ABSTRACT
Biofilm-associated polymicrobial infections, particularly those involving fungi and bacteria, are responsible for significant morbidity and mortality and tend to be challenging to treat. Candida albicans and Staphylococcus aureus specifically are considered leading opportunistic fungal and bacterial pathogens, respectively, mainly due to their ability to form biofilms on catheters and indwelling medical devices. However, the impact of mixed-species biofilm growth on therapy remains largely understudied. In this study, we investigated the influence of C. albicans secreted cell wall polysaccharides on the response of S. aureus to antibacterial agents in biofilm. Results demonstrated significantly enhanced tolerance for S. aureus to drugs in the presence of C. albicans or its secreted cell wall polysaccharide material. Fluorescence confocal time-lapse microscopy revealed impairment of drug diffusion through the mixed biofilm matrix. Using C. albicans mutant strains with modulated cell wall polysaccharide expression, exogenous supplementation, and enzymatic degradation, the C. albicans-secreted β-1,3-glucan cell wall component was identified as the key matrix constituent providing the bacteria with enhanced drug tolerance. Further, antibody labeling demonstrated rapid coating of the bacteria by the C. albicans matrix material. Importantly, via its effect on the fungal biofilm matrix, the antifungal caspofungin sensitized the bacteria to the drugs. Understanding such symbiotic interactions with clinical relevance between microbial species in biofilms will greatly aid in overcoming the limitations of current therapies and in defining potential new targets for treating polymicrobial infections.
IMPORTANCE
The fungus Candida albicans and the bacterium Staphylococcus aureus are important microbial pathogens responsible for the majority of infections in hospitalized patients and are often coisolated from a host. In this study, we demonstrated that when grown together, the fungus provides the bacterium with enhanced tolerance to antimicrobial drugs. This process was mediated by polysaccharides secreted by the fungal cell into the environment. The biofilm matrix formed by these polysaccharides prevented penetration by the drugs and provided the bacteria with protection. Importantly, we show that by inhibiting the production of the fungal polysaccharides, a specific antifungal agent indirectly sensitized the bacteria to antimicrobials. Understanding the therapeutic implications of the interactions between these two diverse microbial species will aid in overcoming the limitations of current therapies and in defining new targets for treating complex polymicrobial infections.
INTRODUCTION
Polymicrobial infections caused by a combination of microorganisms are responsible for significant mortality and morbidity, particularly those associated with biofilms formed on indwelling medical devices (1–3). Biofilms are structured three-dimensional communities of surface-associated microbial populations embedded in a matrix of extracellular polysaccharides, proposed to provide a structural scaffold and protection for biofilm cells (4–6). Therefore, in a biofilm, microbes are afforded a stable environment and can tolerate high concentrations of antimicrobials. The impact of these biofilms on public health is dramatic, as cells released from biofilms can migrate into the bloodstream and cause systemic infections with high mortality (7). Importantly, the increase in drug resistance has provided a strong impetus to understand the mechanisms of the enhanced tolerance of biofilm-associated infections to antimicrobial therapy and particularly polymicrobial infections. Although mixed fungal-bacterial infections tend to be the most complex and challenging to treat, the impact of these interactions on therapy remains largely understudied.
Among the fungal species, Candida albicans is the most common human pathogen, causing diseases ranging from superficial mucosal to life-threatening systemic infections (8–10). The ability of C. albicans to transition from commensal to pathogen is primarily the result of its aptitude for morphologically switching between yeast and hyphal forms (9, 11). In fact, the majority of C. albicans infections are associated with its ability to form biofilms, where adhesion of yeast cells to the substrate is followed by proliferation and hypha formation, resulting in a network of cells embedded in a matrix (7, 12, 13). Candida albicans biofilm matrix is complex, with major polysaccharide constituents being α-mannan, β-1,6-glucan, and β-1,3-glucan (14, 15). Although a relatively minor component, β-1,3-glucan is considered the critical matrix polysaccharide, as extracellular glucan has been linked to biofilm resistance to antifungals (16, 17). In fact, previous studies have shown elevated β-1,3-glucan levels to be characteristic of biofilm cells both in the fungal cell walls and as a secreted form. Of more significance, the increase in β-1,3-glucan secretion by biofilm cells was shown in vivo in animal models of catheter infection and disseminated candidiasis (12). Glucan synthase Fks1p is responsible for the synthesis of cell wall β-1,3-glucan during biofilm growth, and FKS1 disruption was shown to reduce manufacture and deposition of β-1,3-glucan in the biofilm matrix (18). Importantly, using strains with modulated FKS1 expression, a study by Nett et al. (18) demonstrated that reduction in expression rendered biofilms more susceptible to various antifungals, whereas overexpression resulted in increased resistance.
In various niches in the host, C. albicans coexists with various bacterial species, including Staphylococcus aureus (2, 19–21). Although S. aureus primarily exists as a commensal organism, this bacterial pathogen is implicated in a variety of diseases ranging from minor skin infections to more serious invasive diseases and specifically device-associated biofilm infections (22–24). With the emergence of methicillin-resistant S. aureus (MRSA), this ubiquitous pathogen is becoming an even greater therapeutic challenge (25, 26). S. aureus is a poor former of biofilms; however, together with C. albicans, this species forms a substantial biofilm where the fungus creates a scaffold for the bacteria (27–29). Our previous in vitro studies have demonstrated that S. aureus exhibits high affinity to the C. albicans hyphal form, as these species coadhere and interact synergistically in a biofilm. Further, our studies identified the C. albicans hypha-specific adhesin Als3p to be involved in the coadherence process (27).
The growing use of implanted medical devices is another reason why the incidence of Candida and staphylococcal infections has steadily increased, since the majority of these infections are emerging from biofilms formed on medical implants (10, 13, 23, 30, 31). In fact, a recent analysis of cases of endocarditis associated with an implanted device found ~25% of the infections to be polymicrobial, and in another study, 27% of nosocomial C. albicans bloodstream infections were estimated to be polymicrobial (32, 33). Importantly, S. aureus was found to be the third most commonly coisolated species with C. albicans (33). Although numerous studies have reported the coisolation of C. albicans and S. aureus from a multitude of diseases such as periodontitis, denture stomatitis, cystic fibrosis, keratitis, ventilator-associated pneumonia, and urinary tract catheter and burn wound infections, the clinical significance of their interaction in a host remains largely understudied, likely due to lack of suitable animal models (34–38). However, using a mouse model of oral infection, we recently demonstrated that upon onset of oral candidiasis (thrush), mice cocolonized with C. albicans and S. aureus suffered systemic bacterial infection with high morbidity and mortality (39).
The inherent characteristics of biofilms are multifactorial but are largely due to the extracellular matrix encasing the biofilm cells, which prevents drugs and other stresses from penetrating the biofilm. Therefore, S. aureus and C. albicans interactions in mixed biofilm infections may also impact response to antimicrobial therapy (39). Although enhanced in vitro tolerance to antimicrobials in C. albicans and staphylococcus mixed biofilms has been reported, the mechanism and specific factors behind these observations remain undefined (40, 41). To that end, in this study, experiments were designed to demonstrate the impact of C. albicans biofilm matrix and secreted components on the susceptibility of S. aureus to antibacterial drugs in biofilm, focusing on vancomycin, the drug of choice for treatment of MRSA infections. The overall goal of this study is to provide crucial insights into the enhanced tolerance of biofilm-associated polymicrobial infections to antimicrobial therapy.
RESULTS
Comparative assessment of the susceptibility of S. aureus to vancomycin in single and mixed biofilms with C. albicans.
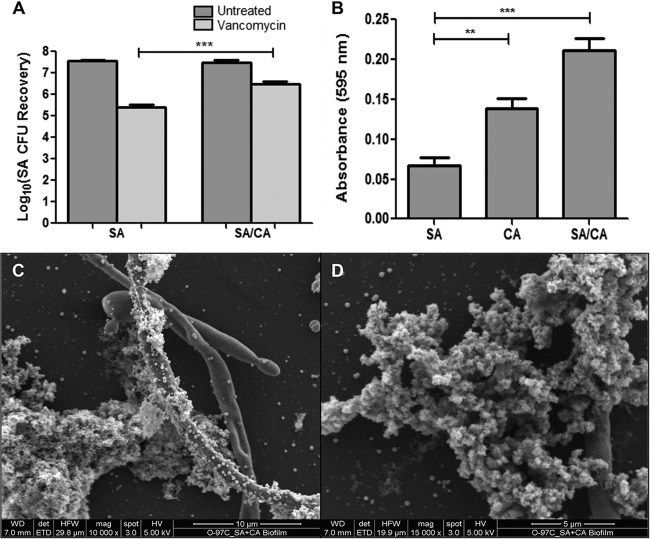
The impact of mixed-species biofilm growth on S. aureus susceptibility to vancomycin was assessed following 24-h treatment of preformed single and mixed biofilms. Based on CFU, results demonstrated a significant increase in S. aureus survival in mixed biofilms following vancomycin treatment compared to survival in single biofilm (Fig. 1A). Analysis of biofilms using crystal violet staining indicated a significant increase in biofilm biomass in mixed biofilms relative to S. aureus biofilm (Fig. 1B). These results were corroborated by scanning electron microscopy (SEM) analysis where images of mixed biofilms revealed a thick matrix, with S. aureus adhering to and forming aggregates around the C. albicans hyphae (Fig. 1C and D).
FIG 1 .
Assessment of relative biomass and vancomycin susceptibility in single S. aureus (SA) and mixed S. aureus and C. albicans (SA/CA) biofilms. (A) Preformed (24-h) S. aureus single and mixed biofilms were treated with vancomycin (800 µg/ml) for an additional 24 h. CFU recovery of S. aureus from both biofilms showed a significant increase in S. aureus recovery from mixed biofilms following vancomycin treatment (***, P < 0.001). (B) To assess biomass, 24-h single- and mixed-species biofilms were treated with crystal violet. Based on absorbance (595 nm), results demonstrated significantly higher biomass for mixed biofilms than for S. aureus biofilm (**, P < 0.01; ***, P < 0.001). Means and standard errors of the means are shown. (C and D) These results were corroborated by SEM analysis where images demonstrated adherence to and clumping of S. aureus around C. albicans hyphae, forming thick biofilm aggregates.
Exogenous supplementation of S. aureus biofilms with C. albicans biofilm matrix material enhances S. aureus tolerance to vancomycin.
To isolate the role of C. albicans biofilm matrix in the enhanced tolerance of S. aureus to vancomycin in mixed biofilms, purified matrix material recovered from C. albicans biofilms was incorporated in susceptibility testing. Assessment of S. aureus viability by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] metabolic assay and CFU recovery demonstrated a significant increase in S. aureus survival in single biofilms formed in the presence of matrix material (Fig. 2). In order to identify the contribution of specific matrix components, similar experiments were also performed where S. aureus biofilms were allowed to form in the presence of glucan or mannan. Results from these experiments demonstrated a comparable increase in survival with vancomycin.
FIG 2 .
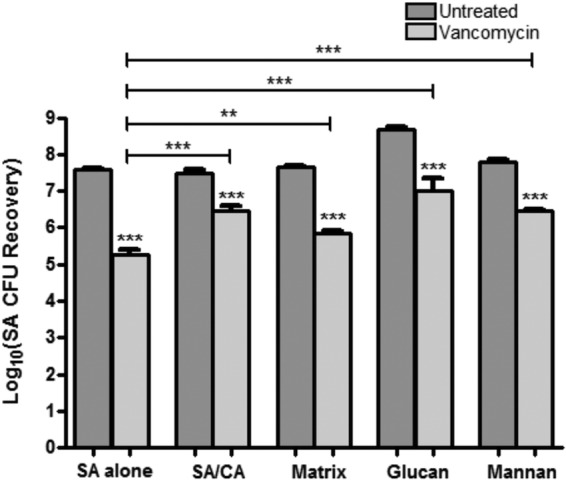
Effect of C. albicans matrix polysaccharides on S. aureus (SA) susceptibility to vancomycin. S. aureus single biofilms were allowed to form in the presence of purified C. albicans (CA) biofilm matrix material (0.02 mg/liter), glucan (1 mg/ml), or mannan (1 mg/ml). S. aureus dual-species biofilms were grown with C. albicans (SA/CA). Preformed biofilms were treated with vancomycin (800 µg/ml) for 24 h. S. aureus CFU recovery from biofilms demonstrated significant increase in S. aureus tolerance to vancomycin in the presence of matrix material, glucan, and mannan. Susceptibility to vancomycin was assessed by CFU counts and corroborated by an MTS assay (**, P < 0.01; ***, P < 0.001). Means and standard errors of the means are shown.
Differential C. albicans β-1,3-glucan but not α-mannan expression modulates vancomycin tolerance in mixed biofilms.
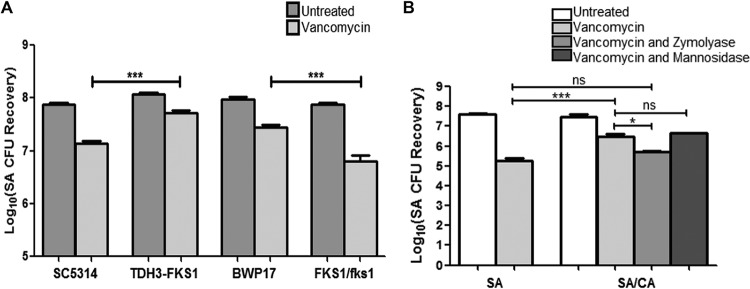
The key role for the β-1,3-glucan matrix component was demonstrated using C. albicans strains with modulated β-1,3-glucan expression in mixed biofilms treated with vancomycin. Compared to their respective reference strains, S. aureus recovery following vancomycin treatment was consistently higher in the presence of the TDH3-FKS1 glucan-overexpressing strain but lower when grown with the FKS1/fks1Δ glucan-deficient heterozygous mutant strain (Fig. 3A). No impact on S. aureus vancomycin susceptibility was seen in mixed biofilms with the mannosylation mutants or the zap1Δ/zap1Δ mutant lacking the zinc response transcription factor Zap1 (a negative regulator of β-1,3-glucan) (data not shown).
FIG 3 .
Effect of C. albicans FKS1 modulation and Zymolyase treatment on S. aureus (SA) susceptibility to vancomycin. (A) Preformed S. aureus single- and mixed-species biofilms with the various C. albicans strains were treated for 48 h with vancomycin (800 µg/ml). Based on CFU recovery, a significant increase in S. aureus survival was seen when grown in mixed biofilms with the TDH3-FKS1 glucan-overexpressing strain compared to its parent strain SC5314, whereas a significant decrease in survival was noted when S. aureus was grown with the glucan-deficient heterozygous FKS1/fks1 mutant strain compared to its parental strain BWP17 (***, P < 0.0001). (B) S. aureus single (SA) and mixed (CA/SA) biofilms were allowed to form for 24 h in the presence of Zymolyase (5 U/ml) or α-mannosidase (2 U/ml) and then treated with vancomycin (800 µg/ml) for 24 h. Based on CFU recovery, although growth with C. albicans in mixed biofilms provided S. aureus with a significant increase in survival with vancomycin, the increase in tolerance was diminished upon treatment with Zymolyase but not with α-mannosidase (*, P < 0.05; ***, P < 0.0001; ns, not significant). Means and standard errors of the means are shown.
β-1,3-Glucanase but not α-mannosidase treatment of mixed biofilms abrogates the enhanced tolerance to vancomycin.
To demonstrate the importance of the C. albicans matrix polysaccharides, experiments were also performed where single- and mixed-species biofilms were treated with vancomycin in the presence of the glucan-degrading enzyme Zymolyase or the mannan-degrading enzyme α-mannosidase. Based on CFU recovery, the increase in S. aureus vancomycin tolerance in mixed biofilms was significantly diminished upon Zymolyase treatment, to a level comparable to that for S. aureus single-species biofilm treated with vancomycin. However, no significant differences were noted with the α-mannosidase treatment (Fig. 3B). Since Zymolyase is known to have contaminating protease activity, enzymatic digestion experiments were also performed using proteinase K; results from these experiments demonstrated no effect for proteinase K on S. aureus response to vancomycin (data not shown).
SEM of S. aureus biofilm architecture supplemented with C. albicans biofilm matrix material and exogenous β-1,3-glucan.
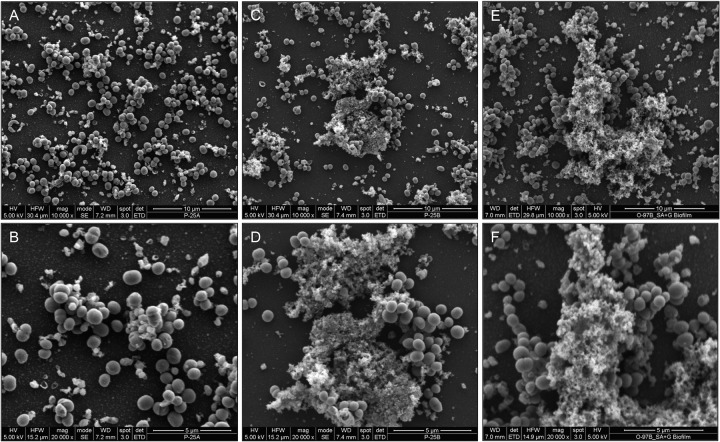
To visualize the structure of the S. aureus biofilm supplemented with C. albicans matrix material, S. aureus biofilms were allowed to form for 24 h in the absence and presence of purified matrix material or glucan, and the formed biofilms were comparatively examined by SEM analysis. Images revealed that where nonsupplemented S. aureus biofilms appeared thin and heterogeneous (Fig. 4A and B), growth in the presence of matrix material (Fig. 4C and D) or β-1,3-glucan (Fig. 4E and F) resulted in significantly increased biofilm mass with considerable aggregation of bacterial cells.
FIG 4 .
Effect of purified matrix and glucan supplementation on S. aureus biofilm architecture using SEM analysis. (A and B) Images of S. aureus biofilms demonstrated a thin and heterogeneous biofilm formed following a 48-h incubation. (C to F) In contrast, when grown in the presence of exogenous matrix material (0.5 mg/ml) (C and D) or glucan (0.25 mg/ml) (E and F), significant increases in biofilm matrix, mass, and bacterial cell clumping were seen.
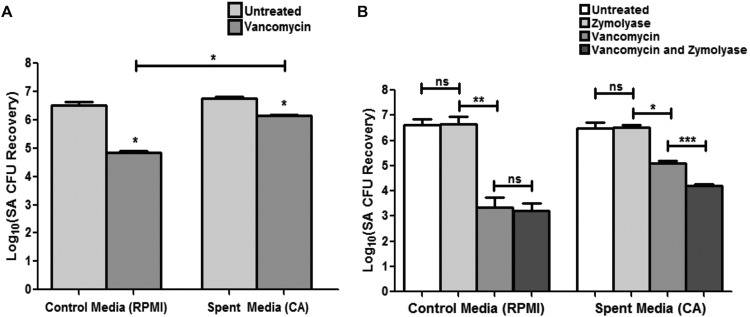
C. albicans spent culture medium provides S. aureus with enhanced tolerance to vancomycin in single-species biofilm.
Since C. albicans cell wall polysaccharides are secreted, experiments were performed where C. albicans spent biofilm culture medium was recovered and used in vancomycin susceptibility testing of S. aureus single biofilms. Results from these experiments demonstrated that C. albicans cell-free spent medium provided S. aureus with significantly enhanced tolerance to vancomycin in the absence of C. albicans in the biofilm (Fig. 5A). To identify the secreted component conferring the tolerance, the spent medium was enzymatically pretreated with Zymolyase or α-mannosidase prior to use in vancomycin susceptibility assays. Results from these experiments demonstrated that, whereas Zymolyase treatment considerably diminished the observed protection in spent medium (Fig. 5B), α-mannosidase treatment had no significant impact on S. aureus susceptibility to vancomycin (data not shown). Zymolyase and α-mannosidase treatment alone did not have any effect on S. aureus viability.
FIG 5 .
Effect of secreted effectors in C. albicans spent medium on S. aureus (SA) vancomycin susceptibility. (A) Supernatant from C. albicans culture medium (spent medium) was collected and used in S. aureus vancomycin susceptibility testing assays. Based on results from S. aureus CFU recovery, growth in C. albicans spent medium significantly enhanced S. aureus vancomycin tolerance compared to growth in control fresh medium (*, P < 0.05). (B) Since C. albicans-produced glucan is secreted into the medium, spent medium was treated with Zymolyase prior to vancomycin susceptibility testing. No significant effect for Zymolyase alone on S. aureus viability was seen; however, S. aureus tolerance to vancomycin was significantly diminished when spent medium was treated with Zymolyase. Results were corroborated with an MTS assay (*, P < 0.05; **, P < 0.001; ***, P < 0.0001; ns, not significant). Means and standard errors of the means are shown.
Inhibition of β-1,3-glucan synthesis in C. albicans compromises the enhanced tolerance to vancomycin in mixed biofilms and of spent medium.
In addition to enzymatic degradation, the antifungal caspofungin, an inhibitor of β-1,3-glucan synthesis in the fungal cell, was used to assess whether its effect on cell wall glucan synthesis impacts S. aureus vancomycin susceptibility. Prior to experiments, the subinhibitory caspofungin concentration on C. albicans was determined using the MTS assay and CFU enumeration to determine its effect on metabolic activity and viability, respectively. Results from the MTS assay indicated a concentration-dependent decrease in C. albicans metabolic activity with caspofungin; however, no effect on viability was noted for these concentrations (Fig. 6A and B). The selected subinhibitory caspofungin concentration (0.5 µg/ml) was subsequently used in mixed biofilm vancomycin susceptibility assays. Based on CFU recovery, results demonstrated a significant decrease in S. aureus survival with vancomycin when mixed biofilms were treated with caspofungin (Fig. 6C). Further, experiments were also performed to determine whether the effect of caspofungin on β-1,3-glucan synthesis affects its secretion and, thus, the protective effect of C. albicans spent medium on S. aureus. Therefore, experiments were also performed where formed C. albicans biofilms were treated with caspofungin prior to recovering the spent culture medium, which was subsequently used in vancomycin susceptibility testing. Results from these experiments similarly demonstrated that caspofungin treatment of C. albicans biofilms significantly diminished the ability of recovered spent medium to confer protection against vancomycin on S. aureus (Fig. 7). Caspofungin did not have any effect on S. aureus.
FIG 6 .
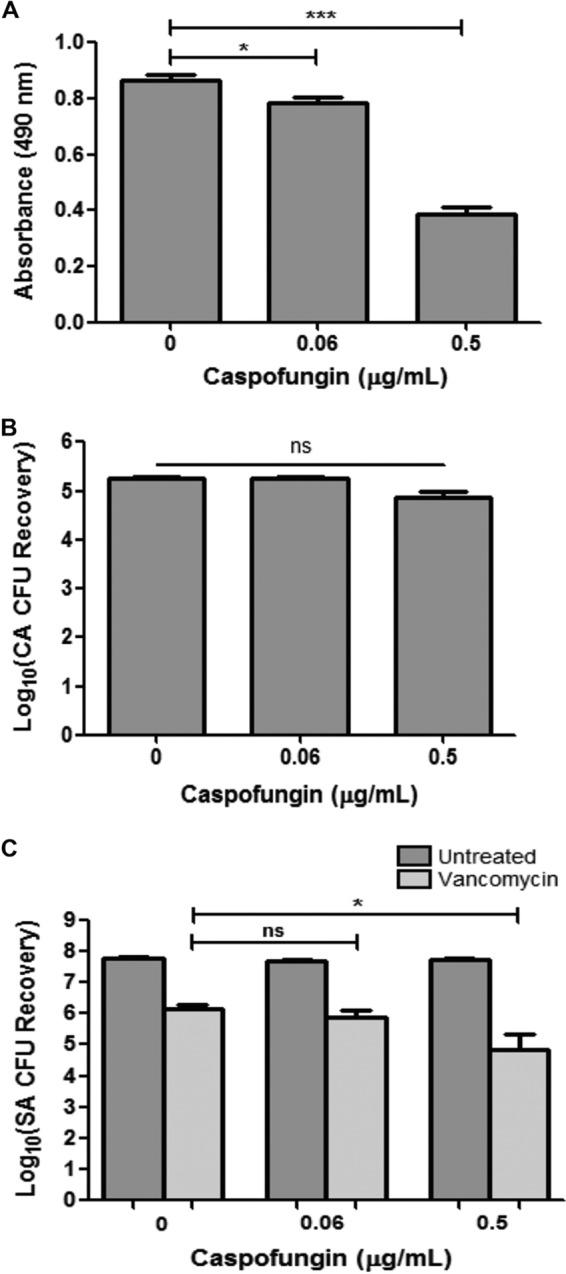
Effect of caspofungin treatment of mixed biofilms on response of S. aureus (SA) to vancomycin. (A) In order to determine the C. albicans (CA) sublethal, inhibitory concentration of caspofungin, susceptibility assays were performed where preformed C. albicans biofilms were treated with caspofungin for 24 h. Results from the MTS assay indicated a concentration-dependent decrease in C. albicans metabolic activity with caspofungin. (B) However, no significant effect on C. albicans viability was noted for these concentrations based on CFU enumeration (*, P < 0.05; ***, P < 0.0001; ns, not significant). (C) Mixed species biofilms were similarly treated with caspofungin and vancomycin (800 µg/ml), and S. aureus viability was assessed by CFU recovery. Results demonstrated a significant decrease in S. aureus survival with vancomycin when mixed biofilms were treated with 0.5 µg/ml caspofungin (*, P < 0.05). Means and standard errors of the means are shown.
FIG 7 .
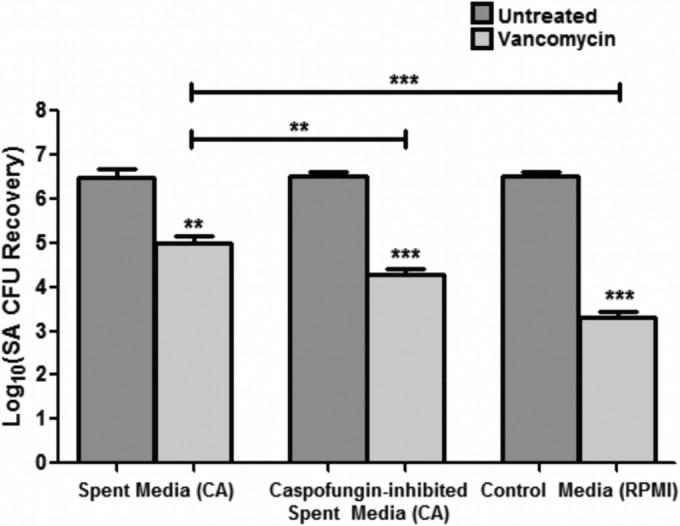
Effect of caspofungin treatment of C. albicans (CA) biofilms on ability of spent culture medium to confer vancomycin tolerance. C. albicans biofilms were treated with caspofungin (0.5 µg/ml) in order to diminish glucan synthesis and secretion. Recovered spent culture medium was then used in vancomycin (400 µg/ml) susceptibility testing of S. aureus (SA) single-species biofilms. Based on S. aureus CFU recovery, results demonstrated that caspofungin treatment of C. albicans biofilms significantly diminished the ability of the spent medium to provide S. aureus with protection against vancomycin. Results were also corroborated by an MTS assay (**, P < 0.01; ***, P < 0.001). Means and standard errors of the means are shown.
Impeded vancomycin diffusion through S. aureus biofilms grown with C. albicans or its purified and secreted matrix material.
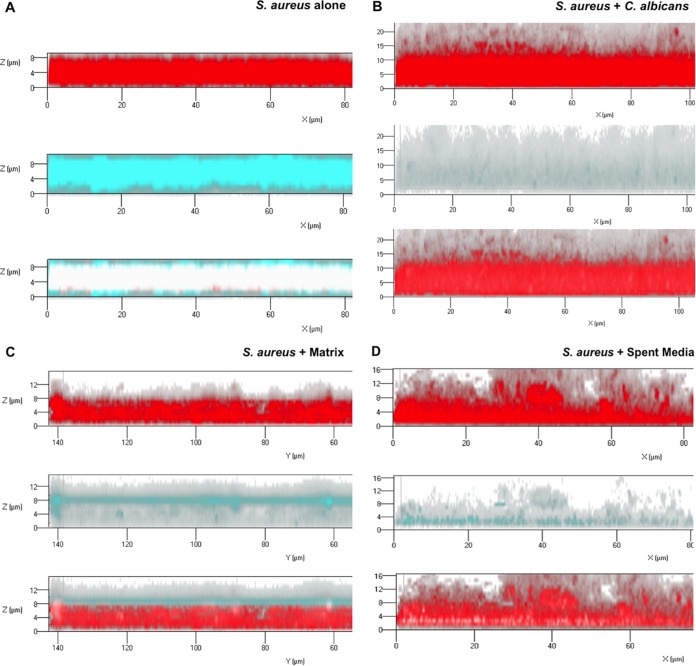
Vancomycin diffusion through single and mixed biofilm matrices was comparatively monitored using fluorescence confocal scanning laser microscopy (CSLM). S. aureus biofilms were grown with C. albicans, with purified C. albicans biofilm matrix material, or in C. albicans spent culture medium with secreted material. Formed biofilms were stained with concanavalin A (ConA) to allow visualization of the polysaccharide matrix (red). To visualize vancomycin diffusion through the matrix, a fluorescently tagged vancomycin compound was used. After 1 h, vancomycin (cyan) had significantly penetrated and diffused throughout the single S. aureus biofilm matrix, reaching the basal layer (Fig. 8A). In contrast, in the mixed biofilms with C. albicans, vancomycin signal was minimally detected, with negligible to no penetration into the matrix seen (Fig. 8B). Similarly, in the biofilms formed with exogenous supplementation with C. albicans matrix material, vancomycin was confined to the outer periphery of the biofilm with minimal to no penetration into the biofilm (Fig. 8C), and in biofilms grown in spent medium, only limited vancomycin penetration into the biofilm was seen (Fig. 8D).
FIG 8 .
Representative CSLM images assessing diffusion of fluorescently labeled vancomycin through S. aureus grown with C. albicans or its derived biofilm matrix material or in spent medium. S. aureus biofilms were grown for 24 h with C. albicans or supplemented with 0.5 mg/ml of purified matrix material. S. aureus biofilms were also grown in C. albicans spent culture medium containing secreted matrix components. Biofilms were stained with ConA for biofilm matrix (red) and fluorescent vancomycin compound (cyan). Following a 1-h diffusion, the biofilms were visualized using CSLM. (A) In the S. aureus monospecies biofilms, vancomycin fully penetrated and diffused throughout the biofilm matrix (white; red/cyan merged), reaching the basal layers. (B) In contrast, in mixed biofilms with C. albicans, minimum vancomycin presence or diffusion was seen. (C) Similarly, in biofilms grown with C. albicans matrix material, vancomycin presence was limited to the surface of the biofilm, with no or minimal penetration into the biofilm. (D) In biofilms grown in spent medium, there was limited penetration of vancomycin into the biofilm matrix and diffused vancomycin was present in greatly reduced concentrations.
S. aureus cell coating by C. albicans purified and secreted biofilm matrix components.
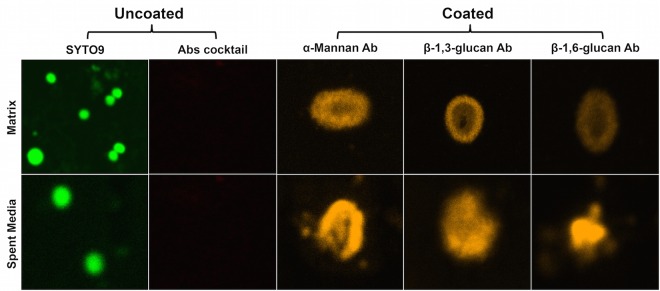
In order to evaluate whether C. albicans matrix polysaccharides also coat the bacterial cells, S. aureus planktonic cell suspension was incubated with C. albicans purified matrix and coating was assessed using monoclonal antibodies specific to each of the C. albicans matrix components. Using fluorescence microscopy, images demonstrated rapid coating of all 3 matrix components (α-mannan, β-1,3-glucan, and β-1,6-glucan) as assessed by the presence of fluorescence around the bacterial cells. S. aureus cells not treated with matrix demonstrated no fluorescence when labeled with antibodies. In addition to purified matrix, to determine whether the secreted matrix material similarly coats the bacterial cells, experiments were also performed where S. aureus was incubated in the C. albicans spent culture medium. Labeling of bacterial cells with the three specific antibodies demonstrated the presence of all matrix components around the surface of S. aureus cells (Fig. 9).
FIG 9 .
Fluorescence microscopy of S. aureus coated with purified and secreted soluble C. albicans biofilm matrix material. S. aureus planktonic cultures were grown for 24 h in the presence of 0.1 mg/ml of purified matrix or in C. albicans spent culture medium. Following washing, cells were treated with α-mannan-, β-1,3-glucan-, and β-1,6-glucan-specific antibodies (Abs) (30 min); stained with a secondary antibody (30 min); and then examined by CSLM. Images revealed the presence of all three matrix components around the bacterial cells when grown with purified matrix material. No fluorescence was detected in control cells from biofilm grown without matrix. Control cells grown in the absence of matrix did not fluoresce but could be visualized by Syto9 staining. Cells were also grown in C. albicans spent culture medium (no matrix supplementation) in order to assess whether secreted matrix components also coat bacterial cells. Similar to what was seen with the purified matrix, cells fluoresced when labeled with the antibodies. Control cells grown in fresh medium did not fluoresce but could be seen by Syto9 staining.
Enhanced tolerance of S. aureus in mixed biofilm is not antibiotic, methicillin resistance, or S. aureus strain dependent.
In order to determine whether the C. albicans-conferred enhanced tolerance of S. aureus in mixed biofilm is exclusive to vancomycin- or methicillin-resistant strains of S. aureus, susceptibility assays were also performed using two additional antibiotics, oxacillin and nafcillin. Further, the susceptibility of a methicillin-susceptible S. aureus (MSSA) strain was also tested against all 3 antibiotics in biofilms with C. albicans. Based on S. aureus CFU recovery, results demonstrated that, similarly to vancomycin, the MRSA strain exhibited enhanced tolerance to both oxacillin and nafcillin in mixed biofilm (see Fig. S1 in the supplemental material). A comparable increase in tolerance to all three antibiotics was also seen with the MSSA strain (data not shown).
DISCUSSION
Traditional therapies for biofilm-associated infections involve removal of infected devices, in addition to multidrug administration that is generally aimed at individual causative agents without consideration for effect on a polymicrobial cause. However, the medical community is recognizing the significance of polymicrobial diseases, and many therapies are now taking into account the cause of these conditions and the repercussions for treatment and prevention (42). Therefore, understanding the physical and molecular interactions between diverse microorganisms will greatly aid in defining new strategies for disrupting these complex mixed infections (20, 30, 33, 43).
Production of extracellular matrix polysaccharides is considered one of the key resistance mechanisms in microbial biofilms, and recent efforts have been focused on understanding the genetic basis for how biofilm matrix production governs drug resistance (16). In exploring the influence of C. albicans biofilm matrix on antifungal susceptibility, studies by Nett et al. (44) demonstrated that the matrix sequestered azole antifungal drugs and prevented them from reaching their target. In this study, we sought to demonstrate that C. albicans matrix may similarly confer protection on other microbial species against their respective antimicrobials when coexisting within mixed biofilms. Specifically, we explored the efficacy of antibacterial drugs against S. aureus in mixed fungal-bacterial biofilms, focusing on the role of the C. albicans β-1,3-glucan matrix component.
Our experiments using C. albicans strains with inhibition or overexpression of FKS1 and concomitant variations in matrix glucan amount decreased or increased S. aureus susceptibility to vancomycin, respectively (Fig. 3A). It is important to note that the strain with decreased FKS1 expression used in our experiments is a heterozygous and not a null mutant, as FKS1 is a vital gene, which makes the observed decrease in vancomycin susceptibility with this strain more significant.
Importantly, by supplementing S. aureus single-species biofilms with exogenous C. albicans biofilm-derived purified matrix material or individual matrix components, we were able to induce the S. aureus resistance phenotype in the absence of C. albicans (Fig. 2). However, experiments probing the role of matrix mannan using the mannan-degrading enzyme α-mannosidase, and various C. albicans mannosylation-deficient mutant strains, yielded no notable difference in S. aureus susceptibility to vancomycin. Further evidence for the role of secreted β-1,3-glucan was demonstrated by findings that C. albicans spent culture medium enhanced S. aureus tolerance to vancomycin; however, the acquired tolerance was significantly diminished when the spent medium was treated with the β-1,3-glucan-degrading enzyme but not the mannan-degrading enzyme (Fig. 3B). These findings are in line of those of Nett et al. (44), where glucanase but not α-mannosidase increased C. albicans susceptibility to the antifungal agent fluconazole. Combined, these findings identify β-1,3-glucan as the key matrix component contributing to S. aureus vancomycin tolerance in mixed biofilms with C. albicans.
Of interest, a study by De Brucker et al. (45) had reported a similar phenomenon involving the tolerance of the Gram-negative bacterial species Escherichia coli to the antibiotic ofloxacin in mixed biofilm, and analogously to our findings, β-1,3-glucan was shown to be involved. These observations are interesting in light of our findings that in mixed biofilm, in addition to vancomycin, S. aureus also exhibited enhanced tolerance to other antibiotics, namely, oxacillin and nafcillin. Importantly, in testing an MSSA strain with all three antibiotics in mixed biofilm, we also demonstrated that the effect of C. albicans on S. aureus susceptibility is not S. aureus strain dependent or associated with methicillin resistance. Of note and in contrast to our findings, the C. albicans zap1Δ/zapΔ mutant strain was shown to provide E. coli with increased, albeit minimal, tolerance to ofloxacin.
The C. albicans zinc response transcription factor Zap1 was identified by Nobile et al. (46) as a negative regulator of β-1,3-glucan, and the zap1Δ/zap1Δ strain was found to produce more soluble β-1,3-glucan in biofilms. However, we found no significant difference in S. aureus tolerance between growth with this mutant and that with the parental strain (data not shown). These discrepancies are intriguing as Zap1 was also shown to govern synthesis of small, secreted molecules involved in quorum sensing (QS) or interspecies communication. Specifically, Zap1 acts as a positive regulator of the accumulation of farnesol, a QS molecule secreted by C. albicans in biofilm, where the zap1Δ/zap1Δ mutant accumulated significantly less farnesol (46). Interestingly, in a previous study, our findings showed that exogenous synthetic farnesol impacted S. aureus susceptibility to various antibiotics, indicating a potential role for this molecule in the C. albicans-mediated modulation of the S. aureus response to vancomycin (47). Hence, it is conceivable to speculate that any potential notable enhanced effect for the zap1Δ/zap1Δ mutant on vancomycin response may have been neutralized by the compromised production and secretion of farnesol by the zap1Δ/zap1Δ mutant. Combined, these observations are of significance as they indicate that QS may also play an important role in mediating the process of enhanced antimicrobial tolerance in biofilm. Therefore, it is important to maintain that although this study focuses on C. albicans biofilm matrix components, polymicrobial biofilm formation and antimicrobial resistance are a multifactorial process involving all microbial partners, and thus, the contribution of S. aureus to the process cannot be disregarded.
One interesting question is whether the C. albicans matrix components, in addition to serving as a barrier, directly bind vancomycin. To explore this possibility, we performed exploratory experiments using the vancomycin antibody in immunoassays to assess polysaccharide binding to vancomycin. However, due to the inherent “stickiness” of the matrix material, these and other experiments were problematic and therefore inconclusive. Nevertheless, the demonstration that the mixed biofilm also conferred protection against other antibacterial agents argues against specific binding to vancomycin being a significant contributor.
Vancomycin is one of the few antibiotics that have remained effective against methicillin-resistant S. aureus, and development of resistance to vancomycin is relatively rare (48). Therefore, the demonstration of the failure of vancomycin to effectively penetrate the dense mixed biofilm matrix, as shown in our diffusion imaging (Fig. 8), carries significant clinical implications, as this process may be indicative of a potential therapeutic outcome in a host with a coinfected indwelling medical device. Of similar clinical relevance is the finding that at concentrations subinhibitory to C. albicans, the antifungal caspofungin sensitized the matrix-embedded S. aureus cells to vancomycin (Fig. 6C).
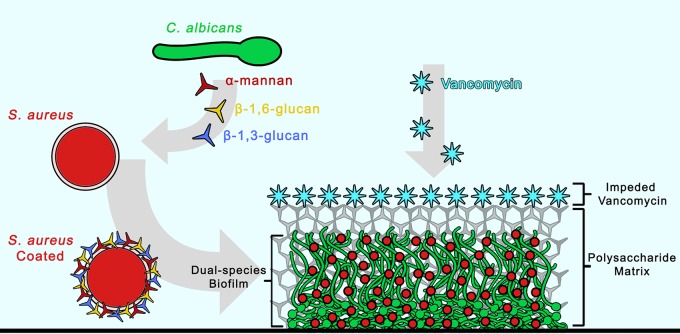
In conclusion, this study provides compelling evidence demonstrating the therapeutic impact of C. albicans cell wall secreted polysaccharide components on a bacterial coinhabitant in a mixed biofilm. To our knowledge, this is the first study utilizing confocal fluorescent time-lapse imaging to visually monitor antimicrobial diffusion through a mixed biofilm. Further, using monoclonal antibodies specific to fungal cell wall components, we demonstrate rapid coating of the bacterial cell by secreted C. albicans cell wall polysaccharides. These novel findings are of significance as they may indicate that in addition to hampering diffusion of antimicrobials in a biofilm, the coating of the bacterial cell by fungal secreted polysaccharides may offer the bacteria added protection by preventing the drug from reaching its cellular target (Fig. 10). Therefore, the combined findings from this study warrant awareness in terms of optimizing and overcoming the limitations of current therapies available to treat resilient polymicrobial infections. Importantly, it is crucial to develop suitable animal models to study these phenomena in vivo, and such investigations are ongoing in our laboratory.
FIG 10 .
Schematic representation of the proposed “barrier model” as a mechanism for the enhanced S. aureus tolerance to vancomycin in mixed biofilm. Based on the combined findings from this study, we propose that the mechanism for the enhanced S. aureus tolerance to vancomycin involves impediment of diffusion of antimicrobials through the mixed biofilm matrix consisting of C. albicans hyphae and secreted cell wall polysaccharides; α-mannan, β-1,3-glucan, and β-1,6-glucan secreted by C. albicans into the mixed biofilm environment adhere to the S. aureus cell surface, coating the outer layer. Concurrently, as the biofilm matures, in addition to providing structure and support to the biofilm, the secreted matrix polysaccharides impede the diffusion of antimicrobials into the biofilm interior. Effectively, the polysaccharide matrix acts as a barrier, sequestering vancomycin at the periphery and preventing it from reaching its target.
MATERIALS AND METHODS
Reagents.
β-1,3-Glucan (laminarin from Laminaria digitata), lyticase from Arthrobacter luteus (Zymolyase), mannan (from Saccharomyces cerevisiae), concanavalin A (from Canavalia ensiformis), α-mannosidase (from jack bean), proteinase K from Tritirachium album, and oxacillin sodium and nafcillin sodium were purchased from Sigma-Aldrich Chemical (St. Louis, MO); the MTS tetrazolium-based proliferation assay was from Promega (Madison, WI); vancomycin hydrochloride was from Hospira Inc. (IL, USA); Syto9 green fluorescent nucleic acid stain and vancomycin dipyrromethene boron difluoride (BODIPY) FL conjugate were from Invitrogen (Grand Island, NY); and FUN1 fungal stain was from Thermo Fisher Scientific (Halethorpe, MD).
Strains and growth conditions.
The standard methicillin-resistant S. aureus (MRSA) strain USA300 and the methicillin-susceptible (MSSA) strain ATCC 29213 were used in these studies (49). The following C. albicans strains were used where indicated: C. albicans reference strains SC5314 (50) and DAY185; heterozygous deletion mutant (FKS1/fks1Δ) constructed from BWP17, FKS1-overexpressing mutant (TDH3-FKS1) with one FKS1 allele under the control of the TDH3 promoter and one allele intact (18); zap1Δ/zap1Δ homozygous deletion strain (CJN1201) and the ZAP1/zap1Δ complemented strain (CJN1193) (46); the mnn4Δ (51) and mnn9Δ (52) mannosylphosphate transferase mutants with reduced phosphomannan and mannan, respectively; and the mnt1Δ and mnt2Δ α-1,2-mannosyltransferase mutants and mnt1Δ/mnt2Δ double mutant (53). C. albicans strains were maintained on yeast-peptone-dextrose (YPD) agar (Difco Laboratories) and grown in YPD broth overnight at 30°C with orbital shaking, and cells were equilibrated in fresh medium to an optical density of absorbance of 1.0 at 600 nm. S. aureus cultures were grown overnight in Trypticase soy broth (TSB) (Difco) at 37°C and then grown in fresh TSB to mid-log phase. Cells were harvested, washed, and resuspended in RPMI 1640 with l-glutamine and HEPES (Invitrogen, Grand Island, NY) and used at final cell densities of 1 × 106 cells/ml.
In vitro single- and mixed-species biofilm formation.
Biofilms were grown in the wells of 96-well polystyrene flat-bottom plates. C. albicans and S. aureus cell suspensions were adjusted to 1 × 106 cells/ml in RPMI medium, and 100 µl of cell suspensions was added to the wells individually or in combination. Plates were incubated for 90 min at 37°C, and then wells were washed twice with phosphate-buffered saline (PBS) to remove nonadherent cells. Fresh medium (200 µl) was added to each well, and biofilms were allowed to form for 24 h at 37°C. Following incubation, wells were washed with PBS.
CV staining of biofilms.
Single and mixed biofilm biomass was quantified using the crystal violet (CV) assay with modifications (54). Biofilms were grown as described above and then washed twice with PBS and air dried at 37°C. Biofilms were stained with 1% aqueous CV solution for 20 min and then washed with sterile water. Plates were air dried, and remaining CV stain was dissolved using 33% acetic acid solution. Following 45 min of destaining, 150 µl of destaining solution was transferred to a new well, and the amount of CV stain was measured with a microtiter plate reader at 595 nm (Titertek; Multiskan MCC1340).
Biofilm vancomycin susceptibility testing.
The impact of C. albicans on the response of S. aureus to vancomycin was assessed in biofilms grown as described above. Mixed biofilms were grown using S. aureus and each of the C. albicans mutant strains for 24 h. Following washing, the wells were supplemented with fresh medium, vancomycin hydrochloride at a final concentration of 800 µg/ml (predetermined based on activity against S. aureus single and mixed biofilms) (see Fig. S2 in the supplemental material) was added, and plates were incubated for an additional 24 h at 37°C. Biofilms were then washed twice with PBS, and 100 µl of PBS was added; cells from the biofilms were recovered by sonication followed by vigorous vortexing and pipetting. Cell suspensions were diluted and plated on C. albicans and S. aureus-specific chromogenic medium (CHROMagar; DRG International, Inc.) for CFU count. Drug-free wells were included as controls. In addition, in order to assess the effect of mixed biofilm growth on S. aureus susceptibility to other antibiotics, experiments were also performed using two additional antibiotics, oxacillin (0 to 480 µg/ml) and nafcillin (0 to 160 µg/ml). Further, susceptibility testing using all 3 antibiotics was performed using an alternate strain of S. aureus that is susceptible to methicillin (MSSA).
MTS assay.
Viability was also assessed using the MTS metabolic assay according to the manufacturer’s directions. Following washing with PBS, 100 µl of PBS was added to the wells followed by 20 µl of MTS reagent and plates were incubated at 37°C until color fully developed. Following color development, colorimetric change at 490 nm (A490) was measured with a microtiter plate reader. On each occasion, reactions were performed in triplicate.
SEM of single- and mixed-species biofilms.
For SEM analysis, S. aureus was grown in single species biofilm with and without exogenous supplementation of purified matrix material (0.5 mg/ml) or glucan (0.25 mg/ml) or with C. albicans in mixed biofilms, on coverslips for 48 h. Coverslips were washed twice with PBS and then fixed in 2% paraformaldehyde, 2.5% glutaraldehyde in phosphate buffer, pH 7.4, for 1 h at room temperature and then at 4°C overnight. Following initial fixation, specimens were washed in three changes of 0.1 M PBS for a total of 30 min, postfixed with 1% osmium tetroxide in PBS for 1 h, and washed again in three changes of buffer. Dehydration of specimens was done using a series of graded ethyl alcohol, 30%, 50%, 70%, 90%, and 100% for 10 min each, and two more changes of 100% ethyl alcohol. Specimens were then chemically dried by immersing them sequentially in 2 parts 100% ethyl alcohol-1 part hexamethyldisilazane (HMDS) (Electron Microscopy Sciences, Fort Washington, PA) for 10 min, 1 part 100% ethyl alcohol-1 part HDMS for 10 min, 1 part 100% ethyl alcohol-2 parts HDMS for 10 min, and then 2 changes for 10 min each with 100% HDMS. Specimens were air dried in a hood overnight, mounted on SEM pin mounts, and sputter coated with 10 to 20 nm of platinum-palladium in a sputter coater (EMS 150T ES). SEM images were captured using a Quanta 200 scanning electron microscope (FEI Co., Hillsboro, OR).
Effect of C. albicans purified biofilm-derived matrix material and β-1,3-glucan and mannan exogenous supplementation on S. aureus susceptibility to vancomycin in single-species biofilm.
C. albicans matrix polysaccharides were extracted from C. albicans biofilms and purified as previously described (55). The recovered matrix material (0.25 mg/ml) was used to supplement S. aureus biofilms, which were allowed to form for 24 h as described above. S. aureus biofilms were also formed in the presence of exogenous β-1,3-glucan (1 mg/ml) or mannan (1 mg/ml) as previously performed (44). Following incubation, biofilms were washed and supplemented with fresh RPMI medium and vancomycin (800 µg/ml), and plates were incubated for an additional 24 h. Following washing, S. aureus viability was assessed using the MTS assay and confirmed by CFU recovery for viability.
Impact of C. albicans β-1,3-glucan and mannan enzymatic degradation on S. aureus susceptibility to vancomycin in mixed biofilms.
Prior to performing experiments, susceptibility of C. albicans to Zymolyase and α-mannosidase was tested to determine the subinhibitory concentrations to be used in mixed biofilm susceptibility testing. Mixed-species biofilms were grown alone or in the presence of glucanase (Zymolyase) (5 U/ml) or α-mannosidase (2 U/ml). Vancomycin (800 µg/ml) was then added alone or in combination with the enzymes, and plates were incubated for an additional 24 h at 37°C. Following incubation, wells were washed three times with PBS and biofilm cells were recovered by sonication and plated for CFU counts. In order to demonstrate lack of contribution of contaminating protease activity in Zymolyase, experiments were also performed using enzymatic digestion with proteinase K.
Impact of inhibition of C. albicans in β-1,3-glucan synthesis on S. aureus susceptibility to vancomycin in mixed biofilms.
Mixed 24-h biofilms were treated with the β-1,3-glucan synthase inhibitor caspofungin at final concentrations of 0, 0.06, and 0.5 µg/ml with and without vancomycin (800 µg/ml), and plates were incubated for an additional 24 h. Following washing, biofilms were sonicated and S. aureus viability was assessed based on CFU counts. Prior to experiments, susceptibility of C. albicans to caspofungin in single species was assessed in order to determine the subinhibitory caspofungin concentration to be used in mixed biofilms. Viability was evaluated using the MTS assay to determine changes in metabolic activity and CFU enumeration for viability.
Effect of C. albicans soluble secreted matrix components on S. aureus susceptibility to vancomycin.
In order investigate the role of C. albicans secreted matrix components on vancomycin susceptibility, cell-free C. albicans biofilm spent culture medium was recovered. Briefly, C. albicans (1 × 106 cells/ml) biofilms were grown in 10 ml of RPMI medium in canted-neck flasks and incubated at 37°C for 48 h. Following incubation, spent culture medium was recovered and filter sterilized through a 0.22-µm pore. The filtered medium was then supplemented 1:1 with fresh RPMI medium (prewarmed to 37°C) and used to grow S. aureus single-species biofilms. Following 24-h incubation, biofilms were washed and then supplemented with fresh medium and vancomycin (400 µg/ml) (predetermined to be subinhibitory in S. aureus single-species biofilm) (see Fig. S2 in the supplemental material) and incubated for an additional 24 h. S. aureus viability was assessed using the MTS assay and based on CFU recovery. To identify β-1,3-glucan as the key secreted component in C. albicans culture medium, experiments were also performed where S. aureus biofilms were cultured in the spent medium (as described above) with the addition of 5 U/ml of the glucan-degrading enzyme glucanase (Zymolyase) for 24 h at 37°C. Following incubation, vancomycin susceptibility testing was performed. Spent medium without glucanase was included as a control. In addition, C. albicans biofilms were grown for 24 h and then treated with the β-1,3-glucan synthesis inhibitor caspofungin (0.5 µg/ml) (subinhibitory dose for C. albicans) for an additional 24 h. Spent medium was then collected and used in vancomycin susceptibility testing of S. aureus single-species biofilms as described above.
Confocal scanning laser microscopy analysis of vancomycin diffusion through single and mixed biofilms.
To visualize the process of vancomycin diffusion through single and mixed biofilms, confocal scanning laser microscopy was performed as based on a previously described method for S. aureus with modifications (48). In addition to monitoring vancomycin diffusion through mixed biofilms, experiments were also performed where S. aureus single biofilms were formed in the presence of exogenous supplementation of purified C. albicans biofilm matrix to isolate the role of the matrix. Further, as matrix components are secreted during C. albicans growth, biofilm spent culture medium was used to grow S. aureus biofilms. For these experiments, biofilms were grown on glass coverslip-bottom dishes (MatTek Co., Ashland, MA) in RPMI medium at 37°C for 24 h. Following incubation, biofilms were gently washed three times with PBS and stained with concanavalin A (ConA) for polysaccharide biofilm matrix (100 µg/ml) (red; 488/545). In order to visualize the diffusion of vancomycin, the fluorescent BODIPY FL conjugate of vancomycin (cyan; 488/650) was added to the biofilms to a final concentration of 1 µg/ml. Biofilms were incubated for 1 h and then washed three times with PBS to remove nonpenetrated vancomycin and observed using a 63× oil immersion objective and a Zeiss 710 confocal microscope. Images were obtained by LSM 5 Image Browser software at a resolution of 512 by 512 pixels, with an average of 8 images per line. To evaluate the structure and size of the biofilms, a series of images at ≤1-µm intervals in the z axis were acquired for the full depth of the biofilm. At least three random fields were visualized for each biofilm, and representative images are presented.
Fluorescence microscopy analysis of S. aureus cell coating with purified and secreted soluble C. albicans biofilm matrix material.
In order to examine whether, in addition to their role in biofilms, purified and secreted matrix components also coat the bacterial cell, S. aureus cells (2 × 107 cells/ml) were incubated with 0.1 mg/ml of the purified matrix material for 30 min at 37°C. S. aureus cells with no matrix treatment were included as controls. In addition to purified matrix, experiments were also performed where S. aureus was exposed to C. albicans spent culture medium in order to determine whether soluble matrix components secreted by C. albicans during biofilm growth similarly coat the bacterial cells. In these experiments, 2 × 107 cells/ml of S. aureus were incubated with 1 ml of C. albicans spent medium for 30 min at 37°C. S. aureus cells incubated in fresh medium were included as a control. Following three washes with PBS, cells were treated with monoclonal antibodies specific to α-mannan (1/100 dilution), β-1,3-glucans (1/6,000 dilution), and β-1,6-glucans (1/4,000 dilution) (produced as previously described) (14) for 1 h at 37°C. Following incubation with primary antibodies, cells were washed three times with PBS and subsequently incubated with a goat anti-mouse IgG Alexa Fluor 488 (orange; 495/519) secondary antibody (1/100 dilution) (Thermo Fisher Scientific) for 30 min at 37°C. Cells were then washed, pelleted, and resuspended in 50 µl PBS. One drop of cell suspension was placed on a slide and covered with a coverslip, and fluorescence was assessed by CSLM. Non-matrix-coated S. aureus cells similarly treated with the antibodies were used as a control. However, as control cells with no matrix did not react with antibodies and therefore were not visible, they were stained with Syto9 nucleic acid stain (10 µM) (green; 488/505) in order to be visualized. Cells were observed using a 63× oil immersion objective and a Zeiss 710 confocal microscope. Images were obtained by LSM 5 Image Browser software at a resolution of 512 by 512 pixels, with an average of 8 images per line. At least three random fields were visualized for each sample, and representative images are presented.
Data analysis.
All experiments were performed on at least 3 separate occasions and in triplicate where applicable, and averages were used to present data. All statistical analysis was performed using GraphPad Prism 5.0 software. The Kruskal-Wallis one-way analysis of variance test was used to compare differences between multiple groups, and Dunn’s multiple-comparison test was used to determine whether the difference between two samples was statistically significant. Student’s unpaired t test was used to compare differences between two samples. P values of ≤0.05 were considered to be significant.
SUPPLEMENTAL MATERIAL
Assessment of S. aureus susceptibility to oxacillin and nafcillin in single S. aureus and mixed (CS) biofilms. Preformed (24-h) S. aureus single and mixed biofilms were treated with either oxacillin (A) or nafcillin (B) for an additional 24 h. CFU recovery of S. aureus from both biofilms showed a significant increase in S. aureus recovery from mixed biofilms following oxacillin or nafcillin treatment (**, P < 0.01; ***, P < 0.001). Means and standard errors of the means are shown. Download
Vancomycin concentration-dependent susceptibility testing in single S. aureus (SA) and mixed S. aureus and C. albicans (SA/CA) biofilms. Preformed (24-h) S. aureus single and mixed biofilms were treated with vancomycin (0 to 1,600 µg/ml) for an additional 24 h. Based on the MTS assay, results demonstrated significant and similar S. aureus killing activities for all vancomycin concentrations tested in single and mixed biofilms. Vancomycin 800-μg/ml (dual-species biofilms) and 400-μg/ml (single-species biofilms) concentrations were arbitrarily chosen for use in subsequent experiments (**, P < 0.01; ***, P < 0.001). Download
ACKNOWLEDGMENTS
We thank Hiram Sanchez for his contribution and Vincent Bruno and Aaron Mitchell for providing us with C. albicans strains.
This work was supported by NIH grant DE14424 to Mary Ann Jabra-Rizk; the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office to Patrick Van Dijck and Mary Ann Jabra-Rizk; the Flemish Science Foundation (FWO), WO.026.11N, to Patrick Van Dijck and Mary Ann Jabra-Rizk; and the FWO postdoctoral fellowships awarded to Sona Kucharíková. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Kong EF, Tsui C, Kucharíková S, Andes D, Van Dijck P, Jabra-Rizk MA. 2016. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 7(5):e01365-16. doi:10.1128/mBio.01365-16.
REFERENCES
- 1.Brogden KA, Guthmiller JM, Taylor CE. 2005. Human polymicrobial infections. Lancet 365:253–255. doi: 10.1016/S0140-6736(05)17745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions in biofilms: impact on pathogenesis and human disease. Clin Microbiol Rev 25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabra-Rizk MA. 2011. Pathogenesis of polymicrobial biofilms. Open Mycol J 5:39–43. doi: 10.2174/1874437001105010039. [DOI] [Google Scholar]
- 4.O’Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Lewis K. 2001. Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghannoum M, Roilides E, Katragkou A, Petraitis V, Walsh TJ. 2015. The role of echinocandins in Candida biofilm–related vascular catheter infections: in vitro and in vivo model systems. Clin Infect Dis 61(Suppl 6):S618–S621. doi: 10.1093/cid/civ815. [DOI] [PubMed] [Google Scholar]
- 7.Finkel JS, Mitchell AP. 2011. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9:109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguly S, Mitchell AP. 2011. Mucosal biofilms of Candida albicans. Curr Opin Microbiol 14:380–385. doi: 10.1016/j.mib.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderone R (ed). 2012. Candida and candidiasis, 2nd ed. ASM Press, Washington, DC. [Google Scholar]
- 10.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauvel M, Nesseir A, Cabral V, Znaidi S, Goyard S, Bachellier-Bassi S, Firon A, Legrand M, Diogo D, Rossignol T, Naulleau C, d’Enfert C. 2012. A versatile overexpression strategy in the pathogenic yeast Candida albicans: identification of regulators of morphogenesis and fitness. PLoS One 7:e45912. doi: 10.1371/journal.pone.0045912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nett J, Andes D. 2006. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol 9:340–345. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Tournu H, Van Dijck P. 2012. Candida biofilms and the host: models and new concepts for eradication. Int J Microbiol 2012:845352 doi: 10.1155/2012/845352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, Mitchell AP, Andes DR. 2015. Community participation in biofilm matrix assembly and function. Proc Natl Acad Sci U S A 112:4092–4097. doi: 10.1073/pnas.1421437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall R, Gow NA. 2013. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol 90:1147–1161. doi: 10.1111/mmi.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. 2012. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog 8:e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taff HT, Mitchell KF, Edward JA, Andes DR. 2013. Mechanisms of Candida biofilm drug resistance. Future Microbiol 8:1325–1337. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nett JE, Crawford K, Marchillo K, Andes DR. 2010. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother 54:3505–3508. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson HF, Barbour ME, Jagger DC, Miles M, Bamford CM, Nobbs AH, Dutton LC, Silverman RJ, McNally L, Vickerman MM, Gill S. 2008. Candida albicans-bacteria interactions in biofilms and disease, p 1–6. University of Bristol Dental School, Bristol, United Kingdom. [Google Scholar]
- 20.Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 299:1–8. doi: 10.1111/j.1574-6968.2009.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog 6:e1000886. doi: 10.1371/journal.ppat.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehm SJ. 2008. Staphylococcus aureus: the new adventures of a legendary pathogen. Cleve Clin J Med 75:177–192. doi: 10.3949/ccjm.75.3.177. [DOI] [PubMed] [Google Scholar]
- 23.Otto M. 2013. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 24.McGavin MJ, Heinrichs DE. 2012. The staphylococci and staphylococcal pathogenesis. Front Cell Infect Microbiol 2:48 doi: 10.3389/fcimb.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakoulas G, Moellering RC. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 46(Suppl 5):S360–S367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 26.Gordon RJ, Lowy FD. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46(Suppl 5):S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters B, Ovchinnikova E, Schlecht L, Hoyer L, Busscher H, van der Mei H, Krom B, Jabra-Rizk MA, Shirtliff M. 2012. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 158:2975–2986. doi: 10.1099/mic.0.062109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. 2010. Microbial interactions and differential protein expression in Staphylococcus aureus and Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol 59:493–503. doi: 10.1111/j.1574-695X.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother 53:3914–3922. doi: 10.1128/AAC.00657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harriott MM, Noverr MC. 2011. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol 19:557–563. doi: 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazzell AL, Chaturvedi AK, Pierce CG, Prasad D, Uppuluri P, Lopez-Ribot JL. 2009. Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J Antimicrob Chemother 64:567–570. doi: 10.1093/jac/dkp242. [DOI] [PubMed] [Google Scholar]
- 32.Chrissoheris MP, Libertin C, Ali RG, Ghantous A, Bekui A, Donohue T. 2009. Endocarditis complicating central venous catheter bloodstream infections: a unique form of health care associated endocarditis. Clin Cardiol 32:E48–E54. doi: 10.1002/clc.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. 2007. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis 59:401–406. doi: 10.1016/j.diagmicrobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Timsit JF, Cheval C, Gachot B, Bruneel F, Wolff M, Carlet J, Regnier B. 2001. Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med 27:640–647. doi: 10.1007/s001340000840. [DOI] [PubMed] [Google Scholar]
- 35.Pate JC, Jones DB, Wilhelmus KR. 2006. Prevalence and spectrum of bacterial co-infection during fungal keratitis. Br J Ophthalmol 90:289–292. doi: 10.1136/bjo.2005.081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tawara Y, Honma K, Naito Y. 1996. Methicillin-resistant Staphylococcus aureus and Candida albicans on denture surfaces. Bull Tokyo Dent Coll 37:119–128. [PubMed] [Google Scholar]
- 37.Gupta N, Haque A, Mukhopadhyay G, Narayan RP, Prasad R. 2005. Interactions between bacteria and Candida in the burn wound. Burns 31:375–378. doi: 10.1016/j.burns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Baena-Monroy T, Moreno-Maldonado V, Franco-Martínez F, Aldape-Barrios B, Quindós G, Sánchez-Vargas LO. 2005. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10(Suppl 1):E27–E39. [PubMed] [Google Scholar]
- 39.Kong EF, Kucharíková S, Van Dijck P, Peters BM, Shirtliff ME, Jabra-Rizk MA. 2015. Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect Immun 83:604–613. doi: 10.1128/IAI.02843-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adam B, Baillie GS, Douglas LJ. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol 51:344–349. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- 42.Sancho S, Artero A, Zaragoza R, Camarena JJ, González R, Nogueira JM. 2012. Impact of nosocomial polymicrobial bloodstream infections on the outcome in critically ill patients. Eur J Clin Microbiol Infect Dis 31:1791–1796. doi: 10.1007/s10096-011-1503-8. [DOI] [PubMed] [Google Scholar]
- 43.Bouza E, Burillo A, Muñoz P, Guinea J, Marín M, Rodríguez-Créixems M. 2013. Mixed bloodstream infections involving bacteria and Candida spp. J Antimicrob Chemother 68:1881–1888. doi: 10.1093/jac/dkt099. [DOI] [PubMed] [Google Scholar]
- 44.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51:510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Brucker K, Tan Y, Vints K, De Cremer K, Braem A, Verstraeten N, Michiels J, Vleugels J, Cammue BP, Thevissen K. 2015. Fungal 1,3-glucan increases ofloxacin tolerance of Escherichia coli in a polymicrobial E. coli/Candida albicans biofilm. Antimicrob Agents Chemother 59:3052–3058. doi: 10.1128/AAC.04650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault J-S, Nantel A, Andes DR, Johnson AD, Mitchell AP. 2009. Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol 7:e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial resistance. Antimicrob Agents Chemother 50:1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira PM, Filipe SR, Tomasz A, Pinho MG. 2007. Fluorescence ratio imaging microscopy shows decreased access of vancomycin to cell wall synthetic sites in vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 51:3627–3633. doi: 10.1128/AAC.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother 64:441–446. doi: 10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- 50.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine 5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 51.Hobson RP, Munro CA, Bates S, MacCallum DM, Cutler JE, Heinsbroek SE, Brown GD, Odds FC, Gow NA. 2004. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem 279:39628–39635. doi: 10.1074/jbc.M405003200. [DOI] [PubMed] [Google Scholar]
- 52.Southard SB, Specht CA, Mishra C, Chen-Weiner J, Robbins PW. 1999. Molecular analysis of the Candida albicans homolog of Saccharomyces cerevisiae MNN9, required for glycosylation of wall mannoproteins. J Bacteriol 181:7439–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munro CA, Bates S, Buurman ET, Hughes HB, Maccallum DM, Bertram G, Atrih A, Ferguson MA, Bain JM, Brand A, Hamilton S, Westwater C, Thomson LM, Brown AJ, Odds FC, Gow NA. 2005. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem 280:1051–1060. doi: 10.1074/jbc.M411413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. 2003. Biofilm forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol 41:2961–2967. doi: 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR. 2014. Novel entries in a fungal biofilm matrix encyclopedia. mBio 5:e01333-14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of S. aureus susceptibility to oxacillin and nafcillin in single S. aureus and mixed (CS) biofilms. Preformed (24-h) S. aureus single and mixed biofilms were treated with either oxacillin (A) or nafcillin (B) for an additional 24 h. CFU recovery of S. aureus from both biofilms showed a significant increase in S. aureus recovery from mixed biofilms following oxacillin or nafcillin treatment (**, P < 0.01; ***, P < 0.001). Means and standard errors of the means are shown. Download
Vancomycin concentration-dependent susceptibility testing in single S. aureus (SA) and mixed S. aureus and C. albicans (SA/CA) biofilms. Preformed (24-h) S. aureus single and mixed biofilms were treated with vancomycin (0 to 1,600 µg/ml) for an additional 24 h. Based on the MTS assay, results demonstrated significant and similar S. aureus killing activities for all vancomycin concentrations tested in single and mixed biofilms. Vancomycin 800-μg/ml (dual-species biofilms) and 400-μg/ml (single-species biofilms) concentrations were arbitrarily chosen for use in subsequent experiments (**, P < 0.01; ***, P < 0.001). Download