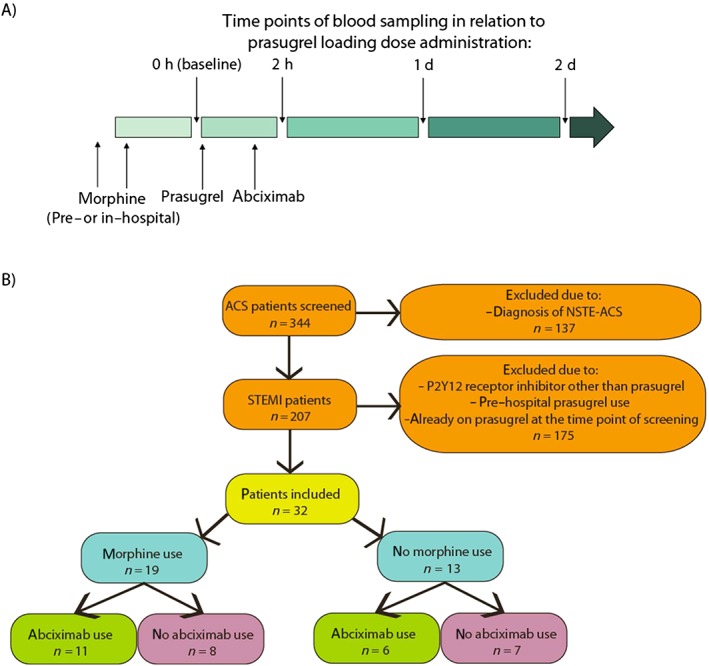
Study design. (A) Time points of drug administration and blood sampling. (B) Flow of participants in the study. ACS, acute coronary syndrome; d, day; NSTE‐ACS, non ST‐elevation ACS
Impedance aggregometry
Whole‐blood aggregation was determined using multiple electrode aggregometry on a new‐generation impedance aggregometer (Multiplate Analyzer, Roche, Munich, Germany). The system detects the change in electrical impedance due to the adhesion and aggregation of platelets on two independent electrode‐set surfaces in the test cuvette, as described 9, 10, 11. We used hirudin as anticoagulant, and adenosine diphosphate (ADP) and arachidonic acid (AA) as agonists. A 1:2 dilution of whole blood anticoagulated with hirudin and 0.9% NaCl was stirred at 37 °C for 3 min in the test cuvettes; ADP (6.4 μM) or AA (0.5 mM) was then added and the increase in electrical impedance was recorded continuously for 6 min 12, 13. The mean values of the two independent determinations were expressed as U (units). According to the position documents, values >46 U were assigned as high on‐treatment platelet reactivity (HTPR) 1, 14, 15.
Study endpoints
The primary endpoint was ADP‐induced platelet aggregation. For descriptive purposes, the incidence of myocardial infarction and major cardiac adverse events (MACE: myocardial infarction, cardiac death and stroke) was recorded and reported during the hospital stay. Myocardial infarction was defined according to the universal definition 16.
Statistical analysis
Based on threefold higher ADP aggregation values in morphine users compared with non–users, 2 h after clopidogrel loading [standard deviation (SD) = 10) 3, we estimated that, with 28 patients (seven patients per group), our study had 80% power to detect significant differences (two‐sided P value <0.05). Normal distribution was tested using the Kolmogorov–Smirnov test. Data were expressed as mean, SD, 95% confidence intervals (CIs), median or interquartile range (IQR), as appropriate. Statistical comparisons were performed using the Mann–Whitney U test, paired Wilcoxon test and χ2 test when applicable. All statistical calculations were performed using the commercially available SPSS Version 21.0 statistical software (IBM, Armonk, NY, USA).
Results
Patient demographics
Patient flow through the study is shown in Figure 1B. Of 344 acute coronary syndrome patients screened, 32 fulfilled the inclusion criteria and were included in the study. The majority of individuals had the typical risk factors associated with STEMI: hypertension (62%), hyperlipidaemia (35%), smoking (86%) and diabetes mellitus (14%) (Table 1). Among included patients, 19 (59%) were treated with intravenous morphine (at doses: 10 mg in 17 patients, 5 mg in one and 15 mg in one) before prasugrel loading. There were no differences in demographic data between patients treated with and without morphine. Abciximab was used in 17 patients (57%) in the catheterization laboratory and its administration was distributed equally between patients treated with and without morphine. All patients underwent a successful primary PCI.
Table 1.
Patient demographics
| Patient demographics | Overall n = 32 | With morphine n = 19 | Without morphine n = 13 | P |
|---|---|---|---|---|
| Age (years) | 60 ± 11 | 58 ± 11 | 63 ± 10 | 0.17 |
| Gender (male) n (%) | 29 (93) | 18 (94) | 11 (84) | 0.69 |
| Risk factors/past medical history n (%) | ||||
| Hypertension | 18 (62) | 10 (56) | 8 (72) | 0.36 |
| Hyperlipidaemia | 10 (35) | 7 (39) | 3 (27) | 0.52 |
| Smoking | 25 (86) | 15 (83) | 10 (91) | 0.57 |
| Family history of CAD | 10 (34) | 9 (50) | 5 (38) | 0.25 |
| Diabetes mellitus | 4 (14) | 3 (17) | 1 (9) | 0.57 |
| Prior PCI | 4 (13) | 3 (18) | 1 (9) | 0.53 |
| Prior myocardial infarction | 6 (21) | 4 (22) | 2 (18) | 0.80 |
| Peripheral arterial occlusive disease | 3 (10) | 1 (6) | 2 (18) | 0.28 |
| Cerebrovascular disease | 1 (3) | 1 (5) | 0 (0) | 0.43 |
| Laboratory data (mean ± SD) | ||||
| Platelet counts (×10 9 l –1 ) | 224 ± 67 | 222 ± 73 | 229 ± 61 | 0.90 |
| C reactive protein (mg dl −1 ) | 0.83 ± 1.12 | 0.63 ± 1.14 | 1.16 ± 1.06 | 0.07 |
| White blood cell count (×10 9 l –1 ) | 12.26 ± 3.56 | 12.27 ± 3.89 | 12.24 ± 3.07 | 0.80 |
| Creatinine (mg dl −1 ) | 0.98 ± 0.27 | 1.02 ± 0.32 | 0.93 ± 0.18 | 0.64 |
| Haemoglobin (g dl −1 ) | 14.9 ± 1.03 | 14.9 ± 0.86 | 14.9 ± 1.33 | 0.55 |
| Fibrinogen (mg dl −1 ) | 384 ± 85 | 382 ± 76 | 386 ± 101 | 0.78 |
| Concomitant medications at 2 h n (%) | ||||
| Aspirin | 32 (100) | 19 (100) | 13 (100) | |
| Proton pump inhibitors | 23 (80) | 15 (83) | 8 (73) | 0.49 |
| β‐blockers | 23 (80) | 15 (83) | 8 (72) | 0.59 |
| Statins | 25 (86) | 16 (90) | 9 (82) | 0.49 |
| Angiotensin‐converting enzyme inhibitors | 23 (80) | 14 (78) | 9 (82) | 0.79 |
| Calcium channel blockers | 3 (10) | 1 (6) | 2 (18) | 0.28 |
| Angiographic data | ||||
| GPIIbIIIa blocker (abciximab) during the PCI | 17 (57) | 11 (60) | 6 (55) | 0.78 |
| Heparin | 32 (100) | 19 (100) | 13 (100) | |
| Bivalirudin | 0 (0) | 0 (0) | 0 (0) | |
| Primary PCI | 32 (100) | 19 (100) | 13 (100) | |
| Number of stents per patient | 1.57 ± 1.19 | 1.79 ± 1.31 | 1.18 ± 0.87 | 0.23 |
| Total stent length (mm) | 35.9636.0 ± 34.051 | 38.4 ± 40.0 | 30.8 ± 16.384 | 0.78 |
Data are reported as mean ± standard deviation (SD), n (number of patients) or percentages. CAD, coronary artery disease; GP, glycoprotein; PCI, percutaneous coronary intervention.
ADP‐induced platelet aggregation
In the overall population, the median level of ADP‐induced platelet aggregation decreased by 66% 2 h after prasugrel loading compared with baseline (27 U, IQR: 15–57 U vs. 80 U, IQR: 73–96, respectively; P < 0.001; Figure 2). Platelet aggregation achieved the lowest level on day 1 after loading (7 U, IQR: 3–12 U; 91% decrease compared with baseline; P < 0.001; 74% decrease compared with value at 2 h; P = 0.001), and stayed in the same range on day 2 after loading (15 U; IQR: 5–21; P = 0.235 compared with day 1; Figure 2).
Figure 2.
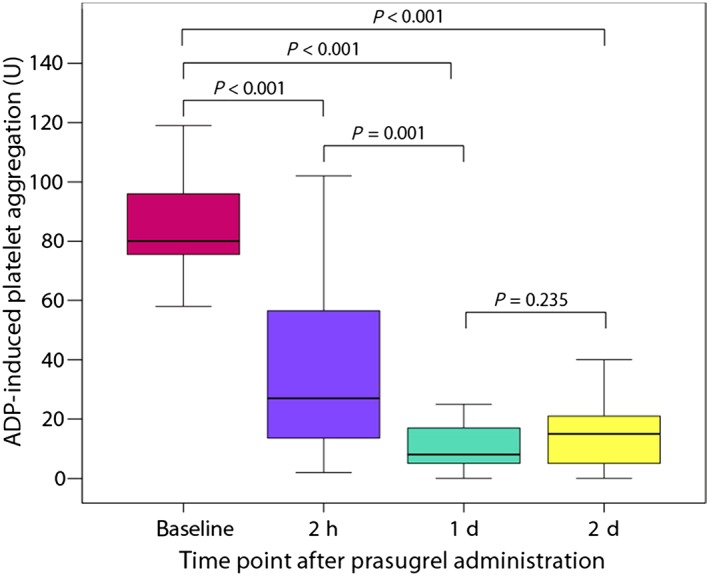
Adenosine diphosphate (ADP)‐induced platelet aggregation assessed by multiple electrode aggregometry in relation to the time of prasugrel loading. d, day
ADP‐induced platelet aggregation in relation to abciximab and morphine use
Patients were stratified into morphine users vs. non‐users, and patients with or without abciximab infusion. The use of morphine was associated with threefold higher median levels of ADP‐induced aggregation at 2 h compared with no morphine use among the non‐abciximab group (68 U; IQR: 50–94 vs. 23 U; IQR: 16–46; P = 0.019; Figure 3). By contrast, the infusion of abciximab appeared to counteract the negative effect of morphine (16 U; IQR: 8–34 vs. 16 U; IQR: 7–41; P = 0.884; Figure 3). This interaction was also prominent when HTPR rates at 2 h were taken into account: while patients who received morphine but not abciximab had an HTPR rate of 88%, the HTPR rate was only 17–20% in the three other groups (P = 0.003; Figure 4). The negative effect of morphine disappeared by day 1 – 2, independent of abciximab use (Figure 5).
Figure 3.
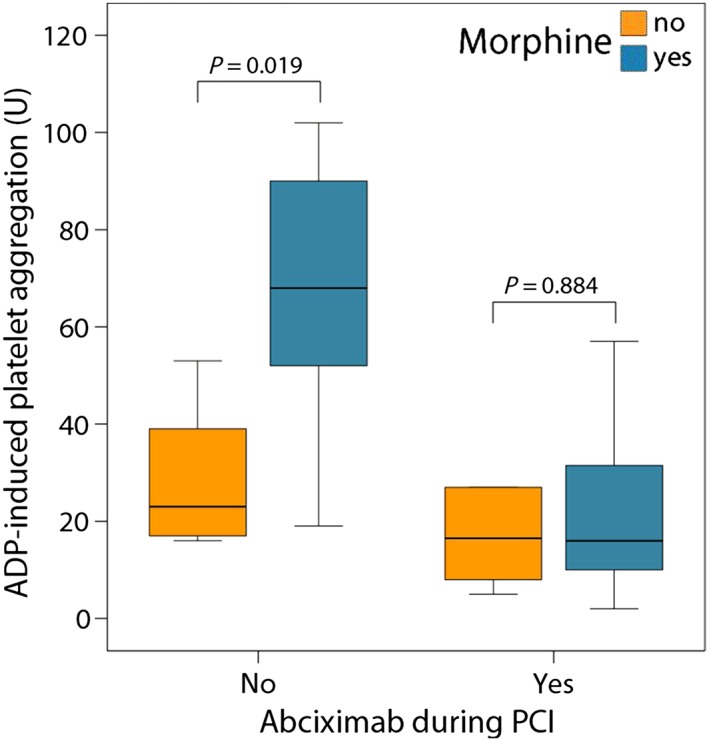
Adenosine diphosphate (ADP)‐induced platelet aggregation values stratified by morphine and abciximab administration, measured 2 h after prasugrel loading
Figure 4.
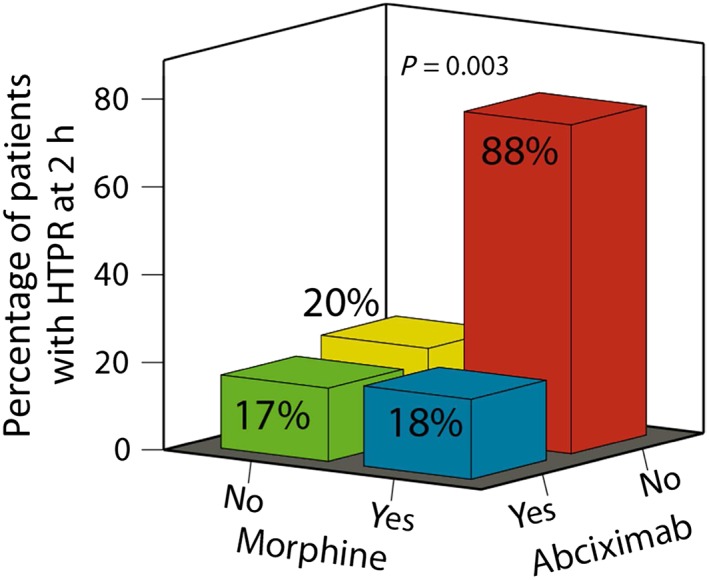
The proportion of patients with high on‐treatment platelet reactivity (HTPR) stratified by morphine and abciximab administration, measured 2 h after prasugrel loading
Figure 5.
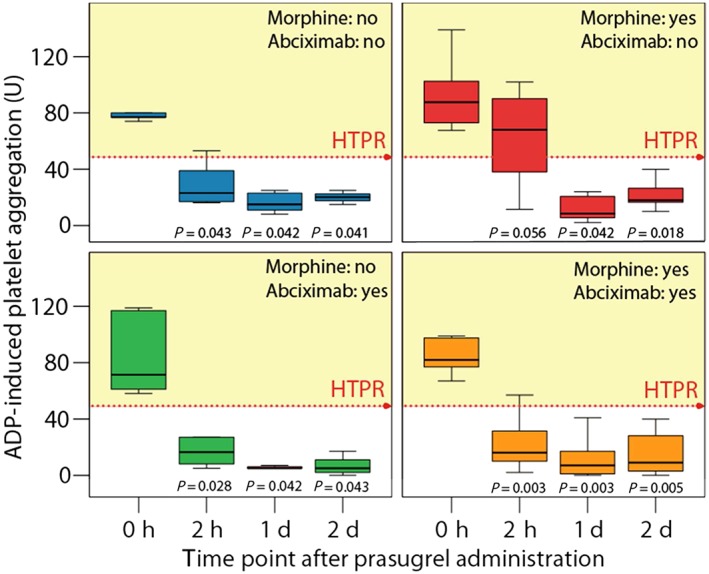
Adenosine diphosphate (ADP)‐induced platelet aggregation stratified by morphine and abciximab administration, measured at various time points after prasugrel loading. HTPR, high on‐treatment platelet reactivity. The P values given are for the comparison between baseline and other time points
AA‐induced platelet aggregation
As expected, morphine did not have an effect on AA‐induced platelet aggregation (data not shown), probably because aspirin given intravenously produces a rapid effect onset in patients with myocardial infarction 17.
In‐hospital outcome
During the hospital stay, no ischaemic events and no major bleeding events occurred. Minimal bleeding events (epistaxis and skin haematoma; n = 9) were reported predominantly in patients who received abciximab (n = 7 vs. n = 2 for abciximab‐ vs. no‐abciximab‐treated patients; P = 0.072).
Discussion
The central findings of the present study investigating the interaction between morphine, abciximab and prasugrel were:
A single dose of intravenous morphine was associated with a delayed onset of prasugrel action.
The morphine–prasugrel interaction was apparent early on (at 2 h) after prasugrel administration, and did not persist longer than 1 day after a single morphine administration.
Abciximab successfully reduced platelet aggregation for primary PCI when morphine was administered concomitantly with prasugrel, and might be a useful bridging strategy to improve platelet inhibition in such patients.
Decreased gastrointestinal absorption is the major limitation to the onset of action of orally administered antiplatelet agents in STEMI patients, and apparently cannot be overcome by increasing the loading doses of P2Y12 receptor inhibitors, as shown for ticagrelor 18, 19. The morphine–P2Y12 receptor inhibitor interaction has been proposed as an explanation for this phenomenon 3, 4, 5, 20, 21. The recently published randomized trial Influence of Morphine on Pharmacokinetics and Pharmacodynamics of Ticagrelor in Patients with Acute Myocardial Infarction (IMPRESSION) confirmed the negative impact of morphine on the pharmacokinetics and antiplatelet action of ticagrelor in patients presenting with an acute myocardial infarction 20. By contrast, 5 mg of morphine primarily reduced and delayed the early bioavailability of ticagrelor in healthy volunteers but had no measurable impact on ticagrelor effects 21. Concordantly, morphine reduced the maximal concentration of the prasugrel active metabolite by only 31% but did not delay the onset of action of prasugrel in healthy subjects 6. By contrast, morphine was associated with a reduction in the effects of prasugrel in patients with myocardial infarction, which has also been found in previous studies 4, 5. Therefore, it seems reasonable that the negative impact of morphine on the antiplatelet effect of ADP receptor blockers is more pronounced in patients with acute myocardial infarction who have compromised gastrointestinal perfusion due to cardiac ischaemia than in healthy volunteers.
The highest rate of HTPR was observed at 2 h after prasugrel loading, which indicates the external validity of our findings 4, 5. Morphine was significantly associated with HTPR, even after adjustment for other variables 5, 20. In line with these findings, the morphine–prasugrel interaction was apparent 2 h after prasugrel administration in the present study, but not beyond day 1, which is also consistent with previous findings 3.
The clinically most important issue is whether the morphine–P2Y12 receptor blocker interaction influences clinical outcomes. The Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC) trial showed that prehospital administration of ticagrelor significantly improved the primary endpoint of ST‐segment resolution only in patients not receiving morphine 22. Despite this interesting finding of a primary negative trial, more studies are needed to confirm the possible negative impact of morphine administration on clinical outcome data in acute myocardial infarction.
The biological effect of morphine on orally administered antiplatelet drugs is likely to be due to the inhibition of gastric emptying 7. In the present study, we demonstrated that the intravenous GPIIb/IIIa receptor inhibitor abciximab can be used as a potential bridging strategy in STEMI patients when rapid and efficient platelet inhibition is required. Our study in prasugrel‐treated STEMI patients indicated that abiciximab sufficiently inhibits platelet aggregation after morphine administration. By contrast, morphine use was associated with an 88% rate of HTPR 2 h after prasugrel intake, when abciximab was not infused. A large body of evidence suggests that HTPR is most common when clopidogrel is used but is also present following treatment with prasugrel and ticagrelor and is associated with thrombotic risk at follow‐up 23, 24, 25, 26, 27, 28, 29. HTPR is also predictive of intra‐ and periprocedural thrombotic events, even under prasugrel therapy in the acute setting with a large thrombus burden 30. Therefore, the rationale for the immediate blocking of platelet aggregation by abciximab infusion as a bridging strategy to overcome the delay of P2Y12 receptor inhibitor action is based on the hypothesis that abciximab inhibits platelet activation downstream by preventing activation of platelet GPIIb/IIIa receptors. This concept has been successfully demonstrated in patients treated with clopidogrel and prasugrel in a pharmacodynamic study 8. In smaller studies, platelet inhibition with GPIIb/IIIa receptor antagonists in individuals with HTPR reduced the incidence of major adverse cardiac events without increasing bleeding 31, 32. Nevertheless, it remains to be shown whether the infusion of a GPIIb/IIIa receptor blocker for immediate platelet inhibition in addition to oral loading with an ADP receptor antagonist may improve the net clinical outcomes, when morphine is used. As bleeding is an independent determinant of short‐ and long‐term mortality 33, 34, use of GPIIb/IIIa receptor blockers may be harmful. In the present study, which was not powered for clinical outcomes, abciximab was associated with more minimal bleeding events. Interestingly, a previous study indicated that patients at high risk of bleeding also had a net benefit from a GPIIb/IIIa receptor blocker 34. It should therefore be explored whether the short‐acting intravenous P2Y12 receptor blocker cangrelor can also be a useful alternative in this setting 35, especially when GPIIb/IIIa receptor blockers are not indicated.
Limitation
The main limitation of the present study was its nonrandomized design. However, despite this limitation, the groups appeared to be reasonably well balanced. In addition, as it would not have been feasible in the setting of acute myocardial infarction, the study did not present the time course of the effect of morphine coadministration on the antiplatelet effect of prasugrel between 2 h postdosing and day 1 after admission. A further limitation of the present study was the fact that we used only one test for the assessment of antiplatelet efficacy – ADP‐induced platelet aggregation.
Conclusion
Morphine use is associated with a delay in the onset of prasugrel action in STEMI patients undergoing primary PCI, resulting in suboptimal inhibition of platelet aggregation for at least 2 h. Such suboptimal inhibition can be overcome efficiently with abciximab.
Competing Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: support from the Austrian Society of Cardiology for the submitted work; JSM received lecture or consultant fees from Daiichi Sankyo, Eli Lilly and Astrazeneca; RDC received fees, honoraria and research funding from Sanofi‐Aventis, Boehringer Ingelheim, Bayer, BMS/Pfizer, Daiichi‐Sankyo, Novartis, Merck and Lilly; BJ reported consultant fees and research funding from AstraZeneca and Bayer; GC received lecture or consultant fees from Daiichi Sankyo and Eli Lilly; IML has relationships with drug companies including AOPOrphan Pharmaceuticals, Actelion, Bayer, Astra‐Zeneca, Servier, Cordis, Medtronic, GSK, Novartis, Pfizer and United Therapeutics, in the previous 3 years. In addition, IML is an investigator in trials involving these companies, with relationships including consultancy service, research grants and membership in scientific advisory boards.
This study was supported by a grant from the Austrian Society of Cardiology.
Contributors
JMS‐M, JK, DA, RC, GC and IML were responsible for the study conception. JMS‐M and IML designed the study. SS was responsible for patient inclusion, and collection of laboratory and patient data; JMS‐M analysed the data. JMS‐M, JK, DA, RC, ELH, BJ and GC interpreted the data. All authors were involved in drafting the manuscript, and contributed to revisions of the draft and approval of the final manuscript.
Siller‐Matula, J. M. , Specht, S. , Kubica, J. , Alexopoulos, D. , De Caterina, R. , Hobl, E. ‐L. , Jilma, B. , Christ, G. , and Lang, I. M. (2016) Abciximab as a bridging strategy to overcome morphine–prasugrel interaction in STEMI patients. Br J Clin Pharmacol, 82: 1343–1350. doi: 10.1111/bcp.13053.
References
- 1. Siller‐Matula JM, Trenk D, Schror K, Gawaz M, Kristensen SD, Storey RF, et al. How to improve the concept of individualised antiplatelet therapy with P2Y12 receptor inhibitors – is an algorithm the answer? Thromb Haemost 2015; 113: 37–52. [DOI] [PubMed] [Google Scholar]
- 2. Siller‐Matula JM, Trenk D, Krahenbuhl S, Michelson AD, Delle‐Karth G. Clinical implications of drug–drug interactions with P2Y12 receptor inhibitors. J Thromb Haemost 2014; 12: 2–13. [DOI] [PubMed] [Google Scholar]
- 3. Hobl EL, Stimpfl T, Ebner J, Schoergenhofer C, Derhaschnig U, Sunder‐Plassmann R, et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double‐blind, placebo‐controlled trial. J Am Coll Cardiol 2014; 63: 630–5. [DOI] [PubMed] [Google Scholar]
- 4. Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, et al. Comparison of prasugrel and ticagrelor loading doses in ST‐segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol 2013; 61: 1601–6. [DOI] [PubMed] [Google Scholar]
- 5. Parodi G, Bellandi B, Xanthopoulou I, Capranzano P, Capodanno D, Valenti R, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST‐elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv 2015; 8: e001593. [DOI] [PubMed] [Google Scholar]
- 6. Hobl EL, Reiter B, Schoergenhofer C, Schwameis M, Derhaschnig U, Lang IM, et al. Morphine interaction with prasugrel: a double‐blind, cross‐over trial in healthy volunteers. Clin Res Cardiol 2016; 105: 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kubica J, Kubica A, Jilma B, Adamski P, Hobl EL, Navarese EP, et al. Impact of morphine on antiplatelet effects of oral P2Y12 receptor inhibitors. Int J Cardiol 2016; 215: 201–8. [DOI] [PubMed] [Google Scholar]
- 8. Christ G, Hafner T, Siller‐Matula JM, Francesconi M, Grohs K, Wilhelm E, et al. Platelet inhibition by abciximab bolus‐only administration and oral ADP receptor antagonist loading in acute coronary syndrome patients: the blocking and bridging strategy. Thromb Res 2013; 132: e36–e41. [DOI] [PubMed] [Google Scholar]
- 9. Siller‐Matula JM, Trenk D, Schror K, Gawaz M, Kristensen SD, Storey RF, et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv 2013; 6: 1111–28. [DOI] [PubMed] [Google Scholar]
- 10. Siller‐Matula JM, Gruber C, Francesconi M, Dechant C, Jilma B, Delle‐Karth G, et al. The net clinical benefit of personalized antiplatelet therapy in patients undergoing percutaneous coronary intervention. Clin Sci (Lond) 2015; 128: 121–30. [DOI] [PubMed] [Google Scholar]
- 11. Siller‐Matula JM, Lang IM, Neunteufl T, Kozinski M, Maurer G, Linkowska K, et al. Interplay between genetic and clinical variables affecting platelet reactivity and cardiac adverse events in patients undergoing percutaneous coronary intervention. PLoS One 2014; 9: e102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christ G, Siller‐Matula JM, Francesconi M, Dechant C, Grohs K, Podczeck‐Schweighofer A. Individualising dual antiplatelet therapy after percutaneous coronary intervention: the IDEAL‐PCI registry. BMJ Open 2014; 4: e005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Komosa A, Siller‐Matula JM, Kowal J, Lesiak M, Siniawski A, Maczynski M, et al. Comparison of the antiplatelet effect of two clopidogrel bisulfate formulations: Plavix and generic‐Egitromb. Platelets 2015; 26: 43–7. [DOI] [PubMed] [Google Scholar]
- 14. Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–73. [DOI] [PubMed] [Google Scholar]
- 15. Kubica A, Kasprzak M, Siller‐Matula J, Kozinski M, Pio Navarese E, Obonska K, et al. Time‐related changes in determinants of antiplatelet effect of clopidogrel in patients after myocardial infarction. Eur J Pharmacol 2014; 742: 47–54. [DOI] [PubMed] [Google Scholar]
- 16. Alpert JS, Thygesen K, Jaffe A, White HD. The universal definition of myocardial infarction: a consensus document: ischaemic heart disease. Heart 2008; 94: 1335–41. [DOI] [PubMed] [Google Scholar]
- 17. Fuchs I, Spiel AO, Frossard M, Derhaschnig U, Riedmuller E, Jilma B. Platelet hyperfunction is decreased by additional aspirin loading in patients presenting with myocardial infarction on daily aspirin therapy. Crit Care Med 2010; 38: 1423–29. [DOI] [PubMed] [Google Scholar]
- 18. Alexopoulos D, Gkizas V, Patsilinakos S, Xanthopoulou I, Angelidis C, Anthopoulos P, et al. Double versus standard loading dose of ticagrelor: onset of antiplatelet action in patients with STEMI undergoing primary PCI. J Am Coll Cardiol 2013; 62: 940–1. [DOI] [PubMed] [Google Scholar]
- 19. Franchi F, Rollini F, Cho JR, Bhatti M, DeGroat C, Ferrante E, et al. Impact of Escalating loading dose regimens of ticagrelor in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of a prospective randomized pharmacokinetic and pharmacodynamic investigation. JACC Cardiovasc Interv 2015; 8: 1457–67. [DOI] [PubMed] [Google Scholar]
- 20. Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Sroka W, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double‐blind, placebo‐controlled IMPRESSION trial. Eur Heart J 2016; 37: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hobl EL, Reiter B, Schoergenhofer C, Schwameis M, Derhaschnig U, Kubica J, et al. Morphine decreases ticagrelor concentrations but not its antiplatelet effects: a randomized trial in healthy volunteers. Eur J Clin Invest 2016; 46: 7–14. [DOI] [PubMed] [Google Scholar]
- 22. Montalescot G, van 't Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, et al. Prehospital ticagrelor in ST‐segment elevation myocardial infarction. N Engl J Med 2014; 371: 1016–27. [DOI] [PubMed] [Google Scholar]
- 23. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, et al. Bleeding and stent thrombosis on P2Y12‐inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015; 36: 1762–71. [DOI] [PubMed] [Google Scholar]
- 24. Droppa M, Tschernow D, Muller KA, Tavlaki E, Karathanos A, Stimpfle F, et al. Evaluation of clinical risk factors to predict high on‐treatment platelet reactivity and outcome in patients with stable coronary artery disease (PREDICT‐STABLE). PLoS One 2015; 10: e0121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siller‐Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle‐Karth G, et al. Personalized antiplatelet treatment after percutaneous coronary intervention: the MADONNA study. Int J Cardiol 2013; 167: 2018–23. [DOI] [PubMed] [Google Scholar]
- 26. Siller‐Matula JM, Hintermeier A, Kastner J, Kreiner G, Maurer G, Kratochwil C, et al. Distribution of clinical events across platelet aggregation values in all‐comers treated with prasugrel and ticagrelor. Vascul Pharmacol 2016; 79: 6–10. [DOI] [PubMed] [Google Scholar]
- 27. Komosa A, Siller‐Matula JM, Lesiak M, Michalak M, Kowal J, Maczynski M, et al. Association between high on‐treatment platelet reactivity and occurrence of cerebral ischemic events in patients undergoing percutaneous coronary intervention. Thromb Res 2016; 138: 49–54. [DOI] [PubMed] [Google Scholar]
- 28. Winter MP, Kozinski M, Kubica J, Aradi D, Siller‐Matula JM. Personalized antiplatelet therapy with P2Y12 receptor inhibitors: benefits and pitfalls. Postepy Kardiol Interwencyjnej 2015; 11: 259–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siller‐Matula JM, Akca B, Neunteufl T, Maurer G, Lang IM, Kreiner G, et al. Inter‐patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets 2016; 27: 373–7. [DOI] [PubMed] [Google Scholar]
- 30. Orban M, Byrne RA, Hausleiter J, Laugwitz KL, Sibbing D. Massive thrombus burden with recurrence of intracoronary thrombosis early after stenting and delayed onset of prasugrel action in a patient with ST‐elevation myocardial infarction and cardiac shock. Thromb Haemost 2011; 106: 555–8. [DOI] [PubMed] [Google Scholar]
- 31. Valgimigli M, Campo G, de Cesare N, Meliga E, Vranckx P, Furgieri A, et al. Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double‐blind, prospective, randomized Tailoring Treatment with Tirofiban in Patients Showing Resistance to Aspirin and/or Resistance to Clopidogrel study. Circulation 2009; 119: 3215–22. [DOI] [PubMed] [Google Scholar]
- 32. Cuisset T, Frere C, Quilici J, Morange PE, Mouret JP, Bali L, et al. Glycoprotein IIb/IIIa inhibitors improve outcome after coronary stenting in clopidogrel nonresponders: a prospective, randomized study. JACC Cardiovasc Interv 2008; 1: 649–53. [DOI] [PubMed] [Google Scholar]
- 33. Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE‐2 Trial. Am J Cardiol 2007; 100: 1364–9. [DOI] [PubMed] [Google Scholar]
- 34. Hermanides RS, Ottervanger JP, ten Berg JM, Gosselink AT, van Houwelingen G, Dambrink JH, et al. Net clinical benefit of prehospital glycoprotein IIb/IIIa inhibitors in patients with ST‐elevation myocardial infarction and high risk of bleeding: effect of tirofiban in patients at high risk of bleeding using CRUSADE bleeding score. J Invasive Cardiol 2012; 24: 84–9. [PubMed] [Google Scholar]
- 35. Kubica J, Kozinski M, Navarese EP, Tantry U, Kubica A, Siller‐Matula JM, et al. Cangrelor: an emerging therapeutic option for patients with coronary artery disease. Curr Med Res Opin 2014; 30: 813–28. [DOI] [PubMed] [Google Scholar]


