Abstract
Group II metabotropic glutamate receptors (mGluR2 and mGluR3) may control relapse of alcohol seeking, but previously available Group II agonists were unable to discriminate between mGluR2 and mGluR3. Here we use AZD8529, a novel positive allosteric mGluR2 modulator, to determine the role of this receptor for alcohol-related behaviors in rats. We assessed the effects of AZD8529 (20 and 40 mg/kg s.c.) on male Wistar rats trained to self-administer 20% alcohol and determined the effects of AZD8529 on self-administration, as well as stress-induced and cue-induced reinstatement of alcohol seeking. The on-target nature of findings was evaluated in Indiana P-rats, a line recently shown to carry a mutation that disrupts the gene encoding mGluR2. The behavioral specificity of AZD8529 was assessed using self-administration of 0.2% saccharin and locomotor activity tests. AZD8529 marginally decreased alcohol self-administration at doses that neither affected 0.2% saccharin self-administration nor locomotor activity. More importantly, cue- but not stress-induced alcohol seeking was blocked by the mGluR2 positive allosteric modulator. This effect of AZD8529 was completely absent in P rats lacking functional mGluR2s, demonstrating the receptor specificity of this effect. Our findings provide evidence for a causal role of mGluR2 in cue-induced relapse to alcohol seeking. They contribute support for the notion that positive allosteric modulators of mGluR2 block relapse-like behavior across different drug categories.
INTRODUCTION
Glutamate, the principal excitatory neurotransmitter in the brain (Meldrum, 2000; Pin and Duvoisin, 1995), is key for the development and maintenance of drug addiction (Gass and Olive, 2008; Kalivas, 2009). Presynaptic metabotropic glutamate receptors (mGluRs) control synaptic release of glutamate (Meldrum, 2000), making them potential targets for novel addiction pharmacotherapies (Heilig and Egli, 2006; Kenny and Markou, 2004; Spooren et al, 2003). Eight subtypes of mGluRs have been identified and classified into three groups (Conn and Pin, 1997; Pin and Duvoisin, 1995; Schoepp et al, 1999). Several reports have indicated that group II (mGluR2 and mGluR3) agonists can affect addiction-related behaviors in animal models. For example, the orthosteric mGluR2/3 agonist LY379268 has been shown to attenuate contextual as well as cue-induced heroin seeking (Bossert et al, 2005; Bossert et al, 2004), cue-induced methamphetamine seeking (Kufahl et al, 2013), incubation of cocaine-craving (Lu et al, 2007), and cue-induced nicotine seeking (Liechti et al, 2007).
Several reports have specifically indicated that glutamatergic transmission is involved in the mediation of alcohol-related behavior. It has, for example, been shown that extracellular glutamate levels in rats are elevated during early ethanol withdrawal in the hippocampus, the dorsal striatum, and the nucleus accumbens (Dahchour and De Witte, 2000, 2003; Rossetti and Carboni, 1995) and that glutamate levels correlate with the intensity of the withdrawal symptoms. This observation was recently extended to humans in a translational study that used magnetic resonance spectroscopy (MRS) to measure glutamate levels. Both alcohol-dependent rats and alcohol-dependent patients showed increased glutamate levels during acute alcohol withdrawal in corresponding prefrontocortical regions (Hermann et al, 2012). Another MRS study indicated that glutamate levels in the anterior cingulate cortex of alcohol-dependent patients continue to increase over 3 weeks following cessation of alcohol use and that the approved alcoholism medication acamprosate prevents this increase (Umhau et al, 2010). Seemingly in agreement with these observations, mGluR2/3 agonists can affect alcohol-related behaviors. Systemic injections of LY379268 reduced alcohol self-administration in Long-Evans (Backstrom and Hyytia, 2005) and Wistar rats (Sidhpura et al, 2010) but not in alcohol-preferring P rats (Rodd et al, 2006).
However, LY379268 and other orthosteric group II mGluR agonists are unable to discriminate between contributions of mGluR2 and mGluR3, respectively, and may also be associated with long-term toxicity. To overcome these limitations, positive allosteric modulators (PAMs) selective for mGluR2 were recently developed (Conn et al, 2009; Cross, 2013; Marino and Conn, 2006). These PAMs exert their effects on mGluR2s through a binding site that is topographically distinct from that bound by the endogenous ligand (Ritzen et al, 2005) and selectively activate the receptor in the presence of glutamate (Schaffhauser et al, 2003). AZD8529 is a new, highly specific PAM that potentiates the effect of glutamate on the mGluR2 with little effect on other mGluR subtypes (Cross, 2013; Justinova et al, 2015). It has recently been shown that AZD8529 decreases nicotine self-administration, nicotine-primed, and cue-induced reinstatement of nicotine seeking in squirrel monkeys (Justinova et al, 2015), as well as incubation of methamphetamine craving in rats (Caprioli et al, 2015). Its effects on self-administration or relapse to other classes of addictive substances are presently unknown.
Here we therefore assessed the effects of AZD8529 on alcohol-related behaviors in rats. We first trained Wistar rats to self-administer 20% alcohol and tested the effects of AZD8529 on alcohol self-administration. We then assessed the effect of AZD8529 on cue-induced, as well as stress-induced, alcohol seeking. Finally, to establish the causal role of mGluR2 in the observed effects, we assessed cue-induced alcohol seeking in alcohol-preferring P rats, a strain that does not express functional mGluR2s owing to a recently discovered single point mutation that introduces a stop codon in the gene encoding this receptor (Zhou et al, 2013).
METHODS
Drugs
AZD8529 (7-methyl-5-(3-piperazin-1-ylmethyl-[1,2,4]oxadiazol-5-yl)-2-(4-trifluoromethoxybenzyl)-2,3-dihydroisoindol-1-one) was obtained from AstraZeneca. AZD8529 was dissolved in saline and pH was adjusted as necessary. The compound was injected subcutaneously at a volume of 1 ml/kg. Doses (20 and 40 mg/kg) were chosen based on prior data (Caprioli et al, 2015). Alcohol solutions were prepared volume/volume in tap water from 95% alcohol.
Subjects
A total of 92 adult male Wistar rats (Charles River, Frederick, MD, USA) weighing 200–225 g at the beginning of the experiments and 16 P rats (Indiana University, Indianapolis, IN, USA) were pair-housed in a temperature- (21 °C) and humidity-controlled environment with a reversed 12 h light–dark cycle. Rats were given free access to chow and tap water for the duration of the experiment. All behavioral testing was conducted during the dark phase of the light–dark cycle. A detailed timeline of experimental events is shown in Supplementary S1. The studies were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Behavioral Equipment
Operant training and testing was in 16 identical operant chambers (Med Associates, St Albans, VT, USA; 30.5 × 29.2 × 24.1 cm3) housed in sound-attenuating cubicles. Each operant chamber was equipped with two retractable levers positioned laterally to a liquid cup receptacle. Locomotor activity was assessed using six identical standard locomotor activity testing chambers (Med Associates; 44.5 × 44.5 × 30.5 cm3).
Alcohol Self-Administration
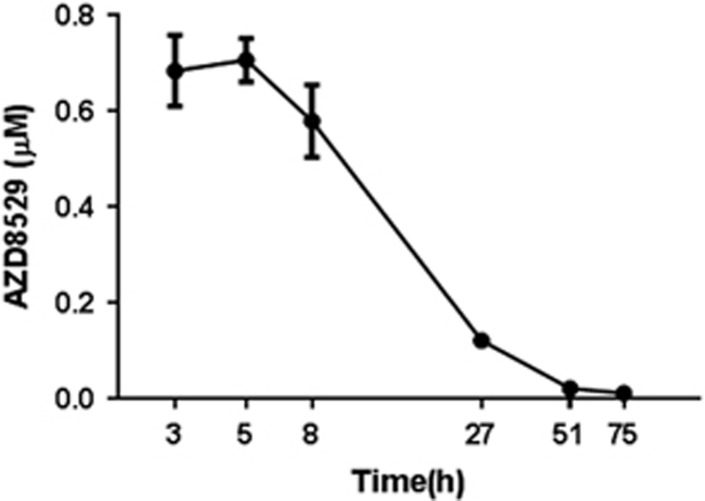
Thirty-two experimentally naive rats (group 1, see Supplementary S1) were trained to self-administer 20% (v/v) alcohol without sucrose/saccharin fading as described (Augier et al, 2016; Augier et al, 2014). Briefly, rats were first trained on a fixed ratio 1 (FR1) schedule to self-administer 20% alcohol during 30 min sessions. Two levers were extended to mark the onset of the session and to signal alcohol availability. Pressing once on the lever associated with alcohol (active) was reinforced by the delivery of a volume of 100 μl of 20% alcohol in water in the adjacent drinking well and initiated a concomitant 5 s time-out period signaled by the illumination of the cue-light above the lever. Responses on the other lever (inactive) were recorded but had no behavioral consequences. During the time-out period, responding had no scheduled consequences. Sessions were conducted 5/6 days a week until stabilization of performance (defined as a minimum of 15 sessions and no change >15% in the total number of reinforcers earned during the last 3 sessions). A total of 20 FR1 sessions were performed. Once a stable self-administration baseline was reached, the fixed ratio was increased and a total of 21 FR2 sessions were performed. Starting on day 42, rats were tested in a balanced/random order in a within-subjects design across one of the three AZD8529 dosing cycles (0, 20, and 40 mg/kg) 3 h before the self-administration session. The timing of injections was chosen based on the pharmacokinetic study (see Figure 1) showing that AZD8529 concentration in the blood is maximal 3–5 h after injection. Between each dosing cycle, rats were allowed to washout the drug with five consecutive self-administration sessions. As a result, at the end of the test, all rats had been injected with each of the three doses.
Figure 1.
AZD8529 concentration is maximum between 3 and 5 h after injection. Plasma concentration–time curve of AZD8529 after a single subcutaneous injection of a 20 mg/kg dose of AZD8529 (n=4). Blood samples were taken 3, 5, 8, 27, 51, and 75 h after injection.
Cue-Induced Reinstatement
After returning to baseline during 4 consecutive sessions on FR2, responding was extinguished during 30 sessions. During extinction, conditions were identical to baseline self-administration except that active lever presses resulted in neither alcohol delivery nor activation of the light cue. Rats were tested for reinstatement if they decreased their active lever presses to <15 during the last 3 stabilized sessions of extinction. Two rats did not achieve this criterion and were excluded from the analysis. For cue-induced reinstatement, a small droplet of alcohol was added on the drinking well associated with alcohol to serve as an odor cue and the test was preceded by 1 min of intermittent presentation of the cue-light (the light was illuminated for 5 s every 20 s). During the test itself, active lever presses resulted in contingent illuminations of the cue-light previously paired with the drug, but alcohol was not delivered.
Stress-Induced Reinstatement
Following the cue-induced reinstatement test, responding was re-extinguished during a total of 20 sessions. To prepare for stress-induced reinstatement, rats were habituated for 15 min in the self-administration chambers immediately preceding extinction sessions for the last 4 days of extinction. For stress-induced reinstatement, rats received 15 min of intermittent footshock (0.5 s shock, 0.6 mA, mean intershock interval 40 s) immediately preceding the reinstatement test, in accordance with previous experiments conducted in our laboratory (Augier et al, 2014). One rat was excluded from the analysis for not meeting extinction criterion.
Progressive Ratio Schedule of Reinforcement
The motivation of the animals to consume alcohol was assessed using a progressive ratio schedule (Hodos, 1961). Conditions were identical to baseline self-administration except that the response requirement to receive a single alcohol reinforcer was increased within session according to the following formula: 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 28, 32, …. The self-administration session terminated once 30 min had elapsed without a reinforcer. The breakpoint is defined as the last completed response requirement during the progressive ratio test.
Saccharin Self-Administration
Conditions were similar to alcohol self-administration. Briefly, rats were trained to self-administer 0.2% saccharin in 30 min sessions under a FR2 5 s time-out schedule of reinforcement. Once a stable self-administration baseline was reached (which occurred after 10 sessions), rats were tested in a balanced/random order in a within-subjects design across one of the three AZD8529 dosing cycles (0, 20, and 40 mg/kg) 3 h before the self-administration session. Rats were allowed to washout the drug for at least four consecutive sessions between each dosing cycle.
Genotype Analysis
Genomic DNAs were isolated from the tails of Wistar (group 1, n=32) and P rats (group 2, n=16) using Maxwell 16 DNA Purification Kit (Promega). The SNP of rGrm2–5340 (G/T) was genotyped using Custom TaqMan SNP Genotyping Assays (assay ID: AHBJ29I) from Applied Biosystems (Foster City, CA, USA). The assay contained a pair of primers and a pair of fluorescent-quenched probes (AHCTAG4_V: 5′-TAGCATCGCAGAGGTG-3′ and AHCTAG4_M: 5′-CATAGCATCTCAGAGGTG-3′). Primers and probes were mixed with TaqMan Universal PCR Master Mix (Applied Biosystems). A total of 4.5 μl of genomic DNA (2.5 ng/μl) was transferred in triplicate to a 384-well plate, each well of which contained 5.5 μl PCR mixtures. The PCR reaction was performed following a protocol provided by ABI. The allele was discriminated by post-PCR plate read on ViiA7 System (Applied Biosystems). Data were processed using the ViiA7 software (Applied Biosystems).
Saccharin Cue-Induced Reinstatement
A new batch of rats (group 3, n=41) was trained under similar conditions to those described above to self-administer 0.2% saccharin in 30 min sessions first under a FR1 5 s time-out schedule of reinforcement (9 sessions) followed by 5 sessions under a FR2 5 s time-out. Responding for saccharin was then extinguished during 15 extinction sessions. Conditions were identical to baseline self-administration except that active lever presses resulted in neither saccharin delivery nor activation of the light cue. For cue-induced reinstatement, a small droplet of saccharin was added to the drinking well associated with saccharin to serve as an odor cue and the test was preceded by 1 min of intermittent presentation of the cue-light (the light was illuminated for 5 s every 20 s). During the test itself, active lever presses resulted in contingent illuminations of the cue-light previously paired with saccharin but no reinforcer delivery.
Locomotor Activity
Locomotor activity was measured in a separate group of rats (group 4, n=15) during 30 min sessions to match the length of the self-administration session. Rats were first tested during three consecutive sessions until their baseline locomotor activity was established. They were then tested in a balanced/random order in a within-subjects design across one of the three AZD8529 dosing cycles (0, 20, and 40 mg/kg) 3 h before the self-administration session. Between each dosing cycle, rats were allowed to washout the drug with at least four consecutive locomotion sessions.
Pharmacokinetic Study
The pharmacokinetic profile of AZD8529 was assessed in a separate group of rats (group 5, n=4). Rats were injected subcutaneously with a dose of 20 mg/kg of the drug and blood samples were taken as described previously (Augier et al, 2016; Augier et al, 2014) at 6 different time points: 3, 5, 8, 27, 51, and 75 h after the injection. Plasma samples were obtained no later than 30 min after blood collection, by centrifugation (2000 g) at 4 °C for 20 min, frozen immediately, and stored at −80 °C. For analysis of AZD8529, samples were diluted in methanol/acetonitrile, recentrifuged, and analyzed directly by LC/MS/MS (AB/MDS Sciex API 4000).
Statistics
The effect of AZD8529 on alcohol self-administration, saccharin self-administration, and locomotion was analyzed using a one-way repeated-measures analysis of variance (one-way RM ANOVA). The effect of AZD8529 on progressive ratio was analyzed using a one-way ANOVA.
The effect of AZD8529 on stress-induced reinstatement, as well as cue-induced reinstatement, was analyzed using a two-way RM ANOVA.
The comparison of the effects of AZD8529 on cue-induced reinstatement in P rats and Wistar rats was analyzed using a three-way RM ANOVA, with genotype (P rats vs Wistar rats) and dose (vehicle vs 40 mg/kg) as independent factors and test conditions (extinction vs reinstatement) as a dependent factor.
Extinction data presented in Figures 3 and 4d were collapsed between groups to improve the readability of these figures. All groups were, however, compared and separately analyzed in the statistical analysis.
All post-hoc analyses were conducted when appropriate using Tukey HSD test.
RESULTS
AZD8529 Marginally Decreases Alcohol Self-Administration
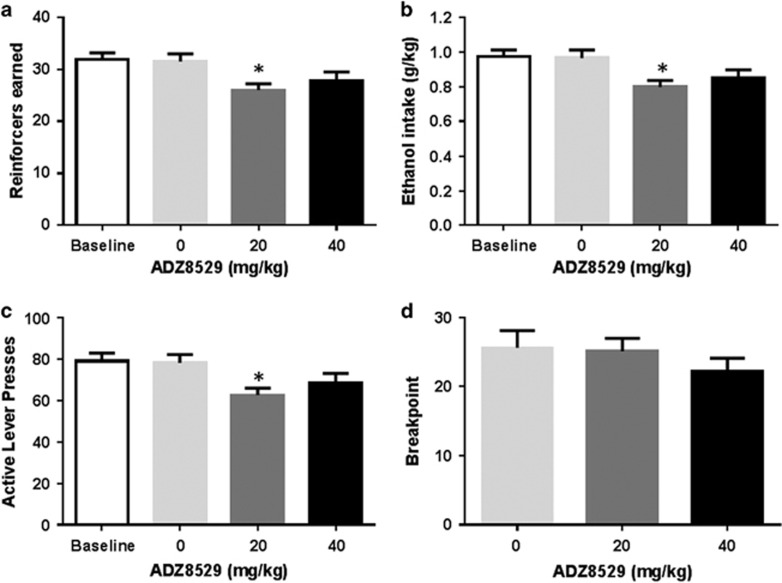
Once operant responding had stabilized, the rats earned a total of 31.9±1.2 reinforcers (corresponding to an average ethanol intake of 0.98±0.03 g/kg) and produced 78.9±3.8 presses on the active lever associated with alcohol (Figure 2a–c, respectively, white bars). One-way RM ANOVA showed that AZD8529 significantly decreased reinforcers (F3,93=7.17, p<0.001, Figure 2a) and total ethanol intake (F3,93=7.13, p<0.001, Figure 2b), as well as active lever presses (F3,93=8.30, p<0.001, Figure 2c) while responses on the inactive lever remained low and unaffected (F3,93=1.05, p=0.37, see Supplementary S2). Moreover, post-hoc analyses indicated a significant effect of the low dose of 20 mg/kg on reinforcers, total ethanol intake, and active responses compared with vehicle (p<0.01) but only a trend to reduce alcohol operant self-administration for the high dose of 40 mg/kg (p=0.054 for active lever presses, p=0.076 for reinforcers and p=0.071 for ethanol intake). The vehicle did not affect self-administration compared with baseline (p=0.99 for both reinforcers and lever presses). Thus AZD8529 significantly affected alcohol self-administration, but the magnitude was modest, with a decrease of only 18% compared with vehicle.
Figure 2.
AZD8529 slightly decreases 20% alcohol self-administration. (a) Mean reinforcers (±SEM) earned during a 30-min FR2 self-administration session of 20% EtOH following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=32) (*p<0.01). (b) Mean ethanol intake (±SEM) during a 30-min FR2 self-administration session of 20% EtOH following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=32) (*p<0.01). (c) Mean active lever presses (±SEM) completed during a 30-min FR2 self-administration session of 20% EtOH following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=32) (*p<0.01). (d) Mean breakpoint (±SEM) reached during a progressive ratio session of 20% EtOH following either saline AZD8529 treatment (20 or 40 mg/kg) (n=10, 11 by groups).
The effect of the AZD8529 on the motivation of the animals to consume alcohol was also tested using a progressive ratio schedule (Hodos, 1961). The statistical analysis revealed that the drug did not affect breakpoint compared with vehicle (F2,29=0.79, p=0.46, Figure 2d).
AZD8529 Potently Blocks Cue-Induced Alcohol Seeking
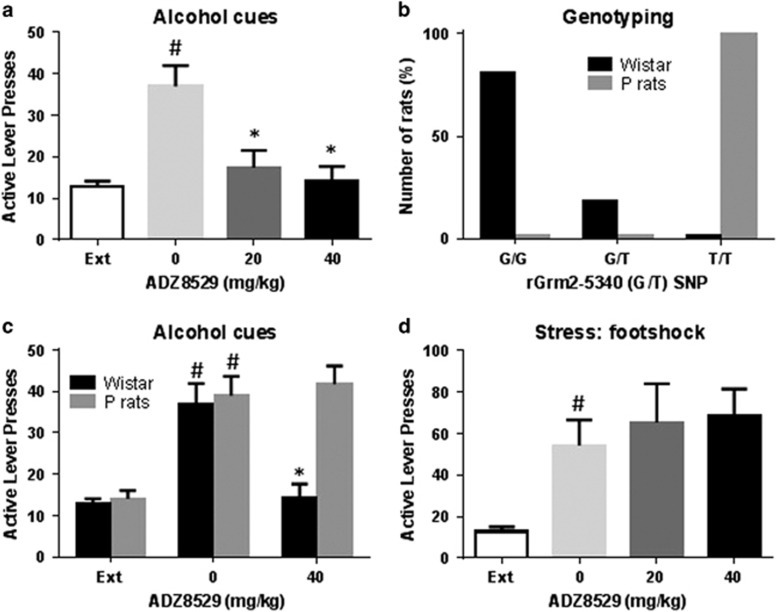
Following extinction, responses on the active lever dropped to a low level (reaching an average of 12.7±1.3 through the 3 last sessions of extinction, Figure 3a). There was no difference in the extinction responding rates between the three groups (all p-values ⩾0.8). There was a significant main effect of test condition, demonstrating a robust reinstatement of responding on the previously alcohol-associated level (F1,26=23.37, p<0.001). This was confirmed on post-hoc analysis, which showed that reintroduction of the alcohol-associated cues led to a robust reinstatement of alcohol seeking in the vehicle group (p<0.001). There was a significant main effect of AZD8529 treatment (F2,26=4.55, p<0.05) and a significant interaction between test condition and treatment (F2,26=5.50, p<0.01). Specifically, post-hoc analysis showed that treatment with AZD8529 blocked cue-induced reinstatement (Figure 3a, p<0.01 for both 20 and 40 mg/kg).
Figure 3.
AZD8529 specifically blocks cue-induced alcohol seeking. (a) Mean number of non-reinforced lever presses (±SEM) during the 30-min test for cue-induced reinstatement following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=10 by groups) (*p<0.01 compared with vehicle, #p<0.001 compared with extinction). (b) Genotyping of the SNP of rGrm2–5340 (G/T) in P rats (n=16) and Wistar rats (n=32). (c) Mean number of non-reinforced lever presses (±SEM) during the 30 min test for cue-induced reinstatement following either saline or AZD8529 treatment (40 mg/kg) in Wistar rats (n=10 by groups) and P rats (n=8 by groups) (*p<0.01, #p<0.001). (d) Mean number of non-reinforced lever presses (±SEM) during the 30-min test for stress-induced reinstatement following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=10, 11 by groups) (#p<0.001 compared with extinction).
Alcohol-Preferring P Rats are Homozygous for the Grm2 407 Stop Codon
Genotyping of SNP of rGrm2–5340 (G/T) showed that all 16 P rats were homozygous for the T-allele of this SNP (Figure 3b), which introduces a stop codon in the gene encoding this receptor, and results in a lack of functional mGluR2 expression (Zhou et al, 2013). In contrast, 26 out of 32 Wistar rats were homozygous for the G-allele, with the remaining 6 rats being heterozygous G/T.
AZD8529 Blockade of Cue-Induced Alcohol Seeking is Mediated Through mGluR2s
To determine target specificity of AZD8529, we therefore examined the effect of 40 mg/kg of AZD8529 on alcohol-preferring P rats, a strain that does not express functional mGluR2 (Zhou et al, 2013). Similarly to outbred Wistar rats, reintroduction of the alcohol-associated cues led to a robust reinstatement of alcohol seeking in the vehicle-treated animals (main effect of the reinstatement test, F1,30=17.26, p<0.001), as confirmed on post-hoc analysis (p<0.001 for both P rats and Wistar rats, Figure 3c). There was in addition a significant main effect of genotype (P rats vs Wistar rats, F1,30=7.57, p<0.01), a significant main effect of AZD8529 treatment (F1,30=4.98, p<0.05), and a significant interaction between test condition, treatment, and genotype (F1,30=7.01, p<0.05). However, as expected, a dose of 40 mg/kg failed to block cue-induced reinstatement only in alcohol-preferring P rats (p=0.99 for P rats vs p<0.001 for Wistar rats compared with their respective vehicles, Figure 3c).
AZD8529 Does not Affect Stress-Induced Alcohol Seeking
Exposure to a stressor (footshock) also produced reinstatement of alcohol seeking (main effect of the reinstatement test, F1,27=37.80, p<0.001), but AZD8529 treatment had no effect on stress-induced reinstatement of alcohol seeking (p⩾0.84 for both doses, Figure 3d).
AZD8529 Does not Affect Operant Responding of Saccharin or Locomotor Activity
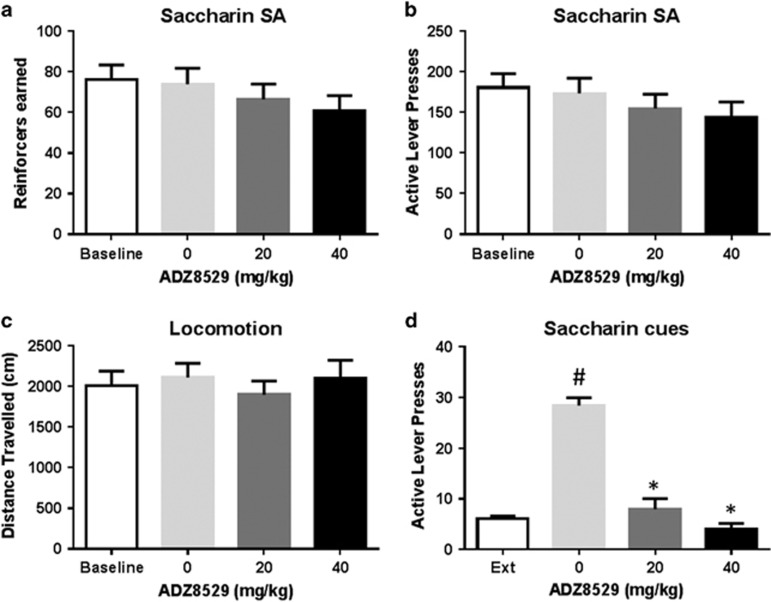
Rats previously trained on FR2 alcohol self-administration quickly acquired FR2 0.2% saccharin self-administration, earning 73.9±7.9 reinforcers by session (Figure 4a) and producing 173.2±18.7 on the active lever (Figure 4b). The statistical analysis showed no difference between baseline responding and the test days (F3,93=1.62, p=0.19 for reinforcers and F3,93=1.78, p=0.16 for response rates). Treatment with AZD8529 neither significantly affected the number of reinforcers earned (p⩾0.28 for both doses) nor the total number of active lever presses produced by the animals (p⩾0.35 for both doses). Additionally, the effect of AZD8529 on locomotor activity was assessed in a separate group of rats. There was no difference between baseline locomotion and the test days (F2,28=1.58, p=0.22) and AZD8529 did not alter locomotor activity at the doses tested (p⩾0.31 for both doses, Figure 4c).
Figure 4.
AZD8529 blocks cue-induced saccharin seeking without affecting 0.2% saccharin operant responding. (a) Mean reinforcers (±SEM) earned during a 30-min FR2 self-administration session of 0.2% saccharin following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=32). (b) Mean active lever presses (±SEM) completed during a 30-min FR2 self-administration session of 0.2% saccharin following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=32). (c) Mean distance travelled (±SEM) following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=15). (d) Mean number of non-reinforced lever presses (±SEM) during the 30-min test for cue-induced reinstatement following either saline or AZD8529 treatment (20 or 40 mg/kg) (n=12–14 by groups) (*p<0.001 compared with vehicle, #p<0.001 compared with extinction).
AZD8529 Potently Blocks Cue-Induced Saccharin Seeking
Finally, to investigate whether the pattern of effects induced by AZD8529 on alcohol-related behavior would generalize to a non-drug reinforcer, we assessed the effect of the mGluR2 PAM on cue-induced saccharin seeking. A new batch of rats was trained to self-administer 0.2% saccharin self-administration (see Supplementary S4). Following acquisition, responding for saccharin was extinguished through extinction sessions and responses on the active lever dropped to an average of 6.2±0.6 through the 3 last sessions of extinction (Figure 4d). Re-exposure to the saccharin-associated cues produced a robust reinstatement of saccharin seeking (main effect of the reinstatement test, F1,37=42.46, p<0.001) only in the vehicle-treated animals, as indicated by post-hoc analysis (p<0.001 for the vehicle group, but p⩾0.78 for both 20 and 40 mg/kg groups when compared with their respective extinction baselines). There was a significant main effect of AZD8529 treatment (F2,37=39.82, p<0.001) and a significant interaction between test condition and treatment (F2,37=46.87, p<0.001). Specifically, post-hoc analysis showed that treatment with AZD8529 blocked cue-induced reinstatement (Figure 4d, p<0.001 for both 20 and 40 mg/kg doses).
DISCUSSION
The key finding of our study is that AZD8529, a novel positive allosteric modulator with high selectivity for mGluR2, potently suppressed alcohol seeking in an established animal model of relapse. AZD8529 also decreased alcohol self-administration, but the magnitude of that effect was marginal and thus of lesser interest. Several control experiments showed that these effects of AZD8529 were not the result of non-specific sedative or otherwise performance-impairing properties of the drug. Specifically, operant responding for a non-drug reinforcer, saccharin, was unaffected by treatment. Furthermore, AZD8529 did not impair locomotor activity at either of the doses tested (20 and 40 mg/kg).
Our findings are in line with prior results obtained with orthosteric group II mGluRs ligands lacking an ability to discriminate between mGluR2 and mGluR3. For example, systemic injections of LY379268, an mGluR2/3 agonist, reduced ethanol intake (Backstrom and Hyytia, 2005) whereas injections of LY341495, an mGluR2/3 antagonist resulted in escalation of alcohol-intake in Wistar rats, although this effect was short-lived (Zhou et al, 2013). Our findings are also in line with reports suggesting that signaling specifically through the mGluR2 subtype accounts for the effects observed with mGluR2/3 agonists. Specifically, the loss of mGluR2 function has been shown to result in escalation of alcohol drinking and increased preference for highly concentrated alcohol solutions (15 and 17%) in Grm2 knockout mice (Zhou et al, 2013). Furthermore, escalation of alcohol self-administration and increased motivation to obtain alcohol is observed following a history of alcohol dependence, and this ‘postdependent' phenotype has been shown to be associated with a marked downregulation of prelimbic mGluR2 expression. Rescue of this molecular deficit specifically in neurons projecting to the nucleus accumbens shell was also able to rescue the behavioral phenotype in these animals (Meinhardt et al, 2013).
We found that the effects of mGluR2 activation on relapse-like behavior in the reinstatement model were highly specific to reinstatement induced by alcohol-associated stimuli, with no effect on stress-induced reinstatement of alcohol seeking. In contrast, Weiss and colleagues (Sidhpura et al, 2010; Zhao et al, 2006) found that treatment with the orthosteric agonist LY379268 decreased both cue- and stress-induced reinstatement of alcohol seeking. Because LY379268 does not discriminate between mGluR2 and mGluR3, the apparent discrepancy between these findings may indicate that mGluR2 and mGluR3 are differentially involved in cue-induced reinstatement of alcohol seeking and stress-induced reinstatement of alcohol seeking, respectively. Alternatively, this may reflect a difference in the way direct agonists and PAMs modulate glutamatergic transmission. Furthermore, while our data show that AZD8529 treatment somewhat lowered the reinforcing value of alcohol, this contribution seems weak in comparison to the effect of the drug on cue-induced alcohol seeking. In accordance with these observations, Sidhpura et al (2010) also found that the agonist LY379268 differentially altered alcohol seeking and the reinforcing properties of alcohol in both non-dependent and dependent rats. Additionally, LY379268 attenuated conditioned reinstatement of cocaine seeking, rather than the acute reinforcing effects of cocaine (Baptista et al, 2004). Similarly, we found that AZD8529 potently blocked cue-induced saccharin seeking, without affecting operant responding for the non-drug reinforcer. Saccharin is highly reinforcing for rats, as witnessed by higher self-administration rates and progressive ratio breakpoints than alcohol (Goodwin et al, 2001). The finding that AZD8529 blocks reinstatement triggered by exposure to both alcohol- and saccharin-associated cues may therefore support a broader utility of this medication for prevention of relapse caused by exposure to drug memories. This effect is nevertheless highly behaviorally specific, as the drug did not influence stress-induced reinstatement.
We were able to establish the causal role of mGluR2 activation in mediating the effects of AZD8529 effects on cue-induced reinstatement of alcohol seeking. Alcohol-preferring P rats, a strain that has been selectively bred from Wistar rats for high alcohol drinking, have been proposed to model important aspects of human alcoholism (Li et al, 1993; Li et al, 1986; McBride and Li, 1998). A recent investigation identified a functional genetic variant in Grm2, the gene that encodes mGluR2, in P rats (Zhou et al, 2013). This variant occurs in position 407 of Grm2, where it introduces a stop codon. All P rats so far examined are homozygous for this allele (*407/*407), which leads to a loss of functional mGluR2 protein expression. In contrast, the frequency of the *407 allele was only 0.086 in the parental Wistar strain, and none of 64 Wistar rats examined by Zhou et al (2013) were *407/*407 homozygotes. In agreement with this work, all 16 P rats used in our study were confirmed to be *407/*407 homozygous for the Grm2 407 stop codon and therefore do not express functional mGluR2. The presence of this deletion completely eliminated the effect of AZD8529 on cue-induced reinstatement of alcohol seeking. This result provides compelling evidence that suppression of cue-induced reinstatement by this drug is solely dependent on mGluR2 potentiation. Of note, Rodd et al (2006) have previously reported the suppression of alcohol seeking in P-rats following administration of the mGluR2/3 receptor agonist LY404039. Given the subsequent findings that P-rats lack functional mGluR2 receptors, these data suggest the possibility that mGluR3 receptors can in part compensate for the null mutation in the P-line.
Our data extend the recent finding indicating that mGluR2 PAMs should be considered as therapeutic candidates to treat drug addiction and, in particular, may prove efficient in preventing relapse. It was recently shown that AZD8529 decreases nicotine self-administration as well as nicotine-primed and cue-induced reinstatement of nicotine seeking in squirrel monkeys (Justinova et al, 2015). Moreover, AZD8529 reduces cue-induced methamphetamine craving in rats in both models of voluntary and forced abstinence from the drug (Caprioli et al, 2015). Additionally, BINA, another mGluR2 PAM, similarly decreases cue-induced cocaine seeking in rats (Jin et al, 2010). Together, these results indicate that positive allosteric modulators of mGluR2 have efficacy in attenuating relapse across a variety of drugs of abuse in animal models and suggest that this class of drug should be considered for relapse prevention in humans.
FUNDING AND DISCLOSURE
We thank AstraZeneca for kindly providing AZD8529. This work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism and funding from the Swedish Research Council. AJC is an AstraZeneca employee. All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary Material
References
- Augier E, Dulman RS, Singley E, Heilig M (2016). A method for evaluating the reinforcing properties of ethanol in rats without water deprivation, saccharin fading or extended access training. J Vis Exp (in press). [DOI] [PMC free article] [PubMed]
- Augier E, Flanigan M, Dulman RS, Pincus A, Schank JR, Rice KC et al (2014). Wistar rats acquire and maintain self-administration of 20% ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology (Berl) 231: 4561–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P (2005). Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol 528: 110–118. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F (2004). Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement vs primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci 24: 4723–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM (2005). The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport 16: 1013–1016. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24: 10726–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R et al (2015). Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry 78: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK (2009). Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237. [DOI] [PubMed] [Google Scholar]
- Cross AJ (2013). AZD8529-an mGluR2 positive allosteric modulator for the treatment of schizophrenia. Neuropsychopharmacology 38: S25. [Google Scholar]
- Dahchour A, De Witte P (2000). Taurine blocks the glutamate increase in the nucleus accumbens microdialysate of ethanol-dependent rats. Pharmacol Biochem Behav 65: 345–350. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P (2003). Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. Eur J Pharmacol 459: 171–178. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF (2008). Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75: 218–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FL, Campisi M, Babinska I, Amit Z (2001). Effects of naltrexone on the intake of ethanol and flavored solutions in rats. Alcohol 25: 9–19. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M (2006). Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther 111: 855–876. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N et al (2012). Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 71: 1015–1021. [DOI] [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R et al (2010). The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology 35: 2021–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ et al (2015). The novel metabotropic glutamate receptor 2 positive allosteric modulator, AZD8529, decreases nicotine self-administration and relapse in squirrel monkeys. Biol Psychiatry 78: 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10: 561–572. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A (2004). The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 25: 265–272. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C et al (2013). Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology 66: 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP (1993). Selective breeding for alcohol preference and associated responses. Behav Genet 23: 163–170. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB, Murphy JM (1986). Studies on an animal model of alcoholism. NIDA Res Monogr 66: 41–49. [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A (2007). Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27: 9077–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y (2007). Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry 61: 591–598. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ (2006). Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol 6: 98–102. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK (1998). Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12: 339–369. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M et al (2013). Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci 33: 2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS (2000). Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130: 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R (1995). The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34: 1–26. [DOI] [PubMed] [Google Scholar]
- Ritzen A, Mathiesen JM, Thomsen C (2005). Molecular pharmacology and therapeutic prospects of metabotropic glutamate receptor allosteric modulators. Basic Clin Pharmacol Toxicol 97: 202–213. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD et al (2006). The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res 171: 207–215. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S (1995). Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 283: 177–183. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C et al (2003). Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol 64: 798–810. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA (1999). Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38: 1431–1476. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R (2010). Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry 67: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R (2003). Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol 14: 257–277. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L et al (2010). Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26: 9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD et al (2013). Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci USA 110: 16963–16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.