Abstract
Individuals differ in their awareness of afferent information from within their bodies, which is typically assessed by a heartbeat perception measure of ‘interoceptive accuracy’ (IAcc). Neural and behavioural correlates of this trait have been investigated, but a theoretical explanation has yet to be presented. Building on recent models that describe interoception within the free energy/predictive coding framework, this paper applies similar principles to IAcc, proposing that individual differences in IAcc depend on ‘precision’ in interoceptive systems, i.e. the relative weight accorded to ‘prior’ representations and ‘prediction errors’ (that part of incoming interoceptive sensation not accounted for by priors), at various levels within the cortical hierarchy and between modalities. Attention has the effect of optimizing precision both within and between sensory modalities. Our central assumption is that people with high IAcc are able, with attention, to prioritize interoception over other sensory modalities and can thus adjust the relative precision of their interoceptive priors and prediction errors, where appropriate, given their personal history. This characterization explains key findings within the interoception literature; links results previously seen as unrelated or contradictory; and may have important implications for understanding cognitive, behavioural and psychopathological consequences of both high and low interoceptive awareness.
This article is part of the themed issue ‘Interoception beyond homeostasis: affect, cognition and mental health’.
Keywords: interoception, interoceptive accuracy, heartbeat perception, free energy, predictive coding
1. Introduction
The free energy principle proposes that living systems are driven to minimize the sum of differences between the sensory sensations they encounter and the sensory inputs predicted by internal models of the world [1]. Perception, action, attention and learning have all been described within this account [1]. It is timely that interoception—defined as afferent information arising from within the body [2]—has recently been placed at the heart of free energy minimization, with the recognition that interoceptive signals provide the organism with the vital maps of its internal states that underpin homeostasis [3–7]. However, recent theoretical models [3,4] have typically not discussed one of the most prominent topics within the interoception literature—namely the considerable variability that individuals display in their ability to call interoceptive signals into awareness and the influence that this variability has on behaviour. The purpose of this paper is to apply the free energy framework to explain ‘interoceptive accuracy’ (IAcc) which is assumed to reflect trait awareness of interoceptive sensations.
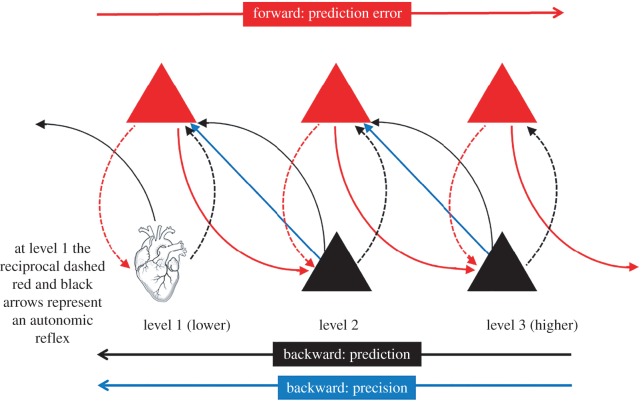
The free energy framework is operationalized under the principles of predictive coding and Bayesian inference [5]. It is assumed that the brain builds internal ‘generative models’, within which ‘prior’ predictions/beliefs about what accounts for the incoming sensory data are updated by ‘prediction errors’ (PEs), which are that part of the data that is not compatible with the prior. These probabilistic predictions are passed, top-down, through hierarchical brain pathways, whereas PEs are passed, bottom-up, for resolution at a higher level, such that the ‘posterior’ prediction at any one level (after updating to accommodate PEs) becomes the prior for the level below (figure 1) [1]. Technically, these priors are known as empirical priors. Empirical priors are posterior beliefs that arise within the hierarchies of the (sensory) data and are therefore prior beliefs that are informed by sensory evidence. For the sake of simplicity, we refer to these as priors. The interoceptive hierarchy in the brain has been described, extending from spinal visceral afferents to subcortical structures and projecting to the amygdala, insula, anterior cingulate and orbitofrontal cortices [7,8]. Although interoceptive predictions and prediction errors must be reconciled at each level of this hierarchy, the insula is assumed to be the principal cortical region in the interoceptive pathway, being activated by all interoceptive and affective stimuli ([9], but see also [7]). Diffusion tensor and functional imaging data indicate that the anterior insula is a hub between brain networks involved in externally directed attention to stimuli in the environment and internally directed attention to one's body [10]. Thus, it is potentially the key region that mediates variability in the influence of interoceptive signals on behaviour [11], which is the trait that IAcc seeks to capture.
Figure 1.
Schematic overview of the hierarchical message passing in the brain that is assumed to underlie predictive coding. Predictions (priors) are illustrated as black lines that project down the levels of the hierarchy, from prediction units (deep pyramidal cells) shown as black triangles. Forward projecting prediction errors are represented by red lines, passing up the hierarchy from prediction error units (superficial pyramidal cells), which are represented by red triangles. Importantly, PEs and predictions occur at every level. The dashed red and black arrows indicate local processing within a level. At level 1, they thus represent an autonomic reflex. Precision, which plays the crucial role of determining the relative weight of the PEs versus the priors, at every level of the hierarchy, passes down the hierarchy and is indicated by the blue arrows. A percept is formed when PE is minimized at all levels within the hierarchy. Adapted from Edwards et al. [6].
Much of interoceptive signalling supports homeostasis without awareness but people are also capable of being aware of interoceptive sensations—either through top-down-directed attention, as in a heartbeat counting task, or as a result of bottom-up salience, such as when perceiving the racing heart that accompanies arousal. Psychological research into interoceptive awareness has focused mainly on objective measures of the accuracy with which we become aware of our heartbeats because of the known role that heart–brain interactions (and concomitant balance between the sympathetic and parasympathetic systems) plays in emotion processing [8]. IAcc is generally measured by one of two types of heartbeat perception tasks. IAcc is assumed to reflect the individual's trait awareness of, and tendency to be influenced by, their interoceptive sensations. Of the two principal heartbeat perception tasks, ‘mental tracking’ involves counting one's heartbeat [12], whereas ‘heartbeat discrimination’ requires judging whether an external signal is synchronous with one's heartbeat [13]. Although it has been suggested that heartbeat counting tasks are confounded by the use of particular strategies and do not reflect awareness of the heartbeat per se [14], there is an extensive research literature linking both types of heartbeat perception measures to a variety of behavioural outcomes [15,16]. This suggests that IAcc does reflect trait awareness of interoceptive sensation and consequent behaviour. Moreover, scores on the two types of heartbeat perception test correlate in individuals with above average IAcc [17], and both measures are related to awareness of gastric cues [18,19]. Except where otherwise stated, this paper cites studies that have measured IAcc using heartbeat counting. The purpose of our model is to contribute to the understanding of mechanisms that potentially underlie variability in IAcc, which may, in turn, clarify its behavioural effects. Here we present a model of IAcc according to the free energy framework, in order to provide a better understanding of the mechanisms that underlie variability in IAcc and, in turn, clarify its behavioural effects. We first outline how the free energy and predictive coding principles provide an account of interoceptive signalling, followed by a discussion of how this can be applied to IAcc. We subsequently link this account with other variables and with behaviour. Finally, we indicate how this model may provide a novel perspective on mental health problems in which IAcc is putatively an underlying cause.
2. ‘Perceptual inference’, ‘precision’ and ‘active inference’ within predictive coding
IAcc depends on forming percepts for heartbeats, although awareness may be at the very borders of conscious perception [20]. Within predictive coding it is assumed that perception is achieved by ‘perceptual inference’, which requires minimization of free energy (equivalent to the sum total of PEs) at every level in the hierarchy, so that the sensory data has been accounted for as fully as possible and a precept is formed [1]. This process applies equally well to interoceptive percepts (by ‘interoceptive inference’) [20] and to percepts that do not reach conscious awareness [21].
Within predictive coding, empirical priors, predictions and associated PEs are all represented in terms of expectations and precisions. (Expectations and precisions correspond to first- and second-order moments of the probabilistic beliefs.) ‘Precision’ refers to the inverse variance associated with each probability distribution and is thus a measure of their relative salience and reliability [1]. Precision operates both within and between modalities. Within any modality, at each level of the hierarchy and taking account of the given context, the brain weighs the relative precision of PEs that inform or revise expectations at higher levels of the hierarchy [21,22].
Figure 2 illustrates this relationship schematically. If PEs are precise relative to a prior (as in figure 2a), this implies that they carry more reliable information than the prior and the likely effect is that they will update the prior, i.e. that the posterior will shift in the direction of the PEs, with increased precision. For example, jumping into a swimming pool on a hot day produces precise PEs that will update the priors for body temperature. The (updated) posterior at any given level becomes a prior for the level below in the hierarchy. A relatively precise prior, by contrast may be impervious to imprecise PEs from the level below. Examples are various visual illusions that depend on precise (overlearned) priors that do not update to incoming sensory data [24]. This is represented by figure 2b.
Figure 2.
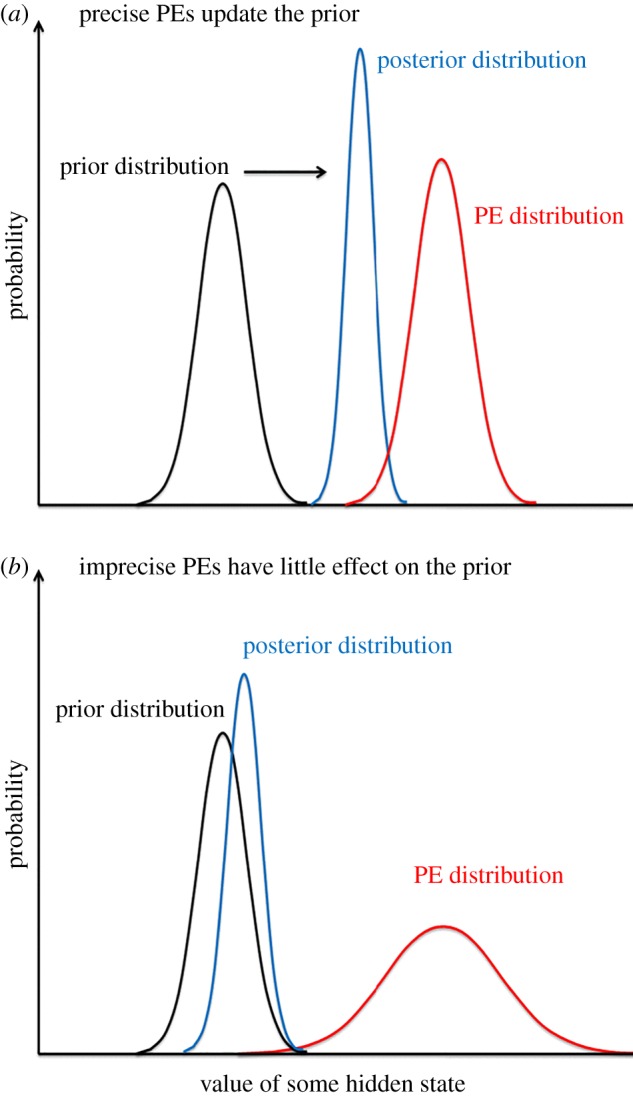
The graphs show Gaussian probability distributions representing, at one particular level in the hierarchy, the descending prior and posterior and the ascending PEs (which arises from the incoming sensory data). These distributions refer to some hidden state of the organism (e.g. some aspect of its interoceptive state) that has to be inferred. The widths of the various distributions correspond to their variance. Precision is the inverse of variance. The relative precision of PEs and prior is crucial in determining the updating of the prior to the posterior. (a) A context in which the precision of the PEs is precise (relative to the prior), so that the posterior is shifted towards the PEs (arrow). (b) By contrast, imprecise PEs have little impact on the prior. The posterior then descends to the level below this in the hierarchy where it becomes, in turn, the empirical prior. Adapted from Adams et al. [23].
Given that precision is always relative and that precepts are usually multimodal, precision plays a similarly crucial role in weighting the available information that arises from various modalities and converges on multimodal association areas in the sensory hierarchy. For example, at night when the precision of vision declines, the relative precision of PEs in other modalities rises, which accounts for our tendency to rely on touch and audition in the dark. The relative precision of interoceptive signals may also increase, which one author has suggested might explain humanity's common fear of ‘bogeymen’ [25]. Importantly, for our model, the relative precision of PEs and priors within and between modalities is constantly being updated [26].
Precise PEs can also lead to ‘active inference’ whereby the organism moves in order to acquire more sensory information with which to confirm or update its priors. It does this by forming a prediction of the proprioceptive consequences of the intended/desired movement. This prediction gives rise to precise proprioceptive PEs that descend through the hierarchy providing motor control, fulfilled at the lowest level by peripheral motor reflexes [1]. Mechanisms equivalent to active inference exist in interoception [7,20]. If there are deviations from the desired (prior) inner state of the body (e.g. there is a fall in body temperature because one jumps into a swimming pool) then the consequent interoceptive PEs may be resolved by updating interoceptive priors (the water soon feels less cold). However, interoceptive PEs can also resolve themselves by engaging peripheral autonomic reflexes (e.g. closing capillaries) in the same way that precise prediction errors enslave classical motor reflex arcs to elicit movement [3,27]. In other words, interoceptive and proprioceptive prediction errors can either ascend into the brain to revise prior beliefs or descend to the periphery to make those prior beliefs come true by engaging reflexes. The relative precision of ascending and descending prediction errors therefore determines whether reflexes are engaged. If interoceptive prediction errors are sufficiently precise they may be resolved through motor action [27] (such as moving to a warmer place) or directly (e.g. by shivering). Thus, perception, action and autonomic response are united within one powerful overarching framework [28].
3. A model of heartbeat perception with a predictive coding framework
Healthy people do not generally perceive their heartbeats in day-to-day experience, despite the strength and pervasiveness of the signal [2]. This potentially surprising phenomenon can readily be explained within predictive coding. When a stimulus is fully predicted, the prior will match the incoming sensory data, there will be no PEs, no updating of priors and consequently no percept. The strength, rhythm and variability of one's heartbeat are constantly present. This implies that the heartbeat is fully predicted by the brain in contexts that have been regularly experienced. Contexts that elicit unexpected changes in heart functioning, on the other hand, may require a response from the organism and are likely to reach awareness. It follows that during a heartbeat perception test, whenever an individual is temporarily able to perceive a heartbeat, sensory information about the heartbeat is not, in that particular moment, being fully predicted by one's priors. The reason for this must lie in the nature of the task, which requires focused top-down attention to the heartbeat, whereas other variables that might affect the heartbeat itself (such as arousal) are held constant.
Crucially for our model, the effect of attention within predictive coding is to optimize the precision of sensory signals, by assigning the best possible relative precision (for that particular individual) between modalities and also between priors and PEs within modalities [29]. It is important to note that the role of attention in the free energy framework is not to promote salience but to optimize precision, i.e. to regulate whether, at a given moment, in any given context or modality, PEs or priors have more weight in determining the percept. Attention optimizes precision by continually fine-tuning the precisions of all priors and all PEs, both in the very short-term as well as over longer time frames. The priors themselves will tend to become more precise, through the updating involved in learning, but this is always subject to change if precise contradictory information (PEs) emerges. Thus, attention does not so much promote salience as optimize salience. This optimization of precision therefore serves the overall goal of reducing PEs and free energy over time [4].
It follows that the ability of individuals to be aware of their heartbeats must depend on their ability to enhance the precision of their interoceptive signals by attending to them. The ability to increase precision in interoceptive systems will be dictated at a higher level in the brain hierarchy (figure 1), where a further prior (not necessarily conscious), about the importance of interoceptive sensation relative to other modalities, will govern the overall precision of interoceptive information. Thus, if the individual uses attention to increase the relative precision of interoception as a modality, then this will have the effect of raising the precision of PEs versus priors within interoceptive systems.
A much-discussed issue within the interoception literature is the extent to which objective measures of IAcc measure the tendency to be influenced by interoceptive signals [17]. Our model assumes that if an individual is able to perceive heartbeats by directed top-down attention during a heartbeat perception task, then the same optimization principles are more likely to apply in daily life, as regards both top-down and bottom-up attention to interoceptive sensations. We assume that the interoceptive experience of people with high IAcc is therefore characterized by the continuous, Bayes optimal, updating of interoceptive priors, at the borders of conscious awareness, which can account for the behaviour associated with trait interoceptive awareness, as discussed below.
We propose that people with lower IAcc, by contrast, are those who are unable to enhance the precision of their interoceptive signals by focused attention during heartbeat perception tasks. This implies that interoception is, for them, a sensory modality with less habitual salience, leading to less frequent updating of priors and hence less flexible adjusting of precision throughout the interoceptive hierarchy. As we review below, this may make them more liable to illusory percepts and/or aberrant beliefs (priors) [30]. It is consistent with our account that although people with low IAcc cannot easily increase the precision of heartbeat with endogenous attention, when their attention to interoception is driven by external stress, for example during physical exercise or emotional arousal, the effect is generally to raise IAcc, regardless of the person's baseline IAcc [2,15].
Our model assumes that during a heartbeat perception task the increased precision of PEs (relative to priors for the heartbeat) causes these PEs to be projected up through the cortical hierarchy where, at some level, the heartbeat can be detected, potentially in the anterior insula [9]. Our model is therefore consistent with the greater cortical activity in the anterior insula that has been observed during both types of heartbeat perception tasks in better (compared with less good) heartbeat perceivers [12,13], as people with higher IAcc experience greater updating of their interoceptive priors—thus over time being able to adjust more easily to any changes to habitual heart parameters. By contrast, we assume that people with lower IAcc are not able to adjust the precision of their interoceptive signals with attention and are thus not as good at perceiving their own heartbeats, at will, during IAcc tests.
Such mechanisms would explain why, during tests of interoception, ‘the threshold level of consciousness reportability constantly fluctuates' [2, p. 81]. Analogously to the process of binocular rivalry [31], we suggest that, as the prediction updates, the heartbeat is temporarily available to awareness until it is once again fully predicted and becomes unavailable to perception, before attention starts the cycle of updating again.
4. Influences on precision
Why the precision accorded to interoceptive signals might differ between individuals has not yet been fully elucidated. Precision depends on the post-synaptic gain of superficial pyramidal cells (the cells that signal PEs) [32]. Acetylcholine and dopamine are thought to determine precision in perception and action, respectively [1], and oxytocin may perform this function in interoception [32]. IAcc, which our model assumes depends on the precision of interoceptive signals, correlates with concentrations of both GABA [33] and glutamate in the insula [34]. Crucially, however, precision is refined by learning [29,35]. In order to minimize free energy (and thus PEs), the brain must continually optimize the relative precisions of PEs and priors, over time and across all sensory modalities and contexts, for the particular individual [1,4]. Our model implies that in people with higher IAcc this optimization involves the prioritizing of interoceptive sensations such that they can be called into awareness, with attention. Potentially high IAcc may, at least in part, result from learned attention to internal bodily changes (interoceptive PEs), relative to other sensory modalities, presumably owing to various neurophysiological and psychosocial parameters in development and during the lifespan.
5. Application of the model to prominent aspects of the literature on interoceptive accuracy
(a). Heartbeat-evoked potentials
Heartbeat perception tasks tap a continuum between day-to-day pre-consciousness and conscious awareness of the heartbeat under focused attention. This suggests that IAcc will be related to the amplitude of heartbeat-evoked potentials (HEPs), which are characteristic waves of cortical activity that accompany the rhythmic activity of the heart, whether or not the heartbeat is consciously perceived. HEPs can be observed with electroencephalography (EEG) as a positive potential shift over right frontocentral electrodes, 200–600 ms after the R-wave of the heartbeat [36]. They have been source localized to the insula and anterior cingulate cortex [37] and are considered to be an index of cortical interoceptive processing, for example being modulated by affective tasks [38,39]. As our model would expect, high IAcc is associated with greater amplitude of HEPs [36]. Moreover, when that amplitude is enhanced by attention, this effect is stronger in people with higher IAcc [40]. It is generally thought that PEs are encoded by superficial pyramidal cells that are the major contributor to neurophysiological responses recorded empirically. This is potentially important, because the amplitude of evoked responses will therefore reflect their precision and the degree to which PEs are afforded more weight or confidence.
(b). Attention to interoception
Several studies have used heartbeat counting to experimentally enhance attention to interoception. For example, a preliminary period of attention to heartbeats enhances blood-oxygen-level dependent (BOLD) activity in the anterior insula during later judgements about emotional faces [41]. Our model assumes that attention increases the precision of PEs associated with the heartbeat, which are then cascaded up through the hierarchy causing activity visible in the anterior insula under fMRI [42]. These results potentially imply that enhanced precision persists for a short period, i.e. that the precision of the prior is down-weighted in this instance for an extended period of time. Interestingly, while people with high IAcc show an increase in BOLD activity in the anterior insula during heartbeat counting, functional connectivity analysis has revealed that, in good heartbeat perceivers only, attention to heartbeats also decreases connectivity between lower and higher levels of the interoceptive hierarchy from the right posterior to the right anterior insula [43]. The authors of this study suggest that ‘an increase in salience may be achieved by decreasing the amount of noise that is transported along this axis’ [43, p. 12]. Our model would suggest that as attention increases the salience of the heartbeat, in people with high IAcc, it diminishes the relative precision of other interoceptive signals within the insula, thus ‘decreasing the amount of noise’. As attention increases the salience of the heartbeat in people with high IAcc, it increases the precision of ascending interoceptive PEs. This would correspond to an increased sensitivity to ascending PEs and high gain on autonomic reflexes. It is important to note that there is no neuronal ‘noise’ in predictive coding; the noise is actually estimated as part of the inference and encoded in terms of expected precision, by synaptic gain.
People with high IAcc (measured by heartbeat discrimination) perform above chance on tests of masked fear conditioning [44]. Our interpretation is that these individuals experience precise PEs associated with the fear-provoking stimuli, so that the interoceptive changes that occur when they orient to the fear cues are likely to update their priors for the heartbeat and facilitate the detection of the fear-provoking trials. Assuming that attention to heartbeat enhances the precision of PEs arising from the heart [30], we would expect masked fear conditioning to be stronger after practice on a heartbeat discrimination task, which has also been reported [45].
(c). Autonomic reactivity
As explained above, our model implies that precise interoceptive PEs can either ascend into the brain to revise prior beliefs (and thus give rise to emotion, as discussed below) or they may descend to the periphery to make those prior beliefs come true by engaging autonomic reflexes. A major implication of our model is therefore that individuals with high IAcc, who experience more updating of interoceptive priors by PEs, will also experience greater autonomic reactivity to emotional stimuli, whenever the effect on the heartbeat of those stimuli is not fully predicted so that they give rise to interoceptive PEs. These PEs will pass down through the cortical levels with the potential to be ultimately resolved by interoceptive active inference in the form of autonomic reflexes [3–5]. In support of this interpretation, a number of studies have demonstrated greater autonomic reactivity in individuals with higher IAcc. They show (i) greater amplitude of respiratory sinus arrhythmia in response to a hand encroaching into peripersonal space [46]; (ii) greater heart rate deceleration when viewing emotional stimuli [47]; and (iii) greater amplitudes of the P300 and slow wave under EEG in response to emotionally arousing pictures [48] (where the P300 is thought to indicate the updating of representations of the current environment). All of these results can be explained if precise interoceptive PEs boost interoceptive processing in people with higher IAcc. Conversely, (iv) individuals with higher IAcc are able to use appraisal to reduce the amplitude of their P300 response to affective stimuli [49], which we would interpret in terms of their precise PEs enabling them to more readily update interoceptive priors associated with these emotional stimuli.
(d). Emotion
The seminal model that first placed interoception within the free energy and predictive coding framework, proposes that emotion results from the brain's interpretation of interoceptive precepts (by ‘interoceptive inference’) [20]. A related definition accounts for emotional valence by suggesting that emotion is the result of changes in free energy, with falling free energy producing positive emotion and vice versa [50]. Our model complements these formulations and extends the latter by suggesting that emotional arousal is dependent on interoceptive precision. A fundamental assumption of our model is that the interoceptive PEs of people with higher IAcc may be cascaded up the interoceptive hierarchy, rather than being suppressed by low-level priors. Given that these interoceptive PEs indicate changes in free energy, we conclude that they will consequently give rise to feelings of generalized physiological arousal and ultimately to specific learned emotions [50]. As a result, we expect people with higher IAcc to report stronger emotional arousal for identical objective changes in physiological arousal. This has been reported in a range of studies, using both types of heartbeat perception task [13,47,48]. Assuming that the interoceptive changes associated with any memory is greater for people with high IAcc, similar mechanisms would account for their enhanced capacity to remember stimuli that alter interoceptive signals, such as heart rate [51].
Our account can also explain why people with higher IAcc are more averse to making errors, given the assumption that the affective significance of making a mistake is recorded as interoceptive PE. For example, IAcc correlates with post-error slowing on the Simon task and with the amplitude of the error-positivity component shown by EEG [52]. This aversion may, in turn, explain why people with high IAcc have greater difficulty inhibiting the tendency to imitate observed task-irrelevant actions [53], presumably the affective significance of the near-errors involved are stronger for them and thus tend to slow their reaction times. Furthermore, a failure to attenuate sensory precision (the context of sensory attenuation) may also result in autonomic forms of echopraxia and emotional contagion [54].
(e). Enhanced self-focus
Attending to self-relevant information temporarily enhances IAcc, but only in those people for whom IAcc is originally low [55]. Our model proposes that such people have difficulty enhancing precision in interoceptive systems by attending to interoceptive cues per se. However, we assume that the self is a multilevel, multimodal construct, continually updated in the brain from all available interacting cues including interoception [3,35]. Precision necessarily varies along this hierarchy [6,24]. If self-focus enhances the precision of a high-level (conscious) prior for the multimodal self, this will affect the precision of priors and PEs at lower levels of the self-hierarchy (including those for the heartbeat itself). In people with high IAcc, this would be unlikely to have any additional effect on heartbeat perception. However, for people with low IAcc, the effect could be to enhance the precision of all self-relevant and self-specifying signals, including interoceptive PEs, thus enabling updating of priors in interoceptive systems and consequent perception of heartbeats.
(f). Body ownership
Individuals with high IAcc are less susceptible to illusory body ownership [16]. In the rubber hand illusion, the participant's hidden hand is stroked synchronously with a fake hand, onto which visual attention is focused. To experience the illusion, participants must form the percept that the prosthetic hand is their own, by minimizing PEs across all available sensory modalities according to their relative precision [35]. The final (illusory) precept depends on the normally high precision of visual and somatosensory PEs (enhanced by attention). However, neither vision nor touch is self-specific. Interoceptive cues, by contrast, provide uniquely self-specifying sensory input. Their importance is indicated by the way the immune system starts to disown the real hand as the illusion takes hold [56]. We suggest that people with high IAcc resist the illusion because they are able to attend to, and thus enhance the precision of, their interoceptive cues during multisensory integration. The fake hand does not have the interoceptive feelings (priors) attached to the true hand. In people with high IAcc, this will set up interoceptive PEs which will serve to update these priors and give rise to interoceptive percepts for the true hand, thus anchoring the sense of body ownership.
A contrasting paradigm dispenses with a prosthetic hand by filming the subject's true hand and replaying this to them, in real time [57]. An ‘interoceptive rubber hand illusion’ is achieved by causing the virtual hand to flush in synchrony with the participant's heartbeat. In this paradigm, it is now the people with high IAcc who experience the greater illusion. This illustrates the crucial effect of context, whereby the interoceptive priors now indicate that the virtual hand is one's own. People with high IAcc (measured by heartbeat discrimination), whom our model assumes are able to raise the precision of interoceptive cues by attention, are now more likely to claim ownership of this virtual hand [57].
(g). Neuroeconomic decision-making and motivation
People vary considerably in their decisions about whether to take risks and also about whether to exert effort [58,59]. Potentially, decision-making and bodily signals are linked and this is reflected in information processing in the insula. It has been shown, for example, that the insula is activated both by predictions (priors) about the risk involved in any decision and also by risk PEs that update these priors [60]. Signals in the insula are seen to gradually increase during both effortful exertion and during subsequent rests [61], suggesting that the insula is encoding changes in bodily state—perhaps reflecting the precision of PEs, which may continuously rise until a threshold is reached that updates the prior and triggers a change in behaviour. Thus, variability in behaviour and insula activity during neuroeconomic decision-making tasks may potentially reflect individual differences in IAcc and thus the influence of interoception on behaviour.
Evidence in support of this is that individuals with higher IAcc work less hard during self-paced exercise [62]. Likewise, for identical objective changes in bodily signals, the choices they make when evaluating risks tend to reflect their bodily changes [15]. Our model explains this in terms of changes in the state of the body, including heart rate and cardiac output, that result from risky behaviours and physical exertion [2]. Assuming that these interoceptive changes have the effect of increasing the precision of PEs relative to priors, our model would predict that when the individual must make a decision the more accumulation there is of precise PEs the greater change there will be in the ‘value’ associated with any given choice. People with high IAcc who (in contrast to those with low IAcc) can raise the precision of their PEs with attention, will more readily accumulate sufficient precision in PEs to update their priors and thus affect their choice behaviour. Thus, we would expect to see greater influence of interoceptive PEs on behaviour in people with higher IAcc and that also such individuals would be less willing to expend physical effort, as borne out by the empirical evidence [15,62].
6. Interoceptive accuracy in clinical disorders
High IAcc is common in panic disorder and anxiety [63]. Conversely, inaccuracy in heartbeat counting has been linked to alexithymia, eating disorders, depression, functional disorders and depersonalization/derealization (see [64] for a review and also [65]). Our model suggests that an individual's ability (IAcc) and tendency (trait interoceptive awareness) to use focused attention to adjust precision in interoceptive systems potentially plays a role in the aetiology of these disorders and may be relevant to their remediation.
The interoceptive priors of healthy people update over time, as the brain seeks to optimize precision [4]. However, what is Bayes optimal for a given individual may give rise to aberrant behaviour if generative models include highly precise priors, at some level of the hierarchy, that are unable to update appropriately to incoming sensory signals [26]. A number of clinical disorders have been characterized in this fashion including schizophrenia [22], somatization [6], depression [7] and autism [32].
Attention and learning play a crucial role in assigning and optimizing precision. Our model proposes that people with high IAcc can increase precision in interoceptive systems with attention because they have higher-level (unconscious) prior beliefs that prioritise interoception and hence allow them to increase precision in interoceptive systems generally and hence raise the precision of interoceptive PEs relative to priors [64]. In some (but not necessarily all such people), this may reflect habits of attention to their internal bodily changes. This could explain why certain individuals are more vulnerable to some disorders but less prone to others. For example, alexithymia, a condition characterized by difficulties in identifying and describing emotion, may be accompanied by low IAcc [66]. Our model implies that sufferers may have highly precise interoceptive priors that do not update appropriately to interoceptive PEs, making it difficult for them to gain awareness of the interoceptive changes that signal affect.
Habits of excessive attention to harmless bodily cues have, however, been proposed as the basis of both panic disorder, which has been linked to higher IAcc [67], and functional disorders, which are associated with lower IAcc [68]. It has accordingly been argued that disorders associated with IAcc may depend fundamentally on cognitive interpretation of the relevance of these sensations, rather than on the availability of the interoceptive sensations per se [69]. These interpretations take the form of stable, precise, high-level cognitive priors (beliefs) that do not update appropriately with learning but instead inappropriately bias attention, resulting in interoceptive generative models in which precise priors, at some level, fail to update. High-level beliefs about future threat to the self, for example, may underpin all anxiety disorders [69], which are more common amongst people with high IAcc [63]. Sufferers (e.g. phobics) typically avoid the anxiety-provoking stimuli, which suggest that their precise but inaccurate beliefs are maintained by avoidance of disconfirming evidence. Our model adds to this explanation by proposing that because people with high IAcc are able to direct their attention to interoceptive cues their internal bodily changes are more likely to reach awareness, predisposing them to anxiety by enhancing the perception of threat. However, although people with high IAcc have the ability to be aware of interoceptive cues under focused attention, this does not necessarily imply that all such individuals habitually misinterpret the significance of such sensation or suffer from anxiety disorders.
There is currently much research interest in therapeutic interventions based on enhanced body awareness that typically ask patients to practice attending to interoceptive sensations [64]. Our model implies that consideration should crucially be paid to whether the patient's IAcc is high or low. For example, the many people with panic disorder who have high IAcc [67] may benefit from paying less attention to the body and more to reducing the precision of high-level beliefs about the danger of real but harmless interoceptive sensations, which other individuals with high IAcc recognize as normal for themselves [64]. However, if an individual with panic disorder has low IAcc [67], their interoceptive sensations are likely to be illusory and for them it may consequently be therapeutic to find ways to improve their ability to adjust precision in low-level interoceptive systems. Likewise, functional disorders are assumed to involve an over-precise prior at some undetermined level of the hierarchy [6]. We suggest that where such patients have low IAcc this potentially indicates that the fault lies with highly precise (but inaccurate) precision in low-level interoceptive sensation, whereas high IAcc would imply that a precise high-level belief may be the cause.
7. Future directions
Our characterization of IAcc in terms of precision in interoceptive systems raises a number of potential research questions. If the precision of interoception PEs can be experimentally enhanced (e.g. by attention to interoception), then we predict that this will result in diminished experience of body illusions. Conversely, if synchronous multisensory stimulation raises the precision of all incoming self-relevant sensory data, changes in body-ownership while experiencing the rubber hand illusion should result in increases in IAcc. Potentially, autonomic reflexes (observable under ECG) are engaged by people with higher IAcc while they resist the rubber hand illusion, as they update interoceptive priors that anchor them to the true hand. Given the involvement of the anterior insula in IAcc and the mid-posterior insula in body-ownership, our model predicts that fMRI during the rubber hand illusion will reveal changes in functional connectivity within the insula during synchronous versus asynchronous visuotactile stimulation and that this will be modulated by the precision of PEs, i.e. by IAcc. We predict that other processes dependent on sensorimotor integration, such as feelings of agency, will be modulated by IAcc. Actions not only produce exteroceptive effects in the world, but they also have crucial interoceptive consequences that support homeostasis. The amplitude of the HEP may also be used to probe interoceptive precision, for example, we expect that this will be modulated by attention to exteroceptive self-relevant cues. Finally, we propose that therapeutic interventions in conditions such as anxiety, somatization and alexithymia will be more effective when tailored to the patient's IAcc.
8. Conclusion
Predictive coding accounts of interoceptive processing have recently been proposed to account for phenomenal consciousness [20] and mental illness [7]. We go beyond these models to propose a novel predictive coding account of interoceptive awareness whereby individual differences can be explained in terms of variations in the ‘precision’ with which interoceptive signals from within the body are represented. Our model characterizes individual differences in ‘IAcc’ (as measured by heartbeat perception) by hypothesizing that higher (versus lower) IAcc arises when the individual is able to use attention to call interoceptive sensation into awareness when needed. This implies the presence of a prior at a higher level that can, when appropriate, prioritize interoceptive sensation over other sensory modalities. The established but sometimes contradictory literature linking IAcc with such variables as autonomic reactivity, emotional experience and body ownership can be readily explained within our model, which may also have implications for clinical conditions associated with both high and low IAcc.
Acknowledgements
M.A.J.A. is supported by an Anniversary Future Leader fellowship from the Biotechnology and Biological Sciences Research Council (BB/M013596/1). A.F. is supported by a European Research Council Starting Investigator Award (ERC-2012-STG GA313755). M.T. is supported by a European Research Council Starting Investigator grant no. (ERC-2010-StG-262853). We thank two anonymous reviewers for their generous contributions to the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The preparation of this paper was supported by ERC-2010-STG-262853 to M.T.
References
- 1.Friston K. 2009. The free-energy principle: a rough guide to the brain? Trends Cogn. Sci. 13, 293–301. ( 10.1016/j.tics.2009.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Ádám G. 2010. Visceral perception. New York, NY: Plenum Press. [Google Scholar]
- 3.Seth AK. 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573. ( 10.1016/j.tics.2013.09.007) [DOI] [PubMed] [Google Scholar]
- 4.Fotopoulou A. 2013. Beyond the reward principle: consciousness as precision seeking. Neuropsychoanalysis 15, 33–38. ( 10.1080/15294145.2013.10773715) [DOI] [Google Scholar]
- 5.Rao RPN, Ballard DH. 1999. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87. ( 10.1038/4580) [DOI] [PubMed] [Google Scholar]
- 6.Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ. 2012. A Bayesian account of ‘hysteria’. Brain 135, 3495–3512. ( 10.1093/brain/aws129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett LF, Simmons WK. 2015. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429. ( 10.1038/nrn3950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critchley HD, Harrison N. 2013. Visceral influences on brain and behavior. Neuron 77, 624–638. ( 10.1016/j.neuron.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 9.Craig AD. 2009. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. ( 10.1007/s00429-010-0262-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farb N, Segal Z, Anderson A. 2012. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex 23, 114–126. ( 10.1093/cercor/bhr385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollatos O, Gramann K, Schandry R. 2007. Neural systems connecting interoceptive awareness and feelings. Hum. Brain Mapp. 28, 9–18. ( 10.1002/hbm.20258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 14.Ring C, Brener J, Knapp K, Mailloux J. 2015. Effects of heartbeat feedback on beliefs about heart rate and heartbeat counting: a cautionary tale about interoceptive awareness. Biol. Psychol. 104, 193–198. ( 10.1016/j.biopsycho.2014.12.010) [DOI] [PubMed] [Google Scholar]
- 15.Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T. 2010. Listening to your heart: how interoception shapes emotion experience and intuitive decision making. Psychol. Sci. 21, 1835–1844. ( 10.1177/0956797610389191) [DOI] [PubMed] [Google Scholar]
- 16.Tsakiris M, Tajadura-Jiménez A, Costantini M. 2011. Just a heartbeat away from one's body: interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B 278, 2470–2476. ( 10.1098/rspb.2010.2547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. 2015. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74. ( 10.1016/j.biopsycho.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Drescher VM. 1980. Perception of gastric contractions and self-control of gastric motility. Psychophysiology 17, 552–558. ( 10.1111/j.1469-8986.1980.tb02296.x) [DOI] [PubMed] [Google Scholar]
- 19.Herbert BM, Muth ER, Pollatos O, Herbert C. 2012. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE 7, e36646 ( 10.1371/journal.pone.0036646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seth AK, Suzuki K, Critchley HD. 2012. An interoceptive predictive coding model of conscious presence. Front. Psychol. 3, 1–16. ( 10.3389/fpsyg.2011.00395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohwy J. 2012. Attention and conscious perception in the hypothesis testing brain. Front. Psychol. 3, 96 ( 10.3389/fpsyg.2012.00096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown H, Adams RA, Parees I, Edwards M, Friston K. 2013. Active inference, sensory attenuation and illusions. Cogn. Process 14, 411–427. ( 10.1007/s10339-013-0571-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams RA, Brown H, Friston K. 2015. Bayesian inference, predictive coding and delusions. Avant V, 51–88. [Google Scholar]
- 24.Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. 2013. The computational anatomy of psychosis. Front. Psychiatry 4, 47 ( 10.3389/fpsyt.2013.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezzulo G. 2014. Why do you fear the bogeyman? An embodied predictive coding model of perceptual inference. Cogn. Affect. Behav. Neurosci. 14, 902–911. ( 10.3758/s13415-013-0227-x) [DOI] [PubMed] [Google Scholar]
- 26.Schwartenbeck P, FitzGerald THB, Mathys C, Dolan R, Wurst F, Kronbichler M, Friston K. 2015. Optimal inference with suboptimal models: addiction and active Bayesian inference. Med. Hypotheses 84, 109–117. ( 10.1016/j.mehy.2014.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu X, Fitzgerald THB. 2014. Interoceptive inference: homeostasis and decision-making. Trends Cogn. Sci. 18, 269–270. ( 10.1016/j.tics.2014.02.001) [DOI] [PubMed] [Google Scholar]
- 28.Pezzulo G, Rigoli F, Friston K. 2015. Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 134, 17–35. ( 10.1016/j.pneurobio.2015.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman H, Friston K. 2010. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 4, 215 ( 10.3389/fnhum.2010.00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J, Summerfield C, Egner T. 2013. Attention sharpens the distinction between expected and unexpected percepts in the visual brain. J. Neurosci. 33, 18 438–18 447. ( 10.1523/JNEUROSCI.3308-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohwy J, Roepstorff A, Friston K. 2008. Predictive coding explains binocular rivalry: an epistemological review. Cognition 108, 687–701. ( 10.1016/j.cognition.2008.05.010) [DOI] [PubMed] [Google Scholar]
- 32.Quattrocki E, Friston K. 2014. Autism, oxytocin and interoception. Neurosci. Biobehav. Rev. 47, 410–430. ( 10.1016/j.neubiorev.2014.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjańska M, Doyon J, Bajbouj M, Northoff G. 2013. GABA in the insula—a predictor of the neural response to interoceptive awareness. Neuroimage 86, 10–18. ( 10.1016/j.neuroimage.2013.04.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernst J, Boker H, Hattenschwiler J, Schupbach D, Northoff G, Seifritz E, Grimm S. 2013. The association of interoceptive awareness and alexithymia with neurotransmitter concentrations in the insula and anterior cingulate. Soc. Cogn. Affect. Neurosci. 9, 857–863. ( 10.1093/scan/nst058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apps MAJ, Tsakiris M. 2014. The free energy self: a predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 41, 85–97. ( 10.1016/j.neubiorev.2013.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollatos O, Schandry R. 2004. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. ( 10.1111/1469-8986.2004.00170.x) [DOI] [PubMed] [Google Scholar]
- 37.Pollatos O, Kirsch W, Schandry R. 2005. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. ( 10.1002/hbm.20121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukushima H, Terasawa Y, Umeda S. 2011. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. Int. J. Psychophysiol. 79, 259–265. ( 10.1016/j.ijpsycho.2010.10.015) [DOI] [PubMed] [Google Scholar]
- 39.Couto B, Adolfi F et al. 2015. Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton. Neurosci. 193, 132–137. ( 10.1016/j.autneu.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Yan H-M, Xu X-G, Han F, Yan Q. 2007. Effect of heartbeat perception on heartbeat evoked potential waves. Neurosci. Bull. 23, 357–362. ( 10.1007/s12264-007-0053-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. 2013. Interoceptive awareness enhances neural activity during empathy. Hum. Brain Mapp. 34, 1615–1624. ( 10.1002/hbm.22014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu X, Hof PR, Friston K, Fan J. 2013. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 521, 3371–3388. ( 10.1002/cne.23368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuehn E, Mueller K, Lohmann G, Schuetz-Bosbach S. 2015. Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Struct. Funct. 221, 1555–1571. ( 10.1007/s00429-015-0989-8) [DOI] [PubMed] [Google Scholar]
- 44.Katkin ES, Wiens S, Ohman A. 2001. Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psychol. Sci. 12, 366–370. ( 10.1111/1467-9280.00368) [DOI] [PubMed] [Google Scholar]
- 45.Raes AK, De Raedt R. 2011. Interoceptive awareness and unaware fear conditioning: are subliminal conditioning effects influenced by the manipulation of visceral self-perception? Conscious Cogn. 20, 1393–1402. ( 10.1016/j.concog.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 46.Ferri F, Ardizzi M, Ambrosecchia M, Gallese V. 2013. Closing the gap between the inside and the outside: interoceptive sensitivity and social distances. PLoS ONE 8, e75758 ( 10.1371/journal.pone.0075758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollatos O, Herbert BM, Matthias E, Schandry R. 2007. Heart rate response after emotional picture presentation is modulated by interoceptive awareness. Int. J. Psychophysiol. 63, 117–124. ( 10.1016/j.ijpsycho.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 48.Herbert BM, Pollatos O, Schandry R. 2007. Interoceptive sensitivity and emotion processing: an EEG study. Int. J. Psychophysiol. 65, 214–227. ( 10.1016/j.ijpsycho.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 49.Füstös J, Gramann K, Herbert BM, Pollatos O. 2012. On the embodiment of emotion regulation: interoceptive awareness facilitates reappraisal. Soc. Cogn. Affect. Neurosci. 8, 911–917. ( 10.1093/scan/nss089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joffily M, Coricelli G. 2013. Emotional valence and the free-energy principle. PLoS Comput. Biol. 9, e1003094 ( 10.1371/journal.pcbi.1003094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werner NS, Peres I, Duschek S, Schandry R. 2010. Implicit memory for emotional words is modulated by cardiac perception. Biol. Psychol. 85, 370–376. ( 10.1016/j.biopsycho.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 52.Sueyoshi T, Sugimoto F, Katayama J, Fukushima H. 2014. Neural correlates of error processing reflect individual differences in interoceptive sensitivity. Int. J. Psychophysiol. 94, 278–286. ( 10.1016/j.ijpsycho.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 53.Ainley V, Brass M, Tsakiris M. 2014. Heartfelt imitation: high interoceptive awareness is linked to greater automatic imitation. Neuropsychologia 60, 21–28. ( 10.1016/j.neuropsychologia.2014.05.010) [DOI] [PubMed] [Google Scholar]
- 54.Grynberg D, Pollatos O. 2015. Perceiving one's body shapes empathy. Physiol. Behav. 140, 54–60. ( 10.1016/j.physbeh.2014.12.026) [DOI] [PubMed] [Google Scholar]
- 55.Ainley V, Maister L, Brokfeld J, Farmer H, Tsakiris M. 2013. More of myself: manipulating interoceptive awareness by heightened attention to bodily and narrative aspects of the self. Conscious Cogn. 22, 1231–1238. ( 10.1016/j.concog.2013.08.004) [DOI] [PubMed] [Google Scholar]
- 56.Barnsley N, Mcauley JH, Mohan R, Dey A, Thomas P, Mosley G. 2012. The rubber hand illusion increases histamine reactivity in the real arm. Curr. Biol. 21, R945–R946. ( 10.1016/j.cub.2011.10.039) [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Garfinkel SN, Critchley HD, Seth AK. 2013. Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia 51, 2909–2917. ( 10.1016/j.neuropsychologia.2013.08.014) [DOI] [PubMed] [Google Scholar]
- 58.Bonnelle V, Veromann KR, Burnett Heyes S, Lo Sterzo E, Manohar S, Husain M. 2015. Characterization of reward and effort mechanisms in apathy. J. Physiol. Paris 109, 16–26. ( 10.1016/j.jphysparis.2014.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Apps MAJ, Grima LL, Manohar S, Husain M. 2015. The role of cognitive effort in subjective reward devaluation and risky decision-making. Sci. Rep. 5, 16880 ( 10.1038/srep16880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preuschoff K, Quartz SR, Bossaerts P. 2008. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 28, 2745–2752. ( 10.1523/JNEUROSCI.4286-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyniel F, Sergent C, Rigoux L, Daunizeau J, Pessiglione M. 2013. Neurocomputational account of how the human brain decides when to have a break. Proc. Natl Acad. Sci. USA 110, 2641–2646. ( 10.1073/pnas.1211925110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herbert BM, Ulbrich P, Schandry R. 2007. Interoceptive sensitivity and physical effort: implications for the self-control of physical load in everyday life. Psychophysiology 44, 194–202. ( 10.1111/j.1469-8986.2007.00493.x) [DOI] [PubMed] [Google Scholar]
- 63.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. 2010. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin. Psychol. Rev. 30, 1–11. ( 10.1016/j.cpr.2009.08.008) [DOI] [PubMed] [Google Scholar]
- 64.Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, Klein AC, Paulus MP, Mehling WE. 2015. Interoception, contemplative practice, and health. Front. Psychol. 6, 886 ( 10.3389/fpsyg.2015.00763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrett LF, Quigley KS, Hamilton P. 2016. An active inference theory of allostasis and interoception in depression. Phil. Trans. R. Soc. B 371, 20160011 ( 10.1098/rstb.2016.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbert BM, Herbert C, Pollatos O. 2011. On the relationship between interoceptive awareness and alexithymia: is interoceptive awareness related to emotional awareness?. J. Pers. 79, 1149–1175. ( 10.1111/j.1467-6494.2011.00717.x) [DOI] [PubMed] [Google Scholar]
- 67.Van der Does AJ, Antony MM, Ehlers A, Barsky AJ. 2000. Heartbeat perception in panic disorder: a reanalysis. Behav. Res. Ther. 38, 47–62. ( 10.1016/S0005-7967(98)00184-3) [DOI] [PubMed] [Google Scholar]
- 68.Schaefer M, Egloff B, Witthöft M. 2012. Is interoceptive awareness really altered in somatoform disorders? Testing competing theories with two paradigms of heartbeat perception. J. Abnorm. Psychol. 121, 719–724. ( 10.1037/a0028509) [DOI] [PubMed] [Google Scholar]
- 69.Paulus MP, Stein MB. 2010. Interoception in anxiety and depression. Brain Struct. Funct. 214, 451–463. ( 10.1007/s00429-010-0258-9) [DOI] [PMC free article] [PubMed] [Google Scholar]