Abstract
Cleavage is a period after fertilization, when a 1-cell embryo starts developing into a multicellular organism. Due to a series of mitotic divisions, the large volume of a fertilized egg is divided into numerous smaller, nucleated cells—blastomeres. Embryos of different phyla divide according to different patterns, but molecular mechanism of these early divisions remains surprisingly conserved. In the present paper, we describe how polarity cues, cytoskeleton and cell-to-cell communication interact with each other to regulate orientation of the early embryonic division planes in model animals such as Caenorhabditis elegans, Drosophila and mouse. We focus particularly on the Par pathway and the actin-driven cytoplasmic flows that accompany it. We also describe a unique interplay between Par proteins and the Hippo pathway in cleavage mammalian embryos. Moreover, we discuss the potential meaning of polarity, cytoplasmic dynamics and cell-to-cell communication as quality biomarkers of human embryos.
Keywords: polarity, par proteins, Hippo signalling, cytoplasmic flow, embryo, C. elegans, Drosophila, mouse, human, preimplantation development
Introduction
Cleavage is a period after fertilization, when a 1-cell embryo starts developing into a multicellular organism. It consists of a series of mitotic divisions, which divide the large volume of a fertilized egg into numerous smaller, nucleated cells—blastomeres.
The pattern of cleavage divisions differs between species. The main factor determining where cleavage can occur, regulating size of the blastomeres and division timings is yolk. In general, blastomeres formed in the relatively yolk-free animal pole are smaller than those on the vegetal, yolk-rich pole. A good example is an amphibian egg, with a moderate vegetal yolk deposition (mesolecithal egg). During cleavage, small blastomeres (micromeres) are formed in the animal pole, whereas big blastomeres (macromeres)—in the vegetal pole. However, as amount of yolk differs between species, the cleavage pattern observed in amphibians is not universal. Mammalian eggs have no yolk (alecithal eggs) and blastomeres created during cleavage are of equal size. At the other extreme are eggs of insects, fish, reptile and birds. Most of their volume is filled with yolk and they undergo a meroblastic (partial cleavage) with cleavage furrows penetrating only a portion of the cytoplasm. In insect (e.g. Drosophila) eggs, yolk is localized in the cell centre and cleavage occurs in the cortical area (i.e. superficial cleavage). Conversely, in fish, reptiles and birds, the cleavage divisions take place only in a small disc of cytoplasm at the animal pole of the egg (discoidal cleavage) (reviewed in Gilbert, 2013).
In spite of this diversity in cleavage patterns, molecular mechanisms regulating early embryonic divisions remain strongly conserved among phyla. Interactions between polarity cues, cytoskeleton and cell-to-cell communication are crucial for cleavage in a wide range of examined species, from Caenorhabditis elegans to mouse. In the present paper, we wish to present these universal mechanisms that together guide embryos through the cleavage and discuss their potential meaning as quality biomarkers of human embryos.
Polarity and spindle orientation
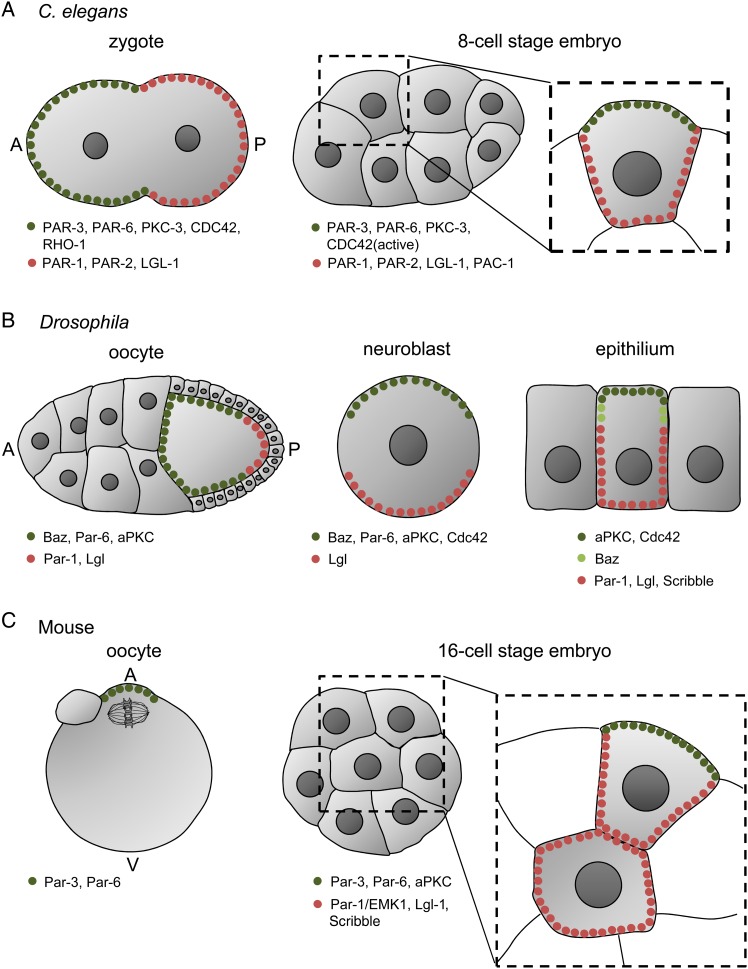
Establishment of cellular polarity is one of the most important events during early embryonic divisions. In most species, including mammals, it enables cells to adopt distinct developmental fates. The main signalling pathway involved in cell polarization, mediated by the PAR (partitioning defective) proteins, was discovered in C. elegans embryos due to its ability to affect asymmetry of the first cleavage division (Kemphues et al., 1988). Before fertilization, PAR-3, PAR-6 and PKC-3 (the nematode homologue of aPKC) are present throughout the egg cortex and PAR-1 and PAR-2 localize in the cytoplasm (Munro and Bowerman, 2009; Nance and Zallen, 2011). After fertilization, PAR proteins and PKC-3 become redistributed in a polarized manner with PAR-1 and 2 found in the cortex above the sperm-derived centrosome (marking it as the posterior pole) (Guo and Kemphues, 1995; Boyd et al., 1996), and PAR-3, PAR-6 and PKC-3 located to the cortex of the opposite, anterior pole (Fig. 1A) (Etemad-Moghadam et al., 1995; Tabuse et al., 1998; Hung and Kemphues, 1999). PAR-4 and PAR-5 in contrast are localized uniformly in the whole cortex of the 1-cell embryo (Watts et al., 2000; Morton et al., 2002). When polarity is already established, Rho GTPases such as CDC42 and RHO1 (homologue of vertebrate RhoA) also become enriched in the anterior pole, participating in polarity maintenance (Aceto et al., 2006; Motegi and Sugimoto, 2006; Schonegg and Hyman, 2006). On the other hand, the posterior pole accumulates the protein LGL-1, reported to act redundantly with PAR-2 to maintain PAR asymmetry (Beatty et al., 2010, 2013; Hoege et al., 2010).
Figure 1.
Polarity in C. elegans, Drosophila and mouse oocytes and embryos. Polarized distribution of PAR proteins and accompanying factors in C. elegans zygote and 8-cell stage embryo (A), Drosophila oocyte, neuroblast and epithelium (B), and mouse oocyte and 16-cell stage embryo (C). In (A) and (B): A, anterior pole; P, posterior pole. In (C): A, animal pole; V, vegetal pole.
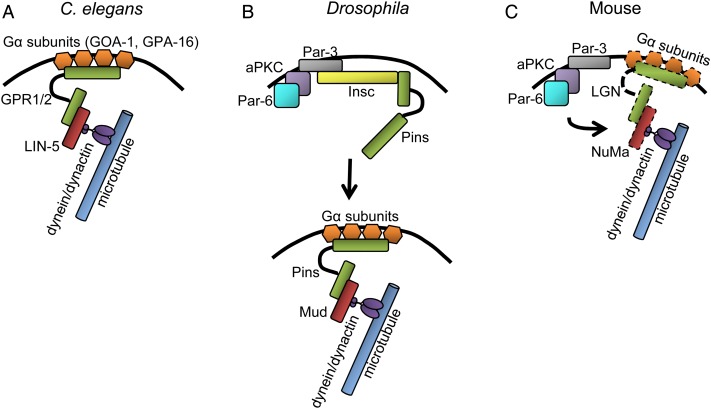
During the first division, the interaction between astral microtubules emanating from the spindle poles and the cortex leads to an asymmetric localization of the spindle, as it is pulled towards the posterior part of the zygote. Due to this dislocation, the first cleavage division results in two daughter cells of unequal size, termed the AB and P1 blastomeres (Nance and Zallen, 2011; Rose and Gönczy, 2014). These cells differ not only in size, but also inherit different complements of cell fate determinants, enabling them to follow distinct developmental paths. It seems that translocation of the spindle depends on a ternary protein complex comprised of two partially redundant protein Gα subunits GOA-1 and GPA-16, two essentially identical proteins containing GoLoco domain, GPR1 and GPR2, and the protein LIN-5 (Fig. 2A) (Gotta and Ahringer, 2001; Colombo et al., 2003; Gotta et al., 2003; Srinivasan et al., 2003; Tsou et al., 2003). As Gα proteins are myristoylated, the entire complex is anchored in the cell membrane. It is still unclear how the asymmetric pulling force is generated by the complex. However, it is probable that it may be related to the slightly asymmetric distribution of the complex components: whereby GOA-1 and GPA-16 are distributed uniformly throughout the cell cortex and LIN-5 is localized equally at both cell poles, GPR1 and 2 are enriched in the posterior region (Miller and Rand, 2000; Gotta and Ahringer, 2001; Colombo et al., 2003; Gotta et al., 2003; Tsou et al., 2003; Afshar et al., 2004, 2005; Park and Rose, 2008). Moreover, asymmetry in GPR1 and 2 localization depends on anterior–posterior polarization (Gotta and Ahringer, 2001; Colombo et al., 2003; Gotta et al., 2003; Srinivasan et al., 2003; Tsou et al., 2003), suggesting that it can be indeed essential for the asymmetry in the pulling forces and translocation of the mitotic spindle. LIN-5 is a homologue of NuMa that can associate in vertebrate cells with dynein, a microtubule motor protein (Merdes et al., 1996; Kotak et al., 2012). Therefore, it is thought that the ternary complex binds through LIN-5 to a dynein/dynactin complex and in consequence can generate pulling force on spindle astral microtubules (Fig. 2A) (Rose and Gönczy, 2014). There is also a possibility that posteriorly directed pulling force is, at least partially, caused by anchoring and stabilization of anterior microtubules by PAR-3 (Labbé et al., 2003). PAR-2 and PAR-3 are known to regulate pulling forces exerted on both sides of the spindle during spindle positioning (Grill et al., 2001); however, as the microtubules are stabilized by anteriorly accumulated PAR-3, the net force is directed towards the more dynamic, posterior pole (Labbé et al., 2003).
Figure 2.
Interplay between PAR proteins and microtubules. Mechanism of a polarized dynein/dynactin-driven force exerted on microtubules in C. elegans (A), Drosophila (B) and mouse (C). Details in the main text. Boxes encircled with a dashed-line symbolize proteins that were not directly confirmed in the PAR proteins–microtubule interactions in the cleavage mouse embryos.
At the 4-cell stage, the pattern of PAR asymmetry changes, as the C. elegans embryo becomes polarized radially. Blastomeres at this stage are named ABa and ABp (derived from AB blastomere) and EMS and P2 (derived from P1 blastomere). Interestingly, in contrast to other model organisms, radial polarization of C. elegans blastomeres does not affect cell fate but rather influences events during early gastrulation (Nance et al., 2003). PAR-3, PAR-6 and PKC-3 are absent at the cell contact sites and accumulate in the contact-free surfaces (Etemad-Moghadam et al., 1995; Hung and Kemphues, 1999; Nance and Priess, 2002; Nance et al., 2003). At the same time, PAR-1 and PAR-2 become enriched in the surfaces between blastomeres (Fig. 1A) (Nance and Priess, 2002; Nance et al., 2003). This process is probably mediated by PKC-3 that localizes in contact-free surfaces in a PAR-3 and PAR-6-dependent manner (Tabuse et al., 1998; Nance et al., 2003) and phosphorylates PAR-2, blocking its cortical binding (Hao et al., 2006). As PAR-1 depends on PAR-2 in its localization, it also cannot localize to the cell apical cortex (Motegi et al., 2011). The localization of the mitotic spindle under such conditions depends, at least partially, on proteins expressed also in the zygote stage. GPR1, GPR2 and LIN-5 regulate spindle position during the asymmetric division of P2 blastomere (Srinivasan et al., 2003; Tsou et al., 2003; Werts et al., 2011), whereas GPA-16 participates in spindle orientation in the asymmetric division of the EMS and symmetric divisions of the ABa and ABp blastomeres (Bergmann et al., 2003; Tsou et al., 2003; Zhang et al., 2008). Interestingly, in the EMS, blastomere spindle position is also regulated by selected components of the Src and Wnt signalling pathways (Schlesinger et al., 1999; Bei et al., 2002; Zhang et al., 2008; Werts et al., 2011; Rose and Gönczy, 2014).
Par proteins also participate in the establishment of polarity in Drosophila embryos, both in single cells and multicellular epithelia. The Par3 homologue, Bazooka (Baz), was discovered in fly embryos in the 1980s (Wieschaus et al., 1984), and later other Par-related components were isolated, including: Par-1, aPKC (homologue of PKC-3), Par-6, Lkb-1 (homologue of PAR-4) and 14-3-3ε and ζ (homologues of PAR-5) (Shulman et al., 2000; Tomancak et al., 2000; Wodarz et al., 2000; Petronczki and Knoblich, 2001; Benton et al., 2002; Martin and St Johnston, 2003). The anterior–posterior axis in Drosophila is formed before fertilization with Baz, Par-6 and aPKC localizing to the anterior and lateral cortex of the oocyte, and Par-1 and Lgl (homologue of C. elegans LGL-1) to the posterior cortex (Fig. 1B) (reviewed in Nance and Zallen, 2011). aPKC phosphorylates Par-1 and Lgl, thus excluding them from the anterior and lateral cortex and restricting their localization to the posterior region (Hurov et al., 2004; Tian and Deng, 2008; Doerflinger et al., 2010). Conversely, Par-1 phosphorylates Baz and excludes it from the posterior domain (Vaccari and Ephrussi, 2002; Benton and St Johnston, 2003).
The Par pathway also participates in polarity establishment during later stages of Drosophila embryonic development, such as in neuroblasts and epithelial cells. In neuroblasts, Baz, aPKC, Par-6 and Cdc42 form an apical domain that is limited, at least in part, by antagonism with basally located Lgl (Fig. 1B) (Prehoda, 2009; Bergstralh et al., 2013). In epithelial cells on the other hand, the apical domain is enriched with aPKC and Cdc42, whereas the basolateral cortex accumulates Par-1, Lgl, Dlg and Scribble. Baz is present in the adherens junction sites, at the boundary between apical and basolateral domains (Fig. 1B) (reviewed in Bergstralh et al., 2013). As in Drosophila oocytes, aPKC also phosphorylates Par-1 and Lgl in epithelial cells, causing their basolateral translocation, whereas Par-1 phosphorylates Baz excluding it from the basolateral cortex (Benton and St Johnston, 2003; Rolls et al., 2003; Betschinger et al., 2005; Nance and Zallen, 2011; Bergstralh et al., 2013).
The Drosophila neuroblast is an ideal model for studying the interplay between polarization, spindle orientation and asymmetric cell division (Fig. 2B). Neuroblasts express Inscuteable (Insc), that localizes apically due to its interaction with aPKC/Par-6/Baz (Schober et al., 1999; Wodarz et al., 1999). Insc is responsible for recruiting the GoLoco domain containing protein, Partner of Inscuteable (Pins, a homologue of vertebrate LGN) to the apical membrane. This interaction promotes spindle orientation along the apical–basal axis. Pins can bind to either Insc or Mud (a homologue of vertebrate NuMa and C. elegans LIN-5) but not to both simultaneously (Culurgioni et al., 2011; Yuzawa et al., 2011; Zhu et al., 2011), suggesting that Insc recruits Pins to the membrane, but then it has to pass it to Mud. This does not necessarily remove Pins from the apical cortex, as Pins may interact with cortical subunits Gα. Once bound to Mud, Pins can exert pulling forces on astral spindle microtubules through dynein/dynactin complex (Fig. 2B). Considerably less attention has been paid to spindle orientation in symmetrically dividing Drosophila epithelial cells; however, it seems that the interaction between aPKC and Pins also plays a role there (Bergstralh et al., 2013).
In mouse, polarity is first established in unfertilized oocytes characterized by the metaphase spindle localized to the animal hemisphere of the oocyte. Chromosomal proximity leads to cortex reorganization in a Ran GTPase-dependent manner and induces actin, myosin 2, Par-3 and Par-6 accumulation above the spindle (Fig. 1C) (Vinot et al., 2004; Duncan et al., 2005; Deng et al., 2007; Ajduk et al., 2013). Several studies suggest that Cdc42, Rac-GTPase and the Mos/MEK/MAP kinase pathway may be also involved in the reorganization of the cortex above the spindle, as they regulate the translocation of the meiotic spindle to the oocyte cortex (Araki et al., 1996; Choi et al., 1996; Verlhac et al., 2000; Tong et al., 2003; Deng et al., 2005; Na and Zernicka-Goetz, 2006; Halet and Carroll, 2007; Yu et al., 2007).
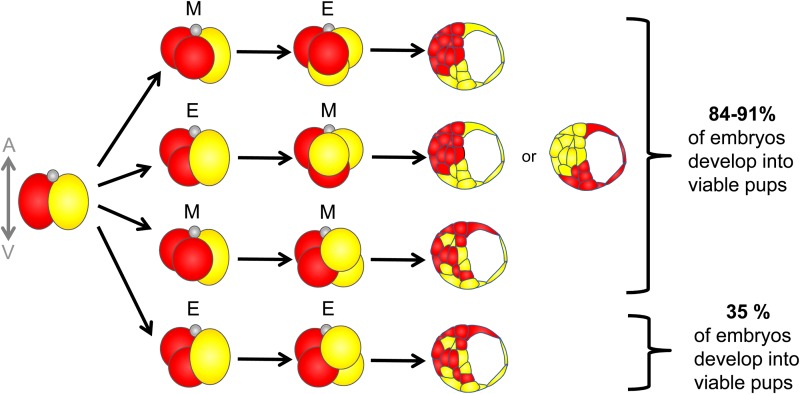
After fertilization, the clear asymmetry observed in oocytes either entirely or largely disappears. However, it is likely that some, unknown yet, components, localized in a polarized way along the animal–vegetal axis, still affect cell divisions and developmental potential of the blastomeres. The first cleavage plane in zygotes is almost exclusively meridional (M), which means it occurs along the animal–vegetal axis (Howlett and Bolton, 1985; Gardner, 1997; Plusa et al., 2002; Piotrowska-Nitsche and Zernicka-Goetz, 2005) resulting in an equal inheritance of cytoplasm from the animal and vegetal zygotic regions between both blastomeres in the 2-cell stage embryo. However, the divisions of the second cleavage can be orientated either meridionally, like in zygotes, or equatorially (E), i.e. perpendicular to the animal–vegetal axis with the animal and vegetal material inherited differentially by different daughter cells (Fig. 3) (Gardner, 2002; Piotrowska-Nitsche and Zernicka-Goetz, 2005). Therefore, and after considering the relative order of timing between the two asynchronous cell divisions, four distinct types of 4-cell stage embryo can be distinguished: ME, EM, MM and EE. It has been shown that the pattern of the second cleavage division, as well as the order in which these divisions occur, affects both fate and developmental potential of the resulting cells (Piotrowska-Nitsche and Zernicka-Goetz, 2005). In the majority of ME embryos, the embryonic part of the blastocyst (so-called inner cell mass, ICM) is built by the progeny of the 2-cell blastomere that divided meridionally. Moreover, the progeny of the E-blastomere that inherited the vegetal material subsequently undertake significantly more symmetric divisions compared with the progeny of other blastomeres (Bischoff et al., 2008), thus preferentially contribute to the mural trophectoderm (i.e. the abembryonic part of the blastocyst; Piotrowska-Nitsche et al., 2005). In comparison, progeny of the E-blastomere containing animal material predominantly populates the boundary zone between ICM and trophectoderm (Piotrowska-Nitsche et al., 2005). EM embryos, on the other hand, have embryonic and abembryonic regions in the blastocyst built mainly by progeny of one of the 2-cell stage blastomere, but this can be either a clone originating from equatorial or meridional division. In contrast, the relationship between division orientation and cell allocation in blastocyst appears completely random in MM and EE embryos (Fig. 3). Importantly, the pattern of segregation and inheritance of animal and vegetal material also correlates with developmental potential of embryos. The majority of embryos in which at least two 4-cell stage blastomeres inherit both animal and vegetal material (i.e. embryos that have at least one meridional division: ME, EM, MM), develop successfully to term, whereas only one-third of embryos in which all 4-cell stage blastomeres have exclusively either animal or vegetal material (EE embryos) give rise to viable pups (Fig. 3) (Piotrowska-Nitsche and Zernicka-Goetz, 2005; Piotrowska-Nitsche et al., 2005).
Figure 3.
Cleavage patterns of mouse embryos at 2- to 4-cell transition. Two-cell stage mouse blastomeres divide either meridionally (M) or equatorially (E) giving rise to four types of embryos: ME, EM, MM and EE. Depending on the cleavage plane, progeny of the blastomeres populate different regions of the blastocyst (for details, see the main text). Moreover, embryos, which underwent at least one meridional division (ME, EM and MM), develop to term significantly more efficiently than EE embryos (data from Piotrowska-Nitsche and Zernicka-Goetz, 2005). A, animal pole; V, vegetal pole.
The exact molecular mechanism behind this cleavage-related asymmetry still remains to be discovered and is the subject of intensive research. Molecules distributed differentially between animal and vegetal part of the mammalian zygote that could be responsible for different cell fate of the blastomeres that inherited them are still unknown. The candidates proposed so far are: hormone leptin, transcription factor STAT3, growth factors TGFβ2 and VEGF, and the apoptosis-associated proteins BCL-X and BAX (Antczak and Van Blerkom, 1997, 1999; Schulz and Roberts, 2011). Unfortunately, apart from asymmetric localization of these proteins, there is no proof for a functional link between them and developmental fate of the blastomeres. It has been found however, that in ME embryos, cells inheriting more vegetal part of the zygote have lower levels of histone H3 R26/17 methylation when compared with the remaining cells at the 4-cell stage (Torres-Padilla et al., 2007). Moreover, it has been shown that blastomeres with high levels of Carm1 methylotransferase, an enzyme responsible for this specific H3 arginine methylation, up-regulate pluripotency markers, such as Nanog and Sox2 and their progeny tends to localize in the ICM (Torres-Padilla et al., 2007; Parfitt and Zernicka-Goetz, 2010). In support of these findings, another study revealed that 8-cell blastomeres originated from equatorial division in ME embryo are characterized by 5-fold higher expression of a trophectoderm marker, Cdx2 (Jedrusik et al., 2008). Furthermore, Plachta et al. (2011) have found that blastomeres at 4-cell and 8-cell stage differ in kinetics of nuclear import/export of another key pluripotency factor Oct4. Cells with a stable nuclear pool of Oct4 take more asymmetric divisions and preferentially develop to ICM, whereas cells with more ‘mobile’ nuclear Oct4 tend to divide symmetrically and differentiate into trophectoderm. Therefore, it is plausible that these differences in Oct4 kinetics originate from the animal–vegetal asymmetries formed initially within the oocyte.
Par-related polarity becomes re-established in mouse embryos at the 8-cell stage, but this time, as in C. elegans, the polarity is radial. During this process, mouse blastomeres increase intercellular adhesion (i.e. undergo compaction) and a clear distinction between apical and basolateral surfaces develops. Similarly to C. elegans and Drosophila cells, the apical region in mouse blastomeres is enriched in Par-6 and aPKC proteins, as well as in F-actin, whereas basolateral parts accumulate Par-1/EMK1. At later stages, the Par-3 protein joins Par-6 and aPKC in the apical domain (Fig. 1C) (Pauken and Capco, 2000; Plusa et al., 2005; Vinot et al., 2005). Other basolateral polarity regulators, such as Lgl-1 and Scribble, are also present in cell contact sites and they seem to be directed to this localization by activity of aPKC and Par6 (Hirate et al., 2013). During 8- to 16- and 16- to 32-cell transitions, a portion of cells divides at the angle that is more perpendicular than parallel to the embryo surface, producing outer polarized cells and inner apolar cells. Both of these cells display different fates, with inner cells forming ICM in the blastocyst stage, and outer cells—trophectoderm (reviewed in Zernicka-Goetz et al., 2009). The different fates of these cells in mammals are defined by differences in cell polarity and in the expression pattern of key transcription factors triggered by both cell position (i.e. inner or outer) and a distinct localization of transcripts [e.g. apically biased localization of Cdx2 mRNA, facilitating its inheritance by the outer cells (Jedrusik et al., 2008; Skamagki et al., 2013)].
A mechanism governing the orientation of division planes in a cleavage mouse embryo remains largely elusive; however, recent papers provide some important insights. It has been shown that division plane correlates in the 8-cell stage blastomere with the position of the nucleus. Blastomeres with nuclei located apically divide almost exclusively symmetrically, whereas blastomeres with nuclei situated in the basolateral region divide either symmetrically or asymmetrically (Ajduk et al., 2014). Apical localization of the nuclei is facilitated by aPKC activity, as overexpression of dominant negative version of aPKC leads to more nuclei relocated basally (Ajduk et al., 2014). Importantly, lack of or diminished aPKC activity also results in an increased frequency of asymmetric divisions or cell displacement from the surface to the inside of the embryo (Plusa et al., 2005; Ajduk et al., 2014) induced by constrictions of the apical actomyosin network (Samarage et al., 2015). Nucleus position seems to be regulated by kinesin and dynein microtubule motor proteins: basal displacement of blastomere nuclei depends on kinesins and is counteracted by dynein-driven forces, and is probably, analogous to the situation in C. elegans and Drosophila embryos. Moreover, aPKC also promotes movement towards the apical cortex (Ajduk et al., 2014). In other mammalian cell systems, aPKC and dynein/dynactin-driven force is exerted on microtubules via proteins such as Gα, LGN and NuMa (reviewed in Siller and Doe, 2009); therefore, it seems plausible that they too may participate during nuclear positioning in 8-cell stage blastomeres (Fig. 2C).
Cytoplasmic flow and a polarity determinants distribution
Establishment of polarity in embryos requires displacement of various cell fate determinants. This redistribution is often mediated by a cytoplasmic flow, a directional cytoplasmic movement dependent on actomyosin cytoskeleton. The mechanism of cytoplasmic flow was examined in detail in C. elegans embryos. Some analogical processes have been also identified in mammalian oocytes and zygotes (Deguchi et al., 2000; Ajduk et al., 2011; Yi et al., 2011a, b), but there is currently no evidence for asymmetric actomyosin flow related to polarity in Drosophila oocytes or embryos. However, a microtubule- and kinesin-dependent cytoplasmic streaming has been identified in Drosophila oocytes. Its function is not completely clear, but the streaming seems to be involved in establishment of the polarity (Glotzer et al., 1997; Serbus et al., 2005; Ganguly et al., 2012).
In C. elegans egg, cytoplasmic flow is induced by fertilization. The egg is filled with a dynamic and contractile actomyosin network, which becomes destabilized at the sperm entry site. It initiates a flow of cortical non-muscle myosin 2 (NMY-2) and F-actin towards the opposite, anterior, pole. PAR-3, PAR-6 and PKC-3, as well as non-PAR proteins that associate with the cytoskeleton, appear to be transported to the anterior by this movement (Munro et al., 2004; Nance and Zallen, 2011). In turn, PAR proteins modulate actomyosin dynamics. PAR-3, PAR-6 and PKC-3 promote the cortical flow (Munro et al., 2004). PAR-2, which localizes to the posterior cortex, inhibits NMY-2 from accumulating there during the flow, and maintains asymmetry by preventing inappropriate, posterior-directed flows (Munro et al., 2004). PAR-4, on the other hand, facilitates actomyosin contractility and its depletion leads to reduced mobility of NMY-2, mislocalization of anterior PAR proteins and defects in cytokinesis (Chartier et al., 2011). It seems that PAR-4 affects actomyosin contractility, and in turn PAR asymmetric distribution, regulating activity of actin cytoskeleton scaffold protein anillin (Chartier et al., 2011). Myosin is also regulated by RHO1 small GTPase, which phosphorylates and activates MLC4, the myosin regulatory light chain subunit (Jenkins et al., 2006). RHO1 is activated by Rho guanine nucleotide exchange factors (RhoGEFs), with ECT-2 being the principal one (Jenkins et al., 2006; Motegi and Sugimoto, 2006; Schonegg and Hyman, 2006), and inhibited by Rho guanosine triphosphatase activating proteins (RhoGAPs), such as CYK-4, RGA3 and 4 (Jenkins et al., 2006; Schmutz et al., 2007; Schonegg et al., 2007).
In later cleavage stages of C. elegans embryo, cortical flow also plays an important role. A rotational cortical flow orthogonal to the anterior–posterior axis occurs during the division of the AB blastomere and positions the cytokinetic midbody remnant of the previous division asymmetrically at the future ventral side of the embryo, participating in establishment of the embryonic dorsoventral axis (Singh and Pohl, 2014).
Actomyosin-dependent cytoplasmic flow has also been described in mouse oocytes and embryos. In unfertilized oocytes, actin filaments flow continuously away from the animal cortex, inducing a cytoplasmic streaming exerting a net pushing force on the spindle towards the cortex. This flow is regulated by Arp2/3 complex, a nucleator of branched actin filaments, which accumulates to a cortical cap above the spindle in a Ran-GTPase-dependent way. Arp2/3 inhibition not only diminishes the flow but also enables a reverse streaming driven by myosin 2-based cortical contraction, moving the spindle away from the cortex (Yi et al., 2011a, b). Therefore, the asymmetric spindle position seems to be maintained by balanced forces governed by the Arp2/3 complex. The asymmetric localization of metaphase spindle is also regulated by Rac GTPase, as its inhibition leads to a displacement of the spindle from the cortex to the central region (Halet and Carroll, 2007).
Actomyosin-induced cytoplasmic streaming has also been identified in mouse oocytes upon fertilization (Deguchi et al., 2000; Ajduk et al., 2011). Sperm entry drastically changes the dynamics of cytoplasmic movements, leading to rhythmical cytoplasmic flows. They are caused by contractions of the actomyosin cytoskeleton triggered by Ca2+ oscillations induced by the sperm. The whole actomyosin cytoskeleton in the zygote contracts during the Ca2+ transients, but, due to its asymmetric distribution (a strong enrichment in the cortical caps above the oocyte and sperm chromosomes), the cytoplasmic movement becomes directional. It has two phases that can be easily distinguished during each Ca2+ transient: first, the cytoplasm moves towards cortical actomyosin caps, and then it retracts (Ajduk et al., 2011). Although similar Ca2+- and actomyosin-dependent cytoplasmic flows occur in ascidians and sea urchins and have been shown to play an important role in the establishment of embryo polarity (Speksnijder et al., 1990; Roegiers et al., 1995; Sardet et al., 2007), their function in mammalian zygotes remains elusive. Actomyosin-driven contractile waves have been also described in 8-cell stage mouse embryo upon compaction. The contractions occur periodically and are regulated by E-cadherin that appears to redirect contractility away from the cell–cell contacts (Maitre et al., 2015). Moreover, a recent study suggests that cortical tension generated by actomyosin contractility is the main factor responsible for internalization of a subset of cells (precursors of the ICM) at 8- to 16-cell stage transition (Samarage et al., 2015).
Cell-to-cell communication as a polarity cue
In many biological systems, polarization events depend on intracellular contacts. Similar relationships can be observed during embryonic development, although their extent differs between species. In C. elegans embryos, cell contacts are necessary for establishing and maintaining polarity, while in mammals (mice)—only for its establishment. In some species, e.g. Xenopus, connections between blastomeres are neither required for setting up nor maintaining cellular polarity (reviewed in Nance, 2014).
In C. elegans embryo, cell-to-cell communication is necessary in radial polarization of somatic blastomeres at the 4-cell stage (Nance, 2014). During early cleavage divisions, cells do not form cell junctions but remain adherent and can receive signals from cell contact sites. E-cadherin HMR-1 together with α-catenin HMP-1, p120 catenin JAC-1, and linker protein PICC-1 recruit the RhoGAP protein PAC-1 (homologue of vertebrate Arghgap 21), a CDC42 inhibitor, to the cell contact sites (Anderson et al., 2008; Klompstra et al., 2015). CDC42 is distributed uniformly in the blastomere cortex, but due to PAC-1 polarized localization, it becomes inactivated in the cell contact surfaces. On the other hand, its activity in the apical cortex is sustained by RhoGEFs such as CGEF-1 and ECT-2 (Kumfer et al., 2010; Chan and Nance, 2013). As only active CDC42 can recruit PAR-6, the localization of PAR-6 and as a consequence PAR-3 and PKC-3 becomes restricted to the apical, cell contact-free, blastomere domain, leaving basolateral cortex enriched only for PAR-1 and -2 proteins (Gotta et al., 2001; Nance et al., 2003; Aceto et al., 2006; Anderson et al., 2008; Nance, 2014).
In Drosophila embryos, the interplay between cell-to-cell communication and polarity has been best examined in the epithelium. Par proteins collaborate with adherens junction proteins (e.g. E-cadherin, α- and β-catenin) to generate the apical–basal polarity. Adherens junctions mark the boundary between apical and basolateral domains and mediate interactions between cells (Nishimura and Takeichi, 2009; Nance and Zallen, 2011). Lack of Baz leads to mislocalization of the adherens junctions that fail to locate to the apicolateral membrane and become distributed throughout the basolateral domain (Müller and Wieschaus, 1996; Harris and Peifer, 2004). Baz may regulate adherens junction placement either directly, as it binds to β-catenin (Wei et al., 2005), or indirectly, e.g. through interactions with the actomyosin cytoskeleton or microtubules (Bertet et al., 2004; Zallen and Wieschaus, 2004; Harris and Peifer, 2007; Simões et al., 2010). Interestingly, Baz function as a modulator of adherens junction localization seems to be well conserved: in C. elegans epithelial cells, PAR-3 also mediates the initial clustering and apical localization of E-cadherin (Achilleos et al., 2010).
In mouse embryos, E-cadherin also plays an important role in the interplay between cell-to-cell communication and polarity. It becomes enriched in a cleavage post-compacted embryo in the contact cell surfaces (Vestweber et al., 1987). When it is depleted, aPKC is no longer restricted to apical surface in outer cells, but appears in all surfaces of these cells (Stephenson et al., 2010). This suggests that like PAC-1 in C. elegans, E-cadherin is necessary to establish PAR asymmetry in mouse. However, since depletion of E-cadherin also hinders cell-to-cell adhesion, it is difficult to interpret the molecular role of E-cadherin unambiguously. E-cadherin could possibly recruit other factors to cell contact regions that participate in the establishment of polarity, or it may be required for cells to sustain sufficient contacts with one another, thus priming a cadherin-independent mechanism.
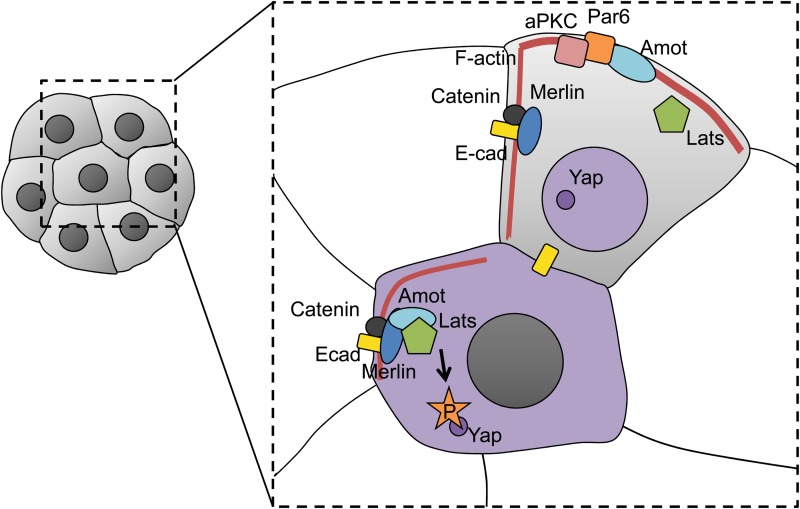
It has been suggested that E-cadherin may co-operate with the Hippo pathway (Hirate et al., 2013; Anani et al, 2014). The Hippo pathway is crucial for the regulation of organ growth and as such, it seems to be well conserved among species, from C. elegans to mammals (reviewed in Halder and Johnson, 2011; Yang and Hata, 2013). However, until now, it has only been shown to function during cleavage in mammalian embryos, where it is responsible for translating intercellular interactions into different cell fates in inner and outer cell populations (Nishioka et al., 2009; Stephenson et al., 2010; Hirate et al., 2013). The Hippo cascade regulates nuclear localization of transcriptional co-activator Yap and in consequence—the expression of Cdx2, a key determinant for differentiation into the trophectoderm lineage. In inner cells, where Hippo signalling occurs, Yap is phosphorylated by Lats protein kinase and localizes to the cytoplasm; in outer cells, where the pathway is inactive, Yap localizes to the nucleus, and regulate target genes promoting the trophoblast cell fate (Fig. 4) (Yagi et al., 2007; Nishioka et al., 2008, 2009; Wicklow et al., 2014). E-cadherin is required to exclude Yap from the nucleus of some inner cells (Nishioka et al., 2009; Stephenson et al., 2010), indicating that it may be needed for Hippo signalling, although it is difficult to discount an indirect requirement for proper cellular adhesion, which also depends on E-cadherin. The association between the cortical protein Angiomontin (Amot), known to activate the Hippo pathway (Hirate et al., 2013; Leung and Zernicka-Goetz, 2013), and E-cadherin may be especially important for asymmetric Hippo signalling (Hirate et al., 2013). In outer cells, Amot is localized at the apical, contact-free surfaces, whereas in inner cells, it is phosphorylated and enriched throughout the cell cortex. Amot binds to actin and E-cadherin, and this interaction is promoted by Nf2/Merlin (Cockburn et al., 2013; Hirate et al., 2013). It seems that a large protein complex of E-cadherin/α- and β-catenin/Merlin/Amot is formed at the contact sites and probably activates Lats kinase responsible for Yap phosphorylation (Gladden et al., 2010; Yi et al., 2011a, b; Hirate et al., 2013). Importantly, Amot colocalizes with E-cadherin only within inner cells, where its function is required, as it is excluded from basolateral surfaces in outer cells (Hirate et al., 2013; Leung and Zernicka-Goetz, 2013).
Figure 4.
Regulation of Hippo pathway in 16-cell stage mouse embryo. Regulation of Yap phosphorylation and nuclear translocation in outer and inner cells. In outer cells, due to a lack of interaction between E-cadherin (E-cad), Merlin, Amot and Lats kinases Yap remains unphosphorylated and can access the nucleus, where it facilitates Cdx2 transcription. In inner cells, interaction between E-cad, Merlin and Amot activates Lats, leading to Yap phosphorylation and its sequestration in the cytoplasm. Details in the main text.
The asymmetric localization of Amot, as well as inner–outer differences in Hippo cascade activity, is determined by the blastomere polarity (Fig. 4). Without Par6 or aPKC, Hippo signalling is active and Yap localizes to the cytoplasm in both inner and outer cells (Hirate et al., 2013). This effect depends on cell adhesion, as dissociated cells lacking Par6 have nuclear Yap, like wild-type dissociated cells. Therefore, both cell polarity and cell adhesion are required for asymmetric Hippo signalling. Interestingly, some cells originating from asymmetric divisions can transiently localize to outer surfaces, even though they appear apolar (Anani et al., 2014). These cells are eventually internalized but can activate Hippo signalling while still on the surface of the embryo, suggesting that cell polarity rather than position regulates Hippo activity. The loss of Par6 or aPKC activity enables Amot to locate in the basolateral cortex in outer cells and therefore to colocalize with E-cadherin (Hirate et al., 2013). It seems likely that restriction of Amot only to the contact-free surfaces in outer cells, through the direct or indirect action of PAR proteins, prevents Amot from associating with E-cadherin at the contact sites, and in consequence inhibits Hippo signalling. Amot sequestration to the apical region in outer cells can be also mediated by actin-binding: Amot can bind actin, which is enriched in the apical cortex of outer blastomeres (Hirate et al., 2013). Additionally, actomyosin may affect the Hippo signalling via Rho and Rock GTPases. Their inhibition not only disrupts Par, Scribble, Lgl-1 and F-actin distribution in blastomeres, but also diminishes Yap nuclear localization in outer cells (Clayton et al., 1999; Liu et al., 2013; Kono et al., 2014).
Importantly, asymmetry in Cdx2 between outer and inner cells is also regulated by the asymmetric localization and inheritance of Cdx2 transcripts. During compaction, Cdx2 transcripts localizes to the apical domain of blastomeres, mirroring asymmetric localization of developmentally important transcripts in other non-mammalian vertebrate and invertebrate embryos. This localization of Cdx2 transcripts contributes to a process whereby Cdx2 expression becomes restricted to outer cells, thus biasing those cells to become trophectoderm (Skamagki et al., 2013). How many other transcripts might become asymmetrically localized and inherited to guide/bias cell fate remains still to be discovered.
Human perspective
As we have shown in the previous paragraphs, Par-related mechanisms regulating the pattern of cleavage divisions are quite conserved among different phyla. Therefore, although there are no direct data on the mechanisms involved in polarity establishment or division plane orientation during cleavage in human embryos, it is highly likely that they are govern by interactions between the cytoskeleton, Par signalling and cell-to-cell communication analogical to those reported in other species. Consequently, a reliable assessment of these parameters may be very beneficial for selection of the highest quality embryos in IVF clinics.
How could this be achieved? One promising approach involves transcriptomic analysis of the embryonic material. Single-cell transcriptome analysis (Guo et al., 2010; Tang et al., 2010) enables development of feasible embryo selection protocols based on RNA analysis. Although at present, there are still not enough data correlating gene expression patterns in oocytes and blastomeres with embryonic developmental potential, the collection of transcriptome information in humans and animal models is in progress (Hamatani et al., 2004; Wang et al., 2004; Zhang et al., 2009; Robert et al., 2011; Vassena et al., 2011; Kakourou et al., 2013). Thus, these data will provide a necessary starting point for the design of appropriate transcriptome-based oocyte/embryo selection procedures. It is very plausible that assessment of the whole transcriptome will not be necessary to distinguish the most viable embryos, and that selected quality markers alone may be sufficient. RNAs of proteins involved in the establishment of polarization, cell-to cell communication and division planes are promising candidates for such biomarkers. Material for the RNA analysis can be obtained either from a biopsy of an oocyte's first polar body or a single blastomeres at 8-cell stage. It has been shown that transcriptome analysis of the polar body reflects well the transcriptome of the oocyte and is harmless for the future embryo (Reich et al., 2011). Since embryonic genome is activated in humans at 4- to 8-cell stage (Dobson et al., 2004; Zhang et al., 2009), maternal transcripts regulate at least first three rounds of cleavage divisions and therefore their analysis might provide important information about developmental potential of the embryo. Eight-cell stage blastomeres, which can be an alternative source of RNA, are biopsied routinely in preimplantation genetic diagnosis (PGD) and screening (PGS) and it has been proved that embryos devoid of a single 8-cell stage blastomere develop to term normally (Harper et al., 2010; Harton et al., 2011; Ajduk and Zernicka-Goetz, 2013).
An alternative approach to assess blastomere polarization and mechanism of cleavage orientation involves time-lapse imaging. Analysis of recordings obtained from automated visualization systems allows the identification of cleavage patterns at 2- to 4-cell stage transition and therefore the distinction of ME, EM, MM and EE embryos. As shown in mouse, such embryos have clearly different developmental potentials (Piotrowska-Nitsche and Zernicka-Goetz, 2005; Piotrowska-Nitsche et al., 2005). Importantly, this observation has been confirmed in humans: embryos with planar morphology (corresponding probably to EE and MM embryos) have significantly reduced rates of blastocyst formation and implantation (Ebner et al., 2012). Time-lapse imaging also helps to assess the exact timing of embryo compaction (a visual sign of blastomere polarization and adhesion). This morphokinetic parameter has been correlated with embryonic developmental potential, particularly the ploidy status: euploid embryos required a significantly shorter time to initiate compaction than aneuploid ones (Campbell et al., 2013). Interestingly, it has also been shown that diagnostic blastomere biopsy (PGD) conducted at the 6- to 8-cell stage delays compaction, indicating that PGD may interfere with embryo polarization and formation of potentially important cell-to-cell contacts (Kirkegaard et al., 2012). However, it remains to be elucidated how delayed polarization caused by the blastomere biopsy may affect further embryonic development, e.g. subsequent embryonic cell differentiation.
Time-lapse imaging also enables functional assessment of another key polarization-related factor, the actomyosin cytoskeleton. Frequent visualization (every 10 s) of mouse zygotic cytoplasm in a period directly after fertilization has identified rhythmic cytoplasmic movements dependent on actin and myosin interactions (Ajduk et al., 2011). Embryos with slow cytoplasmic movement, reflecting poor quality of the actomyosin network, showed lower developmental potential as assessed by the developmental stage and cell number reached after 4 days in culture and their ability to develop to term (Ajduk et al., 2011). Such cytoplasmic flows have also been described in human 1-cell embryos (Swann et al., 2012), although there are still no data correlating cytoplasmic dynamics with developmental quality of the human embryos.
In summary, mechanisms regulating blastomere polarization and orientation of division plane are highly conserved among distinct phyla, indicating that these developmental features are crucial for proper embryonic development. Therefore, it is worth considering whether their assessment, based either on visual cues provided by time-lapse imaging or on molecular analysis, could be incorporated into embryo selection protocols as an additional factor describing embryonic quality.
Author's roles
A.A. and M.Z.-G. prepared the manuscript.
Funding
A.A. is a beneficent of the National Science Centre grant (UMO-2012/07/D/NZ5/04301). M.Z.-G. thanks the Wellcome Trust for supporting the work in her laboratory. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Conflict of interest
The authors do not have any conflict of interest.
Acknowledgements
We would like to thank our colleague Meng Zhu, Dr Isabel Palacios and Dr Alexander W. Bruce for critical reading of the manuscript and their valuable comments. We would like to thank the Wellcome Trust to support the work in MZG laboratory.
References
- Aceto D, Beers M, Kemphues KJ. Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans. Dev Biol 2006;299:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilleos A, Wehman AM, Nance J. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 2010;137:1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gönczy P. RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 2004;119:219–230. [DOI] [PubMed] [Google Scholar]
- Afshar K, Willard FS, Colombo K, Siderovski DP, Gönczy P. Cortical localization of the Galpha protein GPA-16 requires RIC-8 function during C. elegans asymmetric cell division. Development 2005;132:4449–4459. [DOI] [PubMed] [Google Scholar]
- Ajduk A, Zernicka-Goetz M. Quality control of embryo development. Mol Aspects Med 2013;34:903–918. [DOI] [PubMed] [Google Scholar]
- Ajduk A, Ilozue T, Windsor S, Yu Y, Seres KB, Bomphrey RJ, Tom BD, Swann K, Thomas A, Graham C et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun 2011;2:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajduk A, Jedrusik A, Zernicka-Goetz M. Oocyte polarity and its developmental significance. In: Coticchio G, Albertini DF, De Santis L (eds). Oogenesis. London, UK: Springer-Verlag, 2013, 253–264. [Google Scholar]
- Ajduk A, Biswas Shivhare S, Zernicka-Goetz M. The basal position of nuclei is one pre-requisite for asymmetric cell divisions in the early mouse embryo. Dev Biol 2014;392:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anani S, Bhat S, Honma-Yamanaka N, Krawchuk D, Yamanaka Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development 2014;141:2813–2824. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 2008;320:1771–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak M, Van Blerkom J. Oocyte influences on early development: the regulatory proteins leptin and STAT3 are polarized in mouse and human oocytes and differentially distributed within the cells of the preimplantation stage embryo. Mol Hum Reprod 1997;3:1067–1086. [DOI] [PubMed] [Google Scholar]
- Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod 1999;14:429–447. [DOI] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 1996;55:1315–1324. [DOI] [PubMed] [Google Scholar]
- Beatty A, Morton D, Kemphues K. The C. elegans homolog of Drosophila Lethal giant larvae functions redundantly with PAR-2 to maintain polarity in the early embryo. Development 2010;137:3995–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty A, Morton DG, Kemphues K. PAR-2, LGL-1 and the CDC-42 GAP CHIN-1 act in distinct pathways to maintain polarity in the C. elegans embryo. Development 2013;140:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Y, Hogan J, Berkowitz LA, Soto M, Rocheleau CE, Pang KM, Collins J, Mello CC. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev Cell 2002;3:113–125. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 2003;115:691–704. [DOI] [PubMed] [Google Scholar]
- Benton R, Palacios IM, St Johnston D. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev Cell 2002;3:659–671. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lee M, Robertson B, Tsou M-FB, Rose LS, Wood WB. Embryonic handedness choice in C. elegans involves the Galpha protein GPA-16. Development 2003;130:5731–5740. [DOI] [PubMed] [Google Scholar]
- Bergstralh DT, Haack T, St Johnston D. Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R Soc Lond B Biol Sci 2013;368:20130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004;429:667–671. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Eisenhaber F, Knoblich JA. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr Biol 2005;15:276–282. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Parfitt DE, Zernicka-Goetz M. Formation of the embryonic–abembryonic axis of the mouse blastocyst: relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development 2008;135:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd L, Guo S, Levitan D, Stinchcomb DT, Kemphues KJ. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development 1996;122:3075–3084. [DOI] [PubMed] [Google Scholar]
- Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online 2013;26:477–485. [DOI] [PubMed] [Google Scholar]
- Chan E, Nance J. Mechanisms of CDC-42 activation during contact-induced cell polarization. J Cell Sci 2013;126:1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier NT, Salazar Ospina DP, Benkemoun L, Mayer M, Grill SW, Maddox AS, Labbé J-C. PAR-4/LKB1 mobilizes nonmuscle myosin through anillin to regulate C. elegans embryonic polarization and cytokinesis. Curr Biol 2011;21:259–269. [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA 1996;93:7032–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L, Hall A, Johnson MH. A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres. Dev Biol 1999;205:322–331. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Curr Biol 2013;23:1195–1201. [DOI] [PubMed] [Google Scholar]
- Colombo K, Grill SW, Kimple RJ, Willard FS, Siderovski DP, Gönczy P. Translation of polarity cues into asymmetric spindle positioning in Caenorhabditis elegans embryos. Science 2003;300:1957–1961. [DOI] [PubMed] [Google Scholar]
- Culurgioni S, Alfieri A, Pendolino V, Laddomada F, Mapelli M. Inscuteable and NuMA proteins bind competitively to Leu-Gly-Asn repeat-enriched protein (LGN) during asymmetric cell divisions. Proc Natl Acad Sci USA 2011;108:20998–21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Spatiotemporal analysis of Ca(2+) waves in relation to the sperm entry site and animal–vegetal axis during Ca(2+) oscillations in fertilized mouse eggs. Dev Biol 2000;218:299–313. [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol 2005;278:358–366. [DOI] [PubMed] [Google Scholar]
- Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell 2007;12:301–308. [DOI] [PubMed] [Google Scholar]
- Dobson AT, Raja R, Abeyta MJ, Taylor T, Shen S, Haqq C, Pera RA. The unique transcriptome through day 3 of human preimplantation development. Hum Mol Genet 2004;13:1461–1470. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Vogt N, Torres IL, Mirouse V, Koch I, Nüsslein-Volhard C, St Johnston D. Bazooka is required for polarisation of the Drosophila anterior–posterior axis. Development 2010;137:1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Schultz RM, Williams CJ. PAR-3 defines a central subdomain of the cortical actin cap in mouse eggs. Dev Biol 2005;280:38–47. [DOI] [PubMed] [Google Scholar]
- Ebner T, Maurer M, Shebl O, Moser M, Mayer RB, Duba HC, Tews G. Planar embryos have poor prognosis in terms of blastocyst formation and implantation. Reprod Biomed Online 2012;25:267–272. [DOI] [PubMed] [Google Scholar]
- Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 1995;83:743–752. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Williams LS, Palacios IM, Goldstein RE. Cytoplasmic streaming in Drosophila oocytes varies with kinesin activity and correlates with the microtubule cytoskeleton architecture. Proc Natl Acad Sci USA 2012;109:15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal–vegetal axis of the zygote in the mouse. Development 1997;124:289–301. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Experimental analysis of second cleavage in the mouse. Hum Reprod 2002;17:3178–3189. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology, 10th edn Sunderland, USA: Sinauer Associates, Inc., 2013. [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell 2010;19:727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer JB, Saffrich R, Glotzer M, Ephrussi A. Cytoplasmic flows localize injected oskar RNA in Drosophila oocytes. Curr Biol 1997;7:326–337. [DOI] [PubMed] [Google Scholar]
- Gotta M, Ahringer J. Distinct roles for Galpha and Gbetagamma in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nat Cell Biol 2001;3:297–300. [DOI] [PubMed] [Google Scholar]
- Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol 2001;11:482–488. [DOI] [PubMed] [Google Scholar]
- Gotta M, Dong Y, Peterson YK, Lanier SM, Ahringer J. Asymmetrically distributed C. elegans homologs of AGS3/PINS control spindle position in the early embryo. Curr Biol 2003;13:1029–1037. [DOI] [PubMed] [Google Scholar]
- Grill SW, Gönczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 2001;409:630–633. [DOI] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 1995;81:611–620. [DOI] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell 2010;18:675–685. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development 2011;138:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell 2007;12:309–317. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 2004;13:2263–2278. [DOI] [PubMed] [Google Scholar]
- Hao Y, Boyd L, Seydoux G. Stabilization of cell polarity by the C. elegans RING protein PAR-2. Dev Cell 2006;10:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JC, Coonen E, De Rycke M, Harton G, Moutou C, Pehlivan T, Traeger-Synodinos J, Van Rij MC, Goossens V. ESHRE PGD Consortium data collection X: cycles from January to December 2007 with pregnancy follow-up to October 2008. Hum Reprod 2010;25:2685–2707. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol 2004;167:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell 2007;12:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harton GL, Magli MC, Lundin K, Montag M, Lemmen J, Harper JC. ESHRE PGD Consortium/Embryology Special Interest Group—best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum Reprod 2011;26:41–46. [DOI] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K-I, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr Biol 2013;23:1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C, Constantinescu A-T, Schwager A, Goehring NW, Kumar P, Hyman AA. LGL can partition the cortex of one-cell Caenorhabditis elegans embryos into two domains. Curr Biol 2010;20:1296–1303. [DOI] [PubMed] [Google Scholar]
- Howlett SK, Bolton VN. Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J Embryol Exp Morphol 1985;87:175–206. [PubMed] [Google Scholar]
- Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 1999;126:127–135. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 2004;14:736–741. [DOI] [PubMed] [Google Scholar]
- Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, Robson P, Zernicka-Goetz M. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev 2008;22:2692–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science 2006;313:1298–1301. [DOI] [PubMed] [Google Scholar]
- Kakourou G, Jaroudi S, Tulay P, Heath C, Serhal P, Harper JC, Sengupta SB. Investigation of gene expression profiles before and after embryonic genome activation and assessment of functional pathways at the human metaphase II oocyte and blastocyst stage. Fertil Steril 2013;99:803–814. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 1988;52:311–320. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Human embryonic development after blastomere removal: a time-lapse analysis. Hum Reprod 2012;27:97–105. [DOI] [PubMed] [Google Scholar]
- Klompstra D, Anderson DC, Yeh JY, Zilberman Y, Nance J. An instructive role for C. elegans E-cadherin in translating cell contact cues into cortical polarity. Nat Cell Biol 2015;17:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev Biol 2014;394:142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Busso C, Gönczy P. Cortical dynein is critical for proper spindle positioning in human cells. J Cell Biol 2012;199:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfer KT, Cook SJ, Squirrell JM, Eliceiri KW, Peel N, O'Connell KF, White JG. CGEF-1 and CHIN-1 regulate CDC-42 activity during asymmetric division in the Caenorhabditis elegans embryo. Mol Biol Cell 2010;21:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé JC, Maddox PS, Salmon ED, Goldstein B. PAR proteins regulate microtubule dynamics at the cell cortex in C. elegans. Curr Biol 2003;13:707–714. [DOI] [PubMed] [Google Scholar]
- Leung CY, Zernicka-Goetz M. Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nat Commun 2013;4:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wu Z, Shi X, Li W, Liu C, Wang D, Ye X, Liu L, Na J, Cheng H et al. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates Ezrin during eight-cell embryocompaction. Dev Biol 2013;375:13–22. [DOI] [PubMed] [Google Scholar]
- Maitre J-L, Niwayama R, Turlier H, Nédélec F, Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat Cell Biol 2015;17:849–855. [DOI] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature 2003;421:379–384. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996;87:447–458. [DOI] [PubMed] [Google Scholar]
- Miller KG, Rand JB. A role for RIC-8 (Synembryn) and GOA-1 (G(o)alpha) in regulating a subset of centrosome movements during early embryogenesis in Caenorhabditis elegans. Genetics 2000;156:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DG, Shakes DC, Nugent S, Dichoso D, Wang W, Golden A, Kemphues KJ. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Dev Biol 2002;241:47–58. [DOI] [PubMed] [Google Scholar]
- Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol 2006;8:978–985. [DOI] [PubMed] [Google Scholar]
- Motegi F, Zonies S, Hao Y, Cuenca AA, Griffin E, Seydoux G. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat Cell Biol 2011;13:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol 1996;134:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Bowerman B. Cellular symmetry breaking during Caenorhabditis elegans development. Cold Spring Harb Perspect Biol 2009;1:a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell 2004;7:413–424. [DOI] [PubMed] [Google Scholar]
- Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol 2006;16:1249–1254. [DOI] [PubMed] [Google Scholar]
- Nance J. Getting to know your neighbor: cell polarization in early embryos. J Cell Biol 2014;206:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Priess JR. Cell polarity and gastrulation in C. elegans. Development 2002;129:387–397. [DOI] [PubMed] [Google Scholar]
- Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development 2011;138:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 2003;130:5339–5350. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol 2009;89:33–54. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev 2008;125:270–283. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 2009;16:398–410. [DOI] [PubMed] [Google Scholar]
- Parfitt DE, Zernicka-Goetz M. Epigenetic modification affecting expression of cell polarity and cell fate genes to regulate lineage specification in the early mouse embryo. Mol Biol Cell 2010;21:2649–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Rose LS. Dynamic localization of LIN-5 and GPR-1/2 to cortical force generation domains during spindle positioning. Dev Biol 2008;315:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken CM, Capco DG. The expression and stage-specific localization of protein kinase C isotypes during mouse preimplantation development. Dev Biol 2000;223:411–421. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol 2001;3:43–49. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K, Zernicka-Goetz M. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mech Dev 2005;122:487–500. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development 2005;132:479–490. [DOI] [PubMed] [Google Scholar]
- Plachta N, Bollenbach T, Pease S, Fraser SE, Pantazis P. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat Cell Biol 2011;13:117–123. [DOI] [PubMed] [Google Scholar]
- Plusa B, Grabarek JB, Piotrowska K, Glover DM, Zernicka-Goetz M. Site of the previous meiotic division defines cleavage orientation in the mouse embryo. Nat Cell Biol 2002;4:811–815. [DOI] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis A-K, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci 2005;118:505–515. [DOI] [PubMed] [Google Scholar]
- Prehoda KE. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect Biol 2009;1:a001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich A, Klatsky P, Carson S, Wessel G. The transcriptome of a human polar body accurately reflects its sibling oocyte. J Biol Chem 2011;286:40743–40749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Nieminen J, Dufort I, Gagné D, Grant JR, Cagnone G, Plourde D, Nivet AL, Fournier É, Paquet É et al. Combining resources to obtain a comprehensive survey of the bovine embryo transcriptome through deep sequencing and microarrays. Mol Reprod Dev 2011;78:651–664. [DOI] [PubMed] [Google Scholar]
- Roegiers F, McDougall A, Sardet C. The sperm entry point defines the orientation of the calcium-induced contraction wave that directs the first phase of cytoplasmic reorganization in the ascidian egg. Development 1995;121:3457–3466. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih H-P, Lee C-Y, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol 2003;163:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Gönczy P. Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook 2014;1–43. [DOI] [PubMed]
- Samarage CR, White MD, Álvarez YD, Fierro-González JC, Henon Y, Jesudason EC, Bissiere S, Fouras A, Plachta N. Cortical tension allocates the first inner cells of the mammalian embryo. Dev Cell 2015;34:435–447. [DOI] [PubMed] [Google Scholar]
- Sardet C, Paix A, Prodon F, Dru P, Chenevert J. From oocyte to 16-cell stage: cytoplasmic and cortical reorganizations that pattern the ascidian embryo. Dev Dyn 2007;236:1716–1731. [DOI] [PubMed] [Google Scholar]
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev 1999;13:2028–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz C, Stevens J, Spang A. Functions of the novel RhoGAP proteins RGA-3 and RGA-4 in the germ line and in the early embryo of C. elegans. Development 2007;134:3495–3505. [DOI] [PubMed] [Google Scholar]
- Schober M, Schaefer M, Knoblich JA. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature 1999;402:548–551. [DOI] [PubMed] [Google Scholar]
- Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development 2006;133:3507–3516. [DOI] [PubMed] [Google Scholar]
- Schonegg S, Constantinescu AT, Hoege C, Hyman AA. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. Proc Natl Acad Sci USA 2007;104:14976–14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC, Roberts RM. Dynamic changes in leptin distribution in the progression from ovum to blastocyst of the pre-implantation mouse embryo. Reproduction 2011;141:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus LR, Cha B-J, Theurkauf WE, Saxton WM. Dynein and the actin cytoskeleton control kinesin-driven cytoplasmic streaming in Drosophila oocytes. Development 2005;132:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Benton R, St Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 2000;101:377–388. [DOI] [PubMed] [Google Scholar]
- Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol 2009;11:365–374. [DOI] [PubMed] [Google Scholar]
- Simões S, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell 2010;19:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Pohl C. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev Cell 2014;28:253–267. [DOI] [PubMed] [Google Scholar]
- Skamagki M, Wicher KB, Jedrusik A, Ganguly S, Zernicka-Goetz M. Asymmetric localization of Cdx2 mRNA during the first cell-fate decision in early mouse development. Cell Rep 2013;3:442–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speksnijder JE, Sardet C, Jaffe LF. The activation wave of calcium in the ascidian egg and its role in ooplasmic segregation. J Cell Biol 1990;110:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan DG, Fisk RM, Xu H, van den Heuvel S. A complex of LIN-5 and GPR proteins regulates G protein signaling and spindle function in C elegans. Genes Dev 2003;17:1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RO, Yamanaka Y, Rossant J. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 2010;137:3383–3391. [DOI] [PubMed] [Google Scholar]
- Swann K, Windsor S, Campbell K, Elgmati K, Nomikos M, Zernicka-Goetz M, Amso N, Lai FA, Thomas A, Graham C. Phospholipase C-ζ-induced Ca2+ oscillations cause coincident cytoplasmic movements in human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril 2012;97:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 1998;125:3607–3614. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, Lao K, Surani MA. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc 2010;5:516–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian A-G, Deng W-M. Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development 2008;135:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, Ephrussi A. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat Cell Biol 2000;2:458–460. [DOI] [PubMed] [Google Scholar]
- Tong C, Fan HY, Chen DY, Song XF, Schatten H, Sun QY. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtubule organization, asymmetric division and metaphase II arrest. Cell Res 2003;13:375–383. [DOI] [PubMed] [Google Scholar]
- Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 2007;445:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M-FB, Hayashi A, Rose LS. LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development 2003;130:5717–5730. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Ephrussi A. The fusome and microtubules enrich Par-1 in the oocyte, where it effects polarization in conjunction with Par-3, BicD, Egl, and dynein. Curr Biol 2002;12:1524–1528. [DOI] [PubMed] [Google Scholar]
- Vassena R, Boué S, González-Roca E, Aran B, Auer H, Veiga A, Izpisua Belmonte JC. Waves of early transcriptional activation and pluripotency program initiation during human preimplantation development. Development 2011;138:3699–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. Asymmetric division in mouse oocytes: with or without Mos. Curr Biol 2000;10:1303–1306. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Gossler A, Boller K, Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev Biol 1987;124:451–456. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Maro B, Louvet-Vallée S. Two PAR6 proteins become asymmetrically localized during establishment of polarity in mouse oocytes. Curr Biol 2004;14:520–525. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Ohno S, Pawson T, Maro B, Louvet-Vallée S. Asymmetric distribution of PAR proteins in the mouse embryo begins at the 8-cell stage during compaction. Dev Biol 2005;282:307–319. [DOI] [PubMed] [Google Scholar]
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell 2004;6:133–144. [DOI] [PubMed] [Google Scholar]
- Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 2000;127:1467–1475. [DOI] [PubMed] [Google Scholar]
- Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell 2005;8:493–504. [DOI] [PubMed] [Google Scholar]
- Werts AD, Roh-Johnson M, Goldstein B. Dynamic localization of C. elegans TPR-GoLoco proteins mediates mitotic spindle orientation by extrinsic signaling. Development 2011;138:4411–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, Sasaki H, Ralston A. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet 2014;10:e1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Nusslein-Volhard C, Jurgens G. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster III. Zygotic loci on the X-chromosome and fourth chromosome. Roux's Arch Dev Biol 1984;193:296–307. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 1999;402:544–547. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol 2000;150:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development 2007;134:3827–3836. [DOI] [PubMed] [Google Scholar]
- Yang Z, Hata Y. What is the Hippo pathway? Is the Hippo pathway conserved in Caenorhabditis elegans. J Biochem 2013;154:207–209. [DOI] [PubMed] [Google Scholar]
- Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Luna Persson N, Shimono A, Speicher DW, Marmorstein R et al. A tight junction-associated Merlin-Angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 2011. a;19:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 2011. b;13:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L-Z, Xiong B, Gao W-X, Wang C-M, Zhong Z-S, Huo L-J, Wang Q, Hou Y, Liu K, Liu XJ et al. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle 2007;6:330–338. [DOI] [PubMed] [Google Scholar]
- Yuzawa S, Kamakura S, Iwakiri Y, Hayase J, Sumimoto H. Structural basis for interaction between the conserved cell polarity proteins Inscuteable and Leu-Gly-Asn repeat-enriched protein (LGN). Proc Natl Acad Sci USA 2011;108:19210–19215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell 2004;6:343–355. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M, Morris SA, Bruce AW. Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 2009;10:467–477. [DOI] [PubMed] [Google Scholar]
- Zhang H, Skop AR, White JG. Src and Wnt signaling regulate dynactin accumulation to the P2-EMS cell border in C. elegans embryos. J Cell Sci 2008;121:155–161. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zucchelli M, Bruce S, Hambiliki F, Stavreus-Evers A, Levkov L, Skottman H, Kerkela E, Kere J, Hovatta O. Transcriptome profiling of human pre-implantation development. PLoS One 2009;4:e7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wen W, Zheng Z, Shang Y, Wei Z, Xiao Z, Pan Z, Du Q, Wang W, Zhang M. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol Cell 2011;43:418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]