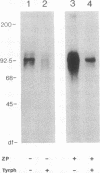
Abstract
A 95-kDa mouse sperm protein has been previously identified as a putative receptor involved in the sperm-egg interactions that lead to fertilization. The ligand for this receptor is the zona pellucida glycoprotein ZP3. This constituent of the oocyte-specific extracellular matrix mediates not only sperm binding to the zona but also triggers acrosomal exocytosis. The latter, also termed the acrosome reaction, is a key regulatory event upon which fertilization is absolutely dependent. Previously, we showed that the 95-kDa protein that binds ZP3 is a substrate for tyrosine kinase, and its phosphotyrosine content increases after sperm-zona pellucida binding. Here, we show the presence of protein tyrosine kinase activity in sperm plasma membranes and in electroeluted 95-kDa protein. The tyrosine kinase activity of the isolated protein is stimulated by solubilized zona pellucida and inhibited by tyrphostin RG-50864, a membrane-permeable tyrosine kinase inhibitor. Furthermore, tyrphostin inhibits zona-triggered acrosomal exocytosis in a dose-dependent manner. These findings indicate that the 95-kDa protein participates in a critical regulatory event of gamete interaction; moreover, our experiments suggest that sperm protein tyrosine kinase may be an excellent target for the control of fertility.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Wassarman P. M. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J Cell Biol. 1986 Apr;102(4):1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980 Jul;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983 Feb;95(2):317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Bunch D. O., Saling P. M. Generation of a mouse sperm membrane fraction with zona receptor activity. Biol Reprod. 1991 Apr;44(4):672–680. doi: 10.1095/biolreprod44.4.672. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Ikari N., Yoshino H., Moses A. C., Flier J. S. Evidence that receptor aggregation may play a role in transmembrane signaling through the insulin-like growth factor-I receptor. Mol Endocrinol. 1988 Sep;2(9):831–837. doi: 10.1210/mend-2-9-831. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leyton L., Robinson A., Saling P. Relationship between the M42 antigen of mouse sperm and the acrosome reaction induced by ZP3. Dev Biol. 1989 Mar;132(1):174–178. doi: 10.1016/0012-1606(89)90215-7. [DOI] [PubMed] [Google Scholar]
- Leyton L., Saling P. 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell. 1989 Jun 30;57(7):1123–1130. doi: 10.1016/0092-8674(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Leyton L., Saling P. Evidence that aggregation of mouse sperm receptors by ZP3 triggers the acrosome reaction. J Cell Biol. 1989 Jun;108(6):2163–2168. doi: 10.1083/jcb.108.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall R. M., Zilberstein A., Gazit A., Gilon C., Levitzki A., Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989 Aug 25;264(24):14503–14509. [PubMed] [Google Scholar]
- O'Brien R. M., Soos M. A., Siddle K. Monoclonal antibodies to the insulin receptor stimulate the intrinsic tyrosine kinase activity by cross-linking receptor molecules. EMBO J. 1987 Dec 20;6(13):4003–4010. doi: 10.1002/j.1460-2075.1987.tb02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H., Akiyama T., Watanabe S., Ito N., Kobori M., Seoda Y. Inhibition of tyrosine protein kinase activity by synthetic isoflavones and flavones. J Antibiot (Tokyo) 1989 Feb;42(2):340–343. doi: 10.7164/antibiotics.42.340. [DOI] [PubMed] [Google Scholar]
- Pawson T. Tyrosine kinases and their interactions with signalling proteins. Curr Opin Genet Dev. 1992 Feb;2(1):4–12. doi: 10.1016/s0959-437x(05)80314-4. [DOI] [PubMed] [Google Scholar]
- Saling P. M. Mammalian sperm interaction with extracellular matrices of the egg. Oxf Rev Reprod Biol. 1989;11:339–388. [PubMed] [Google Scholar]
- Saling P. M., Storey B. T. Mouse gamete interactions during fertilization in vitro. Chlortetracycline as a fluorescent probe for the mouse sperm acrosome reaction. J Cell Biol. 1979 Dec;83(3):544–555. doi: 10.1083/jcb.83.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Ward C. R., Storey B. T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol. 1984 Aug;104(2):287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]