ABSTRACT
The utilization of simple sugars is widespread across all domains of life. In contrast, the breakdown of complex carbohydrates is restricted to a subset of organisms. A regulatory paradigm for integration of complex polysaccharide breakdown with simple sugar utilization was established in the mammalian gut symbiont Bacteroides thetaiotaomicron, whereby sensing of monomeric fructose regulates catabolism of both fructose and polymeric fructans. We now report that a different regulatory paradigm governs utilization of monomeric arabinose and the arabinose polymer arabinan. We establish that (i) arabinan utilization genes are controlled by a transcriptional activator that responds to arabinan and by a transcriptional repressor that responds to arabinose, (ii) arabinose utilization genes are regulated directly by the arabinose-responding repressor but indirectly by the arabinan-responding activator, and (iii) activation of both arabinan and arabinose utilization genes requires a pleiotropic transcriptional regulator necessary for survival in the mammalian gut. Genomic analysis predicts that this paradigm is broadly applicable to the breakdown of other polysaccharides in both B. thetaiotaomicron and other gut Bacteroides spp. The uncovered mechanism enables regulation of polysaccharide utilization genes in response to both the polysaccharide and its breakdown products.
IMPORTANCE
Breakdown of complex polysaccharides derived from “dietary fiber” is achieved by the mammalian gut microbiota. This breakdown creates a critical nutrient source for both the microbiota and its mammalian host. Because the availability of individual polysaccharides fluctuates with variations in the host diet, members of the microbiota strictly control expression of polysaccharide utilization genes. Our findings define a regulatory architecture that controls the breakdown of a polysaccharide by a gut bacterium in response to three distinct signals. This architecture integrates perception of a complex polysaccharide and its monomeric constituent as well as feedback of central metabolism. Moreover, it is broadly applicable to several prominent members of the mammalian gut microbiota. The identified regulatory strategy may contribute to the abundance of gut Bacteroides, despite fluctuations in the host diet.
INTRODUCTION
A well-established paradigm governs the importation and metabolism of simple sugars in a wide range of bacterial species: expression of genes encoding transport and metabolic functions is induced by a specific sugar and prevented by other preferred sugars (1). For example, utilization of arabinose by the bacterium Escherichia coli requires a multisubunit transporter to import arabinose from the periplasm to the cytoplasm and enzymes to shunt arabinose into the pentose phosphate pathway (2). These proteins are encoded within four operons coordinately regulated by AraC, a protein that represses transcription of these operons in the absence of arabinose but activates their transcription when arabinose is present (3). Transcription of the arabinose utilization operons is independently stimulated by the cyclic AMP (cAMP) receptor protein (CRP) (3), a protein allosterically activated by cyclic AMP, the levels of which increase when preferred sugars are absent (1).
By contrast, the ability to import and metabolize complex polysaccharides exhibits a more limited phylogenetic distribution. Complex polysaccharide utilization is prominent in the Bacteroidetes, an abundant phylum within the mammalian gut microbiota (4–6). For example, in Bacteroides thetaiotaomicron, the fructose polymer levan is broken down to fructo-oligosaccharides that are transported into the periplasm and catabolized to monomeric fructose (7). A single regulatory protein activated by fructose promotes transcription both of genes necessary for the transport and degradation of levan and of genes necessary for transporting fructose into the cytoplasm and its entry into glycolysis (7).
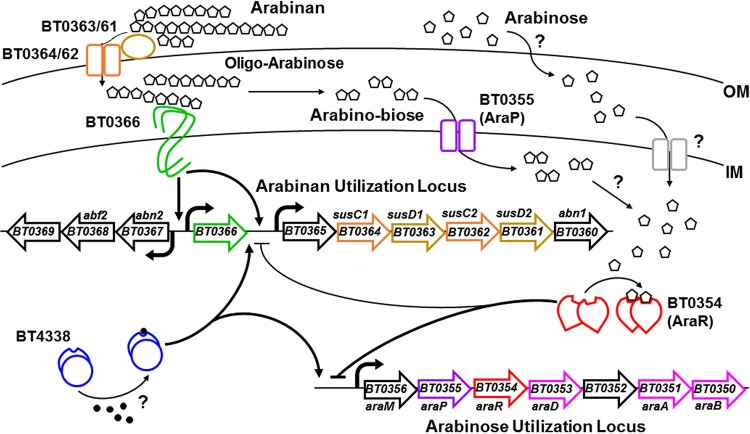
Analysis of the genes implicated in the utilization of the arabinose polymer arabinan and of monomeric arabinose suggests that their control is unlikely to follow the paradigm described for levan and fructose utilization because of the following. First, the sensing domain of the regulatory protein BT0366 binds arabinose polymers of six to eight subunits in length (4). Second, the BT0366 gene is required for growth on arabinan (4, 8) but not arabinose (8) and for transcription of genes within the BT0360-to-BT0369 arabinan polysaccharide utilization locus (PUL) (8). These genes encode proteins predicted to mediate transport and catabolism of arabinan (Fig. 1) (9–11). Third, putative arabinose utilization proteins are encoded within the independently transcribed BT0356-to-BT0350 operon, which specifies proteins thought to transport arabinose into the cytoplasm and convert it to xylulose-5-phosphate, an intermediate in the pentose phosphate pathway (10, 11).
FIG 1 .
Schematic of the regulation of arabinan and arabinose utilization genes in B. thetaiotaomicron. Arabinan is extracellularly bound by two SusD-like proteins and imported into the periplasm by two SusC-like transporters. Large arabinan polymers are broken down into polymers of six to eight subunits in chain length (oligoarabinose) and eventually smaller arabino-oligosaccharides, such as arabinobiose, which are transported into the cytoplasm by AraP. These oligosaccharides are broken down to arabinose in the cytoplasm by an unknown glycoside hydrolase. Oligoarabinose binds to and activates the transcriptional regulator BT0366, which, in turn, promotes transcription of the arabinan utilization genes BT0365 to -60, BT0366, and BT0367 to -69. l-Arabinose is transported into the cell by an unknown mechanism. Cytoplasmic arabinose prevents binding of the transcriptional repressor AraR to the promoters of the arabinan utilization gene BT0365 and the arabinose utilization gene BT0356 (araM). The transcriptional regulator BT4338 is necessary for full activation of arabinan and arabinose utilization genes. The signal controlling the activity of BT4338 is at present unknown. OM, outer membrane; IM, inner membrane.
We now report the genetic basis for transcriptional control of arabinan and arabinose utilization genes. We determine that a regulatory protein activated by oligoarabinose in the periplasm controls transcription not only of arabinan utilization genes directly but also of arabinose utilization genes indirectly, by enabling the generation of arabinose, which allosterically inactivates a repressor of arabinose utilization genes in the cytoplasm. We establish that an inner membrane transporter encoded within the arabinose utilization locus is dispensable for growth on monomeric arabinose but is required for utilization of arabino-oligosaccharides. Furthermore, we uncover a role of a pleiotropic transcriptional regulator in the expression of both arabinan and arabinose utilization genes, and we demonstrate its requirement for the utilization of several carbohydrates. Taken together with the genome analysis of polysaccharide utilization in other Bacteroides species, our findings argue that the use of multiple regulators responding to different signals constitutes a new paradigm for the utilization of complex polysaccharides.
RESULTS
A permease encoded in the arabinose utilization operon is necessary for arabinan catabolism.
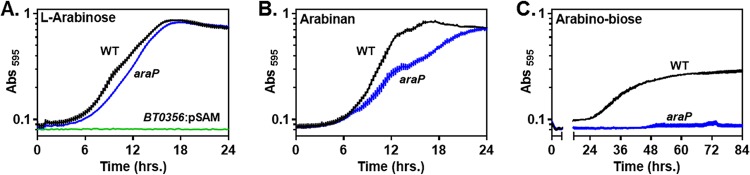
The BT0356-to-BT0350 operon specifies functions necessary for l-arabinose utilization because a polar transposon insertion in the BT0356 gene (Fig. 1) (12) prevented growth on l-arabinose (Fig. 2A) but not on fructose (see Fig. S1A in the supplemental material). Following BT0356 is the BT0355 gene, which encodes a putative permease designated AraP that is proposed to import arabinose from the periplasm into the cytoplasm (10, 11). Surprisingly, deletion of the BT0355 (araP) gene resulted in a very modest decrease in growth rate on l-arabinose compared to the wild-type strain (average growth rate of 0.056 versus 0.061 ΔA595/h; P = 0.27 by Student’s two-tailed t test) (Fig. 2A). These results imply that a transporter other than AraP can import arabinose into the cytoplasm.
FIG 2 .
An araP mutant exhibits a significant growth defect on arabinan. (A) Growth of three B. thetaiotaomicron strains, one harboring a polar transposon insertion in the BT0356 gene (BT0356:pSAM, NS423), one deleted for the araP gene (NS401), and the isogenic wild-type strain (WT, GT23), in minimal medium containing 0.5% arabinose. (B) Growth of isogenic araP strain (NS401) and wild-type B. thetaiotaomicron in minimal medium containing 0.5% arabinan. (C) Growth of isogenic araP strain (NS401) and wild-type B. thetaiotaomicron in minimal medium containing 0.5% arabinobiose. Graphed are the mean and standard error of the mean from at least five independent replicates grown in the same plate.
Because arabinose is generated during the breakdown of arabinose-containing polysaccharides, we hypothesized that araP is necessary for utilization of arabinan, a component of pectin comprised primarily of 1,5-linked arabinosyl residues (13), which is commonly found in the mammalian diet (14). As hypothesized, the araP mutant exhibited a growth defect on arabinan (Fig. 2B). The growth rate of the araP mutant was 0.035 ΔA595/h, significantly lower (P = 2.8 × 10−5 by Student’s two-tailed t test) than the wild-type growth rate of 0.067 ΔA595/h. However, the araP mutant reached the same final optical density as the isogenic wild-type strain by 24 h. By contrast, the araP mutant grew similarly to the wild-type strain in arabinogalactan (see Fig. S1B in the supplemental material), which contains terminal arabinofuranosyl side chains (13).
The results presented above suggested that AraP transports an intermediate in arabinan breakdown. In agreement with this notion, wild-type B. thetaiotaomicron grew on arabinobiose (the 1,5-linked α-l-arabinose disaccharide), albeit after a 24-h delay and to a lower growth yield than in l-arabinose or arabinan; however, the araP mutant did not grow on arabinobiose (Fig. 2C). Taken together, the results presented in this section establish that, despite being encoded in an arabinose utilization operon, AraP is necessary for normal arabinan catabolism and likely transports an arabino-oligosaccharide.
Arabinan promotes transcription of both arabinan and arabinose utilization genes.
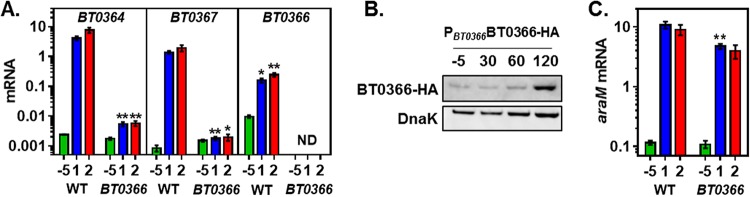
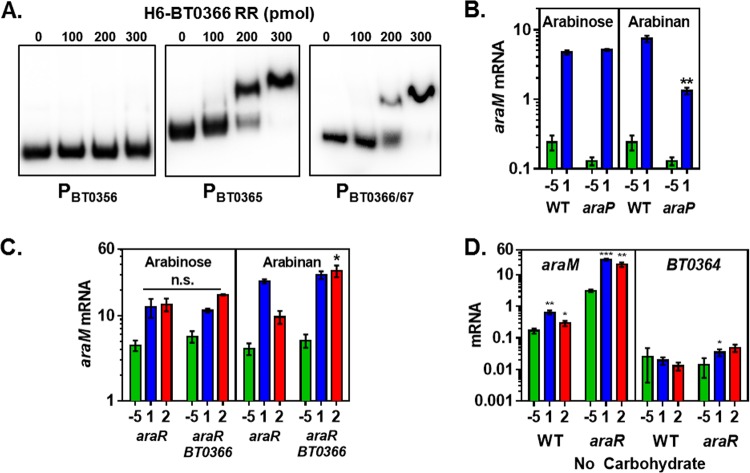
Because genes in the arabinose utilization locus are specifically involved in growth on arabinan (Fig. 2), we reasoned that the transcriptional activator of arabinan utilization genes, BT0366 (8), may control transcription of araP and other genes in the BT0356-to-BT0350 operon. Thus, we examined the mRNA levels of both arabinan and arabinose utilization genes in isogenic wild-type and BT0366 mutant strains following growth to mid-log phase in minimal medium containing 0.5% glucose as the sole carbon source and then switched to medium containing 0.1% arabinan.
The mRNA levels of the arabinan utilization genes BT0364 and BT0367 were >1,500-fold higher in the presence of arabinan than in the presence of glucose (Fig. 3A). Transcriptional activation of BT0367 was absent in the BT0366 mutant (Fig. 3A). By contrast, activation of BT0364 was dramatically decreased, but not abolished, in the BT0366 mutant (Fig. 3A). These results suggest that although BT0364 and BT0367 require BT0366 for complete transcriptional activation by arabinan, they are differentially regulated.
FIG 3 .
Arabinan promotes transcription of arabinan PUL genes and arabinose utilization genes in a BT0366-dependent manner. (A) mRNA levels of the BT0364, BT0367, and BT0366 genes in isogenic BT0366 (GT44) and wild-type (WT, GT23) B. thetaiotaomicron prior to the switch (−5) and after 1 and 2 h of exposure to minimal medium containing 0.1% arabinan. (B) Western blot of crude extracts from a strain specifying an HA-tagged BT0366 protein (NS204) collected from cultures grown to mid-log phase in minimal medium containing 0.5% glucose (−5) or 30, 60, and 120 min after switching to medium containing 0.1% arabinan. Data are representative of three independent experiments, which produced similar results. (C) mRNA levels of the araM gene in isogenic BT0366 mutant (GT44) and wild-type (GT23) B. thetaiotaomicron prior to the switch (−5) and after 1 and 2 h of exposure to minimal medium containing 0.1% arabinan. Graphed are the mean and standard error of the mean from at least three independent experiments. Asterisks indicate significant differences from the wild-type strain for BT0364 and BT0367 expression and significant difference from the −5 sample for BT0366 expression (*, P ≤ 0.05; **, P ≤ 0.01 by two-tailed Student’s t test). Note log scale of y axis in panels A and C.
The mRNA levels of the BT0366 gene were 15-fold higher in arabinose than in glucose (Fig. 3A), which resulted in larger amounts of chromosomally encoded epitope-tagged BT0366 protein (Fig. 3B). These results suggested that the regulator of arabinan utilization genes positively regulates its own transcription.
The mRNA levels of the arabinose utilization gene BT0356 (araM) increased 90-fold in the presence of arabinan (Fig. 3C). However, in contrast to the results obtained with the BT0364 and BT0367 genes (Fig. 3A), deletion of the BT0366 gene decreased araM mRNA levels only 2-fold (Fig. 3C). The increase of araM mRNA levels observed in the BT0366 mutant upon exposure to arabinan (Fig. 3C) appears to result from arabinose-containing polysaccharide contamination, because an ~30-fold increase in araM mRNA levels was still observed in this strain upon exposure to dialyzed arabinan (see Fig. S2A in the supplemental material). Moreover, the BT0366 mutant retained wild-type growth on l-arabinose as the sole carbon source (see Fig. S2B). Cumulatively, these results, which are in agreement with previous reports (4, 8), establish that arabinan promotes transcription of arabinan and arabinose utilization genes and that this activation is strictly dependent on BT0366 for the genes in the arabinan PUL but only moderately dependent on BT0366 for the arabinose utilization genes.
AraR represses transcription of arabinose utilization genes in the absence of arabinose.
Investigation of the in vitro properties of the regulatory protein AraR showed that it binds to DNA sequences located upstream of arabinose utilization gene BT0356 and arabinan utilization gene BT0365 (11) (see Fig. S3 in the supplemental material) and that binding to these DNAs was prevented when l-arabinose was present in the reaction mixture (11), suggesting that l-arabinose is an allosteric regulator of AraR. However, the in vivo function of AraR has remained unknown.
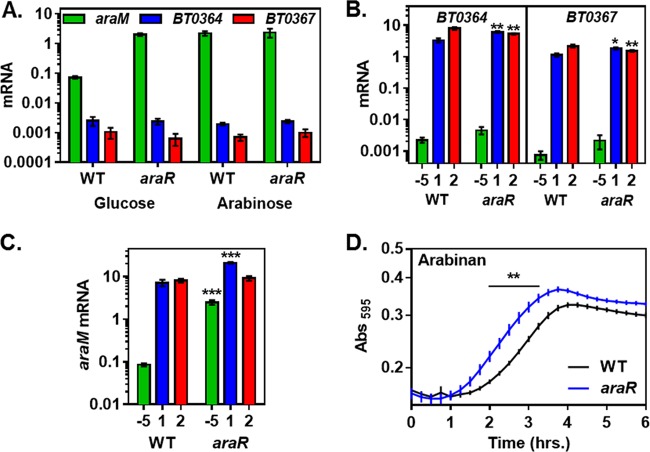
In wild-type B. thetaiotaomicron, araM mRNA levels were ~30-fold higher following growth in arabinose than in glucose (Fig. 4A). By contrast, an araR-deficient mutant displayed the same high mRNA levels during growth on arabinose and on glucose, which were similar to those observed in the wild-type strain grown in the presence of arabinose (Fig. 4A). Complementation of the araR mutant in trans restored araM transcription to the levels displayed by the wild-type strain (see Fig. S4A in the supplemental material). Taken together with the in vitro analysis of the AraR protein (11), our results indicate that AraR directly represses transcription of arabinose utilization genes and that this repression is antagonized by l-arabinose.
FIG 4 .
AraR is a repressor of arabinose and arabinan utilization genes. (A) mRNA levels of the araM, BT0364, and BT0367 genes in isogenic araR (NS367) and wild-type (WT, GT23) B. thetaiotaomicron strains following growth in minimal medium containing 0.5% of either arabinose or glucose. (B) mRNA levels of the arabinan PUL genes BT0364 and BT0367 in isogenic araR (NS367) and wild-type (GT23) B. thetaiotaomicron strains prior to the switch (−5) and after 1 and 2 h of exposure to minimal medium containing 0.1% arabinan. (C) mRNA levels of the arabinose utilization gene araM in isogenic araR (NS367) and wild-type (GT23) B. thetaiotaomicron strains after 1 and 2 h of exposure to minimal medium containing 0.1% arabinan and prior to the switch (−5) to medium containing arabinan. (D) Growth of isogenic araR (NS367) and wild-type B. thetaiotaomicron (GT23) strains after switching from minimal medium containing 0.5% glucose to minimal medium containing 0.1% arabinan. Graphed are the mean and standard error of the mean from at least three independent experiments. Asterisks indicate significant difference from the wild-type strain (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 by two-tailed Student’s t test). Note log scale of y axis in panels A, B, and C.
AraR controls the transcription dynamics of arabinan utilization genes in the presence of arabinan.
Because arabinan catabolism generates arabinose, we wondered whether AraR might also regulate genes in the arabinan PUL, thereby providing feedback based on arabinose levels. However, the mRNA levels of the arabinan utilization genes BT0364 and BT0367 were not affected by inactivation of araR during growth on arabinose or glucose (Fig. 4A). Likewise, the araR mutant grew like the wild-type strain on arabinan (see Fig. S4B in the supplemental material).
The araR mutant exhibited altered expression dynamics of the BT0364 and BT0367 genes when B. thetaiotaomicron was switched from medium containing glucose to medium containing arabinan (Fig. 4B). In the wild-type strain, the mRNA levels of both BT0364 and BT0367 were >2-fold higher at 2 h than at 1 h after exposure to arabinan (Fig. 4B). By contrast, in the araR mutant, the mRNA levels of these two genes decreased between 1 and 2 h (Fig. 4B). These effects extend to genes necessary for the breakdown of arabinogalactan (see Fig. S4C in the supplemental material) but not rhamnogalacturonan I (see Fig. S4D), both of which contain arabinoyl residues. The mRNA levels of the arabinogalactan PUL gene BT0268, which encodes a SusC-like transporter similar to the BT0364 product, were ~1.5-fold higher in the araR mutant than in the isogenic wild-type strain following 1-h exposure to arabinogalactan (see Fig. S4C). By contrast, mRNA levels of the rhamnogalacturonan I PUL SusC-like protein-encoding gene BT4164 were nearly identical in the araR mutant and wild-type strains following exposure to rhamnogalacturonan I (see Fig. S4D). These arabinose-containing polysaccharides promoted an increase in the mRNA levels of the arabinose utilization gene araM (see Fig. S4C and D), presumably because arabinose is generated during their breakdown.
The mRNA levels of araM were only 3-fold higher in the araR mutant than in the wild-type strain 1 h after induction with arabinan, despite 30-fold-higher levels present during growth in glucose (Fig. 4C). However, the araR mutant displayed wild-type araM mRNA levels by 2 h (Fig. 4C). This is likely due to the breakdown of arabinan in the wild-type strain antagonizing AraR. The araR mutant initiated growth significantly faster than the isogenic wild-type strain following a switch from medium containing glucose to medium containing arabinan (Fig. 4D). This effect appears to be specific for arabinan because wild-type and araR strains grew similarly when switched to the unrelated polysaccharide chondroitin sulfate (see Fig. S4E in the supplemental material). Taken together, these results indicate that AraR is a transcriptional repressor that controls the expression kinetics of both arabinose and arabinan breakdown genes when B. thetaiotaomicron encounters arabinan.
The arabinan-responsive BT0366 protein indirectly regulates arabinose utilization genes.
The BT0366 protein appears to control transcription of arabinose utilization genes in the presence of arabinan because the mRNA levels of the araM gene were lower in the BT0366 mutant than in the isogenic wild-type strain (Fig. 3C). BT0366 may exert its regulatory effect directly, by binding to the araM promoter, or indirectly, by activating arabinan breakdown genes, thereby impacting cytoplasmic arabinose levels.
BT0366 does not appear to control araM mRNA levels directly (i.e., by binding to the BT0356-to-BT0350 promoter region), because the purified BT0366 protein did not shift a radiolabeled 207-bp fragment corresponding to the sequence immediately upstream of the araM start codon (see Fig. S3 in the supplemental material) in an electrophoretic mobility shift assay (EMSA) (Fig. 5A). By contrast, the BT0366 protein shifted the positive-control fragments (Fig. 5A) corresponding to the BT0365 promoter and the BT0367-BT0366 intragenic region (see Fig. S3).
FIG 5 .
BT0366 controls expression of arabinose utilization genes but does not bind to the corresponding promoter regions. (A) Electrophoretic mobility shift assay (EMSA) of a fragment of the BT0366 hybrid two-component system harboring the response regulator and DNA-binding domains with DNA fragments located upstream of the BT0365 coding region, intragenic to BT0366-BT0367, and upstream of the araM coding region. (B) mRNA levels of the araM gene in isogenic araP (NS401) and wild-type B. thetaiotaomicron (GT23) strains prior to the switch (−5) and after 1-h exposure to minimal medium containing 0.1% arabinose or 0.1% arabinan. (C) mRNA levels of the araM gene in isogenic araR (NS367) and araR BT0366 (NS422) strains prior to the switch (−5) and after 1- and 2-h exposure to minimal medium containing 0.1% arabinan. (D) mRNA levels of the araM and BT0364 genes in isogenic araR (NS367) and wild-type (GT23) strains after 1- and 2-h exposure to minimal medium lacking a carbohydrate. Graphed are the mean and standard error of the mean from at least three independent experiments. Asterisks indicate significant differences from the wild-type strain in panels B and C and significant difference from the −5 sample in panel D (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 by two-tailed Student’s t test). Note log scale of y axis in panels B, C, and D.
In agreement with the notion that BT0366 controls araM mRNA levels indirectly (i.e., by generating the AraP substrate, which is broken down into arabinose), the araP mutant produced ~5-fold-lower araM mRNA levels than the wild-type strain when bacteria were switched from medium containing glucose to medium containing arabinan (Fig. 5B). The mRNA levels of araM were similar in the araP mutant and wild-type strains when switched to medium containing arabinose (Fig. 5B). As expected, the araP mutant retained wild-type BT0364 mRNA levels upon induction with either arabinose or arabinan (see Fig. S5A in the supplemental material). These results suggest that BT0366 regulates transcription of arabinose utilization genes in the presence of arabinan by controlling the production of arabinose, which likely allosterically inactivates AraR.
The absence of accessible carbohydrates promotes sustained transcription of the arabinose utilization gene araM.
We hypothesized that, when grown in arabinan, an araR BT0366 double mutant would exhibit slightly lower araM mRNA levels than the araR single mutant. This is because the araR mutant exhibited higher araM mRNA levels than the wild-type strain in arabinan (Fig. 4C) and also because the BT0366 mutant displayed 2-fold-lower araM mRNA levels under these conditions (Fig. 3C). To examine this possibility, we measured araM mRNA levels in isogenic araR and araR BT0366 strains following exposure to arabinose or arabinan. The mRNA levels were similar in the two strains in arabinose (Fig. 5C), in agreement with the notion that BT0366 is dispensable for arabinose utilization (see Fig. S3B in the supplemental material).
In arabinan, however, the araR BT0366 double mutant exhibited sustained araM expression compared to the araR single mutant (Fig. 5C). That is to say, araM mRNA levels decreased between 1 and 2 h postinduction in the araR mutant but not in the araR BT0366 double mutant. Because the araR single mutant grew on arabinan (see Fig. S4B in the supplemental material) but the araR BT0366 double mutant did not (see Fig. S5B), the sustained araM expression exhibited by the latter strain may be triggered by lack of growth. In agreement with this notion, araM mRNA levels increased 9-fold in the araR mutant and 3-fold in the wild-type strain following a 1-h exposure to minimal medium lacking a carbohydrate (Fig. 5D). By contrast, BT0364 mRNA levels remained essentially unchanged when bacteria were switched from medium containing glucose to medium lacking a carbohydrate (Fig. 5D). The latter results presumably reflect that in the absence of arabinan, the BT0366 protein does not promote BT0364 transcription. Taken together, these results indicate that transcription of arabinose utilization genes responds to arabinose via AraR and to a signal produced under nutrient-poor conditions.
The global regulator BT4338 controls araM transcription in the presence of arabinan.
In E. coli, transcriptional activation of arabinose utilization genes requires binding of both the AraC-arabinose complex and the CRP-cyclic AMP (cAMP) complex to target promoters (3). The N-terminal region of the B. thetaiotaomicron BT4338 gene product contains a CRP-like effector domain, and its C terminus harbors a helix-turn-helix DNA-binding motif (15). Moreover, a bioinformatics analysis predicted BT4338 binding to the araM promoter region (10). Therefore, we hypothesized that BT4338, originally named MalR for its role in maltose utilization in the absence of the starch utilization regulator SusR (16), operates as an activator of arabinose utilization genes.
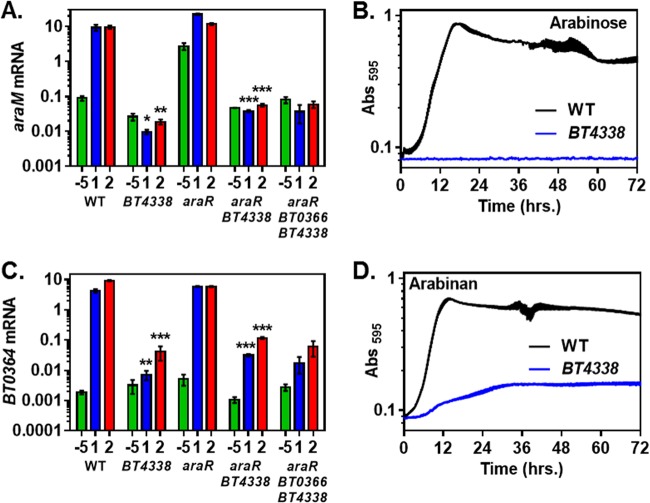
We examined araM mRNA levels in five isogenic strains—wild-type, BT4338, araR, araR BT4338, and araR BT0366 BT4338 strains—following a switch from medium containing glucose to medium containing arabinan. Deletion of BT4338 decreased the basal araM mRNA levels produced in glucose and abolished the induction promoted by arabinan (Fig. 6A). The araR BT4338 double mutant and the araR BT4338 BT0366 triple mutant displayed a similar behavior, though araM mRNA levels were 3- to 4-fold higher than in the BT4338 single mutant (Fig. 6A).
FIG 6 .
The regulatory gene BT4338 is essential for arabinan utilization. (A) mRNA levels of the araM gene in isogenic wild-type (GT23), BT4338 (NS364), araR (NS367), araR BT4338 (NS404), and araR BT0366 BT4338 (NS408) strains prior to the switch (−5) and after 1- and 2-h exposure to minimal medium containing 0.1% arabinan. (B) Growth of isogenic BT4338 (NS364) and wild-type (GT23) strains in minimal medium containing 0.5% arabinose. (C) mRNA levels of the BT0364 gene in isogenic wild-type (GT23), BT4338 (NS364), araR (NS367), araR BT4338 (NS404), and araR BT0366 BT4338 (NS408) strains prior to the switch (−5) and after 1- and 2-h exposure to minimal medium containing 0.1% arabinan. (D) Growth of isogenic BT4338 (NS364) and wild-type (GT23) strains in minimal medium containing 0.5% arabinan. For transcription experiments, the mean and standard error of the mean from at least three independent experiments are graphed. Asterisks indicate significant difference from the background strain containing BT4338 (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 by two-tailed Student’s t test). For growth experiments, graphed are the mean and standard error of the mean from at least five independent replicates grown in the same plate. Note log scale of y axis in panels A and C.
The BT4338 mutant was unable to grow on arabinose (Fig. 6B), reflecting its essential role in transcription of the arabinose utilization gene araM (Fig. 6A). By contrast, the BT4338 mutant reached a wild-type growth yield in the rich tryptone-yeast extract-glucose (TYG) medium, albeit with slightly slower kinetics (see Fig. S6A in the supplemental material). Cumulatively, the results in this section establish that BT4338 is required for transcriptional activation of arabinose utilization genes and that its role is not simply to overcome repression by AraR.
The BT4338 gene is necessary for full transcription of arabinan PUL genes.
Given the critical role that the BT4338 gene plays in transcription of arabinose utilization genes, we investigated whether BT4338 is also required for transcription of arabinan utilization genes. When B. thetaiotaomicron was exposed to arabinan, the mRNA levels of the arabinan PUL gene BT0364 were ~600-fold higher at 1 h and ~225-fold higher at 2 h in the wild-type strain than in the BT4338 mutant (Fig. 6C). The BT4338 araR double mutant displayed 3- to 4-fold-higher BT0364 mRNA levels than the BT4338 single mutant (Fig. 6C), analogous to the behavior of arabinose utilization genes (Fig. 6A). Furthermore, the BT4338 null mutant was defective for growth on arabinan (Fig. 6D). Expression of the BT4338 gene in trans from its native promoter and in single copy restored the ability of the BT4338 mutant strain to grow in both arabinan (see Fig. S6B in the supplemental material) and arabinose (see Fig. S6C), albeit with slightly decreased kinetics. Taken together, these results demonstrate that BT4338 is essential for B. thetaiotaomicron to utilize both monomeric arabinose and its polymeric form arabinan.
The BT4338 gene is required for growth utilizing a variety of carbohydrates.
Because the BT4338 protein has a domain structure similar to that of CRP, we explored the possibility of the BT4338 gene being required for growth on carbohydrates other than arabinose and arabinan (Fig. 6B and D). The BT4338 mutant displayed limited or no growth on arabinogalactan, fucose, glucuronate, N-acetylgalactosamine, polygalacturonic acid, ribose, or xylose (see Fig. S7A to G in the supplemental material). No difference in growth was observed between the BT4338 mutant and the wild-type strain in glucose, heparin, mannose, or N-acetylglucosamine (see Fig. S7H to K). The BT4338 mutant exhibited a longer lag phase than the isogenic wild-type strain in all other carbohydrates tested: amylopectin, chondroitin sulfate, fructose, galactose, galacturonate, maltose, maltotriose, α-mannan, pectic galactan, and rhamnogalacturonan I (see Fig. S7L to V). The difference in lag phase was short, ~2.6 h to an A595 of ≥0.2, in galactose (see Fig. S7L) but extended to ~24 h to an A595 of ≥0.2 in amylopectin (see Fig. S7M) and ~32 h to an A595 of ≥0.2 in galacturonate (see Fig. S7N). Taken together, these results indicate that BT4338 both contributes to wild-type growth kinetics of B. thetaiotaomicron and is essential for growth on a variety of carbohydrates.
DISCUSSION
We have uncovered how a gut bacterium integrates multiple signals to control expression of genes mediating the utilization of a polysaccharide and the monosaccharide derived from that polysaccharide (Fig. 1). The BT0366 hybrid two-component system of B. thetaiotaomicron senses an intermediate in arabinan breakdown in the periplasm and activates transcription of the arabinan PUL, which encodes products that transport arabinan into the cell and degrade it into arabinose.
Arabinose binding to the repressor AraR in the cytoplasm prevents AraR binding to the promoters of genes required for arabinose utilization and a subset of genes within the arabinan PUL (Fig. 4). The regulatory activities of BT0366 and AraR are connected by AraP, which transports arabinobiose (and potentially other arabino-oligosaccharides) originating from arabinan catabolism into the cytoplasm (Fig. 2 and 5B) and is encoded in the arabinose utilization locus (Fig. 1).
We established that BT4338 is a global regulator required for full transcriptional activation of the genes necessary to metabolize both arabinan and arabinose (Fig. 6), utilization of several carbohydrates (see Fig. S7A to G in the supplemental material), and wild-type growth kinetics on other carbohydrates (see Fig. S7L to V). Our in vivo analysis provides direct genetic evidence for regulatory interactions suspected on the basis of biochemical (11) and bioinformatics (10) analyses.
Taken together, our findings establish that B. thetaiotaomicron coordinates the utilization of arabinan, arabinose, and other nutritional signals. These coordinated processes may play a critical role in gut colonization because BT4338 and several genes within the arabinan PUL and arabinose utilization operon are necessary for survival in the murine gut (6, 12).
Bacteria use distinct strategies for arabinan and arabinose utilization.
Arabinan utilization is restricted to a subset of microorganisms, including Aspergillus spp. (17); Gram-positive soil bacteria, such as Bacillus subtilis (18); and mammalian gut Bacteroidetes (4, 10). B. subtilis encodes several endo-arabinases and α-l-arabinofuranosidases that catabolize arabinan to arabinose (18, 19). The genes specifying these enzymes are regulated solely by a distinct AraR protein that senses intracellular arabinose (18).
By contrast, arabinan breakdown in the Gram-negative bacterium B. thetaiotaomicron is regulated by an activator (BT0366) that senses a degradation product of arabinan in the periplasm, a repressor (AraR) that senses arabinose in the cytoplasm, and a global regulator (BT4338) that senses a yet-undescribed signal. The strategy for arabinan breakdown in B. thetaiotaomicron is reminiscent of the enzymatic capabilities of B. subtilis arabinan utilization and the regulatory framework governing arabinose utilization in E. coli, which relies on AraC operating both as an activator and as a repressor (3).
A new regulatory paradigm for polysaccharide utilization in Bacteroides.
We present a new regulatory paradigm governing utilization of arabinan and arabinose that relies on sensing both a degradation product of the polysaccharide and the monosaccharide (Fig. 1). We propose that variations on this paradigm are more common in the Bacteroidetes than the existing paradigm where a single regulator senses monomeric fructose and activates transcription of genes necessary for utilization of both levan and its constituent fructose (7). This is because of the following. (i) The regulator that senses fructose (BT1754) is thus far unique among the Bacteroidetes in sensing a monomeric sugar in the periplasm (7). Indeed, several characterized regulators sense polymers of two to eight monosaccharides in length (4, 20, 21). (ii) Several genes in B. thetaiotaomicron and other Bacteroides spp. are predicted to encode regulators that sense cytoplasmic monosaccharides (10, 11, 22, 23). (iii) The monosaccharides sensed by these regulators are components of complex dietary and mucosal polysaccharides encountered by Bacteroides in the gut (14). (iv) Bioinformatics analysis predicts that some of these regulators bind to promoters of genes not necessary for utilization of the monosaccharide that they sense (10).
The pleiotropic transcriptional regulator BT4338.
BT4338 was previously designated MalR because a transposon insertion in the BT4338 gene in a strain lacking a functional copy of the starch utilization regulator SusR decreased the ability of B. thetaiotaomicron to metabolize maltose and maltotriose (16). The domain structure of the BT4338 protein is similar to that of the Proteobacteria CRP (despite low sequence identity) with an N-terminal CRP/Fnr-like ligand-binding domain and a C-terminal DNA-binding domain (15, 24). CRP also controls expression of genes involved in adhesion (25, 26) and virulence (27, 28) in Proteobacteria. We have now determined that the pleiotropic regulatory protein BT4338 is necessary for utilization of multiple sugars (see Fig. S7 in the supplemental material). Therefore, BT4338 may connect expression of arabinan and arabinose utilization genes to a larger regulatory network that integrates polysaccharide utilization with additional metabolic signals and/or other physiological cues in B. thetaiotaomicron. The identification of the signal(s) controlling the levels and activity of the BT4338 protein may help us understand its critical role in the colonization of the mammalian gut (6).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. thetaiotaomicron strains were derived from strain VPI-5482 (15) and grown under anaerobic conditions at 37°C on brain heart infusion agar supplemented with 10% horse blood and in tryptone-yeast extract-glucose medium containing tetracycline (2 µg/ml), erythromycin (10 µg/ml), gentamicin (200 µg/ml), or 5-fluro-2′-deoxyuridine (FUdR) (200 µg/ml), when needed. All experiments with B. thetaiotaomicron were performed with cells grown anaerobically in minimal medium (9) supplemented with the indicated carbon sources and antibiotics when required. E. coli strains were derived from S17-1 and grown in LB medium containing 100 µg/ml ampicillin. All chemicals were purchased from Sigma except arabinan (sugar beet, P-ARAB), arabinobiose (O-ABI), pectic galactan (P-PGAPT), and rhamnogalacturonan I (P-RHAM1), which were purchased from Megazyme, and beta-d-(−)-fructose (MP Biomedicals). Dialyzed arabinan was prepared by incubating 10 ml of 5% (wt/vol) arabinan within a 3,500-molecular-weight-cutoff (MWCO) Slide-A-Lyzer dialysis cassette (Thermo) in 1.5 liters distilled water overnight at 4°C. All strains and plasmids used in this study are listed in Table S1 in the supplemental material. All oligonucleotides used in this study are listed in Table S2 in the supplemental material.
Strain construction.
Phusion high-fidelity polymerase was used to amplify all DNA fragments, which were ligated into vectors by T4 DNA ligase or NEBuilder HiFi DNA Assembly Master Mix (all products from NEB). Deletion mutants were generated using counterselectable allelic exchange (9).
Growth curve analysis.
Growth of B. thetaiotaomicron strains was examined as follows. Following overnight incubation in tryptone-yeast extract-glucose (TYG) medium, bacteria were subcultured at a 1:500 dilution directly into the indicated medium. Growth proceeded anaerobically and was monitored by A595 measurement in a Tecan Infinite F200 Pro microplate reader. Growth rate was quantified by identifying the absorbance where growth increased by 15% over the baseline (Amin) and the maximum growth immediately after exponential growth (Amax). The time points corresponding to these absorbances, Tmin and Tmax, respectively, were used to calculate growth rate as (Amax − Amin)/(Tmax − Tmin). To examine the effects of the araR mutation on growth adaptation to arabinan, cultures were grown overnight in minimal medium with 0.5% glucose and subcultured 1:50 into the same medium, and cells were grown to an optical density at 600 nm (OD600) of 0.3 to 0.4. Cells were harvested by centrifugation, resuspended in minimal medium lacking a carbon source, and incubated with 0.1% (wt/vol) arabinan or chondroitin sulfate.
Gene expression analysis and quantitative real-time PCR.
Time course gene expression analysis was carried out as described previously (20), with the following modifications. Cells were grown to an OD600 of 0.35 to 0.5 prior to induction. Cells were harvested by centrifugation and resuspended in medium containing the indicated carbon sources. One-milliliter culture samples were collected before (−5-min time point) and at the indicated times after introduction to medium containing the indicated carbon sources. mRNA levels of genes were measured as described previously (29). mRNA levels are represented normalized to a 1,000-fold dilution of 16S rRNA abundance to account for cell density or as a fold change of values obtained from this normalization.
Western blot analysis.
To examine BT0366 hemagglutinin (HA) protein levels, minimal medium cultures (10 ml) were harvested at each time point before (0.5% glucose) and after (0.1% arabinan) induction. Western blotting was performed as described previously (29). Membranes were immunoblotted with anti-HA (Sigma) or anti-E. coli DnaK (Clontech) antibodies.
Electrophoretic mobility shift assays.
Electrophoretic mobility shift assays were carried out as described previously (30), with the following modifications. Fragments were amplified from B. thetaiotaomicron VPI 5482 genomic DNA. The BT0366 response regulator domain used for binding was purified as described previously (29).
SUPPLEMENTAL MATERIAL
The araP gene contributes to growth in arabinose-containing polysaccharides. (A) Growth of the BT0356:pSAM (NS423) and wild-type (GT23) B. thetaiotaomicron strains in minimal medium containing 0.5% fructose. (B) Growth of the araP (NS401) and wild-type (GT23) B. thetaiotaomicron strains in minimal medium containing 0.5% arabinogalactan. Graphed are the mean and standard error of the mean from five independent replicates grown in the same plate. Download
(A) mRNA levels of the araM gene in the BT0366 (GT44) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to minimal medium (MM) containing 0.1% 3.5-kDa dialyzed arabinan. Graphed are the mean and standard error of the mean from three independent experiments. (B) Growth of the BT0366 (GT44) and isogenic wild-type (GT23) strains in minimal medium containing 0.5% arabinose. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Download
Nucleotide sequence of the promoter regions corresponding to the BT0356, BT0365, and BT0366 to -67 genes. Note that the BT0366-to-BT0367 region is in the positive-sense orientation for BT0367. Boxed are the in silico predicted binding sites for AraR (red), BT0366 (green), and BT4338 (blue) (D. A. Ravcheev, A. Godzik. A. L. Osterman, and D. A. Rodionov, BMC Genomics 14:873, 2013, http://dx.doi.org/10.1186/1471-2164-14-873). Boldface indicates the predicted −7 box sequences (E. H. Patel, L. V. Paul, S. Patrick, and V. R. Abratt, Res Microbiol 159:678–684, 2008, http://dx.doi.org/10.1016/j.resmic.2008.09.002). Italics indicate predicted start codons. Brackets indicate the fragments used for electrophoretic mobility shift assays (Fig. 5A). Download
AraR controls expression of arabinose and arabinan utilization genes. (A) mRNA levels of the araM and BT0364 genes in a B. thetaiotaomicron strain deleted for araR complemented with araR expressed from the araM promoter in single copy on the chromosome (araR + pNBU: araR, NS441) or the empty vector (araR + pNBU, NS440) and the isogenic wild-type strain with the empty vector (WT + pNBU, VR86) at steady state in minimal medium (MM) containing 0.5% glucose. Graphed are the mean and standard error of the mean from at least three independent experiments. (B) Growth of the araR (NS367) and isogenic wild-type (GT23) strains in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. (C) mRNA levels of the araM and BT0268 genes in the araR (NS367) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to MM containing 0.1% arabinogalactan. (D) mRNA levels of the araM and BT4164 genes in the araR (NS367) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to MM containing 0.1% rhamnogalacturonan. (E) Growth of araR (NS367) or the isogenic wild-type (GT23) after switching from MM containing 0.5% glucose to MM containing 0.1% chondroitin sulfate. Graphed are the mean and standard error of the mean from at least three independent experiments. Asterisks indicate significant difference from the wild-type strain in panels C and D (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 by two-tailed Student’s t test). Note log scale of y axis in panels A, C, and D. Download
(A) mRNA levels of the BT0364 gene in isogenic araP (NS401) and wild-type B. thetaiotaomicron (WT, GT23) strains prior to the switch (−5) and after 1-h exposure to minimal medium (MM) containing 0.1% arabinose or 0.1% arabinan. Graphed are the mean and standard error of the mean from four independent experiments. (B) Growth of the araR BT0366 (NS422) and isogenic wild-type (GT23) strains in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Note log scale of y axis in panel A. Download
The BT4338 gene is necessary for utilization of arabinose and arabinan. (A) Growth of the BT4338 (NS364) and isogenic wild-type (WT, GT23) strains in tryptone-yeast extract-glucose (TYG) medium. (B) Growth of the BT4338 strain complemented with BT4338 expressed from its native promoter in single copy on the chromosome (BT4338 + pNBU:BT4338, NS433) or the empty vector (BT4338 + pNBU, NS432) and the isogenic wild-type strain with the empty vector (WT + pNBU, VR86) in minimal medium (MM) containing 0.5% arabinose. (C) Growth of the strains listed in panel B in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Download
The BT4338 gene is necessary for growth in a subset of carbohydrates. Growth of the BT4338 (NS364) and isogenic wild-type (GT23) strains in minimal medium containing 0.5% arabinogalactan (A), fucose (B), glucuronate (C), N-acetylgalactosamine (D), polygalacturonic acid (E), ribose (F), xylose (G), glucose (H), heparin (I), mannose (J), N-acetylglucosamine (K), galactose (L), amylopectin (M), galacturonate (N), chondroitin sulfate (O), fructose (P), maltose (Q), maltotriose (R), α-mannan (S), pectic galactan (T), rhamnogalacturonan (U), or rhamnose (V). Graphed are the mean and standard error of the mean from five independent replicates grown in the same plate. Download
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.
ACKNOWLEDGMENTS
We thank Andrew L. Goodman and his laboratory for providing the B. thetaiotaomicron BT0356 transposon insertion mutant.
Funding Statement
This work, including the efforts of Nathan D. Schwalm, was funded by HHS | National Institutes of Health (NIH) (T32 GM007499). This research was supported by funds provided by Yale University to E.A.G.
Footnotes
Citation Schwalm ND, III, Townsend GE, II, Groisman EA. 2016. Multiple signals govern utilization of a polysaccharide in the gut bacterium Bacteroides thetaiotaomicron. mBio 7(5):e01342-16. doi:10.1128/mBio.01342-16.
REFERENCES
- 1.Görke B, Stülke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Zhang T, Wu H. 2014. The transport and mediation mechanisms of the common sugars in Escherichia coli. Biotechnol Adv 32:905–919. doi: 10.1016/j.biotechadv.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Schleif R. 2010. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol Rev 34:779–796. doi: 10.1111/j.1574-6976.2010.00226.x. [DOI] [PubMed] [Google Scholar]
- 4.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI. 2011. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, Gordon JI. 2013. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol 11:e1001637. doi: 10.1371/journal.pbio.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin NW, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, Osterman AL, Gordon JI. 2015. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350:aac5992. doi: 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. 2010. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell 141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch JB, Sonnenburg JL. 2012. Prioritization of a plant polysaccharide over a mucus carbohydrate is enforced by a Bacteroides hybrid two-component system. Mol Microbiol 85:478–491. doi: 10.1111/j.1365-2958.2012.08123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens EC, Chiang HC, Gordon JI. 2008. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravcheev DA, Godzik A, Osterman AL, Rodionov DA. 2013. Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: comparative genomics reconstruction of metabolic and regulatory networks. BMC Genomics 14:873. doi: 10.1186/1471-2164-14-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C, Tesar C, Li X, Kim Y, Rodionov DA, Joachimiak A. 2015. A novel transcriptional regulator of l-arabinose utilization in human gut bacteria. Nucleic Acids Res 43:10546–10559. doi: 10.1093/nar/gkv1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. 2009. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeil M, Darvill AG, Fry SC, Albersheim P. 1984. Structure and function of the primary cell walls of plants. Annu Rev Biochem 53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- 14.Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 16.Cho KH, Cho D, Wang GR, Salyers AA. 2001. New regulatory gene that contributes to control of Bacteroides thetaiotaomicron starch utilization genes. J Bacteriol 183:7198–7205. doi: 10.1128/JB.183.24.7198-7205.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiboth B, Metz B. 2011. Fungal arabinan and l-arabinose metabolism. Appl Microbiol Biotechnol 89:1665–1673. doi: 10.1007/s00253-010-3071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo MP, Inácio JM, Mota LJ, de Sá-Nogueira I. 2004. Transcriptional regulation of genes encoding arabinan-degrading enzymes in Bacillus subtilis. J Bacteriol 186:1287–1296. doi: 10.1128/JB.186.5.1287-1296.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inácio JM, Correia IL, de Sá-Nogueira I. 2008. Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in Bacillus subtilis. Microbiology 154:2719–2729. doi: 10.1099/mic.0.2008/018978-0. [DOI] [PubMed] [Google Scholar]
- 20.Raghavan V, Lowe EC, Townsend GE II, Bolam DN, Groisman EA. 2014. Tuning transcription of nutrient utilization genes to catabolic rate promotes growth in a gut bacterium. Mol Microbiol 93:1010–1025. doi: 10.1111/mmi.12714. [DOI] [PubMed] [Google Scholar]
- 21.Lowe EC, Baslé A, Czjzek M, Firbank SJ, Bolam DN. 2012. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc Natl Acad Sci U S A 109:7298–7303. doi: 10.1073/pnas.1200479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel EH, Paul LV, Patrick S, Abratt VR. 2008. Rhamnose catabolism in Bacteroides thetaiotaomicron is controlled by the positive transcriptional regulator RhaR. Res Microbiol 159:678–684. doi: 10.1016/j.resmic.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. 1999. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci U S A 96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Körner H, Sofia HJ, Zumft WG. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev 27:559–592. doi: 10.1016/S0168-6445(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 25.Espert SM, Elsinghorst EA, Munson GP. 2011. The tib adherence locus of enterotoxigenic Escherichia coli is regulated by cyclic AMP receptor protein. J Bacteriol 193:1369–1376. doi: 10.1128/JB.00288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller CM, Aberg A, Straseviçiene J, Emody L, Uhlin BE, Balsalobre C. 2009. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog 5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skorupski K, Taylor RK. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci U S A 94:265–270. doi: 10.1073/pnas.94.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtiss R III, Kelly SM. 1987. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun 55:3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend GE II, Raghavan V, Zwir I, Groisman EA. 2013. Intramolecular arrangement of sensor and regulator overcomes relaxed specificity in hybrid two-component systems. Proc Natl Acad Sci U S A 110:E161–E169. doi: 10.1073/pnas.1212102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee EJ, Groisman EA. 2010. An antisense RNA that governs the expression kinetics of a multifunctional virulence gene. Mol Microbiol 76:1020–1033. doi: 10.1111/j.1365-2958.2010.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The araP gene contributes to growth in arabinose-containing polysaccharides. (A) Growth of the BT0356:pSAM (NS423) and wild-type (GT23) B. thetaiotaomicron strains in minimal medium containing 0.5% fructose. (B) Growth of the araP (NS401) and wild-type (GT23) B. thetaiotaomicron strains in minimal medium containing 0.5% arabinogalactan. Graphed are the mean and standard error of the mean from five independent replicates grown in the same plate. Download
(A) mRNA levels of the araM gene in the BT0366 (GT44) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to minimal medium (MM) containing 0.1% 3.5-kDa dialyzed arabinan. Graphed are the mean and standard error of the mean from three independent experiments. (B) Growth of the BT0366 (GT44) and isogenic wild-type (GT23) strains in minimal medium containing 0.5% arabinose. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Download
Nucleotide sequence of the promoter regions corresponding to the BT0356, BT0365, and BT0366 to -67 genes. Note that the BT0366-to-BT0367 region is in the positive-sense orientation for BT0367. Boxed are the in silico predicted binding sites for AraR (red), BT0366 (green), and BT4338 (blue) (D. A. Ravcheev, A. Godzik. A. L. Osterman, and D. A. Rodionov, BMC Genomics 14:873, 2013, http://dx.doi.org/10.1186/1471-2164-14-873). Boldface indicates the predicted −7 box sequences (E. H. Patel, L. V. Paul, S. Patrick, and V. R. Abratt, Res Microbiol 159:678–684, 2008, http://dx.doi.org/10.1016/j.resmic.2008.09.002). Italics indicate predicted start codons. Brackets indicate the fragments used for electrophoretic mobility shift assays (Fig. 5A). Download
AraR controls expression of arabinose and arabinan utilization genes. (A) mRNA levels of the araM and BT0364 genes in a B. thetaiotaomicron strain deleted for araR complemented with araR expressed from the araM promoter in single copy on the chromosome (araR + pNBU: araR, NS441) or the empty vector (araR + pNBU, NS440) and the isogenic wild-type strain with the empty vector (WT + pNBU, VR86) at steady state in minimal medium (MM) containing 0.5% glucose. Graphed are the mean and standard error of the mean from at least three independent experiments. (B) Growth of the araR (NS367) and isogenic wild-type (GT23) strains in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. (C) mRNA levels of the araM and BT0268 genes in the araR (NS367) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to MM containing 0.1% arabinogalactan. (D) mRNA levels of the araM and BT4164 genes in the araR (NS367) and isogenic wild-type (GT23) strains prior to the switch (−5) and after 1- and 2-h exposure to MM containing 0.1% rhamnogalacturonan. (E) Growth of araR (NS367) or the isogenic wild-type (GT23) after switching from MM containing 0.5% glucose to MM containing 0.1% chondroitin sulfate. Graphed are the mean and standard error of the mean from at least three independent experiments. Asterisks indicate significant difference from the wild-type strain in panels C and D (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 by two-tailed Student’s t test). Note log scale of y axis in panels A, C, and D. Download
(A) mRNA levels of the BT0364 gene in isogenic araP (NS401) and wild-type B. thetaiotaomicron (WT, GT23) strains prior to the switch (−5) and after 1-h exposure to minimal medium (MM) containing 0.1% arabinose or 0.1% arabinan. Graphed are the mean and standard error of the mean from four independent experiments. (B) Growth of the araR BT0366 (NS422) and isogenic wild-type (GT23) strains in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Note log scale of y axis in panel A. Download
The BT4338 gene is necessary for utilization of arabinose and arabinan. (A) Growth of the BT4338 (NS364) and isogenic wild-type (WT, GT23) strains in tryptone-yeast extract-glucose (TYG) medium. (B) Growth of the BT4338 strain complemented with BT4338 expressed from its native promoter in single copy on the chromosome (BT4338 + pNBU:BT4338, NS433) or the empty vector (BT4338 + pNBU, NS432) and the isogenic wild-type strain with the empty vector (WT + pNBU, VR86) in minimal medium (MM) containing 0.5% arabinose. (C) Growth of the strains listed in panel B in MM containing 0.5% arabinan. Graphed are the mean and standard error of the mean from six independent replicates grown in the same plate. Download
The BT4338 gene is necessary for growth in a subset of carbohydrates. Growth of the BT4338 (NS364) and isogenic wild-type (GT23) strains in minimal medium containing 0.5% arabinogalactan (A), fucose (B), glucuronate (C), N-acetylgalactosamine (D), polygalacturonic acid (E), ribose (F), xylose (G), glucose (H), heparin (I), mannose (J), N-acetylglucosamine (K), galactose (L), amylopectin (M), galacturonate (N), chondroitin sulfate (O), fructose (P), maltose (Q), maltotriose (R), α-mannan (S), pectic galactan (T), rhamnogalacturonan (U), or rhamnose (V). Graphed are the mean and standard error of the mean from five independent replicates grown in the same plate. Download
Bacterial strains and plasmids used in this study.
Oligonucleotides used in this study.