Abstract
FOXP2 is the major gene associated with severe, persistent, developmental speech and language disorders. While studies in the original family in which a FOXP2 mutation was found showed volume reduction and reduced activation in core language and speech networks, there have been no imaging studies of different FOXP2 mutations. We conducted a multimodal MRI study in an eight-year-old boy (A-II) with a de novo FOXP2 intragenic deletion. A-II showed marked bilateral volume reductions in the hippocampus, thalamus, globus pallidus, and caudate nucleus compared with 26 control males (effect sizes from −1 to −3). He showed no detectable functional MRI activity when repeating nonsense words. The hippocampus is implicated for the first time in FOXP2 diseases. We conclude that FOXP2 anomaly is either directly or indirectly associated with atypical development of widespread subcortical networks early in life.
FOXP21 is the only identified gene associated with a severe, and persistent developmental speech and language disorder in the setting of preserved cognition. Its discovery through the study of a multigenerational British family (the KE family2) has allowed scientists to explore the possible molecular pathways associated with dysfunction of speech and language networks in the brain3,4. This body of work has also informed the possible mechanisms linked to the emergence of speech and language skills during human evolution5,6. About 20 individuals with either de novo or familial FOXP2 disruptions that only affect the transcript of FOXP2, and no other surrounding genes, have been reported worldwide. All affected individuals share a common phenotype of childhood apraxia of speech, oral dyspraxia and language impairment7. Most cases present with normal brain MRIs, suggesting that this severe phenotype arises from subtle functional brain anomalies. No advanced brain imaging data have been reported on children with FOXP2 mutations. Studies have focused on imaging adult members of the KE family2,8,9,10, thereby imaging them decades after the emergence of their speech-language impairment. As a result, some of these family-specific findings could be due to compensation strategies, neural reorganization, or both. Altogether, there is a lack of data on how FOXP2 abnormality disrupts human development, affecting brain structure and function in childhood, and the development of white matter connectivity crucial for speech and language.
Here we report multimodal neuroimaging findings in an eight-year-old boy with a unique de novo intragenic FOXP2 deletion, case A-II7, whose speech and language profile is consistent with the previously described FOXP2 phenotype. We aimed to identify early markers of brain dysfunction by combining functional and structural MRI methods. We used whole-brain as well as a priori defined regions of interest analyses and compared A-II’s data to those of age-matched typically developing males. Based on neuroimaging findings from the affected members of the KE family, we hypothesized that A-II would show bilateral grey matter reductions in the head of the caudate nucleus, precentral gyrus, cerebellum, and dorsal inferior frontal gyrus8,11. Grey matter increases were predicted in the angular gyrus and posterior superior temporal gyrus11. We also predicted fMRI underactivity during nonsense word repetition (a phenotypic marker8,12) in the speech related sensorimotor cortex bilaterally, the left rolandic operculum, the left putamen, and Broca’s area (pars opercularis and triangularis)9,10. Finally, we hypothesized atypical microstructure of the arcuate fasciculus/superior longitudinal fasciculus, two white matter tracts involved in the “dorsal” language stream responsible for the transformation of speech sounds into articulatory productions13,14.
Results
Global brain measures are preserved
Whole brain grey matter (mean = 0.857l, 38th percentile; Effect size for the difference = −0.299, 95% CI −0.689 to 0.097) and white matter (mean = 0.389l; Effect size for the difference = −1.268, 95% CI −1.780 to −0.741) volumes for case A-II were within the normal range relative to the control group (mean for grey = 0.877, SD = 0.067; mean for white = 0.460l, SD = 0.056).
Whole-brain method reveals grey matter anomalies in the hippocampus and caudate nucleus
Voxel Based Morphometry (VBM) revealed bilateral reductions (see Fig. 1) in a large cluster within the hippocampus (left peak, cluster size 738 voxels, p = 0.046, FWE correction; right peak, cluster size 324 voxels, p < 0.001 uncorrected) and in the head of caudate nucleus (p < 0.001 uncorrected, but a priori hypothesized). One voxel (peak at 27, 3, −2) within the right putamen was significant at uncorrected level (T = 2.68, p = 0.007, cluster size 61 voxels) but did not survive small volume correction. No bilateral increases were detected at p = 0.05 (Family Wise Error correction or FWE). No significant bilateral reductions or increases were found in any of the 25 control participants when compared to the remainder of the control group.
Figure 1. Bilateral grey matter reductions in A-II relative to the control group.
Reductions in the hippocampus (a) and the head of the caudate nucleus (b) were revealed using VBM after correcting for age and total brain grey matter volume. Results are displayed at p = 0.001, uncorrected for multiple comparisons and superimposed onto the study-specific grey matter template. Left hippocampus: peak at 33, −26, −3; cluster size = 738 voxels, T = 5.77, p = 0.046 FWE; Right hippocampus: peak at −26, −30, −2, cluster size = 324 voxels; T = 4.04, p < 0.001 uncorrected; Left caudate head: peak at −14, −3, 18, cluster size = 46 voxels; T = 3.61, p < 0.001 uncorrected; Right caudate head: peak at 14, −6, 16, cluster size = 158 voxels; T = 4.22, p < 0.001 uncorrected.
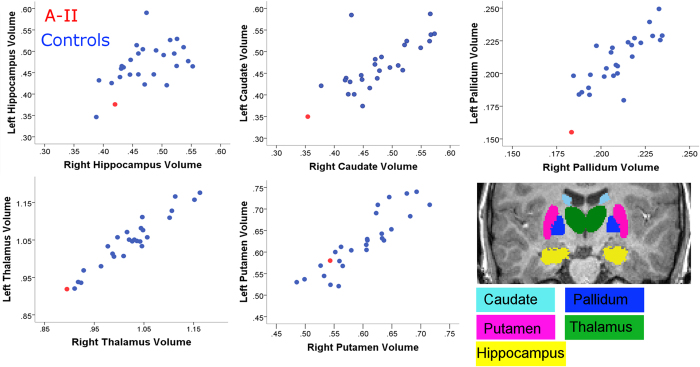
Regional volumetric analysis reveals reductions in the hippocampus and basal ganglia
Bilateral reductions beyond one standard deviation were detected in all regions of interest except the putamen (see Table 1 and Fig. 2). For the globus pallidus and hippocampus, reductions were only statistically significant in the left hemisphere. Values survived correction for multiple comparison in the globus pallidus only. We did not detect any statistically significant volume increases in A-II relative to the control group in any of these a-priori regions of interest.
Table 1. Relative volumes (in percentage of total grey matter) in regions of interest in A-II and in the control group (N = 26).
| Region of interest | Control group mean (SD) | A-II’s value | Estimated percentile | Significance test (one tailed) | Estimated effect size for difference (95% CI) | |
|---|---|---|---|---|---|---|
| Hippocampus | Left | 0.471 (0.047) | 0.376 | 3.63% | t = −1.953; p = 0.036 | −2.021 (−2.939 to −1.079) |
| Right | 0.473 (0.046) | 0.420 | 14.29% | t = −1.113; p = 0.142 | −1.152 (−1.822 to −0.456) | |
| Caudate nucleus | Left | 0.471 (0.056) | 0.350 | 2.85% | t = −2.087; p = 0.029 | −2.161 (−3.123 to −1.175) |
| Right | 0.481 (0.054) | 0.354 | 2.04% | t = −2.272; p = 0.020 | −2.352 (−3.376 to −1.305) | |
| Globus pallidus | Left | 0.210 (0.018) | 0.155 | 0.56% | t = −2.952; p = 0.0056 | −3.056 (−4.319 to −1.773) |
| Right | 0.210 (0.015) | 0.183 | 5.28% | t = −1.739; p = 0.053 | −1.800 (−2.649 to −0.926) | |
| Thalamus | Left | 1.049 (0.068) | 0.919 | 4.38% | t = −1.847; p = 0.044 | −1.912 (−2.795 to −1.004) |
| Right | 1.025 (0.067) | 0.895 | 4.17% | t = −1.875; p = 0.042 | −1.940 (−2.832 to −1.023) | |
| Putamen | Left | 0.624 (0.068) | 0.580 | 27.14% | t = −0.625; p = 0.27 | −0.647 (−1.216 to −0.058) |
| Right | 0.600 (0.060) | 0.543 | 18.77% | t = −0.918; p = 0.19 | −0.950 (−1.574 to −0.301) |
Figure 2. Left and right regional volumes in A-II (red circle) relative to age-matched control males (blue circles).
Volumes are expressed in percentages of total brain grey matter volumes. Insert, example of segmented regions of interest from a control participant (coronal view).
White matter anomalies in speech and language related tracts
No fractional anisotropy (FA) differences between A-II and the control group were statistically significant (Table 2). FA reductions above one standard deviation were however detected for the right dorsal corticobulbar tract, the left arcuate fasciculus, left superior longitudinal fasciculus and left cingulum bundle. FA increases were detected in the right arcuate fasciculus.
Table 2. Mean FA measures derived from tractography in A-II and in the control group.
| Track | Control group mean (SD) | A-II’s value | Estimated percentile | Significance test (one tailed) | Estimated effect size for difference (95% CI) | |
|---|---|---|---|---|---|---|
| Corticospinal tract | Left | 0.393 (0.0146) | 0.387 | 17.67% | t = −0.377; p = 0.35 | −0.390 (−0.928 to 0.161) |
| Right | 0.399 (0.0153) | 0.396 | 43.60% | t = −0.164; p = 0.43 | −0.170 (−0.695 to 0.361) | |
| Corticobulbar tract | Left dorsal | 0.445 (0.0705) | 0.3874 | 22.09% | t = −0.793; p = 0.22 | −0.821 (−1.419 to −0.200) |
| Right dorsal | 0.447 (0.0566) | 0.3614 | 8.37% | t = −1.462; p = 0.084 | −1.513 (−2.279 to −0.722) | |
| Left ventral | 0.396 (0.0475) | 0.3874 | 43.19 | t = −0.175; p = 0.43 | −0.181 (−0.706 to 0.350) | |
| Right ventral | 0.422 (0.0635) | 0.3745 | 24.32% | t = −0.716; p = 0.24 | −0.741 (−1.325 to −0.136) | |
| Arcuate fasciculus | Left | 0.437 (0.0262) | 0.4027 | 11.31% | t = −1.270; p = 0.11 | −1.315 (−2.026 to −0.578) |
| Right | 0.404 (0.0277) | 0.4349 | 66.19% | t = 1.064; p = 0.15 | 1.101 (0.418 to 1.759) | |
| Dorsal (SLF) portion of arcuate | Left | 0.419 (0.0212) | 0.395 | 14.44% | t = −1.106; p = 0.14 | −1.145 (−1.813 to −0.451) |
| Right | 0.400 (0.0188) | 0.4185 | 82.05% | t = 0.947; p = 0.18 | 0.980 (0.325 to 1.611) | |
| Cingulum bundle | Left | 0.348 (0.0241) | 0.3102 | 7.53% | t = −1.527; p = 0.075 | −1.581 (−2.365 to −0.771) |
| Right | 0.335 (0.0299) | 0.3444 | 61.92% | t = 0.310; p = 0.38082 | 0.321 (−0.223 to 0.853) |
Z values that fall one standard deviation below and above the control mean are highlighted in bold.
fMRI activation during nonsense word repetition
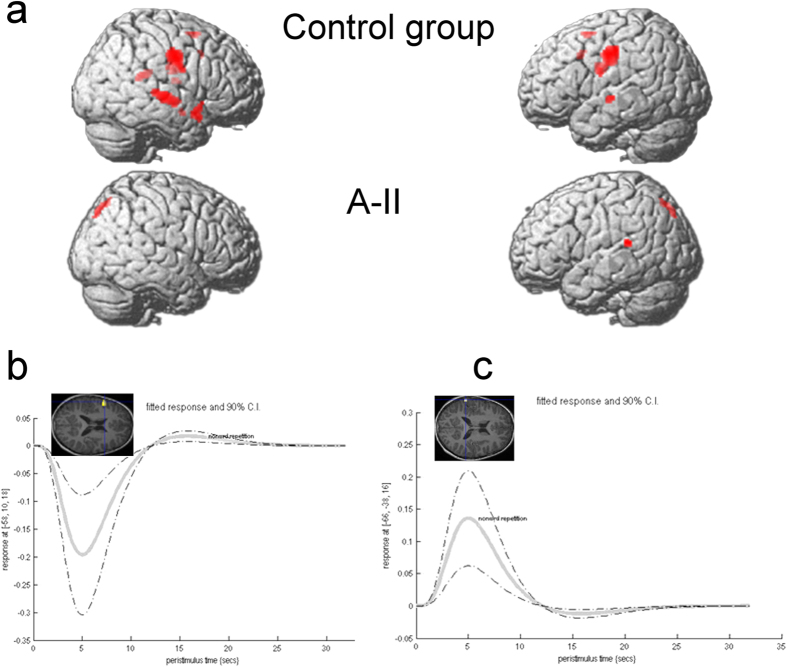
A-II showed no supra-threshold detectable activation during the Nonword repetition vs. Baseline contrast, despite performing the task (Fig. 3). Activation was detected at p = 0.005, uncorrected for multiple comparisons in the left posterior superior temporal gyrus (hypothesized, peak at −66, −38, 16; T = 3.04, p = 0.002, cluster size 17 voxels; see Fig. 2a). The control group showed extensive activation involving the bilateral sensorimotor and superior temporal cortices, as well as the left hemisphere supplementary motor area, anterior cingulate cortex, and pars opercularis (Fig. 3a; see Supplementary Table S1). Bilateral globus pallidus activation was also detected in the control group, but did not survive an extent threshold of 10 voxels (Supplementary Table S1). A-II showed left inferior frontal gyrus activity for the reverse contrast (Baseline vs. Nonword repetition, peak coordinate −58, 10, 18, T = 2.99, p = 0.002, 39 voxels; see Fig. 3b), and superior temporal activation for the Task vs. Baseline contrast at uncorrected threshold (Fig. 3c). No difference between A-II and the control group however survived correction for multiple comparisons (p = 0.05 FWE).
Figure 3. fMRI activation during nonsense word repetition.
(a) Average activation in the control group (top row) and in A-II (bottom row). Results are displayed at an intensity threshold of p = 0.05 family-wise error correction for the group (minimum cluster size 10 voxels) and p = 0.005 uncorrected for multiple comparisons for A-II, and projected onto a single brain rendering. (b) Signal change in the left inferior frontal gyrus peak (insert) shows negative BOLD response in A-II. (c) Signal change in the superior temporal gyrus peak (insert) shows positive BOLD response in A-II.
Two control participants also showed no detectable activation at p = 0.005, and one showed activation only in the left superior temporal gyrus during the Task vs. Baseline contrast. None of these participants showed activation in the pars opercularis for the reverse contrast.
Discussion
Substantial bilateral structural abnormalities in the basal ganglia and hippocampus were detected in an 8 year old boy with the characteristic FOXP2 mutation speech phenotype, providing key insights into the structural correlates of this disease. Brain cortical activity during nonsense word repetition was abnormal. These findings only partly overlap with those of affected members of the KE family, and provide us with early neural markers of FOXP2 dysfunction.
Basal ganglia and thalamus reductions
Both volumetric and VBM results supported our hypothesis of a reduction in the caudate nucleus, a key neural marker in the adult affected KE members2,8,11. Structural reductions15,16 and functional17 anomalies in that region have also been reported in children with language impairment18, indicating a likely link between caudate anomalies and language difficulties. Such a link would be consistent with caudate connectivity with frontal associative, rather than motor, circuits19,20.
Thalamic reductions were detected only with our volumetric approach. Dorsal thalamic reductions had been observed in both affected and unaffected KE members using VBM11, casting doubt on their relevance to the behavioural phenotype. To our knowledge, the thalamus has not been reported as abnormal in children with speech sound disorders or idiopathic childhood apraxia of speech18, and this is therefore a novel finding. As this reduction was only detected using volumetric analysis and did not survive correction for multiple comparisons, replication will be necessary to establish an association between FOXP2 disruption and thalamic changes.
The largest volumetric reductions detected were located in the globus pallidus. Structural anomalies in that region have not been detected using VBM in the affected KE adults or in A-II, but it should be noted that segmentation of this region is problematic due to its low contrast on T1-weighted images21. Inconsistent pallidum results have been reported in speech and language impaired individuals. Pallidum enlargement has even been reported in young adults with developmental language impairment15 and increased fMRI activity has been reported in one study of children with speech sound error (not apraxia of speech)22. Conversely, reduced fMRI activity in the globus pallidus was reported in the affected KE members during a covert language task9. The globus pallidus (internal segment) is a major output structure of the striatum, via the thalamus to the cortex. In adulthood, the globus pallidus and thalamus are closely linked during speech tasks via subcortico-cortical networks involving the supplementary motor area, insula, premotor cortex, and inferior frontal gyrus23. It is therefore plausible that globus pallidus anomaly in childhood would be associated with functional and structural anomalies in these target cortical regions of the speech network later in life.
Altogether, our structural MRI findings point to early abnormal development of the basal ganglia in individuals with FOXP2 dysfunction, consistent with a subset of the proposed original anatomical model of FOXP2 based on expression and neuroimaging studies3. The early role of the basal ganglia in speech and language function is also supported both by recent language models23,24, and by the procedural learning hypothesis for language disorders25,26.
Hippocampal reductions
Bilateral reductions of the hippocampus and surrounded cortices were unexpected and must be interpreted cautiously as only the left peak survived correction for multiple comparisons. No evidence of expression of FOXP2/foxp2 in the mature or developing hippocampus has been previously reported in rodent or human embryo studies27,28 or in the developing monkey brain29. Paradoxically, enlargement of the hippocampus has been reported in young adults with language impairment15. The early role of the hippocampus in language development is unclear, as hippocampal function is traditionally linked to episodic memory skills, which were not tested in A-II. In principle, hippocampal reduction could limit the potential for verbal memory and language development. Recent evidence suggests that declarative memory is a significant predictor of grammar comprehension in children with language impairments, suggesting a compensatory role for this system30. In addition, a recent meta-analysis suggested deficits in verbal declarative memory in individuals with specific language impairment31. The hippocampal reductions reported here could therefore relate to the language phenotype in A-II. They could also however be coincidental, or be an indirect result of gene-environment interactions. The interaction between declarative and procedural memory circuits in developmental communication disorders warrants further investigation, and our findings point to a potential disruption of both in this boy with FOXP2 deletion. However, it should be noted that this view of completely separate memory systems has been recently challenged32 by studies of patients with Parkinson’s disease33,34 and of children with language impairment30.
fMRI findings
Despite performing the task aloud, A-II showed little fMRI activation during nonsense word repetition, and mirrors the findings in the KE family members10. It is intriguing that the peak coordinates of de-activation (negative response) in pars opercularis (−58, 10, 10) were so close to the peak coordinates of underactivation reported in the affected members of the KE family9 during verb generation (−60 12 12). Altogether, we have preliminary evidence that a further marker of FOXP2-related dysfunction could be malfunction in the inferior frontal gyrus, pars opercularis, a region functionally connected to the basal ganglia circuit via the globus pallidus23.
Cross-sectional neuroimaging studies do not allow us to distinguish the brain changes directly caused by FOXP2 mutation and those indirectly affected following interactions with the environment, surrounding genes, and functional reorganization of interacting neural circuits. Based on expression studies and gene function studies3, our current interpretation is that caudate, thalamic and pallidum changes are a direct result of FOXP2 anomaly. Either their structural development, their functional development (via dopaminergic systems), or both, are disrupted. Hippocampus reductions could, by contrast, be the result of behavioural compensation or neural reorganization of circuits, as a result of altered interaction with the environment.
In conclusion, our findings suggest that large subcortical reductions are strong markers of FOXP2 disruption in childhood. A combination of bilateral reduction in the caudate nucleus, globus pallidus, and hippocampus may therefore also be a promising biomarker of severe and long lasting speech and language impairments arising from of other genetic causes. These regions could therefore also serve as targets for novel therapeutic strategies.
Methods
Participants
Case A-II
A-II’s early history and current neuropsychological profile are detailed elsewhere7. In brief, he has a diagnosis of oral motor apraxia, severe childhood apraxia of speech, and dysarthria. Concomitant impairments in receptive and expressive language as well as literacy (<5th centile on all tests) are present. Speech is highly unintelligible due to reduced consonant inventory, imprecise articulation, delayed phonological processes, and atypical error patterns. Both speech and non-speech oromotor functions are impaired (<5th percentile). In contrast, nonverbal intelligence as measured using Performance IQ lies within the average range. A-II was aged 8 years 11 months at the time of scanning.
Our team recently diagnosed a submucous cleft during our research evaluations, despite A-II being seen by community speech pathologists as well as an ear nose and throat surgeon since a young age. He recently had surgery to correct this anomaly, with resultant improvement in speech resonance.
Control groups
A-II’s MRI data were compared to those of 24 typically developing term-born males aged 8 to 11 years recruited via a community prospective longitudinal study35 in metropolitan Melbourne, Australia. These control participants had been followed up between 8 months and 7 years and had never had a diagnosis of hearing deficit, speech or language impairment or delay, neurological symptoms, genetic disorder, or pervasive developmental disorder. Two additional 8-year-old males were recruited via advertisement to widen the age range, using the same exclusion criteria. All 26 control participants were native monolingual English speakers.
Some datasets were either missing or unusable due to excessive motion artefact, and therefore the final number of control participants differs slightly between VBM (N = 26, mean age = 10 years 3 months, SD = 8.3 months, no data excluded) and fMRI (N = 25, mean age = 10 years 2 months) analyses. Age ranged from 8 years 3 months to 11 years 2 months in both cases. As tractography was performed in native space, only a subgroup of males closest in age to A-II was investigated (N = 14 after one dataset was excluded due to excessive motion artefact; mean age 9 years 9 months, SD = 5.5 months, range 8 years 3 months to 10 years 5 months).
MRI acquisition
All MRI data were acquired on a 3T Siemens Skyra scanner (Erlangen, Germany).
Structural MRI
3D T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) images were acquired with 0.9 mm isotropic resolution (TR: 1900 ms; TE: 2.49 ms; flip angle: 9°; matrix size: 256 × 256; FoV: 240 mm).
Diffusion MRI
Diffusion data were acquired with 64 diffusion directions at b = 3000 s/mm2 and 1 b = 0 s/mm2, voxel size = 2.5 mm isotropic, and TR/TE = 6800/110 ms.
Functional MRI
All functional images were acquired aligned along the AC-PC plane using an echo planar imaging (EPI) sequence with whole-brain coverage (TR: 3000 ms; TE: 30 ms; flip angle: 90°; 44 interleaved slices; matrix size: 72 × 72; 3 mm isotropic voxels; FoV: 216 mm).
FMRI nonword repetition task
We used a block design paradigm alternating five blocks of Baseline and five blocks of Task (12 s duration each). The order of presentation was counterbalanced across the group, with 12 participants starting with Baseline, and 14 (including A-II) starting with Task. Participants were instructed to look at a cross presented on a screen throughout each functional MRI scan. During the Task period (“nonword repetition”), participants were required to repeat 35 nonsense words aloud (seven per block) presented via inner ear buds. Participants were given 3 seconds (ISI–5 sec) to provide an overt response to the nonword stimuli. Nonwords were two to five syllables long, with 28 from the Children’s Nonword repetition Test36 and seven from the Nonword Memory test (with permission from the author https://www.york.ac.uk/res/wml/test%20of%20WM.html). During the Baseline period, participants were asked to listen to frequency and duration matched bursts of pink noise (“Pink noise”) generated using the Praat software (www.fon.hum.uva.nl/praat/). Participants practiced the nonword repetition task and completed a mock scan prior to the actual MRI scan. Overt responses were recorded using Audacity software (audacity.sourceforge.net/) via a custom made noise cancelling microphone. This procedure ensured participants were completing the task and allowed for offline scoring of responses.
MRI analysis
VBM
We used VBM37 to identify grey matter differences at the whole-brain level (as used in previous KE family studies8). The results informed the choice of an additional region of interest (the hippocampus) for regional volumetric analyses. T1-weighted datasets were analysed within SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Firstly, data were segmented into grey matter, white matter and cerebrospinal fluid using DARTEL and a custom template generated from all participants. Segmented data were modulated then smoothed using a large smoothing kernel (12 mm FWHM), as recommended for unbalanced designs38 and in particular for single subject analyses. Focal regions of increased and decreased grey matter in A-II were identified using analysis of covariance (ANCOVA) with age and total grey matter volumes as covariates. Equality of variance was assumed, as recommended for single case designs39.
Differences significant at p = 0.05 corrected for family-wise error were reported. Voxels significant at p < 0.001, uncorrected for multiple comparisons were also reported where we had a-priori hypotheses, that is, in the basal ganglia and inferior frontal gyrus11. Small volume correction was used in the caudate nucleus and putamen where we had an a priori hypothesis, using the images generated from volumetric analyses (see below) as masks.
Of note, given our single case design and large smoothing kernel (12 mm, see Methods) for VBM, our methods had reduced sensitivity to detect differences at smaller spatial scales than 12 mm, as is likely to be the case in cortical and cerebellar regions.
Volume extraction in regions of interest
Whole-brain grey and white matter volumes were extracted using SPM12. Volumes from the basal ganglia (a-priori hypothesized) and hippocampus (based on VBM findings) were extracted using FSL FIRST40 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST) which uses automatic subcortical segmentation. Volumes were subsequently adjusted for total grey matter. Segmentation errors were checked in all cases, and manually corrected using published protocols41,42.
Since VBM and direct volumetric analyses are subject to different methodological sensitivities, they may not necessarily result in identical findings. For example, while VBM does reflect absolute volume changes when using modulated data as in the present case, the segmentation of the grey matter structures of interest are achieved by quite different processes. In addition, the fact that VBM is a voxel based method (i.e. statistical tests are performed on a voxel by voxel basis), the requirement for multiple comparison correction is very different in the two approaches, and this is compounded by the above-mentioned need to use a large smoothing kernel for single subject VBM. Nevertheless, there is evidence that the two methods produce similar findings, particularly in subcortical structures in healthy individuals43, and they are therefore regarded as providing complementary information.
Diffusion Weighted Imaging (DWI) tractography
Given the disadvantages of the diffusion tensor model and of deterministic tractography methods for reconstructing fibre tracts44, here we used DWI probabilistic tracking based on constrained spherical deconvolution (CSD using MRtrix45 version 0.2 software). This method has been shown to be particularly advantageous in regions of crossing fibers46. We also used MRtrix to calculate the diffusion tensor and to derive the fractional anisotropy (FA) in all voxels. FA analysis was performed in voxels within tract regions of interest identified from fibre tracking. We examined the FA characteristics of essential speech and language-related pathways, namely (i) the corticobulbar tract (originating in the ventral part of the motor cortex, corresponding to the lips/larynx/tongue representations) and (ii) the direct and dorsal segments of the arcuate fasciculus. The left and right cingulum bundles and corticospinal tracts (originating from the hand region) were used as control tracks, as they were not hypothesized to be related to the phenotype. We used tractography methods previously validated in children with other neurological conditions47,48 (see Supplementary Methods).
fMRI
Individual data were realigned, coregistered, normalized to the MNI template, and smoothed using an 8 mm full width at half maximum kernel within SPM8 using the default parameters. First level analysis involved the comparison of Task (Nonword repetition) vs. Baseline contrasts using motion parameters generated from the realignment process as regressors. Second level analysis involved the comparison of Case-A-II vs. control group in an ANCOVA, with age as covariate of no interest. Equality of variance was assumed.
Given previous report of altered activation in the affected members of the KE family in Broca’s area but not in the superior temporal gyrus9, mean signal change and time series data were extracted from peak voxel within these two regions of interest using the “plot” function within SPM8.
Statistical analysis for volumetric and tractography-derived data
All numerical data were transformed into z-scores calculated using the mean and standard deviations of the control group as recommended for case-control designs. We reported the p value based on a one-tailed one-sample t-test, and provided confidence interval of the effect size and percentiles scores49 calculated using a customized program (www.abdn.ac.uk/~psy086/dept/Single_Case_Effect_Sizes.htm). This calculation assumes non-central t distribution of the control data rather than normal distribution, and therefore takes sample size into account. We estimated that normal distribution was not appropriate in our study as our control sample sizes were moderate, and therefore the risk of Type I error was inflated.
Statistical analysis for whole-brain MRI comparisons
VBM analyses comparing a single participant to a control group can result in false positives. To overcome this, we employed a similar method to that described in Muhlau et al.39 whereby each individual dataset was compared to that of the rest of the control group. For the fMRI analysis, each control’s activation map was examined individually.
Ethics
The study was approved by the Royal Children’s Hospital Human Research Ethics Committee (HREC 27053 and #31225) and carried out in accordance with the provisions of the World Medical Association Declaration of Helsinki. All parents/guardians gave informed consent. Families were reimbursed for travel expenses.
Additional Information
How to cite this article: Liegeois, F. J. et al. Early neuroimaging markers of FOXP2 intragenic deletion. Sci. Rep. 6, 35192; doi: 10.1038/srep35192 (2016).
Supplementary Material
Acknowledgments
We thank A-II and his family for their time. This work was supported by Victorian State Government Operational Infrastructure Support and the Australian Research Council Discovery Project grant (DP120100285) awarded to A.T.M., F.L., I.S., A.C. and M.B. The work is also supported by IRIISS funding to the Walter and Eliza Hall Institute of Medical Research (M.B. and S.T.). The NHMRC of Australia provided further support to A.M. (Career Development Fellowship 607315 and Practitioner Fellowship 1105008) and M.B. (Senior Research Fellowship 1002098 and Program Grant 1054618 to M.B.). F.L.’s research is supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London (London, UK).
Footnotes
Author Contributions F.J.L. and A.T.M. designed the experiment. F.J.L. analysed the tractography, volumetric and fMRI data. A.B. performed VBM analysis and extracted volumetric data under F.J.L.’s supervision. S.J.T. and A.T.M. collected phenotypic data and performed analysis. M.B. and M.H. performed the genetic analysis. F.J.L. and A.T.M. wrote the first draft of the manuscript. All co-authors provided significant input in revising the manuscript.
References
- Lai C. S., Fisher S. E., Hurst J. A., Vargha-Khadem F. & Monaco A. P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523, doi: 10.1038/35097076 (2001). [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F. et al. Neural basis of an inherited speech and language disorder. Proceedings of the National Academy of Sciences of the United States of America 95, 12695–12700 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F., Gadian D. G., Copp A. & Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nature reviews. Neuroscience 6, 131–138, doi: 10.1038/nrn1605 (2005). [DOI] [PubMed] [Google Scholar]
- Fisher S. E. & Scharff C. FOXP2 as a molecular window into speech and language. Trends in genetics: TIG 25, 166–177, doi: 10.1016/j.tig.2009.03.002 (2009). [DOI] [PubMed] [Google Scholar]
- Enard W. FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Current opinion in neurobiology 21, 415–424, doi: 10.1016/j.conb.2011.04.008 (2011). [DOI] [PubMed] [Google Scholar]
- Fisher S. E. & Ridley M. Evolution. Culture, genes, and the human revolution. Science 340, 929–930, doi: 10.1126/science.1236171 (2013). [DOI] [PubMed] [Google Scholar]
- Turner S. J. et al. Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. American journal of medical genetics. Part A 161, 2321–2326, doi: 10.1002/ajmg.a.36055 (2013). [DOI] [PubMed] [Google Scholar]
- Watkins K. E. et al. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain 125, 465–478 (2002). [DOI] [PubMed] [Google Scholar]
- Liegeois F. et al. Language fMRI abnormalities associated with FOXP2 gene mutation. Nature neuroscience 6, 1230–1237, doi: 10.1038/nn1138 (2003). [DOI] [PubMed] [Google Scholar]
- Liegeois F., Morgan A. T., Connelly A. & Vargha-Khadem F. Endophenotypes of FOXP2: dysfunction within the human articulatory network. European journal of paediatric neurology: EJPN: official journal of the European Paediatric Neurology Society 15, 283–288, doi: 10.1016/j.ejpn.2011.04.006 (2011). [DOI] [PubMed] [Google Scholar]
- Belton E., Salmond C. H., Watkins K. E., Vargha-Khadem F. & Gadian D. G. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Human brain mapping 18, 194–200, doi: 10.1002/hbm.10093 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins K. E., Dronkers N. F. & Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain 125, 452–464 (2002). [DOI] [PubMed] [Google Scholar]
- Hickok G. & Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92, 67–99, doi: 10.1016/j.cognition.2003.10.011 (2004). [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Perani D. & Friederici A. D. Dorsal and ventral pathways in language development. Brain and language 127, 289–295, doi: 10.1016/j.bandl.2013.03.001 (2013). [DOI] [PubMed] [Google Scholar]
- Lee J. C., Nopoulos P. C. & Bruce Tomblin J. Abnormal subcortical components of the corticostriatal system in young adults with DLI: a combined structural MRI and DTI study. Neuropsychologia 51, 2154–2161, doi: 10.1016/j.neuropsychologia.2013.07.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock N. A., Bishop D. V., Hardiman M. J., Barry J. G. & Watkins K. E. Co-localisation of abnormal brain structure and function in specific language impairment. Brain and language 120, 310–320, doi: 10.1016/j.bandl.2011.10.006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guibert C. et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia). Brain 134, 3044–3058, doi: 10.1093/brain/awr141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F., Mayes A. & Morgan A. Neural Correlates of Developmental Speech and Language Disorders: Evidence from Neuroimaging. Current developmental disorders reports 1, 215–227, doi: 10.1007/s40474-014-0019-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulos G. P., Tremblay P. & Small S. L. The neostriatum and response selection in overt sentence production: an fMRI study. NeuroImage 82, 53–60, doi: 10.1016/j.neuroimage.2013.05.064 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B. et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. The Journal of neuroscience 28, 7143–7152, doi: 10.1523/jneurosci.1486-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A. C. et al. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human brain mapping 34, 2313–2329, doi: 10.1002/hbm.22068 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. L. et al. Functional brain activation differences in school-age children with speech sound errors: speech and print processing. Journal of speech, language, and hearing research: JSLHR 55, 1068–1082, doi: 10.1044/1092-4388(2011/11-0056) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes J. L. et al. Connectivity of the subthalamic nucleus and globus pallidus pars interna to regions within the speech network: a meta-analytic connectivity study. Human brain mapping 35, 3499–3516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann F., Krugel L. K. & Ehlen F. Functional roles of the thalamus for language capacities. Frontiers in systems neuroscience 7, doi: 10.3389/fnsys.2013.00032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J. A., Conti-Ramsden G., Morgan A. T. & Ullman M. T. Procedural learning deficits in specific language impairment (SLI): A meta-analysis of serial reaction time task performance. Cortex 51, 1–10, doi: 10.1016/j.cortex.2013.10.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M. T. The declarative/procedural model of lexicon and grammar. Journal of psycholinguistic research 30, 37–69 (2001). [DOI] [PubMed] [Google Scholar]
- Lai C. S., Gerrelli D., Monaco A. P., Fisher S. E. & Copp A. J. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain 126, 2455–2462, doi: 10.1093/brain/awg247 (2003). [DOI] [PubMed] [Google Scholar]
- Takahashi K., Liu F. C., Hirokawa K. & Takahashi H. Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. Journal of neuroscience research 73, 61–72, doi: 10.1002/jnr.10638 (2003). [DOI] [PubMed] [Google Scholar]
- Takahashi K. et al. Expression of FOXP2 in the developing monkey forebrain: comparison with the expression of the genes FOXP1, PBX3, and MEIS2. The Journal of comparative neurology 509, 180–189, doi: 10.1002/cne.21740 (2008). [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G., Ullman M. T. & Lum J. A. G. The relation between receptive grammar and procedural, declarative, and working memory in specific language impairment. Frontiers in psychology 6, doi: 10.3389/fpsyg.2015.01090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J. A. G. & Conti-Ramsden G. Long-term memory: A review and meta-analysis of studies of declarative and procedural memory in specific language impairment. Topics in language disorders 33, 282–297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature reviews. Neuroscience 11, 523–532, doi: 10.1038/nrn2850 (2010). [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Tozzi A. & Ghiglieri V. Interaction between basal ganglia and limbic circuits in learning and memory processes. Parkinsonism & related disorders 22 Suppl 1, S65–S68, doi: 10.1016/j.parkreldis.2015.09.017 (2016). [DOI] [PubMed] [Google Scholar]
- Foerde K. & Shohamy D. The role of the basal ganglia in learning and memory: insight from Parkinson’s disease. Neurobiology of learning and memory 96, 624–636, doi: 10.1016/j.nlm.2011.08.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S. et al. Predicting language at 2 years of age: a prospective community study. Pediatrics 120, e1441–e1449, doi: 10.1542/peds.2007-0045 (2007). [DOI] [PubMed] [Google Scholar]
- Gathercole S. E., Willis C. S., Baddeley A. D. & Emslie H. The Children’s Test of Nonword Repetition: a test of phonological working memory. Memory 2, 103–127, doi: 10.1080/09658219408258940 (1994). [DOI] [PubMed] [Google Scholar]
- Ashburner J. & Friston K. J. Voxel-based morphometry–the methods. NeuroImage 11, 805–821, doi: 10.1006/nimg.2000.0582 (2000). [DOI] [PubMed] [Google Scholar]
- Salmond C. H. et al. Distributional assumptions in voxel-based morphometry. NeuroImage 17, 1027–1030 (2002). [PubMed] [Google Scholar]
- Muhlau M. et al. Voxel-based morphometry in individual patients: a pilot study in early Huntington disease. AJNR. American journal of neuroradiology 30, 539–543, doi: 10.3174/ajnr.A1390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S. M., Kennedy D. N. & Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56, 907–922, doi: 10.1016/j.neuroimage.2011.02.046 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardi M. et al. Delphi definition of the EADC-ADNI Harmonized Protocol for hippocampal segmentation on magnetic resonance. Alzheimer’s & dementia 11, 126–138, doi: 10.1016/j.jalz.2014.02.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi J. C. et al. Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry research 163, 279–288, doi: 10.1016/j.pscychresns.2007.07.005 (2008). [DOI] [PubMed] [Google Scholar]
- Focke N. K., Trost S., Paulus W., Falkai P. & Gruber O. Do manual and voxel-based morphometry measure the same? A proof of concept study. Frontiers in psychiatry 5, 39, doi: 10.3389/fpsyt.2014.00039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J. D., Mori S. & Leemans A. Diffusion tensor imaging and beyond. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine 65, 1532–1556, doi: 10.1002/mrm.22924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J. D., Calamante F. & Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology 22 (2012). [Google Scholar]
- Tournier J. D. et al. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. NeuroImage 42, 617–625, doi: 10.1016/j.neuroimage.2008.05.002 (2008). [DOI] [PubMed] [Google Scholar]
- Liegeois F., Tournier J. D., Pigdon L., Connelly A. & Morgan A. T. Corticobulbar tract changes as predictors of dysarthria in childhood brain injury. Neurology 80, 926–932, doi: 10.1212/WNL.0b013e3182840c6d (2013). [DOI] [PubMed] [Google Scholar]
- Liegeois F. J. et al. Pediatric traumatic brain injury: language outcomes and their relationship to the arcuate fasciculus. Brain and language 127, 388–398, doi: 10.1016/j.bandl.2013.05.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. R., Garthwaite P. H. & Porter S. Point and interval estimates of effect sizes for the case-controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards. Cognitive neuropsychology 27, 245–260, doi: 10.1080/02643294.2010.513967 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.