Abstract
Localized stimulation of the human brain to treat neuropsychiatric disorders has been in place for over 20 years. Although these methods have been used to a greater extent for mood and movement disorders, recent work has explored brain stimulation methods as potential treatments for addiction. The rationale behind stimulation therapy in addiction involves reestablishing normal brain function in target regions in an effort to dampen addictive behaviors. In this review, we present the rationale and studies investigating brain stimulation in addiction, including transcranial magnetic stimulation, transcranial direct current stimulation, and deep brain stimulation. Overall, these studies indicate that brain stimulation has an acute effect on craving for drugs and alcohol, but few studies have investigated the effect of brain stimulation on actual drug and alcohol use or relapse. Stimulation therapies may achieve their effect through direct or indirect modulation of brain regions involved in addiction, either acutely or through plastic changes in neuronal transmission. Although these mechanisms are not well understood, further identification of the underlying neurobiology of addiction and rigorous evaluation of brain stimulation methods has the potential for unlocking an effective, long-term treatment of addiction.
INTRODUCTION
There are four main procedures developed to stimulate specific brain regions. These include: (i) transcranial electrical stimulation; (ii) transcranial magnetic stimulation (TMS); (iii) transcranial direct current stimulation (tDCS); and (iv) deep brain stimulation (DBS). Transcranial electrical stimulation delivers an electrical current to the brain across electrodes, and it provided much of the early data on the neurophysiological effects of stimulating cortical regions and the propagation of the stimulus in the central nervous system (Rossini et al, 2015). However, because transcranial electrical stimulation requires a current of several hundred volts, transcranial electrical stimulation was largely replaced by TMS, which uses magnetic pulse to induce an electrical current in the brain. TDCS delivers a very low intensity electrical current (1–2 mA), which is likely too low to initiate action potentials in neurons, but may modulate firing rates by changing the membrane potential of neurons. DBS is currently the only method available to directly stimulate deeper brain regions, but a disadvantage of DBS is the need for surgery and the maintenance of implanted hardware. The purpose of this review is to condense experimental findings from the large and growing literature of brain stimulation and addiction, draw conclusions, and offer our perspective on how to improve its evaluation. We refer the reader to other recently published reviews for additional perspectives of its safety and efficacy (see (Gorelick et al, 2014b; Hadar and Zangen, 2015)).
TRANSCRANIAL MAGNETIC STIMULATION (TMS)
TMS uses electromagnetic induction to generate an electrical current in the brain. The device consists of a conducting coil to produce the current, which induces a brief magnetic field orthogonal to the plane of the coil, which in turn generates an electrical current in the brain. Early TMS coils were circular, and the stimulation delivered was greatest around the circle edge but low in the center. The figure 8 coil was designed to produce a field with the highest intensity at the juncture of the two circles (Rossini et al, 2015).
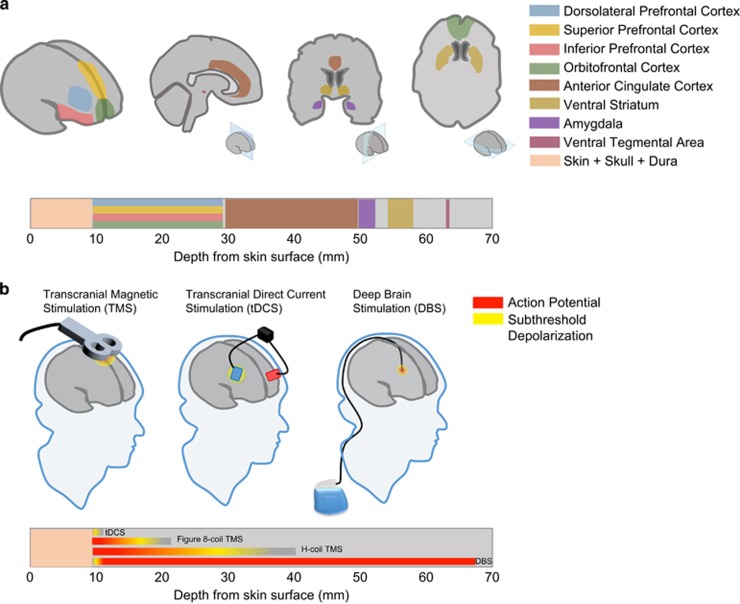
An issue with TMS is the limited depth of brain tissue that can be reached (Figure 1b). The rapid attenuation of the electrical field results in TMS being largely restricted to superficial cortical targets (Deng et al, 2014). As a result, most TMS studies of psychiatric disorders, including addiction, have stimulated the dorsolateral prefrontal cortex (DLPFC). However, given the need to investigate the stimulation of deeper brain regions as therapeutic targets (Figure 1a), there is a demand to develop coils that can reach these targets. Among these is the H coil, designed to stimulate deeper brain structures using spatial summation to reduce attenuation (Roth et al, 2002), although this comes at the cost of reduced focality (Zangen et al, 2005; Huang et al, 2009), so that larger brain regions are stimulated. There are a number of different types of H coils that target different brain regions, and these have been tested clinically in psychiatric and neurologic disorders (Deng et al, 2014).
Figure 1.
Overlap of brain regions to target in addiction with brain stimulation methods. (a) Human brain regions implicated in addiction. Top. Illustration of the major areas of the brain implicated in addiction based on imaging studies. Perspectives are from (left to right) cortical surface, sagittal, coronal, and horizontal perspectives (inset shows approximate view) Bottom. Approximate distances of implicated brain regions from skin surface above frontal and parietal bones. (b) Methods of brain stimulation. Top. Illustrations depicting methods of brain stimulation used in human from left: repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and deep brain stimulation (DBS) with theoretical range of depolarization and action potential level of stimulation from current delivered. Bottom: Theoretical range of direct stimulation shown in distance from skin surface above frontal and parietal bones. Both tDCS and rTMS can stimulate cortical regions, while can DBS reach deeper subcortical structures.
TMS applied to the motor cortex provides a method for investigating the neurophysiology of cortico-spinal connections, because changes in activation can be determined by measuring alterations in the motor evoked potential (MEP). In clinical studies, the intensity of the TMS stimulus is determined by the minimum stimulation required to elicit a reliable MEP in the targeted muscle group. This is assessed using the motor threshold (MT), identified as the intensity delivered to the MT that elicits the contraction of a muscle (such as the hand or calf muscle). The MT is used to determine the intensity of the TMS pulse that will be delivered to the target brain region, such as the DLPFC.
Generally, repetitive TMS (rTMS), in which a series of consecutive stimuli are delivered within a session, is used in clinical research. The frequency of the delivered stimuli can be low frequency (LF, 1 Hz or below) or high frequency (HF, between 5 and 20 Hz), where LF stimulation is inhibitory and HF stimulation increases cortical excitability (Gorelick et al, 2014a). This theory is largely based on studies of cortico-spinal motor output, where HF TMS to the motor cortex increases cortical excitability, whereas LF stimulation reduces the measured MEP, although there are exceptions to this observation (Gorelick et al, 2014a). An additional variation in stimulation frequency, based on observations made using in vitro electrophysiology is called theta burst stimulation (TBS) where 3 pulses at 50 Hz is repeated at 5 Hz. When applied to the cortex, low amplitude continuous TBS (cTBS) produces an inhibitory-like effect, similar to LF continuous stimulation, whereas intermittent TBS (2 s TBS every 10 s) produces a facilitatory effect similar to HF stimulation (Huang et al, 2005; Hanlon et al, 2015b).
Repetitive TMS has been shown to cause lasting effects on physiology and behavior. These actions are believed to follow or approximate traditional phenomena of Hebbian forms of synaptic plasticity including long-term potentiation and long-term depression (Hoogendam et al, 2010). For instance, LF rTMS (~1 Hz) of the human motor cortex can decrease the MEP for over an hour following cessation (Chen et al, 1997; Iyer et al, 2003). HF stimulation with rTMS (10 Hz) can increase the MEP for up to 2 h post cessation (Jung et al, 2008). Interestingly, the persistent effects of HF stimulation can be blocked by NMDA receptor antagonists indicating that a glutamatergic mechanism similar to long-term potentiation is involved (Huang et al, 2008). Imaging studies have supported these findings and demonstrate that cortical stimulation can achieve lasting effects in brain regions that receive efferent connections from the targeted region including the anterior insula (Hanlon et al, 2015b) and the striatum (Cho et al, 2015; Hanlon et al, 2015b) when the PFC is stimulated.
rTMS Studies in Addiction
There have been a series of studies investigating the effect of rTMS in addiction, and these are summarized in Table 1. Most studies used a limited number of sessions (usually 1–2), to investigate acute effects such as craving. Fewer studies used more sessions (10 or more), which are needed to assess rTMS as a potential intervention for drug or alcohol consumption.
Table 1. Transcranial Magnetic Stimulation (TMS).
| Drug | Treatments | n | Target | Stimulation | Outcome measures | Effect | Citation |
|---|---|---|---|---|---|---|---|
| Nicotine | 1 | 11 | L DLPFC | 10,20 Hz, 90,100% MT | Craving | ↓ | Johann et al, 2003 |
| 1 | 16 | L DLPFC | 10 Hz, 100% MT | Cue-induced craving | ↓ | Li et al, 2013a, b | |
| 2 | 14 | L DLPFC | 20 Hz, 90% MT | Craving Ad libitum smoking | No effect ↓ | Eichhammer et al, 2003 | |
| 1 | 14 | L DLPFC | 10 Hz, 90% MT | Cue-induced craving EEG delta | ↓ ↓ | Pripfl et al, 2014 | |
| 1 | 10 | L DLPFC | 1 Hz, 110% MT | Cue-induced craving fMRI: ACC, OFC, VS | ↓ ↓ | Hayashi et al, 2013 | |
| 1 | 15 | SFG SFG MOC | 1 Hz, 90% MT 10 Hz, 90% MT 1, 10 Hz, 90% MT | Cue-induced craving Cue-induced craving Cue-induced craving | No effect ↓ No effect | Rose et al, 2011 | |
| 10 | 48 | L DLPFC | 10 Hz, 100% MT | Cue-induced craving Cigarette consumption | ↓ ↓ | Amiaz et al, 2009 | |
| 20, w therapy | 15 | L,R DLPFC | 20 Hz, 90% MT | Craving Smoking | ↓ No effect | Wing et al, 2012 | |
| 15 | 35 | L DLPFC | 10 Hz, 110% MT | Smoking | ↓ | Prikryl et al, 2014 | |
| 13, h-coil, w/cues | 115 | PFC, insula PFC, insula | 1 Hz, 120% MT 10 Hz, 120% MT | Cigarette consumption Cigarette consumption | No effect ↓ | Dinur-Klein et al, 2014 | |
| Alcohol | 10 | 45 | R DLPFC | 10, Hz, 110% MT | Craving | ↓ | Mishra et al, 2010 |
| 10 | 20 | R and L DLPFC | 10, Hz, 110% MT | Craving | ↓ | Mishra et al, 2015 | |
| 1 | 31 | R DLPFC | 20 Hz, 110% MT | Craving (lab) Craving (home) | No effect No effect | Herremans et al, 2012 | |
| 1 | 29 | R DLPFC | 20 Hz, 110% MT | Craving Response inhibition | No effect ↑ | Herremans et al, 2013 | |
| 1 | 19 | L DLPFC | 20 Hz, 90% MT | Craving Depressive symptoms Alcohol cue attention | No effect No effect ↓ | Hoppner et al, 2011 | |
| 20, h-coil | 11 | MPFC LPFC | 20 Hz, 120% MT | Craving | ↓ | Rapinesi et al, 2015 | |
| 10 | 18 | MPFC | 20 Hz, 120% MT | Craving Depressive symptoms | ↓ ↓ | Ceccanti et al, 2015 | |
| Cocaine | 1 | 6 6 | R DLPFC L DLPFC | 10 Hz, 90% MT 10 Hz, 90% MT | Craving Craving | ↓ No effect | Camprodon et al, 2007 |
| 10 | 36 | L DLPFC | 15 Hz, 100% MT | Craving | ↓ | Politi et al, 2008 | |
| 1 | 11 | MPFC | cTBS, 110% MT | Craving | ↓ | Hanlon et al, 2015a, b | |
| Methamph. | 1 | 10 | L DLPFC | 1 Hz, 100% MT | Craving | ↑ | Li et al, 2013a, b |
Abbreviations: L, left; R, right; cTBS, theta burst simulation; DLPFC, dorsolateral prefrontal cortex; MOC, motor cortex; MT, motor threshold; MPFC, medial prefrontal cortex; PFC, prefrontal cortex; SFG, superior frontal gyrus; VS, ventral striatum.
Nicotine
Among the substance use disorders, nicotine (tobacco) dependence has been the most studied with both acute (1–2 sessions) and repeated (>10 sessions) of rTMS.
Acute effects of rTMS on craving. Six studies have investigated the effect of 1–2 sessions of rTMS, and craving was the primary behavioral measure. Five of these targeted the left DLPFC, and one study stimulated the superior frontal gyrus (see Table 1). Of the studies targeting the left DLPFC, most (4/5) used HF rTMS at 90–110% of MT.
The first study, by Johann et al (2003), compared HF rTMS with sham and showed a reduction in craving for cigarettes. Similar results were reported by Li et al (2013a), who compared a single session of HF rTMS with the left DLPFC (vs sham) on cue-induced craving (smoking vs neutral pictures) and showed that active rTMS reduced craving for cigarettes under both cue conditions. A third study looked at both craving and cigarette smoking and showed that HF rTMS to the left DLPFC had no effect on craving, but did decrease smoking during an ad libitum smoking period following the delivery of rTMS (Eichhammer et al, 2003).
Two of the studies targeting the DLPFC investigated brain function in addition to craving. Pripfl et al (2014) showed that HF rTMS reduced cigarette craving in nicotine-deprived smokers and reduced EEG delta power, a measure of wakefulness that has been implicated in nicotine dependence (Pripfl et al, 2014). The second study delivered LF (1 Hz), in contrast to the other studies, to inhibit the left DLPFC and used fMRI to investigate changes in brain activation (Hayashi et al, 2013). The results showed that one session of LF rTMS reduced craving for cigarettes and reduced the response to craving-related signals in the ventral striatum, medial orbitofrontal cortex, and anterior cingulate (Hayashi et al, 2013).
Of the studies delivering short-term rTMS, one targeted the superior frontal gyrus, and compared high and LF stimulation (Rose et al, 2011). The results showed that craving, elicited by cigarette smoke, was increased following 10 Hz stimulation (compared with the 1 Hz), although cigarette craving was reduced following the neutral cues in the 10 Hz condition (Rose et al, 2011).
Repeated sessions of rTMS. Four studies have investigated the effect of multiple sessions of rTMS as an intervention for cigarette smoking. The first was a randomized, sham-controlled study (n=48) performed with the figure 8 coil targeting the left DLPFC using 10 HF rTMS sessions and a maintenance phase (Amiaz et al, 2009). Craving for cigarettes was elicited with smoking vs neutral cues and cigarette consumption was measured by self-report and urine cotinine levels. Both craving and cigarette smoking were reduced in the active rTMS group, but there was significant subject drop out and a dissipation of the effect during the maintenance phase, suggesting that longer stimulation may be needed.
Studies in smokers with schizophrenia show split results. A significant decrease in cigarette smoking was not seen in a 10-week, randomized, double-blind, sham-controlled trial of 20 sessions of HF rTMS to the DLPFC (with the figure 8 coil) in schizophrenic smokers (Wing et al, 2012). The rTMS was delivered as an adjunctive treatment to transdermal nicotine and group therapy, and whereas the active rTMS group reported a decrease in craving compared with the sham group, no difference in smoking was seen between the two groups. However, another study in smokers with schizophrenia showed that HF rTMS to the DLPFC (figure 8 coil) for 21 sessions reported a reduction in cigarette smoking. Craving was not assessed, and the amount of cigarettes consumed was measured only by subjects' self-report (Prikryl et al, 2014).
Only one study has been published using an H coil to stimulate the insula, ventrolateral prefrontal cortex and the DLPFC bilaterally (Dinur-Klein et al, 2014). This study was a large randomized sham-controlled study (n=115) that compared sham, high and LF stimulation administered for 10 days over 2 weeks followed by three non-consecutive treatments for an additional week. The rTMS was delivered in the context of smoking cues, and smoking was measured by urine cotinine levels and subjects' self-report. The results showed that HF rTMS combined with exposure to smoking cues led to a reduction in smoking of 44% at 3 months (Dinur-Klein et al, 2014).
Overall, the studies of nicotine use disorders show that acute rTMS directed at the DLPFC reduces craving for nicotine. However, some of this work shows that there is a mismatch between craving and actual smoking, indicating that studies investigating cigarette consumption are crucial. Of the studies investigating this, the largest (115 subjects) showed that HF, but not LF, stimulation with the H coil directed at the bilateral insula, ventrolateral prefrontal cortex, and the DLPFC reduced smoking (Dinur-Klein et al, 2014). Similar results were shown in the study using the figure 8 coil delivering HF stimulation to the left DPLFC (Amiaz et al, 2009). Together, these studies indicate that HF rTMS, directed at the structures of the prefrontal cortex, may serve as an effective intervention for nicotine use disorders.
Alcohol
In alcohol use disorders, the majority of studies have investigated craving only, with no randomized controlled trials investigating alcohol intake. These studies have used both the figure 8 coil to target the DLPFC and H coil to stimulate broader regions of the prefrontal cortex.
Five studies targeted the DLPFC with HF rTMS, to investigate craving for alcohol. Mishra et al (2010) performed a single-blind, sham-controlled study of 10 sessions of HF rTMS to the right DLPFC in alcohol-dependent subjects who were abstinent >10 days before starting rTMS. The results showed a greater reduction in craving for alcohol in the active group over the sham condition. In a subsequent study, this same group compared 10 sessions of HF rTMS with the right and left DLPFC and showed that both right and left rTMS reduced craving for alcohol (Mishra et al, 2015).
However, other studies targeting the DLPFC have not shown that rTMS reduces craving in alcohol dependence. Herremans et al (2012) investigated a single session of HF rTMS to the right DLPFC (vs sham), and did not report a difference in craving, measured immediately after the session nor in subjects' regular home environment during the days following rTMS (Herremans et al, 2012). In a second study by this group, using similar methods, active rTMS was shown to improve cognitive performance on the Go-NoGo task, indicating improved response inhibition, though again no change was seen in craving for alcohol (Herremans et al, 2013). Similarly, in alcohol-dependent women, one session of HF rTMS (20 Hz at 90% MT), vs sham, did not produce changes in craving or depressed mood, although this group reported a decrease in attention to alcohol-related cues, measured as attentional eye blink (Hoppner et al, 2011).
Two studies have investigated the H coil, directed at medial and lateral prefrontal cortex. Rapinesi et al (2015) performed a 6-month study in subjects with comorbid dysthymic disorder/alcohol use disorder using bilateral rTMS to stimulate the medial and lateral prefrontal regions (including the orbitofrontal cortex), with a left preference (Roth et al, 2007). The rTMS was used as an adjunctive treatment to pharmacotherapy. The results showed an improvement in depressive symptoms and a reduction in craving for alcohol. Similar results were reported in a pilot study using the H coil directed at the medial prefrontal cortex vs sham, where HF rTMS was shown to decrease craving for alcohol and reported alcohol intake (mean number of drinks per day and drinks on days of maximum alcohol intake) (Ceccanti et al, 2015).
Overall, the data in alcohol dependence are less cohesive than that reported for nicotine use disorders, but there are fewer studies. In addition, most of the studies targeted the right DLPFC, whereas the nicotine studies targeted the left DLPFC. Nonetheless, the studies targeting the DLPFC and craving are more split than those investigating nicotine. Small studies using the H coil, which stimulates broader prefrontal regions, show some promise for using rTMS in this disorder. Indicating that specific stimulation may not be necessary to achieve a therapeutic outcome.
Cocaine and methamphetamine
Three studies have been performed using TMS with the figure 8 coil in cocaine dependence. The first study used two sessions of HF rTMS and showed that craving for cocaine was reduced by rTMS applied to the right, but not the left, DLPFC (Camprodon et al, 2007). In a second larger study, HF rTMS applied to the left DLPFC did reduce cocaine craving in 10 daily sessions (Politi et al, 2008). A sham-controlled, crossover study investigated the effect of low amplitude TBS directed at the mPFC on craving and brain activation using fMRI (Hanlon et al, 2015b). The results showed that TBS to inhibit stimulus evoked brain activity in the medial PFC reduced craving and decreased activity in the striatum and anterior insula (Hanlon et al, 2015b).
Only one study has been performed in methamphetamine dependence comparing a single session of LF (1 Hz) vs sham (Li et al, 2013b). rTMS was delivered to the DLPFC in the presence of drug-related and neutral cues, and the results showed that the LF rTMS increased craving for methamphetamine compared to sham.
Cannabis
The only study we found investigating TMS in cannabis abuse used single and paired pulse TMS to investigate differences in cortical excitability, and did not investigate craving or other measures of drug use (Fitzgerald et al, 2009). Two groups of cannabis users, heavy and light, were compared with controls and both groups showed short interval cortical inhibition, but no difference in other measures of cortical inhibition or cortical excitability.
Other studies have investigated changes in cortical inhibition and excitability in addiction, and these have been recently reviewed (Bunse et al, 2014). Overall, these findings indicate that alcohol dependence is associated with alterations in cortical excitation and inhibition, depending on the duration of abstinence (Bunse et al, 2014). Cocaine abuse is associated with a higher resting MTs and higher intracortical facilitation compared with controls (Bunse et al, 2014; Hanlon et al, 2015a). Given that cortical inhibition and excitability can serve as surrogates for GABA and glutamate signaling, these studies provide some insight into potential alteration in these neurotransmitters (Bunse et al, 2014; Hanlon et al, 2015a).
Considerations
Overall, a number of studies indicate that 1–2 sessions of HF rTMS may be effective in reducing craving in addiction compared with sham, and the strongest evidence for this is in nicotine use disorders. However, most of the short-term rTMS studies in addiction included small numbers of subjects, and there is a degree of variability in the subjects' clinical characteristics, brain regions targeted, and methods for assessing and eliciting craving. In addition, some of these studies are inconsistent for drug or alcohol craving, indicating that studies investigating the effect of rTMS on the consumption of substances are needed.
Randomized controlled studies on cigarette smoking have been performed in nicotine dependence. These studies indicate that HF rTMS reduces smoking, and there is evidence that pairing rTMS with smoking cues may increase the efficacy of rTMS. In addition, these studies indicate that multiple sessions are required, and that maintenance dosing may be needed to sustain the effect.
TRANSCRANIAL DIRECT CURRENT STIMULATION (tDCS)
Transcranial direct current stimulation (tDCS) delivers a low voltage, relatively weak current across an anode and cathode placed on the scalp. The electrical current penetrates the skull to a degree, and there are two mechanisms by which tDCS has been proposed to modulate brain activity: (i) by changing the resting membrane potential of the neurons, where the neurons proximal to the anode are depolarized and those at the cathode are hyperpolarized; and (ii) by modulating synaptic activity in a manner similar to long-term potentiation (at the anode) and long-term depression (at the cathode) (Stagg and Nitsche, 2011). Thus, the modulatory effects are thought to be dependent on the intensity, duration, and direction of the current, where excitability is increased with anodal tDCS and that cathodal tDCS contributes to hyperpolarization and inhibition.
tDCS Studies in Addiction
TDCS has been tested for the treatment for addictive behaviors and the results of these studies are summarized in Table 2.
Table 2. Transcranial Direct Current Stimulation (tDCS).
| Drug | Treatments | n | Target | Stimulation | Outcome measures | Effect | Citation |
|---|---|---|---|---|---|---|---|
| Nicotine | 1, 20 min | 24 | R DLPFC L DLPFC | Anodal. 2 mA Anodal, 2 mA | Cue induced craving Cue induced craving | ↓ ↓ | Fregni et al, 2008 |
| 5, 20 min | 23 | L DLPFC | Anodal, 2 mA | Craving Smoking, self-report | ↓ ↓ | Boggio et al, 2009 | |
| 5, 30 min | 12 | R DLPFC | Anodal, 2 mA | Craving, intent to smoke Craving, desire to smoke Smoking, self-report Carbon monoxide monitor | No effect ↓ ↓ No effect | Fecteau et al, 2014 | |
| 1, 20 min | 24 | L DLPFC | Anodal, 2 mA | Craving Negative affect | No effect ↓ | Xu et al, 2013 | |
| Alcohol | 1, 20 min | 13 | R DLPFC L DLPFC | Anodal, 2 mA Anodal, 2 mA | Cue induced craving Cue induced craving | ↓ ↓ | Boggio et al, 2008 |
| 5, 26 min | 33 | R DLPFC | Anodal, 2 mA | Cue induced craving Anxiety/Depressive sympt Quality of life percept. | No effect No effect ↑ | Klauss et al, 2014 | |
| 5, repetitive 20 min | 13 | L DLPFC | Anodal, 2 mA | Cue induced craving Anxiety/Depressive sympt Relapse | ↓ ↓ ↑ | da Silva et al, 2013 | |
| 1, 10 min. | 41 | L DLPFC R IFG | Anodal, 1 mA Anodal, 1 mA | Craving Craving | ↓ No effect | den Uyl et al, 2015 | |
| Cocaine | 1, 20 min | 36 | R DLPFC L DLPFC | Anodal, 1.5 mA Anodal, 1.5 mA | Risk taking Risk taking | ↓ ↑ | Gorini et al, 2014 |
| 5 repetitive, 20 min | 13 | R DLPFC R DLPFC | Anodal, 2 mA Anodal, 2 mA | Cortical excit. to drug cues | ↓ | Conti et al, 2014a | |
| 1, 20 min | 13 | R DLPFC | Anodal, 2 mA | Ant Cingulate excit | ↓ | Conti et al, 2014a | |
| 5, 20 min | 36 | R DLPFC | Anodal, 2 mA | Craving | ↓ | Batista et al, 2015 | |
| Cannabis | 1, 15 min | 25 | R DLPFC L DLPFC | Anodal, 2 mA Anodal, 2 mA | Risk taking Craving Risk taking Craving | ↑ ↓ ↑ No effect | Boggio et al, 2010 |
Abbreviations: L: left; R, Right; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus.
Nicotine
Studies investigating the effects of tDCS on nicotine dependence have focused on craving and nicotine cues, and the results suggest that tDCS may reduce craving for nicotine, though they are not completely consistent. Fregni et al (2008) performed a randomized, crossover study of smokers using three conditions: sham, anodal tDCS to the left DLPFC, and anodal tDCS to the right DLPFC. Both types of active tDCS reduced craving for nicotine, following cues, compared with sham. A subsequent study by this same group showed that repeated tDCS with the anode positioned at the left DLPFC resulted in a self-reported decrease in craving and smoking, in the active group vs sham (Boggio et al, 2009). In a third study by this group, Fecteau et al (2014), investigated two tDCS conditions (active or sham), where the active condition consisted of the anode at the right DLPFC and cathode at the left DLPFC. This group reported that active tDCS reduced craving on the ‘desire to smoke' scale but not on other measures. Cigarette smoking was decreased when measured as subjects' self-report in a smoking diary, but not when using a carbon monoxide monitor (obtained before stimulation and at day 5 after stimulation). Only one other group has performed a study of anodal stimulation over the left DLPFC (Xu et al, 2013). The results showed improvement in smoking-related negative affect compared with sham, but no effect on cigarette craving.
Alcohol
In alcohol dependence, tDCS shows a less consistent effect on craving. Boggio et al (2008) performed a sham-controlled study in alcohol-dependent subjects (abstinent 10 days) comparing three types of tDCS: (i) sham, (ii) anode at the left DLPFC/cathode right DLPFC, and (ii) anode at the right DLPFC/cathode left DLPFC. Craving was elicited with alcohol-related cues, and the results showed that both types of active tDCS reduced craving compared with sham. However, another study investigated craving for alcohol using a similar type of tDCS (left cathodal/right anodal over the DLPFC), in alcohol-dependent subjects showed no difference in alcohol craving or depression/anxiety symptoms compared with sham (Klauss et al, 2014).
In a third study, da Silva et al (2013) investigated active tDCS (anode over the left DLPFC) vs sham in alcohol-dependent subjects receiving outpatient clinical treatment. Measures of craving, depression/anxiety, and cue response were also obtained. The results showed that active tDCS was associated with a trend toward greater relapse, although depressive symptoms, response to cues, and craving improved with tDCS vs sham.
Finally, a study of tDCS to the DLPFC was compared using tDCS directed at the right inferior frontal gyrus and sham in heavy drinkers that measured craving for alcohol and response bias toward alcohol (den Uyl et al, 2015). The results showed that craving was reduced with tDCS to the DLPFC, but not the inferior frontal gyrus, and that tDCS did not have an effect on bias for alcohol, assessed with an implicit association test.
Cocaine
The tDCS studies in cocaine abuse have mostly focused on cognitive function. Gorini et al (2014) investigated the effect of tDCS in risky choices in cocaine abusers and controls under three conditions: (i) sham; (ii) left anodal/right cathodal stimulation of the DLPFC; (iii) a right anodal/left cathodal stimulation of the DLPFC. The results showed that activation of the DLPFC (both conditions) reduced risky choices on the balloon analog risk task. However, another measure of risk-taking, the game of dice task, showed that right DLPFC anodal stimulation increased safe behavior, while risk-taking behavior increased after left DLPFC anodal stimulation in the cocaine abusers (Gorini et al, 2014).
Conti et al (2014a) investigated the effect on tDCS on cortical excitability using event-related potentials, under neutral and drug cue exposure in cocaine abusers, using bilateral (left cathodal/right anodal) tDCS to the DLPFC vs sham. The results showed that single session tDCS increased current density in response to cues the P3 segment, though the methods for obtaining current density were not explicit. Craving was measured in this study, and no significant change was seen following active tDCS. In a similar study, Conti and Nakamura-Palacios (2014b) administered left cathodal/right anodal or sham stimulation over the DLPFC to cocaine abusers and recorded event-related potentials during drug-related vs neutral cues. Active TDCS was found to decrease activity in N2 EEG component in response to a cocaine cue, whereas activity increased in the sham group.
Most recently, a double-blind randomized clinical trial in cocaine abusers was performed with five sessions of tDCS to the left cathodal/right anodal DLPFC vs sham (Batista et al, 2015). The results showed that craving for cocaine was significantly reduced in the tDCS group after treatment when compared with sham.
Cannabis
One study has looked at tDCS in chronic marijuana smokers comparing: (i) sham; (ii) left anodal/right cathodal tDCS of the DLPFC; and (iii) right anodal/left cathodal tDCS of the DLPFC (Boggio et al, 2010). Craving for marijuana was assessed in addition to risk taking with a task. The results showed that sham was associated with less risky choices compared with the active tDCS but that craving for marijuana was reduced after right anodal/left cathodal DLPFC stimulation compared with sham stimulation.
Considerations
Taken together, the studies investigating the effect of tDCS on substance use disorders are unclear. The studies using craving as an outcome measure are split, with some studies showing an effect while others do not. In addition, whereas tDCS at the anode is thought to increase excitability, while the cathode is inhibitory, many of these studies show the same effect with the anode and cathode switched.
Only three studies measured actual drug or alcohol intake, and these did not show a beneficial effect. One of these investigated alcohol intake, and reported a trend toward greater relapse in the active tDCS group. The other two studies investigated cigarette smoking, and found a decrease in smoking based on the subjects' self-report (smoking diary). However, when objective measures were used (one study, breath carbon monoxide), no effect was seen from active tDCS over sham.
An issue with both tDCS and TMS in treating psychiatric disorders is that of reliability and reproducibility. As described above, many of the studies in addiction include small numbers of subjects, and use different methods with varying outcome measures. However, it should be noted that this process is similar to that establishing rTMS as a treatment for depression (George et al, 2009). The early studies in depression consisted of investigator-initiated studies with small numbers of subjects and variations in methodology, which eventually led to a consensus on rTMS and depression (George et al, 2009).
DEEP BRAIN STIMULATION (DBS)
Deep brain stimulation (DBS) is a surgical procedure where bipolar electrodes are placed into specific brain regions and stimulated through implanted pulse generators. Stimulation parameters are programmable and depend on targeted brain region, disorder, and patient response. Stimulation induces an electric field up to 1 cm depending on specific neural tissue density (Hardesty and Sackeim, 2007). The mechanism of action is somewhat unclear as stimulation has variable actions on cellular physiology; each neural process may be depolarized or hyperpolarized depending on distance from the electrode (McIntyre et al, 2004). In addition, many stimulation variables including brain region, frequency, intensity, and state of neuronal synchronization can influence the direction and duration of its effects on neuronal activity. Although the specific neuronal effect of DBS remain largely debated, it is likely that DBS acts through multiple concurrent mechanisms (reviewed by (Herrington et al, 2016)). Macroscopic theories based on clinical data posit that HF stimulation results in an ablative effect of synchronized neural circuits, but a stimulatory effect in unsynchronized neural circuits (Murrow, 2014).
DBS Studies in Addiction
DBS is currently in use in humans for some neurologic and psychiatric disorders. DBS has not been used extensively in addiction, but there are some preliminary studies. In humans, previous case studies in which patients received DBS for other indications, such as anxiety or mood disorders, have reported that stimulation to the ventral striatum/nucleus accumbens reduced the consumption of substances of abuse, such as alcohol, nicotine, and heroin (Kravitz et al, 2015). There have been some studies and case reports evaluating DBS in addictive disorders (summarized in Table 3).
Table 3. Deep Brain Stimulation (DBS).
| Drug | Treatments | n | Target | Stimulation | Outcome measures | Effect | Citation |
|---|---|---|---|---|---|---|---|
| Nicotine | Chronic for other disorders | 10 | B VS | Range: 130–145 Hz; 90–180 μs, 3–6.5 V | Cessation | 3/10 | Kuhn et al, 2009 |
| Alcohol | Chronic | 5 | B VS | 130 Hz; 90 μS, 4.5 V | Craving Abstinence | ↓ 2/5 | Voges et al, 2013 |
| Chronic | 1 | B VS | 130 Hz; 120 μS, 5.5 V | Alcohol consumption Cognitive control | ↓ ↓ | Kuhn et al, 2011 | |
| Heroin | Chronic | 2 | B VS | Range: 130–140 Hz; 90, 120 μs; 0–5 V | Drug use Anxiety/Depressive sympt | ↓ ↓ | Kuhn et al, 2014 |
Abbreviations: L, left; R, right; B, bilateral; VS, ventral striatum.
Alcohol
Small studies have been conducted specifically using DBS to treat refractory alcohol dependence. Five severe alcohol dependent subjects have been reported in the literature, where bilateral DBS was performed to the ventral striatum (nucleus accumbens) (Muller et al, 2009; Heldmann et al, 2012; Voges et al, 2013). These studies showed that all subjects experienced a reduction in craving and two achieved complete abstinent from alcohol. In another case report of one subject, similar results were reported where the subject improved error processing on cognitive testing and reduced addictive behaviors (Kuhn et al, 2011).
Heroin
A case report of two heroin-dependent subjects who received bilateral DBS to the ventral striatum reported that the subjects experienced an improvement in depressive symptoms and anxiety, and a reduction, though not cessation, in their drug use (Kuhn et al, 2014). This group also reported that 3 of 10 nicotine-dependent subjects who had received DBS to the ventral striatum for the treatment of other disorders were able to quit smoking despite previous failed attempts (Kuhn et al, 2009).
Preclinical work has supported the use of DBS in addictive behaviors. Studies in rodents have shown that brain stimulation of the NAc reduces alcohol self-administration, morphine seeking, and cocaine-seeking and relapse-like behavior (Liu et al, 2008; Vassoler et al, 2008; Knapp et al, 2009; Henderson et al, 2010; Vassoler et al, 2013; Wilden et al, 2014) (further reviewed in (Luigjes et al, 2012) and summarized in Table 4). These studies have also shown effects on addictive behaviors when the prefrontal cortex, lateral hypothalamus, subthalamic nucleus, and lateral habenula have been targeted (Levy et al, 2007; Friedman et al, 2010; Rouaud et al, 2010). A major limitation of these studies are that the electrode and brain structures are difficult to scale from human to rodent, which makes it surprising that nonhuman primate studies have not been attempted.
Table 4. DBS in Animal Models.
| Drug | Treatment | Species | n | Target | Stimulation | Behavioral model | Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| Alcohol | DBS, during session | LE Rat | 4 5 | NAc shell NAc core | 160 Hz; 200 μS; 150 μA | 2BC EtOH consumption (30 min.) 2BC EtOH consumption (30 min.) | ↓ ↓ | Knapp et al, 2009 |
| DBS, 1 h during session 24 h doing session | P Rat | 9 6 | NAc shell NAc shell | 140–150 Hz; 60 μS; 200 μA | 2BC EtOH consumption (1 h) 2BC EtOH preference (1 h) 2BC EtOH consumption (24 h) 2BC EtOH preference (24 h) | No Effect ↓ ↓ ↓ | Henderson et al, 2010 | |
| DBS, during session | P Rat | 4 | NAc shell | 150 Hz; 100 μS; 200 μA | 2BC EtOH consumption (1 h.) | ↓ | Wilden et al, 2014 | |
| Cocaine | DBS, during session | SD Rat | 14 9 7 8 8 7 | LH mPFC | 20–100 Hz; 100 μS; 200–400 μA | Seeking i.v. cocaine PR i.v. cocaine Seeking, PR sucrose Seeking i.v. cocaine PR i.v. cocaine Seeking, PR sucrose | ↓ No effect No effect ↓ ↓ No effect | Levy et al, 2007 |
| DBS, during session | SD Rat | 7 8 8 | NAc shell DS | 160 Hz; 60 μS; 150 μA | Priming induced reinstatement Priming induced reinstatement Food seeking | ↓ No effect No effect | Vassoler et al, 2008 | |
| DBS, during session | LE Rat | 9 14 | STN STN | 130 Hz; 60 μS; 50–130 μA | SA, PR i.v. cocaine SA, PR food | ↓ ↑ | Rouaud et al, 2010 | |
| DBS, 15 min during session | SD Rat | 10 12 30 | R LHb | Low: 10 Hz; 200 μA High: 100 Hz; 200 μA Combined High and Low | SA cocaine SA cocaine SA cocaine, Extinction, Reinstatement | ↑ No effect ↓ | Friedman et al, 2010 | |
| DBS, during session | SD Rat | 5 8 | NAc shell NAc core | 160 Hz; 60 μS; 150 μA | Cocaine Reinstatement | ↓ No effect | Vassoler et al, 2013 | |
| DBS, during session DBS, 1, 7 days prior to session | Mouse | 7 7 4–6 8, 12 | NAc shell NAc core mPFC NAc shell | 130 Hz; 90 μS; 50 μA 2–130 Hz; 90 μS; 50 μA 2–130 Hz; 90 μS; 50 μA 12 Hz; 90 μS; 50 μA w/ D1 antagonist | Locomotor sensitization Locomotor sensitization Locomotor sensitization Locomotor sensitization | ↓ No effect No effect ↓ | Creed et al, 2015 | |
| Morphine | DBS, intermittent | SD Rat | 10 | L NAc core | Range: 130 Hz; 210 μs; 200–500 μA | CPP | ↓ | Liu et al, 2008 |
Abbreviations: L, Left, R, right, LE, Long–Evans; P, alcohol preferring; SD, Sprague-Dawley; LH, lateral hypothalamus; LHb, lateral habenula; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; STN, subthalamic nucleus; 2BC, 2-bottle choice; EtOH, ethanol; i.v., intravenous; i.p., intraperitoneal; PR, progressive ratio; CPP, conditioned place preference.
Considerations
The data using DBS in addiction are limited, and shows uneven results, with some subjects, but not the majority, reducing or stopping their addictive behaviors. However, these studies are small, and owing to invasive nature, the implantation of stimulation devices is only performed in the most refractory of patients. Preclinical work supports the use of DBS in modulating addictive behaviors and provides a chance to better understand its therapeutic mechanisms.
Comparison of Stimulation Techniques
Currently, there are no existing clinical or preclinical studies that directly compare the effectiveness of brain stimulation methods; however, it is clear that each method has practical advantages and disadvantages. For instance, there are many brain regions implicated in addiction such as the nucleus accumbens and amygdala that are not accessible to direct stimulation from tDCS or rTMS (Figure 1). It is also unknown whether continuous stimulation, not yet feasible with rTMS, would be required to achieve a long-term therapeutic effect. Finally, it is unclear whether focal stimulation as provided by DBS is more effective than widespread stimulation by rTMS. In practice, these questions may be answered on an individual basis where noninvasive stimulation therapies like rTMS and tDCS would be initially attempted and that if there is no response, DBS could be a last resort step to treat addictive disorders in resistant patients.
TARGETING PLASTIC MECHANISMS IN ADDICTION
The progression to addiction is thought to be driven by maladaptive changes in the neural circuitry of reward learning and response inhibition (Kelley, 2004). Such changes have been identified in animal models and occur as a result of the repeated pharmacological effects of abused substances in the context of drug administration (Luscher, 2013). It is believed that chronic, excessive drug use causes an overlearning of the environmental and internal cues associated with drug use as compared with natural rewards (Di Chiara and Imperato, 1988; Hyman et al, 2006). Additional studies have shown that the cortical circuitry that regulates behavioral flexibility and the inhibition of drug-seeking behavior can undergo adaptations as well (Kalivas et al, 2005; Everitt, 2014). The motivation for drugs following extended drug exposure is believed to driven by a shift from positive to negative reinforcement (Koob and Le Moal, 1997) and as a result, drug-associated cues become increasingly salient and can drive drug-seeking behaviors despite negative consequences (Schultz, 2011). The potentiation of drug reward systems and an attenuation of brain systems underlying response inhibition are thought to be mediated by long-term changes in synaptic plasticity and intrinsic excitability of neurons (Kauer and Malenka, 2007; Stuber et al, 2010; Kourrich et al, 2015).
If addictive disorders are mediated by adaptations that are truly plastic, then it should be possible to reverse them if affected circuits can be correctly identified and targeted. Evidence from animal models suggests this may be possible. Physiological measurements of synaptic strengthening including long-term potentiation and its surrogate, an increase in glutamate receptor AMPA/NMDA receptor ratio can be induced in the rodent ventral tegmental area by acute exposures to many drugs of abuse including cocaine, alcohol, nicotine, morphine, and benzodiazepines indicating common neural substrates (Ungless et al, 2001; Saal et al, 2003; Tan et al, 2010). Long-term glutamatergic adaptations can be observed in the NAc following chronic cocaine, alcohol, and nicotine exposure (Mameli et al, 2009; Gipson et al, 2013; Wang et al, 2015). Behavioral data from drug-exposed mice has further supported a role of glutamate neurotransmission in the NAc in addictive behaviors. For example, cue-induced seeking of nicotine, cocaine, and alcohol can be blocked by genetic and pharmacological inhibition of glutamate receptors in the NAc (Anderson et al, 2008; Conrad et al, 2008; Schroeder et al, 2008; Gipson et al, 2013). Intriguingly, increased AMPA/NMDA ratio in VTA and NAc neurons observed during withdrawal from cocaine can be reversed through the induction of metabotropic glutamate receptor-long-term depression which results in a redistribution of AMPAR subunits in the synapse (Mameli et al, 2007; Creed et al, 2015). The ability to block or reverse potentiated circuitry that regulates addictive behaviors demonstrates the therapeutic potential of targeting plastic mechanisms in addiction.
In recent years, emerging strategies like optogenetics that can bi-directionally modulate specifically targeted neural circuits have advanced our understanding of the circuits underlying addictive behaviors (Aston-Jones and Deisseroth, 2013). These techniques have also demonstrated the potential to reverse drug-induced neuroadaptations and subsequent drug-seeking behavior. For instance, Chen et al (2013) demonstrated that the intrinsic excitability of the prefrontal cortex in compulsive drug-seeking animals is profoundly reduced, and that this effect could be reversed with optogenetic stimulation of prelimbic neurons. Similarly, Pascoli et al showed that increased AMPA/NMDA ratio in the NAc that underlies cocaine sensitization can be reversed by low-frequency optogenetic stimulation of corticostriatal circuitry through a depotentiation of cortical inputs on NAc neurons (Pascoli et al, 2012; Pascoli et al, 2014). As optogenetics is not available for human use, recent work has focused on the use of DBS to mimic the effects of optogenetic manipulation, and a recent study showed that LF DBS, in the presence of a dopamine receptor 1 antagonist, reversed the cocaine-induced physiological changes in NAc neurons and blocked locomotor sensitization 1 week after treatment (Creed et al, 2015). These data highlight the translatable potential of findings from emerging technologies like optogenetics into stimulation therapies as well as the capability to combine pharmacological treatment with brain stimulation to reverse drug-induced plasticity and addictive behavior (Luscher et al, 2015).
In summary, brain stimulation modalities are being explored as treatments for addiction as pharmacological treatments have not been as successful as previously hoped. Whereas brain stimulation techniques have proven to be effective in treating a variety of neurological and psychiatric disorders, there are few randomized controlled clinical trials in addiction. The studies described above indicate that brain stimulation, may have an effect on craving, but more studies that measure drug or alcohol intake are needed. Furthermore, optimization of stimulation strategies based on the known biology of the disorder will help realize the potential of stimulation therapies for addictive disorders.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
FUNDING SOURCE: MCS is supported by AA023531. DM is supported by DA026525-05 and DA034433.
References
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104: 653–660. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE et al (2008). CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11: 344–353. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Deisseroth K (2013). Recent advances in optogenetics and pharmacogenetics. Brain Res 1511: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM (2015). A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tdcs in patients with crack-cocaine dependence. Int J Neuropsychopharmacol 18: pii: pyv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F (2009). Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 463: 82–86. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A et al (2008). Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 92: 55–60. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F (2010). Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112: 220–225. [DOI] [PubMed] [Google Scholar]
- Bunse T, Wobrock T, Strube W, Padberg F, Palm U, Falkai P et al (2014). Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimul 7: 158–169. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A (2007). One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend 86: 91–94. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A et al (2015). Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol 93: 283–290. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW et al (2013). Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496: 359–362. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M et al (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403. [DOI] [PubMed] [Google Scholar]
- Cho SS, Koshimori Y, Aminian K, Obeso I, Rusjan P, Lang AE et al (2015). Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology 40: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti CL, Moscon JA, Fregni F, Nitsche MA, Nakamura-Palacios EM (2014. a). Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int J Neuropsychopharmacol 17: 1465–1475. [DOI] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM (2014. b). Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul 7: 130–132. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Luscher C (2015). Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science (New York, NY 347: 659–664. [DOI] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F et al (2013). Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 107: 493–502. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW (2015). Transcranial direct current stimulation, implicit alcohol associations and craving. Biol Psychol 105: 37–42. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV (2014). Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol 125: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M et al (2014). Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 76: 742–749. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N et al (2003). High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry 64: 951–953. [DOI] [PubMed] [Google Scholar]
- Everitt BJ (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci 40: 2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D et al (2014). Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend 140: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Williams S, Daskalakis ZJ (2009). A transcranial magnetic stimulation study of the effects of cannabis use on motor cortical inhibition and excitability. Neuropsychopharmacology 34: 2368–2375. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS (2008). Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 69: 32–40. [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E et al (2010). Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology 59: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Padberg F, Schlaepfer TE, O'Reardon JP, Fitzgerald PB, Nahas ZH et al (2009). Controversy: Repetitive transcranial magnetic stimulation or transcranial direct current stimulation shows efficacy in treating psychiatric diseases (depression, mania, schizophrenia, obsessive-complusive disorder, panic, posttraumatic stress disorder). Brain Stimul 2: 14–21. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME et al (2013). Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA 110: 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS (2014. a). Transcranial magnetic stimulation in the treatment of substance addiction. Ann NY Acad Sci 1327: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS (2014. b). Transcranial magnetic stimulation in the treatment of substance addiction. Ann NY Acad Sci 1327: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G (2014). Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci 8: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar A, Zangen A. Brain stimulation as a novel technique for craving management and the treatment of addiction. Wilson SJ (ed). The Wiley Handbook on the Cognitive Neuroscience of Addiction. John Wiley & Sons, Ltd: Chichester, UK, 2015. Wiley Online, Vol 16. [Google Scholar]
- Hanlon CA, DeVries W, Dowdle LT, West JA, Siekman B, Li X et al (2015. a). A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: Integrating TMS and functional MRI data. Drug Alcohol Depend 157: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW et al (2015. b). What goes up, can come down: Novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res 1628: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardesty DE, Sackeim HA (2007). Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry 61: 831–835. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ko JH, Strafella AP, Dagher A (2013). Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci USA 110: 4422–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmann M, Berding G, Voges J, Bogerts B, Galazky I, Muller U et al (2012). Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS One 7: e36572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC (2010). Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus 29: E12. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L et al (2012). No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend 120: 209–213. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C (2013). Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol 48: 552–557. [DOI] [PubMed] [Google Scholar]
- Herrington TM, Cheng JJ, Eskandar EN (2016). Mechanisms of deep brain stimulation. J Neurophysiol 115: 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3: 95–118. [DOI] [PubMed] [Google Scholar]
- Hoppner J, Broese T, Wendler L, Berger C, Thome J (2011). Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry 12 (Suppl 1): 57–62. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005). Theta burst stimulation of the human motor cortex. Neuron 45: 201–206. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS (2008). Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 18: 563–570. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W et al (2009). Consensus: New methodologies for brain stimulation. Brain Stimul 2: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM (2003). Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 10867–10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann M, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G et al (2003). [Transcranial magnetic stimulation for nicotine dependence]. Psychiatrische Praxis 30 Suppl 2: S129–S131. [PubMed] [Google Scholar]
- Jung SH, Shin JE, Jeong YS, Shin HI (2008). Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol 119: 71–79. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J (2005). Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45: 647–650. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC (2007). Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858. [DOI] [PubMed] [Google Scholar]
- Kelley AE (2004). Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179. [DOI] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, de Almeida Correia Santos G, Fregni F, Nitsche MA et al (2014). A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 17: 1793–1803. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C (2009). Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav 92: 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science New York, NY 278: 52–58. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A (2015). Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci 16: 173–184. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tomasi D, LeBlanc KH, Baler R, Volkow ND, Bonci A et al (2015). Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res 1628: 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH et al (2009). Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res 15: 196–201. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D et al (2011). Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol 16: 620–623. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Moller M, Treppmann JF, Bartsch C, Lenartz D, Gruendler TO et al (2014). Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry 19: 145–146. [DOI] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A (2007). Repeated electrical stimulation of reward-related brain regions affects cocaine but not ‘natural' reinforcement. J Neurosci 27: 14179–14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA et al (2013. a). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 73: 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT et al (2013. b). Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol Depend 133: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Jin J, Tang JS, Sun WX, Jia H, Yang XP et al (2008). Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol 13: 40–46. [DOI] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R et al (2012). Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry 17: 572–583. [DOI] [PubMed] [Google Scholar]
- Luscher C (2013). Drug-evoked synaptic plasticity causing addictive behavior. J Neurosci 33: 17641–17646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Pascoli V, Creed M (2015). Optogenetic dissection of neural circuitry: from synaptic causalities to blue prints for novel treatments of behavioral diseases. Curr Opin Neurobiol 35: 95–100. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C (2007). Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317: 530–533. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R et al (2009). Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12: 1036–1041. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL (2004). Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115: 1239–1248. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK (2010). Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105: 49–55. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, Nizamie SH (2015). Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J Neuropsychiatry Clin Neurosci 27: e54–e59. [DOI] [PubMed] [Google Scholar]
- Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M et al (2009). Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry 42: 288–291. [DOI] [PubMed] [Google Scholar]
- Murrow RW (2014). Penfield's Prediction: A Mechanism for Deep Brain Stimulation. Front Neurol 5: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Luscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509: 459–464. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Luscher C (2012). Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481: 71–75. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E (2008). Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict 17: 345–346. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Ustohal L, Kucerova HP, Kasparek T, Jarkovsky J, Hublova V et al (2014). Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 49: 30–35. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C (2014). Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul 7: 226–233. [DOI] [PubMed] [Google Scholar]
- Rapinesi C, Bersani FS, Kotzalidis GD, Imperatori C, Del Casale A, Di Pietro S et al (2015). Maintenance deep transcranial magnetic stimulation sessions are associated with reduced depressive relapses in patients with unipolar or bipolar depression. Front Neurol 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud'homme X, Krystal AD (2011). Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry 70: 794–799. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R et al (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Y, Amir A, Levkovitz Y, Zangen A (2007). Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J Clin Neurophysiol 24: 31–38. [DOI] [PubMed] [Google Scholar]
- Roth Y, Zangen A, Hallett M (2002). A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol 19: 361–370. [DOI] [PubMed] [Google Scholar]
- Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci USA 107: 1196–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW (2008). Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology 55: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2011). Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron 69: 603–617. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist 17: 37–53. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A (2010). Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci 3: 3–27. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM et al (2010). Neural bases for addictive properties of benzodiazepines. Nature 463: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C et al (2008). Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci 28: 8735–8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O et al (2013). Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci 33: 14446–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges J, Muller U, Bogerts B, Munte T, Heinze HJ (2013). Deep brain stimulation surgery for alcohol addiction. World Neurosurg 80: S28 e21–S28 e31. [DOI] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H et al (2015). Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum. J Neurosci 35: 11634–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden JA, Qing KY, Hauser SR, McBride WJ, Irazoqui PP, Rodd ZA (2014). Reduced ethanol consumption by alcohol-preferring (P) rats following pharmacological silencing and deep brain stimulation of the nucleus accumbens shell. J Neurosurg 120: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Bacher I, Wu BS, Daskalakis ZJ, George TP (2012). High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res 139: 264–266. [DOI] [PubMed] [Google Scholar]
- Xu J, Fregni F, Brody AL, Rahman AS (2013). Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry 4: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M (2005). Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 116: 775–779. [DOI] [PubMed] [Google Scholar]