Abstract
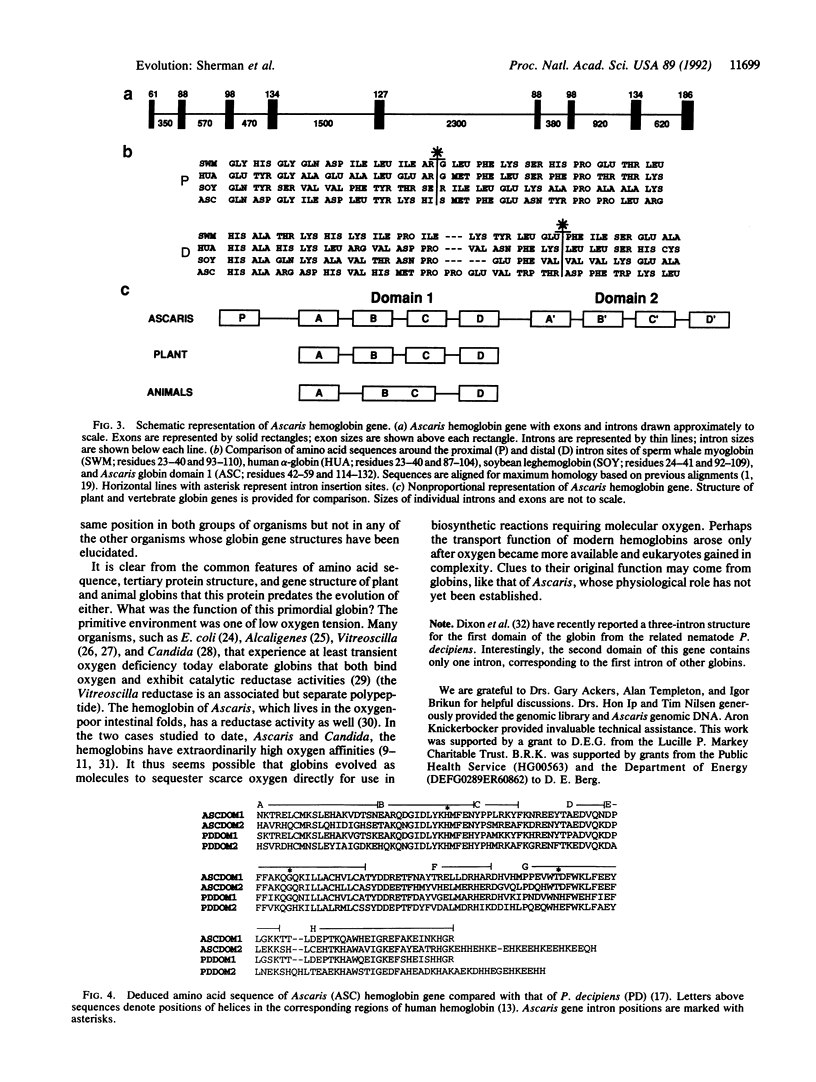
Animal globin genes have two introns at strictly conserved positions, while plant globin genes have both of these as well as an additional, central intron. It has been proposed that a common ancestor gene had three introns, one of which was subsequently lost from animal but not plant globin genes. We have elucidated the cDNA sequence and gene structure of a hemoglobin from the parasitic nematode Ascaris suum and found a plant-like central intron, providing strong evidence for a three-intron ancestor of modern globin genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford D., Chothia C., Lesk A. M. Determinants of a protein fold. Unique features of the globin amino acid sequences. J Mol Biol. 1987 Jul 5;196(1):199–216. doi: 10.1016/0022-2836(87)90521-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown G. G., Lee J. S., Brisson N., Verma D. P. The evolution of a plant globin gene family. J Mol Evol. 1984;21(1):19–32. doi: 10.1007/BF02100624. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Darawshe S., Tsafadyah Y., Daniel E. Quaternary structure of erythrocruorin from the nematode Ascaris suum. Evidence for unsaturated haem-binding sites. Biochem J. 1987 Mar 15;242(3):689–694. doi: 10.1042/bj2420689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baere I., Liu L., Moens L., Van Beeumen J., Gielens C., Richelle J., Trotman C., Finch J., Gerstein M., Perutz M. Polar zipper sequence in the high-affinity hemoglobin of Ascaris suum: amino acid sequence and structural interpretation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4638–4642. doi: 10.1073/pnas.89.10.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikshit K. L., Spaulding D., Braun A., Webster D. A. Oxygen inhibition of globin gene transcription and bacterial haemoglobin synthesis in Vitreoscilla. J Gen Microbiol. 1989 Oct;135(10):2601–2609. doi: 10.1099/00221287-135-10-2601. [DOI] [PubMed] [Google Scholar]
- Dixon B., Walker B., Kimmins W., Pohajdak B. A nematode hemoglobin gene contains an intron previously thought to be unique to plants. J Mol Evol. 1992 Aug;35(2):131–136. doi: 10.1007/BF00183224. [DOI] [PubMed] [Google Scholar]
- Dixon B., Walker B., Kimmins W., Pohajdak B. Isolation and sequencing of a cDNA for an unusual hemoglobin from the parasitic nematode Pseudoterranova decipiens. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5655–5659. doi: 10.1073/pnas.88.13.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Smith M. H. Rates of reaction of Ascaris haemoglobins with ligands. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):206–214. doi: 10.1098/rspb.1965.0067. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Go M. Correlation of DNA exonic regions with protein structural units in haemoglobin. Nature. 1981 May 7;291(5810):90–92. doi: 10.1038/291090a0. [DOI] [PubMed] [Google Scholar]
- Jhiang S. M., Garey J. R., Riggs A. F. Exon-intron organization in genes of earthworm and vertebrate globins. Science. 1988 Apr 15;240(4850):334–336. doi: 10.1126/science.2832953. [DOI] [PubMed] [Google Scholar]
- Naito Y., Riggs C. K., Vandergon T. L., Riggs A. F. Origin of a "bridge" intron in the gene for a two-domain globin. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6672–6676. doi: 10.1073/pnas.88.15.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Wittenberg J. B. The hemoglobin of Ascaris perienteric fluid. 3. Equilibria with oxygen and carbon monoxide. Biochim Biophys Acta. 1965 Dec 16;111(2):503–511. doi: 10.1016/0304-4165(65)90060-7. [DOI] [PubMed] [Google Scholar]
- Oshino R., Asakura T., Takio K., Oshino N., Chance B., Hagihara B. Purification and molecular properties of yeast hemoglobin. Eur J Biochem. 1973 Nov 15;39(2):581–590. doi: 10.1111/j.1432-1033.1973.tb03157.x. [DOI] [PubMed] [Google Scholar]
- Oshino R., Asakura T., Tamura M., Oshino N., Chance B. Yeast hemoglobin-reductase complex. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1055–1060. doi: 10.1016/s0006-291x(72)80079-2. [DOI] [PubMed] [Google Scholar]
- Vanfleteren J. R., Evers E. A., Van de Werken G., Van Beeumen J. J. The primary structure of cytochrome c from the nematode Caenorhabditis elegans. Biochem J. 1990 Nov 1;271(3):613–620. doi: 10.1042/bj2710613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S. G., Armarego W. L., Shaw D. C., Lilley P. E., Dixon N. E., Poole R. K. Isolation and nucleotide sequence of the hmp gene that encodes a haemoglobin-like protein in Escherichia coli K-12. Mol Gen Genet. 1991 Apr;226(1-2):49–58. doi: 10.1007/BF00273586. [DOI] [PubMed] [Google Scholar]
- Viglierchio D. R., Gortz J. H. Hemoglobin derivatives of the zooparasitic nematode Anisakis physeteris and the sperm whale host. Exp Parasitol. 1972 Oct;32(2):211–216. doi: 10.1016/0014-4894(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Matsubara H., Webster D. A. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. 1986 Jul 31-Aug 6Nature. 322(6078):481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- Zhu H., Riggs A. F. Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5015–5019. doi: 10.1073/pnas.89.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]