Abstract
Interoception is a complex process encompassing multiple dimensions, such as accuracy, learning and awareness. Here, we examined whether each of those dimensions relies on specialized neural regions distributed throughout the vast interoceptive network. To this end, we obtained relevant measures of cardiac interoception in healthy subjects and patients offering contrastive lesion models of neurodegeneration and focal brain damage: behavioural variant fronto-temporal dementia (bvFTD), Alzheimer's disease (AD) and fronto-insular stroke. Neural correlates of the three dimensions were examined through structural and functional resting-state imaging, and online measurements of the heart-evoked potential (HEP). The three patient groups presented deficits in interoceptive accuracy, associated with insular damage, connectivity alterations and abnormal HEP modulations. Interoceptive learning was differentially impaired in AD patients, evidencing a key role of memory networks in this skill. Interoceptive awareness results showed that bvFTD and AD patients overestimated their performance; this pattern was related to abnormalities in anterior regions and associated networks sub-serving metacognitive processes, and probably linked to well-established insight deficits in dementia. Our findings indicate how damage to specific hubs in a broad fronto-temporo-insular network differentially compromises interoceptive dimensions, and how such disturbances affect widespread connections beyond those critical hubs. This is the first study in which a multiple lesion model reveals fine-grained alterations of body sensing, offering new theoretical insights into neuroanatomical foundations of interoceptive dimensions.
This article is part of the themed issue ‘Interoception beyond homeostasis: affect, cognition and mental health’.
Keywords: interoception, learning, awareness, neurodegenerative diseases, stroke
1. Introduction
Interoception is the ability to sense autonomic changes via viscero-cortical pathways [1,2]. While research on this domain has greatly illuminated normal [3] and pathological [4,5] processes, it has not fully exploited the possibilities of the lesion model approach, which allows establishing direct connections between brain lesions and behaviour [6,7]. By including two contrastive lesion models, such as focal stroke and early neurodegeneration [8,9], we aim to reveal critical links between affected brain regions and interoceptive performance. To this end, we measured behavioural, neuroimaging, and electrophysiological correlates of cardiac interoception in patients with behavioural variant fronto-temporal dementia (bvFTD, a condition with early compromise of fronto-insular-temporal structures), early stage Alzheimer's disease (AD, which includes posterior and temporal atrophy), and fronto-insular stroke (FIS). Such conditions may offer novel insights into interoception, because relevant evidence is scant in neurological disorders, and null in dementias.
Cardiac interoception tasks, which assess sensing of one's own heartbeats [5,10,11], offer robust evidence on three relevant dimensions: accuracy (behavioural precision in tracking cardiac signals [3]), learning (improvement of behavioural accuracy after feedback [11]), and awareness (metacognitive processes underlying confidence about one's own performance [3]). These dimensions rely on distributed networks critically engaging the insular cortex (IC), the anterior cingulate cortex (ACC) and the somatosensory cortex (SC) [2,12], while interactions between interoceptive and high-level functions are mediated by IC projections to the ACC, the orbitofrontal cortex (OFC), the amygdala and the hippocampus (HP) [3,4,11,13–18].
First, as shown in structural and functional studies on interoceptive accuracy, task precision and online performance are associated with IC, ACC and SC hubs [12]. Additionally, the heart-evoked potential (HEP) is a cortical marker of cardiac monitoring which is modulated by attention to one's own heartbeats (expressed by a negative deflection that peaks in a 200–500 ms window after the R-wave) [19,20], and is mainly originated in the IC and the ACC [1,2,12]. HEP modulation amplitude is larger in subjects with high interoceptive accuracy [11,19,21,22] and could be enhanced by training [23]. In addition, the HEP is attenuated in neuropsychiatric patients [20] and such an alteration is associated with interoceptive deficits [5,17,18]. Moreover, phasic signals from individual heartbeats are related to memory circuits [24]. As all such mechanisms are to some extent compromised in our three patient groups, we hypothesized they would all present impairments in interoceptive accuracy and associated cortical measures.
Second, regarding interoceptive learning, cortical and intracranial recordings show that post-feedback behavioural improvements are associated with activity modulations in the IC and the frontal cortex [11]. However, whole-brain neuroimaging analyses may reveal other regions related to impairments in this dimension. Specifically, the crucial role of the HP, adjacent temporal structures and frontal cortices in memory and learning [25] suggests that such a skill should be distinctively compromised in AD patients, as reported in many other domains.
Finally, interoceptive awareness has been associated with the ACC, IC, prefrontal cortex (PFC, Brodmann area 10 (BA10) [26,27]) and OFC [28,29]. Although this metacognitive dimension has not been examined in neurological populations, impaired awareness and diminished insight are core features of dementia [30,31]. Thus, we predicted that bvFTD and AD would be worse than controls at estimating self-performance.
Previous evidence aligns with the notion of brain hubs as a biologically costly anatomical structure, which supports higher communication rates and information processing [32]. Given the elevated metabolic rate and centrality of hubs, damage to them could disrupt important functional networks, causing both general deficits in cognitive functions and specific brain disorders [32,33]. The differential compromise of hubs in our samples (temporal and posterior in AD, fronto-insular, in FIS, and fronto-temporal in bvFTD) offers a unique opportunity to dissociate brain networks within interoception.
In sum, for interoceptive accuracy, bvFTD and FIS are expected to perform worse than controls due to damage of critical interoceptive regions; instead, for AD, we predicted that performance would depend on the extent of atrophy of the IC and other subsidiary areas that could support this process (e.g. HP). Interoceptive learning should be impaired only in AD as a result of degeneration of the HP and adjacent temporal structures. Damage to these regions, together with frontal-related areas (OFC) that play a key role in learning and memory processes, could distinctively compromise this dimension. Regarding interoceptive awareness, we hypothesized that both patient groups with dementia would estimate self-performance worse than controls as a result of reduced insight and impaired metacognition, mainly associated with fronto-temporal damage. Finally, we expected the disruption of interoceptive networks to extend beyond critical areas, also compromising relevant long-range connections. To our knowledge, this is the first study to assess the structural, functional and dynamical brain signatures of interoceptive dimensions by comparing differential lesion models of neurodegeneration and focal stroke.
2. Material and methods
Given the journal's space limitations, several methods and results sections are provided in the electronic supplementary material.
(a). Participants
Ninety-nine subjects participated in the study. We recruited three patient samples that fulfilled our inclusion criteria [34,35]: 18 with probable bvFTD, 21 with AD, 18 featuring non-haemorrhagic FIS, and 42 healthy subjects. All participants provided signed informed consent in accordance with the Declaration of Helsinki.
Patients with bvFTD were diagnosed following current revised criteria [34]. BvFTD is an early onset dementia [36], which involves social and behavioural impairments [37–39] associated with fronto-temporo-insular atrophy on MRI (or frontal hypoperfusion in PET recordings). We excluded all patients who gave signs of other forms of dementia (e.g. primary progressive aphasia and amyotrophic lateral sclerosis). The resulting sample offers a unique model of fronto-insular compromise, which includes critical areas for each interoceptive dimension.
AD patients were diagnosed following NINCDS-ADRDA criteria [35,40]. They presented memory and language deficits and early atrophy in the temporal lobes, parietal regions [41,42], and, in some cases, in the IC [43]. Patients with logopenic progressive aphasia and atypical forms of AD (e.g. posterior cortical atrophy), were not included. The posterior atrophy characteristic of AD provides a contrasting model relative to frontal neurodegeneration in bvFTD. This model thus allowed us to explore other areas (e.g. HP, TP) likely to be implicated in specific interoceptive dimensions.
FIS patients presented non-haemorrhagic, fronto-insular lesions provoked by stroke. They were evaluated at least six months post-stroke (the time needed for stability of the lesion and presentation of clinical symptoms). A direct comparison of these patients with bvFTD patients [8,9] may reveal convergent areas that contribute to interoceptive dimensions (which may or may not be differentially compromised by the distinctive aetiology of each condition).
The combined study of bvFTD, AD and FIS may reveal specific and unspecific brain regions related to interoceptive dimensions. In particular, bvFTD and FIS patients provide a convergent lesion model to assess the key critical regions underlying interoception at large. The AD group provides a contrastive lesion model to explore areas which are not commonly involved in interoception but which do play a role in learning and memory mechanisms relevant to our target domain.
(b). Heartbeat detection task
We used a validated heartbeat detection (HBD) task [5,17,18,44,45] involving three conditions (figure 1). First, participants tapped a keyboard to follow their own heartbeats with no feedback. This afforded a measure of interoceptive accuracy. Next, they performed the same task while receiving feedback via a stethoscope (feedback control condition). In line with previous studies [11], auditory feedback was delivered through a stethoscope, which was held by the participants themselves with the left hand, while they tapped on the keyboard with the right hand. Finally, they were asked to follow their heartbeats again without external cues, which allowed measuring interoceptive learning. The first and third conditions were repeated twice for 2 min. Additionally, to measure interoceptive awareness [11], at the end of each condition we had participants rate their confidence in their performance from 1 (not confident at all) to 9 (fully confident). For more details, see electronic supplementary material, §1.2.
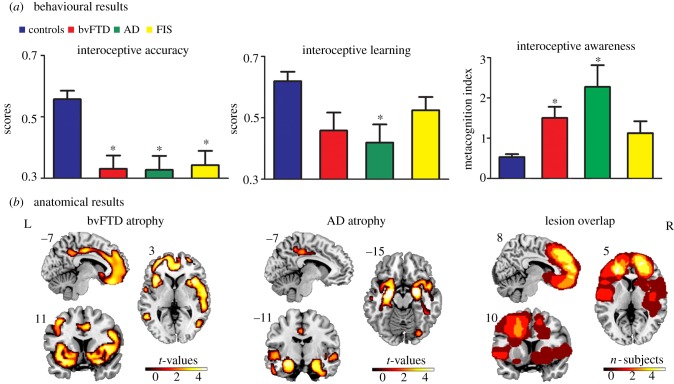
Figure 1.
Behavioural and anatomical results. (a) Performance of all four groups in each interoceptive dimension. Interoceptive (accuracy and learning) scores vary between 0 and 1, with higher scores indicating better performance. For awareness, scores nearer zero mean better metacognition. (b) Atrophy of bvFTD and AD patients (VBM) compared to controls (p < 0.001, extent threshold = 50 voxels) and lesion overlap in FIS patients. The asterisk (*) indicates significant differences relative to controls. L = left; R = right.
(i). Electroencephalography recordings
During the HBD task, electroencephalography (EEG) signals were recorded with a Biosemi Active-two 128-channel system at 1024 Hz, resampled offline at 256 Hz. Two Ag/Ag-Cl adhesive electrodes placed in lead-II positions were included to record ECG data. EEG data from 47 participants (9 bvFTD, 9 AD, 9 FIS and 20 controls) complied with the requisites for adequate preprocessing analysis (no strong movements or artefacts, strong signal-to-noise ratio, and a trial rejection rate below 30%). Only recordings from the interoceptive accuracy condition were reported. For EEG preprocessing details, see electronic supplementary material, §1.2.1.
(ii). Heart-evoked potential analysis
The HEP is obtained by sampling EEG epochs time-locked to the ECG-R-wave. It consists of a negative deflection in central and frontal electrodes [5] within a 200–400 ms window post-R wave [11,46]. As this component is modulated by attention to heartbeats [46], it indexes interoceptive processes without involvement of learning mechanisms. Thus, following previous studies [17,46], we measured this potential only during the interoceptive accuracy condition, around an extended frontal region of interest (ROI) encompassing 33 electrodes (figure 2), focusing on the maximum HEP modulation described over frontal electrodes [46]. We also assessed three separate four-electrode ROIs in frontal–right, –left and –central topographies. To control the well-known cardiac artefact, cardiac components were subtracted via ICA. See electronic supplementary material for details of HEP preprocessing (§1.2.1) and HEP results in separate ROIs (§2.3).
Figure 2.
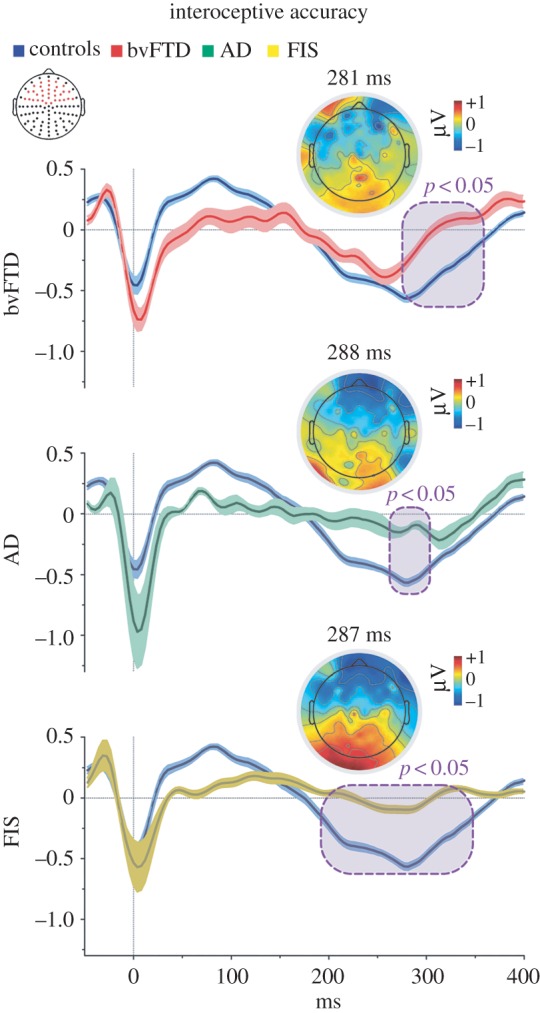
HEP modulation in the interoceptive accuracy condition. Point-by-point ERP comparison between controls and patient groups via the Monte Carlo permutation test. Purple boxes indicate p < 0.05 (a minimum extension of five consecutive points was selected as criteria to graph clusters). Shadowed bars around potentials indicate s.e.m. Scalp topography shows the differences in amplitude (microvolts) between controls and each patient group. Red dots in the channel location diagram illustrate the electrodes included in the fronto-central ROI.
As in previous cardiac interoception studies [44], ERP data were compared among conditions via Monte Carlo permutation tests [47] combined with bootstrapping from 100 ms to 400 ms (to cover typical HEP latencies [11,19]). This simple method offers a straightforward solution for multiple comparison problems and does not depend on multiple comparisons correction or Gaussian assumptions about data probability distribution [48] (see electronic supplementary material, §1.2.2).
(c). Image analysis
(i). Image acquisition
We obtained 82 MRI recordings (structural and resting-state fMRI) from 15 bvFTD, 16 AD and 16 stroke patients, alongside 35 controls (several patients were excluded from this protocol due to claustrophobia or excessive movement). Subjects were scanned in a 1.5 T Phillips Intera scanner with a standard head coil. We used a T1-weighted spin echo sequence that covered the whole brain. T2 and FLAIR sequences were acquired to improve lesion/atrophy detection in each group.
Following previous reports [49–52], during a 10-min fMRI session, participants were instructed not to think about anything in particular, to keep their eyes closed, and avoid moving and falling asleep. We chose the closed-eyes modality to avoid highly noisy signals coming from the visual cortex [53] and to facilitate attention during interoceptive processing [54,55]. Moreover, this time window enabled us to obtain enough signal points for data analysis and to ensure that the patients would go through the whole protocol (see electronic supplementary material, §1.3.1).
(ii). Structural image analysis
Voxel-based morphometry (VBM) was performed to account for global atrophy patterns in bvFTD and AD patients. Images were preprocessed with the DARTEL Toolbox from Statistical Parametric Mapping software (SPM12) and analysed following previous procedures [56,57]; see electronic supplementary material, §1.3.2.
To establish the whole-brain atrophy pattern of bvFTD and AD patients, their VBM preprocessing results were compared with those of controls via t-tests. Total intracranial volume was used as a covariate to discard the influence of brain-size differences (whole-brain analysis, p < 0.001 uncorrected [58], extent threshold = 50 voxels). Next, multiple regression analyses were performed to assess the relation between HBD performance and grey matter volume in specific regions. First, three groups (controls, bvFTD, AD) were included in the whole-brain analysis to obtain all interoceptive areas (FIS were excluded given that stroke requires a specific analysis detailed in the next section). Both patients and controls were included in each analysis to increase behavioural variance and statistical power [59,60]. Then, a controls-bvFTD and a controls-AD regression was conducted to identify interoceptive regions specific to each patient group (whole-brain analysis, p < 0.001 uncorrected [59], extent threshold = 50 voxels).
(iii). Lesion mapping analysis
Lesion masks were manually traced in native patient spaces according to visible damage on T1 and T2 scans. All masks were normalized to MNI space and then overlapped to obtain the lesion map. T1 images from FIS patients and controls were segmented, and each lesion mask was used for cost-function masking for normalization to the MNI template. This procedure prevents lesions from biasing the transformations applied [61].
For each HBD task condition, results of the all-groups-regression statistical maps were used to construct binary masks. We analysed the association between task performance and grey matter volume in each mask region using Spearman's correlations. As our sample size was small, α-values were set at p < 0.05. These results are considered exploratory and complementary to VBM analyses (for details, see electronic supplementary material, §1.3.3).
(iv). Functional image analysis
To improve fMRI analysis of high-motion subjects, functional images were tested on the Artifact Repair toolbox for SPM8 [8,62]. We excluded recordings with movements greater than 3 mm and/or rotation movements higher than 3°. Motion parameters did not differ among groups (see electronic supplementary material, §1.3.4). Following previous reports of functional connectivity in stroke and neurodegeneration [8,63], images were preprocessed with the Data Processing Assistant for Resting-State fMRI (DPARSF). For details, see electronic supplementary material, §1.3.4. To examine associations between functional connectivity and behaviour, we built 5 mm spherical ROIs from the largest VBM-all-regression cluster peaks for the three dimensions (for detailed coordinates, see electronic supplementary material, table S6). For each participant, we extracted the BOLD signal time-course from the voxels within each seed region. To obtain a functional connectivity map, we then correlated these data to every voxel in the brain using Pearson's correlation coefficient [52]. We used the SPM multiple regression module to explore associations between performance and connectivity maps. We first performed an analysis including all four groups. To improve statistical power, we replicated our approach to VBM-regression analyses [59,60]. Then, the connectivity maps of bvFTD-controls, AD-controls and FIS-controls were correlated with behavioural results (whole-brain analysis, p < 0.001 uncorrected [58], extent threshold = 10 voxels: recommended threshold given the increased voxel size of fMRI [64]).
3. Results
Demographic information and performance was compared between groups using ANOVA and Tukey post hoc comparisons. Gender and handedness were analysed with Pearson's χ2 tests. To ensure robustness in our findings, all results were covaried with age and educational level; only significant results surviving covariance are reported.
(a). Demographic results
Each patient group was similar to the control group in gender, handedness, age, formal education and body mass index—the latter measure was included given its impact on interoception (see electronic supplementary material, table S1).
(b). Behavioural results
First, interoceptive accuracy was affected in all patient groups (F3,95 = 11.06, p < 0.001). Post hoc comparisons (Tukey HSD, MS = 0.03, d.f. = 95) revealed significantly worse performance for bvFTD, AD and FIS patients than controls (all p < 0.001, figure 1). Second, the feedback condition yielded no between-group differences (F3,95 = 2.07, p = 0.11), indicating that all subjects were paying attention and could follow their own heartbeats with an external aid. Third, interoceptive learning scores also revealed differences among groups (F3,95 = 4.50, p < 0.01). Post hoc comparisons revealed that only AD patients were impaired in this dimension relative to controls (Tukey HSD, MS = 0.05, d.f. = 95, AD: p < 0.01, figure 1). Finally, interoceptive awareness was also impaired in patients relative to controls (F3,95 = 7.83, p < 0.001), but post hoc comparisons (Tukey HSD, MS = 1.94, d.f. = 95) showed that deficits appeared only in bvFTD (p < 0.05) and AD (p < 0.001); for details, see electronic supplementary material, §2.2, table S2.
(c). Heart-evoked potential results
Relative to the three patient groups, controls exhibited more negative HEP modulations during the interoceptive accuracy condition. This occurred in the frontal ROI, within the characteristic HEP time window (from 200 to 400 ms, figure 2) [11,46]. Notably, similar effects emerged when ROIs were separately considered (left, central and right electrodes; all p < 0.05; electronic supplementary material, figure S1). For details, see electronic supplementary material, §2.3, table S3.
(d). Imaging results
(i). Atrophy patterns and lesion overlap
In line with previous studies [49,65], bvFTD patients, relative to controls, showed fronto-insulo-temporal atrophy including superior and middle frontal gyrus, amygdala, parahippocampus (pHP), IC, gyrus rectus, mid cingulate cortex (MCC), putamen, superior temporal gyrus, among other areas. As expected, atrophy in the AD group was confined to temporal and posterior regions [66], including the amygdala, the temporal pole, the HP, the precuneus, the inferior temporal gyrus and the fusiform gyrus. Lesion overlap in the FIS sample showed fronto-insular lobe damage, with the IC and the ACC being most severely compromised (figure 1; electronic supplementary material, §2.4.1, tables S4 and S5 for detailed areas of atrophy).
(ii). Structural association with interoceptive dimensions
All groups (except FIS). Each dimension showed positive correlations with grey matter volume in different sets of regions. For interoceptive accuracy, these included frontal regions (inferior frontal gyrus (IFG), MCC), IC, temporal regions (superior temporal gyrus, fusiform gyrus, pHP, HP), and parietal cortices. For interoceptive learning, these encompassed temporal (HP, fusiform gyrus, superior gyrus) and frontal (IFG, MCC) structures. For interoceptive awareness, the regions were superior temporal gyrus, temporal pole, IFG, amygdala, ACC, HP and pHP. For details relating to each patient group, see figure 3 and electronic supplementary material, §2.4.2, tables S6–S8.
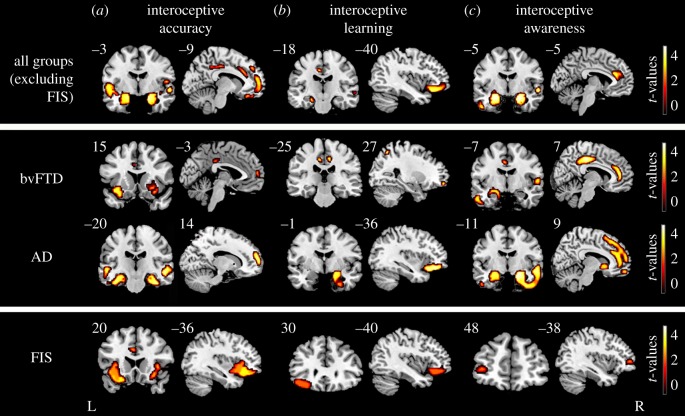
Figure 3.
Structural association with interoceptive dimensions. A regression analysis considering bvFTD-AD-controls was performed to explore associations between HBD performance with grey matter volume (whole-brain analysis, top). Regressions considering controls-bvFTD and controls-AD were conducted to identify interoceptive regions specific to each patient group (middle) (all p < 0.001, extent threshold = 50 voxels). For FIS patients, Spearman's correlations were performed between task performance and masked grey matter (p < 0.05, bottom). L = left; R = right.
BvFTD patients. In bvFTD patients, positive correlations between grey matter volume and performance involved fronto-temporal and posterior regions, as detailed below. For interoceptive accuracy, these included IC and MCC as well as the pHP, the HP, middle temporal gyrus (MTG), the fusiform gyrus and parietal cortices. For interoceptive learning, the regions comprised the MCC and the middle frontal, parietal and fusiform gyri. For interoceptive awareness, correlations were found with the MCC, pHP, amygdala and temporal and parietal regions.
AD patients. Interoceptive accuracy was positively related to temporal (HP, superior and middle gyrus, pHP, fusiform gyrus) and frontal (middle and IFG) regions. Correlations with interoceptive learning involved the temporal pole and the pHP together with the IFG, superior and MTG. Regions associated with interoceptive awareness included the pHP, temporal pole, IFG, PFC, supplementary motor area, ACC and inferior temporal gyrus.
FIS patients. Interoceptive accuracy in FIS patients positively correlated with the left ACC, the left frontal inferior OFC and bilateral IC. Only the left inferior orbital cortex was implicated in interoceptive learning. Finally, we found a positive correlation between interoceptive awareness and the left middle frontal cortex.
(iii). Functional connectivity associations with interoceptive dimensions
All groups. For interoceptive accuracy, performance was positively associated with connectivity in the IC, Rolandic operculum, superior, middle and IFG, postcentral gyrus and temporal structures (superior temporal gyrus, HP, pHP, temporal pole and MTG). Interoceptive learning correlated with connectivity in the inferior and middle frontal gyri, pHP, HP, temporal pole, superior temporal gyrus and IC. Finally, interoceptive awareness correlated with connectivity in the IFG, HP and pHP (figure 4). For details relating to each patient group, see electronic supplementary material, §2.4.3, tables S9–S12.
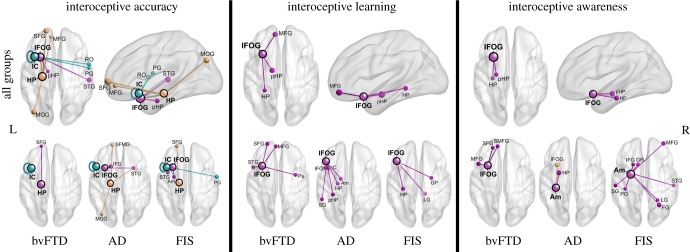
Figure 4.
Functional networks associated with interoceptive dimensions. A regression analysis considering bvFTD-AD-FIS-controls was performed to explore associations between HBD performance and functional connectivity in particular nodes (p < 0.001, top panels). Regressions considering bvFTD, AD and FIS were conducted to identify interoceptive networks specific to each patient group (bottom). The seeds are marked with black circles. The size of the node indicates the t-value and the edge and node colour shows the network to which the node belongs. IFOG = inferior frontal orbital gyrus; IFG = inferior frontal gyrus; SFG = superior frontal gyrus; MFG = medial frontal gyrus; SMFG = superior medial frontal gyrus (B10); RO = Rolandic opeculum; PG = postcentral gyrus; IC = insular cortex; SG = supramarginal gyrus; STG = superior temporal gyrus; Pu = putamen; Am = amygdala; HP = hippocampus; pHP = parahippocampus; LG = lingual gyrus; FG = fusiform gyrus; MOG = middle occipital gyrus; L = left, R = right.
bvFTD. Interoceptive accuracy was associated with connectivity in the IC, the superior frontal gyrus and the HP. Interoceptive learning was related with connectivity in the temporal lobe, inferior, middle and superior frontal gyri, and putamen. Finally, interoceptive awareness correlated with connectivity in the inferior, middle and superior frontal gyri (including PFC).
AD. Associations between connectivity and performance involved IC, inferior and superior frontal gyri, temporal pole and middle occipital gyrus, for interoceptive accuracy; IFG, HP, amygdala, pHP, IC and supramarginal gyrus for interoceptive learning; and HP, amygdala and IFG for interoceptive awareness.
FIS. Interoceptive accuracy was associated with connectivity in the IC, inferior and superior frontal gyri, amygdala, HP and postcentral gyrus. For interoceptive learning, positive associations with connectivity concerned the HP, lingual gyrus and globus pallidus. Finally, interoceptive awareness correlated with connectivity in the amygdala, lingual gyrus, middle and IFG, gyrus rectus, supramarginal gyrus, postcentral gyrus and superior temporal gyrus.
4. Discussion
We have assessed the neural correlates of interoceptive accuracy, learning and awareness in samples with damage to different hubs of an extended interoceptive network. Convergent behavioural, neuroanatomical, electrophysiological and functional connectivity results showed that each dimension relied on partially overlapping but specialized mechanisms. Moreover, behavioural disturbances in those dimensions were associated not only with compromise in specific regions, but also with long-range connectivity disruptions beyond those critical loci.
(a). Interoceptive accuracy
Interoceptive accuracy relied on a widely distributed network. Performance in all groups was explained by grey matter volume in fronto-temporal regions, including the insular and cingulate cortices, which are key nodes of interoception [1,2,12]. Moreover, the patients' deficits were related to altered electrophysiological and anatomo-functional patterns. First, HEP modulations were attenuated across patient groups. This finding aligns with previous evidence that such alterations correlate with behavioural interoceptive deficits [5,18]. Also, it indicates that difficulties to allocate attention to visceral signals are not confined to psychiatric conditions [67] with diffuse brain abnormalities, and may actually result from relatively circumscribed brain damage. Second, the association of impaired performance with fronto-temporal-insular and fronto-insular damage (in bvFTD and FIS, respectively) corroborates the central role of the IC networks in sensing body states, and of the cingulate and frontal cortices in the integration of interoceptive information [1,2,12]. In the case of AD, interoceptive deficits were associated with hippocampal and temporal atrophy. As such damage is consistently associated with impaired memory mechanisms, it seems that cardiac interoception may also rely on them. In fact, phasic signals from individual heartbeats are related to such circuits, and participants with high interoceptive accuracy perform better in subliminal learning and memory tasks [24]. This finding further stresses the multidimensional nature of interoception.
Above and beyond those critical regions, interoceptive accuracy also relies on widely distributed network activity. Behavioural performance was associated with functional connectivity between fronto-temporo-insular hubs in all groups. Moreover, abnormal temporo-frontal and IC–IC connections in each patient group highlighted the crucial role of the IC as a hub in this dimension [1,2,12], while showing that its damage can also disrupt the widespread flow of information beyond specifically compromised regions [32].
Finally, note that abnormalities in the networks sub-serving interoceptive accuracy resulted from either neurodegeneration or focal brain lesions. Thus, not only does this domain depend on the full integrity of a broad network cutting across anterior and posterior hubs, but it can also be similarly affected irrespective of the underlying physiopathology. This observation, together with evidence of interoceptive accuracy impairments in conditions without a specific locus of brain damage [5,67], confirms the widely distributed and multidimensional nature of its putative mechanisms. In sum, interoceptive accuracy seems to rely on complex interactions among hubs, which span the overall interoceptive network and participate in other functional domains.
(b). Interoceptive learning
The networks related to interoceptive learning were less widely distributed. Performance was related to frontal (inferior and superior frontal gyrus and MCC) and temporal (superior temporal gyrus and HP) structures. Nevertheless, behavioural impairments were observed only in AD patients, and VBM results confirmed their association with temporal structures involved in general learning and memory functions [25].
The absence of deficits in bvFTD and FIS suggests that fronto-insular regions are not crucially engaged by this dimension. However, they are not irrelevant to it. Indeed, all groups' long-range connections between frontal (middle and IFG, IC) and temporal (HP, pHP, temporal pole) structures were associated with their performance.
In the case of AD, alterations involved long-range connections linking temporal structures (e.g. HP, pHP, amygdala) with frontal cortices related to impaired interoceptive learning. This network plays a key role in learning, memory and multimodal association processes, mediated by hippocampal connections with the pHP, the OFC and the IC [25]. Thus, while interoceptive learning seems to depend specifically on temporo-hippocampal structures, damage to the latter appears to disrupt its widespread connections with relevant frontal hubs. In brief, although this dimension calls on interoceptive mechanisms proper, it seems to rely more critically on general memory and learning skills.
(c). Interoceptive awareness
Finally, interoceptive awareness also seems to engage widespread regions associated with self-awareness, error monitoring, metacognition and confidence. Performance in all groups was associated with grey matter volume in frontal (IFG, ACC) and temporal (HP, pHP, amygdala, superior temporal gyrus, temporal pole) structures. More particularly, altered interoceptive awareness in bvFTD and AD was associated with cingulate, prefrontal and fronto-temporal cortices. All these regions are related to self-awareness in both populations and in healthy controls [68], and to error monitoring mechanisms sub-served by the ACC [69]. Moreover, functional connectivity between the IFG, HP and pHP was associated with interoceptive awareness in all groups. Alterations of this dimension in both neurodegenerative conditions were associated with abnormal OFC-PFC-frontal areas and altered OFC-HP connectivity.
The PFC—and, in particular BA10—is a key node of metacognition, responsible for monitoring and controlling task performance, mostly dependent on posterior and temporal structures [27]. On the other hand, interoceptive awareness and metacognition are intimately related. Focus on internal visceral signals and constant monitoring and feedback updating of internal predictions are important for the construction of subjective feeling states, and their disruption could explain the patients' behavioural failures [11,70,71]. In this sense, previous reports show that BA10 (i) supports emotional processing of internal states [72], (ii) is related to information retrieval and prospective memory [72] and (iii) could contribute to anosognosia, a typical symptom of dementias [30]. Our results converge with these findings, showing that interoceptive awareness is affected in bvFTD patients, with major compromise of connections between PFC and OFC hubs. Those deficits could reflect involvement of more specific aspects of metacognition, such as confidence judgement functions related to the OFC [29]. In addition, temporal atrophy and disruptions of temporal-frontal connectivity in AD could impact the metacognitive feedback circuit to PFC and give rise to the observed behavioural impairments.
Previous lesion studies have further evidenced the crucial role of the PFC in metacognition during memory and perceptual tasks [27,30,73]. However, damage to the PFC could lead to different patterns of metacognitive performance depending on the domain evaluated [73], which underscores the importance of assessing interoception in particular. Regarding FIS patients, the absence of deficits merits attention, given that bvFTD patients featured a similar pattern of brain damage. Such a discrepancy may reflect the impact of different physiopathological mechanisms in each condition [8]. Also, beyond localized damage, FIS patients (as opposed to both bvFTD and AD patients) presented sparse bilateral long-range connections associated with preserved performance. In this sense, interoceptive awareness deficits seemed to follow from bilateral damage, which characterizes neurodegenerative diseases (bvFTD and AD) [49], but did not emerge subsequent to unilateral stroke. The unaffected regions may have allowed for functional reorganization and plasticity processes, crucial for the integrity of awareness. This would not be possible in neurodegenerative diseases, given that candidate compensation areas would become progressively damaged as time passes [74]. Future research should explore the potential role of plastic brain changes in interoception and the relation of impaired body awareness and anosognosia in neurodegeneration.
In sum, interoceptive awareness engages distributed hubs, which may be compromised following bilateral long-range atrophy. Also, this network may be highly susceptible to functional reorganization in stroke. Future research should explore the potential role of plastic brain changes in interoception and the relation of impaired body awareness and anosognosia.
5. Limitations
Our study featured a number of limitations. First, the size of our patient samples was relatively small; however, it proved similar to those of prior studies [51]. Also, this caveat was counteracted by the strict control of several demographic, clinical and lesion/atrophy variables. Second, although our hypotheses are based on differential neurocognitive domains affecting basic interoceptive processes (e.g. memory and metacognition), we were unable to include additional tests to tap them directly. Further studies should include social and cognitive variables in the main analyses to control for the effects of cognitive dysfunction. For instance, according to the social context network model [37,75], damage to interoceptive hubs in bvFTD may affect salience attribution during social cognition deficits (e.g. [57,76–78]). Moreover, interoceptive tasks and the HEP have been linked to emotion and social cognition [5,79]. This connection can be directly tested by parametrizing interactions between interoception and social cognition performance. In addition, as in any study assessing neurodegeneration, a possible effect of atrophy in the regression analyses cannot be ruled out. However, each dimension worked as a control condition, demonstrating that the differential structural patterns were indeed associated with the groups' performance. Finally, a common strategy in studies of neurodegenerative diseases consists of including both patients and controls in the same analysis to increase behavioural variance and statistical power [60,80]. However, studies combining groups of patients and controls must be conducted with caution and future research should more precisely compare the role of the resulting structures.
6. Conclusion
To our knowledge, this is the first study to assess the correlates of cardiac interoceptive dimensions with differential models of neuroanatomical damage. By combining neuroimaging and electrophysiological measures, we revealed functional specializations for three aspects of interoception (accuracy, learning and awareness). The crucial role of frontal-temporo-insular networks was highlighted by impairments associated not only with damage to specific loci, but also with widespread connectivity abnormalities. More generally, our findings speak to the importance of neural mappings of bodily states and metacognitive monitoring in the construal of subjective feeling states and the deployment of daily behavioural impairments [2]. In the future, further studies could assess the interaction among these interoceptive dimensions by testing hypotheses from hierarchical predictive coding for interoceptive levels [70,81] indexed by insulo-fronto-temporal network dynamics. This agenda opens novel pathways to understand how interoceptive mechanisms are functionally organized across cortical and subcortical regions, and how they can be specifically disrupted by varied forms of brain damage.
Supplementary Material
Supplementary Material
Ethics
The study protocol was approved by the institutional Ethics Committee (no. 2012–0412).
Data accessibility
The dataset supporting this article and summarized in the supplementary material is available upon request (corresponding author: aibanez@ineco.org.ar)
Authors' contributions
A.I., I.G.-C. and L.S. designed the study. I.G.-C., L.S., G.F., A.Y., S.E., M.M. and P.S. carried out the experiments. I.G.-C., L.S., L.d.l.F. and S.B. analysed the experiments. I.G.-C., L.S., A.M.G. and A.I. wrote the paper. A.S., F.K., J.F., C.R., J.B., T.T., S.E., D.H. and F.M. contributed to the clinic aspects of the paper. A.I., I.G.-C. and L.S. conceived the study and wrote the final paper, together with the other authors. All authors have approved the manuscript.
Competing interests
We have no competing interests.
Funding
A.I., I.G.-C., L.S., L.d.l.F., G.F., S.B., A.Y., M.M., P.S., A.M.G., F.M., D.H. and T.T. are funded by CONICET, CONICYT/FONDECYT Regular (1130920 and 1140114), FONCyT-PICT 2012-0412 and FONCyT-PICT 2012-1309. This work was also partially supported by FONDAP 15150012 and the INECO Foundation.
References
- 1.Critchley HD, Harrison NA. 2013. Visceral influences on brain and behavior. Neuron 77, 624–638. ( 10.1016/j.neuron.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 2.Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 3.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. 2015. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74. ( 10.1016/j.biopsycho.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 4.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. 2009. The pathways of interoceptive awareness. Nat. Neurosci. 12, 1494–1496. ( 10.1038/nn.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couto B, et al. 2014. The man who feels two hearts: the different pathways of interoception. Soc. Cogn. Affect. Neurosci. 9, 1253–1260. ( 10.1093/scan/nst108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rorden C, Karnath HO. 2004. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat. Rev. Neurosci. 5, 813–819. ( 10.1038/nrn1521) [DOI] [PubMed] [Google Scholar]
- 7.Melloni M, et al. In press Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain. ( 10.1093/brain/aww1231) [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Cordero I, et al. 2015. Stroke and neurodegeneration induce different connectivity aberrations in the insula. Stroke 46, 2673–2677. ( 10.1161/STROKEAHA.115.009598) [DOI] [PubMed] [Google Scholar]
- 9.Baez S, et al. 2014. Comparing moral judgments of patients with frontotemporal dementia and frontal stroke. JAMA Neurol. 71, 1172–1176. ( 10.1001/jamaneurol.2014.347) [DOI] [PubMed] [Google Scholar]
- 10.Schandry R. 1981. Heart beat perception and emotional experience. Psychophysiology 18, 483–488. ( 10.1111/j.1469-8986.1981.tb02486.x) [DOI] [PubMed] [Google Scholar]
- 11.Canales-Johnson A, et al. 2015. Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb. Cortex 25, 4490–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 13.Bechara A, Naqvi N. 2004. Listening to your heart: interoceptive awareness as a gateway to feeling. Nat. Neurosci. 7, 102–103. ( 10.1038/nn0204-102) [DOI] [PubMed] [Google Scholar]
- 14.Damasio AR. 1996. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Phil. Trans. R. Soc. Lond. B 351, 1413–1420. ( 10.1098/rstb.1996.0125) [DOI] [PubMed] [Google Scholar]
- 15.Werner NS, Schweitzer N, Meindl T, Duschek S, Kambeitz J, Schandry R. 2013. Interoceptive awareness moderates neural activity during decision-making. Biol. Psychol. 94, 498–506. ( 10.1016/j.biopsycho.2013.09.002) [DOI] [PubMed] [Google Scholar]
- 16.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. ( 10.1523/jneurosci.5587-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couto B, et al. 2015. Disentangling interoception: insights from focal strokes affecting the perception of external and internal milieus. Front. Psychol. 6, 503 ( 10.3389/fpsyg.2015.00503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedeno L, et al. 2014. How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE 9, e98769 ( 10.1371/journal.pone.0098769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollatos O, Schandry R. 2004. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. ( 10.111/1469-8986.2004.00170.x) [DOI] [PubMed] [Google Scholar]
- 20.Schandry R, Montoya P. 1996. Event-related brain potentials and the processing of cardiac activity. Biol. Psychol. 42, 75–85. ( 10.1016/0301-0511(95)05147-3) [DOI] [PubMed] [Google Scholar]
- 21.Yuan H, Yan HM, Xu XG, Han F, Yan Q. 2007. Effect of heartbeat perception on heartbeat evoked potential waves. Neurosci. Bull. 23, 357–362. ( 10.1007/s12264-007-0053-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollatos O, Kirsch W, Schandry R. 2005. On the relationship between interoceptive awareness, emotional experience, and brain processes. Brain Res. Cogn. Brain Res. 25, 948–962. ( 10.1016/j.cogbrainres.2005.09.019) [DOI] [PubMed] [Google Scholar]
- 23.Schandry R, Weitkunat R. 1990. Enhancement of heartbeat-related brain potentials through cardiac awareness training. Int. J. Neurosci. 53, 243–253. ( 10.3109/00207459008986611) [DOI] [PubMed] [Google Scholar]
- 24.Garfinkel SN, Critchley HD. 2016. Threat and the body: how the heart supports fear processing. Trends Cogn. Sci. 20, 34–46. ( 10.1016/j.tics.2015.10.005) [DOI] [PubMed] [Google Scholar]
- 25.Squire LR, Zola-Morgan S. 1991. The medial temporal lobe memory system. Science 253, 1380–1386. ( 10.1126/science.1896849) [DOI] [PubMed] [Google Scholar]
- 26.Fleming SM, Dolan RJ. 2012. The neural basis of metacognitive ability. Phil. Trans. R. Soc. B 367, 1338–1349. ( 10.1098/rstb.2011.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baird B, Smallwood J, Gorgolewski KJ, Margulies DS. 2013. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J. Neurosci. 33, 16 657–16 665. ( 10.1523/JNEUROSCI.0786-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD. 2009. How do you feel now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 29.Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. 2014. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron 84, 190–201. ( 10.1016/j.neuron.2014.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen HJ, Alcantar O, Zakrzewski J, Shimamura AP, Neuhaus J, Miller BL. 2014. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer's disease. Neuropsychology 28, 436–447. ( 10.1037/neu0000012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornberger M, Yew B, Gilardoni S, Mioshi E, Gleichgerrcht E, Manes F, Hodges JR. 2014. Ventromedial-frontopolar prefrontal cortex atrophy correlates with insight loss in frontotemporal dementia and Alzheimer's disease. Hum. Brain Mapp. 35, 616–626. ( 10.1002/hbm.22200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET. 2014. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137, 2382–2395. ( 10.1093/brain/awu132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696. ( 10.1016/j.tics.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 34.Rascovsky K, et al. 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. ( 10.1093/brain/awr179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKhann GM, et al. 2011. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 7, 263–269. ( 10.1016/j.jalz.2011.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratnavalli E, Brayne C, Dawson K, Hodges JR. 2002. The prevalence of frontotemporal dementia. Neurology 58, 1615–1621. ( 10.1212/WNL.58.11.1615) [DOI] [PubMed] [Google Scholar]
- 37.Ibanez A, Manes F. 2012. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 78, 1354–1362. ( 10.1212/WNL.0b013e3182518375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neary D, et al. 1998. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. ( 10.1212/WNL.51.6.1546) [DOI] [PubMed] [Google Scholar]
- 39.Piguet O, Hornberger M, Mioshi E, Hodges JR. 2011. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 10, 162–172. ( 10.1016/S1474-4422(10)70299-4) [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944. ( 10.1212/WNL.34.7.939) [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. ( 10.1007/BF00308809) [DOI] [PubMed] [Google Scholar]
- 42.Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF. 2006. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res. 146, 243–249. ( 10.1016/j.pscychresns.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 43.Bonthius DJ, Solodkin A, Van Hoesen GW. 2005. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J. Neuropathol. Exp. Neurol. 64, 910–922. ( 10.1097/01.jnen.0000182983.87106.d1) [DOI] [PubMed] [Google Scholar]
- 44.Yoris A, et al. 2015. The roles of interoceptive sensitivity and metacognitive interoception in panic. Behav. Brain Funct. 11, 14 ( 10.1186/s12993-015-0058-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melloni M, et al. 2013. Preliminary evidence about the effects of meditation on interoceptive sensitivity and social cognition. Behav. Brain Funct. 9, 47 ( 10.1186/1744-9081-9-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollatos O, Kirsch W, Schandry R. 2005. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. ( 10.1002/hbm.20121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manly BFJ. 1997. Randomization, bootstrap and Monte Carlo methods in biology. London, UK: Chapman & Hall/CRC. [Google Scholar]
- 48.Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25. ( 10.1002/hbm.1058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. 2009. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52. ( 10.1016/j.neuron.2009.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, et al. 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133, 1352–1367. ( 10.1093/brain/awq075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day GS, Farb NA, Tang-Wai DF, Masellis M, Black SE, Freedman M, Pollock BG, Chow TW. 2013. Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol. 70, 1249–1253. ( 10.1001/jamaneurol.2013.3258) [DOI] [PubMed] [Google Scholar]
- 52.Tuladhar AM, Snaphaan L, Shumskaya E, Rijpkema M, Fernandez G, Norris DG, de Leeuw FE. 2013. Default mode network connectivity in stroke patients. PLoS ONE 8, e66556 ( 10.1371/journal.pone.0066556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou Q, Miao X, Liu D, Wang DJ, Zhuo Y, Gao JH. 2015. Reliability comparison of spontaneous brain activities between BOLD and CBF contrasts in eyes-open and eyes-closed resting states. Neuroimage 121, 91–105. ( 10.1016/j.neuroimage.2015.07.044) [DOI] [PubMed] [Google Scholar]
- 54.Xu P, et al. 2014. Different topological organization of human brain functional networks with eyes open versus eyes closed. Neuroimage 90, 246–255. ( 10.1016/j.neuroimage.2013.12.060) [DOI] [PubMed] [Google Scholar]
- 55.Wang XH, Li L, Xu T, Ding Z. 2015. Investigating the temporal patterns within and between intrinsic connectivity networks under eyes-open and eyes-closed resting states: a dynamical functional connectivity study based on phase synchronization. PLoS ONE 10, e0140300 ( 10.1371/journal.pone.0140300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashburner J, Friston KJ. 2000. Voxel-based morphometry—the methods. Neuroimage 11, 805–821. ( 10.1006/nimg.2000.0582) [DOI] [PubMed] [Google Scholar]
- 57.Couto B, Manes F, Montanes P, Matallana D, Reyes P, Velasquez M, Yoris A, Baez S, Ibanez A. 2013. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front. Hum. Neurosci. 7, 467 ( 10.3389/fnhum.2013.00467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irish M, Piguet O, Hodges JR, Hornberger M. 2014. Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer's disease. Hum. Brain Mapp. 35, 1422–1435. ( 10.1002/hbm.22263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sollberger M, Rankin KP, Miller BL. 2010. Social cognition. Continuum (Minneap. Minn.) 16, 69–85. ( 10.1212/01.CON.0000368261.15544.7c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, Hodges JR, Hornberger M. 2016. Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain 139, 204–216. ( 10.1093/brain/awv315) [DOI] [PubMed] [Google Scholar]
- 61.Brett M, Leff AP, Rorden C, Ashburner J. 2001. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14, 486–500. ( 10.1006/nimg.2001.0845) [DOI] [PubMed] [Google Scholar]
- 62.Bruno JL, Garrett AS, Quintin EM, Mazaika PK, Reiss AL. 2014. Aberrant face and gaze habituation in fragile x syndrome. Am. J. Psychiatry 171, 1099–1106. ( 10.1176/appi.ajp.2014.13111464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sedeno L, et al. 2016. Brain network organization and social executive performance in frontotemporal dementia. J. Int. Neuropsychol. Soc. 22, 250–262. ( 10.1017/S1355617715000703) [DOI] [PubMed] [Google Scholar]
- 64.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. 2009. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 4, 143–157. ( 10.1093/scan/nsp007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. 2006. Structural anatomy of empathy in neurodegenerative disease. Brain 129, 2945–2956. ( 10.1093/brain/awl254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. 2011. Neurostructural predictors of Alzheimer's disease: a meta-analysis of VBM studies. Neurobiol. Aging 32, 1733–1741. ( 10.1016/j.neurobiolaging.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 67.Muller LE, Schulz A, Andermann M, Gabel A, Gescher DM, Spohn A, Herpertz SC, Bertsch K. 2015. Cortical representation of afferent bodily signals in borderline personality disorder: neural correlates and relationship to emotional dysregulation. JAMA Psychiatry 72, 1077–1086. ( 10.1001/jamapsychiatry.2015.1252) [DOI] [PubMed] [Google Scholar]
- 68.Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. 2014. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain 137, 2368–2381. ( 10.1093/brain/awu161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–749. ( 10.1126/science.280.5364.747) [DOI] [PubMed] [Google Scholar]
- 70.Seth AK. 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573. ( 10.1016/j.tics.2013.09.007) [DOI] [PubMed] [Google Scholar]
- 71.Barttfeld P, Wicker B, McAleer P, Belin P, Cojan Y, Graziano M, Leiguarda R, Sigman M. 2013. Distinct patterns of functional brain connectivity correlate with objective performance and subjective beliefs. Proc. Natl Acad. Sci. USA 110, 11 577–11 582. ( 10.1073/pnas.1301353110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramnani N, Owen AM. 2004. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5, 184–194. ( 10.1038/nrn1343) [DOI] [PubMed] [Google Scholar]
- 73.Fleming SM, Ryu J, Golfinos JG, Blackmon KE. 2014. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain 137, 2811–2822. ( 10.1093/brain/awu221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mesulam MM. 1999. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron 24, 521–529. ( 10.1016/S0896-6273(00)81109-5) [DOI] [PubMed] [Google Scholar]
- 75.Baez S, García AM, Ibanez A. In press. The social context network model in psychiatric and neurological diseases. Curr. Top Behav. Neurosci. ( 10.1007/7854_2016_443) [DOI] [PubMed] [Google Scholar]
- 76.Baez S, et al. 2016. Integration of intention and outcome for moral judgment in frontotemporal dementia: brain structural signatures. Neurodegener. Dis. 16, 206–217. ( 10.1159/000441918) [DOI] [PubMed] [Google Scholar]
- 77.Baez S, et al. 2014. Primary empathy deficits in frontotemporal dementia. Front. Aging Neurosci. 6 ( 10.3389/fnagi.2014.00262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baez S, Morales JP, Slachevsky A, Torralva T, Matus C, Manes F, Ibanez A. 2016. Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex 75, 20–32. ( 10.1016/j.cortex.2015.11.007) [DOI] [PubMed] [Google Scholar]
- 79.Couto B, et al. 2015. Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton. Neurosci. 193, 132–137. ( 10.1016/j.autneu.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 80.Sollberger M, et al. 2009. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia 47, 2812–2827. ( 10.1016/j.neuropsychologia.2009.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. 2004. Interoceptive sensitivity and self-reports of emotional experience. J. Pers. Soc. Psychol. 87, 684–697. ( 10.1037/0022-3514.87.5.684) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article and summarized in the supplementary material is available upon request (corresponding author: aibanez@ineco.org.ar)