Abstract
Context:
Laryngeal mask airway (LMA) Classic™ has an inflatable cuff while i-gel™ has a noninflatable cuff made of thermoplastic elastomer.
Aims:
To compare ease of insertion, number, and duration of insertion attempts among the two device. Secondary objectives were to evaluate the hemodynamic response and SpO2 during device insertion and during maintenance of general anesthesia.
Settings and Design:
This study was conducted as randomized observational study in a teaching hospital.
Subjects and Methods:
One hundred American Society of Anesthesiologists I and II, patients posted for surgery under general anesthesia were divided in two groups of fifty each. LMA Classic™ and i-gel™. Ease of insertion, duration of insertion, hemodynamic data, and episodes of hypoxia during insertion, 1, 3 and 5 min for 30 min, during removal and 1 min after removal.
Statistical Analysis Used:
Descriptive analyses were expressed as a mean ± standard deviation. Independent t-test used for parametric data, Chi-square test for nonparametric data and hemodynamic data were analyzed using repeated measures ANOVA to find statistical difference within the groups.
Results:
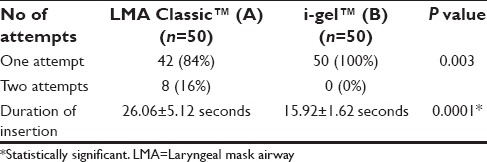
Devices were easy to insert, the mean duration of insertion attempts was 15.92 ± 1.62 s in the i-gel™ group, while it was 26.06 ± 5.12 s in the LMA Classic™ group, was statistically significant (P = 0.0001).
Conclusions:
Successful and shorter duration of insertion, with less hemodynamic response makes i-gel™ a suitable alternative to LMA Classic™ during general anesthesia.
Keywords: Diastolic blood pressure, heart rate, i-gel™ ease of insertion, laryngeal mask airway Classic™, systolic blood pressure
INTRODUCTION
Maintenance of airway is an integral part of general anesthesia. The major responsibility of an anesthesiologist is to provide adequate ventilation to the patient. There are wide variety of supraglottic airway devices available which are used for surgeries requiring general anesthesia, so as to avoid the hemodynamic response associated with endotracheal intubation.[1]
Once the device is inserted, the cuff needs to be inflated to create a seal around the peri-laryngeal tissues. This is likely to cause tissue enema, venous congestion, and nerve injury.[2,3,4] Depending on the material from which the cuff is manufactured, they may absorb anesthetic gases, which can lead to increased mucosal pressure.[5] In addition, it could lead to the potential risk of aspiration persists with the use of laryngeal mask airway (LMA) Classic™.[6]
The other second generation newer airway device i-gel™ is a new supraglottic airway device designed to fit the peri-laryngeal and hypo-pharyngeal structures without the use of an inflatable cuff, made of a thermoplastic elastomer (styrene ethylene butadiene styrene) with a soft durometer (hardness) and gel like, provides a seal in patients with a wide range of anatomical variation. The claimed potential advantages include ease of insertion and use with minimal tissue compression and congestion, airway complications and stability following insertion. A previous anatomical study in cadavers has shown that the i-gel™ is capable of achieving a good peri-laryngeal seal without the requirement for an inflatable cuff.[7] It also has features designed to allow a gastric tube to be passed into the stomach.
As there are very few studies comparing the LMA Classic™ and i-gel™ where the device insertion was done by final year postgraduate students under the supervision of anesthesia consultants in a tertiary hospital, especially in South Indian population, we proposed to assess the ease of insertion and hemodynamic changes of these two devices in this population. The primary aim of the study was to compare ease of insertion, number and duration of insertion attempts among the two devices. Secondary objectives were to evaluate the hemodynamic response and SpO2 during device insertion and during maintenance of general anesthesia.
SUBJECTS AND METHODS
This study was conducted as randomized observational study in a Tertiary Teaching Hospital in South India. After getting ethical committee approval IEC/RC/11/66 and written informed consent, 100 adult patients aged between 18 and 50 years of either sex and American Society of Anesthesiologists (ASA) I and II, with Mallampati Grades I and II admitted in our hospital undergoing elective surgery under general anesthesia (≤30 min) were enrolled in the study. Patients with hypertension, obesity, pregnancy and gastro esophageal reflex disease, history of any cardiovascular and renal disease were excluded from the study.
One hundred patients were randomly allotted into two groups each based on the computer-generated codes, Group A for (LMA Classic™) and Group B for (i-gel™). Preanesthetic assessment was done for all the patients fasting for 8 h before surgery and premedicated with tablet lorazepam 0.025 mg/kg, the night prior to the surgery as well as in the morning of the surgery, 1 h prior to the procedure, an intravenous access was established and slow infusion of crystalloids was commenced. Noninvasive monitors like electrocardiogram, Noninvasive blood pressure (BP), pulse oximetry were instituted and baseline values of heart rate (HR), BP, oxygen saturation SpO2 were recorded. While preoxygenating using 100% oxygen, intravenous injection glycopyrrolate 10 µg/kg, midazolam 0.03 mg/kg, fentanyl 1 µg/kg were administered. The size of the airway device used was decided based on the patient's body weight, according to the manufacturer's recommendation. For the LMA Classic™, size 3 was used when the patient's weight <50 kg, size 4 for patients weighing 50–70 kg and size 5 for patients weighing >70 kg. Whereas for i-gel™, size 3 was used for patients weighing between 30 and 60 kg, and size 4 for patients weighing between 50 and 90 kg. Both devices were lubricated on the tip and the posterior surface as recommended by the manufacturers and the LMA Classic™ was fully deflated prior to insertion, using the LMA Classic™ cuff deflator.
Anesthesia was induced intravenously with 1% propanol (2 mg/kg) (2 ml of preservative free lignocaine 2% was added to 10 ml of propofol). Once an adequate depth of anesthesia was achieved (loss of eye lash reflex), LMA Classic™ or i-gel™ was inserted by a final year postgraduate student under the supervision of experienced anesthetist. We ensured the safety of our patients by training the postgraduate students to insert the devices successful on an airway mannequin before conducting the pilot study. Ease of insertion, number, and duration of insertion attempts were assessed by the insertion attempts (<2 attempts - good, >2 attempts - poor) and duration (time from picking up the device until attaching it to the breathing system in seconds). The LMA Classic™ is introduced by the classic method introduced by Dr. Archie Brain. Following insertion of the LMA Classic™, air was introduced into the cuff for a good seal as recommended by the manufacturers. A successful airway placement was confirmed by bilateral symmetrical chest movement, square waveform on capnography.
Anesthesia was maintained with controlled intermittent positive pressure ventilation using oxygen and nitrous oxide (50–50%), halothane (0.5–1.2%), atracurium 0.5 mg/kg. Serial HR, BP, SpO2 monitoring were done at the time of insertion, 1, 3, and 5 min following insertion and thereafter every 5 min till the end of the procedure and were recorded. Patients were reversed with injection neostigmine 60 µg/kg and injection glycopyrrolate 5 µg/kg. The device was removed when the reflexes were restored and the patient was able to respond to oral commands (eye opening, mouth opening). HR, BP, SpO2 at the time of removal and 1 min after removal was also noted.
Statistical methods
The sample size was calculated based on previous studies where the standard deviation (SD) of 4.9 in Group 1 and SD of 17.7 in Group 2 mean difference = (Group 1 mean) − (Group 2 mean) Mean difference[8] = −9.58 duration of insertion attempts. Confidence interval (two-sided) 95% and power of 95% and alpha set at 0.05. Our sample size was 48 patients in each group. Mean difference = (Group A mean) − (Group B mean) Mean difference[8] = −9.58 duration of insertion attempts. All data were recorded in Microsoft excel chart, and statistical analysis was done by Statistical Package for Social Sciences (SPSS Statistics for Windows, Version 17.0. SPSS Inc., Chicago) software version 17. Descriptive analysis was expressed as a mean ± SD. Independent t-test was used for parametric data, Chi-square test for nonparametric data and hemodynamic data were analyzed using repeated measures ANOVA to find the statistical difference within the groups.
RESULTS
The following observations were made during the course of the study. The demographic profile was comparable among both groups [Table 1]. The ASA physical status and airway assessment data [Tables 2 and 3] between the two groups were comparable, and there is no significant difference between the two groups. i-gel™ was inserted in all the study population at the first attempt itself (100%). While with LMA Classic™ it was 84% and second attempt in 16% of the study population, which was statistically significant with a P = 0.003 [Table 4]. The mean duration of insertion attempts was 15.92 ± 1.62 s in the i-gel™ group, while it was 26.06 ± 5.12 s in the LMA Classic™ group, this was statistically significant P = 0.0001 [Table 4].
Table 1.
Sex distribution of the study groups i-gel™ and LMA Classic™
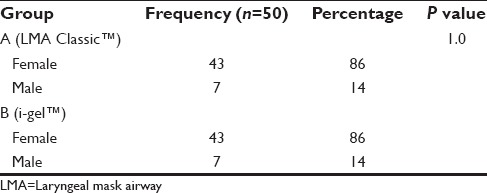
Table 2.
ASA physical status of patients in the study group i-gel™ and LMA Classic™

Table 3.
Airway assessment of patients in the study groups i-gel™ and LMA Classic™
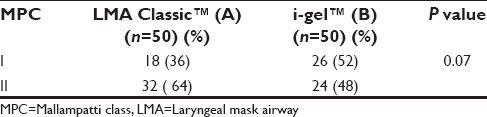
Table 4.
Comparison of ease of insertion and duration of insertion of i-gel™ and LMA Classic™ in the study groups
No significant change in HR and BP was observed at baseline between the study groups. There was significant difference in HR and BP during insertion and at 1, 3, 5, 10, 15, 20, 25 min postinsertion as well as during removal between the two groups. However, this increase in HR was higher in LMA Classic™ group when compared to the i-gel™ group at the abovementioned time with significant P < 0.0001 [Figures 1–3].
Figure 1.
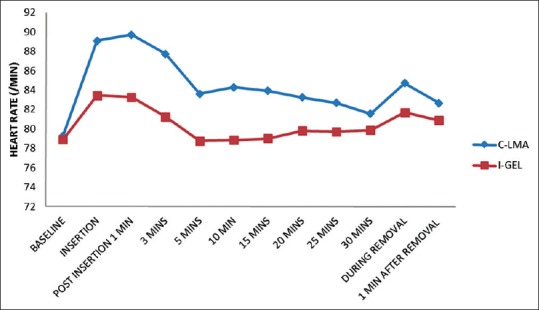
Heart rate variation at different time intervals between i-gel™ and LMA Classic™. Significant difference in heart rate between the groups at 3 min to 25 min postinsertion = 0.0001
Figure 3.
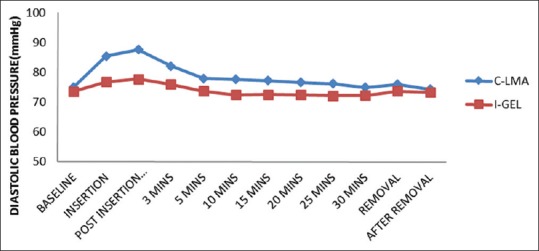
Diastolic blood pressure variation at different time intervals between i-gel™ and LMA Classic™. Significant difference in Diastolic blood pressure between the groups at 3 min to 25min post-insertion = 0.0001
Figure 2.
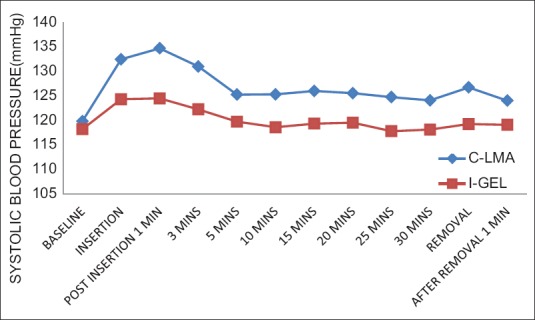
Systolic blood pressure variation at different time intervals between i-gel™ and LMA Classic™. Significant difference in Systolic blood pressure between the groups at 3 min to 25 min postinsertion = 0.0001
No episodes of desaturation were noted with both the groups during insertion, maintenance, and removal of the airway device.
DISCUSSION
In our study we compared two supraglottic airway devices LMA Classic™ and i-gel™, in relation to ease of insertion, duration of insertion, hemodynamic responses, and SpO2 changes.
We observed that both the devices were easy to insert <two attempts, but the success rate in the first attempt was 100% with i-gel™ and 84% with LMA Classic™, which is statistically significant (P = 0.003). Singh et al. and Siddiqui et al. also reported similar findings for i-gel™.[9,10] Revi et al.[11] observed ease of insertion was more with i-gel™ 96% (24/25) compared to ProSeal LMA™ 80% (20/25) and LMA Classic™ 88% (22/25). But the results were not statistically significant (P = 0.194).[11] Radhika et al.[12] observed the higher rate of failure of i-gel™ insertion can be attributed to the overlap in size selection according to body weight as recommended by the manufacturer. Saran et al.[13] have also observed a similar problem with size selection of i-gel™ in pediatric patients. The 100% success rate in insertion of i-gel™ in our study can be attributed to the prior training received by the final year postgraduate students under the supervision of anesthesia consultants in handling the supraglottic airway devices.
The duration of insertion time was significantly longer with LMA Classic™ compared to i-gel™. The median insertion time of 16 s has been reported with i-gel™. Helmy et al.,[14] Reza Hashemian et al.,[15] and Chauhan et al.[8] observed significantly lower insertion times with i-gel™. Since no cuff inflation is required in the i-gel™, time required to achieve an effective airway was shorter, and does not require an introducer, the device can be simply pushed into place.[16,17,18] The mean insertion time observed by Durrani et al.[19] was statistically insignificant. Theiler et al.[20] have attributed the longer insertion time of i-gel™, due to the bulky design of the airway device.
There are conflicting data about the hemodynamic responses to LMA Classic™ and i-gel™. In our study the baseline mean HR and BP values were comparable and not clinically significant The HR for the first 25 min after insertion of LMA Classic™ was persistently high from the baseline when compared to i-gel™ and clinically significant P = 0.0001. Jindal et al.[21] and Atef et al.[22] observed increase in heart and BP in LMA Classic™ group compared to i-gel™. These studies correlated with our study.[23,24,25] Revi et al.[11] observed no significant difference in hemodynamic data 1 min after insertion of devices among the three groups. Radhika et al.[12] attributed the increase in hemodynamic to the minimal sympathetic response caused by inflation of the cuff in LMA Classic™ group.
In our study there were no episodes of desaturation (SpO2 <95%) with both the groups during insertion, maintenance and removal of the airway device. In a study published by Atef on comparative study between i-gel™ and LMA Classic™ in eighty patients who were scheduled for surgery under general anesthesia maintaining spontaneous ventilation, there was no significant difference between both the groups SpO2.[25]
Limitation of our study was it was single center and we have not used flexible intubating fiberscope for assessing the airway placement position. The devices were inserted by final year postgraduate students, after prior training, thus the results of ease of insertion and duration of insertion were similar to experienced personal.
CONCLUSIONS
Successful and shorter duration of insertion, with less hemodynamic response, makes i-gel™ a suitable alternative to LMA Classic™ during general anesthesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Richez B, Saltel L, Banchereau F, Torrielli R, Cros AM. A new single use supraglottic airway device with a noninflatable cuff and an esophageal vent: An observational study of the i-gel. Anesth Analg. 2008;106:1137–9. doi: 10.1213/ane.0b013e318164f062. [DOI] [PubMed] [Google Scholar]
- 2.Stewart A, Lindsay WA. Bilateral hypoglossal nerve injury following the use of the laryngeal mask airway. Anaesthesia. 2002;57:264–5. doi: 10.1046/j.1365-2044.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 3.Twigg S, Brown JM, Williams R. Swelling and cyanosis of the tongue associated with use of a laryngeal mask airway. Anaesth Intensive Care. 2000;28:449–50. doi: 10.1177/0310057X0002800417. [DOI] [PubMed] [Google Scholar]
- 4.Ouellette RG. The effect of nitrous oxide on laryngeal mask cuff pressure. AANA J. 2000;68:411–4. [PubMed] [Google Scholar]
- 5.Levitan RM, Kinkle WC. Initial anatomic investigations of the I-gel airway: A novel supraglottic airway without inflatable cuff. Anaesthesia. 2005;60:1022–6. doi: 10.1111/j.1365-2044.2005.04258.x. [DOI] [PubMed] [Google Scholar]
- 6.Brimacombe JR, Berry AM, Brain AI. The laryngeal mask airway. Anesthesiol Clin North Am. 1995;13:411–37. [Google Scholar]
- 7.Jackson KM, Cook TM. Evaluation of four airway training manikins as patient simulators for the insertion of eight types of supraglottic airway devices. Anaesthesia. 2007;62:388–93. doi: 10.1111/j.1365-2044.2007.04983.x. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan G, Nayar P, Seth A, Gupta K, Panwar M, Agrawal N. Comparison of clinical performance of the I-gel with LMA ProSeal. J Anaesthesiol Clin Pharmacol. 2013;29:56–60. doi: 10.4103/0970-9185.105798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Yadav MK, Marahatta SB, Shrestha BL. Randomized crossover comparison of the laryngeal mask airway classic with i-gel laryngeal mask airway in the management of difficult airway in post burn neck contracture patients. Indian J Anaesth. 2012;56:348–52. doi: 10.4103/0019-5049.100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui AS, Raees US, Siddiqui SZ, Haider S, Raza SA. Comparison of performance and safety of I-gel with laryngeal mask airway (classic) for general anaesthesia with controlled ventilation. Anaesth Pain Intensive Care. 2010;14:17–20. [Google Scholar]
- 11.Revi N, Harikishore, Puthur B, Ershad A comparitive study on cardiovascular response and ease of insertion in classical laryngeal mask airway, ProSeal laryngeal mask airway and I-gel during surgery under general anaesthesia. J Evid Based Med Healthc. 2015;2:3039–46. [Google Scholar]
- 12.Radhika KS, Sripriya R, Ravishankar M, Hemanth Kumar VR, Jaya V, Parthasarathy S. Assessment of suitability of i-gel and laryngeal mask airway-supreme for controlled ventilation in anesthetized paralyzed patients: A prospective randomized trial. Anesth Essays Res. 2016;10:88–93. doi: 10.4103/0259-1162.167849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saran S, Mishra SK, Badhe AS, Vasudevan A, Elakkumanan LB, Mishra G. Comparison of i-gel supraglottic airway and LMA-ProSeal™ in pediatric patients under controlled ventilation. J Anaesthesiol Clin Pharmacol. 2014;30:195–8. doi: 10.4103/0970-9185.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmy AM, Atef HM, EI-Taher EM, Henidak AM. Comparative study between I-gel, a new supraglottic airway device and classical laryngeal mask airway in anaesthetized spontaneously ventilated patients. Saudi J Anaesth. 2010;4:131–6. doi: 10.4103/1658-354X.71250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reza Hashemian SM, Nouraei N, Razavi SS, Zaker E, Jafari A, Eftekhari P, et al. Comparison of i-gel™ and laryngeal mask airway in anesthetized paralyzed patients. Int J Crit Illn Inj Sci. 2014;4:288–92. doi: 10.4103/2229-5151.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh I, Gupta M, Tandon M. Comparison of clinical performance of I-Gel with LMA-ProSeal in elective surgeries. Indian J Anaesth. 2009;53:302–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Gabbott DA, Beringer R. The igel supraglottic airway: A potential role for resuscitation? Resuscitation. 2007;73:161–2. doi: 10.1016/j.resuscitation.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Uppal V, Gangaiah S, Fletcher G, Kinsella J. Randomized crossover comparison between the I-gel and the LMA-Unique in anaesthetized, paralysed adults. Br J Anaesth. 2009;103:882–5. doi: 10.1093/bja/aep292. [DOI] [PubMed] [Google Scholar]
- 19.Durrani HD, Butt KJ, Sadaf S, Rehan A, Khan AM, Umar A. Comparison of LMA classic and I-gel in anesthetized, spontaneously breathing patients during elective surgical procedures. Anaesth Pain Intensive Care. 2013;17:274–8. [Google Scholar]
- 20.Theiler LG, Kleine-Brueggeney M, Kaiser D, Urwyler N, Luyet C, Vogt A, et al. Crossover comparison of the laryngeal mask supreme and the i-gel in simulated difficult airway scenario in anesthetized patients. Anesthesiology. 2009;111:55–62. doi: 10.1097/ALN.0b013e3181a4c6b9. [DOI] [PubMed] [Google Scholar]
- 21.Jindal P, Rizvi A, Sharma JP. Is I-gel a new revolution among supraglottic airway devices? – A comparative evaluation. Middle East J Anaesthesiol. 2009;20:53–8. [PubMed] [Google Scholar]
- 22.Atef HM, Fattah SA, Gaffer ME, Al Rahman AA. Perfusion index versus non-invasive hemodynamic parameters during insertion of i-gel, classic laryngeal mask airway and endotracheal tube. Indian J Anaesth. 2013;57:156–62. doi: 10.4103/0019-5049.111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali A, Ali L, Sheikh NA, Siddique SA. Airway device; Comparison of I-gel supraglotic with laryngeal mask airway for ease of insertion. Professional Med J Dec. 2010;17:643–7. [Google Scholar]
- 24.Jindal P, Rizvi AA, Khurana G, Sharma P. Safety and efficacy of insertion of supraglottic devices in anaesthtised patients by first time users. South Afr J Anaesthesiol Analg. 2010;16:23–6. [Google Scholar]
- 25.Atef HM, Helmy AM, El-Taher EM, Henidak AM. Comparative study between I-gel, a new supraglottic airway device, and classical laryngeal mask airway in anesthetized spontaneously ventilated patients. Middle East J Anaesthesiol. 2012;21:583–90. [PubMed] [Google Scholar]


