Abstract
We briefly review the evidence for distinct neuroanatomical substrates that underlie interoception in humans, and we explain how they substantialize feelings from the body (in the insular cortex) that are conjoined with homeostatic motivations that guide adaptive behaviours (in the cingulate cortex). This hierarchical sensorimotor architecture coincides with the limbic cortical architecture that underlies emotions, and thus we regard interoceptive feelings and their conjoint motivations as homeostatic emotions. We describe how bivalent feelings, emotions and sympathovagal balance can be organized and regulated efficiently in the bicameral forebrain as asymmetric positive/negative, approach/avoidance and parasympathetic/sympathetic components. We provide original evidence supporting this organization from studies of cardiorespiratory vagal activity in monkeys and functional imaging studies in healthy humans showing activation modulated by paced breathing and passively viewed emotional images. The neuroanatomical architecture of interoception provides deep insight into the functional organization of all emotional feelings and behaviours in humans.
This article is part of the themed issue ‘Interoception beyond homeostasis: affect, cognition and mental health’.
Keywords: anterior cingulate, insula, vagus, asymmetry, respiration
1. Introduction
The neurophysiological term ‘interoception’ was introduced 100 years ago to signify sensory input from receptors located inside of the body, as opposed to cutaneous receptors that are activated by external stimuli (exteroception) [1]. Neuroanatomists of the same era categorized ‘visceral’ and ‘somatic’ neurons in the vertebrate spinal cord and medulla based on embryological and morphological evidence. However, the defining characteristics of these conceptual categories could not be distinguished more centrally in the brain. Consequently, for many years the term ‘interoception’ meant simply ‘visceral sensory input’ [2].
In 2002, a redefinition of interoception as ‘the physiological sense of the condition of the body’ was prompted by the identification of a phylogenetically unique ascending pathway to cortex in monkeys that conveys homeostatic sensory activity from all bodily tissues [3]. Considerable convergent evidence now supports this proposal and the broader concept of a homeostatic sensorimotor hierarchy that coincides with the ‘central autonomic network’ [4,5], the ‘emotional motor system’ [6] and much of the classical ‘limbic system’ ([7] see also [8]). It has further been proposed that it critically underpins both human awareness and mental illness [9,10].
We first briefly summarize the neuroanatomical organization of this homeostatic sensorimotor system and its role in feelings, emotional (or adaptive) behaviour and asymmetric autonomic and emotional regulation. Next, we describe original findings in monkeys that localize cardiorespiratory (vagal) sensory activity in the interoceptive insular cortex anterior to spinal input. We present functional imaging (fMRI) evidence in humans showing that, during alternate epochs of slow and fast breathing, sympathovagal balance and activation of the homeostatic sensory (insular) and motor (cingulate) cortices are significantly enhanced, in tandem and asymmetrically, while subjects passively view pleasant or unpleasant images. We relate these and other findings to the concept of coupled asymmetric regulation of feelings, emotions and sympathovagal balance in the homeostatic sensorimotor model of emotion. Finally, we highlight the explanation that this opponent organization offers for the crucial significance of sympathovagal balance for both emotional and cardiorespiratory health [11–13].
2. The ascending interoceptive pathway
In cats and macaque monkeys, spinal lamina I neurons thought traditionally to mediate feelings of ‘pain and temperature’ were found intermixed with neurons selectively responsive to stimuli that produce various bodily feelings in humans, including itch, sensual touch, and muscle and visceral sensations. Altogether, these neurons represent physiological conditions in the different tissue compartments of the body itself [3]. This interoceptive activity is needed for homeostasis, the process that dynamically maintains an optimal balance in the living body across all conditions at all times through neural, endocrinological and behavioural functions. The neural effector of homeostasis is the autonomic nervous system (ANS). Significantly, modern evidence has demonstrated that the homeostatic sensorimotor (i.e. interoceptive, ‘visceral’) neural elements and the skeletal sensorimotor (i.e. exteroceptive, ‘somatic’) neural elements originate embryologically from two ancient and distinct gene regulatory networks in all vertebrates [10].
The functional anatomical characteristics of lamina I neurons confirm that they are the second-order homeostatic sensory neurons [14,15]. Physiologically and anatomically distinct classes of lamina I neurons receive input selectively from distinct subsets of interoceptive small-diameter primary sensory fibres and constitute discrete sensory channels or ‘labelled lines’, which is comparable to other sensory systems (e.g. mechanoreception, vision) and makes sense as a developmentally energy-efficient pattern of organization. Their spinal projections specifically target the autonomic motor columns, and their brainstem projections target the cardiorespiratory/homeostatic regions, which in return send descending projections to the spinal autonomic columns. This hierarchical homeostatic sensorimotor architecture is capped in all mammals by bidirectional connections with the hypothalamus, the main homeostatic integration region of the forebrain, which sends descending terminations to exactly the same brainstem regions and, in the spinal cord, exclusively to the autonomic motor columns and lamina I [14,15].
In anthropoid primates, a direct interoceptive pathway to thalamus and cortex is provided by spinal (and trigeminal) lamina I neurons, together with a parallel component that conveys visceral and cranial homeostatic sensory activity and ascends directly from the lower brainstem. The interoceptive relay nuclei in the thalamus (the posterior and basal portions of the ventral medial nucleus, VMpo and VMb) are aligned with a topographic gradient distinct from that of the exteroceptive substrates. This novel pathway terminates topographically in a discrete isocortical area at the dorsal margin of the posterior insula in both monkeys and humans [16]; it is not present in cats or rats. Comparative neuroanatomical evidence (see the electronic supplementary material, figure S1, S2) supports convergent functional evidence indicating that this pathway is most highly developed in humans and substantializes all affective bodily feelings, including cool, warm, pricking pain, burning pain, itch, muscle ache and C-fibre affective (sensual) touch, as well as taste, hunger, thirst, ‘air hunger’, bowel distension, urge to urinate and so on [9,10]. Interoceptive integration in the hyperallometrically enlarged human anterior insular cortex [17] supports emotional feelings, which in our model are generated as if they were feelings from the body and are grounded on the feeling of being alive that emerges from the integration of homeostatic sensory and motor (pre-autonomic) activity in the middle insula (‘homeostatic sentience’; [10]).
Importantly, the interoceptive pathway terminates also in the cingulate motor cortex [18,19]. Together, the insular and cingulate cortices constitute the homeostatic sensorimotor cortex that surmounts the ancient homeostatic hierarchy in the spinal cord, brainstem and hypothalamus [3,7,10]. (We infer from evolutionary studies that they may have originated as a unified region in the earliest mammals, but were split into ‘orbital’ sensory and ‘cingulate’ motor portions by the expansion of prefrontal cortex [20,21].) The insular and cingulate cortices are conjointly activated during all affective bodily feelings [10]. In fact, considerable evidence supports the assertion that they are uniquely activated in humans during all feelings and all emotions, and further, that they function as a ‘core control network’ that coordinates, mediates and switches activity between networks across the entire brain [10,22]. Indeed, clinical studies now suggest that the insular and cingulate cortices bilaterally are crucial brain areas that are abnormal in an array of mental disorders [23].
Thus, the bilateral insular and cingulate cortical areas together serve as the homeostatic/emotional/limbic sensorimotor neocortex and provide adaptive (homeostatic) control of both the body and the brain. This sensorimotor architecture fits with considerable evidence suggesting that feelings (and awareness) are engendered in the insular cortex, while motivations (and agency) are engendered in the cingulate cortex; this congruence is also consonant with the definition of an emotion as the combination of a feeling and a concurrent motivation that is accompanied by obligatory autonomic sequelae [24]. This suggests that affective feelings from the body and their conjoint motivations and autonomic effects can be viewed as emotions that signal adaptive homeostatic responses to the needs of our body, or homeostatic emotions [10]. (The ‘primordial emotions’ of Denton [25] are conceptually similar; see also [26].) From this perspective, emotional feelings and their concomitant motivations signal adaptive responses to needs that are represented by patterns of activity in the brain, which correspond with social, cognitive, or fictive circumstances. In all instances, such adaptive responses flexibly balance anticipated outcomes (based on hereditary and learned associations encoded in the sensorimotor hierarchy) with the optimal utilization of energy, which is the critical evolutionary and homeostatic arbiter.
Temperature sensation provides a good example of a homeostatic emotion. We normally regard this as a discriminative cutaneous sensory capacity. However, with each thermal sensation we feel an obligatory hedonic affect (pleasantness or unpleasantness, unless neutral), and that affective feeling occurs along with a behavioural thermoregulatory motivation, which guides an adaptive, energy-efficient response to an interoceptive sensory challenge that the automatic (sub-cortical) homeostatic mechanisms cannot rectify. Critically, the valence of that affective feeling depends directly on the body's thermoregulatory needs [27–29]; for example, the same cool glass of water that feels wonderful if you are overheated would feel awful if you were chilled.
3. Opponent homeostatic/emotional control in the bicameral forebrain
Ample evidence now suggests that a bivalent, asymmetric control system is manifested in the bicameral vertebrate forebrain, such that sympathetic activity, negative affect, avoidance behaviour and energy expenditure are operationalized predominantly in the right forebrain while parasympathetic activity, positive affect, approach behaviour and energy nourishment are operationalized predominantly in the left forebrain, with opponent interactions between the two sides [9,10,30]. This asymmetry is supported by evidence from several different fields: ethological studies (left, ‘routine’; right, ‘challenging’ behaviours [31,32]); clinical studies (Wada test: left forebrain anesthesia releases depressed mood; right, euphoria [33]); physiological results (left, bradycardia, control of heart rate variability (HRV); right, tachycardia, control of tonic heart rate [5,34]); and psychophysiological findings (EEG activation: left, approach, positive affect, increased vagal tone; right, avoidance, negative affect, cortisol release [35–37]). Notably, opponent or asymmetric mechanisms are ubiquitous in biology, probably because they provide optimal energy-efficient control [38].
Yet, various notions of forebrain organization are being studied, and until recently, there was almost no evidence of such lateralization in human imaging studies. The recent demonstration of a corresponding asymmetry in dopamine receptor binding and behavioural orientation bias provided the first neuroanatomical evidence of this asymmetry in humans [39,40]. Direct evidence of asymmetric activation in the amygdala, anterior insular cortex and cingulate cortex was identified by two meta-analyses of fMRI studies of emotional tasks: during positive emotional conditions, strong activation occurred almost exclusively on the left side, while activation on the right side occurred only during negative emotional conditions [41,42]. Activation during negative conditions was also observed on the left side; this bilateral activity confounds recognition of this asymmetry but is consistent with opponent regulation and the survival value of being able to generate rapid escape behaviours with either hemisphere.
Examples of activation on the left side in association with negative events or the right side with positive events could also be consistent with the mechanism of opponent regulation. Several psychophysiological studies associated EEG activation of the left forebrain not only with positive affect and parasympathetic tone, but also with regulatory control of negative affect and inhibition of sympathetic cortisol release (see [10, p. 269]), in part because the onset was not coincident but rather immediately followed the stimulus event.
Two recent clinical reports provide direct support for left hemisphere dominance in the control of parasympathetic outflow in humans. One study in patients with fronto-temporal dementia found that leftward compared with rightward asymmetry in the structural and functional loss in the insular and anterior cingulate regions predicted lower parasympathetic control of the heart [43]. The other study in a broad sample showed that individuals with leftward asymmetry in high-frequency (23–36 Hz) EEG activity had lower resting heart rate and higher baroreflex sensitivity, while those with rightward asymmetry showed the opposite pattern [44].
Clinically depressed patients, who experience extreme negative affect, display comparable emotional and autonomic asymmetry, with hyperactivity in the right hemisphere and hypoactivity in the left hemisphere [45] that is directly related both to clinical severity [46,47] and to sympathovagal (im)balance [48]. Consonant findings were reported in patients with treatment-resistant depression, in whom decreased right and increased left activity was produced by therapeutically effective vagus nerve stimulation (VNS) [49–54], while patients with the highest levels of activity in the right anterior insula did not respond to VNS [55] or to antidepressant medications [56]. We believe that the key inference from these and many other findings is that complementary activity in the left and right insula and cingulate cortices is important for both emotional well-being and for autonomic (sympathovagal) balance.
4. Is there a direct vagal-activated projection to interoceptive cortex?
The ascending lamina I pathway in the monkey conveys spinal homeostatic sensory activity that complements motor activity in the sympathetic division of the ANS. The identification of this unforeseen projection naturally raised the question of whether a parallel direct projection conveys homeostatic sensory activity from the visceral and cranial sources that are innervated by the parasympathetic division of the ANS. This question is especially important for our understanding of the role of this system in sympathovagal balance and its linkage with feelings and emotion.
Visceral and cranial homeostatic sensory activity is mediated by input from the vagus and other nerves to the nucleus of the solitary tract (NTS) in the lower medulla. In rodents, such activity is conveyed to the forebrain indirectly, by way of the parabrachial nucleus (PB) in the upper brainstem. Intriguingly, a novel direct projection to thalamus from the NTS was described in the monkey in 1980 [57]; however, it was interpreted as a specific gustatory pathway, in part, because it was found to originate entirely from the rostral third of the NTS, which contains nearly exclusively gustatory neurons. Physiological evidence indicates clearly that visceral and cardiorespiratory vagal inputs activate insular cortex in rat, monkey and human, but whether a direct ascending vagal-activated pathway from NTS to interoceptive cortex exists in primates is unknown [58–60].
Evidence addressing this question was obtained during a series of vagal-activation studies in macaque monkeys in Craig's laboratory [61,62] and reported previously in abstracts [63,64]. Microelectrode recordings were used to identify vagal-evoked potentials and vagal-activated neurons in the thalamus under anaesthesia (details in the electronic supplementary material). Micro-injections of anterograde tracers made at vagal-activated foci in VMb produced labelling of homolateral middle-layer cortical projections that were consistently located in two regions (figure 1): within the walls and fundus of the anterior portion of the superior limiting sulcus, which is the portion of interoceptive cortex anterior to the spinal lamina I-recipient portion (area Idfa [65]); and, at the inferior precentral sulcus (area 3a) at the most anterior and lateral end of the primary sensorimotor area. These two projections parallel the dual gustatory projections from VMb that were reported earlier in the monkey (also called VPMpc; [66,67]) but are focused more medially in area 3a and more posteriorly in area Idfa. (Neurons in VMpo also have an ancillary projection to area 3a; see [10] for discussion.) Retrograde tracer injections at vagal-activated foci in VMb produced dense labelling of neurons ipsilaterally in the rostral third of NTS (its gustatory portion), as in the earlier study [57], but there was also modest cell labelling in the middle portion and sparse labelling in the caudal (commissural) portion of NTS (see the electronic supplementary material, figure S3), where vagal sensory fibre terminations predominate [57,58]. Since we consistently found the focus of vagal activation in the middle of VMb, just anterior to the gustatory neurons in its most posterior portion [57,61], the additional labelling in both the anterograde and retrograde tracing experiments suggests that vagal homeostatic sensory input to interoceptive cortex may include direct ascending projections in primates too.
Figure 1.
Vagal cortical projection targets. (a) Tracer injections were made at vagal-evoked potential foci, which were generally located in the middle of VMb in different cases (colour coded). (b) One example (STM 158) is shown: the dark reaction product identifies the location of the WGA*HRP injection. (c) Dense middle-layer terminal projections (red) were found in two cortical regions: anterior insula (in the fundus of the superior limiting sulcus, focused posterior to its anterior end) and ventral precentral cortex (near the fundus of the inferior precentral sulcus, approx. 2–4 mm anterolateral to the end of the central sulcus). Two examples (STM158, STM172) are illustrated and a darkfield photomicrograph of the terminal labelling in each cortical region is shown. These illustrations are taken from Ito and Craig [64]. The inset (top right) shows the current architectural map of the macaque insula, taken directly from Craig [10] and modified from Evrard et al. [65]. WGA*HRP, wheat germ agglutinin-conjugated HRP.
5. Does breathing facilitate asymmetric emotional processing?
Breathing and autonomic function are tightly linked. Voluntary reduction of breathing rate enhances parasympathetic activity and reduces sympathetic activity. Slow breathing increases respiratory sinus arrhythmia (RSA) [68,69] and the high-frequency component of HRV [70], while it decreases chemoreflex sensitivity and increases baroreflex sensitivity [71]. These actions all indicate increased parasympathetic (vagal) tone. Slow breathing also attenuates stress-related increases in skin conductance and finger pulse volume [72,73], which indicate reduced sympathetic activity [74]. Clinically, ANS function and emotional well-being are closely related [75]. In particular, yogic breathing was reported to improve clinical depression [76], and focused-attention Zen breathing reduced negative feelings [77]. In a prior study in Craig's lab, slow breathing reduced ratings of heat pain intensity and unpleasantness, and notably, it produced significant reductions in overall levels of negative affect [78].
In the lateralization model described above, the left anterior insula and anterior cingulate are associated predominantly with parasympathetic activity, positive emotions and ‘energy enrichment’, whereas the right anterior insula and anterior cingulate are associated predominantly with sympathetic activity, negative emotions and ‘energy expenditure’. The evidence of increased parasympathetic tone and reduced negative affect during controlled slow breathing suggests that slow breathing might facilitate positive affect associated with left forebrain activity, whereas fast breathing might facilitate negative affect and right forebrain activity. This proposal offers a simple test of the opponent homeostatic model of emotion.
We used passive exposure to positive and negative affective images to probe affective state—without engaging active emotional processing or appraisal—during the different autonomic conditions associated with slow or fast breathing. Specifically, this fMRI study examined activation within bilateral insula and anterior cingulate cortices during passive viewing of positive versus negative images within alternate epochs of controlled slow and fast breathing. Based on the lateralization model, we hypothesized that a positive affective state induced by slow breathing would facilitate activation of the left anterior insula and left cingulate during passive viewing of pleasant images (International Affective Picture System (IAPS); normed ratings for pleasant images: 7.32 ± 2.06), whereas a negative emotional state induced by fast breathing would facilitate activation of the right anterior insula and right cingulate during passive viewing of unpleasant images (from IAPS; normed ratings for unpleasant images: 2.24 ± 2.67).
The subjects performed a paced breathing task (see details in the electronic supplementary material, figure S4) in the scanner by synchronizing their breathing with the audible inhalation and exhalation of a professional yoga instructor performing a breathing exercise at a digitally synchronized slow (5 breaths min−1) or fast (20 breaths min−1) pace. Each individual's heart rate, HRV and respiration were continuously recorded. Subjects' breathing rates were nearly perfectly correlated with the expected breathing rates (mean ± s.e.m.: r = 0.995 ± 0.03, p < 0.01). The mean heart rate across subjects was significantly lower during slow compared with fast-breathing epochs (mean ± s.e.m.: 60.96 ± 0.36 beats min−1 (slow), 62.14 ± 0.36 beats min−1 (fast), p < 0.01, t = 3.39). Likewise, the overall HRV (figure 2, bottom right; as measured by SDNN) was significantly higher during slow compared with fast-breathing epochs (mean ± s.e.m.: 93.72 ± 2.53 (slow), 78.07 ± 2.53 (fast), p < 0.01, t = 6.4). Both of these findings signify increased parasympathetic modulation of heart rate during slow breathing [68–70]. Slight yet significant decreases in heart rate were also noted during viewing of pleasant compared with unpleasant images (mean ± s.e.m.: 61.5 ± 0.1 (pleasant), 62 ± 0.1 (unpleasant), p < 0.05, t = 2.5).
Figure 2.
Positive versus negative images during slow (a) and fast (b) controlled breathing. (a) Brain regions showing significant activation for the positive versus negative images during slow (5 breaths min−1) controlled breathing. A significant positive correlation (Spearman ρ, scatter plot) was found between left anterior insula activation during viewing of positive images and slow breathing and increased HRV (as measured by SDNN) during slow breathing. (b) Brain regions showing significant activation for the negative versus positive images during fast (20 breaths min−1) controlled breathing. Significant differences in the overall HRV measured by SDNN between slow- and fast-breathing epochs are demonstrated in the bar graph on the bottom right (median ± 95% CI). Left = right. ACC, anterior cingulate cortex. See text for details on significant activations; *** < 0.001.
The analysis of the fMRI data compared the blood-oxygen-level dependent (BOLD) signals during the display of positive images to the signals during the display of negative images in the regions of interest (ROIs) defined in the insular and cingulate cortices separately for both the epochs of slow breathing and the epochs of fast breathing. This balanced design mitigates the global differences in BOLD signal inevitably caused by the changes in blood CO2 level at different respiration rates [79,80]. No significant differences in CO2 level were observed between trials in which positive and negative images were displayed during epochs of either slow or fast breathing (ps > 0.05; see the electronic supplementary material for details). In epochs of slow breathing, greater BOLD signal was observed during passive viewing of positive images than during negative images in the left mid- and anterior insula (peak XYZ: −39/5/15, 320 mm3, t14 = 3.2) and left anterior cingulate (XYZ: −9/30/16, 320 mm3, t14 = 2.4), consistent with our hypothesis. Conversely, in epochs of fast breathing, increased BOLD signal was observed during passive viewing of negative compared to positive images in the right anterior cingulate cortex (peak XYZ: 6/32/20, 320 mm3, t14 = 2.8; figure 2). Critically, the increase in activation in the left insula during positive images and slow breathing was significantly correlated with increased HRV across subjects (Spearman ρ = 0.7 p < 0.01; figure 2, scatter plot).
In both slow- and fast-breathing epochs, no significant decreases were observed. That suggests that the autonomic state induced by slow breathing preferentially modulated positive affect (associated with passive viewing of positive images), while the autonomic state induced by fast breathing preferentially modulated negative affect (associated with passive viewing of negative images). Although we did not directly measure subjects' emotional experience in the current work in order to minimize attentional and appraisal influences on the induced affect, the observed brain activations and the correlated changes in heart rate and HRV support the inference that a positive affective state was induced by slow breathing and a negative affective state was induced by fast breathing in this study, consistent with the measured affective changes in our prior study [78].
Direct comparisons of concurrent activations on the left and right sides showed significantly asymmetric effects in the anterior cingulate cortex, as demonstrated in the conjunction map in figure 3. Specifically, in epochs of fast breathing, the right ACC was significantly more activated during passive viewing of negative images than it was during positive images, and that right ACC activation was significantly greater than the concurrent activity in the left ACC (p < 0.05, left bar graph). Conversely, in epochs of slow breathing the left ACC was significantly more activated during passive viewing of positive than negative images, and that activation was significantly greater than the concurrent activity on the right side (p < 0.05, right bar graph). Similar laterality comparisons of concurrent activations in the insula showed similar relationships but did not reach criterion (data not shown, p = 0.2).
Figure 3.
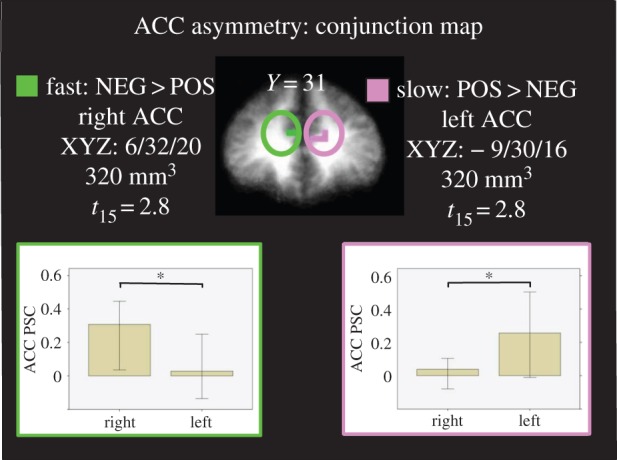
Conjunction map between negative versus positive images and fast breathing and positive versus negative images and slow breathing within the anterior cingulate cortex. Bar graphs (median ± 95% CI) indicate that increased right ACC during fast breathing and negative versus positive images was specific to the right ACC, while increased left ACC activation during slow breathing and positive versus negative images was specific to the left ACC. Left = right; * < 0.05; PSC, percent signal change.
Thus, we observed increased insular and cingulate activation in the left hemisphere in association with positive images during slow breathing, and increased cingulate activation in the right hemisphere in association with negative images during fast breathing. In addition, both slow breathing and pleasant images were significantly associated with reduced heart rate, and further, the interoceptive brain area activated by exposure to positively valenced images during slow breathing, the left insula, showed significant positive correlation with the increased HRV (i.e. parasympathetic tone) associated with slow breathing. Taken together, these results provide neurobiological evidence signifying asymmetric forebrain loci that are important for the interoceptive (respiratory) modulation of emotional affect and sympathovagal balance, which is consistent with the homeostatic model of adaptive (emotional) behaviour presented above.
6. Summary and conclusion
Interactions between the sympathetic and parasympathetic nervous systems are complex yet are important for healthy heart and healthy mind. We have provided evidence for the concept of coupled asymmetric regulation of feelings, emotions and sympathovagal balance in the homeostatic sensorimotor model of emotion. Our findings are consistent with the idea that the insular cortex in humans does not simply process interoceptive activity but rather integrates and modulates cardiovascular, respiratory and emotional signals in parallel bilaterally in order to create an integrated emotional experience.
First, our experimental findings in monkeys showed evidence consistent with a direct projection of cardiorespiratory vagal activity to the primary interoceptive cortex. It makes sense that homeostatic sensory input from both sympathetically and parasympathetically innervated sources ascend directly to primary interoceptive cortex in humans because that provides complete high-fidelity sensory information for the asymmetric regulation of sympathovagal balance. However, evidence in humans should be obtained and several confounding issues need to be addressed. The earlier studies reported that the NTS and PB projections to VMb in the monkey are almost entirely ipsilateral [57,81]), while, by contrast, we recorded strong vagal-evoked activity bilaterally in the monkey thalamus following stimulation of the left or right vagus nerve [62], consistent with evidence for bilateral PB projections in the rat [82]. We also found that the strongest vagal-evoked potential in the monkey thalamus is not in VMb but rather in the medially adjacent parafascicular nucleus, which projects to the rostral striatum [61,62], a finding that also was not seen by the earlier studies. In addition, the organization of these projections needs closer examination because: (i) trigeminal lamina I input to PB is mainly ipsilateral but spinal lamina I input is contralateral; (ii) the gustatory projection from posterior VMb (or VPMpc) to anterior Idfa is counter to the overall topography; and (iii) the right and left vagus nerves are themselves asymmetric (e.g. [83]).
Second, we provided initial evidence for asymmetric facilitation of affective processing in humans by voluntary manipulation of homeostatic processing. Although the present results were obtained with a modest sample size and analyses restricted to a priori ROIs, our observations contribute to accumulating literature supporting the integration of interoceptive and emotional activity, and the regulation of sympathovagal balance in the insular and cingulate cortices of the human brain [11–13,84]. These observations support practical behavioural methods that human beings have used for millennia to modulate emotional experience, such as meditative breathing. Nevertheless, the complexity of these interrelationships is emphasized by reports that slow breathing, guided by rhythmic (hexameter) speech or by Zen meditation, can also produce pronounced cardiorespiratory synchronization associated with optimal tissue oxygenation, with improved mood and with increases in both high- and low-frequency HRV [85,86]. Future studies that examine the coupling between interoceptive and affective activity would do well to focus on the asymmetric role of the anterior insula and anterior cingulate cortices in the regulation of sympathovagal and emotional balance.
Supplementary Material
Acknowledgements
We are grateful to Drs P. R. Hof, S. I. Ito, K. Krout, K. Semendeferi, C. C. Sherwood and A. N. Simmons for direct contributions to the original experiments described.
Ethics
All subjects gave written consent to participate in this study, which was approved by the University of California San Diego Human Research Protection Program. Research using monkeys was performed with the approval of the Institutional Animal Care and Use Committee of the Barrow Neurological Institute.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
Both authors made substantial contributions to the experiments described above and to the present report, and both authors approve of the version to be published.
Competing interests
We have no competing interests.
Funding
This work was supported in part by I01-CX-000816 from the U.S. Department of Veterans Affairs CS R&D Service and by the Barrow Neurological Foundation, with contributions by the National Chimpanzee Brain Resource (NINDS grant NS092988 to C. C. Sherwood et al.) and grant no. AG014308 (to J. M. Erwin for support of primate histological archives).
References
- 1.Sherrington CS. 1900. Cutaneous sensations. Textb. Physiol. 2, 920–1001. [Google Scholar]
- 2.Cameron OG. 2002. Visceral sensory neuroscience: interoception. New York, NY: Oxford University Press. [Google Scholar]
- 3.Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE. 1993. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 68, 988–1001. ( 10.1016/S0025-6196(12)62272-1) [DOI] [PubMed] [Google Scholar]
- 5.Cechetto DF, Shoemaker JK. 2009. Functional neuroanatomy of autonomic regulation. Neuroimage 47, 795–803. ( 10.1016/j.neuroimage.2009.05.024) [DOI] [PubMed] [Google Scholar]
- 6.Holstege G. 1992. The emotional motor system. Eur. J. Morphol. 30, 67–79. [PubMed] [Google Scholar]
- 7.Heimer L, Van Hoesen GW. 2006. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 30, 126–147. ( 10.1016/j.neubiorev.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 8.Pessoa L, Hof PR. 2015. From Paul Broca's great limbic lobe to the limbic system. J. Comp. Neurol. 523, 2495–2500. ( 10.1002/cne.23840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. 2015. How do you feel?: an interoceptive moment with your neurobiological self. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Citron FM, Gray MA, Critchley HD, Weekes BS, Ferstl EC. 2014. Emotional valence and arousal affect reading in an interactive way: neuroimaging evidence for an approach-withdrawal framework. Neuropsychologia 56, 79–89. ( 10.1016/j.neuropsychologia.2014.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith R, Baxter LC, Thayer JF, Lane RD. 2016. Disentangling introspective and exteroceptive attentional control from emotional appraisal in depression using fMRI: a preliminary study. Psychiatry Res. 248, 39–47. ( 10.1016/j.pscychresns.2016.01.009) [DOI] [PubMed] [Google Scholar]
- 13.Oppenheimer S, Cechetto D. 2016. The insular cortex and the regulation of cardiac function. Compr. Physiol. 6, 1081–1133. ( 10.1002/cphy.c140076) [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. 2003. Pain mechanisms: labeled lines versus convergence in central processing. Annu. Rev. Neurosci. 26, 1–30. ( 10.1146/annurev.neuro.26.041002.131022) [DOI] [PubMed] [Google Scholar]
- 15.Ma Q. 2010. Labeled lines meet and talk: population coding of somatic sensations. J. Clin. Invest. 120, 3773–3778. ( 10.1172/JCI43426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig AD. 2014. Topographically organized projection to posterior insular cortex from the posterior portion of the ventral medial nucleus in the long-tailed macaque monkey. J. Comp. Neurol. 522, 36–63. ( 10.1002/cne.23425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauernfeind AL, et al. 2013. A volumetric comparison of the insular cortex and its subregions in primates. J. Hum. Evol. 64, 263–279. ( 10.1016/j.jhevol.2012.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dum RP, Levinthal DJ, Strick PL. 2009. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J. Neurosci. 29, 14 223–14 235. ( 10.1523/JNEUROSCI.3398-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig AD. 2004. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J. Comp. Neurol. 477, 119–148. ( 10.1002/cne.20240) [DOI] [PubMed] [Google Scholar]
- 20.Kaas JH. 2013. The evolution of brains from early mammals to humans. Wiley Interdiscip. Rev. Cogn. Sci. 4, 33–45. ( 10.1002/wcs.1206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Striedter G. 2005. Principles of brain evolution, pp. 307–309. Sunderland, MA: Sinauer. [Google Scholar]
- 22.Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. ( 10.1007/s00429-010-0262-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodkind M, et al. 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315. ( 10.1001/jamapsychiatry.2014.2206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolls ET. 1999. The brain and emotion. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Denton D, McKinley M, Farrell M, Egan G. 2009. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cogn. 18, 500–514. ( 10.1016/j.concog.2008.06.009) [DOI] [PubMed] [Google Scholar]
- 26.Damasio AR. 1994. Descartes' error New York, NY: Avon Books. [Google Scholar]
- 27.Cabanac M, Massonnet B, Belaiche R. 1972. Preferred skin temperature as a function of internal and mean skin temperature. J. Appl. Physiol. 33, 699–703. [DOI] [PubMed] [Google Scholar]
- 28.Mower GD. 1976. Perceived intensity of peripheral thermal stimuli is independent of internal body temperature. J. Comp. Physiol. Psychol. 90, 1152 ( 10.1037/h0077284) [DOI] [PubMed] [Google Scholar]
- 29.Strigo IA, Carli F, Bushnell MC. 2000. Effect of ambient temperature on human pain and temperature perception. Anesthesiology 92, 699–707. ( 10.1097/00000542-200003000-00014) [DOI] [PubMed] [Google Scholar]
- 30.Craig AD. 2005. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn. Sci. 9, 566–571. ( 10.1016/j.tics.2005.10.005) [DOI] [PubMed] [Google Scholar]
- 31.MacNeilage PF, Rogers LJ, Vallortigara G. 2009. Origins of the left & right brain. Sci. Am. 301, 60 ( 10.1038/scientificamerican0709-60) [DOI] [PubMed] [Google Scholar]
- 32.Rogers LJ, Vallortigara G, Andrew RJ. 2013. Divided brains: the biology and behaviour of brain asymmetries. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Heilman KM. 2000. Emotional experience: a neurological model. Cogn. Neurosci. Emot. 2000, 328–344. [Google Scholar]
- 34.Gianaros PJ, Van Der Veen FM, Jennings JR. 2004. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 41, 521–530. ( 10.1111/1469-8986.2004.00179.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson RJ, Shackman AJ, Maxwell JS. 2004. Asymmetries in face and brain related to emotion. Trends Cogn. Sci. 8, 389–391. ( 10.1016/j.tics.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 36.Wittling W, Block A, Genzel S, Schweiger E. 1998. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia 36, 461–468. ( 10.1016/S0028-3932(97)00129-2) [DOI] [PubMed] [Google Scholar]
- 37.Wittling W, Block A, Schweiger E, Genzel S. 1998. Hemisphere asymmetry in sympathetic control of the human myocardium. Brain Cogn. 38, 17–35. ( 10.1006/brcg.1998.1000) [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Buckner RL, Liu H. 2014. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J. Neurosci. 34, 12 341–12 352. ( 10.1523/JNEUROSCI.0787-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomer R, Slagter HA, Christian BT, Fox AS, King CR, Murali D, Davidson RJ. 2013. Dopamine asymmetries predict orienting bias in healthy individuals. Cereb. Cortex 23, 2899–2904. ( 10.1093/cercor/bhs277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomer R, Slagter HA, Christian BT, Fox AS, King CR, Murali D, Davidson RJ et al. 2014. Love to win or hate to lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. J. Cogn. Neurosci. 26, 1039–1048. ( 10.1162/jocn_a_00544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens JS, Hamann S. 2012. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia 50, 1578–1593. ( 10.1016/j.neuropsychologia.2012.03.011) [DOI] [PubMed] [Google Scholar]
- 42.Duerden EG, Arsalidou M, Lee M, Taylor MJ. 2013. Lateralization of affective processing in the insula. Neuroimage 78, 159–175. ( 10.1016/j.neuroimage.2013.04.014) [DOI] [PubMed] [Google Scholar]
- 43.Guo CC, et al. 2016. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc. Natl Acad. Sci. USA 113, E2430–E2439. ( 10.1073/pnas.1519019113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tegeler CH, Shaltout HA, Tegeler CL, Gerdes L, Lee SW. 2015. Rightward dominance in temporal high-frequency electrical asymmetry corresponds to higher resting heart rate and lower baroreflex sensitivity in a heterogeneous population. Brain Behav. 5, e00343 ( 10.1002/brb3.343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht D. 2010. Depression and the hyperactive right-hemisphere. Neurosci. Res. 68, 77–87. ( 10.1016/j.neures.2010.06.013) [DOI] [PubMed] [Google Scholar]
- 46.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. 2008. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol. Psychiatry 63, 369–376. ( 10.1016/j.biopsych.2007.05.033) [DOI] [PubMed] [Google Scholar]
- 47.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 76, 258–266. ( 10.1016/j.biopsych.2013.11.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tegeler CH, Lee SW, Shaltout HA. 2014. Significance of right anterior insula activity for mental health intervention. JAMA Psychiatry 71, 336 ( 10.1001/jamapsychiatry.2013.3507) [DOI] [PubMed] [Google Scholar]
- 49.Nahas Z, et al. 2007. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 32, 1649–1660. ( 10.1038/sj.npp.1301288) [DOI] [PubMed] [Google Scholar]
- 50.Conway CR, et al. 2013. Association of cerebral metabolic activity changes with vagus nerve stimulation antidepressant response in treatment-resistant depression. Brain Stimulation 6, 788–797. ( 10.1016/j.brs.2012.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borckardt JJ, Anderson B, Kozel FA, Nahas Z, Smith AR, Thomas KJ, Kose S, George MS. 2006. Acute and long-term VNS effects on pain perception in a case of treatment-resistant depression. Neurocase 12, 216–220. ( 10.1080/13554790600788094) [DOI] [PubMed] [Google Scholar]
- 52.Kosel M, Brockmann H, Frick C, Zobel A, Schlaepfer TE. 2011. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Res. 191, 153–159. ( 10.1016/j.pscychresns.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 53.Pardo JV, et al. 2008. Chronic vagus nerve stimulation for treatment-resistant depression decreases resting ventromedial prefrontal glucose metabolism. Neuroimage 42, 879–889. ( 10.1016/j.neuroimage.2008.04.267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narayanan JT, Watts R, Haddad N, Labar DR, Li PM, Filippi CG. 2002. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia 43, 1509–1514. ( 10.1046/j.1528-1157.2002.16102.x) [DOI] [PubMed] [Google Scholar]
- 55.Conway CR, et al. 2012. Pretreatment cerebral metabolic activity correlates with antidepressant efficacy of vagus nerve stimulation in treatment-resistant major depression: a potential marker for response? J. Affect Disord. 139, 283–290. ( 10.1016/j.jad.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. 2008. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol. Psychiatry 63, 1171–1177. ( 10.1016/j.biopsych.2007.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckstead RM, Morse JR, Norgren R. 1980. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J. Comp. Neurol. 190, 259–282. ( 10.1002/cne.901900205) [DOI] [PubMed] [Google Scholar]
- 58.Saper CB. 2002. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 25, 433–469. ( 10.1146/annurev.neuro.25.032502.111311) [DOI] [PubMed] [Google Scholar]
- 59.Penfield W, Faulk ME. 1955. The insula: further observations on its function. Brain 78, 445–470. ( 10.1093/brain/78.4.445) [DOI] [PubMed] [Google Scholar]
- 60.Bachman DS, Hallowitz RA, MacLean PD. 1977. Effects of vagal volleys and serotonin on units of cingulate cortex in monkeys. Brain Res. 130, 253–269. ( 10.1016/0006-8993(77)90274-8) [DOI] [PubMed] [Google Scholar]
- 61.Ito S, Craig AD. 2008. Striatal projections of the vagal-responsive region of the thalamic parafascicular nucleus in macaque monkeys. J. Comp. Neurol. 506, 301–327. ( 10.1002/cne.21513) [DOI] [PubMed] [Google Scholar]
- 62.Ito S, Craig AD. 2005. Vagal-evoked activity in the parafascicular nucleus of the primate thalamus. J. Neurophysiol. 94, 2976–2982. ( 10.1152/jn.00235.2005) [DOI] [PubMed] [Google Scholar]
- 63.Ito S, Craig AD. 2008. Thalamocortical projections of the vagus-responsive region of the basal part of the ventral medial nucleus in monkeys. Soc. Neurosci. Abstract Online, 364.10 http://www.sfn.org/annual-meeting/past-and-future-annual-meetings. [Google Scholar]
- 64.Ito S, Craig AD. 2007. Afferent projections to the vagus-responsive region of the thalamic parafascicular nucleus in monkeys. Soc. Neurosci. Abstract Online, 417.11 http://www.sfn.org/annual-meeting/past-and-future-annual-meetings. [DOI] [PubMed] [Google Scholar]
- 65.Evrard HC, Logothetis NK, Craig AD. 2014. Modular architectonic organization of the insula in the macaque monkey. J. Comp. Neurol. 522, 64–97. ( 10.1002/cne.23436) [DOI] [PubMed] [Google Scholar]
- 66.Norgren R, Hajnal A, Mungarndee SS. 2006. Gustatory reward and the nucleus accumbens. Physiol. Behav. 89, 531–535. ( 10.1016/j.physbeh.2006.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pritchard TC, Hamilton RB, Morse JR, Norgren R. 1986. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J. Comp. Neurol. 244, 213–228. ( 10.1002/cne.902440208) [DOI] [PubMed] [Google Scholar]
- 68.Bernardi L, Gabutti A, Porta C, Spicuzza L. 2001. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J. Hypertens. 19, 2221–2229. ( 10.1097/00004872-200112000-00016) [DOI] [PubMed] [Google Scholar]
- 69.Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. 2001. Modulatory effects of respiration. Auton. Neurosci. 90, 47–56. ( 10.1016/S1566-0702(01)00267-3) [DOI] [PubMed] [Google Scholar]
- 70.Sakakibara M, Hayano J. 1996. Effect of slowed respiration on cardiac parasympathetic response to threat. Psychosom. Med. 58, 32–37. ( 10.1097/00006842-199601000-00006) [DOI] [PubMed] [Google Scholar]
- 71.Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L. 2000. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet 356, 1495–1496. ( 10.1016/S0140-6736(00)02881-6) [DOI] [PubMed] [Google Scholar]
- 72.McCaul KD, Solomon S, Holmes DS. 1979. Effects of paced respiration and expectations on physiological and psychological responses to threat. J. Pers. Soc. Psychol. 37, 564–571. ( 10.1037/0022-3514.37.4.564) [DOI] [PubMed] [Google Scholar]
- 73.Harris VA, Katkin ES, Lick JR, Habberfield T. 1976. Paced respiration as a technique for the modification of autonomic response to stress. Psychophysiology 13, 386–391. ( 10.1111/j.1469-8986.1976.tb00850.x) [DOI] [PubMed] [Google Scholar]
- 74.Oneda B, Ortega KC, Gusmao JL, Araujo TG, Mion D Jr. 2010. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens. Res. 33, 708–712. ( 10.1038/hr.2010.74) [DOI] [PubMed] [Google Scholar]
- 75.Thayer JF, Brosschot JF. 2005. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 30, 1050–1058. ( 10.1016/j.psyneuen.2005.04.014) [DOI] [PubMed] [Google Scholar]
- 76.Brown RP, Gerbarg PL. 2005. Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression. Part II--clinical applications and guidelines. J. Altern. Complement Med. 11, 711–717. ( 10.1089/acm.2005.11.711) [DOI] [PubMed] [Google Scholar]
- 77.Yu X, Fumoto M, Nakatani Y, Sekiyama T, Kikuchi H, Seki Y, Sato-Suzuki I, Arita H. 2011. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. Int. J. Psychophysiol. 80, 103–111. ( 10.1016/j.ijpsycho.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 78.Zautra AJ, Fasman R, Davis MC, Craig AD. 2010. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain 149, 12–18. ( 10.1016/j.pain.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 79.Giardino ND, Friedman SD, Dager SR. 2007. Anxiety, respiration, and cerebral blood flow: implications for functional brain imaging. Compr. Psychiatry 48, 103–112. ( 10.1016/j.comppsych.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Birn RM, Diamond JB, Smith MA, Bandettini PA. 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548. ( 10.1016/j.neuroimage.2006.02.048) [DOI] [PubMed] [Google Scholar]
- 81.Pritchard TC, Hamilton RB, Norgren R. 2000. Projections of the parabrachial nucleus in the old world monkey. Exp. Neurol. 165, 101–117. ( 10.1006/exnr.2000.7450) [DOI] [PubMed] [Google Scholar]
- 82.Krout KE, Loewy AD. 2000. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 428, 475–494. ( 10.1002/1096-9861(20001218)428:3%3C475::AID-CNE6%3E3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 83.Rogers RC, Hermann GE. 1983. Central connections of the hepatic branch of the vagus nerve: a horseradish peroxidase histochemical study. J. Auton. Nerv. Syst. 7, 165–174. ( 10.1016/0165-1838(83)90044-9) [DOI] [PubMed] [Google Scholar]
- 84.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. 2011. Brain systems for baroreflex suppression during stress in humans. Hum. Brain Mapp. 33, 1700–1716. ( 10.1002/hbm.21315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cysarz D, Bussing A. 2005. Cardiorespiratory synchronization during Zen meditation. Eur. J. Appl. Physiol. 95, 88–95. ( 10.1007/s00421-005-1379-3) [DOI] [PubMed] [Google Scholar]
- 86.Cysarz D, von Bonin D, Lackner H, Heusser P, Moser M, Bettermann H. 2004. Oscillations of heart rate and respiration synchronize during poetry recitation. Am. J. Physiol. Heart Circ. Physiol. 287, H579–H587. ( 10.1152/ajpheart.01131.2003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.




