Abstract
Despite the revolution in recent decades regarding monoamine involvement in the management of major depressive disorder (MDD), the biological mechanisms underlying this psychiatric disorder are still poorly understood. Currently available treatments require long time courses to establish antidepressant response and a significant percentage of people are refractory to single drug or combination drug treatment. These issues, and recent findings demonstrating the involvement of synaptic plasticity in the pathophysiological mechanisms of MDD, are encouraging researchers to explore the molecular mechanisms underlying psychiatric disease in more depth. The discovery of the rapid antidepressant effect exerted by glutamatergic and cholinergic agents highlights the mammalian target of rapamycin (mTOR) pathway as a critical pathway that contributes to the efficacy of these pharmacological agents in clinical and pre‐clinical research. The mTOR pathway is a downstream intracellular signal that transmits information after the direct activation of α‐amino‐3‐hydroxyl‐5‐methyl‐4‐isoxazole‐propionate (AMPA) and neurotrophic factor receptors. Activation of these receptors is hypothesized to be one of the major axes involved in the synthesis of synaptogenic proteins underlying synaptic plasticity and critical to both the rapid and delayed effects exerted by classic antidepressants. This review focuses on the involvement of mTOR in the pathophysiology of depression and on molecular mechanisms involved in the activity of emerging and classic antidepressant agents.
Keywords: antidepressants, ketamine, major depressive disorder, mTOR
Introduction
In 2004 depression ranked third among non‐fatal disease global disability burden and is expected to attain first place by 2030 1. Major depressive disorder (MDD) is a serious and recurrent disorder, linked to diminished quality of life, increased morbidity, increased mortality and economic burden 2, 3. The pathogenesis of depression and mechanisms of action of current antidepressant drugs are not yet clear 4. Although the activity of common antidepressant drugs involves acutely the monoaminergic system, the therapeutic effect occurs only after chronic treatment and appears to be under poorly understood changes in cellular biochemical mechanisms. Evidence indicates that neurotrophic and neurogenic factors mediate neural adaptations involved in the late therapeutic responses after chronic treatment with classical antidepressants 5, 6, 7.
Recent research has provided emerging theories on the pathophysiology of depression and possible mechanisms of action of antidepressants that consider the neurobiological processes underlying neuronal and synaptic plasticity and converging factors 8, 9. Importantly, recent research urges development of improved treatments, given that available treatments are effective in fewer than 50% of people with MDD and antidepressants require weeks to months for therapeutic effect 10, 11. To this end, recent research has focused on the mTOR signalling as a pathway underlying the effects of emerging agents that relieve depression faster than classic antidepressants. This review will focus on cellular mechanisms described in preclinical and clinical studies and agents that promote antidepressant effects via activity in the mTOR signalling pathway.
Mammalian target of rapamycin (mTOR)
Target of rapamycin (TOR) is a highly conserved serine/threonine kinase. Two distinct protein complexes,TOR complex 1 (TORC1) and TORC2, regulate important functions such as cell growth and metabolism 12. Rapamycin activity at the TOR complexes was identified from mutations in the budding yeast Saccharomyces cerevisiae, with certain mutations making the complexes resistant to the inhibitory properties of rapamycin. In the TORC1 complex, rapamycin binds to FKBP12 to form a FKBP12‐rapamycin complex and thereby inhibit TORC1 activity 13, 14. Rapamycin allosterically inhibits TORC1 activity, possibly by blocking interactions with regulatory proteins via steric hindrance or conformational changes 15. The upstream activators of mTOR signalling are protein kinase B (PKB/Akt) and extracellular signal‐related kinase (ERK), which inhibit tuberous sclerosis (TSC1 and TSC2) complexes, which are inhibitors of mTOR 16. The activation of glycogen synthase kinase‐3 (GSK‐3) leads to increase on TSC1/2 activity, thus inhibiting the mTOR pathway 16. The downstream targets of mammalian TOR (mTOR) are the ribosomal protein S6 kinases (S6Ks) and the eukaryotic initiation factor 4E (eIF4E)‐binding proteins (4E‐BP). These downstream proteins regulate protein biosynthesis 17. S6K presents inhibitory function on the kinases of eukaryotic elongation factor 2 (eEF2), whose phosphorylation inhibits protein translation 1. Stimuli inducing dephosphorylation of eEF2 increases translation and the underlying dephosphorylation process is a target for blockade by rapamycin, implying it to be an effect also mediated through mTOR 18. In addition to protein synthesis, mTOR is being studied as an important signalling pathway in several other homeostasis and cell survival processes inherent in the homeostatic and extreme living conditions of cells [reviewed in 15].
mTOR and brain physiology
Activation of the mTOR signalling pathway is implicated in many physiological processes of the nervous system, including neurogenesis, axonal sprouting, dendritic spine growth, ionic and receptor channel expression, axonal regeneration and myelination. A large number of physiological processes regulated by mTOR underlie higher nervous system functions such as neuronal excitability and survival, cognition, feeding behaviour and control of circadian rhythm 17. Studies have shown that mTOR signalling is involved in various important aspects of the hippocampal dendritic tree, such as an increase in the size and maturation of dendrites, as well as in dendritic growth stimulated by activity 19. In addition, the coordinated development of dendrite size, shape and dendritic complexity also are underlying the mTOR pathway 20. The downstream 4E‐BP2 proteins, mTOR targets and translation repressor, are important regulators of long term potentiation phenomena and are critical to the process of hippocampal synaptic plasticity and memory 21.
Considering the important physiological mechanisms in the brain, it is reasonable to hypothesize that changes in mTOR signalling are involved in various pathologies of the nervous system and psychiatric disorders, including MDD 22, 23, 24.
Modulators, receptors and mTOR signalling
In addition to stress and stimuli contributions from energetic and homeostatic status, several modulators, such as neurotransmitters, hormones, growth factors and receptors, are involved in the activation or inhibition of mTORC1 signalling 25. Factors involved in synaptic plasticity and neurogenesis, such as brain‐derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), insulin and insulin‐like growth factor 1(IGF1), bind to tyrosine kinase receptors and are activators of the mTORC1 pathway 17, 25, 26. Research has shown that BDNF, through tropomyosin‐related kinase B (TrkB) receptor, increases the rate of protein synthesis by increasing the unphosphorylated eukaryotic elongation factor 2 (eEF2) protein in primary cortical neurons 27 and hippocampal neurons 28. Other studies have also shown that BDNF activates the mTOR cascade via 4E‐BPs and S6Ks proteins thereby increasing protein synthesis in neuronal dendrites 29. Therefore, the role of BDNF in protein synthesis and neuronal plasticity seems to involve an initiation and elongation translation process of the downstream mechanisms in the mTOR pathway.
Other major neurotransmitters and receptors involved in the regulation of neuronal plasticity and behavioural functions activate the mTOR pathway. The μ‐opioid receptor, which underlies the analgesic and addictive properties of morphine, acts through mTOR translational pathways 30. Metabotropic glutamate receptors of group I (group I mGluRs), involved in synaptic plasticity and important neural functions, also activate mTOR signalling 31, 32. In addition to the metabotropic receptors, many recent studies are focusing on mTOR signalling through ionotropic, α‐amino‐3‐hydroxyl‐5‐methyl‐4‐isoxazole‐propionate (AMPA) receptors, indicating that AMPA glutamatergic activation triggers the synaptogenic process by activating the mTOR pathway 33, 34, 35.
The sustained activation of the N‐methyl‐D‐aspartate receptor (NMDA) receptor provides inhibitory action on the mTORC1 signalling activity, resulting in reduced protein synthesis 36. NMDA activation is associated with dephosphorylation of extracellular signal‐related kinase (ERK) protein kinase B (PKB/Akt) 37 and activation of glycogen synthase kinase‐3 (GSK‐3) 38. PKB/Akt proteins and ERK inhibit tuberous sclerosis (TSC1 and TSC2) complexes, which are inhibitors of mTOR 37, 39. Therefore, PKB/Akt and ERK activate the mTOR signalling pathway, while GSK3 activity leads to increased TSC1/2 activity, thus inhibiting the mTOR pathway 35. Hypofunction of the NMDA receptor caused by antagonists leads to synaptogenic effects 40 and increased AMPA activation in the prefrontal cortex (PFC) 41.
In neurons of Aplysia, serotonin decreases phosphorylation of the eEF2, increases synaptic 4EBPs phosphorylation and increases protein translation and synaptic strength 42, 43. Serotonin also seems to activate synaptic plasticity through protein mechanisms of initiation and elongation in protein translation processes by the mTOR pathway. On the other hand, chronic treatment with the selective serotonergic re‐uptake inhibitor (SSRI) fluoxetine increased phosphorylation of the eEF2 in the hippocampus, PFC and the dentate gyrus, while phosphorylation of eIF4E increased only in dentate gyrus 44. Thus, increased chronic serotonergic activity in regions involved with MDD seems to involve temporally and spatially different mechanisms, which initially appears paradoxical considering that eEF2 phosphorylation is related to reduction of protein translation 45. However, eEF2 phosphorylation is also related to an increased translation of critical proteins involved in subcellular regional organization of the translational machinery 46.
Scopolamine is a muscarinic cholinergic receptor (mAChR) antagonist with rapid‐onset antidepressant effects in clinical trials 47, 48, 49. Rats treated with scopolamine have synaptogenesis in the prefrontal cortex (PFC) associated with rapid activation of mTORC1 signalling 50.
mTOR signalling and depression
The mTOR signalling pathway is impaired in the PFC of individuals diagnosed with MDD. The protein levels of the translation initiation step of the mTOR pathway, namely p70S6K and eIF4B, were reduced in post mortem brains of depressed people. A reduction of phosphorylated eIF4B was also observed in the brains of these subjects, indicating a reduction in mTOR/p70S6K/eIF4B pathway function 51. In a post mortem study of individuals previously diagnosed with MDD, researchers observed a reduction in kinase activity of Akt and increased GSK3β enzyme in the ventral prefrontal cortex 52. Another mTOR upstream regulator, the RDD1 (regulated in development and DNA damage responses‐1) protein, which stabilizes the TSC1 and TSC2, increased in the post mortem PFC of MDD patients. The same authors also observed that RDD1 is involved on depressive‐like behaviour and synaptic plasticity impairment that occurs in the PFC of rats subjected to chronic stress. In addition to RDD1, phosphorylation of S6K and 4EBP in the PFC were also reduced in chronically stressed rats 53. Preclinical studies utilizing animal models of depression reported decreased mTOR brain activation. A similar reduction in the phosphatidylinositol 3‐kinase (PI3K)/Akt/mTOR signalling pathway has been described in PFC and amygdala of stressed rats 54. In studies with rodents exposed to chronic unpredictable stress, researchers observed depression‐like behaviour and a reduction in phosphorylation levels of mTOR and phospho‐p70S6K in the PFC, hippocampus and amygdala 54, 55, 56. It was shown that depressive‐like behaviour induced by chronic stress in rats was reversed quickly by ketamine and Yueju, a medicinal herb 57. Also, there was an increase in the levels of phosphorylated upstream and downstream targets of the mTOR pathway, such as ERK, Akt, S6K and 4E‐BP 57. Thus, these and other results indicate that translational losses underlying to MDD are not necessarily due to complex deficiency, but to upstream and downstream targets of the mTOR signalling pathway. Immobilization stress also decreases BDNF expression in parallel to reduced phosphorylation of mTOR and p70S6K in the hippocampus of rats 58. In vitro methods replicate these findings, since cultured primary cortical neurons from mice presenting depression‐like behaviour after chronic corticosterone treatment have a reduction in mTOR activity 59.
Several researchers have shown that mTOR signalling is an important mechanism underlying the activation and function of AMPA receptors in synaptogenesis 33, 34. Adult rats exposed to a chronic mild stress protocol had increased anhedonia and reduced AMPA receptor expression in the PFC and nucleus accumbens and decreased neurogenesis and BDNF levels in the hippocampus 60. Other studies have shown that the antidepressant‐like effect of ketamine occurred along with an increase in the AMPA receptor activity, as well as levels of phosphorylated mTOR and expression of BDNF in the hippocampus and PFC of rats. The same authors also found that blocking AMPA receptors prevented the expression of BDNF, phosphorylated mTOR and antidepressant‐like response, suggesting that the AMPA receptor plays an important role in convergence of mTOR signalling, BDNF expression and antidepressant response 61.
Involvement of mTOR in depression treatment
Activation of the mTOR pathway seems to underlie the antidepressant effects of NMDA receptor antagonists and other classic antidepressants 62. Classic antidepressants, such as escitalopram, paroxetine, and tranylcypromine, increase levels of phosphorylated mTOR and phosphorylated forms of the upstream regulators Akt and ERK in rat hippocampal cultures. In addition, these antidepressants also increase synaptic protein levels and growth of hippocampal dendrites 63. Several studies have shown that the glutamatergic non‐selective NMDA receptor antagonist ketamine exerts a rapid and prolonged antidepressant effect after acute administration in humans 64, 65, 66 and in animal models of depression 35, 40, 67. Ketamine has synaptogenic effects that are seen to be mediated via disinhibition of glutamatergic transmission on the AMPA receptors. The spontaneous activity of GABAergic interneurons is decreased by ketamine in the PFC of rats and, consequently, the firing rate of glutamatergic pyramidal neurons are increased 41. Some authors suggested that NMDA receptor antagonists block spontaneous GABAergic activity, resulting thereby in disinhibition of glutamate transmission on the AMPA receptors 34. On the other hand, a direct effect on cortical neurons is hypothesized from evidence that the function of ketamine seems to be happening in mechanisms underlying to tonic activity of NMDAR receptors in pyramidal neurons (see Miller et al. 68, for a detailed review about the theories of inhibition and disinhibition by ketamine). In addition, ketamine has been shown effective in people resistant to treatment with classic antidepressants 40, 66, 69, 70. Ketamine, at doses that induce antidepressant‐like effects in animals, rapidly increased mTOR signalling, BDNF levels, and structural and functional plasticity in the PFC and hippocampus 35, 61, 71, 72, 73. The action of ketamine occurred on ERK, PKB/Akt and signalling pathways of growth factors linked to the activation of mTOR. The antidepressant effect exerted by ketamine requires the inhibition of GSK‐3 71, 72, which inhibits mTOR signalling 37, 39. Importantly, inhibition of GSK‐3 by lithium or agents that preferentially inhibit the GSK‐3β potentiated and increased the duration of antidepressants and synaptogenic effects of the ketamine, through activation of the mTORC1 72. It is also important to note that activation of these mTORC signalling pathways was blocked by an inhibitor of AMPA activity, emphasizing the intermediation of the AMPA glutamate receptor in the action of ketamine 33, 40. Treatment with ketamine and Ro 25–6981, a glutamate NMDA receptor 2B (GluN2B) antagonist in a rodent chronic stress model led to a sustained reversal of depressive‐like behaviours, reduction of synaptic proteins and density and decreased excitatory postsynaptic currents in PFC via mTOR 74. Moreover, other authors observed that Ro 25–6981 induces an antidepressant effect with fewer side effects than ketamine and other non‐specific NMDA antagonists 75. Another NMDA GluN2B antagonist, CP‐101 606, had a rapid antidepressant effect in humans with treatment‐refractory MDD 76. Miller and colleagues 77 demonstrated that antagonism or suppression of subunit GluN2B of the NMDA receptor promoted mTOR‐dependent antidepressant‐like effects and increased translation and synaptic plasticity in cortical neurons. The authors argue that GluN2B has a more effective function in inhibiting mTOR function and limiting protein synthesis and may therefore be a target of rapid antidepressant actions of ketamine. Based on other studies, these researchers also argue that AMPA activation may be a mechanism involved in responses affected by blocking the function GluN2B. It is important to note that greater glutamate affinity occurs with GluN2 subunits of NMDA during activation while the co‐agonist glycine has a higher affinity for GluN1 subunits 78. On the other hand, an inverse engagement of the GluN2B subunit in brain regions of animals subjected to the chronic stress protocol was observed 79. Another study showed that the expression of the GluN2B subunit did not change, while the GluN1 subunit was increased in the PFC of mice subjected to chronic stress 57. Thus, these apparent paradoxical findings need to be elucidated, observing specific aspects, such as stress protocols, regional differences with respect to subunit expression and composition, among other aspects inherent in MDD.
AMPA availability and activation with subsequent mTOR signalling are also required for the antidepressant effect elicited by sarcosine, a substance that increases the availability of glycine in the synaptic cleft and NMDA receptor activity. Therefore, glutamatergic function goes beyond the antagonistic effect of ketamine and other compounds at the NMDA receptor. These data indicate that mTOR signalling through the activation of AMPA is the framework of the mechanisms involved in synaptic plasticity and antidepressant activity of drugs involved in ionotropic glutamatergic neurotransmission 80. It is worth noting that NMDA antagonism appears to reduce the spontaneous activity of GABAergic interneurons in the PFC and consequently disinhibits pyramidal glutamatergic activity. This activity is proposed to increase the cortical excitability and glutamate levels and consequently the activation of AMPA (Figure 1) 33, 41. Anticonvulsant drugs with antidepressant properties, such as lamotrigine 81, 82 and riluzole 81, also increase traffic and availability of the AMPA receptor in the membrane of hippocampal neurons 81. This phenomenon can therefore increase mTOR signalling and potentially underlies plasticity changes involved in anticonvulsant and antidepressant effects. However, there is conflict in the field about what specific mechanisms underlie drugs that increase AMPA/mTOR activity but exhibit timing differences in antidepressant effect 80.
Figure 1.
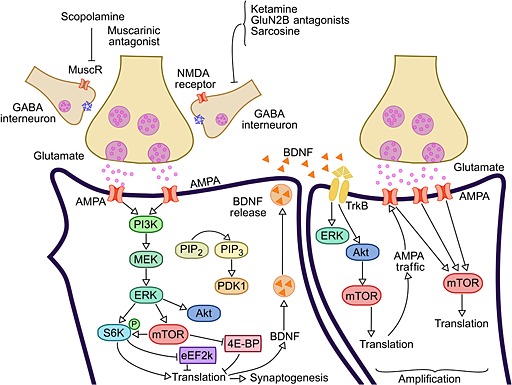
Fast action antidepressants activate mTOR. NMDA and muscarinic receptors antagonists, as well as compounds that act on AMPA receptors activate the mTOR signalling pathway. The activated mTOR triggers a translational machinery, increasing protein synthesis involved with synaptogenesis. Among other synaptogenic molecules, BDNF is released and activates TrkB receptor, whose signalling activates mTOR pathway. Thus, BDNF increases synthesis and traffic of AMPA receptors, thereby increasing their availability and mTOR signalling. This positive feedback loop between AMPA and BDNF seems to be a mechanism involved in the amplification of synaptic plasticity, as well as for the rapid, robust and durable therapeutic antidepressant response. BDNF, brain‐derived neurotrophic factor; mTOR, mammalian target of rapamycin; NMDA, N‐methyl‐daspartate; AMPA, α‐amino‐3‐hydroxyl‐5‐methyl‐4‐isoxazole‐propionate; PI3K, phosphatidylinositol‐3‐kinase; MuscR, muscarinic receptor; ERK, extracellular signal‐regulated kinase; MEK, mitogen‐activated protein kinase; Akt, protein kinase B (PKB); PIP2, phosphatidylinositol biphosphate; PIP3, phosphatidylinositol triphosphate; PDK1, phosphoinositide‐dependent kinase‐1; S6K, ribosomal protein S6 kinase; eEF2K, eukaryotic elongation factor‐2 kinase; 4E‐BP, eukaryotic initiation factor 4E (eIF4E)‐binding proteins
As observed by Autry and colleagues 83 the antidepressant‐like action of ketamine and other NMDA antagonist, MK‐801, requires the function of BDNF. The same authors found that ketamine increased levels of BDNF protein without changing mRNA expression, indicating that the ketamine and other NMDA antagonists trigger translational machinery. Going further, the researchers showed that the antidepressant‐like action of ketamine and other NMDA antagonists requires AMPA activation, dephosphorylation of eEF2 and increased synthesis of BDNF by translational processes. Considering that BDNF activates the mTOR cascade and increases the unphosphorylated form of eEF2 it is important to note that the mechanisms fired from the AMPA activation, at least with respect to BDNF, may form a positive feedback loop 27, 28. In addition to the mTOR pathway, the action of BDNF involves other mechanisms that underlie synaptic plasticity and antidepressant effects, such as activation of mitogen‐activated protein kinases (MAPK) 84, 85, as well as expression of elevated of phosphorylated cAMP response element‐binding protein (pCREB) in the hippocampus of adult mice 86. Thus, the amplification mechanism by BDNF involves other signalling pathways, including the transcriptional machinery. Therefore, the translational machinery after AMPA activation can become broad and persistent and potentially underlie the rapid, robust and long acting antidepressant response from NMDA antagonists (Figure 1).
mTOR signalling as a consequence of AMPA receptor activity is also shown as a convergence path from cholinergic neurotransmission in MDD. Scopolamine, a muscarinic cholinergic receptor (mAChR) antagonist exerts rapid‐onset antidepressant effects in clinical trials 47, 48, 49 and synaptogenesis in the PFC of rats, together with rapid activation of mTORC1 signalling 50. These effects were antagonized by AMPA blockers, suggesting a shared action of glutamatergic neurotransmitter similar to NMDA antagonism by ketamine (Figure 1) 50.
The expression of VEGF and its receptor Flk‐1 increases in the hippocampus after treatment with classic antidepressants such as SSRIs and norepinephrine selective re‐uptake inhibitors (NSRIs) and is involved in neurogenesis and antidepressant‐like effect induced by these drugs and electroconvulsive seizure 87. VEGF activates cell proliferation through the mTORC1 signalling pathway 25, 26. These results suggest that behavioural and neurogenic action of classic antidepressants occurs, at least in part, via activation of VEGF receptors and downstream mTOR signalling.
Ketamine in combination with other agents that cause synergistic effects is a potential strategy to increase antidepressant effects and reduce possible adverse effects resulting from chronic administration of ketamine. Thus, some compounds in parallel with the expansion of the effects of ketamine also activate the mTOR pathway 35, 73, 83, 88.
Discussion and conclusion
Research shows that classic antidepressants quickly increase levels of monoamines in the synapses, but the antidepressant effect is delayed. It is important that classic antidepressants increase the expression and function of neurotrophic factors and activate mTOR. However, drugs that quickly activate mTOR, such as NMDA and muscarinic antagonists, Yueju, among other compounds, have a quick and long lasting antidepressant effect, even if the activation of mTOR is transient. Thus, it is important to determine what mechanisms mediate the delayed effect of classic drugs and compare those with the mechanisms utilized by drugs, which quickly activate mTOR signalling. It is important to note that chronically administered classic antidepressants increase the expression of BDNF and VEGF (Figure 2) 87, 89, 90, 91, 92, wherein the antidepressant effect seems to coincide with the increased levels of these factors 92. The quick, potent and long lasting effects of drugs that activate mTOR is related mainly to increased activation of the AMPA receptors with subsequent and rapid mTOR activation. It is also important that mTOR activity induced by AMPA activation leads to an increase in BDNF expression. In addition, BDNF activates mTOR signalling and increases AMPA receptor expression and function 83, thereby may be building a positive feedback loop and thus amplifing mechanisms related to synaptic plasticity and antidepressant behavioural response. However, the connections between BDNF, mTOR and AMPA, in both directions require further studies. At the same time it is also important to note that the striking effect of agents that activate mTOR signalling and induce rapid antidepressant activity in treatment‐refractory people may result from a culmination of mechanisms on which classic antidepressants have no direct activity. If this is the case, how might we find mechanisms that could infer differences between treatments? Because classic antidepressants seem to require an increase in the expression of synaptogenic factors after chronic treatment, and hence culminate in mTOR activation, is interesting to note that some routes suffer genetic variants that confer resistance to treatment, as in the case of genetic polymorphisms for the serotonin transporter and BDNF (Figure 2) 7, 93, 94, 95. Thus, synaptic plasticity as well as the antidepressant response may suffer delays and losses when the treatment is by these routes.
Figure 2.
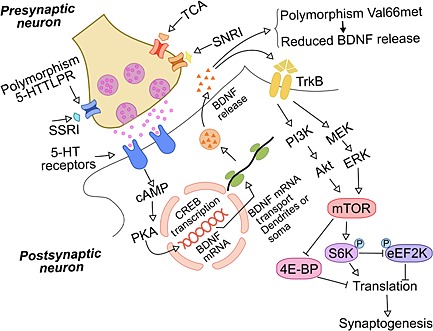
Mechanisms involved in the delay of therapeutic response to classic antidepressants in MDD patients. The classic antidepressants, which inhibit the re‐uptake of monoamines, increase the availability of serotonin, norepinephrine and dopamine in the synaptic cleft. The activation of serotonergic receptors (example above) triggers the internal cellular signalling that leads to CREB activity and BDNF transcription. The synthesis and release of BDNF from the transcription process activates TrkB receptors and downstream mTOR signalling pathway, which coincides with the therapeutic response. Activated mTOR triggers the translational machinery, increasing protein synthesis involved in synaptogenesis. Genetic polymorphisms of the serotonin transporter and BDNF are involved in resistance to classical antidepressant treatment in some people. These genetic traits may be at least one of the possible mechanisms related to refractory to classical treatments. MDD, major depressive disorder; 5‐HTTLPR, serotonin‐transporter‐linked polymorphic region; Val66met BDNF single‐nucleotide polymorphism; SSRI, selective serotonin re‐uptake inhibitors; SNRI, serotonin‐norepinephrine re‐uptake inhibitors; TCA, tricyclic antidepressants; BDNF, brain‐derived neurotrophic factor; TrkB, tropomyosin‐related kinase B receptor; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol‐3‐kinase; ERK, extracellular signal‐regulated kinase; MEK, mitogen‐activated protein kinase; Akt, protein kinase B (PKB); S6K, ribosomal protein S6 kinase; eEF2K, eukaryotic elongation factor‐2 kinase; 4E‐BP, eukaryotic initiation factor 4E (eIF4E)‐binding proteins; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CREB, cAMP response element‐binding protein
These issues from the literature show that mTOR is a key signalling pathway related to the effectiveness of antidepressants and its activation culminates in neuroplasticity and behavioural responses critical to antidepressant treatments. Therefore, agents that interfere more directly on the mTOR pathway are targets that should be of particular interest for new treatments for MDD. However, possible side effects from powerful and persistent increase of mTOR activity, which can induce cell proliferation and tumours 96, as well as effects on other mechanisms induced by agents already known, for example, psychotic symptoms from higher concentrations of ketamine 97 and others side effects are features that require further research strategies. Thus, research for more selective drugs targeting certain receptor subunits, as well as association of compounds that cause synergistic antidepressant effects are strategies that will contribute to the advancement in knowledge of neurobiological mechanisms and the discovery of more effective treatments 35, 73.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The Center for Translational Psychiatry (USA) is funded by the Department of Psychiatry and Behavioral Sciences, The University of Texas Medical School at Houston. Laboratory of Neurosciences (Brazil) is one of the centres of the National Institute for Molecular Medicine (INCT‐MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). Its research is supported by grants from CNPq (JQ, GZR), FAPESC (JQ), InstitutoCérebro e Mente (JQ) and UNESC (JQ). JQ is a 1A CNPq Research Fellow.ZMI and HMA are holders of a CAPES studentship and COA is holder of a CNPq. We thank Leandro D.V. Soares for making the figures.
Ignácio, Z. M. , Réus, G. Z. , Arent, C. O. , Abelaira, H. M. , Pitcher, M. R. , and Quevedo, J. (2016) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol, 82: 1280–1290. doi: 10.1111/bcp.12845.
References
- 1. World Health Organization . The Global Burden of Disease: 2004 Update. Geneva: WHO, 2008. [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 2003; 289: 3095–105. [DOI] [PubMed] [Google Scholar]
- 3. Spijker J, Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Functional disability and depression in the general population. Results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand 2004; 110: 208–14. [DOI] [PubMed] [Google Scholar]
- 4. Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, Faull K, Whitelegge J, Andrews AM, Loo J, Way B, Nelson SF, Horvath S, Lebowitz BD. Biomarkers to predict antidepressant response. Curr Psychiatry Rep 2010; 12: 553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt HD, Banasr M, Duman RS. Future antidepressant targets: neurotrophic factors and related signaling cascades. Drug Discov Today Ther Strateg 2008; 5: 151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodes E, Hill‐Smith TE, Lucki I. Fluoxetine treatment induces dose dependent alterations in depression associated behavior and neural plasticity in female mice. Neurosci Lett 2010; 484: 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ignácio ZM, Réus GZ, Abelaira HM, Quevedo J. Epigenetic and epistatic interactions between serotonin transporter and brain‐derived neurotrophic factor genetic polymorphism: insights in depression. Neuroscience 2014; 275: 455–68. [DOI] [PubMed] [Google Scholar]
- 8. Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008; 7: 426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zarate CA Jr, Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp Neurol 2008; 211: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002; 34: 13–25. [DOI] [PubMed] [Google Scholar]
- 11. Trivedi MH, Fava, Wisniewski SR , Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores‐Wilson K, Rush AJ, STAR*D Study Team . Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006; 354: 1243–52. [DOI] [PubMed] [Google Scholar]
- 12. Wullschleger S, Loewith R, Hall MN. mTOR signaling in growth and metabolism. Cell 2012; 124: 471–84. [DOI] [PubMed] [Google Scholar]
- 13. Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991; 253: 905–9. [DOI] [PubMed] [Google Scholar]
- 14. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 2010; 33: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004; 23: 3151–71. [DOI] [PubMed] [Google Scholar]
- 16. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006; 126: 955–68. [DOI] [PubMed] [Google Scholar]
- 17. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149: 274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Browne GJ, Proud CG. A novel mTOR‐regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol 2004; 24: 2986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide‐3'‐kinase‐Akt‐mammalian target of rapamycin pathway. J Neurosci 2005; 25: 11300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by ras‐PI3K‐Akt‐mTOR and ras‐MAPK signaling pathways. J Neurosci 2005; 25: 11288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E‐BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci 2005; 25: 9581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garelick MG, Kennedy BK. TOR on the brain. Exp Gerontol 2011; 46: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abelaira HM, Réus GZ, Neotti MV, Quevedo J. The role of mTOR in depression and antidepressant responses. Life Sci 2014; 101: 10–4. [DOI] [PubMed] [Google Scholar]
- 24. Maiese K Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res 2014; 9: 1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci 2014; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, Kim EJ, Choi JS, Kim S, Rhim H, Kaang BK, Son H. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal 2008; 20: 714–25. [DOI] [PubMed] [Google Scholar]
- 27. Takei N, Kawamura M, Ishizuka Y, Kakiya N, Inamura N, Namba H, Nawa H. Brain‐derived neurotrophic factor enhances the basal rate of protein synthesis by increasing active eukaryotic elongation factor 2 levels and promoting translation elongation in cortical neurons. J Biol Chem 2009; 284: 26340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manadas B, Santos AR, Szabadfi K, Gomes JR, Garbis SD, Fountoulakis M, Duarte CB. BDNF‐induced changes in the expression of the translation machinery in hippocampal neurons: protein levels and dendritic mRNA. J Proteome Res 2009; 8: 4536–52. [DOI] [PubMed] [Google Scholar]
- 29. Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain‐derived neurotrophic factor induces mammalian target of rapamycin‐dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 2004; 24: 9760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu‐opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem 1998; 273: 23534–41. [DOI] [PubMed] [Google Scholar]
- 31. Page G, Khidir FA, Pain S, Barrier L, Fauconneau B, Guillard O, Piriou A, Hugon J. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal‐regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int 2006; 49: 413–21. [DOI] [PubMed] [Google Scholar]
- 32. Baudry M, Greget R, Pernot F, Bouteiller J‐M, Xiaoning B. Roles of group I metabotropic glutamate receptors under physiological conditions and in neurodegeneration. WIREs Membr Transp Signal 2012; 1: 523–2. [Google Scholar]
- 33. Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR‐dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012; 62: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl) 2013; 230: 291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation‐dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci 2007; 27: 449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burket JA, Benson AD, Tang AH, Deutsch SI. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog Neuropsychopharmacol Biol Psychiatry 2015; 60: 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 2007; 53: 703–17. [DOI] [PubMed] [Google Scholar]
- 39. Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid‐acting antidepressants. Biol Psychiatry 2013; 73: 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeng S, Zarate CA Jr, Du J, Schloesser RJ, Mccammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid receptors. Biol Psychiatry 2008; 63: 349–52. [DOI] [PubMed] [Google Scholar]
- 41. Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 2007; 27: 11496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carroll M, Warren O, Fan X, Sossin WS. 5‐HT stimulates eEF2 dephosphorylation in a rapamycin‐sensitive manner in Aplysia neurites. J Neurochem 2004; 90: 1464–76. [DOI] [PubMed] [Google Scholar]
- 43. Carroll M, Dyer J, Sossin WS. Serotonin increases phosphorylation of synaptic 4EBP through TOR, but eukaryotic initiation factor 4E levels do not limit somatic cap‐dependent translation in aplysia neurons. Mol Cell Biol 2006; 26: 8586–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dagestad G, Kuipers SD, Messaoudi E, Bramham CR. Chronic fluoxetine induces region‐specific changes in translation factor eIF4E and eEF2 activity in the rat brain. Eur J Neurosci 2006; 23: 2814–8. [DOI] [PubMed] [Google Scholar]
- 45. Chotiner JK, Khorasani H, Nairn AC, O'Dell TJ, Watson JB. Adenylyl cyclase‐dependent form of chemical long‐term potentiation triggers translational regulation at the elongation step. Neuroscience 2003; 116: 743–52. [DOI] [PubMed] [Google Scholar]
- 46. Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Håvik B, Ying SW, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF‐induced LTP in vivo: evidence for compartment‐specific translation control. J Neurochem 2006; 99: 1328–37. [DOI] [PubMed] [Google Scholar]
- 47. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo‐controlled clinical trial. Arch Gen Psychiatry 2006; 63: 1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo‐controlled clinical trial. Biol Psychiatry 2010; 67: 432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry 2013; 73: 1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74: 742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase‐3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry 2007; 61: 240–5. [DOI] [PubMed] [Google Scholar]
- 53. Ota KT, Liu RJ, Voleti B, Maldonado‐Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, DiLeone RJ, Rex C, Aghajanian GK, Duman RS. REDD1 is essential for stress‐induced synaptic loss and depressive behavior. Nat Med 2014; 20: 531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry 2013; 40: 240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu W, Wang S, Liu M, Shi H, Zhang R, Liu J, Ding Z, Lu L. Glycine site N‐methyl‐D‐aspartate receptor antagonist 7‐CTKA produces rapid antidepressant‐like effects in male rats. J Psychiatry Neurosci 2013; 38: 306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ, Zhang HT, Cravatt BF, Liu QS. Monoacylglycerol lipase inhibition blocks chronic stress‐induced depressive‐like behaviors via activation of mTOR signaling. Neuropsychopharmacology 2014; 39: 1763–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang J, Xue W, Xia B, Ren L, Tao W, Chen C, Zhang H, Wu R, Wang Q, Wu H, Duan J, Chen G. Involvement of normalized NMDA receptor and mTOR‐related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep 2015; 5: 13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fang ZH, Lee CH, Seo MK, Cho H, Lee JG, Lee BJ, Park SW, Kim YH. Effect of treadmill exercise on the BDNF‐mediated pathway in the hippocampus of stressed rats. Neurosci Res 2013; 76: 187–94. [DOI] [PubMed] [Google Scholar]
- 59. Howell KR, Kutiyanawalla A, Pillai A. Long‐term continuous corticosterone treatment decreases VEGF receptor‐2 expression in frontal cortex. PLoS One 2011; 6: : e20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toth E, Gersner R, Wilf‐Yarkoni A, Raizel H, Dar DE, Richter‐Levin G, Levit O, Zangen A. Age‐dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem 2008; 107: 522–32. [DOI] [PubMed] [Google Scholar]
- 61. Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine‐induced antidepressant effects are associated with AMPA receptors‐mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 2014; 29: 419–23. [DOI] [PubMed] [Google Scholar]
- 62. Karolewicz B, Cetin M, Aricioglu F. Beyond the glutamate N‐methyl D‐aspartate receptor in major depressive disorder: the mTOR signaling pathway. Bull Clin Psychopharmacol 2011; 21: 1–6. [Google Scholar]
- 63. Park SW, Lee JG, Seo MK, Lee CH, Cho HY, Lee BJ, Seol W, Kim YH. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int J Neuropsychopharmacol 2014; 17: 1831–46. [DOI] [PubMed] [Google Scholar]
- 64. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–4. [DOI] [PubMed] [Google Scholar]
- 65. Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N‐methyl‐D‐aspartate antagonist in treatment‐resistant major depression. Arch Gen Psychiatry 2006; 63: 856–64. [DOI] [PubMed] [Google Scholar]
- 66. Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado‐Vieira R, Baumann J, Mallinger AG, Zarate CA Jr Rapid decrease in depressive symptoms with an N‐methyl‐d‐aspartate antagonist in ECT‐resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant‐like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–11. [DOI] [PubMed] [Google Scholar]
- 68. Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 2016; 100: 17–26. [DOI] [PubMed] [Google Scholar]
- 69. Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado‐Vieira R, Manji HK, Zarate CA Jr A randomized add‐on trial of an N‐methyl‐D‐aspartate antagonist in treatment‐resistant bipolar depression. Arch Gen Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer‐term antidepressant effects of repeated ketamine infusions in treatment‐resistant major depression. Biol Psychiatry 2013; 74: 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase‐3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 2011; 16: 1068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK‐3 inhibition potentiates the synaptogenic and antidepressant‐like effects of subthreshold doses of ketamine. Neuropsychopharmacology 2013; 38: 2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci 2013; 118: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N‐methyl‐D‐aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lima‐Ojeda JM, Vogt MA, Pfeiffer N, Dormann C, Köhr G, Sprengel R, Gass P, Inta D. Pharmacological blockade of GluN2B‐containing NMDA receptors induces antidepressant‐like effects lacking psychotomimetic action and neurotoxicity in the perinatal and adult rodent brain. Prog Neuropsychopharmacol Biol Psychiatry 2013; 45: 28–33. [DOI] [PubMed] [Google Scholar]
- 76. Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N‐methyl‐D‐aspartate antagonist, CP‐101, 606, in patients with treatment‐refractory major depressive disorder. J Clin Psychopharmacol 2008; 28: 631–7. [DOI] [PubMed] [Google Scholar]
- 77. Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ. GluN2B‐containing NMDA receptors regulate depression‐like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 2014; 3: : e0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cull‐Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001; 11: 327–35. [DOI] [PubMed] [Google Scholar]
- 79. Jiang B, Wang W, Wang F, Hu ZL, Xiao JL, Yang S, Zhang J, Peng XZ, Wang JH, Chen JG. The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol Psychiatry 2013; 74: 145–55.79. [DOI] [PubMed] [Google Scholar]
- 80. Chen KT, Tsai MH, Wu CH, Jou MJ, Wei IH, Huang CC. AMPA receptor‐mTOR activation is required for the antidepressant‐like effects of sarcosine during the forced swim test in rats: insertion of AMPA receptor may play a role. Front Behav Neurosci 2015; 9: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 2007; 32: 793–802. [DOI] [PubMed] [Google Scholar]
- 82. Abelaira HM, Réus GZ, Ribeiro KF, Steckert AV, Mina F, Rosa DV, Santana CV, Romano‐Silva MA, Dal‐Pizzol F, Quevedo J. Effects of lamotrigine on behavior, oxidative parameters and signaling cascades in rats exposed to the chronic mild stress model. Neurosci Res 2013; 75: 324–30. [DOI] [PubMed] [Google Scholar]
- 83. Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alonso M, Medina JH, Pozzo‐Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem 2004; 11: 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain‐derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22: 3251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant‐like effects in cellular and behavioral models. Neuropsychopharmacology 2010; 35: 2378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Warner‐Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 2007; 104: 4647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang C, Li WY, Yu HY, Gao ZQ, Liu XL, Zhou ZQ, Yang JJ. Tramadol pretreatment enhances ketamine‐induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J Biomed Biotechnol 2012; 2012: 175619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, ErnforsP CE. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant‐inducedbehavioral effects. J Neurosci 2003; 23: 349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain‐derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 2004; 101: 10827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Duman RS, Monteggia LM. A neurotrophic model for stress‐related mood disorders. Biol Psychiatry 2006; 59: 1116–27. [DOI] [PubMed] [Google Scholar]
- 92. Réus GZ, Dos Santos MA, Abelaira HM, Ribeiro KF, Petronilho F, Vuolo F, Colpo GD, Pfaffenseller B, Kapczinski F, Dal‐Pizzol F, Quevedo J. Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav Brain Res 2013; 242: 40–6. [DOI] [PubMed] [Google Scholar]
- 93. Murphy GM, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry 2004; 1: 1163–9. [DOI] [PubMed] [Google Scholar]
- 94. Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao C‐J, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxietyrelated behavior. Science 2006; 314: 140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta‐analysis of serotonin transporter gene promoter polymorphism (5‐HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 2007; 12: 247–57. [DOI] [PubMed] [Google Scholar]
- 96. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK‐801, and amphetamine on regional brain 2‐deoxyglucose uptake in freely moving mice. Neuropsychopharmacology 2000; 22: 400–12. [DOI] [PubMed] [Google Scholar]


