Abstract
Early life stress (ELS) is highly related to the development of psychiatric illnesses in adulthood, including substance use disorders. A recent body of literature suggests that long-lasting changes in the epigenome may be a mechanism by which experiences early in life can alter neurobiological and behavioral phenotypes in adulthood. In this study, we replicate our previous findings that ELS, in the form of prolonged maternal separation, increases adult methamphetamine self-administration (SA) in male rats as compared with handled controls. In addition, we show new evidence that both ELS and methamphetamine SA alter the expression of the epigenetic regulator methyl CpG-binding protein 2 (MeCP2) in key brain reward regions, particularly in the nucleus accumbens (NAc) core. In turn, viral-mediated knockdown of MeCP2 expression in the NAc core reduces methamphetamine SA, as well as saccharin intake. Furthermore, NAc core MeCP2 knockdown reduces methamphetamine, but not saccharin, SA on a progressive ratio schedule of reinforcement. These data suggest that NAc core MeCP2 may be recruited by both ELS and methamphetamine SA and promote the development of certain aspects of drug abuse-related behavior. Taken together, functional interactions between ELS, methamphetamine SA, and the expression of MeCP2 in the NAc may represent novel mechanisms that can ultimately be targeted for intervention in individuals with adverse early life experiences who are at risk for developing substance use disorders.
INTRODUCTION
Substance use disorders (SUDs) are thought to develop, in part, from the ability of chronic drug exposure to modulate gene expression and function in reward-related neurocircuitries (Chao and Nestler, 2004). Specifically, these drug-induced changes are believed to be the result of perturbations in the neuronal epigenome of brain reward-related regions (Robison and Nestler, 2011). Methyl CpG-binding protein 2 (MeCP2) is a DNA-binding protein that regulates several epigenetic processes and has been linked to various neuropsychiatric disorders, including SUDs (Feng and Nestler, 2010). Cocaine administration increases MeCP2 expression in multiple brain regions in rats (Cassel et al, 2006; Host et al, 2011; Im et al, 2010). In addition, continuous ethanol exposure upregulates MeCP2 expression, whereas ethanol withdrawal downregulates expression in vitro (Liyanage et al, 2015). In turn, manipulation of MeCP2 expression in the striatum and central amygdala alters both psychostimulant- and morphine-abuse-related behavior, respectively (Deng et al, 2010; Im et al, 2010; Zhang et al, 2014; Hou et al, 2015). Furthermore, global reductions in MeCP2 expression through heterozygous deletion of one of the MeCP2 alleles reduced ethanol sensitivity and intake behavior in mice (Repunte-Canonigo et al, 2014). Possible neurobiological mechanisms by which MeCP2 exerts its effects on addiction-related behaviors include regulation of dendritic spine density, number of GABAergic synapses, and expression of brain-derived neurotrophic factor via homeostatic interactions with microRNA-212 (Deng et al, 2010; Im et al, 2010). Collectively, these studies suggest that drugs of abuse alter MeCP2 expression, which in turn influences the reinforcing and rewarding properties of abused drugs. What remains to be determined is whether known risk factors for SUDs, such as early life stress (ELS), may specifically prime the epigenome through alterations in MeCP2 expression toward increased vulnerability to developing SUDs.
Prior exposure to ELS is implicated in several psychiatric conditions, including mood and anxiety-related disorders. ELS has widespread effects on brain development by dysregulating the neuroendocrine stress response, sympathetic, monoamine, oxytocin, and immune systems (for review, see De Bellis and Zisk, 2014). Yet, the possible mechanisms by which ELS can modulate complex neural systems and behavior in adulthood were unknown until recently. Seminal work by Weaver et al (2004) demonstrated that specific patterns of maternal care during early life in rats led to persistent DNA methylation changes, along with altered behavioral and neuroendocrine phenotypes in adulthood. Interestingly, MeCP2 contributes to ELS-dependent epigenetic programming of hypothalamo-pituitary-adrenal axis regulatory genes (eg, Crh, Avp, and Pomc) in stress-related brain regions (Murgatroyd et al, 2009; Wang et al, 2014; Wu et al, 2014). Together, these studies suggest that ELS epigenetically affects stress neurocircuitry resulting in altered physiological and behavioral phenotypes in adulthood. Given that hypothalamo-pituitary-adrenal axis dysregulation is involved in drug reinforcement and reward (Kosten and Ambrosio, 2002), ELS may indirectly influence vulnerability toward addictive behaviors through this mechanism.
Although much work has focused on ELS and epigenetic programming of stress neurocircuitry, few studies have investigated whether ELS directly alters epigenetic landscape in the brain reward circuitry. For instance, recent studies have examined MeCP2 expression in the striatum following ELS, but produced conflicting results. Specifically, it has been shown that ELS induces increased MeCP2 expression in the striatum (Tesone-Coelho et al, 2013), but others have not observed such differences (Romano-López et al, 2012). We previously found that ELS increased methamphetamine (METH) self-administration (SA) as compared with handled control animals, and a history of ELS and high METH intake was negatively correlated with nucleus accumbens (NAc) core MeCP2 expression following METH withdrawal (Lewis et al, 2013). Here, we sought to replicate our ELS findings on METH SA in adulthood, and to investigate the effects of ELS, METH SA, and their interaction on MeCP2 NAc expression prior to withdrawal. We predicted that both ELS and METH SA would increase MeCP2 expression in the striatum. On the basis of findings of these experiments, we next sought to examine the specific role of NAc core MeCP2 in METH SA using a virally mediated knockdown approach. We also examined whether this manipulation would affect intake of the non-drug reinforcer saccharin, and predicted that NAc core MeCP2 knockdown would attenuate the reinforcing properties of METH, but not saccharin.
MATERIALS AND METHODS
Animals
Pregnant Long Evans dams were purchased from Charles River Laboratories (Hollister, CA) for Experiment 1 and arrived on gestational day 12. Litters were randomly assigned to one of the conditions: maternal separation for 180 min per day (ELS-METH or ELS-Saline) or maternal separation for 15 min per day (Handled Control (HC)-METH or HC-Saline). Separation procedures were carried out from PND2–14. During PND15–20, litters were left undisturbed, weaned on PND21 into same sex group housing, and pair-housed with a sibling on PND45. One to two pups per litter were used for statistical analysis. Females were not used for the remainder of the study. For Experiment 2, male Long Evans rats (Charles River Laboratories, San Diego, CA) arrived on PND45 (175–200 g) and were allowed to acclimate to the colony room environment for 5–7 days before virus infusion surgeries.
Intravenous Catheter Surgery
For Experiment 1 and 2, rats were implanted with intravenous catheters into the jugular vein on PND60 (±1). All rats were anesthetized with isoflurane (2% v/v, Butler Schein Animal Health, Dublin, OH) vaporized in oxygen at a flow rate of 2 l/min. Rats received pre-incision injections of buprenorphine (0.05 mg/kg, s.c.) and meloxicam (1 mg/kg, s.c.). Surgical sites were shaved and cleaned with 1% iodine. A small incision was made in order to isolate the right or left jugular vein. A sterile catheter made of silastic tubing (Dow Corning, Midland, MI) was filled with heparinized saline and inserted 2.5 cm into the vein. The catheter was secured to the surrounding tissue with sutures, and the opposite end of the catheter was tunneled subcutaneously to the dorsum where it exited the skin between the scapulae. A mesh collar was attached to a threaded vascular access port (Plastics One, Roanoke, VA). The wound was then treated with 0.2 ml bupivacaine (0.25% v/v), closed with nylon sutures, and treated with topical antibiotic and analgesic ointments. The access port was sealed with Tygon tubing closed at one end and a threaded protective cap. Rats were given small portions of sweetened cereal to facilitate postsurgical rehabilitation. Following surgical procedures, rats were allowed 5 days minimum of recovery and received daily intravenous infusions of 0.1 ml Timentin (66.6 mg/ml i.v. in heparinized saline) to minimize infections and maintain catheter patency. Patency was periodically tested with an i.v. injection of 0.1 ml sodium methohexital (10 mg/ml) and observation of brief loss in muscle tone.
Methamphetamine SA
In Experiment 1, beginning on PND67, rats received 2-h daily SA sessions whereby an active operant response resulted in delivery of METH (0.05 mg/kg/infusion, delivered in a volume of 0.06 ml over a 2-s period) on a fixed ratio (FR) 1 schedule of reinforcement. No prior operant training preceded METH SA. Each infusion was accompanied by concurrent illumination of a stimulus light and presentation of an auditory stimulus for 2 s. Delivery of each infusion was followed by a 20-s timeout period, during which additional active responses were recorded, but did not produce drug infusions. Inactive operant responses were recorded, but did not produce any consequences. Saline-yoked controls were randomly assigned to a METH SA rat and received yoked i.v. infusions of saline (0.06 ml/infusion) along with the light/tone/house light stimulus complex. For yoked controls, operant responses were recorded but produced no consequences.
In Experiment 2, rats underwent 6-h daily SA sessions whereby an active operant response resulted in delivery of METH (0.05 mg/kg/infusion) on an FR1 schedule of reinforcement. After earning 10 or more infusions in two consecutive days, rats progressed to an FR3 and then an FR5 schedule of reinforcement for the remainder of training. Each infusion was accompanied by concurrent presentation of the stimulus complex, as described in Experiment 1. Following SA training, animals underwent 2 days of progressive ratio testing, in which the number of active operant responses required to obtain each subsequent infusion of METH increased exponentially (ie, 1, 2, 4, 7, 10, 15, etc. Richardson and Roberts, 1996). Following progressive ratio testing, baseline operant responding was re-established during two METH SA sessions on an FR1 schedule. Rats then underwent extinction training for eight daily sessions, where all operant responses produced no programmed consequences. Following extinction, rats were tested for drug-induced reinstatement by receiving a priming injection of METH (1 mg/kg, i.p.) 30 min prior to placement into the operant chamber and responding was recorded during 2-h sessions.
Saccharin SA and Reinstatement
In Experiment 2, following a single 12-h operant training session on an FR1 schedule of reinforcement, male rats underwent 30-min daily SA sessions whereby an active operant response activated the syringe pump to deliver ~45 μl of a liquid solution (0.4% w/v saccharin) over a 1.5-s period on an FR1 schedule of reinforcement. Rats underwent identical training and testing procedures as those receiving METH, with the exception of drug-induced reinstatement. Instead, rats in this group underwent cue-induced reinstatement 30-min sessions, whereby an active operant response produced the tone and light cue previously presented with saccharin reinforcement, but no saccharin solution was delivered.
Motor Activity
Motor activity was examined using a Rotorat apparatus that consisted of a stainless steel bowl (40.6 cm diameter × 25.4 cm height; model ENV-500, Med Associates, St Albans, VT) surrounded by clear acrylic walls. A spring tether attached to a rotational sensor was suspended from the center of the apparatus. Attached to the tether was a zip-tie collar that was loosely placed around the neck of the rat and secured with alligator clips. The rotational sensor recorded 2°, 180°, and 360° movements during 30-min sessions. For METH-induced or spontaneous rotational motor tests, rats received either a METH (1 mg/kg, i.p.) or saline injection 30 min prior to placement in the apparatus, respectively.
Tissue Preparation, Immunohistochemistry, and Image Analysis
For Experiment 1, immediately following the last day of SA, rats were placed under deep anesthesia using isoflurane and transcardially perfused with ice-cold phosphate-buffered saline (PBS), followed by ice-cold 4% w/v paraformaldehyde. Brains were quickly removed and stored in 4% paraformaldehyde in 4 °C before transferring to a 30% w/v sucrose solution prior to cryosectioning at 35 μm thickness using a Leica CM1900 cryostat.
For immunohistochemical detection of MeCP2, sections were rinsed 3 × 10 min in PBS containing 0.1% v/v Tween 20 (PBST) followed by incubation in PBST containing 5% v/v normal donkey serum for 1 h. Sections were then incubated overnight under gentle agitation at 4 °C in PBST containing a rabbit anti-MeCP2 polyclonal antibody (1:200; Thermo Scientific, Grand Island, NY) and then rinsed 3 × 10 min in PBS. Sections were then incubated in PBS containing AlexaFluor 488-conjugated donkey anti-rabbit IgG secondary antisera (1:200; Jackson ImmunoResearch, West Grove, PA) and then rinsed 3 × 10 min in PBS. Sections were mounted on microscope slides using VectaShield mounting media (Vector Labs, Burlingame, CA), cover-slipped, and stored in darkness until imaging.
Sections were visualized at × 200 magnification and captured using a Hamamatsu Digital Camera (Hamamatsu City, Japan) attached to an Olympus BX53 microscope. Resolution and exposure settings remained consistent for all samples. MeCP2 immunoreactivity (IR) was quantified using ImageJ software (National Institutes of Health, Bethesda, MD) by observers blind to treatment conditions. All images were subjected to Inversion and Intermodes auto-thresholding. Cell counts were completed using the Analyze Particles option with the pixel size set to 50–300 and circularity set to 0.5–1.0. A total of six sample areas were counted for each animal (ie, 1 sample area × 2 hemispheres × 3 sections per region). Counts from all six sample areas from a particular region were averaged to provide a mean number of immunoreactive cells per animal that was used for statistical analysis. MeCP2 IR was measured in the NAc core per 0.63 mm2, in the NAc shell per 0.28 mm2, and in the medial and lateral dorsal striatum per 0.22 mm2.
Virus Design and Infusions
For Experiment 2, an shRNA directed against rat MeCP2 mRNA (Mecp2 shRNA, target sequence: ctaaagtag, start position 450, sense strand: 5′-tgcctttcgctctaaagtagttcaagagactactttaga-gcgaaaggcttttttc-3′, antisense strand: 5′-tcgagaaaaaagcctttcgctctaaagtagtctcttgaactactttagagcg-aaaggca-3′) was synthesized by Virovek (Hayward, CA). This sequence was chosen based on previous work demonstrating optimal knockdown of MeCP2 expression in comparison with several other shRNA sequences (Jin et al, 2008). The shRNA was incorporated into a vector containing the U6 pol III and cytomegalovirus (CMV) promoters along with the coding sequence for green fluorescent protein (GFP), and packaged into an adeno-associated virus (AAV; serotype 9, 2.25 × 1013 particles/ml). The resulting viral vector (AAV9-U6-Mecp2-shRNA-CMV-GFP; sh-MeCP2) was suspended in PBS containing 0.001% pluronic F-68 and filter sterilized. An empty virus lacking the shRNA sequence (AAV9-U6-CMV-GFP; 2.25 × 1013 particles/ml; sh-Control) served as a control.
For intracranial virus infusions, rats were anesthetized with isoflurane (2% v/v) vaporized in oxygen at a flow rate of 2 l/min and placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA). The scalp was shaved and surgical area cleaned with ethanol and 1% iodine before a ~2 cm incision was made to expose the skull. Guide cannulae (26 G, Plastics One, Roanoke, VA) were placed bilaterally in the NAc core with the following stereotaxic coordinates (in mm from bregma and skull surface): anterior +2.5, lateral±1.5, ventral −6.0 (see Figure 3). AAV vectors were infused at a volume of 2 μl/side over 10 min, after which the injector was left in place for an additional 10 min to allow for virus diffusion. Skull holes were filled with bone wax, and the scalp was sutured before treatment with topical antibiotic and analgesic ointments. Following surgery, animals remained single housed in their home cage for 2 weeks prior to behavioral testing.
Immunoblotting
To avoid possible influence of METH on MeCP2 expression, we used tissue from drug-naive saline-yoked animals to verify shRNA-mediated MeCP2 knockdown. Frozen brains were placed into a rat brain matrix (Kent Scientific, Torrington, CT) and sliced into 1-mm thick coronal sections. NAc core tissue punches (1 mm diameter) from both hemispheres were collected from 2 to 3 coronal sections and were placed in a neuronal protein extraction reagent (N-PER, G-Biosciences, USA) containing a protease inhibitor cocktail. Tissue was homogenized with a Branson sonicator (Danbury, CT) and centrifuged at 10 000 g at 4 °C for 10 min. The supernatant was then stored at −80 °C until further analysis. Protein concentrations were determined using bicinchoninic acid protein assays (Bio-Rad, Hercules, CA). For immunoblot analysis, samples (30 μg of protein per lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 4–12% RunBlue gels (Expedeon). Proteins were transferred to nitrocellulose membranes, pre-blocked with PBS containing 0.1% (v/v) Tween 20 and 5% (w/v) nonfat dried milk powder for 1 h before overnight incubation with the following primary polyclonal antibodies: rabbit anti-MeCP2 (Abcam; 1:100 dilution) and as a loading control, rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam; 1:10 000 dilution). Membranes were then washed and incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG antisera (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:1500 dilution) for 1 h, and immunoreactive bands were detected by enhanced chemiluminescence (Western Lightning Plus) and exposure to Kodak Bio-Max films. Developed films were scanned and analyzed using ImageJ software. For each sample, the optical density of the MeCP2 band was divided by the corresponding GAPDH band to yield a MeCP2/GAPDH ratio.
To further evaluate the specificity of the shRNA viral constructs, separate groups of treatment-naive animals were infused with one of the two viruses into the NAc, and qRT-PCR was performed to assess mRNA levels of MeCP2 and genes with partially homologous sequences to those complementary to the sh-MeCP2 sequence (see Supplementary Figure S1 and Supplementary Methods).
Statistical Analysis
For Experiment 1, effects of ELS on METH SA behavior were analyzed by a mixed-factor ANOVA with ELS history as a between-subjects factor and day as a within-subjects factor. Dependent variables included active and inactive operant responses, and infusions. To examine the effects of ELS history, METH SA, and their interaction on MeCP2 immunolabeled cells, a 2 × 2 multivariate analysis of variance was performed for each region with ELS history (ie, ELS and HC) and drug (ie, METH or yoked saline) as between-subjects factors. Significant interactions were followed by tests for simple effects and Fisher's LSD post hoc analysis. For Experiment 2, independent-samples Student t-tests were used to test differences in mRNA and protein expression levels in virus-infused animals, as well as for progressive ratio responding and intake. Mixed-factors ANOVAs were used to examine differences between groups across days during METH and saccharin SA, with virus group as a between-subjects factor and day as a within-subjects factor. For extinction and reinstatement data, we calculated the average active operant responses for the final 2 days of SA and final 2 days of extinction and then performed a mixed-factor ANOVA to compare virus groups (ie, between-subjects factor) across SA, extinction, and reinstatement (ie, within-subjects factor of day). Significant interactions were followed by tests for simple effects using repeated-measures one-way ANOVAs and post hoc LSD tests, where appropriate. For all data in Experiments 1 and 2, statistical significance was considered p<0.05.
RESULTS
Experiment 1
Effects of ELS on methamphetamine SA
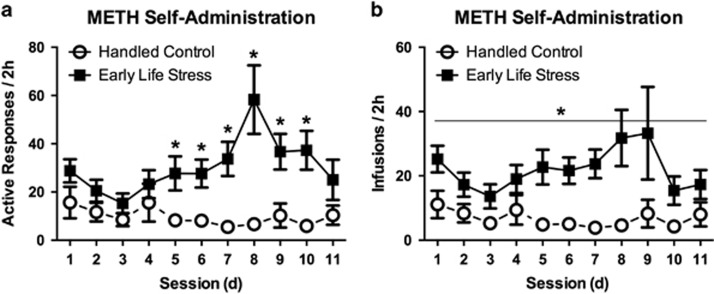
Mixed-factor ANOVA revealed a significant main effect of rearing condition on the number of active responses and infusions per session (F(1, 16)=9.59, p=0.007; F(1, 16)=10.88, p=0.005, respectively), but no main effect of day for either measures (p's>0.05). We found a significant interaction between rearing condition and day for active responses (F(1, 16)=5.891, p=0.027), but not for infusions (p>0.05). Tests for simple effects revealed significant group differences on days 5–10 for active responses (Figure 1a). Overall, the ELS group exhibited greater active responses and received more infusions than the HC group (Figure 1). Mixed ANOVA revealed no significant interaction or main effects for inactive responses between groups.
Figure 1.
Effect of early life stress on methamphetamine self-administration. ELS-exposed rats (n=10) exhibited greater active responses (a) and intake (b) during the daily 2-h SA sessions compared with the Handled Control group (n=8). *p<0.05 vs Handled Control. Error bars indicate SEM.
Effects of ELS and/or METH SA on MeCP2 IR
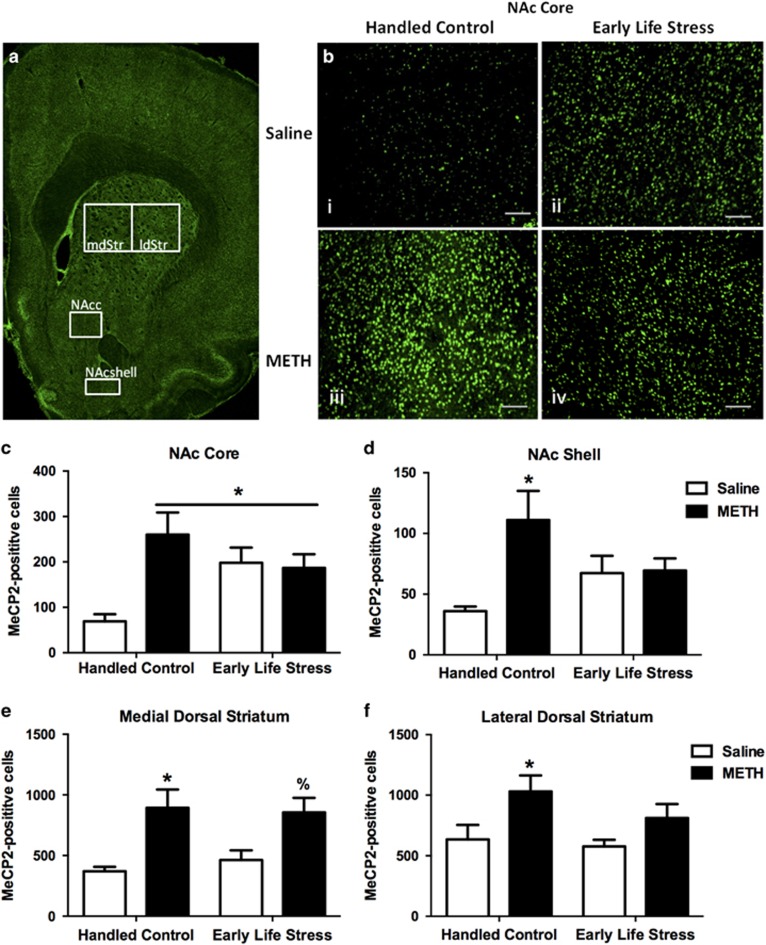
We tested whether ELS and METH SA resulted in significant changes in the number of MeCP2-expressing cells in the striatum (Figure 2a). There was a significant omnibus multivariate analysis of variance for NAc core (F(3, 23)=4.89, p=0.01), NAc shell (F(3, 23)=3.98, p=0.02), medial dorsal striatum (F(3, 23)=5.86, p=0.005), and lateral dorsal striatum (F(3, 23)=3.29, p=0.04). In the NAc core, METH SA increased the number of MeCP2-labeled cells compared with saline in the HC condition (p=0.01), but not in the ELS condition. ELS increased NAc core MeCP2 IR compared with HCs in the saline condition (p=0.01) (Figure 2b and c). In the NAc shell, METH SA increased MeCP2 IR compared with saline in the HC condition (p=0.01), but not in the ELS condition (Figure 2d). There was no effect of ELS on NAc shell MeCP2 IR compared with HCs in the saline conditions. In the medial dorsal striatum, METH SA increased MeCP2 IR compared with saline in the HC condition (p=0.005) and in the ELS condition (p=0.01) (Figure 2e). There was no effect of ELS on medial dorsal striatum MeCP2 IR compared with HCs in the saline conditions. In the lateral dorsal striatum, METH SA increased MeCP2 IR compared with saline in the HC condition (p=0.02), but not in the ELS condition (Figure 2f). There was no effect of ELS on lateral dorsal striatum MeCP2 IR compared with HCs in the saline conditions. These data demonstrate that METH SA leads to significant increases in the number of MeCP2-labeled cells in both the NAc and the dorsal striatum of HC rats. Interestingly, ELS had a much more selective effect. Of the regions analyzed, ELS alone elevated MeCP2 expression in the NAc core relative to HC and further elevated the response to METH SA in the medial dorsal striatum.
Figure 2.
Effect of ELS and METH SA on MeCP2 expression in the striatum. (a) Representative hemisection with schematic of sample areas analyzed for MeCP2 immunoreactive labeling, including the medial dorsal striatum (mdStr), lateral dorsal striatum (ldStr), nucleus accumbens core (NAcc), and nucleus accumbens shell (NAcshell). (b) Representative photomicrographs of MeCP2 immunoreactive labeling in the NAc core of: (i) Handled Control-Saline, (ii) Early Life Stress-Saline, (iii) Handled Control-METH, and (iv) Early Life Stress-METH. Scale bars=90 μm. Average MeCP2-positive cell counts in the NAc core (c), NAc shell (d), medial dorsal striatum (e), and the lateral dorsal striatum (f). Handled Control-Saline (n=5), Handled Control-METH (n=5), Early Life Stress-Saline (n=6), and Early Life Stress-METH (n=7). *p<0.05 vs Handled Control-Saline. %p<0.05 vs ELS-Saline. Error bars indicate SEM.
Experiment 2
Verification of MeCP2 knockdown in the NAc core
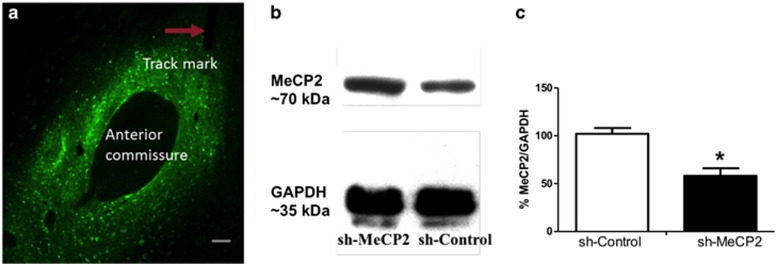
Animals who received infusions of the sh-MeCP2 AAV vector exhibited decreased NAc core MeCP2 protein levels compared with animals who received the sh-Control vector (t(6)=4.66, p=0.003). Control GAPDH levels were not different across groups (Figure 3). In separate groups of animals, qRT-PCR analyses also confirmed a significant knockdown of MeCP2 mRNA levels (t(4)=2.14, p=0.049, one-tailed), whereas mRNA expression levels of four genes with homologous sequences to those complementary to the sh-MeCP2 sequence were unchanged (p-values>0.05; see Supplementary Figure S1).
Figure 3.
Verification of virus placement and MeCP2 knockdown. (a) Representative photomicrograph of expression of the reporter gene GFP and injector track mark in the NAc core following virus infusion. Scale bar=130 μm. (b) Representative immunoblot bands for MeCP2 and GAPDH expression in NAc core tissue from rats receiving either the AAV-empty control virus (sh-Control) or AAV-MeCP2-shRNA (sh-MeCP2), as determined by western blot. (c) Mean percentage (±SEM) of MeCP2/GAPDH band density in the NAc core of sh-Control (n=4) and sh-MeCP2 (n=4) infused animals. *p<0.01.
Effects of MeCP2 knockdown on methamphetamine SA, extinction, and reinstatement
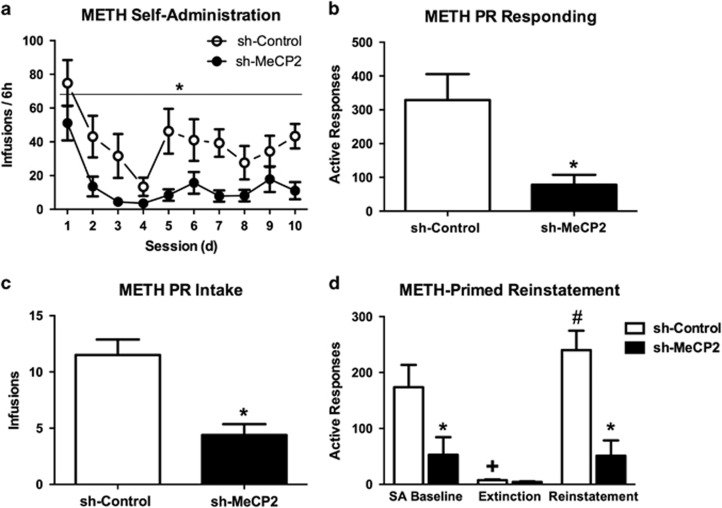
We observed a significant main effect of virus on the number of METH infusions per session (F(1, 15)=16.77, p=0.001), and a significant effect of day (F(1, 15)=6.32, p=0.02), but no significant interaction between the two factors (F(1, 15)=0.0, p=0.997). The sh-MeCP2 group obtained fewer infusions than the sh-Control group (Figure 4a). The sh-MeCP2 group also exhibited reduced motivation for METH under a progressive ratio schedule, as measured by active responses (t(13)=3.56, p=0.003) (Figure 4b) and reinforcements (t(13)=4.49, p=0.001) (Figure 4c) averaged over 2 days of testing compared with the sh-Control group. Group differences were also observed when progressive ratio responding was analyzed separately on each day of testing (all p's<0.05 for active lever presses and infusions obtained on day 1 and day 2 in sh-MeCP2-shRNA vs sh-control).
Figure 4.
Effect of NAc core MeCP2 knockdown on METH SA, extinction, and reinstatement. (a) Average number of infusions per 6-h METH SA (0.05 mg/kg/infusion) session in sh-Control (n=8) and sh-MeCP2 (n=9). Average number of operant responses (b) and infusions (c) earned under a progressive ratio schedule of METH reinforcement (0.05 mg/kg/infusion). Sessions were terminated when no infusions were earned for 2 h. sh-Control (n=6) and sh-MeCP2 (n=9). *p<0.05 vs sh-Control. (d) Average number of active responses during the last 2 days of both SA (ie, SA Baseline) and Extinction, and during METH-primed Reinstatement in sh-Control (n=5) and sh-MeCP2 (n=8) infused rats. For METH-primed reinstatement, rats received METH injections (1 mg/kg, i.p.) 15 min prior to placement in the operant chamber. *p<0.05 vs sh-Control.+p<0.05 vs SA Baseline. #p<0.05 vs Extinction. Error bars indicate SEM.
Mixed-factors ANOVA with the mean active responses during the final 2 days of both SA and extinction, and during METH-primed reinstatement produced significant main effects of day (F(1, 11)=13.69, p<0.001) and virus (F(1, 11)=22.84, p<0.001), as well as a significant interaction (F(1, 11)=5.64, p=0.01). Post hoc LSD tests for each virus group revealed the sh-Control group exhibited significantly reduced active responding during extinction compared with SA (p=0.001) (Figure 4d), and significantly increased active responding during reinstatement compared with extinction (p=0.001). Whereas, the sh-MeCP2 group did not exhibit changes in active responding during extinction (p=0.17) or reinstatement (p=0.19). Upon examining group differences during each test phase, post hoc LSD tests revealed that the sh-MeCP2 group had significantly reduced active responding compared with the sh-Control group during SA (p=0.003) (Figure 4d) and reinstatement (p<0.001), but not during extinction (p=0.936). However, the groups did not differ in the degree that reinstatement returned levels of active responding to their respective SA baseline (ie, reinstatement/SA active responses) (t(11)=0.83, p=0.426). Collectively, these results implicate NAc core MeCP2 expression in the reinforcing and motivational properties of METH in male rats.
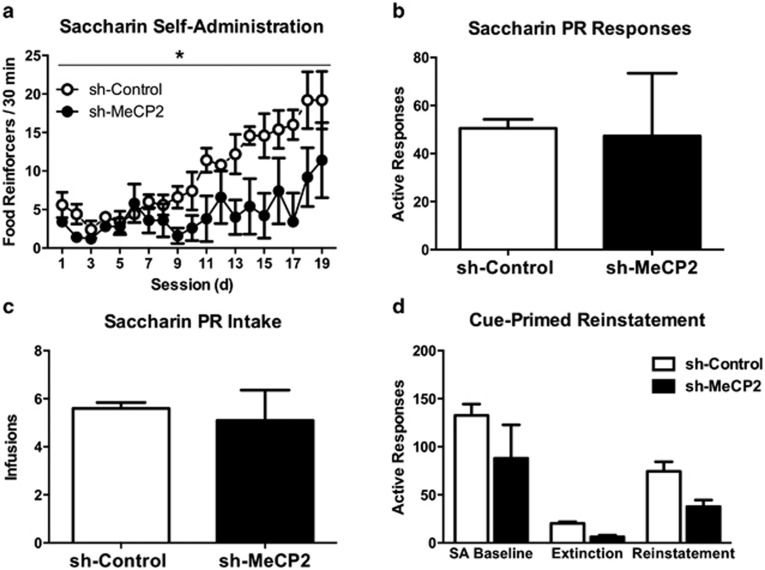
Effects of MeCP2 knockdown on saccharin SA, extinction, and reinstatement
We observed a significant main effect of virus on the number of saccharin reinforcers obtained per session (F(1, 8)=6.52, p=0.034), a significant within-subjects effect of days (F(1, 8)=22.49, p=0.001), and a trend toward a significant interaction (F(1, 8)=4.99, p=0.056). The sh-Control group received more saccharin reinforcers than the sh-MeCP2 group (Figure 5a). The sh-Control and sh-MeCP2 groups did not differ in motivation for saccharin under a progressive ratio schedule as measured by active responses and reinforcements (Figure 5b and c).
Figure 5.
Effect of NAc core MeCP2 knockdown on saccharin intake, extinction, and reinstatement. (a) Average number of saccharin reinforcements earned per 30-min SA session in sh-Control and sh-MeCP2 infused animals. *p⩽0.05 vs sh-Control. Average total number of active responses (b) and reinforcers (c) earned during progressive ratio tests following saccharin SA training. (d) Average number of active responses during the last 2 days of both SA (ie, SA Baseline) and extinction, and during cue-induced reinstatement. sh-Control (n=5) and sh-MeCP2 (n=5). Error bars indicate SEM.
Mixed-factors ANOVA with the mean active responses during the final 2 days of both SA and extinction, and during cue reinstatement produced a significant main effect of day (F(1, 8)=23.98, p<0.001) (Figure 5d), but no significant main effect of virus (F(1, 8)=4.24, p=0.073) or interaction (F(1, 8)=0.64, p=0.54). The groups also did not differ in the degree that reinstatement returned levels of active responses to their respective SA baseline (ie, reinstatement/SA active responses) (t(8)=0.78, p=0.777). Collectively, these results suggest that MeCP2 knockdown in the NAc core differentially mediates the initial reinforcing properties of saccharin, extinction learning, and cue-induced saccharin-seeking behavior.
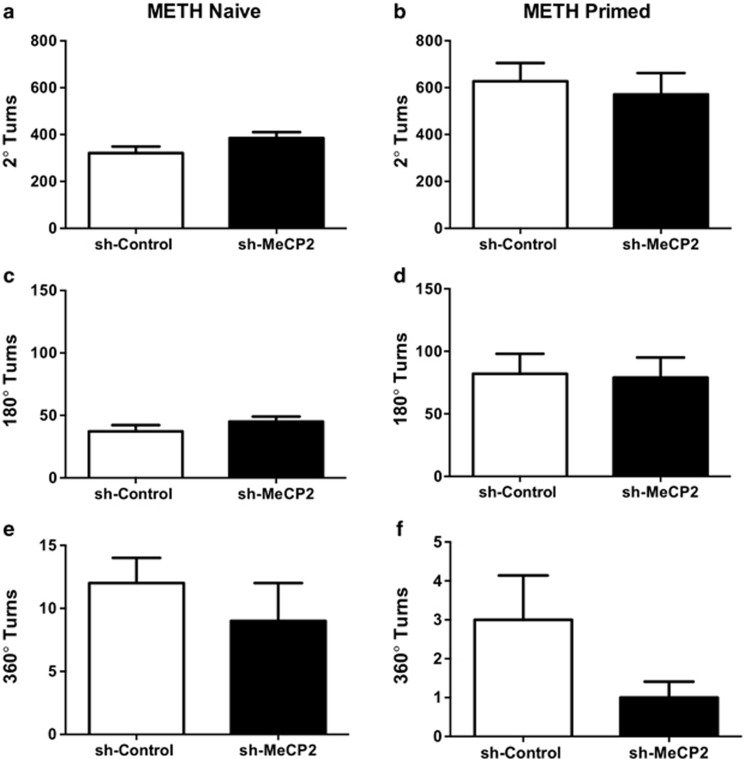
Rotational motor activity
Though we detected significant loss of MeCP2 expression, no difference was found between sh-Control and sh-MeCP2 groups on spontaneous rotational motor activity measured in 2°, 90°, and 360° turns (Figure 6a, c, and e). In addition, no difference was found between sh-Control and sh-MeCP2 groups in METH-primed rotational motor activity measured in 360°, 90°, and 2° turns (Figure 6b, d, and f).
Figure 6.
Effect of NAc core MeCP2 knockdown on spontaneous and METH-induced motor activity. No significant differences between sh-Control and sh-MeCP2 were observed in rotational motor activity in METH-naive rats and METH-primed rats (1 mg/kg, i.p.) during motor activity testing performed 1 week post-reinstatement testing in sh-Control (n=6) and sh-MeCP2 (n=9). Motor activity was measured as average 2° (a and b), 180° (c and d), and 360° (e and f) turns. Error bars indicate SEM.
DISCUSSION
Whereas past work has demonstrated that ELS leads to epigenetic alterations in the hypothalamo-pituitary-adrenal axis, here, we present evidence of ELS producing alterations in the expression of MeCP2 in specific brain reward-related regions. Specifically, we found that both ELS and METH SA alone increased MeCP2 expression in the NAc core. In turn, NAc core MeCP2 knockdown reduced the reinforcing effects of both METH and the non-drug reinforcer saccharin in rats without a history of ELS. Furthermore, NAc core MeCP2 knockdown selectively decreased the motivation for METH, but not saccharin, under a progressive ratio schedule of reinforcement and during reinstatement. These results suggest that both METH and ELS can produce similar changes in brain epigenetic activity and altering these specific changes can directly affect METH abuse-related behavior.
In order to investigate potential epigenetic mechanisms underlying ELS-induced increased vulnerability, we focused on striatal MeCP2 because of its established role in drug reward and reinforcement (Deng et al, 2010; Im et al, 2010; Zhang et al, 2014; Liyanage et al, 2015; Hou et al, 2015). The present study replicated our previous findings of ELS-induced increases in METH SA (Lewis et al, 2013; Lewis et al, 2015), which is also consistent with other drugs of abuse (Vazquez et al, 2005; Moffett et al, 2007). We also present novel findings that ELS alone increases MeCP2 expression in the NAc core, but not the NAc shell or the dorsal striatum. In addition, we found that METH SA alone increases MeCP2 expression throughout the ventral and dorsal striatum in handled control animals, consistent with previous findings (Cassel et al, 2004; Im et al, 2010; Host et al, 2011). Interestingly, in animals with ELS, METH SA increased MeCP2 solely in the medial dorsal striatum. This effect is possibly due to the relatively low MeCP2 levels in the ELS-saline group (Figure 2e), which produced a greater likelihood of observing an increase, whereas in other striatal subregions, this group had relatively higher levels that may have led to a ceiling effect. One potential reason for the differential effects across brain regions is that initial drug exposure stimulates dopamine transmission in the NAc shell, and after repeated drug exposure, this effect extends to the NAc core (Di Chiara, 2002). Moreover, it is thought that the transition from voluntary drug use to compulsive drug use mirrors a shift from ventral to dorsal striatal control over drug seeking (Everitt and Robbins, 2013). Taken together, these data suggest that ELS may modulate the epigenetic profile and epigenetic response to METH in the striatum, thus potentially altering the progression from casual to compulsive use in this vulnerable population.
Given that both ELS and METH SA alone increases MeCP2 expression in the NAc core (Experiment 1), we sought to determine whether NAc core MeCP2 expression influences METH and saccharin SA. We found that viral-mediated knockdown of MeCP2 expression in the NAc core reduces both METH and saccharin intake on an FR schedule of reinforcement. Interestingly, although METH intake was affected during the early stages of acquisition, we observed greater differences between the virus groups during the later sessions for saccharin reinforcement (Figure 5a). In addition, NAc core MeCP2 knockdown decreased motivation for METH, but not saccharin, on a progressive ratio schedule of reinforcement and during reinstatement. This suggests that NAc core MeCP2 expression differentially mediates motivation for METH compared with non-drug reinforcers.
Currently, the functional role of MeCP2 in ELS-induced drug vulnerability is unknown. In Experiment 1, our data suggest that ELS and METH SA both increase NAc core MeCP2 expression. However, because we directly examined the role of MeCP2 on METH abuse-related behavior in non-stressed animals, we are unable to conclude that MeCP2 knockdown would have the same effects in animals with a history of ELS. However, this does not preclude the possibility that ELS and METH SA converge on the same epigenetic process to induce increased addiction-like behavior. Therefore, future research should be pursued to parse out the downstream effects of MeCP2 that are specifically involved in ELS and/or METH SA.
Previous research suggests a multitude of genes regulated by MeCP2 produce changes in drug intake and drug-seeking behavior (Deng et al, 2010; Im et al, 2010; Jayanthi et al, 2013; Zhang et al, 2014; Deng et al, 2014; Hou et al, 2015). For example, MeCP2 is implicated in pain-induced opioid-seeking behavior in rats through regulating GluA1 expression in the central amygdala (Hou et al, 2015). MeCP2 also represses expression of the histone dimethyltransferase G9a in the central amygdala, leading to an increase in brain-derived neurotrophic factor expression that, in turn, increases morphine-conditioned reward (Zhang et al, 2014). In addition, MeCP2 knockdown in the dorsal striatum reduces cocaine intake, and this effect is by indirect regulation of brain-derived neurotrophic factor expression through direct interactions with microRNA-212 (Im et al, 2010). Other studies have shown that reductions in AMPA receptor levels in striatal neurons by chronic METH exposure is partly mediated by MeCP2 recruitment (Jayanthi et al, 2013), and that psychostimulant-induced phosphorylation of MeCP2 regulates behavioral responses to both amphetamine and cocaine (Deng et al, 2010, 2014). Future research is needed to determine the specific genes regulated by MeCP2 in the NAc core that are responsible for producing the changes in METH intake and motivation reported in the present study.
We previously reported that ELS-exposed rats exhibit increased METH SA compared with handled controls and exhibit reduced MeCP2 expression in the NAc core (Lewis et al, 2013). Given that the brain tissue in our previous study was collected more than 2 weeks following the last METH exposure, it is possible that the observed decrease in MeCP2 expression was due to METH withdrawal rather than METH intake per se. This effect is consistent with previous findings (Liyanage et al, 2015), and METH withdrawal has been shown to alter the expression of several transcription factors (Cadet et al, 2014). In the present study, tissue was collected immediately following SA, and we did not observe a difference in NAc core MeCP2 expression between the ELS-exposed rats and handled controls in the METH condition. These data suggest a highly dynamic association between METH SA and NAc core MeCP2 expression dependent on temporal parameters and ELS exposure. Future research is needed to further delineate this relationship.
One limitation of Experiment 1 was that we did not examine whether ELS impacts motivation for METH on a progressive ratio schedule of reinforcement, which would be interesting given our robust effects of NAc core MeCP2 knockdown on progressive ratio measures in Experiment 2. However, others have found an enhancement in motivation to self-administer cocaine in adulthood following ELS (Zhang et al, 2005; Kosten et al, 2006), suggesting that similar effects would likely occur with METH. A limitation of Experiment 2 was the potential for off-target shRNA effects because an empty control vector was utilized in lieu of a scrambled control sequence, as has been similarly performed by other investigators (Jin et al, 2008; Im et al, 2010). However, we utilized qRT-PCR to specifically test for off-target effects on expression levels of genes with sequences partially homologous to those complementary of the sh-MeCP2 vector and found no change in expression, but confirmed a selective knockdown of MECP2 expression (see Supplementary Data and Supplementary Figure S1).
In conclusion, our findings suggest individual epigenetic responses to METH may differ depending on ELS history. Furthermore, epigenetic regulation of gene expression by MeCP2 may differentially influence the motivational effects of METH and natural reinforcement. Future research examining the role of MeCP2 in regulating specific target genes, synaptic plasticity, and its role in other brain regions and specific cell types is warranted. Our results add to the growing body of literature demonstrating that ELS induces specific molecular effects in brain reward circuitry. This body of literature is translationally relevant, as ELS is a known risk factor for SUDs (De Bellis, 2002; Enoch, 2011). Further elucidating these mechanisms may inform future pharmacological and behavioral treatments aiming to reverse, or modulate, high-risk epigenetic landscapes.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
Candace R Lewis*, PhD, Arizona State University, has no competing interests to disclose. Ryan Bastle, PhD, Arizona State University, has received funding from NIH DA035069 and has no competing interests to disclose. Tawny B Manning, BA, Arizona State University, has no competing interests to disclose. Sarah M Himes, Arizona State University, has no competing interests to disclose. Paulette Fennig, BS, Arizona State University, has no competing interests to disclose. Phoebe R Conrad, Arizona State University, has no competing interests to disclose. Jenna Colwell, Arizona State University, has no competing interests to disclose. Broc A Pagni, BS, Arizona State University, has no competing interests to disclose. Lyndsay A Hess, BS, Arizona State University, has no competing interests to disclose. Caitlin G Matekel, Arizona State University, has no competing interests to disclose. Jason M Newbern, PhD, Arizona State University, has received funding from NIH-NINDS R00-NS076661 and has no competing interests to disclose. M Foster Olive, PhD, Arizona State University, has received funding from NIH DA025606 and has no competing interests to disclose.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2014). Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: Evidence from a long-access self-administration model in the rat. Mol Neurobiol 51: 696–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich J et al (2006). Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol 70: 487–492. [DOI] [PubMed] [Google Scholar]
- Cassel S, Revel MO, Kelche C, Zwiller J (2004). Expression of the methyl-CpG-binding protein MeCP2 in rat brain. An ontogenetic study. Neurobiol Dis 15: 206–211. [DOI] [PubMed] [Google Scholar]
- Chao J, Nestler EJ (2004). Molecular neurobiology of drug addiction. Annu Rev Med 55: 113–132. [DOI] [PubMed] [Google Scholar]
- De Bellis MD (2002). Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 27: 155–170. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A (2014). The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am 23: 185–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE (2010). MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci 13: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng JV, Wan Y, Wang X, Cohen S, Wetsel WC, Greenberg ME et al (2014). MeCP2 phosphorylation limits psychostimulant-induced behavioral and neuronal plasticity. J Neurosci 34: 4519–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G (2002). Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res 137: 75–114. [DOI] [PubMed] [Google Scholar]
- Enoch MA (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 214: 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2013). From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37: 1946–1954. [DOI] [PubMed] [Google Scholar]
- Feng J, Nestler EJ (2010). MecP2 and drug addiction. Nat Neurosci 13: 1039–1041. [DOI] [PubMed] [Google Scholar]
- Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J (2011). Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. J Psychopharmaco (Oxf) 25: 222–229. [DOI] [PubMed] [Google Scholar]
- Hou YY, Cai YQ, Pan ZZ (2015). Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of glua1 in rat central amygdala. J Neurosci 35: 3689–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ (2010). MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13: 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Chen B, Britt JP, Kourrich S, Yau HJ et al (2013). Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Bao X, Wang H, Pan H, Zhang Y, Wu X (2008). RNAi-induced down-regulation of Mecp2 expression in the rat brain. Int J Dev Neurosci 26: 457–465. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E (2002). HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 27: 35–69. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P (2006). Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology 31: 70–76. [DOI] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L, Olive MF (2013). The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front Psychiatry 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Tomek SE, Hernandez R, Manning T, Olive MF (2015). Early life stress and chronic variable stress in adulthood interact to influence methamphetamine self-administration in male rats. Behav Pharmacol 27: 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage VR, Zachariah RM, Davie JR, Rastegar M (2015). Ethanol deregulates Mecp2/MeCP2 in differentiating neural stem cells via interplay between 5-methylcytosine and 5-hydroxymethylcytosine at the Mecp2 regulatory elements. Exp Neurol 265: 102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ (2007). Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D et al (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 12: 1559–1566. [DOI] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Chen J, Lefebvre C, Kawamura T, Kreifeldt M, Basson O et al (2014). MeCP2 regulates ethanol sensitivity and intake. Addict Biol 19: 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ (2011). Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12: 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-López A, Méndez-Díaz M, Ruiz-Contreras AE, Carrisoza R, Prospéro-García O (2012). Maternal separation and proclivity for ethanol intake: a potential role of the endocannabinoid system in rats. Neuroscience 223: 296–304. [DOI] [PubMed] [Google Scholar]
- Tesone-Coelho C, Morel LJ, Bhatt J, Estevez L, Naudon L, Giros B et al (2013). Vulnerability to opiate intake in maternally deprived rats: implication of MeCP2 and of histone acetylation. Addict Biol 20: 120–131. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Daugé V (2005). Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci 25: 4453–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q et al (2014). Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLoS ONE 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Diorio J, Seckl JR, Szyf M, Meaney MJ (2004). Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci 1024: 182–212. [DOI] [PubMed] [Google Scholar]
- Wu Y, Patchev AV, Daniel G, Almeida OF, Spengler D (2014). Early-life stress reduces DNA methylation of the Pomc gene in male mice. Endocrinology 155: 1751–1762. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA (2005). Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology 177: 391–399. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ, Pan ZZ (2014). MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci 34: 9076–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.