Abstract
Interoception is the ability to perceive one's internal body state including visceral sensations. Heart-focused interoception has received particular attention, in part due to a readily available task for behavioural assessment, but also due to accumulating evidence for a significant role in emotional experience, decision-making and clinical disorders such as anxiety and depression. Improved understanding of the underlying neural correlates is important to promote development of anatomical-functional models and suitable intervention strategies. In the present meta-analysis, nine studies reporting neural activity associated with interoceptive attentiveness (i.e. focused attention to a particular interoceptive signal for a given time interval) to one's heartbeat were submitted to a multilevel kernel density analysis. The findings corroborated an extended network associated with heart-focused interoceptive attentiveness including the posterior right and left insula, right claustrum, precentral gyrus and medial frontal gyrus. Right-hemispheric dominance emphasizes non-verbal information processing with the posterior insula presumably serving as the major gateway for cardioception. Prefrontal neural activity may reflect both top-down attention deployment and processing of feed-forward cardioceptive information, possibly orchestrated via the claustrum.
This article is part of the themed issue ‘Interoception beyond homeostasis: affect, cognition and mental health’.
Keywords: interoception, meta-analysis, functional magnetic resonance imaging, brain activity, cardioception, heartbeat detection task
1. Introduction
Interoception is the ability to perceive the state of one's internal body including the viscera as opposed to exteroception, which is perceiving stimuli of the external environment, and proprioception, which is perceiving posture and position of one's own body parts [1]. This article follows this definition, although it should be noted that others have defined interoception as including both proprio- and visceroception (i.e. sensing one's inner organs) [2].
The idea that central nervous system representations of psychophysiology comprise a basis for emotional feelings and may guide behaviour dates back to the James–Lange theory [3]. While Lange focused on cardiac reactivity James considered any autonomic function as a possible source of emotion. Current neurobiological research has revived this perspective, arguing that emotion may be a kind of interoceptive inference. From that point of view, emotions emerge from active analysis of physiological reactivity [4].
Recently, interoception has been conceptually refined by differentiating dimensions of interoception [5,6]. Firstly, interoceptive sensibility may be assessed via self-report of subjective sensitivity to interoceptive signals, specifically targeting self-reported belief in interoceptive aptitude. Examples are the awareness sub-scale of the body perception questionnaire (BPQ) [7] or the multidimensional assessment of interoceptive awareness questionnaire (MAIA) [8], but also average confidence ratings regarding one's interoceptive sensitivity may be used. Secondly, interoceptive sensitivity may be operationalized with behavioural tests of interoceptive accuracy, for example with the commonly used heartbeat detection task [9]. Maybe the easy implementation but also the possibility to judge individual performance against an absolute and objective reference score obtained from the electrocardiogram has fostered the wide application of this task in empirical research on interoception. Thirdly, metacognitive awareness, which may be assessed with ratings of confidence regarding one's objective interoceptive accuracy, is considered to provide insight into one's interoceptive aptitude. First evidence suggests that these dimensions are rather independent [6]. Therefore, it appears necessary to consider which of these aspects are relevant in a particular interoceptive task (IT) in order to identify brain correlates specifically associated with these dimensions.
High heart-focused interoceptive accuracy/sensitivity has been associated with increased emotional intensity [10], has been shown to support memory [11,12] and adaptive decision-making [13–15], and has predicted increased emotional intensity and reactivity to emotional pictures [16].1 Furthermore, it has been suggested that mood and anxiety disorders may root in issues related to interoception [17]. This view is supported by accumulating evidence in the domain of anxiety disorders [18] and depression [19,20]. For patients with panic disorder, interoceptive cues may be threatening or confusing. This could explain why high heart-focused interoceptive accuracy/sensitivity was associated with impaired intuitive decision-making in these patients [21].
The insula has been suggested as the primary neural correlate of interoception [22]. Peripheral nervous system afferents project contralateral information regarding somatic homeostasis to posterior granular and mid-dysgranular regions of the insula [23] via lamina I and the ventromedial nucleus (sympathetic afferents) and via the nucleus of the solitary tract and the ventromedial thalamic nucleus for sympathetic afferents [22]. At this level, an embodied representation of interoception is expected [24]. The anterior insula is interconnected with prefrontal cortical (e.g. anterior cingulate and orbitofronal cortex) and limbic structures (e.g. amygdala), and has been linked to tasks requiring cognitive control for identifying a signal against a noisy background [25]. Neural activity in the anterior insula may also reflect uncertainty and valence evaluation associated with such tasks [26]. Hence, on an axis from caudal to rostral, interoceptive information is thought to be represented increasingly abstractly, with the anterior agranular insula as a centre for interoceptive awareness [22,25], relating emotional salience and valence to adjacent structures such as the amygdala, anterior cingulate cortex, the orbitofrontal cortex and the ventral striatum [27,28]. Others have differentiated between dorsal and ventral anterior insula, being associated with cognitive and emotional processing, respectively [24].
While much of this knowledge comes from animal research, this view is also supported by research on humans. Meta-analyses of neural activity assessed with magnetic resonance imaging have parcellated the insula into various functional domains, suggesting a particular role of the mid-insula for interoception [29,30]. Concurrently, interoception of respiratory distress, thirst, heartbeat, as well as distension of the oesophagus, stomach, bladder or rectum have been associated with increased insula activation [22,31]. Damage to the insula by contrast hampers interoception [32].
From the view that interoception mediates between the perception of somatic states and domains that are highly relevant for successful coping with the challenges of everyday life, such as intuition, emotion and (decision) behaviour, it follows that improved understanding of the underlying functional anatomy may aid understanding and interventions for a wide array of issues including maladaptive response to somatic cues in panic disorder [21,33], somatoform disorder [34,35], dissociative disorders [36–38] or eating disorders [39,40]. Maladaptive stress reactivity in terms of cognitive and emotional response to issues in processing interoceptive information may contribute to poor somatic health [41].
Despite these interesting perspectives and detailed knowledge about wiring and functional organization of the insula, only a few studies have in fact examined brain activation associated with tasks involving interoception in humans, although functional magnetic resonance imaging (fMRI) serves as a suitable tool with particular advantages regarding spatial resolution and the possibility to locate sub-cortical brain correlates. Therefore, it appears desirable to increase our knowledge about the location of functional correlates of interoception and the potential involvement of adjacent regions.
However, recent findings indicate that the functional specificity of insular subdivisions clearly goes beyond the commonly accepted tripartite division into dorsal anterior, ventral anterior and posterior insula [42]. Another study has examined brain correlates of modality-specific physiological activation [43]. The findings further support the notion that afferent information from different organs is processed in specific subdivisions of the insula, respectively. Considering the high functional heterogeneity of the insula [42], current models could be improved by considering modality-specific brain correlates of interoception. Perhaps, vulnerability with regards to organ-specific information processing could explain comorbidity between somatic disease and psycho-social issues. For example, issues in heart-focused interoception may reveal a neural basis for the high comorbidity between chronic heart failure and affective disorders such as anxiety and depression [44,45].
Altogether, it appears promising for advancing current human functional-anatomical models of interoception to identify brain areas (i) specifically associated with separate modalities such as heart-focused interoception and (ii) to examine closely which particular aspects or dimensions of interoception are reflected in a particular experimental operationalization (i.e. IT).
Therefore, I have performed the first meta-analysis on studies reporting blood-oxygen-level dependent (BOLD) fMRI activation in brain areas associated with the specific modality of heart-focused interoception (i.e. cardioception). In order to optimize conceptual clarity of this approach, I have evaluated characteristics of the interoceptive and respective control tasks based on the model suggested by Garfinkel and co-workers [5,6].
2. Material and methods
(a). Study selection
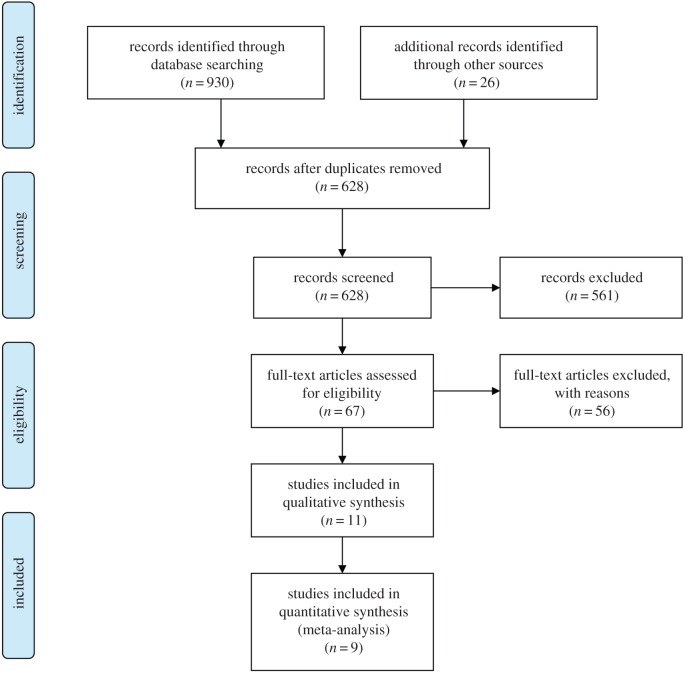
In order to identify fMRI studies concerned with brain activity related to interoception, a literature search with the search-term ‘(interoception or cardioception) and (fMRI or fMRT or ‘brain activation’)’ was conducted with no initial restrictions regarding type of publication or time-frame in the following databases (no. of matching entries in parentheses)2: WorldCat (520), Web of Science (172), PubMed (135), PsycNet (63), pubPsych (32) and Psyndex (8). The combined list was reduced in line with current guidelines (see figure 1 for a flow diagram according to PRISMA guidelines [46]). After removal of duplicates, studies were identified (i) that reported standard coordinates for neural activity across the entire brain (i.e. excluding studies that focused on limited regions of the brain), (ii) that were associated with performing a heart-focused interoception task (IT) as compared to a control task (CT), (iii) that were assessed via BOLD fMRI contrast of IT > CT. In total, 561 of the 628 screened studies were excluded from the list, when it was evident from the title that they (i) did not report fMRI data, (ii) did not examine human participants, (iii) did not follow an experimental protocol involving an IT or (iv) reported group comparisons only. For the remaining set of 67 studies, full-text articles were considered in detail. In 49 of these articles, the focus was not on brain activation associated with an IT as compared to either baseline or a CT. Only one reported the relevant IT > CT contrast for a single modality other than cardioception, namely respiration monitoring [25], and was therefore omitted from the meta-analysis. In seven studies, the IT was—at least partly—concerned with cardioception and would have allowed computation of an IT > CT contrast, but brain correlates of cardioceptive attentiveness were not reported and the respective data could not be obtained from the authors upon request. Finally, one study opted for execution of a heart-focused IT in the scanner environment [47]. This study was also not included in the current meta-analysis because the IT and CT involved demands that differed strongly from all the other studies.
Figure 1.
Flow diagram according to PRISMA guidelines [46] depicting the flow of information through the different phases of the literature search for the current meta-analysis. See the text for details regarding reasons for inclusions and exclusions. (Online version in colour.)
For the remaining set of nine studies, characteristics of the interoceptive and control tasks were evaluated considering the model introduced by Garfinkel and co-workers [5,6] as described above in order to aid the discussion and interpretation of the results of the meta-analysis as compared to individual studies' findings (see table 1).
Table 1.
Overview of fMRI study details reporting neural activity associated with interoception. When no laterality is indicated, this means bilateral activation was found to be significant. MDD, major depressive disorder; HC, healthy controls; BII, blood-injection-injury; SP, spider phobia; HP, high-perceiver; LP, low-perceiver; IT, interoception task; CT, control task; TR, fMRI repetition time; B, magnetic field strength of scanner; T, Tesla; FWHM, full width half maximum of fMRI smoothing kernel; BA, Brodmann area. Note that findings listed for contrast IT > CT for studies by Avery and co-workers [19] and Wiebking & Northoff [48] reflect the HC subsample (see the text for details).
| index | citation | sample: size (female) | age: M (s.d.) | IT | CT | task timing | TR | no. of volumes per IT | B, coil | FWHM | resampled voxel size | hypotheses | brain correlates of significant neural activity in fMRI BOLD contrast IT > CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Avery et al. [19] | MDD: 20 (13) HC: 20 (12) |
MDD: 36 (9) HC: 33 (7) |
focus on intensity of sensations from the heart | count how often the word ‘TARGET’ on a screen switches to lowercase | 9 min run of 10 s randomly alternating IT versus CT and interleaved with null events (M = 6.7 s) | 2500 ms | approx. 60 | 3 T, 32-channel head-coil | 6 mm | 1.75 × 1.75 × 1.75 mm | mid-insula | right dorsal mid-insula |
| 2 | Caseras et al. [49] | SP: 16 (2) BII: 18 (2) HC: 18 (2) |
SP: 21.62 (2.57) BII: 22.78 (2.62) HC: 21.72 (2.80) |
attend / press button for each perceived / counted heartbeat | press button for each detected target in series of sounds | 20 s of attending followed by 10 s of button pressing, alternated five times with 30 s CT plus 30 s fixation-cross baseline at beginning and end | 2000 ms | 75 | 1.5 T, n.a. | 5 mm | n.a. | anterior insula, dorsal anterior cingulate | insula, dorsal anterior cingulate (BA 32), supplementary motor cortex (BA 6), thalamus, right superior temporal lobe (BA 22), right somatosensory cortex (BA 3) |
| 3 | Critchley et al. [50] | 17 (9) | n.a. | judge whether tones correspond to heartbeats or are delayed | judge whether one in 10 tones has different pitch | IT and CT trials in randomized order; total duration reflecting individual resting heart rate but less than 15 min | 2520 ms | approx. 180 | 1.5 T, n.a. | 12 mm | n.a. | (right anterior) insula, oribitofrontal cortex | inferior parietal lobule/post central gyrus, lateral somatomotor cortex, insula, insula/operculum, inferior frontal gyrus, dorsal anterior cingulate, supplementary motor cortex |
| 4 | Kuehn et al. [51] | HP: 12 (4) LP: 11 (5) |
n.a. but well matched | monitor one's own heartbeat while 60 Hz tones are presented concurrently at random times; 60 Hz tone volume was individually adjusted to make stimuli ‘just perceivable’ | monitor 60 Hz tones presented at random times in the presence a slow background tone; 60 Hz tone volume was individually adjusted to make stimuli ‘just perceivable’ | 16 trials consisting of 3 s instructions followed by 35 s either IT or CT in pseudo-random order (no condition presented more than twice in a row), followed by rating and 6–7 s pause | 2000 ms | 140 | 3 T, 12-channel head-coil | 8 mm | n.a. | right anterior insula, right frontal operculum, anterior cingulate cortices, somatomotor areas | frontal operculum and right precentral gyrus (including BA 6), right anterior insula, right parietal operculum (secondary somatosensory cortex), right inferior frontal cortex (BA 44) |
| 5 | Pollatos et al. [52] | 20 (0) | 26.8 (3.7) | count number of perceived heartbeats | count number of tones | 4 runs filled with 32 s of counting followed by 16 s for reporting by button press | 4000 ms | 68 | 1.5 T, n. a. | 8 mm | n.a. | (right) insula, (dorsal) cingulate, somatomotor and prefrontal cortex | insula (BA 13), inferior/middle frontal gyrus (BA 10/46), right medial frontal gyrus (BA 6)/dorsal cingulate gyrus (BA 24), right thalamus (ventral), right inferior parietal lobule (40), left middle/inferior temporal gyrus (BA 37) |
| 6 | Simmons et al. [24] | 14 (8) | 29 (range: 21–43) | perceive any sensation from the heart | count how often an ‘O’ appears at random intervals | 4 runs of 7.5 min filled by 15 s of IT alternating with CT and null events (M = 6.14 s) | 2000 ms | approx. 170 | 3 T, 21-channel head-coil | 6 mm | 2 × 2 × 2 mm | insula | right mid-insula, left postcentral gyrus, right insula and precentral gyrus, right postcentral gyrus, left precuneus, left middle frontal gyrus, right dorsal insula, right cuneus, right inferior parietal lobule, right inferior frontal gyrus, left precentral gyrus, left postcentral gyrus; (dorsal mid-insula characterized by granular cortex and ventral and anterior to the dorsal insula cluster, in the dysgranular insula) |
| 7 | Wiebking et al. [20] | MDD: 17 (11) HC: 17 (11) |
MDD: 41.88 (12.1) HC: 37.59 (12.84) |
count number of perceived heartbeats | count number of tones | 4 runs of 9.6 min with 9–13 s of counting followed by 4 s for reporting the counted number (48 repetitions per condition in total) | 2000 ms | approx. 264 | 3 T, 8-channel head-coil | 6 mm | 2 × 2 × 2 mm | anterior insula | anterior and mid-insula |
| 8 | Wiebking et al. [48] | MDD: 16 (11) HC: 30 (11) |
MDD: 41.19 (11.78) HC: 33.73 (11.62) |
count number of perceived heartbeats | count number of tones | 4 runs containing 12 trials per condition (48 repetitions per condition in total), 9–13 s each with IT or CT in pseudo-randomized order | 2000 ms | approx. 264 | 3 T, 8-channel head-coil | 5 mm | 2 × 2 × 2 mm | insula | claustrum, medial frontal gyrus (BA 6), left superior frontal gyrus (BA 6), left middle frontal gyrus (BA 6), superior temporal gyrus (BA 22), left cingulate gyrus (BA 24), right inferior parietal lobule (BA 40), left superior temporal gyrus (BA 42) |
| 9 | Zaki et al. [53] | 16 (11) | 19.10 (1.72) | press button for each perceived heartbeat (in half of the trials while concurrent repeating tones are present) | press button for each perceived tone (matching the individual participants’ heart rate with added 25% variance) | 2 runs of 300 s with six 30 s blocks | 2000 ms | 150 | 1.5 T, n.a. | 6 mm | n.a. | anterior insula | right anterior insula/inferior frontal operculum, dorsomedial prefrontal cortex, medial cingulate, right medial frontal gyrus, hypothalamus |
Deactivation in response to IT was not scrutinized in the current meta-analysis, because negative BOLD responses were only reported in three studies [50,52,54].
(b). Classification of studies by interoceptive task
All studies included in this meta-analysis have compared neural activity associated with an IT requiring focused attention to sensations from the heart to neural activity associated with a CT requiring attention to exteroceptive stimuli. However, the primary focus of most studies was not this basic comparison. For the current analysis, I ignored further contrasts reported in these articles. With regards to the implementation of both IT and CT, there was considerable variation across studies.
According to the model introduced by Garfinkel and co-workers [5,6], four studies [20,48,51,52] have assessed interoceptive accuracy/sensitivity using minor variants of the heartbeat detection task originally coined by Schandry [9]. In these studies' tasks, the participants had to count their heartbeats in given time intervals of variable lengths. Two studies simply required the participants to focus their attention on sensations from the heart [19,24], a task-variant that is not well-determined by the model suggested by Garfinkel and co-workers [5]. I propose the term attentiveness as a suitable descriptor for focused attention to a particular interoceptive signal for a given time interval that is to the best of my knowledge unambiguous with regards to the existing literature on interoception. Similar to interoceptive accuracy/sensitivity, performance in this task will depend on both top-down resource allocation for conscious registration of relevant events and clarity of bottom-up interoceptive signals against interoceptive background noise, probably mediated by recruitment of strategies like filtering, template matching, and sequence monitoring.
Avery and co-workers [19] asked participants to rate the intensity of sensations after 50 and 100% of trials. Similarly, Simmons and co-workers [24] asked the participants after 50 and 100% of trials to rate from 1 to 7 how fast their heart had been beating. Kuehn and co-workers [51] asked how well participants were able to solve the task on a scale ranging from 0 to 4. This adds a cognitive component to the tasks and the ratings may be influenced by one's interoceptive sensibility, as defined above.
Three studies have used IT that may be considered as in-between interoceptive attentiveness and interoceptive accuracy/sensitivity. In one study [50], participants were asked to attend to visual or auditory signals which were tied to their individual heartbeats, but either minimally delayed (less than 150 ms, i.e. almost in sync), or more or less out of sync (≈ 500 ms delay). After 10 signals, the participants had to judge whether the signal was in sync or not. From this basic set-up, eight highly similar task variants were derived and applied with the aim of computing a more pure effect of interoception. In another study [54], participants were alerted by a visual cue to attend to their heartbeat for 20 s, followed by a 10 s period where they pressed a button for each detected heartbeat. In a third study [55], participants were asked to tap a button each time they perceived their heartbeat. Despite these variations, all three tasks required tracking of individual heartbeats just as in the four studies assessing interoceptive accuracy/sensitivity with the heartbeat detection task [20,48,51,52].
To summarize, all studies' IT involved interoceptive attentiveness towards sensations from one's heart, which includes attention to individual heartbeats. Three studies involved a mental focus on perceiving and detecting single heartbeats [50,54,55]. Four studies [20,48,51,52] closely matched the heartbeat detection task coined by Schandry [9], which is used for assessing interoceptive accuracy/sensitivity. However, it is important to note that strictly speaking, none of the contrasts of IT > CT reported in these studies reflected interoceptive accuracy/sensitivity, because (i) accuracy was not assessed together with fMRI activation (i.e. outside of the scanner) and (ii) only two studies reported in which of the identified areas brain activity actually correlated with accuracy scores [50,52]. Hence, brain activation identified in this contrast reflects all aspects associated with carrying out the IT as compared to the CT.
(c). The role of control tasks
In fMRI studies, neural activation associated with the effect of interest is identified by subtracting activation that is related to general or unspecific neural processes that might confound the signal of interest, measured during the execution of a CT. Ideally, control and experimental tasks differ in all but the specific effect of interest. fMRI studies investigating neural activity during an IT might primarily want to control for (i) general effects of counting and (ii) neural effects of external cues compared to internal stimuli. Subtracting those confounding effects in subsequent fMRI analysis from neural activity during the IT (contrasting IT > CT) will mirror neural activity that is closely related to the effects of the IT relative to the CT. In the following section, the CTs used in studies submitted to the current meta-analysis have been critically considered in order to identify the potential impact of particular CT characteristics on the outcome.
In the four studies using variants of the heartbeat detection task [20,48,51,52], participants had to count (all) tones presented in a given time interval. This interval was of variable length in three of the studies [20,48,52], and had a fixed length of 35 s in one study [51]. In two of these four studies [20,48], the presentation frequency of the tones was adapted to correspond to each participant's heart rate, but onset times of the tones were jittered to avoid synchrony with the actual heartbeat. Avery and co-workers [19] used a visual CT, where participants had to count how often the word ‘TARGET’ on a screen switched to lowercase in a given time interval. Participants in the study by Simmons and co-workers [24] had to count how often an ‘O’ appeared randomly in a given time interval. Apart from using a different modality, these tasks were rather similar to the tone counting tasks of the four previously mentioned studies [20,48,51,52].
Interoceptive accuracy/sensitivity as assessed by heartbeat detection tasks involves focused attention to a body signal. Hence, performance is confounded with cognitive capacity and ability [21,56]. Both Caseras and co-workers [54] and Zaki and co-workers [55] aimed to minimize effects of this confound by the particular design of their CT, where participants had to press a button for each detected target in a series of external sound events for a given time interval. Zaki and co-workers [55] added a third condition where participants had to count heartbeats during concurrent presentation of sounds, allowing for removal of the additional brain activation related to discriminating exteroceptive signals from noise rather than to interoception per se from the contrast of interest. Critchley and co-workers [50] used a similar CT where participants had to evaluate, via button press, whether a series of externally presented tones contained a number of identical tones or whether one of these tones was presented with a different pitch.
Note that in three studies [19,24,51], IT and CT trials closed with rating (see above). As noted above, this adds a cognitive component. However, for the most part associated activation will be cancelled out in the contrast IT > CT because similar brain activation will be induced in both trial types.
Although, task details varied greatly, two main types of CT can be distinguished. While both types of tasks required attention to a series of exteroceptive signals, one set of CT required vigilant attention towards all signals within a given time interval, while another set of CT required identification of rare or singular events within a series of similar other events.
(d). Study samples
The set of studies submitted to the current meta-analysis further varied considerably with regards to the participants. Most of the studies used healthy adult participants with an upper boundary of 43 years; hence, findings may not be valid for older populations. Apart from one study that only included male participants [52], all samples included both sexes (see table 1 for details). One study compared individuals with phobia regarding blood-injection-injury or spiders to healthy controls [54]. In this study, blocks regarding interoception/exteroception were counter-balanced across participants with a 7 min event related phobia symptom provocation task in which phobia-related images where shown. This task may have produced carry-over effects, as increased state anxiety is associated with increased interoceptive accuracy/sensitivity [18,57]. However, this task also preceded the CT. Hence, differences between CT and IT should still reflect neural activity relevant for interoceptive attentiveness and accuracy/sensitivity, and for the most part, such carry-over effects will be cancelled out in the contrast of interest (IT > CT).
Several studies compared patients with a clinical diagnosis of major depression (MDD) to healthy participants. It has been shown that interoceptive accuracy/sensitivity is increased in patients with moderate depression, but decreased in patients with severe depression when compared with healthy controls [58,59]. For the study by Avery and co-workers [19], the contrast of IT > CT did not show any effects for the MDD subgroup; hence, the available data are rather representative for the subgroup of 20 healthy participants. Note that the respective sample size has been adjusted accordingly in the current analysis (for details see the electronic supplementary material). In the study by Wiebking and co-workers [20], the depressed participants had increased scores (total and all but the awareness subscale) on the BPQ [7] used to assess interoceptive sensibility, and they had reduced activation of the insula when performing the IT [20]. I decided to include the data in the current meta-analysis although only findings for the combined sample were reported, because brain areas associated with interoception in healthy participants matched brain areas in depressed individuals, suggesting a quantitative rather than a qualitative difference. For the study by Wiebking & Northoff [48], data from the subgroup of control participants without MDD could be obtained from the authors, hence no data from the MDD subgroup was entered in into the current meta-analysis, and sample size was adjusted accordingly. In one study [51], participants with high cardioceptive accuracy, so-called high-perceivers (HP, see table 1) with an accuracy score of 0.87 and low-perceivers (LP, see table 1) with an accuracy score of 0.54 were identified with the heartbeat detection task [9]. Significant findings were only obtained and reported for the HP subgroup. Hence, in the current meta-analysis, only data from the HP sample was included (also see electronic supplementary material).
(e). Details regarding meta-analysis
In order to identify brain regions consistently activated in a given set of fMRI contrasts as extracted from the studies described above, I have employed multilevel kernel density analysis (MKDA) [60,61]. MKDA is similar to other voxel-based methods [62,63] and the activation likelihood estimate method (ALE) [64,65] with one notable exception: the ALE method computes the probability for at least one peak of activation to fall within a particular voxel, based on joint probability across individual peaks. Accordingly, the null hypothesis is that in none of the studies was a particular voxel activated. By contrast, MKDA identifies voxels with density of reported peaks exceeding chance level by computing spatial consistency among reported peaks. Hence, MKDA tests the null hypothesis that spatial distribution of peaks is random.
To conduct MKDA, peak coordinates for the basic contrast identifying neural activity associated with interoception as compared to the CT (i.e. the contrast IT > CT) were extracted from the individuals studies. Next, all peak coordinates (absolute and relative maxima for each cluster) were converted into a common stereotactic system that is Montreal Neurological Institute (MNI) space and these peaks were plotted onto a canonical brain (avg152T1.img; SPM, Wellcome Department of Imaging Neuroscience). Note that MKDA uses a spatial resolution of 2 × 2 × 2 mm per voxel. Each peak was convolved with a 10 mm three-dimensional Gaussian kernel. This reduces potential bias introduced by studies with many close-by peaks, because the peaks will contribute to one overlapping sphere with maximum activity of 1, regardless of the number of contributing peaks, instead of being counted as if they were from independent observations (i.e. studies). Therefore, MKDA is more conservative than common alternative approaches such as ALE. Furthermore, MKDA respects the multilevel aspect of the data by treating peaks as random rather than fixed.
Next, a summary density map representing the observed activation for a specific fMRI contrast by taking a weighted average of the spheres from each study was computed. The respective weighting factors were computed by multiplying the square-root of N (sample size) with 0.75 for fixed-effects versus 1.00 for random-effects analysis [61,66]. Note that all studies used in the current meta-analysis applied random-effects models for second-level analysis. At this stage, a density map was created where each voxel included a weighed proportion of contrasts that activated within 10 mm of that voxel. To generate a comparison model with random spatial organization of voxel-associated density across white and grey matter of the standard brain, a Monte Carlo simulation with 15 000 iterations per analysis was carried out [67]. Significant activation was then identified by comparing density per voxel as derived from the study set to the Monte Carlo simulation (i.e. null hypothesis) to identify voxels with an above-chance number of activation coordinates.
First, MKDA identifies proximate clusters (i.e. inside the 10 mm kernel region) of contiguous voxels according to a ‘height-based’ threshold. The proportions of contrasts in these voxels exceed the maximum expected over the entire brain by chance. In other words, the chance of making a Type I error at any single voxel is less than or equal to 0.05. Note that this results in family-wise error rate (FWER) correction. Second, MKDA identifies incremental clusters outside the 10 mm of the clusters for the height-based threshold with ‘extent-based’ thresholds that are FWER-corrected for spatial extent at p < 0.05 and meet primary alpha levels of p < 0.01 or p < 0.05. Basically, this test answers the question how many contiguous voxels would be required until this cluster meets the more stringent criterion of a whole-brain corrected threshold? Next, a combined map of voxels meeting both height- and extent-criterion was computed, and the active voxels were clustered using SPM8 contiguity assessment procedures (spm_clusters.m; Wellcome Department of Imaging Neuroscience).
Finally, anatomical localization of the clusters was identified by converting cluster coordinates into Talairach space and consulting a standard brain atlas [68] via the Talairach Daemon [69,70] set to identify the nearest grey matter. For ease of use, all coordinates are reported in MNI space nonetheless.
3. Results
To reveal neural activity that emerged consistently across studies employing task variants that involved cardioceptive attentiveness as defined above, a meta-analysis was conducted including nine studies reporting the BOLD fMRI contrast of interest (IT > CT).
Contiguous clusters of voxels meeting the height-based threshold with proportions of contrasts greater than or equal to 40.11% (632 voxels) at p < 0.05 (FWER-corrected), as well as local maxima within these clusters, are reported in table 2.
Table 2.
MNI coordinates (x, y, z), no. of voxels per contiguous cluster, volume in cubic millimetres and Z-test statistic (max. proportion of activating studies) peaks and local maxima for sub-clusters identified according to the MKDA ‘height-based’ threshold, see text for details. Columns for level 1 (hemisphere), level 2 (lobes), and level 3 (sub-lobar structures) of the Talairach-Daemon classification system provide additional details. The respective Brodmann area (BA) has been noted where applicable.
| sub-cluster and respective local maxima | x | y | z | voxels | volume (mm3) | maxStat | level 1 | level 2 | level 3 | BA | range (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | 8 | 2 | 520 | 4160 | 0.73 | right cerebrum | sub-lobar | insula | 13 | 1 |
| 1a | 46 | 8 | 0 | 117 | — | 0.63 | right cerebrum | sub-lobar | insula | 13 | 1 |
| 1b | 42 | 2 | 2 | 138 | — | 0.73 | right cerebrum | sub-lobar | claustrum | — | 1 |
| 1c | 38 | 10 | 4 | 224 | — | 0.63 | right cerebrum | sub-lobar | insula | 13 | 1 |
| 1d | 56 | 2 | 6 | 41 | — | 0.56 | right cerebrum | frontal lobe | precentral gyrus | 44 | 1 |
| 2 | −40 | 10 | 0 | 86 | 688 | 0.47 | left cerebrum | sub-lobar | insula | 13 | 2 |
| 3 | 52 | −22 | 24 | 84 | 672 | 0.42 | right cerebrum | sub-lobar | insula | 13 | 3 |
| 4 | 6 | −4 | 54 | 2 | 16 | 0.50 | right cerebrum | frontal lobe | medial frontal gyrus | 6 | 1 |
According to ‘extent-based’ criteria, incremental clusters (i.e. outside the 10 mm kernel region) of more than 1498 contiguous voxels with proportions of contrasts more than or equal to 16.67% were considered significant at p < 0.01 (FWER-corrected, one-tailed), and clusters of more than 402 contiguous voxels with proportions of contrasts more than or equal to 24.74% were considered significant at p < 0.05 (FWER-corrected, one-tailed; see tables 3 and 4, for details, respectively).
Table 3.
MNI coordinates, no. of voxels per contiguous cluster, volume in cubic millimetres and Z-test statistic (max. proportion of activating studies) peaks for sub-clusters for incremental clusters (i.e. outside the 10 mm of the clusters reported in table 2) identified according to MKDA ‘extent-based’ thresholds at a primary alpha level of p < 0.01, see text for details. No additional local maxima were found. Columns for level 1 (hemisphere), level 2 (lobes), and level 3 (sub-lobar structures) of the Talairach-Daemon classification system provide additional details. The respective Brodmann area (BA) has been noted where applicable.
| sub-cluster | x | y | z | voxels | volume (mm3) | maxStat | level 1 | level 2 | level 3 | BA | range (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 2 | 2 | 522 | 4176 | 0.40 | right cerebrum | frontal lobe | precentral gyrus | 44 | 0 |
| 2 | 34 | 16 | 10 | 153 | 1224 | 0.37 | right cerebrum | sub-lobar | claustrum | — | 2 |
Table 4.
MNI coordinates, no. of voxels per contiguous cluster, volume in cubic millimetres and Z-test statistic (max. proportion of activating studies) peaks for sub-clusters for further incremental clusters (i.e. outside the 10 mm of the clusters reported in table 2, and not included in table 3) identified according to MKDA ‘extent-based’ thresholds at a primary alpha level of p < 0.05, see text for details. No additional local maxima were found. Columns for level 1 (hemisphere), level 2 (lobes), and level 3 (sub-lobar structures) of the Talairach-Daemon classification system provide additional details. The respective Brodmann area (BA) has been noted where applicable.
| sub-cluster | x | y | z | voxels | volume (mm3) | maxStat | level 1 | level 2 | level 3 | BA | range (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 22 | 2 | 39 | 312 | 0.20 | right cerebrum | frontal lobe | precentral gyrus | 44 | 0 |
| 2 | 12 | −12 | 56 | 1789 | 14 312 | 0.39 | right cerebrum | frontal lobe | medial frontal gyrus | 6 | 3 |
A functional-anatomical evaluation with the Talairach-Daemon classification system on level 1 (hemisphere), level 2 (lobes) and level 3 (sub-lobar structures) was based on the peak coordinates of identified clusters and sub-clusters and revealed that most significant peaks were located in the right hemisphere in the overall region of the telencephalon (see additional details in tables 2–4).
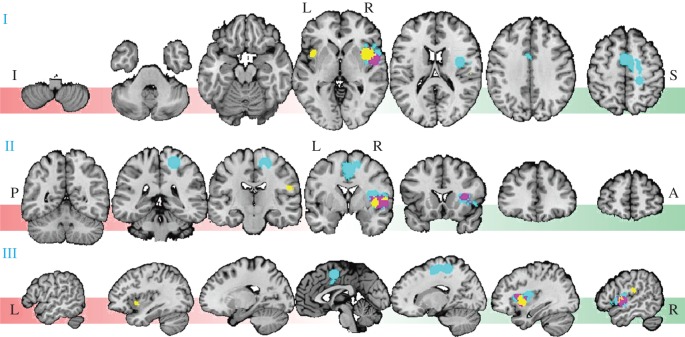
Figure 2 shows a summary of significant voxels in a representative set of brain slices.
Figure 2.
Results of MKDA meta-analysis for neural activity associated with cardioceptive attentiveness (I, axial; II, coronal; III, sagittal view). Slices at 18 mm distance were superimposed on the standard SPM8 high resolution anatomical image SPM8_colin27T1_seg.img and show all identified brain areas representatively. Yellow voxels indicate activation meeting the ‘height-based’ threshold (p < 0.05, whole-brain family-wise error (FWER) corrected). Incremental activation outside the 10 mm kernel region (FWER-corrected for spatial extent at p < 0.05) is shown in magenta and turquoise with primary alpha levels at p < 0.01 and p < 0.05, respectively (FWER-corrected, one-tailed). Coordinates mark cluster peaks in MNI space. I, inferior; S, superior; P, posterior, A, anterior; L, left; R, right.
4. Discussion
In this study, I have conducted a meta-analysis (MKDA) in order to identify brain activity associated with an IT as compared to a CT in a set of nine studies reporting BOLD fMRI contrast (IT > CT).
(a). Brain areas associated with heart-focused interoception
As expected, the findings of the current meta-analysis corroborate that neural activation in the insula (bilateral, cluster peak in BA 13) is associated with processing heart-focused interoceptive attentiveness probably strongly including aspects of interoceptive accuracy/sensitivity. In line with this view, BA 13 has been associated with perceiving visceral information and has been called the interoceptive cortex [22], which has been shown to be activated when participants focus on interoceptive information from various modalities such as temperature, touch, pain, forced respiration and isometric exercise, itching after cutaneous histamine injection, as well as distension of the oesophagus, stomach, bladder or rectum [22,31].
Cytoarchitectonic examination of the insula allows differentiation of anterior-ventral agranular, middle dysgranular and posterior granular parts. While automated analysis [29] has suggested further subparts of the granular insula (area Ig1 and rostral to it, area Ig2), a more recent study performing manual analysis did not confirm such subdivision [71]. In humans, BA 13 is considered part of the posterior granular insula, comprising a bridge between lateral and medial layers of the brain [72]. This corroborates previous reports associating interoception with posterior and mid-insular regions [24,29].
Predictive coding theory has suggested that pyramidal neurons of the supra-granular layers of the granular insular cortex compute differences between predicted and received sensory signals, and may send the respective prediction-error signal back to deep layers of agranular cortical regions [73]. Other intermixed pyramidal neurons are considered for tuning the gain on predictions and prediction errors dynamically in order to modulate the precision weight associated with a particular prediction error. This weight is supposed to depend on the confidence in descending predictions or the reliability of an incoming sensory signal such as heartbeats. Accordingly, BA 13 may serve as a basic evaluator of cardioceptive input comparing one's internal state to predicted effects of action including attempts for regulating physiological homeostasis. Cardioceptive accuracy/sensitivity may therefore correlate with the reliable registration and evaluation of an incoming cardiac afferent signal. Hence, it may be relevant for setting precision weights affecting transmission strength of the respective information and adjusting the priors for subsequent predictions. Organ-specific vulnerability, for example, in terms of high precision weights could be responsible for the development of affective disorders in patients experiencing cardiac arrhythmia. Interoceptive attentiveness may more broadly support optimal resource allocation for the comparison of such information against predictions. This could affect the quality of interoceptive information available for intuition-based decision-making and might affect or even hamper the development of somatic markers, which appears to be an issue in patients with panic disorder [21].
Several of the studies included in the current meta-analysis have found neural activation associated with cardioceptive attentiveness in the anterior insula [20,50,54,55]. Only two studies have reported correlations between scores reflecting interoceptive accuracy/sensitivity obtained with variants of the above-mentioned heartbeat detection task [9], control-task performance as covariate and regions where activity was enhanced by interoceptive attentiveness relative to the CT (contrast IT > CT) [50,52]. Strongest relationships with accuracy were reported for the right anterior insula/opercular cortex (R = 0.62, MNI coordinates: 34, 20, 4) and right anterior insula (R = 0.56, p < 0.01, MNI coordinates: 33, 14, 14) in the two studies, respectively. Further significant correlations with cardioceptive accuracy/sensitivity were found in inferior parietal lobule and the medial frontal gyrus extending into the cingulate gyrus in one study with male participants only [52]. If task demands associated with accuracy are not needed during cardioceptive attentiveness, this could explain why activation in the anterior insula did not reach significance in the current meta-analysis, as accuracy was only featured in a subset of studies. However, it is notable that coordinate transformation into Talairach space and search via the Talairach Daemon allocates the above-mentioned coordinates to the claustrum (for further discussion in the context of this area, see below). In addition, it should be considered that both studies also reported correlations of accuracy scores with negative emotion/anxiety, and negative emotion/anxiety also correlated with neural activation in the anterior insula. This is in line with further studies associating activation in the anterior insula with anxiety, worrying about self-aversive events, risk and the anticipation of punishment, disgust, guilt, sadness or fear [29,74–81]. One of the studies in the current meta-analysis included MDD patients [20]. Another one included participants with phobia and exposed them to phobia-related stimuli [54]. Negative emotion may therefore explain anterior insula activation in these studies. Of note, anxiety also has been shown to inflate accuracy scores in the heartbeat detection task leading to artificially high sensitivity not necessarily rooted in better detection performance [33]. Further studies support the view that the anterior insula is involved in focusing one's attention on detecting salient or deviant external stimuli [82–85]. The IT in two of these studies [54,55] required pressing a button for each detected heartbeat. Another study's IT required judging whether tones corresponded to heartbeats or whether they were delayed. Compared with IT in other studies, these tasks involve monitoring external events. In addition, they may exert particularly high attentional demand. Both could account for higher anterior insula involvement. More studies are required for a comparative analysis that would allow examining whether such task characteristics explain variation in anterior insula recruitment as well as the lack of significant activation in the current meta-analysis. Nevertheless, the findings of our current meta-analysis highlight the robustness of posterior insular activation during cardioceptive attentiveness across such variations.
Further neural activation was confirmed in the right claustrum, a small thin and jagged structure located posterior to the insula, projecting back and forth to nearly all cortical regions [86]. Already Brodmann considered the claustrum as a likely extension of cortical layer VI [87] and more recent perspectives suggest the claustrum may comprise a seventh layer of the insular cortex [88]. Cardioception involves focused attention for detecting discrete specific events in an ongoing stream of concurrent noise (i.e. other visceral and body signals). This would also closely match demand characteristics associated with cardioceptive accuracy, and matches the suggested role of the claustrum for multimodal coordination of widespread ipsilateral and contralateral cortical regions supporting the experience conscious perception, cognition and action [86] with a particular role in orchestrating regions controlling attention [89]. Although other sources claim that the claustrum may comprise separate unimodal processing regions [90], the widespread connectivity of the area suggests that interoceptive information may be fed forward to many adjacent areas and may thus exert concerted moderating influence on processes performed in higher order cortical regions. It is still under debate whether the claustrum also guides sub-cortical regions such as the basal ganglia, caudate nucleus, putamen and globus pallidus [91]. It is notable that none of the studies in the current meta-analysis had explicit hypotheses regarding the claustrum. One reason may be that it is hard to assess such a thin structure via fMRI specifically (e.g. as a region of interest) but it is to be hoped that increasing magnetic field strength, improved control of movement artefact and, last but not least, fibre tractography will allow detailed examination of the role of this interesting structure for interoception in the near future. This will also improve differentiating the particular roles of the anterior insula versus the claustrum.
Further activation was found in the right medial frontal gyrus (BA 6). This area includes the agranular medial part of the gyrus frontalis superior, lacking the internal granular layer IV. BA 6 has been associated with high-level executive functioning and processes relevant for decision-making [92,93]. Right hemisphere BA 6 is said to be relevant for sequential movements, related error monitoring and control, specifically aiding decisions about equality versus difference. While these functions appear useful for the performance in both IT and CT, task demands of the IT are usually considered to be higher. Therefore, the IT may require stronger activation in BA 6, which would explain activation in the contrast IT > CT. To corroborate this interpretation, it would be interesting to see whether neural activation in BA 6 varies with task difficulty. Yet the two studies aiming for particular control of this issue also found activation in BA 6 [54,55]. This suggests that BA 6 may also be concerned with IT-specific issues.
The right precentral gyrus (BA 44), also known as the pars opercularis of the inferior frontal gyrus borders the insula and is considered relevant for phonological and syntactic processing as well as music perception [94]. Other studies have associated emotional expression, verbal understanding, language accentuation, generating melodies, as well as behaviour monitoring and inhibition [22], in particular selective response suppression in go/no-go tasks [95], with this area. The IT of detecting individual heartbeats shares many characteristics with this functional description, involving decoding of an on-going complex stream of information into relevant versus irrelevant discrete events. As strategies for cardioception vary, it should also be noted that the three studies using a heartbeat detection task used tone detection as CT. This may have implicitly led participants to using auditory strategies in the IT. Furthermore, BA 44 activation in the IT > CT contrast may reflect selective suppression of response to events other than the actual heartbeat. Finally, the specific task of identifying heartbeats while scanner noise and vibration distract may reflect processing of whether a signal matches a template or not. Comparing a variety of CT and asking participants about their interoceptive strategies could improve our understanding of this issue.
(b). On hemispheric specialization
With regards to hemispheric specialization, it is noteworthy that the current meta-analysis indicates right-hemispheric dominance for processing heart-related interoceptive information. Although only three of the nine individual fMRI studies have tentatively suggested right-hemispheric dominance in their hypotheses [50–52], outcomes of several studies already provide evidence for a stronger association of interoceptive accuracy/sensitivity with right-insular activation. In the study by Critchley and co-workers [50], accuracy of heartbeat perception correlated with the BOLD response most strongly in the right anterior insula/opercular cortex, but this could also be explained by subjective anxiety symptoms (see above). Caseras and co-workers [54] reported that only right-sided insula activity correlated with interoceptive accuracy/sensitivity, while left-sided activity was linked to increased state and trait anxiety as assessed with the Spielberger Trait and State Anxiety Inventory [96]. Kuehn and co-workers [51] found only right-hemispheric activation in participants with high cardioceptive accuracy. Further evidence for right-insular dominance for interoception comes from electroencephalography- (EEG-) based dipole source-localization studies [97,98]. EEG studies on the so-called heartbeat-evoked potential also have shown greater amplitudes over right electrode positions for subjects with high cardioceptive accuracy/sensitivity [99,100]. Altogether, it is striking that aspects of accuracy are quite prominent in these studies.
The right-sided dominance of interoceptive information processing may instead suggest non-verbal information processing which may partly explain why interoception is often described as gut-feeling rather than explicit knowledge. The right-insular cortex has also been considered particularly important for cerebral regulation of cardiac functions [101,102]. For example, after strokes involving the right-insular cortex the risk of cardio-autonomic dysfunction was enhanced [103], and right-sided stroke was associated with reduced heart rate variability [104]. While (rest and digest related) parasympathetic activity is re-represented in the left (or dominant) insula, (distress related) sympathetic activity is re-represented in the right (or non-dominant) hemisphere [22]. A review regarding the role of interoception in anxiety disorders [18] suggests particularly strong links to panic disorder, where hypervigilant processing and biased evaluation of heart-related symptoms is at the core of both psychopathology and therapeutic approaches. Altogether, this opens interesting perspectives on the role of interoception with regards to (chronic) stress, as this affects the balance between parasympathetic and sympathetic nervous system activation. In this regard, it appears promising to focus on laterality and sub-cortical connectivity of neural activation associated with interactions of interoception and different levels of state and trait anxiety.
(c). Tasks and methods
As reviewed above, considerable variation exists in the study set with regards to task characteristics as well as measurement and analysis features. It may be argued that this heterogeneity hampers the meta-analysis. However, it may also be considered a particular strength of a meta-analysis to reveal which areas are consistently activated across the IT > CT contrasts obtained from individual studies. Interpretation of the current findings should therefore consider such characteristics. Consistently, the studies' IT required cardioceptive attentiveness involving focused attention to one's heartbeat for a given time interval and/or cardioceptive accuracy/sensitivity involving exact counting of perceived heartbeats in a given time interval, and the CT required focused attention to discrete (external) tones uncorrelated to one's heartbeat. It should be noted that variation in the design of CTs lead to variable degrees of control of attentional demand characteristics in the IT > CT contrast. Most studies' CTs simply required attention to target stimuli. Other studies' CTs also involved discrimination of rare targets against a series of concurrent tones. Brain activation identified in the current meta-analysis may therefore reflect aspects of attentional demand characteristics not controlled by the particular CTs of individual studies.
Summarizing table 1 provides further information for correct interpretation of the current findings. First of all, it is notable, that several studies failed to provide all details necessary for precise replication of both study protocol and analysis. The number of volumes per task had to be estimated in five studies, coil-type was not reported in four studies, and resampled voxel size was only reported in four studies. Second, it should be noted that smoothing kernel varied greatly from 5 to 12 mm full width at half maximum (FWHM). Kernel size can have profound effects on outcomes, and an FWHM of at least 8 mm has been suggested as optimal for group inference [105]. In the current meta-analysis, one could roughly conclude that findings of studies with larger smoothing kernel may be overrepresented while studies with smaller kernel may be underrepresented. In the current meta-analysis, a kernel of 6 mm FWHM was used most often. Replication may benefit from choosing similar kernel size. With regards to field strength, the study set is well balanced with four studies using a 1.5 Tesla scanner, and four studies using a 3 Tesla scanner; 3 Tesla can be considered to be the current gold standard for psychological fMRI experiments, because lower field strength leads to reduced detection power whereas higher field strength involves increasing issues due to movement artefacts. Henceforth, the 3 Tesla studies could be weighted in more strongly when interpreting the results of the current meta-analysis.
When considering further task details, it is important to note that variation in measurement and task features or sample characteristics is unfortunately confounded in any pair of studies one might compare. The limited available data also renders statistical comparison of sub-sets of studies rather questionable as they would comprise three or four studies at the most, and variations other than the feature of interest would not be well balanced across the compared study sets. Hence, it would be impossible to evaluate the basis for differences in brain activity identified in such a comparison.
Considering that the MKDA approach relies on location of cluster peaks and sample size to compute meta-analysis further underlines that the results most likely reflect large commonalities between studies rather than being affected by subtle detail.
(d). Limitations and recommendations
The number of available BOLD fMRI studies with human participants on interoception is rather limited. Almost all of these studies have applied IT concerned with cardioception. Therefore, comparative meta-analysis of neural activation associated with different target organs is currently not possible. Studies investigating interoception of target organs other than the heart also deviate in many procedural aspects. Therefore, stringent comparison is rendered rather futile due to confounders. In addition, IT and CT are not always conceptually clear with regards to which aspects of interoception are examined. In this regard, the recently introduced model by Garfinkel and co-workers [5,6] may provide a valuable tool. The current findings can also not differentiate more general from heart-specific aspects of interoceptive information processing. To improve upon this situation, it would be helpful if studies would at least uniformly report findings of whole-brain analysis regarding the basic contrast IT > CT. A comparative meta-analysis across a variety of tasks with the unique commonality of particular target organs might solve these issues, when more studies become available.
With regard to the subset of studies using versions of the heartbeat detection task [9], it is important to note that none of these studies has assessed interoceptive accuracy/sensitivity directly in the scanner. Therefore, results of the current meta-analysis instead reflect all aspects involved in cardioception rather than the aspect of accuracy/sensitivity alone. Of note, neural activity correlated with interoceptive accuracy/sensitivity assessed outside the scanner only in some of the brain areas identified in the contrast IT > CT (see above). Yet a recent study has developed a task for assessing interoceptive accuracy/sensitivity in the scanner. The authors found similar significant neural activity to the current meta-analysis including the right middle frontal gyrus, right inferior frontal operculum, bilateral insular cortices, left inferior frontal triangular gyrus, left superior middle frontal gyrus and bilateral inferior parietal gyri [47]. Cluster peak coordinates particularly pointed out the posterior right insula (BA 13) and the left claustrum. Connectivity analysis in this study further highlighted involvement of prefrontal areas. These findings suggest that brain areas associated with assessing heart-focused interoceptive accuracy/sensitivity largely overlap with brain areas relevant for cardioceptive attentiveness as identified in the current meta-analysis. Future reviews and meta-analyses would however greatly profit if all studies assessing specific aspects of interoception would report correlations between brain areas identified in the basic contrast IT > CT.
Owing to the limited availability of data, it is currently also not possible to consider whether neural activation truly reflects enhanced information processing or inhibition. To this end, it would be helpful if more studies reported data on deactivation related to interoception. Together with appropriate baselines, as for example introduced by Critchley and co-workers [50], this could help to systematically approach this issue.
Finally, the great variation in candidate regions and hypotheses in the current set of studies is remarkable. This underlines the need for further refinement of functional-anatomical models underlying interoception. A meta-analysis is one approach for identifying particularly important structures by revealing the most consistently active areas across variations of task details, samples and experiments. However, the current approach ignores a huge amount of information that could be exploited if original raw data were shared, for example, in open databases available online (e.g. www.openfMRI.org or www.nitric.org).
5. Conclusion and outlook
Findings of the current meta-analysis on nine studies reporting BOLD fMRI contrast regarding cardioceptive attentiveness (i.e. focused attention to one's heartbeat for a given time interval) and/or cardioceptive accuracy (i.e. exact counting of perceived heartbeats in a given time interval) as compared to a CT corroborate and extend established models of interoceptive processing [22]. The MKDA meta-analysis has revealed a complex network involving the posterior (granular) insula (BA 13), the claustrum, as well as temporal and frontal areas, highlighting right-hemispheric dominance of cardioception. This may reflect non-verbal information processing involving sequence monitoring and features of acoustic event detection that may be applied to identification of individual heartbeats. Notably, the claustrum has not been considered in individual studies but emerged as an important brain area in the meta-analysis, possibly orchestrating top-down attention deployment and processing of feed-forward cardioceptive information related to prefrontal neural activity. Although some of the individual studies have emphasized the role of the anterior insula [20,50,54,55], the current findings suggest that this area may rather be concerned with evaluative aspects of interoception and probably negative or anxious emotion. Further research is required on different targets organs (e.g. stomach or bladder) and specific aspects of interoception such as interoceptive sensibility and metacognitive awareness in order to further improve our understanding of the neural correlates of interoception.
Supplementary Material
Acknowledgements
The author thanks Verena Murowski for her valuable assistance during initial analysis, as well as Christine Wiebking and Stefan Sütterlin for their helpful review and comments regarding an early draft version of the manuscript.
Endnote
Please note that the original authors may have used deviating terminology and sometimes have used the same labels with deviating definitions. The terminology in the current manuscript has been unified in accord with the definitions of Garfinkel and co-workers [5,6].
The reference list is available upon request from the corresponding author.
Competing interests
The author has no competing interests.
Funding
During manuscript preparation, S.M.S. was supported by the Federal Ministry of Education and Research, project 01EO1004.
References
- 1.Sherrington CS. 1948. The integrative action of the nervous system. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Vaitl D. 1996. Interoception. Biol. Psychol. 42, 1–27. ( 10.1016/0301-0511(95)05144-9) [DOI] [PubMed] [Google Scholar]
- 3.Lange CG, James W. 1967. The emotions. London, UK: Hafner Publishing Co (edited by Knight Dunlap, Reprinted). [Google Scholar]
- 4.Seth AK, Critchley HD. 2013. Extending predictive processing to the body: emotion as interoceptive inference. Behav. Brain Sci. 36, 227–228. ( 10.1017/S0140525X12002270) [DOI] [PubMed] [Google Scholar]
- 5.Garfinkel SN, Critchley HD. 2013. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: ‘Anterior insular cortex mediates bodily sensibility and social anxiety’ by Terasawa et al. (2012). Social Cogn. Affective Neurosci. 8, 231–234. ( 10.1093/scan/nss140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. 2015. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74. ( 10.1016/j.biopsycho.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 7.Porges S. 1993. Body perception questionnaire: Laboratory of development assessment. College Park, MD: University of Maryland. [Google Scholar]
- 8.Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. 2012. The multidimensional assessment of interoceptive awareness (MAIA). PLoS ONE 7, e48230 ( 10.1371/journal.pone.0048230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schandry R. 1981. Heart beat perception and emotional experience. Psychophysiology 18, 483–488. ( 10.1111/j.1469-8986.1981.tb02486.x) [DOI] [PubMed] [Google Scholar]
- 10.Wiens S, Mezzacappa ES, Katkin ES. 2000. Heartbeat detection and the experience of emotions. Cogn. Emotion 14, 417–427. ( 10.1080/026999300378905) [DOI] [Google Scholar]
- 11.Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD. 2013. What the heart forgets: cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology 50, 505–512. ( 10.1111/psyp.12039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werner NS, Peres I, Duschek S, Schandry R. 2010. Implicit memory for emotional words is modulated by cardiac perception. Biol. Psychol. 85, 370–376. ( 10.1016/j.biopsycho.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 13.Werner NS, Jung K, Duschek S, Schandry R. 2009. Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology 46, 1123–1129. ( 10.1111/j.1469-8986.2009.00855.x) [DOI] [PubMed] [Google Scholar]
- 14.Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T. 2010. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychol. Sci. 21, 1835–1844. ( 10.1177/0956797610389191) [DOI] [PubMed] [Google Scholar]
- 15.Werner NS, Schweitzer N, Meindl T, Duschek S, Kambeitz J, Schandry R. 2013. Interoceptive awareness moderates neural activity during decision-making. Biol. Psychol. 94, 498–506. ( 10.1016/j.biopsycho.2013.09.002) [DOI] [PubMed] [Google Scholar]
- 16.Herbert BM, Pollatos O, Flor H, Enck P, Schandry R. 2010. Cardiac awareness and autonomic cardiac reactivity during emotional picture viewing and mental stress. Psychophysiology 47, 342–354. ( 10.1111/j.1469-8986.2009.00931.x) [DOI] [PubMed] [Google Scholar]
- 17.Paulus MP, Stein MB. 2010. Interoception in anxiety and depression. Brain Struct. Funct. 214, 451–463. ( 10.1007/s00429-010-0258-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. 2010. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin. Psychol. Rev. 30, 1–11. ( 10.1016/j.cpr.2009.08.008) [DOI] [PubMed] [Google Scholar]
- 19.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 76, 258–266. ( 10.1016/j.biopsych.2013.11.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. 2010. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed ‘material me’. World J. Biol. Psychiatry 11, 538–549. ( 10.3109/15622970903563794) [DOI] [PubMed] [Google Scholar]
- 21.Wölk J, Sütterlin S, Koch S, Vögele C, Schulz SM. 2014. Enhanced cardiac perception predicts impaired performance in the Iowa Gambling Task in patients with panic disorder. Brain Behav. 4, 238–246. ( 10.1002/brb3.206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig AD. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 23.Flynn FG, Benson DF, Ardila A. 1999. Anatomy of the insula—functional and clinical correlates. Aphasiology 13, 55–78. ( 10.1080/026870399402325) [DOI] [Google Scholar]
- 24.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. 2013. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum. Brain Mapp. 34, 2944–2958. ( 10.1002/hbm.22113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farb NA, Segal ZV, Anderson AK. 2013. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex 23, 114–126. ( 10.1093/cercor/bhr385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer T, Critchley HD, Preuschoff K. 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334–340. ( 10.1016/j.tics.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 27.Mesulam MM, Mufson EJ. 1982. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J. Comp. Neurol. 212, 1–22. ( 10.1002/cne.902120102) [DOI] [PubMed] [Google Scholar]
- 28.Ongur D, Price JL. 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219. ( 10.1093/cercor/10.3.206) [DOI] [PubMed] [Google Scholar]
- 29.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. 2010. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. ( 10.1007/s00429-010-0255-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. 2012. A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage 61, 1129–1142. ( 10.1016/j.neuroimage.2012.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig AD. 2009. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. ( 10.1038/nrn2555) [DOI] [PubMed] [Google Scholar]
- 32.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. 2009. The pathways of interoceptive awareness. Nat. Neurosci. 12, 1494–1496. ( 10.1038/nn.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlers A, Breuer P. 1996. How good are patients with panic disorder at perceiving their heartbeats? Biol. Psychol. 42, 165–182. ( 10.1016/0301-0511(95)05153-8) [DOI] [PubMed] [Google Scholar]
- 34.Bogaerts K, Van Eylen L, Li W, Bresseleers J, Van Diest I, De Peuter S, Stans L, Decramer M, Van den Bergh O. 2010. Distorted symptom perception in patients with medically unexplained symptoms. J. Abnorm. Psychol. 119, 226–234. ( 10.1037/a0017780) [DOI] [PubMed] [Google Scholar]
- 35.Pollatos O, Werner NS, Duschek S, Schandry R, Matthias E, Traut-Mattausch E, Herbert BM. 2011. Differential effects of alexithymia subscales on autonomic reactivity and anxiety during social stress. J. Psychosom. Res. 70, 525–533. ( 10.1016/j.jpsychores.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 36.Michal M, Reuchlein B, Adler J, Reiner I, Beutel ME, Vogele C, Schachinger H, Schulz A. 2014. Striking discrepancy of anomalous body experiences with normal interoceptive accuracy in depersonalization-derealization disorder. PLoS ONE 9, e89823 ( 10.1371/journal.pone.0089823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedeno L, et al. 2014. How do you feel when you can't feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS ONE 9, e98769 ( 10.1371/journal.pone.0098769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz A, Koster S, Beutel ME, Schachinger H, Vogele C, Rost S, Rauh M, Michal M. 2015. Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: neurophysiological evidence for impaired cortical representation of bodily signals. Psychosom. Med. 77, 506–516. ( 10.1097/PSY.0000000000000195) [DOI] [PubMed] [Google Scholar]
- 39.Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schopf V, Kopietz R, Wiesmann M, Schandry R. 2008. Reduced perception of bodily signals in anorexia nervosa. Eat. Behav. 9, 381–388. ( 10.1016/j.eatbeh.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 40.Herbert BM, Pollatos O. 2014. Attenuated interoceptive sensitivity in overweight and obese individuals. Eat. Behav. 15, 445–448. ( 10.1016/j.eatbeh.2014.06.002) [DOI] [PubMed] [Google Scholar]
- 41.Schulz A, Vögele C. 2015. Interoception and stress. Front. Psychol. 6, 993 ( 10.3389/fpsyg.2015.00993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uddin LQ, Kinnison J, Pessoa L, Anderson ML. 2014. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J. Cogn. Neurosci. 26, 16–27. ( 10.1162/jocn_a_00462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Critchley HD, Garfinkel SN. 2015. Interactions between visceral afferent signaling and stimulus processing. Front. Neurosci. 9, 286 ( 10.3389/fnins.2015.00286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapa DW, Akintade B, Son H, Woltz P, Hunt D, Friedmann E, Hartung MK, Thomas SA. 2014. Pathophysiological relationships between heart failure and depression and anxiety. Crit. Care Nurse 34, 14–24; quiz 25 ( 10.4037/ccn2014938) [DOI] [PubMed] [Google Scholar]
- 45.Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O'Connor C, Sketch MH. 2013. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J. Am. Heart Assoc. 2, e000068 ( 10.1161/JAHA.112.000068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleint NI, Wittchen HU, Lueken U. 2015. Probing the interoceptive network by listening to heartbeats: an fMRI study. PLoS ONE 10, e0133164 ( 10.1371/journal.pone.0133164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiebking C, Northoff G. 2015. Neural activity during interoceptive awareness and its associations with alexithymia: an fMRI study in major depressive disorder and non-psychiatric controls. Front. Psychol. 6, 589 ( 10.3389/fpsyg.2015.00589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caseras X, Murphy K, Mataix-Cols D, Lopez-Sola M, Soriano-Mas C, Ortriz H, Pujol J, Torrubia R. 2013. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum. Brain Mapp. 34, 1220–1229. ( 10.1002/hbm.21503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 51.Kuehn E, Mueller K, Lohmann G, Schuetz-Bosbach S. 2016. Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Struct. Funct. 221, 1555–1571. ( 10.1007/s00429-015-0989-8) [DOI] [PubMed] [Google Scholar]
- 52.Pollatos O, Schandry R, Auer DP, Kaufmann C. 2007. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 1141, 178–187. ( 10.1016/j.brainres.2007.01.026) [DOI] [PubMed] [Google Scholar]
- 53.Zaki J, Davis JI, Ochsner KN. 2012. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62, 493–499. ( 10.1016/j.neuroimage.2012.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caseras X, Murphy K, Mataix-Cols D, Lopez-Sola M, Soriano-Mas C, Ortriz H, Pujol J, Torrubia R. 2013. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum. Brain Mapp. 34, 1220–1229. ( 10.1002/hbm.21503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaki J, Davis JI, Ochsner KN. 2012. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62, 493–499. ( 10.1016/j.neuroimage.2012.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barttfeld P, Wicker B, McAleer P, Belin P, Cojan Y, Graziano M, Leiguarda R, Sigman M. 2013. Distinct patterns of functional brain connectivity correlate with objective performance and subjective beliefs. Proc. Natl Acad. Sci. USA 110, 11 577–11 582. ( 10.1073/pnas.1301353110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naring GW, van der Staak CP. 1995. Perception of heart rate and blood pressure: the role of alexithymia and anxiety. Psychother. Psychosom. 63, 193–200. ( 10.1159/000288959) [DOI] [PubMed] [Google Scholar]
- 58.Dunn BD, Dalgleish T, Ogilvie AD, Lawrence AD. 2007. Heartbeat perception in depression. Behav. Res. Ther. 45, 1921–1930. ( 10.1016/j.brat.2006.09.008) [DOI] [PubMed] [Google Scholar]
- 59.Harshaw C. 2015. Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol. Bull. 141, 311–363. ( 10.1037/a0038101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. 2009. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. NeuroImage 45, S210–S221. ( 10.1016/j.neuroimage.2008.10.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wager TD, Lindquist M, Kaplan L. 2007. Meta-analysis of functional neuroimaging data: current and future directions. Social Cogn. Affective Neurosci. 2, 150–158. ( 10.1093/scan/nsm015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. 2005. BrainMap taxonomy of experimental design: description and evaluation. Hum. Brain Mapp. 25, 185–198. ( 10.1002/hbm.20141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox PT, Laird AR, Eickhoff SB, Lancaster JL, Fox M, Uecker AM. 2013. User manual for GingerALE 2.3. San Antonio, TX: Research Imaging Institute, UT Health Science Center. [Google Scholar]
- 64.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage 16, 765–780. ( 10.1006/nimg.2002.1131) [DOI] [PubMed] [Google Scholar]
- 65.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. ( 10.1002/hbm.20718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barrett LF, Lindquist K, Bliss-Moreau E, Kaplan L. 2008. Documentation for multi-level kernel density analysis (MKDA): Tor Wager's Meta-Analytic procedures for neuroimaging findings. http://wagerlab.colorado.edu/files/tools/MetaDocumentation111908.pdf (accessed 21 June 2016). [Google Scholar]
- 67.Nee DE, Wager TD, Jonides J. 2007. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affective Behav. Neurosci. 7, 1–17. ( 10.3758/CABN.7.1.1) [DOI] [PubMed] [Google Scholar]
- 68.Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme. [Google Scholar]
- 69.Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. 1997. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum. Brain Mapp. 5, 238–242. ( 10.1002/(SICI)1097-0193(1997)5:4%3C238::AID-HBM6%3E3.0.CO;2-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lancaster JL, et al. 2000. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 10, 120–131. ( 10.1002/1097-0193(200007)10:3%3C120::AID-HBM30%3E3.0.CO;2-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morel A, Gallay MN, Baechler A, Wyss M, Gallay DS. 2013. The human insula: architectonic organization and postmortem MRI registration. Neuroscience 236, 117–135. ( 10.1016/j.neuroscience.2012.12.076) [DOI] [PubMed] [Google Scholar]
- 72.National Primate Research Center, University of Washington. BrainInfo. http://braininfo.rprc.washington.edu/centraldirectory.aspx?ID=1009 (accessed 21 June 2016).
- 73.Barrett LF, Simmons WK. 2015. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429. ( 10.1038/nrn3950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jabbi M, Bastiaansen J, Keysers C. 2008. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS ONE 3, e2939 ( 10.1371/journal.pone.0002939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. 2000. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry 48, 30–42. ( 10.1016/S0006-3223(00)00874-X) [DOI] [PubMed] [Google Scholar]
- 76.Mathews A, Yiend J, Lawrence AD. 2004. Individual differences in the modulation of fear-related brain activation by attentional control. J. Cogn. Neurosci. 16, 1683–1694. ( 10.1162/0898929042947810) [DOI] [PubMed] [Google Scholar]
- 77.Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL. 2000. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol. Psychiatry 48, 43–50. ( 10.1016/S0006-3223(00)00251-1) [DOI] [PubMed] [Google Scholar]
- 78.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. 2003. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655–664. ( 10.1016/S0896-6273(03)00679-2) [DOI] [PubMed] [Google Scholar]
- 79.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. 2003. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage 19, 1439–1448. ( 10.1016/S1053-8119(03)00251-9) [DOI] [PubMed] [Google Scholar]
- 80.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. 2006. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol. Psychiatry 60, 402–409. ( 10.1016/j.biopsych.2006.04.038) [DOI] [PubMed] [Google Scholar]
- 81.Hoehn-Saric R, Schlund MW, Wong SH. 2004. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 131, 11–21. ( 10.1016/j.pscychresns.2004.02.003) [DOI] [PubMed] [Google Scholar]
- 82.Menon V, Uddin LQ. 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. ( 10.1007/s00429-010-0262-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. 2010. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 214, 669–680. ( 10.1007/s00429-010-0260-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crottaz-Herbette S, Menon V. 2006. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. 18, 766–780. ( 10.1162/jocn.2006.18.5.766) [DOI] [PubMed] [Google Scholar]
- 85.Downar J, Crawley AP, Mikulis DJ, Davis KD. 2002. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 87, 615–620. ( 10.1152/jn.00636.2001) [DOI] [PubMed] [Google Scholar]
- 86.Crick FC, Koch C. 2005. What is the function of the claustrum? Phil. Trans. R. Soc. B 360, 1271–1279. ( 10.1098/rstb.2005.1661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brodmann K. 1905. Beitraege zur histologischen lokalisation der grosshirnrinde: dritte mitteilung: die rindenfelder der niederen affen. J. Psychol. Neurol. 4, 177–226. [Google Scholar]
- 88.Tanne-Gariepy J, Boussaoud D, Rouiller EM. 2002. Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J. Comp. Neurol. 454, 140–157. ( 10.1002/cne.10425) [DOI] [PubMed] [Google Scholar]
- 89.Smith JB, Alloway KD. 2010. Functional specificity of claustrum connections in the rat: interhemispheric communication between specific parts of motor cortex. J. Neurosci. 30,16 832–16 844. ( 10.1523/JNEUROSCI.4438-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Remedios R, Logothetis NK, Kayser C. 2010. Unimodal responses prevail within the multisensory claustrum. J. Neurosci. 30,12 902–12 907. ( 10.1523/JNEUROSCI.2937-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milardi D, et al. 2015. Cortical and subcortical connections of the human claustrum revealed in vivo by constrained spherical deconvolution tractography. Cereb. Cortex 25, 406–414. ( 10.1093/cercor/bht231) [DOI] [PubMed] [Google Scholar]
- 92.Siedentopf DC. 2005. Cortexareale und ihre funktionelle Bedeutung. http://www.fmri-easy.de/start1.htm (accessed 21 June 2016). [Google Scholar]
- 93.Talati A, Hirsch J. 2005. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on ‘what,’ ‘when,’ and ‘where’ related information: an fMRI study. J. Cogn. Neurosci. 17, 981–993. ( 10.1162/0898929054475226) [DOI] [PubMed] [Google Scholar]
- 94.Brown S, Martinez MJ, Parsons LM. 2006. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur. J. Neurosci. 23, 2791–2803. ( 10.1111/j.1460-9568.2006.04785.x) [DOI] [PubMed] [Google Scholar]
- 95.Forstmann BU, van den Wildenberg WP, Ridderinkhof KR. 2008. Neural mechanisms, temporal dynamics, and individual differences in interference control. J. Cogn. Neurosci. 20, 1854–1865. ( 10.1162/jocn.2008.20122) [DOI] [PubMed] [Google Scholar]
- 96.Laux L, Glanzmann P, Schaffner P, Spielberger CD. 1981. Das State-Trait-Angstinventar (STAI) State-Trait Anxiety Inventory (STAI)]. Weinheim, Germany: Beltz Test. [Google Scholar]
- 97.Pollatos O, Kirsch W, Schandry R. 2005. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum. Brain Mapp. 26, 54–64. ( 10.1002/hbm.20121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan H, Yan HM, Xu XG, Han F, Yan Q. 2007. Effect of heartbeat perception on heartbeat evoked potential waves. Neurosci. Bull. 23, 357–362. ( 10.1007/s12264-007-0053-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pollatos O, Schandry R. 2004. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 41, 476–482. ( 10.111/1469-8986.2004.00170.x) [DOI] [PubMed] [Google Scholar]
- 100.Schandry R, Bestler M. 1995. The influence of cardiodynamic performance on heartbeat perception. In Interoception and cardiovascular processes (eds Vaitl D, Schandry R), pp. 223–259. Frankfurt, Germany: Peter Lang. [Google Scholar]
- 101.Leopold C, Schandry R. 2001. The heartbeat-evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clin. Neurophysiol. 112, 674–682. ( 10.1016/S1388-2457(01)00480-1) [DOI] [PubMed] [Google Scholar]
- 102.Riordan H, Squires NK, Brener J. 1990. Cardio-cortical potentials: electrophysiological evidence for visceral perception. Psychophysiology 27, S59 ( 10.1111/j.1469-8986.1990.tb02374.x) [DOI] [Google Scholar]
- 103.Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. 2004. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport 15, 357–361. ( 10.1097/00001756-200402090-00029) [DOI] [PubMed] [Google Scholar]
- 104.Naver HK, Blomstrand C, Wallin BG. 1996. Reduced heart rate variability after right-sided stroke. Stroke 27, 247–251. ( 10.1161/01.STR.27.2.247) [DOI] [PubMed] [Google Scholar]
- 105.Mikl M, Marecek R, Hlustik P, Pavlicova M, Drastich A, Chlebus P, Brazdil M, Krupa P. 2008. Effects of spatial smoothing on fMRI group inferences. Magn. Reson. Imaging 26, 490–503. ( 10.1016/j.mri.2007.08.006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.