Abstract
Heterozygous kinase domain mutations or homozygous extracellular domain mutations in FGFR1 have been reported to cause Hartsfield syndrome (HS), which is characterized by the triad of holoprosencephaly, ectrodactyly and cleft lip/palate. To date, more than 200 mutations in FGFR1 have been described; however, only 10 HS-associated mutations have been reported thus far. We describe a case of typical HS with hypogonadotropic hypogonadism (HH) harboring a novel heterozygous mutation, p.His253Pro, in the extracellular domain of FGFR1. This is the first report of an HS-associated heterozygous mutation located in the extracellular domain of FGFR1, thus expanding our understanding of the phenotypic features and further developmental course associated with FGFR1 mutations.
Hartsfield syndrome (HS; OMIM #615465) is characterized by the triad of holoprosencephaly (HPE), ectrodactyly and cleft lip/palate, and more than 15 cases have been reported to date.1–11 However, the causative gene responsible for HS remained unknown until recently, when Simonis et al.9 identified homozygous or heterozygous mutations in the fibroblast growth factor receptor 1 (FGFR1) in several cases of HS. Since this discovery, 10 mutations in FGFR1 have been reported in HS patients.9–11 Here, we describe a case of typical HS (HPE, ectrodactyly and cleft lip/palate) harboring a novel heterozygous p.His253Pro mutation in the extracellular domain of FGFR1.
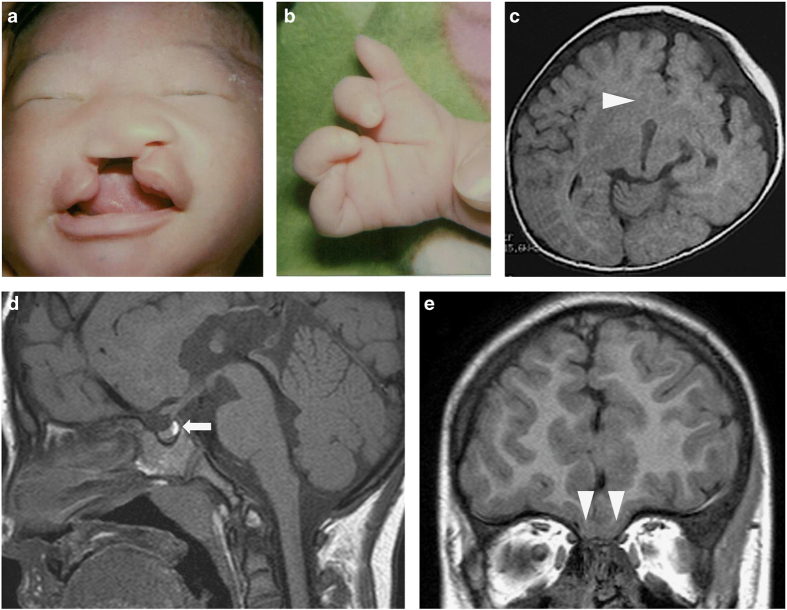
The details of the clinical course in this patient’s early childhood were described by Takenouchi et al.8 previously. In brief, he was born at 42 weeks of gestation with a constellation of malformations, including a cleft lip and palate (Figure 1a), a depressed nasal bridge, bilateral ectrodactyly of the hands (Figure 1b) and a micropenis (2.0 cm) with undescended testes. Brain magnetic resonance imaging (MRI) at the age of 3 months revealed semilobar HPE (Figure 1c). Owing to his midline defect and micropenis, his pituitary gland function was evaluated at the age of 3 months. The secretions of luteinizing hormone and follicle-stimulating hormone (FSH) in response to gonadotropin-releasing hormone (GnRH) were decreased (Table 1) in the period of ‘mini-puberty,’ indicating hypogonadotropic hypogonadism (HH). Brain MRI at the age of 5 years showed no abnormalities in the pituitary gland (Figure 1d), whereas the absence of the olfactory bulb and fusion of the olfactory gyri were shown (Figure 1e).
Figure 1.
Clinical and radiographic features of the patient. (a) Photographs of the patient as a neonate. (b) Photographs of the patient’s hands. The patient’s left hand as a neonate is shown. Both hands had four fingers with deep gaps between the second and third digits. (c) Axial image of the brain MRI at 3 months of age. The fusion of the caudate nuclei is shown (arrowhead). (d and e) Sagittal image (d) and coronal image (e) of the brain MRI at 5 years of age showing no abnormalities in the pituitary gland (arrow) and the absence of olfactory bulbs and olfactory gyri (arrowhead). MRI, magnetic resonance image.
Table 1. Endocrinological findings in the patient.
| Stimulus |
3 Months |
11 Years |
Reference |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | Peak | Basal | Peak | Basal | Peak | ||||
| GH (ng/ml) | Insulin | 3.9 | → | 11.8 | >6 | ||||
| LH (mIU/ml) | GnRH | <0.2 | → | 0.9 | <0.1 | → | 0.43 | 0.17–1.63a | 13.11–25.15a |
| FSH (mIU/ml) | GnRH | <0.2 | → | 0.9 | 1.10 | → | 2.24 | 2.12–5.24a | 5.75–13.25a |
| ACTH (pg/ml) | Insulin | 28 | → | 440 | 9.8–27.3 | 28–130.5 | |||
| Cortisol (μg/dl) | Insulin | 24.9 | → | 44.1 | 5–20 | 19.8b | |||
| TSH (mIU/ml) | TRH | 2.42 | → | 20.04 | 10–35 | ||||
| PRL (ng/ml) | TRH | 6.0 | → | 137.0 | 1.7–15.4 | Increase two times | |||
| IGF-1 (ng/ml) | 32.4 | 11–149c | |||||||
| Free T4 (ng/dl) | 1.3 | 1.01–1.95 | |||||||
| Testosterone (ng/ml) | <0.1 | <0.03 | |||||||
The conversion factors to the SI unit are as follows: GH 1.0 (μg/l), TSH 1.0 (mIU/l), LH 1.0 (IU/l), FSH 1.0 (IU/l), testosterone, 0.035 (nmol/l), prolactin 1.0 (μg/l), ACTH 0.22 (pmol/l), cortisol 27.59 (nmol/l), IGF-I 0.131 (nmol/l), free T4 12.87 (pmol/l) and free T3, 1.54 (pmol/l).
Abbreviations: ACTH, adrenocorticotropin; FSH, follicle-stimulating hormone; GH, growth hormone; GnRH, gonadotropin-releasing hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; PRL, prolactin; TRH, thyrotropin-releasing hormone; TSH, thyroid-stimulating hormone.
Reference data of pubertal (Tanner stage II-III) Japanese boys.
Reference data of UK children (younger than 10 years).
Reference data of Japanese boys (0 years old).
At the age of 11 years, he showed bilateral undescended testes, no pubic hair (P1) and a micropenis (3 cm). His height was 136.9 cm (−1.1 s.d.) and his weight was 33.6 kg (−0.6 s.d.). Hormonal assays revealed very low plasma testosterone levels. The HH diagnosis was confirmed again by a GnRH-stimulating test (Table 1). Ultrasonography and MRI revealed that bilateral testes were undetectable. After recombinant FSH pretreatment, gonadotropin replacement therapy was started at the age of 13 years and 9 months. Bilateral testes became detectable (right: 1.8×0.8 cm, left: 1.4×0.6 cm) in inguinal canals, and bilateral orchiopexy was performed at the age of 14 years. During his last examination at the age of 14 years and 2 months, his height was 149.3 cm (−2.2 s.d.) and his weight was 45.8 kg (−0.8 s.d.).
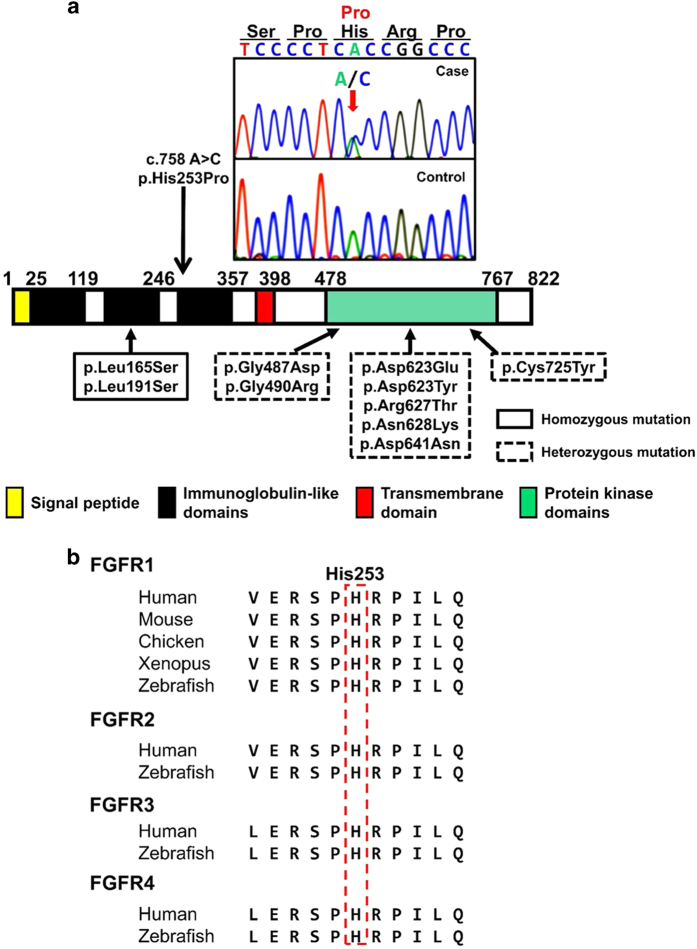
This study was approved by the Institutional Review Board of Tokyo Metropolitan Children’s Medical Center (H25–73). We checked all coding exons and flanking introns of FGFR1, and found a novel de novo heterozygous c.758A>C transition (p.His253Pro) in the patient (Figure 2a). This sequence variation was absent from all selected databases, including the dbSNP, 1000 Genomes Project, Exome Variant Server, NHLBI Exome Sequencing Project and Human Genetic Variation Database for the Japanese population databases. His253 is located at the third immunoglobulin-like domain in the extracellular region of FGFR1 (Figure 2a) and is an evolutionarily conserved residue in all FGFR families (Figure 2b).
Figure 2.
Identification of the sequence variation of FGFR1. (a) Partial sequence of PCR product and schematic diagrams of the FGFR1 protein. The chromatogram exhibits a heterozygous substitution of proline (CCC) in place of histidine (CAC) at codon 253, which is located at the amino terminus of the third immunoglobulin-like domain in the extracellular region of FGFR1. The red arrow indicates the mutated nucleotide. A summary of the reported 10 missense mutations identified in Hartsfield syndrome patients is presented. (b) His253 is a highly conserved amino acid in all four FGFRs. FGFR, fibroblast growth factor receptor.
Because both of the two reported HS-associated mutations in the extracellular domain of FGFR1 are homozygous,9 we performed multiplex ligation-dependent probe amplification (MLPA) analyses (SALSA MLPA KIT P133; MRC-Holland, Amsterdam, the Netherlands) to determine whether the patient had exon-level deletion or duplication of FGFR1 in another allele, with negative results.
We also analyzed all coding exons and flanking introns of SHH, GLI2, SIX3, TGIF1 and FGF8, which are the genes responsible for HPE, using PCR and direct sequencing. No mutation was found in these genes.
To date, more than 200 mutations in FGFR1 have been described; however, only 10 HS-associated mutations have been reported thus far. Among these 10 mutations, 8 were heterozygous within the intracellular protein kinase domain (p.Gly487Asp, p.Gly490Arg, p. Asp623Glu, p.Asp623Tyr, p.Arg627Thr, p.Asn628Lys, p.Asp641Asn and p.Cys725Tyr), whereas the remaining 2 were homozygous and were located in the second immunoglobulin-like domain in the extracellular region (p.Leu165Ser and p.Leu191Ser).9–11 Therefore, the possible mutation discovered in the present case, p.His253Pro, could be the first reported heterozygous HS-associated mutation located in the extracellular domain of FGFR1.
Most of the mutations at the third immunoglobulin-like domain in the extracellular domain of FGFR2 cause craniosynostosis syndromes, such as Crouzon syndrome (e.g., p.Ser252Leu, p.His254Tyr and p.Pro263Leu) or Apert syndrome (p.Ser252Trp and p.Pro253Arg). Notably, the mutation p.His254Tyr in FGFR2, which affects the 254th histidine residue (aligned with the 253rd histidine residue in FGFR1 (Figure 2b)), causes Crouzon syndrome,12 indicating the pathogenesis of p.His253Pro in FGFR1.
Using zebrafish overexpression assays, Hong et al.11 recently demonstrated that kinase domain mutations in FGFR1, resulting in HS/HPE phenotypes, showed dominant-negative effects, which were associated with the clinical severity of HS. Although the functional significance of our heterozygous mutation remains unknown, a de novo substitution in an evolutionarily highly conserved amino acid is likely to be pathogenic. However, further studies are necessary to clarify the contribution of heterozygous extracellular domain mutations in FGFR1 to the development of HS.
In summary, we describe a patient with typical HS harboring a novel de novo heterozygous sequence variation in FGFR1. This is the first report of an HS-associated heterozygous mutation located in the extracellular domain of FGFR1, thus expanding our understanding of the phenotypic features and further developmental course associated with FGFR1 mutations.
Acknowledgments
We thank the patient and his family for participation in this study. We also thank Kazue Kinoshita for technical assistance. This work was supported by a Grant from the Japan Society for the Promotion of Science (16K10007 to MT), Yamaguchi Endocrine Research Foundation (to MT) and Takeda Science Foundation (to MT) and was partly supported by a grant from the Foundation for Growth Science, Japan (to MT).
Footnotes
The authors declare no conflict of interest.
References
- Hartsfield J, Bixler D, DeMeyer W. Syndrome identification case report 119. Hypertelorism associated with holoprosencephaly and ectrodactyly. Clin Dysmorphol 1984; 2: 27–31. [Google Scholar]
- Young ID, Zuccollo JM, Barrow M, Fowlie A. Holoprosencephaly, telecanthus and ectrodactyly: a second case. Clin Dysmorphol 1992; 1: 47–51. [PubMed] [Google Scholar]
- Imaizumi K, Ishii T, Masuno M, Kuroki Y. Association of holoprosencephaly, ectrodactyly, cleft lip/cleft palate and hypertelorism: a possible third case. Clin Dysmorphol 1998; 7: 213–216. [DOI] [PubMed] [Google Scholar]
- Abdel-Meguid N, Ashour AM. Holoprosencephaly and split hand/foot: an additional case with this rare association. Clin Dysmorphol 2001; 10: 277–279. [DOI] [PubMed] [Google Scholar]
- König R, Beeg T, Tariverdian G, Scheffer H, Bitter K. Holoprosencephaly, bilateral cleft lip and palate and ectrodactyly: another case and follow up. Clin Dysmorphol 2003; 12: 221–225. [DOI] [PubMed] [Google Scholar]
- Vilain C, Mortier G, Van Vliet G, Dubourg C, Heinrichs C, de Silva D et al. Hartsfield holoprosencephaly-ectrodactyly syndrome in five male patients: further delineation and review. Am J Med Genet A 2009; 149A: 1476–1481. [DOI] [PubMed] [Google Scholar]
- Corona-Rivera A, Corona-Rivera JR, Bobadilla-Morales L, Garcia-Cobian TA, Corona-Rivera E. Holoprosencephaly, hypertelorism, and ectrodactyly in a boy with an apparently balanced de novo t(2;4) (q14.2;q35). Am J Med Genet 2000; 90: 423–426. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Okuno H, Kosaki R, Ariyasu D, Torii C, Momoshima S et al. Microduplication of Xq24 and Hartsfield syndrome with holoprosencephaly, ectrodactyly, and clefting. Am J Med Genet A 2012; 158A: 2537–2541. [DOI] [PubMed] [Google Scholar]
- Simonis N, Migeotte I, Lambert N, Perazzolo C, de Silva D, Dimitrov B et al. FGFR1 mutations cause Hartsfield syndrome, the unique association of holoprosencephaly and ectrodactyly. J Med Genet 2013; 50: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija R, Kirmani S, Wang X, Ferber MJ, Wieben ED, Lazaridis KN et al. Novel de novo heterozygous FGFR1 mutation in two siblings with Hartsfield syndrome: a case of gonadal mosaicism. Am J Med Genet A 2014; 164A: 2356–2359. [DOI] [PubMed] [Google Scholar]
- Hong S, Hu P, Marino J, Hufnagel SB, Hopkin RJ, Toromanović A et al. Dominant-negative kinase domain mutations in FGFR1 can explain the clinical severity of Hartsfield syndrome. Hum Mol Genet 2016. e-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Chun K, Teebi AS, Azimi C, Steele L, Ray PN. Screening of patients with craniosynostosis: molecular strategy. Am J Med Genet A 2003; 120A: 470–473. [DOI] [PubMed] [Google Scholar]
Data Citations
- Takagi Masaki.HGV Database. 2016. 10.6084/m9.figshare.hgv.873. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Takagi Masaki.HGV Database. 2016. 10.6084/m9.figshare.hgv.873. [DOI]