Abstract
Background:
Multimodal analgesia (MMA) by synergy with volatile anesthetics minimizes their use thus decreasing operation theater pollution and greenhouse gas emission.
Aims:
To estimate minimum alveolar concentration (MAC) requirement of isoflurane (ISO) for skin incision with use of MMA in the study group versus conventional regime in the control group for a constant bispectral index (BIS). To observe the side effects of analgesic drugs administered in the study.
Settings and Design:
Forty-two patients of American Society of Anesthesiologist Class I and II scheduled for lumbar spine surgery were included in this prospective, randomized, double-blind, clinical study. They were randomly allocated into two groups of 21 each.
Materials and Methods:
Group A (MMA group/study group) received injections diclofenac sodium, paracetamol, clonidine, and fentanyl and local infiltration (bupivacaine with adrenaline). Group B (conventional regime group/control group) received injections paracetamol and fentanyl and local infiltration (saline with adrenaline). Preemptive analgesia was practiced in the study. The MAC of ISO for skin incision was documented.
Statistical Analysis Used:
Independent sample t-test: To compare MACISO for skin incision between the two groups. One sample t-test: To compare the standard mean concentration with the means of the two groups. Chi-square test: To compare adverse effects between the groups. P < 5% was considered statistically significant.
Results:
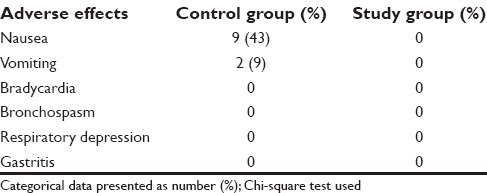
The MACISO requirement was significantly lower in the study group at the time of skin incision for BIS of 50–55 compared to the control group (P < 0.001). Post extubation, 43% had nausea and 9% had vomiting in the control group. None of the patients in either group had intraoperative awareness.
Conclusion:
We conclude that preemptive MMA has synergistic effect with ISO. It effectively reduces MACISO to skin incision to a greater degree.
Keywords: Analgesia: multimodal analgesia, bispectral index, inhalation anesthetics: isoflurane, minimum alveolar concentration
INTRODUCTION
Evidence suggests that trace amounts of inhaled anesthetics carry risk for operation theater (OT) personnel[1] and also impact greenhouse gas emissions.[2] Although scavenging systems have minimized OT pollution, excess gases vented to the atmosphere, still contribute to environmental hazard.[3] Considering this fact, there is a need to reduce the quantity of inhalational agents used intraoperatively. In tertiary hospitals to some extent, it is taken care of as workstations have facilities for low flow anesthesia[3] and the use of target-controlled inhalational anesthesia, an anesthesia delivery system available in the newer anesthesia machines.[4] However, for this to reach all centers where anesthesia is practiced is not foreseeable in the near future.
To minimize the use of inhalational agents intraoperatively, we hypothesized that the hypnotic component of multimodal analgesia (MMA) will reduce the minimum alveolar concentration (MAC) of isoflurane (ISO) for skin incision at a constant bispectral index (BIS).
MMA is a scientific approach to treat acute pain, where all four elements of pain processing are targeted with drugs of different pharmacologic actions. The four elements are (1) transduction, (2) transmission, (3) modulation, and (4) perception. Traditionally, analgesic therapies targeted only one or two elements of the pain pathway.[5]
MAC is a robust quantitative measurement used as the standard for determining potency of an anesthetic gas. It is defined as the MAC at sea level of inhaled anesthetic required to prevent apparently purposeful movement in 50% of patients in response to surgical incision (MAC-50%: 1).[6] Other relevant MAC derivatives are MAC-95%: 1.2–1.3 times MAC and MAC-BAR: 1.5 times MAC (MAC that blunts adrenergic responses to noxious stimuli/skin incision).[1,6,7]
In clinical practice, the components of balanced anesthesia (unconsciousness, analgesia, and immobility) are monitored by the heart rate (HR) and blood pressure (BP).[8] Changes in these parameters signal an “imbalance” in balanced anesthesia,[8] which is detrimental to vital organs.[7] BIS monitoring reflects the degree of hypnotic component of anesthesia. It is added as a “vital sign for consciousness” which correlates with depth of hypnosis in balanced anesthesia.[8,9] It is a numerical index ranging from 100 (awake) – 0 (isoelectric electroencephalogram [EEG]) of the processed EEG. Values of 40–60 have been recommended for general anesthesia.[10]
MATERIALS AND METHODS
This randomized, prospective, double-blind, clinical study was carried out in a Tertiary Care Centre from June 2011 to May 2013. After obtaining approval from Institutional Review Board, 42 patients with informed written consent scheduled for lumbar spine surgery were included in the study. Patients aged between 20 and 65 years of either sex with body mass index of 18–30 belonging to American Society of Anesthesiologists (ASA) physical status I and II were included in the study. Pregnant women, patients with bronchial asthma and known drug allergy were excluded from the study. Patients were randomly allocated into two groups (n = 21), Group A and Group B, by allocation sequence generated by computer-generated random number table.
Group A (MMA group/study group) received injections diclofenac sodium - 75 mg, paracetamol - 1 g, clonidine - 0.75 µg/kg, and fentanyl - 3 µg/kg and local infiltration (bupivacaine 0.25% 20 ml with adrenaline 1:1000, 2 drops). Group B (conventional regime group/control group) received injections paracetamol - 1 g and fentanyl - 3 µg/kg and local infiltration (saline 20 ml with adrenaline 1:1000, 2 drops). Study drugs were prepared by assigned postgraduate students who were not part of study design.
After confirming the preanesthetic evaluation and consent, in the induction room, the following monitors were connected: electrocardiogram, noninvasive BP, SpO2 (O2 saturation of hemoglobin), BIS, end-tidal CO2, and train of four (TOF: neuromuscular monitor) for monitoring throughout the surgery. Intravenous cannula was inserted for drug and fluid administration. Oxygen was administered by O2 mask. Intravenously, injections midazolam 0.03 mg/kg, ondansetron 4 mg, and glycopyrrolate 0.2 mg were administered to all the patients. Preemptive analgesia was practiced: analgesics being administered prior to induction, providing sufficient time for the onset of action of drugs. Following preoxygenation with 100% O2 for 3 min, 2 min prior to induction, injection fentanyl 3 µg/kg was given to all the patients followed by induction with injection propofol and tracheal intubation facilitated with injection atracurium 0.5 mg/kg. Anesthesia was maintained with ISO in 60% N2O and O2, injection atracurium and injection morphine. HR and BP were maintained within ± 10% of the baseline values at skin incision and intraoperatively. End-tidal ISO concentration was continuously monitored using an infrared gas analyzer. The MACISO was titrated to achieve BIS of 50–55 which was maintained for 15 min before skin incision, after painting and draping[6,11] and MACISO for skin incision was documented. At the conclusion of surgery, all patients were allowed to recover spontaneously to TOF of T2. Residual neuromuscular blockade was reversed with injection glycopyrrolate 10 µg/kg and injection neostigmine 50 µg/kg. Tracheal extubation was done when the following criteria were met: BIS: 85–88 and TOF T4/T1 ratio ≥0.9.
Adverse effects of analgesic drugs such as intraoperative bradycardia (opioid, clonidine) bronchospasm, gastritis (NSAID’s), postoperative nausea, vomiting, and respiratory depression (opioid) were noted.
Statistics
This study was nested within a study whose objective was to assess “the effect of multimodal preoperative analgesia on stress response to surgery and postoperative pain score” in patients undergoing lumbar spine surgery. For this study, the sample size estimated was 21 patients in each group with α =0.05 and β =0.20 to observe a difference of 4.3 µg/dl in serum cortisol level between the groups. The distribution of all continuous data was examined using QQ plots. Normally distributed data are presented as mean ± standard deviation. Normally distributed data of MACISO for skin incision between the two groups (control and study group) were compared using independent sample t-test. One sample t-test was used to compare the standard mean concentration with the means of the study and control groups, P < 5% being considered statistically significant. Adverse effects of the drugs were compared between the groups using Chi-square test and presented as number percentage. Data were analyzed using SPSS software (SPSS Inc., Release 2009, PASW Statistics for Windows, Version 18.0. Chicago, IL, USA).
RESULTS
The demographic data in both the groups were comparable [Table 1]. The MACISO requirement was significantly higher in the control groups at the time of skin incision for BIS of 50–55 (P < 0.001) compared to the study group [Table 2]. MACISO mean was reduced both in the control group (6%) and the study group (49%) compared to the standard mean but was significantly reduced in the study group P < 0.001 [Table 3]. Postextubation, 43% had nausea and 9% had vomiting in the control group; however, no adverse effects were noted in the study group [Table 4]. None of the patients in either group had intraoperative awareness.
Table 1.
Patient demographic data
Table 2.
Minimum alveolar concentration for skin incision (n=21 per group)

Table 3.
Comparison of control group and study group mean with standard mean (n=21 per group)
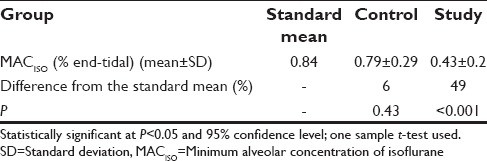
Table 4.
Comparison of adverse effects between control group and study group (n=21 per group)
DISCUSSION
MMA has gained importance with the rising number of fast-track surgeries[12] and its efficacy in attenuating postoperative pain.[13,14,15] In this study, the hypnotic effect of MMA drugs on MACISO to skin incision was determined during general anesthesia with BIS monitoring. The study is currently supported by MAC sparing effects of various analgesic drugs in trials on animals.[16,17,18,19] In patients, MAC of inhalational agents is studied with two-stage or three-stage incremental Infusion of analgesic drugs with estimation of plasma concentration.[20,21,22] Although a BIS of 60 is acceptable with a combination of volatile anesthetics and opioids,[8] we maintained BIS at 50–55 as intraoperative awareness has been documented at values higher than this.[10] The MACISO was titrated to achieve BIS of 50–55 and steady-state was maintained for 15 min before skin incision for alveolar, arterial, and brain partial pressures to equilibrate.[23] In the study, ISO was used as it is a “gold standard” anesthetic.[1]
MACISO in O2 is 1.17% end-tidal, MACISO in 60–70% N2O with O2 is 0.56% end-tidal, and MAC-BARISO in N2O with O2 is 0.84% end-tidal (standard mean).[1] MACISO for skin incision was significantly reduced in the study group to 0.43% end-tidal compared to 0.79% end-tidal in the control group (P < 0.001) [Table 2]. In pairwise comparison with standard mean of MAC-BARISO, MACISO values in the study group were decreased by 49% when compared with 6% in the control group which was clinically significant (P < 0.001) [Table 3]. It is clear from the observed results that there is no significant difference between standard mean and the control group (P = 0.43). In accordance with the hypothesis of the study, the results support the fact that MMA has anesthetic interaction with ISO in reducing MACISO for skin incision. The results of the study are consistent with previous reports that showed a decrease in MACISO after administration of either fentanyl,[3,17] ketamine,[18] dexmedetomidine,[16] lignocaine, or combination of these drugs.[19] Studies are required to assess the synergistic effect of MMA with MAC of inhalational agents without N2O, which could contribute to decreased use of inhalational anesthetics. Thomasy et al. have discussed in their study that MAC varies with different fentanyl doses in different species, for example, plasma fentanyl values of 6–10 ng/ml in dogs reduces MAC by 53%, humans by 82%, and horse and swine with fentanyl of 13.31 ng/ml by 18%.[17] They have concluded that therapeutic range of fentanyl consistently reduces anesthetic requirement. More studies are required with serum drug levels with various doses of fentanyl in different types of surgery as part of MMA regime for appropriate drug dosing. In a study by Rioja et al., MACISO in control group was 1.46 whereas with dexmedetomidine MACISO was 0.83 in rats, which was statistically significant (P < 0.01).[16] In a study by Gutierrez-Blanco et al., MAC was titrated based on clinical signs and autonomic responses to surgical stimuli with ISO in 100% O2 in dogs. They found MAC requirement at the beginning of the skin incision in control group was 1.74, whereas with fentanyl, it was 1.1, with lignocaine 1.35, dexmedetomidine 1.16, and combination of lignocaine-ketamine-dexmedetomidine 1.01. They also found significant difference in MAC values within the groups compared with baseline values (P < 0.05). Similarly, there was a significant difference when each group was compared with control group (P < 0.05).[19] Many studies are required with permutation and combination in MMA regime with available drugs targeting all four elements of the pain pathway to improve recovery profile, shorten both OT and postanesthesia care unit stay, reduce OT pollution, in turn greenhouse gas emission, and avert the side effects of higher doses of drugs.
CONCLUSION
We conclude that preemptive MMA has synergistic effect with ISO. It effectively reduces MACISO to skin incision to a greater degree. Hypnotic component of MMA has to be considered intraoperatively to surmount “imbalance” in balanced anesthesia. Further studies in distinct surgery with different combinations of drugs in MMA are needed to support the findings of the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ebret TJ, Schmid PG. Inhaled anesthetics. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, editors. Clinical Anaesthesia. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 413–43. [Google Scholar]
- 2.Tay S, Weinberg L, Peyton P, Story D, Briedis J. Financial and environmental costs of manual versus automated control of end-tidal gas concentrations. Anaesth Intensive Care. 2013;41:95–101. doi: 10.1177/0310057X1304100116. [DOI] [PubMed] [Google Scholar]
- 3.Riutort KT, Brockwell RC, Brull SJ, Andrews J. The anesthesia workstations and delivery systems. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, editors. Clinical Anaesthesia. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 644–94. [Google Scholar]
- 4.Potdar MP, Kamat LL, Save MP. Cost efficiency of target-controlled inhalational anesthesia. J Anaesthesiol Clin Pharmacol. 2014;30:222–7. doi: 10.4103/0970-9185.130026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macres SM, Moore PG, Fishman SM. Acute pain management. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, editors. Clinical Anaesthesia. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 1473–504. [Google Scholar]
- 6.Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: Ongoing relevance and clinical utility. Anaesthesia. 2013;68:512–22. doi: 10.1111/anae.12168. [DOI] [PubMed] [Google Scholar]
- 7.Roizen MF, Horrigan RW, Frazer BM. Anesthetic doses blocking adrenergic (stress) and cardiovascular responses to incision – MAC BAR. Anesthesiology. 1981;54:390–8. doi: 10.1097/00000542-198105000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kurata J. Deep hypnosis as a sign of “imbalance” in balanced anesthesia. Anesth Analg. 2010;110:663–5. doi: 10.1213/ANE.0b013e3181c30fa0. [DOI] [PubMed] [Google Scholar]
- 9.Chan MT, Gin T. What does the bispectral EEG index monitor? Eur J Anaesthesiol. 2000;17:146–8. doi: 10.1046/j.1365-2346.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 10.Bowdle TA. Can we prevent recall during anesthesia? In: Fleisher LA, editor. Evidence -Based Practice of Anesthesiology. 3rd ed. Philadelphia: Saunders; 2013. pp. 332–7. [Google Scholar]
- 11.Quasha AL, Eger EI, 2nd, Tinker JH. Determination and applications of MAC. Anesthesiology. 1980;53:315–34. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 12.White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: From multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–96. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 13.Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001;13:524–39. doi: 10.1016/s0952-8180(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 14.White PF. Multimodal analgesia: Its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9:76–82. [PubMed] [Google Scholar]
- 15.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107(Suppl 1):i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 16.Rioja E, Santos M, Martínez Taboada F, Ibancovichi JA, Tendillo FJ. Cardiorespiratory and minimum alveolar concentration sparing effects of a continuous intravenous infusion of dexmedetomidine in halothane or isoflurane-anaesthetized rats. Lab Anim. 2006;40:9–15. doi: 10.1258/002367706775404363. [DOI] [PubMed] [Google Scholar]
- 17.Thomasy SM, Steffey EP, Mama KR, Solano A, Stanley SD. The effects of i.v. fentanyl administration on the minimum alveolar concentration of isoflurane in horses. Br J Anaesth. 2006;97:232–7. doi: 10.1093/bja/ael116. [DOI] [PubMed] [Google Scholar]
- 18.Gianotti G, Valverde A, Johnson R, Sinclair M, Gibson T, Dyson DH. Influence of prior determination of baseline minimum alveolar concentration (MAC) of isoflurane on the effect of ketamine on MAC in dogs. Can J Vet Res. 2014;78:207–13. [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez-Blanco E, Victoria-Mora JM, Ibancovichi-Camarillo JA, Sauri-Arceo CH, Bolio-González ME, Acevedo-Arcique CM, et al. Evaluation of the isoflurane-sparing effects of fentanyl, lidocaine, ketamine, dexmedetomidine, or the combination lidocaine-ketamine-dexmedetomidine during ovariohysterectomy in dogs. Vet Anaesth Analg. 2013;40:599–609. doi: 10.1111/vaa.12079. [DOI] [PubMed] [Google Scholar]
- 20.Melvin MA, Johnson BH, Quasha AL, Eger EI 3rd. Induction of anesthesia with midazolam decreases halothane MAC in humans. Anesthesiology. 1982;57:238–41. doi: 10.1097/00000542-198209000-00018. [DOI] [PubMed] [Google Scholar]
- 21.McEwan AI, Smith C, Dyar O, Goodman D, Smith LR, Glass PS. Isoflurane minimum alveolar concentration reduction by fentanyl. Anesthesiology. 1993;78:864–9. doi: 10.1097/00000542-199305000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Aantaa R, Jaakola ML, Kallio A, Kanto J. Reduction of the minimum alveolar concentration of isoflurane by dexmedetomidine. Anesthesiology. 1997;86:1055–60. doi: 10.1097/00000542-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Dwyer R, Bennett HL, Eger EI 2nd, Heilbron D. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology. 1992;77:888–98. doi: 10.1097/00000542-199211000-00009. [DOI] [PubMed] [Google Scholar]


