Abstract
Aims and Objectives:
Supplementation of dexmedetomidine produces a dose-dependent sedation, anxiolysis and analgesia without respiratory depression. This study was conducted to evaluate the possible effect of dexmedetomidine as an adjuvant to levobupivacaine for supraclavicular brachial plexus block in upper limb surgery.
Settings and Design:
Tertiary care institute, Department of Anaesthesiology and Intensive Care, a placebo-controlled study.
Materials and Methods:
After obtaining Ethical Committee approval, a randomized, double-blind, placebo-controlled study was conducted on sixty American Society of Anesthesiologists physical status I and II patients in the age group of 18–60 years, divided randomly into two groups, Group I received 30 ml of 0.5% levobupivacaine with 1 ml of isotonic sodium chloride solution and Group II received 30 ml of 0.5% levobupivacaine and 1 ml (100 mcg) of dexmedetomidine for supraclavicular brachial plexus block. The onset and duration of sensory and motor blockade, duration of analgesia (DOA) and any adverse effects were noted. At the end of the study, data were compiled and analyzed using appropriate statistical tests. The value of P < 0.05 was considered significant.
Results:
Demographic profile was comparable in both the groups. The time to onset of sensory and motor block was 10.54 ± 2.333 min and 12.21 ± 2.529 min in Group I while it was 3.24 ± 0.951 min and 2.83 ± 1.197 min in Group II, respectively. The duration of sensory and motor block was 7.79 ± 2.007 h and 9.18 ± 1.701 h in Group I, and it was 16.31 ± 2.606 h and 17.52 ± 2.098 h in Group II, respectively. The DOA was 678.68 ± 20.492 min in Group I and 1273.79 ± 83.139 min in Group II. On statistical comparison, these values were highly significant (P < 0.001). Side effects such as nausea, vomiting, hypoxemia, pruritis, or urinary retention were not observed in either of the groups.
Conclusion:
Dexmedetomidine shortens the onset time for sensory and motor block significantly and prolongs DOA as well when used with levobupivacaine for supraclavicular brachial plexus block, without increasing the incidence of any adverse effects.
Keywords: Dexmedetomidine, levobupivacaine, supraclavicular brachial plexus block
INTRODUCTION
In modern anesthesia practice, more stress is laid upon using safer and newer anesthesia drugs. The addition of adjuvant not only augments the anesthetic action of the drug but also reduces the dose required thus improving the safety margin. Levobupivacaine is a new local anesthetic drug which has established itself as a safer anesthetic.[1] There are only few studies which have further tried to assess its safety and effectiveness in regional anesthesia when admixed with adjuvants. However, no single drug can be considered as an ideal adjuvant to the local anesthetic. In a quest to find a better alternative, numerous research studies have been done but with varying success. The focus keeps on shifting to newer adjuvants in daily anesthesia practice to fulfil these desirable goals of regional anesthesia.
Dexmedetomidine, an alpha-2 receptor agonist, is increasingly being used nowadays for regional anesthesia and intravenous (i.v.) anesthesia (Bier's block),[2,3,4,5] attenuation of pressor response, i.v. sedation and analgesia for mechanically ventilated patients in Intensive Care Units,[6,7,8] and nonintubated patients for surgical and other procedures.[9] Its use in peripheral nerve blocks has only recently been described.[10,11,12] This study was designed by the addition of dexmedetomidine to levobupivacaine for supraclavicular brachial plexus block and to see its possible effects on sensory and motor blockade as well as any effect on duration of analgesia (DOA).
MATERIALS AND METHODS
After the approval of the Hospital Ethics Committee, written informed consent was taken from all the patients. Sixty American Society of Anesthesiologists physical status I and II patients, in the age group of 18–60 years undergoing upper limb surgery, under supraclavicular brachial plexus block were included in this prospective, randomized, double-blind, placebo-controlled trial. Patients with previous nerve deformity or brachial plexus injury, severe liver or kidney disease, patients having opposite side pneumothorax or collapsed lung, patients posted for bilateral upper limb surgeries, hypersensitivity to amide local anesthetics, local infection, coagulopathy, and uncooperative or unwilling patients were excluded from the study. Assuming, a 30 min difference in prolongation of sensory analgesia and taking the power of study at 90% by keeping type I error (α = 0.05) and type II error (β) at 0.1, the sample size was calculated at 28 patients in each group. We enrolled 30 patients in each group for the better validation of study results. Patients were randomly allocated by computer generated randomization number into two groups, of 30 each by allocating 60 coded slips. Group I (n = 30) received 30 ml of 0.5% levobupivacaine with 1 ml of isotonic sodium chloride solution while Group II (n = 30) received 30 ml of 0.5% levobupivacaine and 1 ml (100 µg) of dexmedetomidine [Figure 1].
Figure 1.
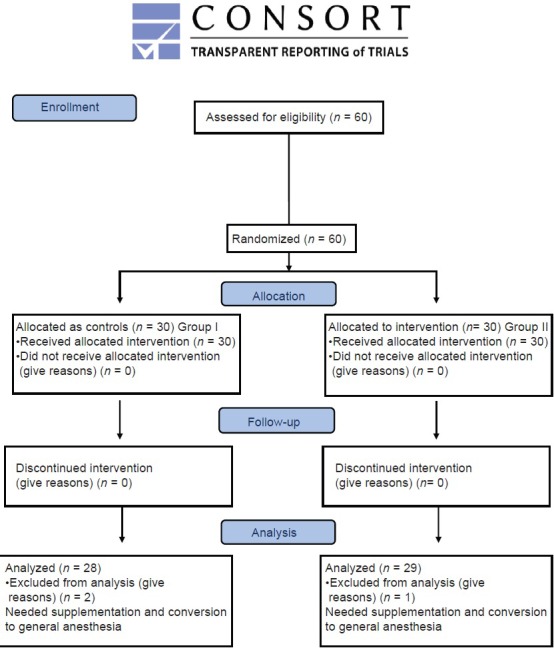
CONSORT 2010 flow diagram
A senior anesthesiologist who was well versed in brachial plexus block technique performed all the procedures, and he was blinded to the study drug solutions. The drug solution was prepared in two partially covered separate syringes for every case by an anesthesia technician who was also unaware of the study design.
On shifting the patients to preoperative room, the basal heart rate (HR), noninvasive arterial systolic blood pressure (SBP), diastolic blood pressure (DBP), and peripheral oxygen saturation (SpO2) were recorded. A 20-gauge, i.v. cannula was inserted in nonoperating arm and lactated Ringer's solution started at 5 ml/kg/h.
For administration of supraclavicular brachial plexus block, the patients were placed in the supine position with adduction of the arm to be anesthetized and head extended and turned away from the side to be blocked. The medial and lateral borders of the clavicle were identified as the first rib generally lies beneath the midpoint of clavicle. The landmarks were confirmed by sliding down the fingers in the interscalene groove until the arterial pulsation of the subclavian artery felt. A skin wheal was raised 0.5–1 cm posterior to the midpoint of the clavicle and a 22-gauge, short bevelled nerve stimulating needle inserted in a caudal, slightly medial and posterior direction. The needle was connected to the negative lead of the nerve locator, preset in the motor testing mode with a current setting of 2–3 mA and the patient's arm observed. When the patient got a distal contraction of the upper limb, the current was reduced to 0.6 mA. After observing the contractions, at this reading also, the drug solution was injected.
Sensory block was assessed by loss of sensation to pinprick in the midline using a 22-gauge blunt hypodermic needle every minute using Hollmen scale (1 - normal sensation of pinprick, 2 - pin prick felt as sharp pointed but weaker compared with the same area in the other limb, 3 - pin prick recognized as touch with blunt object, and 4 - no perception of pin prick).
A sensory block of scale 3 was considered as an endpoint for surgery. The onset of sensory block was taken as the time from injection of drug to Hollmen sensory scale of 2. Duration of sensory block was taken as the time elapsed between performing the block to regression of sensory block and scale of ≤2. Motor block was assessed using Hollmen scale (1 - normal muscle action, 2 - slightly weak muscle action, 3 - very weak muscular action, and 4 - complete loss of muscle action).
The test was performed every minute until scale 2. A motor block of scale 3 was considered as an endpoint for the surgery. The onset of motor block was taken as the time from injection of drug to Hollmen motor scale of 2. Duration of motor block was taken as time elapsed between performing block to regression of motor scale and lower degree.
If there was sparing of dermatomes in the region of surgery in any patient, the block was supplemented with midazolam (0.05 mg/kg) and ketamine (0.5 mg/kg). In patients with lesser degree of block or uncooperative patients, general anesthesia was supplemented.
Postoperative pain was assessed by visual analog scale (VAS) at 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 18 h, and 24 h after surgery. Whenever VAS score reached ≥4, rescue analgesia was given in the form of i.v. injection diclofenac sodium 75 mg. Time to the first dose of diclofenac sodium postoperatively was noted.
VAS score was determined as 0 - no pain, 1–3 - mild pain, 4–7 - moderate pain, and 8–10 - severe pain. HR, SBP, and DBP were also recorded at 0, 3, 6, 9, 15, 20, 25, 30, 45, 60, 75, 90, and 120 min. Sedation score was assessed according to the Ramsay sedation scale from 1 to 6 as follows: 1 = Anxious, agitated, restless; 2 = cooperative, oriented, tranquil; 3 = responds to commands only; 4 = brisk response to light glabellar tap or loud noise; 5 = sluggish response to light glabellar tap or loud noise; and 6 = no response. Adverse effects comprised hypotension (i.e., 20% decrease relative to baseline), bradycardia (i.e., 20% decrease relative to baseline), nausea, vomiting, and hypoxemia (SpO2 <90%). The decoding of the groups was done at the end of the study followed by statistical analysis of the results. After completion of the study, the results were statistically analyzed using Chi-square test for nonparametric data and Student unpaired t-test for parametric data for inter-group comparison. Statistical analysis was done using SPSS III Statistics for Windows, Version 17.0. (Chicago: SPSS Inc). The value of P < 0.05 was considered significant and P < 0.001 as highly significant.
RESULTS
The demographic data were comparable in both the groups [Table 1]. The mean onset time for sensory and motor blocks in Group I 10.54 ± 2.333 and 12.21 ± 2.529 min, and for Group II was 3.24 ± 0.951and 3.83 ± 1.197 min, respectively. The mean duration time for sensory and motor components of Group I was 7.79 ± 2.007 and 9.18 ± 1.701 h while in Group II the corresponding values were 16.31 ± 2.606 and 17.52 ± 2.098 h, respectively. On statistical analysis, the difference was statistically highly significant (P < 0.001) [Table 2]. The mean DOA for Group I was 678.68 ± 20.492 while in Group II patients, it was 1273.79 ± 83.139 min [Table 2]. DOA was significantly longer in Group II than Group I (P < 0.001). HR, SBP, and DBP in Group II were significantly lower than in Group I (P < 0.001) intraoperatively [Tables 3 and 4]. Among the adverse effects, hypotension was seen in 22 patients and bradycardia in 26 patients of Group II. SBP was never <20% from baseline. Fall in pulse rate was more than 20% from baseline in one patient of Group II [Tables 5 and 6]. The mean Ramsay sedation score was 3.90 ± 0.618 in Group II and 2.00 in Group I. Side effects such as nausea, vomiting, hypoxemia, pruritis, or urinary retention were not observed in either of the groups [Table 5]. Two failure cases were found in Group I and one failure case were found in Group II. Therefore, statistical analysis was applied on 28 patients in Group I and 29 patients in Group II.
Table 1.
Demographic data: Age and sex distribution in both groups

Table 2.
Block characteristics in both groups
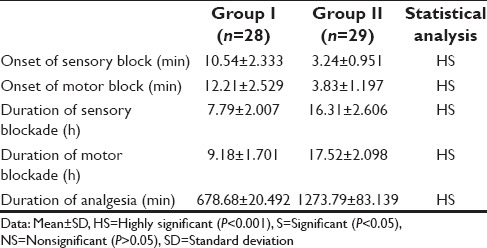
Table 3.
Pulse rate at various time intervals intraoperatively
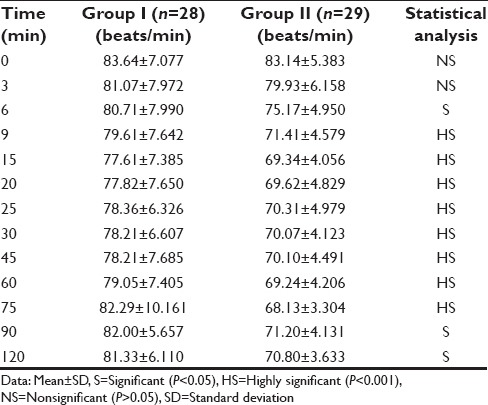
Table 4.
Mean systolic blood pressure and mean diastolic blood pressure at various time intervals intraoperatively

Table 5.
Incidence of adverse effects
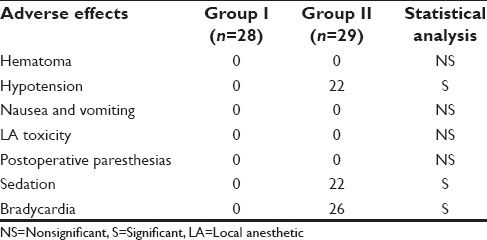
Table 6.
Maximum fall in pulse rate below 20% baseline
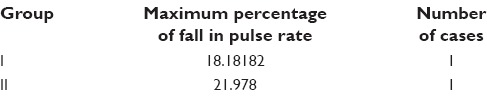
DISCUSSION
Dexmedetomidine, the pharmacologically active d-isomer of medetomidine is a highly specific and selective α2 adrenoceptor agonist with α2:α1 binding selectivity ratio of 1620:1 as compared to 220:1 for clonidine, thus decreasing the unwanted side effects of α1 receptors.[13,14,15,16] Studies have shown that presynaptic activation of α2 adrenoceptor in central nervous system inhibits the release of norepinephrine, terminating the propagation of pain signals, and their postsynaptic activation inhibits sympathetic activity, thereby decreasing HR and BP.[17,18]
In our study, it was observed that the addition of dexmedetomidine to levobupivacaine significantly shortens the sensory and motor blockade onset time (P < 0.001) This was in accordance with the study of Esmaoglu et al.[10], who also concluded that sensory and motor block onset time were significantly shorter in levobupivacaine and dexmedetomidine group than the levobupivacaine group and the difference was statistically significant. Our results were in disagreement with the studies by Kaygusuz et al.[19], who observed shortening of sensory block onset time whereas no shortening of motor onset time. This could be due to use of lower dose of dexmedetomidine in their studies.
In this study, duration of sensory block and motor block was significantly (P < 0.001) more in patients of Group II as compared to Group I. This increased duration of motor block could be because of direct impairment of excitatory amino acid release from spinal interneurons, the results were in accordance with a study by Agarwal et al.,[20]. The DOA in Group II was significantly prolonged as compared to Group I; this finding was in agreement to the studies by Esmaoglu et al.[10] and Agarwal et al.[20], who also stated that DOA was prolonged by adding 100 µg of dexmedetomidine to 0.5% levobupivacaine in axillary brachial plexus block.
C4 dermatome was spared in all patients of both the groups. Both groups were similar statistically, in dermatomal spread of the anesthetic. Similar results were shown by Cox et al.[21] and Vainionpää et al.[22]
Among the adverse effects, hypotension was seen in 22 patients and bradycardia in 26 patients of Group II. SBP was never <20% from the baseline value, so no treatment was given. Fall in pulse rate was more than 20% from baseline in one patient of Group II who responded well to atropine. The decrease in blood pressure is due to the inhibition of central sympathetic outflow. The presynaptic alpha-2 receptors are also stimulated by dexmedetomidine, thereby decreasing norepinephrine release and causing a fall in blood pressure and HR. In our study, intraoperative complications in both the groups were not statistically significant. There was not a single episode of respiratory depression in any of the groups. Agarwal et al.,[20] also showed similar results in his study.
We found that there was one patient in Group II and two patients in Group I who needed supplementation and conversion to general anesthesia.
The limitation of our study was that we did not biochemically analyze the blood concentration of levobupivacaine and dexmedetomidine which would have further supported our conclusions. More randomized trials need to be conducted to verify the findings of our study.
CONCLUSION
To conclude, we found that dexmedetomidine, when added to levobupivacaine for supraclavicular brachial plexus block, shortens the onset time for sensory and motor blocks and prolongs its duration. The significantly prolonged DOA obviates the need for any additional analgesics. The added advantage of conscious sedation, hemodynamic stability, and minimal side effects makes it a potential adjuvant for nerve blocks.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bajwa SJ, Kaur J. Clinical profile of levobupivacaine in regional anesthesia: A systematic review. J Anaesthesiol Clin Pharmacol. 2013;29:530–9. doi: 10.4103/0970-9185.119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esmaoglu A, Mizrak A, Akin A, Turk Y, Boyaci A. Addition of dexmedetomidine to lidocaine for intravenous regional anaesthesia. Eur J Anaesthesiol. 2005;22:447–51. doi: 10.1017/s0265021505000761. [DOI] [PubMed] [Google Scholar]
- 3.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5:365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abosedira MA. Adding clonidine or dexmedetomidine to lignocaine during Biers block: A comparative study. J Med Sci. 2008;8:660–4. [Google Scholar]
- 5.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamtz J, Singer M. Importance of patient orientation and rousability as components of intensive care unit sedation. In: Maze M, Morrison P, editors. Redefining Sedation. London, UK: The Royal Society of Medicine Ltd; 1998. pp. 23–9. [Google Scholar]
- 7.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensive Care Med. 2004;30:2188–96. doi: 10.1007/s00134-004-2417-z. [DOI] [PubMed] [Google Scholar]
- 9.Shukry M, Miller JA. Update on dexmedetomidine: Use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag. 2010;6:111–21. doi: 10.2147/tcrm.s5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 11.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 12.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–83. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 16.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res Clin Exp. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30:36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox CR, Faccenda KA, Gilhooly C, Bannister J, Scott NB, Morrison LM. Extradural S(-)-bupivacaine: Comparison with racemic RS-bupivacaine. Br J Anaesth. 1998;80:289–93. doi: 10.1093/bja/80.3.289. [DOI] [PubMed] [Google Scholar]
- 22.Vainionpää VA, Haavisto ET, Huha TM, Korpi KJ, Nuutinen LS, Hollmén AI, et al. A clinical and pharmacokinetic comparison of ropivacaine and bupivacaine in axillary plexus block. Anesth Analg. 1995;81:534–8. doi: 10.1097/00000539-199509000-00019. [DOI] [PubMed] [Google Scholar]


