Abstract
The processing and perception of individual internal bodily signals (interoception) has been differentiated to comprise different levels and processes involved. The so-called heartbeat-evoked potential (HEP) offers an additional possibility to examine automatic processing of cardiac signals. Knowledge on neural structures potentially supporting different facets of interoception is still sparse. One way to get insights into neuroanatomical function is to manipulate the activity of different brain structures. In this study, we used repetitive transcranial magnetic stimulation (rTMS) and a continuous theta-burst protocol to inhibit specific central locations of the interoceptive network including the right anterior insula and the right somatosensory cortices and assessed effects on interoceptive facets and the HEP in 18 male participants. Main results were that inhibiting anterior insula resulted in a significant decline in cardiac and respiratory interoceptive accuracy (IAc) and in a consistent decrease in perception confidence. Continuous theta-burst stimulation (cTBS) over somatosensory cortices reduced only cardiac IAc and affected perception confidence. Inhibiting right anterior insula and right somatosensory cortices increased interoceptive sensibility and reduced the HEP amplitude over frontocentral locations. Our findings strongly suggest that cTBS is an effective tool to investigate the neural network supporting interoceptive processes.
This article is part of the themed issue ‘Interoception beyond homeostasis: affect, cognition and mental health’.
Keywords: interoception, TMS, insula, somatosensory cortex, interoceptive accuracy, HEP
1. Introduction
Interoception is understood as the sensing and representation of signals concerning the internal state of the body [1,2]. As a general concept, interoception includes two forms of perception: proprioception (signals from the skin and musculoskeletal apparatus) and visceroception (signals from the inner organs such as heart rate, breath and hunger). Garfinkel & Critchley [3] first emphasized the importance of differentiating between different facets of interoceptive processing, suggesting taking the following into account: objective interoceptive accuracy (IAc; e.g. behavioural testing such as performance on heartbeat perception tests), metacognitive awareness (e.g. confidence-accuracy correspondence) and subjective interoceptive sensibility (e.g. as assessed via self-report questionnaires, e.g. body perception questionnaire). In a recent study, Garfinkel and co-workers [4] investigated these three levels of processing and could demonstrate that significant correlations between dimensions emerged only within the subgroup of individuals with higher IAc. They suggest that the relative balance of accuracy, sensibility and awareness dimensions could explain cognitive, emotional and clinical associations of interoceptive ability.
Referring to the study of Garfinkel et al. [4], IAc might be the core ability within the construct of interoception underpinning other interoceptive measures. Individuals differ substantially in measures of IAc, the ability to consciously perceive signals arising from the body. Measuring a person's ability to perceive and accurately report one's heartbeats at rest is often used to quantify these differences [1,5–9]. Other methods exist targeting the gastrointestinal or respiratory system [10–14], but only a few studies have investigated interoceptive abilities across different organ systems [10,15], suggesting that interoceptive abilities are associated across modalities to a certain extent.
The neural substrates of the distinct facets of interoceptive processing are still poorly understood. The insular cortex plays a major role as a cortical projection area of viscerosensory input [16]. Receiving direct viscerosensory input from the thalamus, the posterior and mid insular cortices are implicated as the primary viscerosensory cortices. However, other cortical (e.g. ventromedial prefrontal cortex, anterior cingulate cortex, somatosensory cortices) and subcortical (e.g. ventral striatum) regions as parts of the ‘interoceptive neural network’ also receive ascending interoceptive inputs to support the regulation of physiology and behaviour [17]. Khalsa et al. [18] further emphasized that next to insula and anterior cingulate cortices, primary and secondary somatosensory cortices innervating the skin of the chest area are also essential for the self-report of cardiac sensations. Pollatos et al. [19] showed that IAc as assessed by the heartbeat perception task according to Schandry [9] was positively correlated with blood-oxygen-level dependent (BOLD) activity at right insula, inferior parietal lobule and the medial frontal gyrus extending into the cingulate gyrus. Critchley et al. [5] used a heartbeat discrimination task and could demonstrate that interoceptive attention was associated with activation in anterior insula, lateral somatomotor and adjacent parietal cortices, anterior cingulate and supplementary motor cortices. IAc was strongest related to activity in right anterior insula/operculum. Importantly, the authors also examined interoceptive sensibility using questionnaire data and found positive correlations of subscales of the body perception questionnaire with anterior insula activity. Till now, existing research has been mainly based on correlational findings, and the effects of causal manipulation of brain activity in interoceptive areas by means of brain stimulation remain unknown.
Therefore, transcranial magnetic stimulation (TMS) is a way to experimentally manipulate brain activity in regions associated with certain aspects of interoceptive signal processing that might also be used as an innovative technique in several clinical groups characterized by alternations of interoceptive processing such as depression, eating disorders or anxiety disorders [6,12,20–23].
The so-called heartbeat-evoked potential (HEP) is another methodology for examining the cortical processing of cardiac afferent signals and assessing individual differences in interoceptive processing. HEPs are related to the automatic processing of cardiac information, and were initially thought to be independent of attentional processes involved in perceiving or detecting internal signals. The measurement of HEPs uses methods analogous to other evoked potentials. Typically, electrocortical (encephalographic, EEG) activity is averaged to individual heartbeats, using the electrocardiogram (ECG) R-wave [24]. Individual differences in HEP amplitude are related to IAc, as shown using heartbeat counting tasks [8,25–27]. The sources of the HEP have been localized to insular, somatosensory, rostral cingulate and prefrontal cortices [7]. Because afferent signals from the cardiovascular system continuously reach cortical structures, HEP amplitudes assessed during rest can be interpreted as indicators of central representation of cardiac interoceptive signals independent of overt attention or conscious awareness of cardiac sensations [28]. Therefore, the HEPs are one way to objectively measure one basic aspect of automatic afferent interoceptive processing.
In this context, two recent models of neural structures serving interoceptive processes and their connections to emotions are of great interest. A recent multihierarchical model of emotion processing proposed by Smith & Lane [29] suggests distinctions between discrete body features represented in somatosensory cortices, posterior insula, nucleus of the tractus solitarii, hypothalamic nuclei and the parabrachial nucleus, while so-called whole-body patterns are represented in the mid and anterior insula. Emotional concepts then occur by appraisal mechanisms involving other brain regions such as the anterior cingulate and the medial prefrontal cortices that refer to this mapping of the body-state. It is a multistage interoceptive/somatosensory process by which these body state patterns are detected and assigned conceptual emotional meaning [29]. These assumptions are in accordance with other studies showing that the insular cortex and the anterior cingulate cortex (ACC) play crucial roles in connecting interoceptive processes and emotions [24]. Their activation was found to be modulated by cognitive [30] and emotional factors in several studies [31,32]. This view is also in accordance with recent findings from interoception research [33,34] suggesting that subjective appraisal of interoceptive signals reflect processes independent of interoceptive perception accuracy and are related to mechanisms of self-regulation and top-down control.
A second branch of models refers to assumptions of predictive coding with respect to interoceptive processing. Friston et al. [35–37] suggested that proprioceptive and kinaesthetic perceptions of the body arise from predictions through the same computational principles of active inference as do visual and auditory perceptions of the external world. Comparable ideas on predictive coding have been summarized and used by Seth et al. [38] in order to outline how interoception is associated with conscious presence and its disturbances. The core concept of this model is that a sense of presence arises when informative interoceptive prediction signals are successfully matched to inputs, so that prediction errors are suppressed. Brain areas contributing to interoceptive predictive coding include specific brainstem, subcortical and cortical (insular, orbitofrontal, anterior cingulate) regions. Seth et al. [38] suggest that these regions potentially form at least a loose hierarchy: posterior and mid insula support the primary cortical representation of interoceptive signals, with the anterior insula operating as a comparator error module (see [5,39,40]).
Up to now, only a few studies exist testing whether damage or disturbance of critical neural structures involved in interoceptive processing leads to assumed deficits. Ronchi et al. [41] demonstrated in one patient that IAc as measured by the heartbeat detection task by Schandry was decreased after right insula resection. Terasawa and co-workers [42] also found reduced IAc in patients suffering from insula damage. Up to today, there is no study that has investigated whether an experimental approach using TMS might affect interoception comprising different aspects and levels of interoceptive processing.
Here, we investigated whether interoceptive processes were disturbed by TMS. Depending on the parameters of stimulation, it has been suggested that repetitive TMS may either increase or decrease the excitability of the targeted cortical structure (see [43–45]). As Huang and co-workers [44] summarized, several studies have shown that when delivered over the primary motor cortex (M1), continuous theta-burst stimulation (cTBS) decreases M1 excitability when delivered in a continuous fashion. Eighteen healthy young male participants received either cTBS over right insular and right somatosensory cortices or sham stimulation over occipital areas before performing heartbeat detection and a respiratory load estimation task. Furthermore, the HEP during heartbeat perception (as the most objective marker of automatic processing of cardiac information), assessed after stimulation, was also examined. We hypothesized that cTBS over somatosensory and insular cortices would yield a decrease in accuracy of interoceptive signal processing. We also hypothesized a deflation of the HEP amplitude after somatosensory and insula stimulation. An alternation of the emotional aspects of interoceptive signals was hypothesized after insula inhibition only. A modulation of interoceptive sensibility was assumed after insula stimulation, as this facet refers especially to ‘whole-body’ patterns. Whether confidence of interoceptive signal processing is affected by cTBS over insula and somatosensory cortices should be assessed in an exploratory fashion.
2. Methods
(a). Participants
Eighteen healthy male participants were recruited by advertisement at the University of Ulm. Mean age was 23.9 years (s.d. 2.9). In order to reduce variance owing to menstrual cycle [46,47], we recruited males only. The sample was not selected with respect to any other criteria. They gave written informed consent prior to the study and received compensation of €60. Exclusion criteria were history of brain injury, past or current psychiatric or neurological disorders, severe somatic illness or intake of medication as assessed by anamnestic questionnaire. Experiments were conducted in accordance with the Declaration of Helsinki with the approval of the local ethics committee.
(b). Procedure
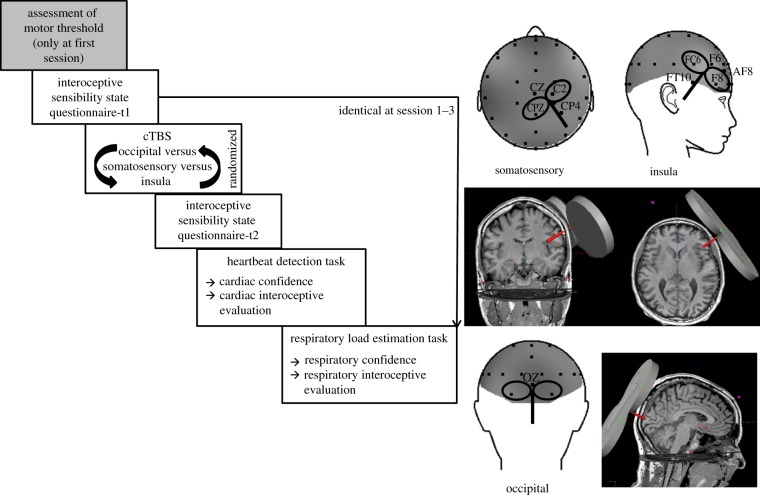
Participants were informed about the study by staff and they received written information about the experiment. At each data collection time, participants were examined at the laboratories of the Clinical and Health Psychology department in Ulm. First, there was a screening session in which the tasks used in the stimulation sessions were introduced and they were made familiar with the TMS procedure. At this screening, exclusion criteria and important side effects of cTBS were explained. From 20 participants screened, 18 took part in the main experiment. At the first session of the main experiment, the participants were seated in a sound-attenuated chamber, which was connected to the neighbouring equipment room by intercom. Passive electrodes for EEG (32 channels using a cap, see below), ECG, respiration (belt), electromyography (EMG) (first dorsal interosseous, FDI; muscle of the left hand) and electrooculograms (EOG) were attached and individual resting motor threshold was assessed over left primary motor cortex using a PowerMAG 100 stimulator (Mag & More, Munich, Germany) with biphasic pulses and a figure-of-eight coil (PMD70). First, optimal cortical representation of FDI muscle (motor hot spot) was assessed by determining a coil position over the EEG cap yielding the highest amplitude of the motor evoked potential (MEP). Resting motor threshold (MEPs > 100 µV in at least 5/10 trials, cf. [48]) was determined, and cTBS intensity was calibrated to 80% of resting motor threshold in accordance with former studies [49]. This value was then used for all stimulation protocols of the participant including the second and third experimental session. All three experimental sessions were identical to the first one except for the fact that the motor threshold was assessed only at the first session. The stimulation site (right insular, right somatosensory cortices or central occipital cortex as sham location) was selected using a randomized protocol for all three experimental sessions, i.e. each participant was stimulated three times in a repeated measurements design with identical test protocols. The experimental procedure is depicted in figure 1.
Figure 1.
Experimental procedure. Anatomical details of the sites of stimulation are depicted using EEG positions and structural MRI pictures together with TMS target locations.
Coil position for each stimulation site was located on the basis of the 10/20 system supplied by the EEG cap (see below). Optimal coil positions with respect to the 10/20 system were derived in the following way: TMS neuronavigation (PowerMag View) was used with standard head model, and the positions derived were validated in two subjects prior to the experimental sessions using their structural magnetic resonance imaging (MRI) images while wearing an EEG cap to define coil positions targeting the three stimulation sites (see also [50,51]). Target areas of stimulation were obtained using MRI structural information and an MRI atlas as depicted in figure 1.
(a) The right anterior insula was reached with a coil position over frontotemporal locations building a triangle corresponding to EEG positions FT10, F8, FC6, pointing to FT8 (F6/AF8). (b) The primary and secondary somatosensory cortices, chest/trunk location, were reached with the centre of the coil within a triangle corresponding to EEG positions CPZ, C2 and CP4, directed to CZ. Primary and secondary somatosensory cortices of the trunk are not distinguishable by means of TMS navigation. (c) Central occipital cortex (V2/V3) was reached with the coil placed over OZ, directed straight to the horizontal plane. In all experimental sessions, the coil was placed according to these EEG coordinates. Coil positioning and TMS target areas are depicted in figure 1.
The experimental design is summarized in figure 1 and was the following: first, participants had to rest for 5 min, while baseline measures (heart rate, breathing) were recorded with a sampling rate of 1000 Hz. They then filled in an interoceptive sensibility state questionnaire designed to assess the momentary subjective perception of bodily sensations focusing on the perception of five bodily systems (heart beat, respiratory functions, trembling, temperature/sweating, gastrointestinal sensations; see examples in the electronic supplementary material, Methods section).
Next, the cTBS took place with 600 pulses applied in 40 s (bursts of three pulses at 50 Hz every 200 ms (5 Hz)) over one of three locations according to the random scheme. After stimulation, participants were asked to fill in the interoceptive sensibility state questionnaire once again. Then, the heartbeat detection task took place. During the first heartbeat perception task, EEG was also assessed and later used for HEP analyses (see below). Four heartbeat counting phases of the mental tracking method were used (see [8,9] and also the electronic supplementary material). After each trial, they were asked to report their confidence of their estimation using a nine-point Likert scale, where one endpoint was labelled ‘no confidence at all’, and the other endpoint was ‘complete confidence’ (see [4]). At the end of the task, the participants were asked to evaluate their feelings during heartbeat perception (cardiac interoceptive evaluation; see the electronic supplementary material).
IAc was calculated as the mean heartbeat perception score according to the following transformation
IAc scores typically range from 0 to 1. Higher scores indicate small differences between the counted and recorded heartbeat and consequently a better IAc.
The respiratory load estimation task took place next. As no standard test for IAc in the respiratory system exists, we designed a task similar to the one used by Petersen et al. [11,12]. The task manipulated breathing effort via respiratory load by switching the diameter of a breathing device (Pari PEP® S-system) resulting in varying expiratory resistance (diameters 1–5 mm, eight levels, in 0.5 mm steps diameter change). Participants were instructed to maximally expire with constant flow as naturally as possible and not to apply high levels of effort or force in doing so; as the resistance levels applied were rather low, the impact of breathing flow or effort on performance is expected to be negligible. Participants were asked to judge respiratory effort on an eight-point Likert scale, using the highest and lowest loads as anchors. Several training intervals were used to make the participants familiar with the range of the scale used. The test then used six trials with varying diameters. A respiratory accuracy score was calculated analogous to the heartbeat perception task, using the weighted difference scores of the six trials according to the following formula
Identical to the heartbeat detection, participants rated confidence after each trial. At the end of the task, participants reported valence, arousal and anxiety when attending to their respiratory functions.
Afterwards, another paradigm was used that will be not reported here. The first session lasted about 2 h, the second and third sessions lasted about 90 min.
(c). Psychophysiological recording
During the heartbeat perception task, EEG activity was recorded continuously from 32 leads (FP1, FPZ, FP2, AF3, AFZ, AF4, F7, F5, Fz, F6, F8, FC5, FCZ, FC6, C5, C1, CZ, C2, C6, CP5, CP1, CPZ, CP2, CP6, P5, PZ, P6, PO3, PO4, O1, OZ, O2), using the Easy-Cap electrode system (Falk Minow Services, Germany) with non-polarizable passive Ag/AgCl electrodes at equidistant positions. Cz served as a reference and the ground electrode was attached at electrode position F4. Horizontal and vertical EOGs were recorded. Impedances were maintained below 5 kΩ. The signals were amplified using an active amplifier system (Brain Products, Germany) and digitized at a sampling rate of 1000 Hz. ECG was measured with non-polarizable Ag/AgCl electrodes placed at the right clavicula and left chest. ECG, EMG and respiration were recorded equivalent to the EEG using an amplifier system (Brain Products, Germany) and were digitized at a sampling rate of 1000 Hz.
All steps of EEG analysis were conducted with the Brain Vision Analyzer v. 2.1 (Brain Products, Germany). ECG R-waves were detected either automatically online or offline semiautomatically in ECG raw data for later EEG averaging. EEG was visually inspected and filtered (0.05–20 Hz). EEG data were examined for ocular, muscular and other sources of artefacts. Trials were rejected from the analysis if the voltage exceeded ± 50 µV ms−1 in any channel. EEG data were segmented relative to the detected R-wave triggers, whereby segments were built in epochs ranging from 200 ms before the R-wave trigger to 800 ms after the R-wave trigger. After baseline correction (200 ms pre R-wave trigger as baseline), average HEP waves were computed of R-triggered EEG segments. HEP amplitude analyses were conducted in accordance with former studies, using a time window of 400–600 ms after R-wave during which the electric field artefact is assumed to be negligible (see [52,53]).
(d). Data analyses
Data analyses were performed with the program SPSS (v. 22). Referring to the cardiac and respiratory system, separate repeated-measures ANOVAs were calculated with the factors location (I, S, O) examining IAc, confidence and interoceptive evaluation (valence, arousal, anxiety). Furthermore, interoceptive sensibility was examined with the factors location (I, S, O) and time (pre-stimulation, post-stimulation). For HEP analyses, we selected frontocentral electrodes with highest HEP amplitudes for statistical evaluation and conducted repeated-measures ANOVAs with the factors location (I, S, O) and electrode. Statistical significance levels reported correspond to p-values less than 0.05, 0.01 and 0.001, respectively. In the Results section, uncorrected F-values are reported together with the Greenhouse–Geiser epsilon values and corrected degrees of freedom. In case of multiple comparisons, we used the Bonferroni correction to adjust the alpha errors.
3. Results
(a). Cardiac interoceptive accuracy
The mean obtained heartbeat perception scores for the three locations are summarized in figure 2 depicting the locations I, S and O. We observed a significant main effect of location (F2,34 = 6.67; p < 0.01; η2 = 0.28; ɛ = 0.80): post hoc Bonferroni corrected comparisons revealed that IAc was significantly lower after stimulation over right insula as well as over right somatosensory cortices when compared with occipital stimulation (ps < 0.05).
Figure 2.
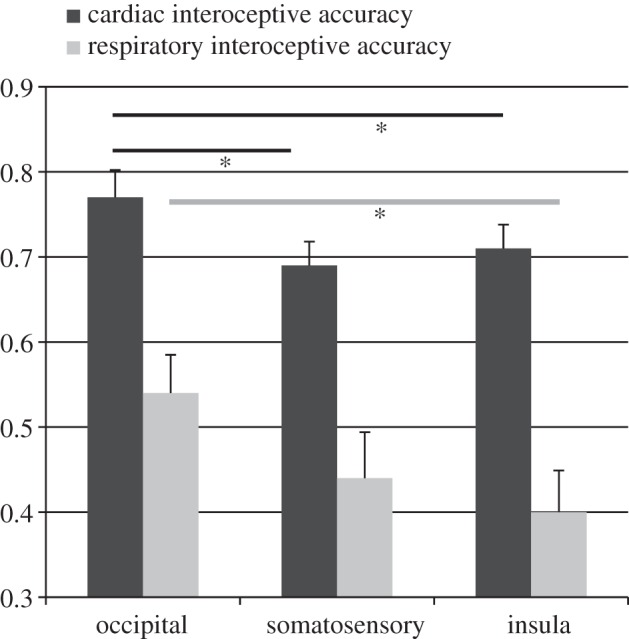
Cardiac and respiratory interoceptive accuracy (n = 18; mean scores ± s.e.m.) contrasting the three cTBS locations. (Asterisks represent a significant difference; *p < 0.05.)
(b). Cardiac confidence
Figure 3 summarizes the mean obtained confidence scores for the three locations. We observed a significant main effect of location (F2,34 = 6.36; p < 0.01; η2 = 0.27; ɛ = 0.87): confidence was significantly lower after insula stimulation when compared with somatosensory stimulation (p < 0.05). No other significant differences were observed.
Figure 3.
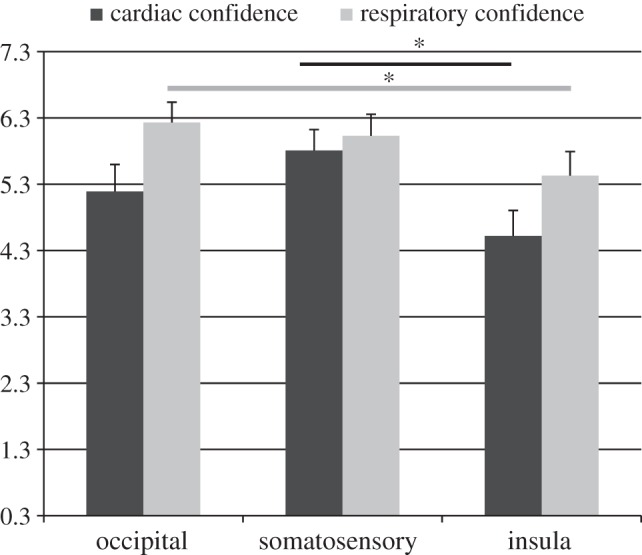
Cardiac and respiratory confidence (n = 18; mean scores ± s.e.m.) contrasting the three cTBS locations. (Asterisks represent a significant difference; *p < 0.05.)
(c). Cardiac interoceptive evaluation
Valence, arousal and anxiety during heartbeat perception are summarized in table 1. Referring to anxiety, a significant main effect of location (F2,34 = 6.36; p < 0.01; η2 = 0.27; ɛ = 0.87) showed that after insula cTBS anxiety was significantly higher when compared with both other locations.
Table 1.
Subjective evaluation of cardiac (c) and respiratory (r) perception contrasting the three cTBS locations (*p < 0.05).
| occipital mean (s.d.) |
somatosensory mean (s.d.) |
insula mean (s.d.) |
ANOVA main effect location |
|||||
|---|---|---|---|---|---|---|---|---|
| c | r | c | r | c | r | c | r | |
| valence (range 1–9) | 7.03 (1.41) | 6.85 (1.26) | 6.68 (1.62) | 6.67 (1.45) | 6.53 (1.63) | 6.67 (1.56) | F2,34 = 2.12; p = 0.14 | F2,34 = 0.34; p = 0.70 |
| arousal (range 1–9) | 1.88 (0.88) | 1.64 (0.56) | 1.78 (0.90) | 1.91 (1.07) | 2.33 (1.47) | 2.20 (1.24) | F2,34 = 2.09; p = 0.15 | F2,34 = 2.41; p = 0.11 |
| anxiety (range 1–9) | 1.01 (0.06) | 1.15 (0.36) | 1.28 (0.54) | 1.37 (0.82) | 1.53 (0.86) | 1.44 (0.37) | F2,34 = 4.09* η2 = 0.19; ɛ = 0.61 | F2,34 = 1.76; p = 0.19 |
(d). Respiratory interoceptive accuracy
Respiratory IAc scores are depicted in figure 2. We observed a significant main effect of location (F2,34 = 3.44; p < 0.05; η2 = 0.17; ɛ = 0.58). Post hoc Bonferroni corrected comparisons revealed that IAc was significantly lower after stimulation over right insula when compared with occipital stimulation (p < 0.05), whereas the observed smaller IAc after somatosensory cTBS did not reach significance.
Cardiac and respiratory IAc were positively correlated after cTBS over somatosensory cortex (r = 0.39, p < 0.05), whereas correlation coefficients for the other locations were not significant (occipital: r = 0.18, p = 0.24; insula: r = 0.25, p = 0.16).
(e). Respiratory confidence
Figure 3 summarizes the mean obtained confidence scores for the three locations. We observed a significant main effect of location (F2,34 = 6.14; p < 0.05; η2 = 0.27; ɛ = 0.77): confidence was significantly lower after cTBS over right anterior insula when compared with occipital stimulation (p < 0.05).
(f). Respiratory interoceptive evaluation
Valence, arousal and anxiety during respiratory load estimation are summarized in table 1. No significant effects were observed.
(g). Interoceptive sensibility
Mean obtained IS scores are summarized in figure 4. We observed a significant main effect of time (F1,17 = 9.84; p < 0.01; η2 = 0.37; ɛ = 0.84) and a significant interaction for location × time (F2,34 = 4.99; p < 0.05; η2 = 0.23; ɛ = 0.61). Post hoc tests showed that mean IS increased only after insula and somatosensory stimulation when compared with before cTBS stimulation (I: F1,17 = 19.95; p < 0.001; η2 = 0.54; ɛ = 0.99; S: F1,17 = 8.46; p < 0.01; η2 = 0.33; ɛ = 0.78).
Figure 4.
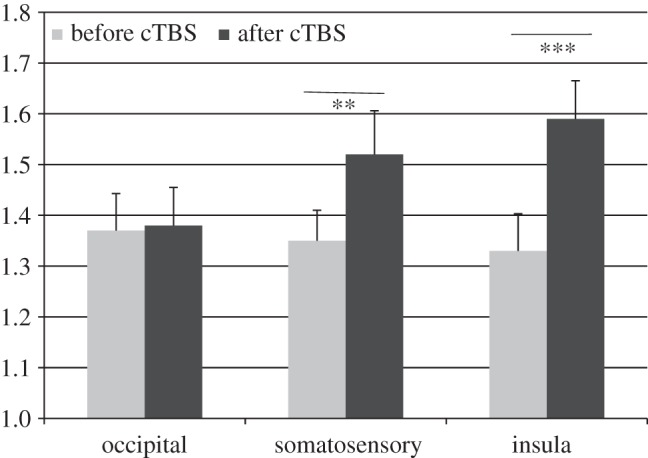
Group (n = 18) average (±s.e.m.) effect of cTBS on interoceptive sensibility as assessed by state questionnaire. (Asterisks represent a significant change in subjective report; **p < 0.01; ***p < 0.001.)
(h). Heartbeat-evoked potentials
Mean HEPs distribution and HEP amplitudes (at right frontocentral and central locations) are depicted in figure 5 contrasting the three cTBS locations.
Figure 5.
(a) HEP amplitudes at right frontocentral and central locations (FC6, C6). (b) HEP scalp distribution at 500 ms post R-wave. (c) HEP distribution over 19 electrodes.
Owing to technical problems, only 15 complete datasets could be included. After visual inspection of the data, we selected FC6 and C6 (in accordance with former research also reporting the most pronounced HEP amplitudes at frontocentral and central locations [8,54]) as the electrodes for statistical analyses.
The repeated-measures ANOVA yielded significant main effects for location (F2,28 = 3.95; p < 0.05; η2 = 0.22; ɛ = 0.57). Post hoc Bonferroni corrected comparisons revealed that the HEP amplitude was significantly lower after stimulation over right insula when compared with occipital stimulation (p < 0.05), whereas the observed smaller HEP amplitude after somatosensory cTBS when compared with occipital stimulation did not reach significance (trend level at C6: p = 0.08).
4. Discussion
This study showed differential effects for IAc, interoceptive sensibility, subjective feelings and confidence judgements evoked by cTBS of right anterior insular and right somatosensory cortices (S1–S2, chest region). As hypothesized, compared with inhibition of occipital cortices as a reference condition, cTBS of right anterior insula and right somatosensory cortices resulted in a significant decline in cardiac IAc, while only cTBS of right anterior insula significantly reduced respiratory IAc. Cardiac and respiratory accuracy were positively correlated after cTBS over the somatosensory location, whereas for the two remaining locations, correlation coefficients were not significant. Larger sample sizes are needed to support our interpretation that similar to observed correlations between perception accuracy in the cardiovascular and gastrointestinal system [10,15], there is a comparable, but weaker association between these two inner systems.
Whether confidence of interoceptive signal processing is affected by cTBS over insula and somatosensory cortices was assessed in an exploratory fashion. Interestingly, analyses of confidence judgements of participants' perception accuracy demonstrated that individuals' confidence judgements were reduced after anterior insula stimulation for both cardiac and respiratory signals, whereas somatosensory inhibition affected only cardiac confidence in the opposite direction, i.e. here participants were more convinced of having accurately perceived their heartbeats although objective perception accuracy declined. These results suggest that while inhibition of anterior insula reduces both IAc and confidence judgements in a consistent way, inhibition of somatosensory cortices is associated with a dissociation of these processes. This interpretation is in accordance with assumptions proposed by Garfinkel and co-workers [4] highlighting that the relative balance of accuracy, sensibility and awareness dimensions could account for cognitive, emotional and clinical associations of interoceptive ability.
Going beyond IAc, cTBS of right anterior insula and right somatosensory cortices led to an immediate increase in interoceptive sensibility as assessed by self-report. As former studies found a correlation between insula activation and sensibility [5], and this facet of interoceptive processing refers especially to the idea of ‘whole-body’ patterns as introduced by Smith & Lane [29], a modulation of interoceptive sensibility was assumed to occur after insula stimulation. The fact that cTBS inhibiting somatosensory cortices also modulated interoceptive sensibility in the same direction as cTBS over right anterior insula highlights that, within the assumed hierarchical structure of interoception, hampering a region processing a discrete body feature leads to greater ‘noise’ in the whole system. Referring to predictive coding [38], the anterior insula is the key error module that might detect such ‘noise’ in the system. This might then lead to an increase of attentional processes examining ongoing discrete body sensations and therefore explain why our participants were more aware of a variety of bodily sensations including cardiac and respiratory functions, but also trembling, temperature/sweating and gastrointestinal sensations. The latter underscores that effects of cTBS definitely occurred and affected bodily sensations.
As hypothesized, an alternation of the emotional aspects of interoceptive signals as captured by the interoceptive evaluation was modulated only in relation to anxiety after inhibition of anterior insula. This underpins its pivotal role for processes related to anticipation of aversive stimuli or anxiety disorders [55]. According to the model of Smith & Lane [29], it might be argued that inhibiting anterior insula functions could have primarily disturbed ‘whole-body patterns’ and therefore, the reduction of IAc becomes evident or conscious to the participants, leading to a corresponding decline in metacognitive beliefs of perception security. In a similar way, together with models of ‘interoceptive prediction error’, this could explain the increases of self-reported subjective feelings of anxiety and arousal and more aversive feelings after inhibition of anterior insula. Considering the role of the insula in integrating bodily representations, affective and cognitive representations and expectations, disturbance of its function should evoke an incoherence or mismatch of the patterns of ongoing real-time basic body feedbacks, subjective feelings and beliefs about it, as well as predictions of bodily reactions based on experience. This disturbance of the interoceptive network in its basic function, as subserved by the insula, may thus induce feelings of general discomfort, arousal and anxiety, i.e. feelings of a vague conscious notion that an otherwise intact interplay between these processes and its neuroanatomical basis has been disturbed.
Our results support the notion that the anterior insula is also a key structure for metacognitive awareness of interoceptive processes. This interpretation is in accordance with Farb et al. [56], who suggested that anterior insula activity during interoception may reflect endogenous attention demands associated with manipulation of interoception at the level of conscious awareness. Additionally, there is evidence that the insular cortex supports different levels of representation of current and predictive states and that anterior insula activity may also reflect feelings of general uncertainty during interoception [57]. In the EPIC model of Barrett & Simmons [58], interoceptive perception is largely a construction of beliefs that are kept in check by the actual state of the body. This is achieved by an ‘interoceptive system’ in the frontal cortex including the cingulate and the anterior insula [58] and could explain why anterior insula inhibition could also affect the whole interoceptive system including the beliefs about one's accuracy.
This also fits with assumptions of the interoceptive predictive coding model of conscious presence in which the anterior insula is the likely locus of a key neural comparator mechanism [38]. This view might also explain why an inhibition of somatosensory cortices does not systematically reduce confidence as demonstrated for cardiac and respiratory signals in this study. Leaving the anterior insula function intact does not compromise the basic integration of ‘whole-body’ representations, including feelings of general discomfort as described above; however, it disturbs another process that is possibly related to the evaluation/appraisal and expectations of and the matching to on-going discrete bodily reactions that have been suggested to be associated with somatosensory cortices [29]. Furthermore, tactile input from the chest might play a role in the detection of heartbeat. While it is clear that the mechanical contraction of the myocardium generates the stimuli for heartbeat detection, the convergence of cardiac with somatosensory afferences during the transmission of information results in subjects localizing cardiac sensations in those parts of the body that share afferent trajectories with the heart.
Because cutaneous sensations are more distinctly represented than visceral sensations and therefore more discriminable, sensations originating in the viscera are often referred to the skin [59]. Therefore, hampering somatosensory input from the chest region might affect cardiac perception to a greater extent than is the case for respiratory perception. Favouring this assumption, Khalsa et al. [18] demonstrated that somatosensory afferents from the skin can also contribute to cardiac judgements. Another reason why effects on the respiratory load estimation task and related measures of confidence and evaluation were less pronounced might refer to the fact that we applied a fixed order (figure 1), with assessment of the respiratory system always after the cardiovascular system. Although the prior parts of the experiment were rather short (about 8–10 min), and though there are several studies showing that cTBS effects last at least 20 min or significantly longer [44,60], it cannot be ruled out that the effects of cTBS were already diminishing when compared with the assessment of interoceptive processes in the cardiovascular system.
Following Smith & Lane [29], discrete body features are represented in somatosensory cortices and disturbing these discrete features might affect the relative balance of accuracy, sensibility and confidence dimensions. Favouring this assumption, both insula and somatosensory stimulation led to an increase of interoceptive sensibility as assessed by questionnaire. The HEP as a marker quantifying basic processes of automatic cardiac signal processing was found to be reduced after inhibition of anterior insula. We suppose that the HEP primarily mirrored bottom-up guided automatic cardiac signal processing [7]. As sources of the HEP are located in insular, somatosensory, cingulate and prefrontal cortices (see [7]), both cTBS locations might affect these HEP generators. The observed HEP modulation goes along with a reduction of IAc on a behavioural level for cardiac signals.
An additional hint that the interplay between different levels of interoceptive processes was affected might be the fact that cardiac IAc was not correlated to the HEP amplitude at central and frontocentral locations as observed in some former studies [8]. Mirroring behavioural results, effects on the HEP amplitude were more pronounced after insula cTBS suggesting that at least during overt attention to one's heartbeats—there was no baseline or rest condition after cTBS to compare with—this region might be more crucial for the integration of cardiac signals as inhibition of right somatosensory cortices affected the HEP amplitude to a lower extent. This assumption confirms ideas that the ability to be ‘aware’ of interoceptive signals is thought to depend upon further propagation of interoceptive signals to the anterior insula, which has been argued to reflect the integration of afferent physiological signals with higher-order contextual information [17,61], thereby becoming consciously accessible, enabling a subjective affective experience or feeling state [2].
There are several issues to be critically discussed in this study. One essential point is that there is no guarantee of reaching the anterior insula with a TMS coil positioned over the skull. We would argue that the effects obtained seem to be specific enough and in accordance with proposed theoretical models to say that anterior parts of the right insula were indeed targeted. Second, further support stems from research on the auditory cortex where the topographic situation is directly comparable to the attempts to stimulate primary auditory cortex in the case of tinnitus. It has been shown that chronic tinnitus with a functional correlate in primary auditory cortex can be modulated by rTMS [62,63], despite the larger distance of the coil to the primary auditory cortex (planum temporale [64]). Because we compared effects from the frontal stimulation site with two other sites, we are sure that the frontal effects are not unspecific but related to the particular stimulation site, at least close to the anterior insula. An important limitation refers to the fact that there were no baseline assessments of the HEP and the IAc tasks before the respective stimulation of brain areas. As we already used a repeated measurements design with three sessions per participant, we wanted to reduce effects of repetition on all tasks used. Other experimental set-ups assessing only one or two locations in each participant and using a between-group design might be used in future to address this issue.
An important limitation refers to the fact that IAc in this opportunistic sample was higher than in other studies [65–67]; after occipital stimulation, the observed mean score was 0.77 when compared with, for example, 0.69 observed in a large sample of more than 400 healthy participants [66]. A cause for this might be that we assessed only male participants who have higher IAc in some studies [1,68]. It might also be the case that the TMS procedure itself increased stress and sympathetic activation which then, in turn, could facilitate heartbeat detection. Other studies could show that a sympathetic increase as induced by short-term food deprivation [33] or physical stress [69] increased IAc. Future studies should therefore use more participants with a greater distribution of IAc to examine whether TMS effects interact with the absolute level of IAc as found for the interplay of different interoceptive levels in a former study [4]. We nevertheless argue that changes in interoceptive processes on various levels, as well as modulation of the HEP amplitude, are favouring the interpretation that also in a sample with substantially lower IAc similar effects are to be expected. Another limitation is that this study concentrated on right lateral effects of cTBS on structures of the interoceptive network. This can be explained by the fact that right anterior insula has been identified as the most significant site that is strongly related to IAc [5,19]. The right-hemisphere anterior insular cortex provides one cortical focus for the integration of exteroceptive and interoceptive signals [17,70] and this region has been proposed as a neural substrate for self-awareness in the form of the ‘material me’ [71]. This may be most relevant for effects of interoceptive metacognition and interoceptive sensibility, and their relation to effects of IAc, as there is ample evidence for hemispheric laterality of emotion processing and cognitive functions as well as interhemispheric interplay [72].
In conclusion, this is the first study that has demonstrated that cTBS inhibiting right anterior insula and right somatosensory cortices efficiently disturbs different facets of interoceptive processing. Inhibiting anterior insula resulted in a significant decline in cardiac and respiratory IAc and in a coherent decrease in perception security. cTBS over somatosensory cortices reduced cardiac IAc and affected metacognitive awareness. Inhibiting right anterior insula and right somatosensory cortices increased interoceptive sensibility. The HEP, as marker of automatic cardiac signal processing, was decreased after cTBS over right anterior insula. Our findings strongly suggest that cTBS is indeed an effective tool to investigate the neural network supporting interoceptive processes. However, it remains to be further elucidated why cardiac and respiratory perception did not perfectly match and how long TMS effects on interoceptive signal processing remain detectable. Obviously, future studies should include a broader range of interoceptive tasks as well as a longer observation interval and should also consider potential differential effects of hemispheric laterality when investigating the function of interoceptive target regions of the brain via stimulation techniques. As interoceptive processes are altered in several psychopathologic syndromes [6,12,21–23], brain stimulation techniques are a promising tool for interventions. While cTBS is used to inhibit certain brain regions, other TMS protocols could be applied to improve the access to bodily sensations and empower concomitant therapeutic approaches as demonstrated for various disorders, e.g. in the decrease of relapse rates in depression [73]. More clinical research is needed to further elucidate future therapeutic avenues.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Immo Curio for his support in setting up the laboratory devices and connecting them. We also thank David Scholz for his support in programming the experimental design, and Julia Braun and Viktoria Probst for their help in data assessment and participants' recruitment.
Ethics
Ethical approval by the local ethics board was obtained. Written informed consent was obtained.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
O.P., B.H., T.K. and S.M. substantially contributed to conception, design and acquisition of the data. O.P. and S.M. analysed the data. O.P., B.H., T.K. and S.M. interpreted the data, drafted the manuscript and approved the version submitted.
Competing interests
We hereby state that we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Cameron OG. 2001. Interoception: the inside story – a model for psychosomatic processes. Psychosom. Med. 63, 697–710. ( 10.1097/00006842-200109000-00001) [DOI] [PubMed] [Google Scholar]
- 2.Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 3.Garfinkel SN, Critchley HD. 2013. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: anterior insular cortex mediates bodily sensibility and social anxiety by Terasawa et al. (2012). Soc. Cogn. Affect. Neurosci. 8, 231–234. ( 10.1093/scan/nss140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. 2015. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74. ( 10.1016/j.biopsycho.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 5.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 6.Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. 2007. Heartbeat perception in depression. Behav. Res. Ther. 45, 1921–1930. ( 10.1016/j.brat.2006.09.008) [DOI] [PubMed] [Google Scholar]
- 7.Pollatos O, Kirsch W, Schandry R. 2005. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. ( 10.1002/hbm.20121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollatos O, Schandry R. 2004. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. ( 10.1111/1469-8986.2004.00170.x) [DOI] [PubMed] [Google Scholar]
- 9.Schandry R. 1981. Heart beat perception and emotional experience. Psychophysiology 18, 483–488. ( 10.1111/j.1469-8986.1981.tb02486.x) [DOI] [PubMed] [Google Scholar]
- 10.Herbert BM, Muth ER, Pollatos O, Herbert C. 2012. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS ONE 7, e36646 ( 10.1371/journal.pone.0036646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen S, Schroijen M, Mölders C, Zenker S, van den Bergh O. 2014. Categorical interoception: perceptual organization of sensations from inside. Psychol. Sci. 25, 1059–1066. ( 10.1177/0956797613519110) [DOI] [PubMed] [Google Scholar]
- 12.Petersen S, Van Staeyen K, Vögele C, von Leupoldt A, van den Bergh O. 2015. Interoception and symptom reporting: disentangling accuracy and bias. Front. Psychol. 6, 732 ( 10.3389/fpsyg.2015.00732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroijen M, Fantoni S, Rivera C, Vervliet B, Schruers K, van den Bergh O, van Diest I. 2016. Defensive activation to (un)predictable interoceptive threat: the NPU respiratory threat test (NPUr). Psychophysiology 53, 905–913. ( 10.1111/psyp.12621) [DOI] [PubMed] [Google Scholar]
- 14.Vlemincx E, van Diest I, van den Bergh O. 2015. Emotion, sighing, and respiratory variability. Psychophysiology 52, 657–666. ( 10.1111/psyp.12396) [DOI] [PubMed] [Google Scholar]
- 15.Whitehead WE, Drescher VM. 1980. Perception of gastric contractions and self-control of gastric motility. Psychophysiology 17, 552–558. ( 10.1111/j.1469-8986.1980.tb02296.x) [DOI] [PubMed] [Google Scholar]
- 16.Craig AD. 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505. ( 10.1016/S0959-4388(03)00090-4) [DOI] [PubMed] [Google Scholar]
- 17.Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 18.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. 2009. The pathways of interoceptive awareness. Nat. Neurosci. 12, 1494–1496. ( 10.1038/nn.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollatos O, Schandry R, Auer DP, Kaufmann C. 2007. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 1141, 178–187. ( 10.1016/j.brainres.2007.01.026) [DOI] [PubMed] [Google Scholar]
- 20.Pollatos O, Georgiou E. 2016. Normal interoceptive accuracy in women with bulimia nervosa. Psychiatry Res. 240, 328–332. ( 10.1016/j.psychres.2016.04.072) [DOI] [PubMed] [Google Scholar]
- 21.Pollatos O, Herbert BM, Wankner S, Dietel A, Wachsmuth C, Henningsen P, Sack M. 2011. Autonomic imbalance is associated with reduced facial recognition in somatoform disorders. J. Psychosom. Res. 71, 232–239. ( 10.1016/j.jpsychores.2011.03.012) [DOI] [PubMed] [Google Scholar]
- 22.Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schöpf V, Kopietz R, Wiesmann M, Schandry R. 2008. Reduced perception of bodily signals in anorexia nervosa. Eat. Behav. 9, 381–388. ( 10.1016/j.eatbeh.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 23.Van der Does AJW, Antony MM, Ehlers A, Barsky AJ. 2000. Heartbeat perception in panic disorder: a reanalysis. Behav. Res. Ther. 38, 47–62. ( 10.1016/S0005-7967(98)00184-3) [DOI] [PubMed] [Google Scholar]
- 24.Pollatos O, Gramann K, Schandry R. 2007. Neural systems connecting interoceptive awareness and feelings. Hum. Brain Mapp. 28, 9–18. ( 10.1002/hbm.20258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katkin ES, Cestaro VL, Weitkunat R. 1991. Individual differences in cortical evoked potentials as a function of heartbeat detection ability. Int. J. Neurosci. 61, 269–276. ( 10.3109/00207459108990745) [DOI] [PubMed] [Google Scholar]
- 26.Montoya P, Schandry R, Müller A. 1993. Heart-beat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr. Clin. Neurophysiol. 88, 163–172. ( 10.1016/0168-5597(93)90001-6) [DOI] [PubMed] [Google Scholar]
- 27.Schulz A, Ferreira de Sa DS, Dierolf AM, Lutz A, van Dyck Z, Vögele C, Schächinger H. 2014. Short-term food deprivation increases amplitudes of heartbeat-evoked potentials. Psychophysiology 52, 695–703. ( 10.1111/psyp.12388) [DOI] [PubMed] [Google Scholar]
- 28.Schulz A, Strelzyk F, Ferreira de Sa DS, Naumann E, Vögele C, Schächinger H. 2013. Cortisol rapidly affects amplitudes of heartbeat-evoked brain potentials—implications for the contribution of stress to an altered perception of physical sensations? Psychoneuroendocrinology 38, 2686–2693. ( 10.1016/j.psyneuen.2013.06.027) [DOI] [PubMed] [Google Scholar]
- 29.Smith R, Lane RD. 2015. The neural basis of one's own conscious and unconscious emotional states. Neurosci. Biobehav. Rev. 57, 1–29. ( 10.1016/j.neubiorev.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 30.Matthias E, Schandry R, Duschek S, Finke K, Pollatos O. 2007. Interoceptive awareness modulates attentional processing of visual stimuli. Int. J. Psychophysiol. 72, 154–159. ( 10.1016/j.ijpsycho.2008.12.001) [DOI] [PubMed] [Google Scholar]
- 31.Terasawa Y, Fukushima H, Umeda S. 2013. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Hum Brain Mapp. 34, 598–612. ( 10.1002/hbm.21458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollatos O, Kirsch W, Schandry R. 2005. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cogn. Brain Res. 25, 948–962. ( 10.1016/j.cogbrainres.2005.09.019) [DOI] [PubMed] [Google Scholar]
- 33.Herbert BM, Herbert C, Pollatos O, Weimer K, Enck P, Sauer H, Zipfel S. 2012. Effects of short-term food deprivation on interoceptive awareness, feelings and autonomic cardiac activity. Biol. Psychol. 89, 71–79. ( 10.1016/j.biopsycho.2011.09.004) [DOI] [PubMed] [Google Scholar]
- 34.Herbert BM, Blechert J, Hautzinger M, Matthias E, Herbert C. 2013. Intuitive eating is associated with interoceptive sensitivity. Effects on body mass index? Appetite 70, 22–30. ( 10.1016/j.appet.2013.06.082) [DOI] [PubMed] [Google Scholar]
- 35.Friston K, Schwartenbeck P, Fitzgerald T, Moutoussis M, Behrens T, Dolan RJ. 2013. The anatomy of choice: active inference and agency. Front. Hum. Neurosci. 7, 598 ( 10.3389/fnhum.2013.00598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friston KJ, Stephan KE, Montague R, Dolan RJ. 2014. Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry 1, 148–158. ( 10.1016/S2215-0366(14)70275-5) [DOI] [PubMed] [Google Scholar]
- 37.Quattrocki E, Friston K. 2014. Autism, oxytocin and interoception. Neurosci. Biobehav. Rev. 47, 410–430. ( 10.1016/j.neubiorev.2014.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seth AK, Suzuki K, Critchley HD. 2012. An interoceptive predictive coding model of conscious presence. Front. Psychol. 2, 395 ( 10.3389/fpsyg.2011.00395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Critchley HD, Mathias CJ, Dolan RJ. 2001. Neuroanatomical basis for first- and second-order representations of bodily states. Nat. Neurosci. 4, 207–212. ( 10.1038/84048) [DOI] [PubMed] [Google Scholar]
- 40.Preuschoff K, Quartz SR, Bossaerts P. 2008. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 28, 2745–2752. ( 10.1523/JNEUROSCI.4286-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronchi R, Bello-Ruiz J, Lukowska M, Herbelin B, Cabrilo I, Schaller K, Blanke O. 2015. Right insular damage decreases heartbeat awareness and alters cardio-visual effects on bodily self-consciousness. Neuropsychologia 70, 11–20. ( 10.1016/j.neuropsychologia.2015.02.010) [DOI] [PubMed] [Google Scholar]
- 42.Terasawa Y, Kurosaki Y, Ibata Y, Moriguchi Y, Umeda S. 2015. Attenuated sensitivity to the emotions of others by insular lesion. Front. Psychol. 6, 1314 ( 10.3389/fpsyg.2015.01314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Lazzaro V, et al. 2005. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J. Physiol. 565, 945–950. ( 10.1113/jphysiol.2005.087288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang G, Mouraux A. 2015. MEP latencies predict the neuromodulatory effect of CTBS delivered to the ipsilateral and contralateral sensorimotor cortex. PLoS ONE 10, e0133893 ( 10.1371/journal.pone.0133893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. 2005. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. ( 10.1016/j.neuron.2004.12.033) [DOI] [PubMed] [Google Scholar]
- 46.Farage MA, Osborn TW, MacLean AB. 2008. Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch. Gynecol. Obstetrics 278, 299–307. ( 10.1007/s00404-008-0708-2) [DOI] [PubMed] [Google Scholar]
- 47.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. 2002. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 51, 599–603. ( 10.1002/ana.10180) [DOI] [PubMed] [Google Scholar]
- 48.Rossini PM, et al. 2015. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107. ( 10.1016/j.clinph.2015.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallesi A, Shallice T, Walsh V. 2007. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb. Cortex 17, 466–474. ( 10.1093/cercor/bhj163) [DOI] [PubMed] [Google Scholar]
- 50.Herwig U, Satrapi P, Schonfeldt-Leucona C. 2003. Using the International 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 16, 95–99. ( 10.1023/B:BRAT.0000006333.93597.9d) [DOI] [PubMed] [Google Scholar]
- 51.Jurcak V, Okamoto M, Singh A, Dan I. 2005. Virtual 10–20 measurement on MR images for inter-modal linking of transcranial and tomographic neuroimaging methods. Neuroimage 26, 1184–1192. ( 10.1016/j.neuroimage.2005.03.021) [DOI] [PubMed] [Google Scholar]
- 52.Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, Brull D, Critchley HD. 2007. A cortical potential reflecting cardiac function. Proc. Natl Acad. Sci. USA 104, 6818–6823. ( 10.1073/pnas.0609509104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Immanuel SA, Pamula Y, Kohler M, Martin J, Kennedy D, Nalivaiko E, Saint DA, Baumert M. 2014. Heartbeat evoked potentials during sleep and daytime behavior in children with sleep disordered breathing. Am. J. Respir. Crit. Care Med. 190, 1149–1157. ( 10.1164/rccm.201405-0920OC) [DOI] [PubMed] [Google Scholar]
- 54.Fukushima H, Terasawa Y, Umeda S. 2011. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. Int. J. Psychophysiol. 79, 259–265. ( 10.1016/j.ijpsycho.2010.10.015) [DOI] [PubMed] [Google Scholar]
- 55.Paulus MP, Stein MB. 2006. An insular view of anxiety. Biol. Psychiatry 60, 383–387. ( 10.1016/j.biopsych.2006.03.042) [DOI] [PubMed] [Google Scholar]
- 56.Farb NAS, Chapman HA, Anderson AK. 2013. Emotions: form follows function. Curr. Opin. Neurobiol. 23, 393–398. ( 10.1016/j.conb.2013.01.015) [DOI] [PubMed] [Google Scholar]
- 57.Singer T, Critchley HD, Preuschoff K. 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. ( 10.1016/j.tics.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 58.Barrett L, Simmons WK. 2015. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429. ( 10.1038/nrn3950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brener J, Ring C. 1995. Perception and heart beat detection. In From the heart to the brain: the psychophysiology of circulation–brain interaction (eds Vaitl D, Schandry R), pp. 193–221. Frankfurt, Germany: Peter Lang GmbH. [Google Scholar]
- 60.Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang Y-Z, Rothwell JC. 2007. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin. Neurophysiol. 118, 1033–1043. ( 10.1016/j.clinph.2007.02.003) [DOI] [PubMed] [Google Scholar]
- 61.Critchley HD, Rotshtein P, Nagai Y, O'Doherty J, Mathias CJ, Dolan RJ. 2005. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage 24, 751–762. ( 10.1016/j.neuroimage.2004.10.013) [DOI] [PubMed] [Google Scholar]
- 62.Plewnia C. 2011. Brain stimulation: new vistas for the exploration and treatment of tinnitus. CNS Neurosci. Ther. 17, 449–461. ( 10.1111/j.1755-5949.2010.00169.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plewnia C, Reimold M, Najib A, Brehm B, Reischl G, Plontke SK, Gerloff C. 2007. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum. Brain Mapp. 28, 238–246. ( 10.1002/hbm.20270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langguth B, Zowe M, Landgrebe M, Sand P, Kleinjung T, Binder H, Hajak G, Eichhammer P. 2006. Transcranial magnetic stimulation for the treatment of tinnitus: a new coil positioning method and first results. Brain Topogr. 18, 241–247. ( 10.1007/s10548-006-0002-1) [DOI] [PubMed] [Google Scholar]
- 65.Ainley V, Tajadura-Jiminez A, Fotopoulou A, Tsakiris M. 2012. Looking into myself: changes in interoceptive sensitivity during mirror self-observation. Psychophysiology 49, 1672–1676. ( 10.1111/j.1469-8986.2012.01468.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kever A, Pollatos O, Vermeulen N, Grynberg D. 2015. Interoceptive sensitivity facilitates both antecedent- and response-focused emotion regulation strategies. Pers. Ind. Differ. 87, 20–23. ( 10.1016/j.paid.2015.07.014) [DOI] [Google Scholar]
- 67.Tsakiris M, Jimenez AT, Costantini M. 2011. Just a heartbeat away from one's body: interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B 278, 2470–2476. ( 10.1098/rspb.2010.2547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koch A, Pollatos O. 2014. Cardiac sensitivity in children: sex differences and its relationship to parameters of emotional processing. Psychophysiology 51, 932–941. ( 10.1111/psyp.12233) [DOI] [PubMed] [Google Scholar]
- 69.Pollatos O, Herbert BM, Kaufmann C, Auer DP, Schandry R. 2007. Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. Int. J. Psychophysiol. 65, 167–173. ( 10.1016/j.ijpsycho.2007.03.005) [DOI] [PubMed] [Google Scholar]
- 70.Critchley HD, Harrison NA. 2013. Visceral influences on brain and behavior. Neuron 77, 624–638. ( 10.1016/j.neuron.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 71.Craig AD. 2009. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Phil. Trans. R. Soc. B 364, 1933–1942. ( 10.1098/rstb.2009.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grimshaw GM, Carmel D. 2014. An asymmetric inhibition model of hemispheric differences in emotional processing. Front. Psychol. 5, 489 ( 10.3389/fpsyg.2014.00489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rapinesi C, et al. 2015. Maintenance deep transcranial magnetic stimulation sessions is associated with reduced depressive relapses in patients with unipolar or bipolar depression. Front. Neurol. 6, 16 ( 10.3389/fneur.2015.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.