Abstract
Background:
Loss of teeth affects the individual's health. Many factors determine the need to wear a removable dental prosthesis. Due to nature of design, age and lack of awareness, prosthesis often are neglected to maintain in an aseptic condition leading to microbial contamination. This provided an impetus for the present study with the aim of determining the microbial contamination of removable dental prosthesis.
Methodology:
Total, 45 patients wearing removable dental prosthesis were randomly selected. Patients were divided into three groups as per duration of usage since 1 month, 6 month and ≥1 year. Sterile cotton swab moistened with phosphate buffer saline (PBS) was used to collect swab from the fitting areas of prosthesis. Swab samples was inserted immediately into the sterile tube containing 1 ml of PBS solution, 10 μl PBS is inoculated on the blood agar and MacConkey agar plates using spread plate technique. Samples were cultured and incubated at 37°C for 48 h. Calibrated microbiologist isolated, identified and counted microorganisms using colony counter. Depending on the nature of data, statistical analysis was done applying Kruskal–Wallis test, Mann–Whitney U-test and Chi-square test.
Result:
Streptococcus species and Staphylococcus aureus were the common microorganisms isolated in all three groups and was statistically significant at P <0.05. Candida albicans, Diptheroid, Escherichia coli, Micrococcus species were isolated from Group II and Group III.
Conclusion:
There is a linear increase in microbial contamination of removable dental prosthesis as the duration of usage increases and might increase the susceptibility of individuals' to many diseases.
Keywords: Denture, microbial contamination, removable dental prosthesis
INTRODUCTION
India had an approximately 7.7% geriatric population[1] and estimated to increase considerably by the year 2025.[2] Factors associated with old age such as reduced salivary flow rate, impaired quality, and quantity, lowered immunity and impaired host defense may aggravate the process of the degradation of the oral tissues. In this regard, loss of teeth in the elderly is a major concern.
Replacing missing tooth helps individual to chew, maintain muscle tone, restore or improve the patient's ability to speak and pronounce words better, and give the patient self-esteem to overcome the social stigma thus enabling individual to enjoy the quality of life.
Though development of implant prosthesis has increased recently, the demand for partial and full dentures is still very high. For those who cannot afford fixed dentures due to very small amount of teeth left, as well as for financial reasons, a removable dental prosthesis remains the only viable solution.[3,4]
Complete denture and removable partial denture are the most common dental prosthesis to replace the missing teeth among many individuals. The removable dental prosthesis is dental restorations that can be removed by the patient when not in use. It has been reported that <50% and 13% of individuals are wearing complete dentures and removable partial dentures.[1] Care of dentures and the mucosal tissues of the edentulous mouth are very important for overall health, especially among older individuals.
The introduction of the removable prosthesis may change the oral ecology either quantitatively or qualitatively, such as increasing the total amount of oral microorganisms, or increasing a certain part of the oral microflora.[5]
It has been observed that the majority of denture wearers do not pay necessary attention to the cleanliness. This may be due to decreasing manual abilities due to an advanced age, the nature of design, lack of awareness, improper storage, and failure to maintain asepsis of dental prosthesis that leads to the growth of microbial agents and formation of biofilms, which are reservoirs of infection.[6]
Different studies have suggested that oral bacteria may be risk factors for a number of prevalent systemic diseases. Oral bacteria have been implicated in bacterial endocarditis, aspiration pneumonia, gastrointestinal infection and chronic obstructive pulmonary disease, and dental prosthesis offers a reservoir for microorganisms associated with these infections.[7] Lesions of the oral mucosa associated with the wearing of removable dentures may represent acute or chronic reactions to microbial denture plaque, a reaction to constituents of the denture base material, or a mechanical denture injury. They include denture stomatitis, angular cheilitis, traumatic ulcers; denture irritation hyperplasia, flabby ridges, and oral carcinomas.[8] In addition, there may be greater social consequences of mouth malodor due to the unclean oral prosthesis.
Many studies have been conducted to understand the changes in oral micro flora before and after insertion of the dental prosthesis.[9,10,11] However, sparse literature is available to assess the microbial contamination of removable prosthesis as per the duration of usage. Thus, the aim of this study was to determine microbial contamination of removable dental prosthesis with the objective of assessing microbial contamination at different interval of usage.
MATERIALS AND METHODS
Study design and setting
The present study is a randomized, three group parallel study among 45 patients aged between 42 and 80 years wearing removable dental prosthesis that fulfilled the following selection criteria. Institutional Ethical Review Board approval was obtained before the start of the study.
Selection criteria
Inclusion criteria
Patient using removable partial denture or complete denture regularly.
Exclusion criteria
Patients with systemic disease
Subjects who were prescribed antibiotics or other medications from last 3 to 6 months.
Method of collection of data
Sample size
A sample of 45 patients wearing removable dental prosthesis was included in the study, 14 were females, and 31 were males. Thirty-four complete denture wearers and 14 removable partial denture wearers were randomly selected.
Study procedure
Complete clinical history was taken, and intraoral examination was performed. Individuals with medications and eliciting subjective symptoms of any systemic/oral disease were excluded.
Randomization
Patients having similar criteria with respect to the period of usage of the removable dental prosthesis were divided into three different groups with 15 subjects in each group.
Group I: Patient using removable dental prosthesis since 1 month
Group II: Patient using removable dental prosthesis since 6 month
Group III: Patient using removable dental prosthesis more than 1 year.
Examination procedure
While collecting samples strict aseptic measures were followed, swab method was employed to collect the samples by the principal investigator (VN). Sterile cotton-tip swab moisten with phosphate buffer saline (PBS) was rubbed on the fitting surface of dental prosthesis to obtain the samples, swab samples was inserted immediately in the sterile tube containing 1 ml of PBS solution[12] 0.85% maintained at pH - 7–7.2, stored at room temperature as the transport media and sent to microbiology laboratory within 2 h for microbiological analysis at Department of Microbiology at ACPM Medical College and Hospital, Dhule District, Maharashtra, India.
The collected swab sample was manually shaken vigorously to facilitate the equal dispersion of microorganisms; then this PBS was inoculated onto the blood agar and MacConkey agar plates using spread plate technique. Inoculation in this technique is done using a bent glass rod (spreader), 0.1 ml of PBS is placed in the center of the plate using a sterile pipette. The glass rod is sterilized by first dipping it into a 70% alcohol solution and then passing it quickly through the Bunsen burner flame. When all the alcohol has burned off, and the rod has air-cooled, the spreader is placed in contact with the inoculum on the surface of the plate and positioned to allow the inoculum to run evenly along the length of the spreader. Even pressure is applied to the spreader, and the plate is spun, by hand.[13]
The culture plates were incubated for 48 h at 37°C and observed for microbial growth. Smear from the colonies was prepared and stained by Gram's stain.
Identification of microorganisms was done by biochemical tests such as coagulase, catalase, oxidase, sugar fermentation with acid and gas production (triple sugar iron), methyl red test, Voges–Proskaure test, test for indole production, H2S production, citrate utilization, and urease test, germ tube test were performed from isolated Candida colonies to confirm C. albicans.
Microorganisms' were identified and counted by calibrated microbiologist using colony counter. The data were tabulated as CFU/µl.
Statistical analysis
Categorical variables were reported using number and percentages [Table 1]. Differences in continuous and categorical variables between two groups of patients were assessed by Mann–Whitney U-test and Chi-square test, respectively [Table 2]. Differences in continuous variables between three groups were assessed using Kruskal–Wallis test. Continuous variables were reported using median and inter-quartile range [Table 3]. Statistical Package of Social Sciences version 17.0. software (SPSS, Chicago). All the analyses were considered statistically significant at 5% level (P < 0.05).
Table 1.
Percentage distribution of different microorganisms’ isolated from number of individuals at regular interval usage of removable dental prosthesis
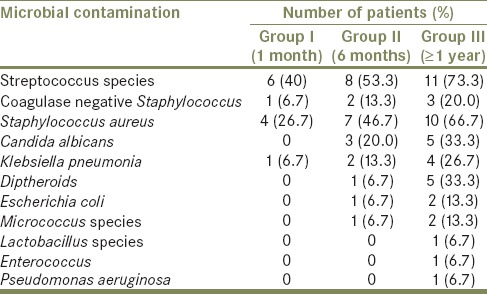
Table 2.
Intergroup comparison between different microorganisms according to its presence
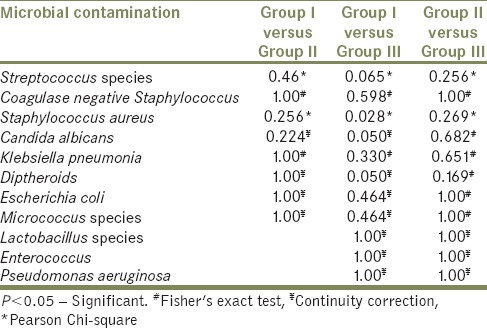
Table 3.
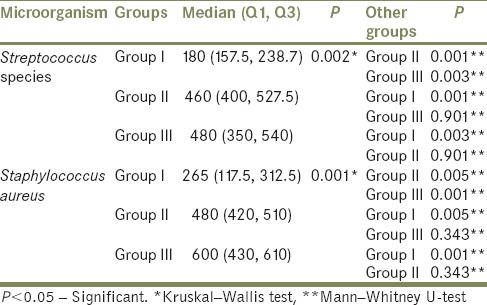
Intergroup comparisons between Streptococcus species and Staphylococcus aureus according to its colony count
RESULTS
The present study revealed there was an increase in the microbial contamination as the duration of usage increased. A higher density of all the other isolated organisms was seen among individuals using dental prosthesis more than 1 year as compared to other groups [Table 1]. The presence of microorganisms also increased as the duration of usage increased which was not statistically significant (P > 0.05) [Table 2].
There was statistically significant difference only between Group I and Group III with respect to Staphylococcus aureus (P = 0.028) [Table 2]. Streptococcus species and S. aureus showed the highest positive culture among the isolated microorganisms in all three groups, and the colony counts were statistically significant between all three groups [Table 3 and Graph 1].
Graph 1.
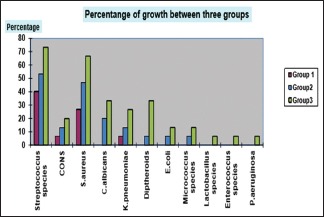
Percentage distribution of microbial growth between three different groups
C. albicans, Escherichia coli, Diptheroids, Micrococcus was absent in 1 month of usage but found increasing after 6 month and 1 year of usage [Table 1]. Klebsiella pneumonia and coagulase negative Staphylococcus showed presence in all groups as the duration of usage increased but was not statistically significant (P > 0.05) [Table 2]. Lactobacillus species, Pseudomonas aeruginosa and Enterococcus was mostly recovered from individuals using dental prosthesis ≥1 year [Table 1].
DISCUSSION
Affordability, accessibility, acceptability, and increased awareness have contributed toward a high percentage of individuals opting to replace the missing natural tooth by various methods. Due to the nature of design and to give rest to the supporting hard and soft tissue it is advised to remove the removable dental prosthesis during the night and when not in use. The liner materials such as cold cured acrylic, polyvinyl siloxanes, or acrylics containing plasticizers are used to construct for the removable dental prosthesis. These are much more porous than heat cured acrylics leading to contamination and if overlooked can cause many ill effects.[14] Thus, investigating their microbial contamination is important and justifiable.
The aim of the current study was to assess and identify the microbial contamination of removable dental prosthesis and compare the presence of microorganisms that are able to survive on the dental prosthesis at different interval of usage. The results of this study revealed that microbial contamination does occur in the removable dental prosthesis as the duration of usage increased. The cultivable flora of the removable dental prosthesis showed a complex bacterial community. Microbial contamination consisted of normal oral flora along with both pathogenic and opportunistic microorganisms when examined, including a wide range of Gram-negative bacteria, Gram-positive bacteria, and fungi. This confirms the possibility of microbial contamination and removable dental prosthesis act as reservoirs that harbor a mixed species of bacterial bio film.[15]
Several studies have been conducted to determine the change in the oral micro flora of individuals wearing a removable dental prosthesis.[8,9,10,11,12,15,16] However, very few literature are available on microbial contamination of removable dental prosthesis at different interval of usage. In the present study, Streptococcus species and S. aureus were the common and highest microorganisms isolated in all three groups. The proportions of S. aureus and Streptococcus species contamination also increased as the duration of usage increased, perhaps age and denture wear might have contributed as seen in earlier studies.[17] This observation is similar with previous studies on the salivary flora of edentulous and dentate person[18,19] and the denture plaque of edentulous individuals.[20]
The presence of species often associated with caries such as Lactobacillus species was found in Group III among patients wearing a removable partial denture. Lactobacillus represents 0.1% of the total salivary flora, a critical concentration of 105 CFU/ml of saliva is necessary for the detection of lactobacilli on the surface of enamel.[21] This might be the reason for isolation of Lactobacillus species in this study.
In the present study C. albicans also showed presence as the duration of usage increased. C. albicans were isolated from dental prosthesis wearing more than 6 months in comparison with 1 month of usage. Denture stomatitis is known as the most common oral disorder and is reported in about 67% of complete denture wearers resulting from the attachment and colonization of Candida on the hard surfaces of the denture with poor maintenance.[10,15] Other studies have shown Candida incorporation into biofilms covering different biomaterials such as dentures: These biofilms may be an increased risk factor for invasive candidiasis when the host immune system is compromised.[22]
Interestingly, co-aggregation studies have shown that C. albicans colonization can be aided by primary colonizers such as Streptococcus species.[23] The denture especially denture base acrylic resin is easily colonized by oral endogenous bacteria and Candida species and eventually by extra-oral species such as Staphylococcus species. This microbial reservoir can be responsible for denture-related stomatitis and aspiration pneumonia, a life-threatening infection; especially in geriatric patients.[6] Improper maintenance and long duration usage of removable dental prosthesis might also be responsible for C. albicans isolation in our study.
In our study, we isolated Enterococcus only from the third group in which duration of usage is ≥1 year. Studies have shown their isolation increased markedly as individuals became more debilitated and hospitalized,[24] whereas in our study subjects were healthy, and lived relatively independent lives. This calls the need to understand the exact reason in future studies.
Few potential respiratory pathogens were also isolated from dental prosthesis including, K. pneumonia and Pseudomonas aeuroginosa.[25] The patient's hands could be considered as a possible vector in isolation of E. coli that is known to cause diarrhea, urinary tract infections, septicemia.[26] This might be due to unhygienic air environment at home and use of contaminated water used to store dental prosthesis. Diptheroids were isolated from the second and third group after 6 months of usage. Probably dental prosthesis stored outside the oral cavity acts as a nidus for air contamination with respect to Diptheroids that calls for the attention of aseptic storage of prosthesis.[27]
Anaerobic organisms require the anaerobic environment to grow and survive, however due to the design of the prosthesis it is exposed to an aerobic environment quite frequently hence we overlooked the possible microbial contamination of anaerobic microorganisms and didn't test for the same which is an inherent limitation of this study.[28]
Thus, in conjunction with other factors, such as denture's hygiene, surface roughness, design and type of metal used in the prosthesis and age,[17] reduction in salivary flow rate could be considered as a significant reason in the change in the oral flora contributing for microbial contamination of prosthesis.[29]
A considerable number of patients use a dental prosthesis for a whole day,[30] prolonged usage of the same dental prosthesis for several years as well as the low frequency of cleaning,[31,32] furthers the occurrence of denture stomatitis. The greatest percentage of patients cleaned their dentures using a toothbrush with toothpaste,[31] which is not recommended due to the chances of micro-abrasions.[3] Increased surface roughness and complicated topography shows higher affinity to microbes than smoother surfaces and subsequently increased the difficulty in complete removal of the biofilm by mechanical cleansing.[33,34,35,36] Furthermore, the crevices created by the roughness generate shelters for the bacteria, so they have time for securing their attachment to the pellicle.
In this study certain individuals, with the presence of oral lesions could have altered the integrity of the host defense and disturbed the stability of the resident oral microflora leading to the increased likelihood of colonization by potentially pathogenic species resulting in the microbial contamination of removable dental prosthesis.
Limitations of the study
Off late, digital colony counter are available to determine the colony forming units that might give an accurate number of colony counts. In the present study, colony counting was done using manual colony counter due to the feasibility. Hence exact microbial load might not have been estimated. However, the study gives an insight of possible count of microbial contamination as the duration of usage increases.
The present study, only healthy volunteers free of oral and systemic diseases were selected. However, the effect of systemic disease on microbial contamination of dental prosthesis remained unknown. Further studies have to be conducted to assess the microbial contamination of dental prosthesis on debilitated individuals to know the pathogenicity of microorganisms and to evaluate the occurrence of any infection in the subjects following the use of a contaminated prosthesis.
Age and gender might play a role in microbial contamination of dental prosthesis. However, age of the individuals and gender were not considered in the present study. The oral hygiene practice of each individuals was not noted which need to be considered in further studies.
CONCLUSION
Thus, the study proved that there is a linear increase in microbial contamination as the duration of usage increased. These changes may persist and could result in plaque formation with considerable pathogenic microorganisms in denture users. This contaminated prosthesis may affect oral health adversely. Thus, it is imperative that factors such as the effect of microbial contamination of dental prosthesis are investigated and evaluated. This is to assure and maintain a healthy oral function and environment. Clinically, one should attempt to monitor such changes. The dentures of even healthy individuals must be considered as possible sources of pathogenic microorganisms. Regular denture maintenance and decontamination should be done to prevent and control microbial contamination of removable dental prosthesis.
Recommendation
During the fabrication of the dentures and removable dental prosthesis, dentists should strive to reduce surface roughness, select a design that will minimize tissue injury and implement a strict oral hygiene regimen. An effective denture hygiene and decontamination is recommended to control denture microbial biofilm to overcome associated oral and systemic diseases. The prosthesis should always be stored and maintained in a hygienic environment. It is recommended that patients should be educated with regards to prosthesis hygiene and regular follow-ups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Karuna Ahire, Faculty of Department of Microbiology, ACPM Medical College, Dhule, Maharashtra, India for providing necessary facilities to conduct this study and Miss. Nivya George, Biostatistician, St. John's Research Institute, Bengaluru, Karnataka, India for helping with statistical analysis. Special thanks to Ambili Kuttan, Microbiologist for helping me in the study.
REFERENCES
- 1.Shah N, Parkash H, Sunderam KR. Edentulousness, denture wear and denture needs of Indian elderly – A community-based study. J Oral Rehabil. 2004;31:467–76. doi: 10.1111/j.1365-2842.2004.01260.x. [DOI] [PubMed] [Google Scholar]
- 2.Ngatia EM, Gathece LW, Macigo FG, Mulli TK, Mutara LN, Wagaiyu EG. Nutritional and oral health status of an elderly population in Nairobi. East Afr Med J. 2008;85:378–85. doi: 10.4314/eamj.v85i8.9655. [DOI] [PubMed] [Google Scholar]
- 3.Kar S, Tripathi A. Prevalence of type of removable dentures in elderly citizens in Northern India. J Contemp Dent. 2015;5:76–9. [Google Scholar]
- 4.Krawczyk J, Bożyk A, Kiworkowa-Rączkowska E, Berger M, Bakalczuk M, Szkutnik J, et al. Hygiene, ways of storage and lifetime of removable dentures. J Pre-Clin Clin Res. 2015;9:54–6. [Google Scholar]
- 5.Ghamrawy EE. Quantitative changes in dental plaque formation related to removable partial dentures. J Oral Rehabil. 1976;3:115–20. doi: 10.1111/j.1365-2842.1976.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 6.Gornitsky M, ParadisI I, Landaverde G, Malo AM, Velly AM. A clinical and microbiological evaluation of denture cleansers for geriatric patients in long-term care institutions. J Can Dent Assoc. 2002;68:39–45. [PubMed] [Google Scholar]
- 7.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budtz-Jørgensen E. Oral mucosal lesions associated with the wearing of removable dentures. J Oral Pathol. 1981;10:65–80. doi: 10.1111/j.1600-0714.1981.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mobeireek A. Qualitative changes in oral flora before and after the insertion of removable prosthesis. Pak Oral Dent J. 2003;23:51–6. [Google Scholar]
- 10.Abdul-Kareem SA. Changes in oral flora of newly edentulous patients, before and after complete dentures insertion. J Bagh Coll Dent. 2012;24:65–9. [Google Scholar]
- 11.Jafari AA, Fallah-Tafti A, Fattahi-Bafghi A, Arzy B. The comparison of predominant oral micro-flora in subjects with and without complete denture referred to Yazd dentistry department. J Community Health Res. 2014;3:195–203. [Google Scholar]
- 12.Williams DW, Chamary N, Lewis MA, Milward PJ, McAndrew R. Microbial contamination of removable prosthodontic appliances from laboratories and impact of clinical storage. Br Dent J. 2011;211:163–6. doi: 10.1038/sj.bdj.2011.675. [DOI] [PubMed] [Google Scholar]
- 13.Noce L, Di Giovanni D, Putnins EE. An evaluation of sampling and laboratory procedures for determination of heterotrophic plate counts in dental unit waterlines. J Can Dent Assoc. 2000;66:262. [PubMed] [Google Scholar]
- 14.Øilo M, Bakken V. Biofilm and dental biomaterials. Materials. 2015;8:2887–900. [Google Scholar]
- 15.Daniluk T, Fiedoruk K, Sciepuk M, Zaremba ML, Rozkiewicz D, Cylwik-Rokicka D, et al. Aerobic bacteria in the oral cavity of patients with removable dentures. Adv Med Sci. 2006;51(Suppl 1):86–90. [PubMed] [Google Scholar]
- 16.Mizugai H, Isogai E, Hirose K, Chiba I. Effect of denture wearing on occurrence of Candida species in the oral cavity. J Appl Res. 2007;7:250–4. [Google Scholar]
- 17.Marsh PD, Percival RS, Challacombe SJ. The influence of denture-wearing and age on the oral microflora. J Dent Res. 1992;71:1374–81. doi: 10.1177/00220345920710070501. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson J, Söderholm G, Almfeldt I. Prevalence of Streptococcus sanguis and Streptococcus mutans in the mouth of persons wearing full-dentures. Arch Oral Biol. 1969;14:243–9. doi: 10.1016/0003-9969(69)90226-x. [DOI] [PubMed] [Google Scholar]
- 19.Gordon DF, Jong BB. Indigenous flora from human saliva. Appl Microbiol. 1968;16:428–9. doi: 10.1128/am.16.2.428-429.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding SD, Wilson M, Dickinson C, Howlett J, Hobkirk J. The cultivable microflora of denture plaque from patients with denture-induced stomatitis. Microb Ecol Health Dis. 1991;4:149–57. [Google Scholar]
- 21.Van Houte J, Green DB. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974;9:624–30. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahariz M, Loeb I, Courtois P. Oral candidiasis and dentures. Rev Stomatol Chir Maxillofac. 2010;111:74–8. doi: 10.1016/j.stomax.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson HF, Lala HC, Shepherd MG. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect Immun. 1990;58:1429–36. doi: 10.1128/iai.58.5.1429-1436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tillotson JR, Finland M. Bacterial colonization and clinical superinfection of the respiratory tract complicating antibiotic treatment of pneumonia. J Infect Dis. 1969;119:597–624. doi: 10.1093/infdis/119.6.597. [DOI] [PubMed] [Google Scholar]
- 25.Sogi SH, Subbareddy VV, Kiran SN. Contamination of toothbrush at different time intervals and effectiveness of various disinfecting solutions in reducing the contamination of toothbrush. J Indian Soc Pedod Prev Dent. 2002;20:81–5. [PubMed] [Google Scholar]
- 26.Karibasappa GN, Nagesh L, Sujatha BK. Assessment of microbial contamination of toothbrush head: An in vitro study. Indian J Dent Res. 2011;22:2–5. doi: 10.4103/0970-9290.79965. [DOI] [PubMed] [Google Scholar]
- 27.Yassin MF, Almouqatea S. Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int J Environ Sci Technol. 2010;7:535–44. [Google Scholar]
- 28.Rolfe RD, Hentges DJ, Campbell BJ, Barrett JT. Factors related to the oxygen tolerance of anaerobic bacteria. Appl Environ Microbiol. 1978;36:306–13. doi: 10.1128/aem.36.2.306-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samaranayake LP, McCourtie J, MacFarlane TW. Factors affecting the in-vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980;25:611–5. doi: 10.1016/0003-9969(80)90076-x. [DOI] [PubMed] [Google Scholar]
- 30.Dikbas I, Koksal T, Calikkocaoglu S. Investigation of the cleanliness of dentures in a university hospital. Int J Prosthodont. 2006;19:294–8. [PubMed] [Google Scholar]
- 31.Peracini A, Andrade IM, Paranhos Hde F, Silva CH, de Souza RF. Behaviors and hygiene habits of complete denture wearers. Braz Dent J. 2010;21:247–52. doi: 10.1590/s0103-64402010000300013. [DOI] [PubMed] [Google Scholar]
- 32.Coelho CM, Sousa YT, Daré AM. Denture-related oral mucosal lesions in a Brazilian school of dentistry. J Oral Rehabil. 2004;31:135–9. doi: 10.1111/j.1365-2842.2004.01115.x. [DOI] [PubMed] [Google Scholar]
- 33.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent Mater. 1997;13:258–69. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 34.Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: A review of the literature. Int J Oral Maxillofac Implants. 2009;24:616–26. [PubMed] [Google Scholar]
- 35.Aykent F, Yondem I, Ozyesil AG, Gunal SK, Avunduk MC, Ozkan S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J Prosthet Dent. 2010;103:221–7. doi: 10.1016/S0022-3913(10)60034-0. [DOI] [PubMed] [Google Scholar]
- 36.Morgan TD, Wilson M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J Appl Microbiol. 2001;91:47–53. doi: 10.1046/j.1365-2672.2001.01338.x. [DOI] [PubMed] [Google Scholar]


