Abstract
Although several efforts have been made in the search for genetic and epigenetic patterns linked to diseases, a comprehensive explanation of the mechanisms underlying pathological phenotypic plasticity is still far from being clarified. Oxidative stress and inflammation are two of the major triggers of the epigenetic alterations occurring in chronic pathologies, such as neurodegenerative diseases. In fact, over the last decade, remarkable progress has been made to realize that chronic, low-grade inflammation is one of the major risk factor underlying brain aging. Accumulated data strongly suggest that phytochemicals from fruits, vegetables, herbs, and spices may exert relevant immunomodulatory and/or anti-inflammatory activities in the context of brain aging. Starting by the evidence that a common denominator of aging and chronic degenerative diseases is represented by inflammation, and that several dietary phytochemicals are able to potentially interfere with and regulate the normal function of cells, in particular neuronal components, aim of this review is to summarize recent studies on neuroinflammaging processes and proofs indicating that specific phytochemicals may act as positive modulators of neuroinflammatory events. In addition, critical pathways involved in mediating phytochemicals effects on neuroinflammaging were discussed, exploring the real impact of these compounds in preserving brain health before the onset of symptoms leading to inflammatory neurodegeneration and cognitive decline.
Keywords: antioxidants, Nfr2, resveratrol, sirtuins, curcumin
Introduction
In the last decades the increasing aging with consequent raise in chronic degenerative diseases has led to an augmented investigation of the environmental factors involved in their origin and progression.
Although several efforts have been made in the search for genetic and epigenetic patterns linked to diseases, a comprehensive explanation of the mechanisms underlying pathological phenotypic plasticity is still far from being clarified (Babenko et al., 2012). Epigenetic control of the gene expression has been recognized as a key player in producing rapid adaptation to changing environmental conditions both within a single lifespan as well as across multiple generations. These mechanisms are particularly applied to the brain, which is capable of changing readily in response to experience throughout a lifetime (Babenko et al., 2012).
Epigenetics is a branch of biology, which studies how changes in gene expression occur without modifications in the DNA sequence (Choudhuri, 2011). Such changes can be induced by environmental factors, and can be highly stable, including those resulting from genetic imprinting, or dynamic, including those associated with memory (Lardenoije et al., 2015). Oxidative stress and inflammation are two of the major triggers of the epigenetic alterations occurring in chronic pathologies, such as neurodegenerative diseases, especially in elderly population, already characterized by modifications of the normal homeostasis of organs and systems.
Starting by the evidence that a common denominator of aging and chronic degenerative diseases is represented by inflammation, and that several dietary phytochemicals are able to potentially interfere with and regulate the normal function of cells (Scapagnini et al., 2014), in particular neuronal components, aim of this review is to summarize recent studies on the neuroinflammaging processes indicating that specific phytochemicals may act as positive modulators of neuroinflammatory events. In addition, pathways involved in mediating phytochemicals effects on neuroinflammaging were discussed, exploring the real impact of dietary phytochemicals in preserving brain health before the onset of symptoms leading to inflammatory neurodegeneration and cognitive decline.
Features of neuroimmunoaging
Over the last decade, remarkable progress has been made to realize that chronic, low-grade inflammation is one of the major risk factor underlying brain aging. During their life the cells progressively impair the ability to defend themselves from stress stimuli and, as a consequence, there is an accumulation of oxidative damages in all cell constituents (Corbi et al., 2008; Bianco et al., 2013; Lardenoije et al., 2015; Conti et al., 2015, 2016).
Growing evidence suggests that the brain and immune system are intricately connected and crosstalk to maintain homeostasis.
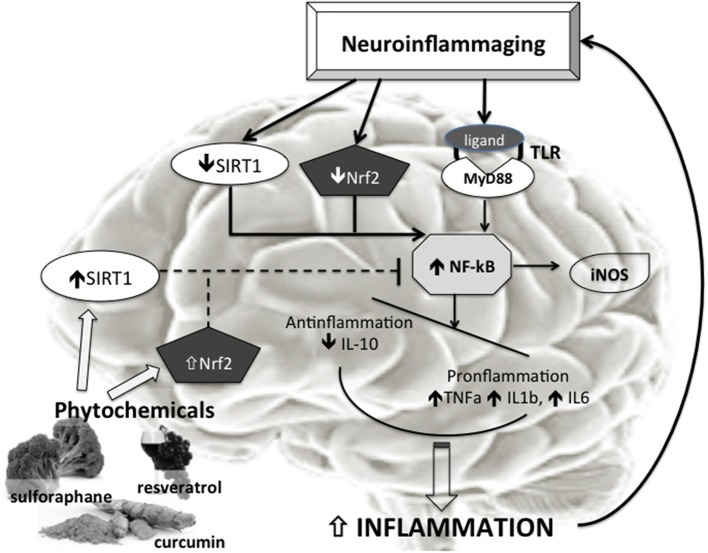
Aging is associated with aberrant inflammatory responses in human brains (Lu et al., 2004; Cribbs et al., 2012). Specifically, basal levels of proinflammatory cytokines are elevated with aging (Sierra et al., 2007), whereas anti-inflammatory mediators are reduced (Ye and Johnson, 1999). In addition, other components involved in innate immune responses, such as the complement (C) pathway, the toll-like receptor (TLR) signaling, and the inflammasome activation, are also upregulated as the brain ages (Cribbs et al., 2012; Cho et al., 2015). In fact, during aging the brain shows an imbalance between pro-and anti-inflammatory cytokine levels (Figure 1).
Figure 1.
Phytochemicals effects on Neurooinflammaging. Neuroimmunoinflammaging is characterized by reduced SIRT1 and Nfr2 activity with consequent increased NF-κB activation. The increased NF-κB activation, also trough Tool Like Receptors (TLR), induces in turn raised proinflammatory factors such as TNFa, IL1b, IL6, iNOS. The disequilibrium between anti- (IL10) and pro-inflammatory molecules determines increased inflammation, and a vicious circle is established that sustains neuroinflammaging. The phytochemicals (like curcumin, resveratrol, sulphurane, etc.) inducing increase in Nrf2 and SIRT1 activity could be able to inhibit the NF-κB activation and then to break the vicious circle ending the progression of the brain aging.
It has been demonstrated both in humans and animal models that aging is associated with decreased levels of interleukin 10 (IL10) (Ye and Johnson, 2001), and increased levels of tumor necrosis factor alpha (TNFa) and IL1b in the nervous system (Lukiw, 2004; Streit et al., 2004), as well as IL6 in plasma (Ye and Johnson, 2001; Godbout and Johnson, 2004). In addition, increased levels odtransforming growth factor b1 (TGFb1) mRNA, a key regulatory cytokine, has been observed in the brain of aged mice and rats (Bye et al., 2001). At the same time, several changes induced by an aged micro-environment, such as increased systemic inflammation, increased permeability of the blood-brain barrier (BBB), and degeneration of neurons and other brain cells, could contribute to the production of Radical Oxygen Species (ROS), thus generating oxidative stress. It has been proposed that BBB permeability increases in aged animals (Blau et al., 2012; Enciu et al., 2013), facilitating perhaps infiltration by monocytes releasing mitochondria-generated ROS. According to this hypothesis, an age-related increase in the number of CD11bC and CD45 cells, compatible with infiltrated monocytes, has been reported in the brain of aged rats (Blau et al., 2012). Likewise, expression levels of chemotactic molecules, such as interferon-inducible protein 10 (IIP10) and monocyte chemotactic protein-1 (MCP-1), are increased in the hippocampal region (Blau et al., 2012; Von Bernhardi et al., 2015).
With normal aging, the immunophenotype of microglia is characterized by up-regulation of glial activation markers including Major Histocompatibility Complex II (MHC II) and CD11b, a finding reported in several species including human post-mortem tissue, rodent, canine, and non-human primates (Tafti et al., 1996; Sheffield and Berman, 1998). This up-regulation of MHCII occurs also at the mRNA level (Frank et al., 2006). Importantly, MHCII is expressed at very low levels on microglia of younger animals under basal conditions (Perry, 1998), providing a clear baseline to detect aging-related changes in microglia immunophenotype. Increased MHCII could result from aging-induced increases in microglia number, or from increases in permicroglial cell expression. Although only few studies are available, they support the idea of increased permicroglial cell expression, and therefore sensitization (Barrientos et al., 2015). Despite these commonalities, the role of the immune system in aging and neurodegenerative disease remains unclear (Lucin and Wyss-Coray, 2009).
Microglia are the resident immune cells of the brain, endowed with numerous receptors capable of detecting physiological disturbances. When neurons are injured as a result of aging or neurodegeneration, microglia become activated via the release of adenosine triphosphate (ATP), neurotransmitters, growth factors or cytokines, ion changes in the local environment, or loss of inhibitor molecules displayed by healthy neurons (Hanisch and Kettenmann, 2007).
The increase in expression of multiple TLRs in the aging brain (Letiembre et al., 2007; Berchtold et al., 2008) may generate a hypersensitive state of glia and neurons and thus magnify potential injury. Stimulation of TLRs induces a signaling cascade, culminating in the activation of nuclear factor κB (NFκB) and subsequent transcriptional activation of numerous proinflammatory genes, encoding cytokines, chemokines, complement proteins, enzymes [such as cyclooxygenase 2 (COX-2) and Inducible nitric oxide synthase (iNOS)], adhesion molecules, and immune receptors (Nguyen et al., 2004). Exactly how neuronal TLRs promote neurodegeneration and the identity of their ligands is currently unclear.
However, aging results in a significant increase in glial activation, complement factors, inflammatory mediators, and brain atrophy (West et al., 1994; Streit et al., 2008). Microarrays of aged human and mouse brains showed that genes related to cellular stress and inflammation increase with age while genes related to synaptic function/transport, growth factors, and trophic support decrease (Lee et al., 2000). These changes suggest that neurons encounter increased challenges with age but receive reduced support. Neurogenesis also decreases with age, possibly as a result of factors secreted by activated microglia (i.e., IL-6). To date, it is unclear the reason of increased inflammation during aging. However, genetic studies suggest an important role of Deoxyribonucleic Acid (DNA), because DNA bases are particularly vulnerable to oxidative stress damage leading to important inflammatory alterations (Bianco et al., 2013). Also unclear is to what extent aging affects the responsiveness of microglia or their potential to contribute to neuronal loss. Despite morphological and phenotypic changes that indicate microglial activation, it has been proposed that microglia may actually become dysfunctional and enter a senescent state with age (Streit et al., 2008). Such a state may cause microglia to secrete diminished levels of neurotrophic factors and downregulate phagocytic function. This phenomenon, associated with increased secretion of inflammatory mediators, may lead to neuronal loss and inefficient clearance of toxic protein aggregates in neurodegenerative disease (Lucin and Wyss-Coray, 2009). Finally, it should be also highlighted that mounting evidence indicates that epigenetic mechanisms play a significant role in shaping environmental influences on brain and behavior (Kosik et al., 2012).
Nrf2 and sirtuins pathway involved in neuroimmunoaging
Although definitive mechanisms are still to be elucidated, the pro-inflammatory phenotype of senescent cells, coupled with the up-regulation of the inflammatory response with increasing age, has been found to play a role in the initiation and progression of age-related diseases such as Alzheimer's disease (Cevenini et al., 2013; Patel et al., 2015).
A large body of evidence has highlighted a role of class III histone deacetylases, named sirtuins, in neurodegenerative processes (Kim et al., 2007; Vang et al., 2011; Baur et al., 2012). Sirtuin 1 (SIRT1), the main characterized molecule of sirtuins family, regulates immune responses via NF-κB signaling and in this way also controls the ROS production (Salminen et al., 2013).
The NF-κB signaling is a crucial pathway of immune defense system and an inducer of inflammatory responses (Vallabhapurapu and Karin, 2009). The NF-κB system is involved in many housekeeping and survival functions during cellular stress e.g., by controlling apoptosis, proliferation, and energy metabolism (Karin and Lin, 2002; Perkins, 2007; Johnson and Perkins, 2012). Both SIRT1 and oxidative stress are known to be able to regulate NF-κB signaling and are crucially involved in the maintenance of cellular homeostasis (Yeung et al., 2004; Morgan and Liu, 2011). Moreover, several studies demonstrated that NF-κB signaling is activated during aging (Helenius et al., 2001; Csiszar et al., 2008).
The crosstalk between oxidative stress and inflammation is a complex process and there are studies reporting that ROS can stimulate inflammation via the activation of inflammasomes and the production of IL-1β and IL-18 cytokines, which subsequently trigger inflammatory responses (Kitazawa et al., 2005; Heneka and O'Banion, 2007; Salminen et al., 2013). Many studies have also demonstrated that SIRT1 is a potent intracellular inhibitor of oxidative stress and inflammatory responses (Rajendran et al., 2011; Salminen et al., 2011). In particular, SIRT1 is a powerful inhibitor of NF-κB signaling and thus it suppresses inflammation (Yeung et al., 2004; Salminen et al., 2008a). Many downstream targets of SIRT1 also repress inflammatory responses, e.g., AMP-activated protein kinase (AMPK) (Salminen et al., 2011) and Forkhead box O (FoxO) factors (Lin et al., 2004), by inhibiting the NF-κB signaling. Yeung et al. (2004) revealed that SIRT1 performs its antinflammatory activity by deacetylating the Lys310 residue of v-rel avian reticuloendotheliosis viral oncogene homolog A/p65 (RelA/p65) component, thus inhibiting the transactivation capacity of the NF-κB complex. Recently Cho et al. (2015) showed that SIRT1 levels in microglia exhibit an age-dependent decline, and microglial SIRT1 deficiency leads to cognitive decline in normal aging. The authors suggested that aging-induced SIRT1 deficiency in microglia could initiate epigenetic alterations on IL-1beta, leading to its enhanced expression that is associated with impairments in memory and related cognitive decline.
Moreover, there is a growing body of evidence suggesting that activation of SIRT1 and other sirtuins can protect neurons in experimental models of neurodegenerative disorders (Duan, 2013). In particular Min et al. (2010) demonstrated that tau protein, that stabilizes microtubules, is acetylated and tau acetylation prevents degradation of phosphorylated tau (p-tau). Hyperphosphorylation of the tau protein can result in the self-assembly of tangles of paired helical filaments and straight filaments, which are involved in the pathogenesis of Alzheimer's disease, and other tauopathies (Alonso et al., 2001). Deleting SIRT1 enhanced levels of acetylated-tau and pathogenic forms of p-tau, probably by blocking proteasome-mediated degradation. These results indicate that SIRT1 can prevent the formation of neurofibrillary tangles (Min et al., 2010; Lee et al., 2014). In primary cortical cultures, overexpression of SIRT1 in microglia protected against amyloid beta toxicity, most likely by inhibiting NF-κB signaling (Chen et al., 2005). SIRT1 could also protect against cellular senescence by inactivating NF-κB (Rovillain et al., 2011; Tilstra et al., 2012) or deacetylating the FOXO3 transcription factor (Yao et al., 2012). In addition, SIRT1 could also enhance the T helper 2 (Th2) lymphocytes responses in dendritic cells (Legutko et al., 2011).
In the Wallerian degeneration slow (Wlds) mouse model, SIRT1 activation protects axons against neuronal injury (Dali-Youcef et al., 2007). Decreasing SIRT1 activity reduces the axonal protection originally observed, whereas SIRT1 activation by resveratrol decreases the axonal degeneration after neuronal injury (Suzuki and Koike, 2007). This suggests that the neuroprotection in the Wild mouse model is achieved by increasing the neuronal nicotinamide adenine dinucleotide (NAD+) reserve and/or SIRT1 activity (Dali-Youcef et al., 2007). Also the inhibition of sirtuin 2 (SIRT2) rescued a-synuclein toxicity and modified inclusion morphology in a cellular model of Parkinson's disease and genetic inhibition of SIRT2 via small interfering RNA similarly rescued a-synuclein toxicity (Outeiro et al., 2007). Furthermore, the inhibitors protected against dopaminergic cell death both in vitro and in a Drosophila model of Parkinson's disease, suggesting another link between neurodegeneration and aging (Outeiro et al., 2007). In addition, SIRT1 activation significantly decreases neuronal cell death induced by amyloid-beta peptides through inhibition of NF-κB signaling (Dali-Youcef et al., 2007). In particular SIRT1 deacetylates retinoic acid receptor beta (RARb) and activates a disintegrin and metalloprotease domain (ADAM) 10 transcription, leading to upregulated Amyloid Precursor Protein (APP) processing by a-secretase, resulting in reduced production of Amyloid beta (Aβ) peptide (Donmez et al., 2010). Thanks to these evidences, SIRT1, as well as the other sirtuins, is now considered a promising therapeutic option for neurological syndromes, such as Alzheimer, Parkinson, and Huntington's disease (Donmez et al., 2010; Jeong et al., 2012), and in general for the control and progression of the neuroimmunoaging.
Another key molecule involved in neuroimmunoaging is represented by Nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Emerging evidence suggests that Nrf2 may play an important role in the regulation of brain inflammation, and some studies have suggested that Nrf2 has an antagonistic effect with the NF-κB pathway, which is considered a hallmark of inflammation (Liu et al., 2008; Djordjevic et al., 2015). Nrf2 is a member of the Cap‘n’Collar family of transcription factors that bind to nuclear factor erythroid derived 2 (NF-E2) binding sites (GCTGAGTCA) that are essential for the regulation of erythroid specific genes. Nrf2 is expressed in a wide range of tissues, many of which are sites of expression for phase 2 detoxification genes (Dinkova-Kostova et al., 2002) and targeted for ubiquitination and proteasomal degradation via binding to a cytosolic repressor protein, Kelch-like ECH associated protein 1 (Keap1) (McMahon et al., 2006). The principle of the Nrf2 system is to keep Nrf2 protein low under normal conditions with the possibility of rapid induction in case of a sudden increase in oxidation status in the cell. This is achieved by constitutive synthesis and degradation of Nrf2 with the possibility of rapid redirection of Nrf2 to the nucleus. (Sandberg et al., 2014). There is now overwhelming amount of experimental evidence that Nrf2 serves as a master regulator of the antioxidants involved in cellular defenses against various electrophiles and oxidants (Kobayashi and Yamamoto, 2006; Calabrese et al., 2008). Indeed new findings connect Nrf2 also to expression of other types of protective proteins such as brain derived neurotrophic factor (BDNF) (Sakata et al., 2012), the anti-apoptotic B-cell lymphoma 2 (BCL-2) (Niture and Jaiswal, 2012), the anti-inflammatory interleukin (IL)-10, the mitochondrial transcription (co)-factors NRF-1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Piantadosi et al., 2011).
The relation between Nrf2 and NF-κB is not well characterized but the identification of NF-κB binding sites in the promoter region of the Nrf2 gene suggests cross talk between these two regulators of inflammatory processes (Nair et al., 2008). The NF-κB subunit p65 has been shown to function as a negative regulator of Nrf2 activation either by depriving cAMP response element-binding protein (CBP) from NRF2 or by recruitment of histone deacetylase 3 (HDAC3), causing local histone hypoacetylation and down-regulation of Nrf2-antioxidant responsive element (Nrf2-ARE) signaling (Liu et al., 2008). Yu et al. (2011) have shown that p65 decreased Nrf2 binding to its cognate DNA sequences and enhanced Nrf2 ubiquitination, then providing direct evidence that the interaction of nuclear factor p65 with Keap1 is critical for NF-κB repressing Nrf2-ARE pathway. Moreover, the N-terminal region of p65 was necessary for both the interaction with Keap1 and its transcriptional suppression activity, and nuclear translocation of Keap1 was augmented by p65. The authors concluded that taken together, these findings suggest that NF-κB signaling inhibits Nrf2-ARE pathway through the interaction of p65 and Keap1 (Yu et al., 2011). Further, activation of NRF2 in response to lipopolysaccharide (LPS) has been suggested to be dependent on the key innate immunity-regulating adaptor protein Myeloid differentiation primary response gene 88 (MyD88) (Kim et al., 2011). In particular Kim et al. (2011) demonstrated that treatment of macrophages with LPS activates Nrf2. Interestingly, the authors found that Nrf2 is activated in a MyD88 dependent fashion without the involvement of ROS. These results suggest the possibility that Nrf2 activated by inflammatory stimuli can be a mechanism that contributes to decreasing excessive inflammatory response (Kim et al., 2011). A study demonstrated that Nrf2 knockout mice were hypersensitive to the neuroinflammation induced by LPS, indicative of an increase in microglial cells, and in the inflammation markers iNOS, IL-6, and TNF-α, compared with the hippocampi of wild-type littermates (Innamorato et al., 2008). Activation of NRF2 could also be achieved via the increase in peripheral IGF-1 that enters the brain after exercise (Cotman et al., 2007; Sandberg et al., 2014).
Recently Rojo et al. (2010) showed that Nrf2-deficient mice exhibited more astrogliosis and microgliosis. Inflammation markers characteristic of classical microglial activation, COX-2, iNOS, IL-6, and TNF-α were also increased and, at the same time, anti-inflammatory markers attributable to alternative microglial activation, such as FIZZ-1 and IL-4 were decreased. These results were confirmed in microglial cultures, further demonstrating a role of Nrf2 in tuning balance between classical and alternative microglial activation (Rojo et al., 2010).
Aging drives a long-lasting sub-ventricular zone impairment at least in part via reduced Nrf2-mediated tolerance to inflammation and oxidative stress associated with dysfunctional astrocyte–microglial dialogue, in turn interrupting key molecular signaling mechanisms finely regulating sub-ventricular cell homeostasis (L'Episcopo et al., 2013). In particular, when “primed” microglia of aged mice become hyperactivated upon a second hit, the generation of highly toxic mediators in the face of impaired antioxidant self-protective neuroprogenitor cell response dramatically inhibits neurogenesis, suggesting that glial age is of critical importance in directing promotion vs. inhibition of neurogenesis. Interestingly, with age, the exaggerated microglial activation can impair an astrocyte's ability to express critical antioxidant, anti-inflammatory, and neurogenic factors, thereby resulting in an overall reduction of glial proneurogenic capacities (L'Episcopo et al., 2013). These processes may disrupt the cross talk between two pivotal pathways in sub-ventricular zone, the NrF2/ Fosfoinositide 3-chinasi/Protein kinase B (Nrf2/PI3-K/Akt) and the Drosophila melanogaster wingless gene/receptor Frizzled/β-catenin (Wnt/Fzd/β-catenin) signaling cascades, involved in cell survival, proliferation, and/or differentiation. The manipulation of these age-related Nrf2 pathways at middle age is associated with significant Dopaminergic neuroprotection (L'Episcopo et al., 2013). A study also demonstrated that direct intrahippocampal gene delivery of Nrf2, by a lentiviral vector, results in a reduction in spatial learning deficits in aged mice (Kanninen et al., 2009). In particular memory improvement in the mice after Nrf2 transduction shifts the balance between soluble and insoluble Aβ toward an insoluble Aβ pool without concomitant change in total brain Aβ burden. Nrf2 gene transfer was associated with reduction in astrocytic but not microglial activation and induction of Nrf2 target gene Heme Oxygenase 1 (HO-1), indicating overall activation of the Nrf2-ARE pathway in hippocampal neurons 6 months after injection (Kanninen et al., 2009). Based on this body of emerging evidence it seems that in many cases the beneficial effects of low doses of phytochemicals rely on their ability to activate the Nrf2/ARE and sirtuins pathways.
Mechanisms of dietary phytochemicals in neuroimmunoaging
Dietary phytochemicals include a large group of no-nutrients compounds from a wide range of plant-derived foods and chemical classes. Several plant-based extracts and chemicals are supposed to have beneficial effects on human brain function. The potential effect of these molecules is linked to the common ancestry, which has provided some phytochemicals of conserved cellular processes, including the similarities in most pathways for synthesis and breakdown of proteins, nucleic acids, carbohydrates, and lipids (Kennedy and Wightman, 2011). In fact, some molecules that function as neurochemicals within the mammalian central nervous system (CNS) are ubiquitous across all eukaryotes (Kawashima et al., 2007).
At a molecular level, signaling molecules and pathways are preserved in both plants and animals (Kushiro et al., 2003). For instance, multiple aspects of cellular and redox signaling are conserved (Dalle-Donne et al., 2009), including similar gene expression in response to cellular stressors, which are regulated by common transcription factors (Scandalios, 2005).
The basis for the use of polyphenol-rich nutritional supplements as a modulator of age-related cognitive decline is the age-related increase in oxidative stress (Morris et al., 2006; Craft et al., 2012) and low-grade inflammation.
Often the beneficial effects of phytochemicals are supposed to be due to their intrinsic antioxidant and antinflammatory properties (Murugaiyah and Mattson, 2015).
At low doses, phytochemicals have beneficial or stimulatory effects on animal cells, whereas in high amounts can be toxic. This is an example of “hormesis” (Mattson, 2008; Lee et al., 2014). Hormetic phytochemicals such as resveratrol, sulforaphane, curcumin, catechins, allicin, and hypericin are reported to activate adaptive stress response signaling pathways that increase cellular resistance to injury and disease (Mattson and Cheng, 2006).
Also neuroactive phytochemicals present in commonly consumed fruits, vegetables, and nuts are generally well tolerated (Wöll et al., 2013; Lee et al., 2014). These phytochemicals are hormetic substances because they can be toxic in high amounts, but are beneficial in the lower amounts usually consumed (Mattson, 2015). In this context, although controversial data are available on the capacity of these compounds to cross the BBB and bioavailability continues to be highlighted as a major concern, hormetic dose-response model has important biological and clinical implications, including activation of neuroprotective stress response pathways at low concentrations (Schaffer and Halliwell, 2012; Davinelli et al., 2016).
Their effects are represented as a biphasic dose–response curve, with the first phase being a positive/beneficial effect and the second phase with a progressively negative/toxic effect (Calabrese et al., 2007; Mattson et al., 2007).
Recent findings suggest that adaptive cellular stress responses to phytochemicals are mediated via some of the same pathways that mediate responses to energy restriction and exercise (Mattson, 2012; Milisav et al., 2012). Commonly consumed phytochemicals are able to induce mild stress in neural cells, enhancing the ability of nervous system to cope with stress, and then promoting optimal function and longevity of the nervous system. As with exercise and energy restriction, intake of neurohormetic phytochemicals typically occurs on intermittent basis, which provides a “recovery period” that allows cells to repair and growth (Mattson, 2015). Examples include pathways that signal via Nrf2, SIRT1, and AMPK (Menendez et al., 2013; Misra et al., 2013). Activation of one or more of these signaling pathways that evolved to defend cells against potentially toxic phytochemicals appears to be a major reason why ingestion of these substances can protect neurons against injury and disease (Calabrese et al., 2008; Murugaiyah and Mattson, 2015).
Some phytochemicals potentially useful in neuroprotection
Several studies indicate that antioxidants, e.g., dietary polyphenols, can inhibit inflammation, and in particular the terpenoids, are able to inhibit NF-κB signaling and thus repress inflammation (Rahman et al., 2006; Salminen et al., 2008b).
In this context, there are many mutual interactions and a delicate balance exists between SIRT1 and ROS signaling which provoke context-dependent responses to autophagic flux and inflammation (Salminen et al., 2013).
Phytochemicals like curcumin, resveratrol, terpenoids, epigallocatechin-3-gallate (EGCG), and isothiocyanates share common properties and play an important role to activate the phase II detoxifying and antioxidant enzymes like HO-1, glutathione peroxidase (GSH-Px), and glutathione-S-transferase (GST) by targeting the common transcription factor Nrf2 (Liby et al., 2007; Tosetti et al., 2009).
Recent findings suggest that several phytochemicals exhibit biphasic dose responses on cells with low doses activating signaling pathways that result in increased expression of genes encoding survival proteins, as in the case of the Keap1/Nrf2/ARE pathway activated by curcumin and NAD/NADH-sirtuin-1 activated by resveratrol.
To underline the role of the dietetic components in modifying cellular mechanisms, recently Morrison et al. (2010) showed as in 20-month old male mice fed either ‘western diet’ (41% fat), very high fat lard diet (60% fat), or corresponding control diets for 16 weeks, only the high fat lard diet increased age-related oxidative damage and impaired retention in the behavioral test. This selective increase in oxidative damage and cognitive decline was also associated with a decline in Nrf2 levels and activity, suggesting a potential role for decreased antioxidant response. Then the authors suggested that impaired Nrf2 signaling and increased cerebral oxidative stress as mechanisms underlying High Fat Diet-induced declines in cognitive performance in the aged brain (Morrison et al., 2010).
Ferulic acid
Ferulic acid (FA) is commonly found in fruits and vegetables such as tomatoes, sweet corn, and rice (Srinivasan et al., 2007). It has been reported that this compound decreases the levels of inflammatory mediators (prostaglandin E2 and TNF-α) (Ou et al., 2003), and iNOS expression and function (Tetsuka et al., 1996). In vivo, long-term administration of FA effectively protects against Aβ toxicity by inhibiting microglial activation (Kim et al., 2004).
Kanaski et al. (2002) reported that FA protects against free radical mediated changes in the conformation of synaptosomal membrane proteins. The long-term administration of FA at a dose of 300 μM effectively protects against Aβ toxicity by inhibiting microglial activation in vivo (Kim et al., 2004). Moreover, Sultana et al. (2005) showed that also at lower doses of 10–50 μM significantly protects against Aβ toxicity by modulating oxidative stress directly and by inducing protecting genes in hippocampal cultures, also exerting neuroprotective effects by up-regulation of protective enzymes, such as Hemeoxygenase-1 (HO-1) and heat shock protein 70 (Hsp70) (Scapagnini et al., 2004; Srinivasan et al., 2007) then suggesting a control by Nrf2 and sirtuins on the FA effects. More recently Mori et al. (2013) demonstrated that also orally administration of FA for 6 months improved behavioral impairment, mitigated cerebral amyloidosis, and inhibited APP metabolism by reducing β-site APP cleaving enzyme 1 (BACE1) expression and β-secretase activity in an accelerated mouse model of cerebral amyloidosis. Supporting results from cultured mutant human APP-overexpressing murine neuron-like cells revealed FA dose-dependent reduction of various Aβ species and inhibition of β-secretase cleavage. FA also ameliorated neuroinflammation in including β-amyloid plaque-associated gliosis and expression of the proinflammatory cytokines, TNF-α, and IL-1β. Lastly, mRNA expression of three oxidative stress markers [superoxide dismutase 1 (SOD1), catalase (CAT), and GSH-Px 1] was decreased in FA-treated mice, providing support for long-term FA dietary supplementation as a therapeutic strategy (Mori et al., 2013).
Green tea
The consumption of green tea has recently attracted much attention in the occidental culture because of its beneficial effects, such as protection of dopaminergic neurons from damage induced by 6-hydroxydopamine in a rat model of Parkinson's disease (Guo et al., 2007); reduction of mutant huntingtin misfolding and neurotoxicity in Huntington's disease models (Ehrnhoefer et al., 2006); direct protection of neurons against Aβ toxicity (Bastianetto et al., 2000); protection against Aβ-induced cognitive impairment in a rat model relevant to Alzheimer's disease (Haque et al., 2008). Moreover, a study by Wu et al. (2006) reports that one of its component, epigallocatechin gallate (EGCG), up-regulates HO-1 expression by activation of the Nrf2-ARE pathway in endothelial cell, conferring resistance against Hydrogen peroxide (H2O2) induced cell death, suggesting a hormetic mechanism of action (Wu et al., 2006).
It has been demonstrated that EGCG selectively protects cultured rat cerebellar granule neurons from oxidative stress (Schroeder et al., 2009).
More recently Obregon et al. have confirmed that oral administration of EGCG promotes cleavage of APP into α-CTF and soluble APP-α (Obregon et al., 2006). These cleavage events are associated with elevated α-secretase cleavage activity and are also positively correlated with activation of ADAM10, a key candidate α-secretase (Rezai-Zadeh et al., 2008), then suggesting a role of sirtuins in mediating EGCG effects (Donmez et al., 2010; Jeong et al., 2012).
Blueberry and strawberry
Evidences showed that plant extracts, from mulberry, strawberry, and blueberry, contain antioxidants, which are able to induce the antioxidant defense system and improve memory deterioration in aging animals (Shih et al., 2010).
Supplementation of the diet of 19 month-old rats with strawberry, blueberry or spinach extracts for 8 weeks resulted in the reversal of age-related deficits in several neuronal and behavioral parameters (Joseph et al., 1999). Blueberry supplementation prevented learning and memory deficits in a mouse model of Alzheimer's disease (Joseph et al., 2003). In addition, dietary supplementation with blueberry extract increased the survival of dopamine-producing neurons in a model relevant to Parkinson's disease therapy (McGuire et al., 2006). Moreover, blueberries and strawberries counter the deleterious effects of irradiation by reducing oxidative stress and inflammation, thereby improving neuronal signaling, preventing the accumulation of disease-related proteins such as tau in the hippocampus of irradiated rats (Poulose et al., 2014).
Indeed Andres-Lacueva et al. (2005) examined whether different classes of polyphenols could be found in brain areas associated with cognitive performance following blueberry (BB) supplementation. Thus, 19 months old F344 rats were fed a control or 2% BB diet for 8–10 weeks and tested in the Morris Water Maze (MWM), a measure of spatial learning and memory. Several anthocyanins were found in the cerebellum, cortex, hippocampus, or striatum of the BB supplemented rats, but not the controls. Correlational analyses revealed a relationship between MWM performance in BB rats and the total number of anthocyanin compounds found in the cortex, suggesting that these compounds may deliver their antioxidant and signaling modifying capabilities centrally.
To support and clarify the antioxidants effects of this compound recently Çoban et al. (2015) investigated the consequence of whole fresh BB treatment at different percentages on oxidative stress in age-related brain damage model. The study showed that BB treatments, especially BB at higher percentage reduced malondialdehyde and Protein C levels and acetylcholinesterase activity and elevated glutathione (GSH) levels and GSH-Px activity, diminishing apoptosis and ameliorating histopathological findings in the brain of rats treated with D-galactose (GAL). The authors concluded that BB partially prevented the shift toward an imbalanced pro-oxidative status and apoptosis together with histopathological amelioration by acting as an antioxidant (radical scavenger) itself in GAL-treated rats (Çoban et al., 2015).
It has been reported that long-term treatment with blueberry has also a neuroprotective effect in attenuating cerebral ischemia/reperfusion (I/R) injury. Zhou et al. (2015) showed that 24 h after I/R, pterostilbene (a major component of blueberry) dose-dependently improved neurological function, reduced brain infarct volume, and alleviated brain oedema. The most effective dose was 10 mg/kg; the therapeutic time window was within 1 h after I/R and treatment immediately after reperfusion showed the best protective effect. The protective effect was further confirmed by the results that post-ischemic treatment with pterostilbene (10 mg/kg) significantly improved motor function, alleviated BBB disruption, increased neurons survival and reduced cell apoptosis in cortical penumbra after cerebral I/R. The authors also found that pterostilbene (10 mg/kg) significantly reversed the increased content of malondialdehyde and the decreased activity of superoxide dismutase in the ipsilateral hemisphere, with decrease of the oxidative stress markers 4-hydroxynonenal and 8-hydroxyguanosine positive cells in the cortical penumbra. Then pterostilbene dose- and time-dependently exerts a neuroprotective effect against acute cerebral I/R injury (Zhou et al., 2015) and its antioxidant action is mediated by the increased expression of Nrf2 (Saw et al., 2014).
Curcumin
Curcumin, the principal curcuminoid and the most active component in turmeric, is a biologically active phytochemical.
Several beneficial effects of curcumin for the nervous system have been reported. In an animal model of stroke curcumin treatment protected neurons against ischemic cell death and ameliorated behavioral deficits (Wang et al., 2005). A hormetic mechanism of action of curcumin is suggested from studies showing that levels of expression of the stress response protein HO-1 were increased in cultured hippocampal neurons treated with curcumin (Scapagnini et al., 2006). Moreover, curcumin has been shown to reverse chronic stress-induced impairment of hippocampal neurogenesis and increase expression of BDNF in an animal model of depression (Xu et al., 2007). At non-toxic concentrations, curcumin induces HO-1 expression by activating the Nrf2/ARE pathway both in vitro (Pae et al., 2007) and in vivo (Farombi et al., 2008).
Several studies also showed that curcumin interacts with NF-κB, and through this interaction exerts protective function also in the regulation of T-cell-mediated immunity (Kou et al., 2013). Recently González-Reyes et al. (2013) identified curcumin as a neuroprotector against hemin-induced damage in primary cultures of cerebellar granule neurons of rats. Hemin, the oxidized form of heme, is a highly reactive compound that induces cellular injury. Pre-treatment of the neurons with 5–30 μM curcumin increased by 2.3–4.9-fold HO-1 expression and by 5.6–14.3-fold GSH levels. Moreover, 15 μM curcumin attenuated by 55% the increase in ROS production, by 94% the reduction of GSH/glutathione disulfide ratio, and by 49% the cell death induced by hemin. Furthermore, it was found that curcumin was capable of Nrf2 translocation into the nucleus, suggesting that the pre-treatment with curcumin induces Nrf2 and an antioxidant response that may play an important role in the protective effect of this antioxidant against hemin-induced neuronal death (González-Reyes et al., 2013).
In rodents and human cells, curcumin-induced HO-1 overexpression was correlated with production of mitochondrial ROS, activation of transcription factors Nrf2 and NF-κB, induction of Mitogen-activated protein kinase (MAPK) p38 and inhibition of phosphatase activity (Andreadi et al., 2006; McNally et al., 2007). Moreover, curcumin is an activator of Nrf2 (Moi et al., 1994) by changing specific highly reactive cysteine residues of Keap1 (Dinkova-Kostova et al., 2002, 2005), with consequent lost ability of Keap1 to target Nrf2 for degradation, which then undergoes nuclear translocation. By using an Alzheimer transgenic mouse model (Tg2576), Lim et al. (2001) shown that dietary curcumin in vitro, inhibited aggregation as well as disaggregated fibrillary Amyloid beta (Aβ). In vivo studies showed that curcumin injected peripherally into aged mice crossed the BBB and directly bound small β-amyloid species to block aggregation and fibril formation in vitro and in vivo. These data suggest that low dose curcumin effectively disaggregates Aβ as well as prevents fibril and oligomer formation, supporting the rationale for curcumin use in clinical trials (Lim et al., 2001).
More recently, Garcia-Alloza et al. (2007) in transgenic APPswe/PS1dE9 mice demonstrated that curcumin, given intravenously for 7 days, crosses the BBB, binds to β-amyloid deposits in the brain and accelerates their rate of clearance (Garcia-Alloza et al., 2007).
Curcumin was also demonstrated to exert a neuroprotective effect in rats who underwent ischemia/reperfusion injury and this effect has been related to the direct scavenger effect of curcumin as well as to a curcumin-induced interference with the apoptotic machinery, increase in antioxidant molecules (GSH) and enzymes such as CAT and SOD (Al-Omar et al., 2006; Calabrese et al., 2008).
Sulforaphane
Sulforaphane (SFN), a phytochemical present in high amounts in cruciferous vegetables such as broccoli, is known to activate the Nrf2-ARE stress response pathway in rodent brains and microvasculature and by this to reduce brain damages in a traumatic brain injury model (Zhao et al., 2007). Sulforaphane has been reported to protect cultured neurons against oxidative stress (Kraft et al., 2004), and dopaminergic neurons against mitochondrial toxins (Han et al., 2007; Son et al., 2008). This compound administration initiated at 1 h post-cortical impact injury has been shown to improve cognitive function, in particular spatial learning and memory, and to reduce working memory dysfunction (Dash et al., 2009).
In a model of neonatal hypoxia-ischemia, pretreatment with SFN increased the expression of Nrf2 and HO-1 in the mouse brain and reduced infarct ratio (Ping et al., 2010). Numerous other non-nutrients contained in food and plants have been ascribed to the list of Nrf2 activators, and among these several food-contained antioxidant polyphenols. One of the most important aspects of current polyphenol research is the focus on the neuroprotective capacity endowed by these molecules that seems to be due mostly to their ability to activate different defensive molecular pathways, instead to involve just their intrinsic antioxidant properties (Scapagnini et al., 2011).
In this regard, it has been recently demonstrated the critical role of Nrf2/HO-1 activation by some of these neuroprotective compounds, providing insight into the possible therapeutic significance of a closely related group of polyphenols against neurodegenerative disorders and cognitive decline (Scapagnini et al., 2011).
Resveratrol
Neuroprotective effects of resveratrol have been reported by several different studies, in particular on beta-amyloid-induced oxidative cell death (Jang and Surh, 2003) and against several different insults on dopaminergic neurons of midbrain slice cultures (Okawara et al., 2007).
In particular in cultured rat pheochromocytoma (PC12) cells Resveratrol attenuated Aβ-induced cytotoxicity, apoptotic features, and intracellular ROS accumulation. Moreover, Aβ transiently induced activation of NF-κB was suppressed by resveratrol pretreatment (Jang and Surh, 2003) suggesting a key role of the NF-κB inflammatory pathway in the Aβ deposition and a possible therapeutic function of resveratrol in mediating neuroprotection.
Resveratrol protects cortical neurons from oxidative stress-induced injury (Zhuang et al., 2003), and suppress alcohol-induced cognitive deficits and neuronal apoptosis (Tiwari and Chopra, 2013). In addition, resveratrol has been found to reduce the production of IL-1 beta and TNF-alpha induced by LPS or Aβ in the microglia (Capiralla et al., 2012; Zhong et al., 2012). Further studies showed that the powerful neuroprotective effect of resveratrol has also been confirmed in neurodegenerative disorders, such as Parkinson's disease, Alzheimer's disease (Albani et al., 2009), and in traumatic brain injury (Ates et al., 2007; Zhang et al., 2015).
Prozorovski et al. (2008) found that the treatment of neural progenitor cells (NPCs) with resveratrol mimicked oxidizing conditions and increased differentiation of NPCs toward astrocytes through a mechanism that requires Sirt1 (Prozorovski et al., 2008). Indeed subtle alterations of the redox state, found in different brain pathologies, regulate the fate of mouse NPCs through SIRT1. Mild oxidation or direct activation of SIRT1 suppressed proliferation of NPCs and directed their differentiation toward the astroglial lineage at the expense of the neuronal lineage, whereas reducing conditions had the opposite effect. Under oxidative conditions in vitro and in vivo, Sirt1 was upregulated in NPCs, bound to the transcription factor Hes1 and subsequently inhibited pro-neuronal Mash1. In response to brain injury, NPCs differentiate preferentially into astrocytes rather than neurons. Excessive astrocyte expansion, known as astrogliosis, can prevent growth of neurons and interfere with proper damage repair. Therefore, the ability to direct differentiation of NPCs may be useful in protecting the brain against inflammatory diseases, such as multiple sclerosis, which involve astrogliosis (Prozorovski et al., 2008).
Indeed resveratrol was shown to affect the activity of SIRT1 in vitro on depending to the nature of the substrate for deacetylation (Baur and Sinclair, 2006). It has been reported that the SIRT1 agonist resveratrol protects C. elegans neurons expressing a fragment of the Huntington disease-associated protein huntingtin and mammalian neurons from mutant polyglutamine cytotoxicity in a HdhQ111 knock-in mouse model of Huntington disease (Dali-Youcef et al., 2007).
Moreover, resveratrol had no effect on the binding of NF-κB proteins to the DNA, but it blocked the TNF-induced translocation of p65 subunit of NF-κB and reporter gene transcription. Similarly, the activation of c-Jun N-terminal kinases (JNK) and its upstream MAPK are inhibited by resveratrol, which may explain the mechanism of suppression of AP-1 by resveratrol (Rahman et al., 2006).
Recently Zhang et al. (2015) investigated the potential role of resveratrol in attenuating hypoxia-induced neurotoxicity via its anti-inflammatory actions through in vitro models of the BV-2 microglial cell line and primary microglia. The authors found that resveratrol significantly inhibited hypoxia-induced microglial activation and reduced subsequent release of pro-inflammatory factors. In addition, resveratrol inhibited the hypoxia-induced degradation of I kappa B-alpha (IκB-alpha) and phosphorylation of p65 NF-κB protein. Importantly, treating primary cortical neurons with conditioned medium (CM) from hypoxia-stimulated microglia induced neuronal apoptosis, which was reversed by CM co-treated with resveratrol. Taken together, the results of this study suggest that resveratrol exerts neuroprotection against hypoxia-induced neurotoxicity through its anti-inflammatory effects in microglia. These effects were mediated, at least in part, by suppressing the activation of NF-κB, extracellular-signal-regulated kinases (ERK), and JNK/MAPK signaling pathways (Zhang et al., 2015).
Although several studies reported an efficacy of these compounds in animal model and in vitro, few plant-based products have been assessed in methodologically adequate human trials (Kennedy and Wightman, 2011), and clinical experiments have often failed to demonstrate any convincing therapeutic potency of these compounds (Berger et al., 2012).
Dietary phytochemicals on cognitive performance in human studies
Accumulated data strongly suggest that phytochemicals from fruits, vegetables, herbs, and spices may exert relevant immunomodulatory and/or anti-inflammatory activities in the context of brain aging. The benefits of these substances for the cognitive health of older adults have been reported in several studies (Davinelli et al., 2015). In a recent review Shukitt-Hale (2012) highlighted the potential benefits of blueberries as a compound to impact age-related changes in neuronal aging. Additionally, Devore et al. (2012) shown that greater self-reported intakes of blueberries and strawberries were associated with slower rates of cognitive decline. Although several evidences point toward the beneficial effects of these substances, limitations of these researches include the use of correlational data as well as the lack of assessment of the bioavailability of these polyphenolic compounds from diets (Rowland et al., 2000).
Commenges et al. (2000) demonstrated that the intake of flavonoids in 1367 subjects over 65 years old was inversely associated with the risk of dementia at a 5-years follow-up. Recently Small et al. (2014) conducted a double-blind, placebo-controlled clinical trial using a pill-based nutraceutical (NT-020) that contained blueberry, carnosine, green tea, vitamin D3, and Biovin to evaluate the impact on changes in cognitive functioning. One hundred and five cognitively intact adults aged 65–85 years of age were randomized to receive NT-020 (n = 52) or a placebo (n = 53). Participants were tested with a battery of cognitive performance tests that were classified into six broad domains (episodic memory, processing speed, verbal ability, working memory, executive functioning, and complex speed) at baseline and 2 months later. The results indicated that persons taking NT-020 improved significantly on two measures of processing speed across the 2-month test period compared to persons on the placebo whose performance did not change. The authors concluded that the results were promising and suggest the potential for interventions like these to improve the cognitive health of older adults (Small et al., 2014).
Indeed these results have been confirmed by recent evidence by Rabassa et al. (2015). In the context of the Invecchiare in Chianti (InCHIANTI), a cohort study with 3 years of follow-up, the authors assessed the total urinary polyphenol (TUP) and the total dietary polyphenol (TDP) concentrations in 652 individuals without dementia aged 65 and older, and assessed cognition using the Mini-Mental State Examination (MMSE) and Trail-Making Test (TMT) at baseline and after 3 years of follow-up. Higher TUP levels were associated with lower risk of substantial cognitive decline on the MMSE and on the TMT-A, in a logistic regression model adjusted for baseline cognitive score and potential confounding factors. These findings showed that high concentrations of polyphenols were associated with lower risk of substantial cognitive decline in an older population studied over a 3-year period, suggesting a protective effect against cognitive impairment (Rabassa et al., 2015).
In a prospective study conducted among Japanese Americans living in the King County of Washington, Dai et al. (2006) found that frequent drinking of fruit and vegetable juices was associated with a substantially decreased risk of Alzheimer's disease, with an inverse association stronger after adjustments for potential confounding factors, and evident in all strata of selected variables. These findings suggest that fruit and vegetable juices may play an important role in delaying the onset of Alzheimer's disease (Dai et al., 2006).
Krikorian et al. (2010a,b) investigated the effects of daily consumption of wild blueberry juice in a sample of nine older adults with early memory changes. At 12 weeks, improved paired associate learning and word list recall were observed. In addition, there were trends suggesting reduced depressive symptoms and lower glucose levels. Instead, twelve older adults with memory decline but not dementia were enrolled in a randomized, placebo-controlled, double blind trial with Concord grape juice supplementation for 12 weeks (Butchart et al., 2011). The authors observed significant improvement in a measure of verbal learning and non-significant enhancement of verbal and spatial recall. There was no appreciable effect of the intervention on depressive symptoms and no effect on weight or waist circumference. Then these findings suggested that supplementation with Concord grape juice may enhance cognitive function for older adults with early memory decline.
In a more recent study (Devore et al., 2012), performed on 16,010 women aged ≥70 years, greater intakes of blueberries and strawberries were associated with slower rates of cognitive decline after adjusting for multiple potential confounders. Berry intake appeared to delay cognitive aging by up to 2.5 years. Additionally, in further supporting evidence, greater intakes of anthocyanidins and total flavonoids were associated with slower rates of cognitive decline in elder women (Devore et al., 2012).
On the other hand Butchart et al. (2011) investigated the same issue but with control for possible confounding factors as prior intelligence quotient (IQ). In a cross-sectional survey of 1091 men and women born in 1936, in which IQ was measured at age 11 years, at the age of 70 years, participants carried out various neuropsychological tests and completed a Food Frequency Questionnaire. Total fruit, citrus fruits, apple, and tea intakes were initially found to be associated with better scores in a variety of cognitive tests, but the associations were no longer statistically significant after adjusting for confounding factors, including childhood IQ, not supporting a role for flavonoids in the prevention of cognitive decline in later life (Butchart et al., 2011).
However, in all of these studies, no specific information on long-term dietary habits was available, while, long-term diet is likely to be most relevant for cognitive decline (Devore et al., 2012).
An epidemiological study (Ringman et al., 2012) suggested that curcumin, as one of the most prevalent nutritional and medicinal compounds used by the Indian population, is responsible for the reduced (4.4-fold) prevalence of AD in India compared to United States.
As seen above, although there are many experimental in vitro and in vivo evidence of the efficacy of curcumin in the prevention of neurodegeneration, at present very few human studies have been performed, which have shown some utility of this compound.
Ringman et al. (2012) performed a 24-week randomized, double blind, placebo-controlled study of curcumin with an open-label extension to 48 weeks. Thirty-six persons with mild-to-moderate AD were randomized to receive placebo, 2 g/day, or 4 g/day of oral curcumin for 24 weeks. For weeks 24 through 48, subjects that were receiving curcumin continued with the same dose, while subjects previously receiving placebo were randomized in a 1:1 ratio to 2 g/day or 4 g/day. At the end of the study no differences were found between treatment groups in clinical or biomarker efficacy measures (Ringman et al., 2012).
Indeed Cox et al. (2015) in a randomized, double blind, placebo-controlled trial examined the acute (1 and 3 h after a single dose), chronic (4 weeks), and acute-on-chronic (1 and 3 h after single dose following chronic treatment) effects of solid lipid curcumin formulation (400 mg) on cognitive function, mood and blood biomarkers in 60 healthy adults aged 60–85. One hour after administration curcumin significantly improved performance on sustained attention and working memory tasks, compared with placebo. Working memory and mood (general fatigue and change in state calmness, contentedness and fatigue induced by psychological stress) were significantly better following chronic treatment. A significant acute-on-chronic treatment effect on alertness and contentedness was also observed (Cox et al., 2015).
All together these results highlight the need for further investigation on the potential cognitive benefits of curcumin, especially in elderly, and the importance of the dose and method of administration.
Although the plant-derived polyphenol resveratrol has been shown to increase memory performance in primates, also for this compound interventional studies in older humans are lacking.
In a study by Kennedy et al. (2010) the effects of oral resveratrol on cognitive performance and localized cerebral blood flow variables in healthy human adults were assessed. In this very interesting randomized, double blind, placebo-controlled, crossover study, 22 healthy adults received placebo and 2 doses (250 and 500 mg) of trans-resveratrol in counterbalanced order on separate days. After a 45-min resting absorption period, the participants performed a selection of cognitive tasks that activate the frontal cortex for an additional 36 min. Cerebral blood flow and hemodynamic, as indexed by concentration changes in oxygenated and deoxygenated hemoglobin, were assessed in the frontal cortex throughout the post-treatment period with the use of near-infrared spectroscopy. The presence of resveratrol and its conjugates in plasma was confirmed by HPLC after the same doses in a separate cohort (n = 9). Resveratrol administration resulted in dose-dependent increases in cerebral blood flow during task performance. There was also an increase in deoxyhaemoglobin after both doses of resveratrol, which suggested enhanced oxygen extraction that became apparent toward the end of the 45-min absorption phase and was sustained throughout task performance. Cognitive function was not affected. Resveratrol metabolites were present in plasma throughout the cognitive task period, suggesting that single doses of orally administered resveratrol can modulate cerebral blood flow variables (Kennedy et al., 2010).
Witte et al. (2014) tested whether supplementation of resveratrol would enhance memory performance in older adults and addressed potential mechanisms underlying this effect. Twenty-three healthy overweight older individuals treated for 26 weeks with 200 mg/d resveratrol were compared to 23 participants that received placebo. Before and after the intervention/control period, subjects underwent memory tasks and neuroimaging to assess volume, microstructure, and functional connectivity (FC) of the hippocampus, a key region implicated in memory functions. In addition, anthropometry, glucose and lipid metabolism, inflammation, neurotrophic factors, and vascular parameters were assayed. The authors observed a significant effect of resveratrol on retention of words over 30 min compared with placebo. In addition, resveratrol led to significant increases in hippocampal FC, and the increases in FC between the left posterior hippocampus and the medial prefrontal cortex correlated with increases in retention scores. Then these finding could offer the basis for novel strategies to maintain brain health during aging (Witte et al., 2014).
Conclusions
Despite the translational gap between basic and clinical research, the current understanding of the molecular interactions between phytochemicals, immune function, and inflammatory response could help in designing effective nutritional strategies to delay brain aging and improve cognitive function.
Although, as described, many studies demonstrate the efficacy in vitro and in vivo of phytochemicals (Table 1) in the prevention and treatment of cognitive disorders, even few evidence of their efficacy are available in humans, and especially still significant differences in the protocols used, dosages and in the different way of administration. Therefore, it seems that these results do not allow to finalize, which is the real efficacy of these compounds to prevent and to prevent and delay neuroinflammation associated with aging. Further research, mainly conducted with randomized controlled trials, should be performed in humans to determine the real role that phytochemicals can play in the prevention and treatment of neuroinflammaging.
Table 1.
Summary of phytochemicals with their food origin, effects in brain, studies demostrating this effects, the models used and their capability to cross the Blood-Brain Barrier (BBB).
| Phytochemicals | Food origin | Effects in neuroprotection | Methods | References | Blood-Brain Barrier passage |
|---|---|---|---|---|---|
| Ferulic acid | Tomatoes |
 Prostaglandin E2 and TNF-α, Prostaglandin E2 and TNF-α, |
in vitro cromatography | Ou et al., 2003 | Ferulic acid was transported across a model Blood-Brain Barrier (BBB). After administration of Shunaoxin pills, ferulic acid was rapidly absorbed and distributed in brain. |
| Sweet corn, rice, |
 iNOS expression and function iNOS expression and function |
Primary mesangial cell cultures | Tetsuka et al., 1996 | ||
| Wheat oats, barley grain, | Inhibits microglial activation | Imprinting Control Region (ICR) strain mice | Kim et al., 2004 | ||
| Chinese water chestnut, pineapple | Protects against changes in the conformation of synaptosomal membrane proteins | Cultured neuronal cells | Kanaski et al., 2002 | Wu et al., 2014 | |
| Seeds of coffee, apple | Protects against Aβ toxicity directly and by inducing protecting genes | Hippocampal cultures | Sultana et al., 2005 | ||
| Artichoke, peanut |
 Hemeoxygenase-1 and heat shock protein 70 Hemeoxygenase-1 and heat shock protein 70 |
Rat astrocytes and neurons | Scapagnini et al., 2004 | ||
| Orange, navy bean | Ameliorated neuroinflammation in including β-amyloid plaque-associated gliosis and expression of TNF-α and IL-1β and  mRNA expression of superoxide dismutase 1, catalase, and GSH-Px 1 mRNA expression of superoxide dismutase 1, catalase, and GSH-Px 1 |
Mouse model of cerebral amyloidosis mutant human transgenes and in vitro mutant human APP-overexpressing murine N2a neuron-like cells. | Mori et al., 2013 | ||
| Epigallocatechin gallate (EGCG) | Black tea, green tea | Protects dopaminergic neurons from damage induced by 6-hydroxydopamine | Unilateral 6-hydroxydopamine (6-OHDA)-treated rat model of Parkinson's disease | Guo et al., 2007 | The level of EGCG found in the major organs was found to be ~1/10 that found in the serum. Most interestingly, this includes the brain, suggesting that EGCG passes through the blood-brain barrier. |
| Oolong teas, carob flour |
 Mutant huntingtin misfolding and neurotoxicity Mutant huntingtin misfolding and neurotoxicity |
Transgenic flies and yeast cultures | Ehrnhoefer et al., 2006 | ||
| Pecans, filberts, hazelnuts | Protects directly neurons against Aβ toxicity | Mixed (glial/neuronal) hippocampal cultured cells from E19 fetuses obtained from Sprague-Dawley rats | Bastianetto et al., 2000 | Smith, 2011 | |
| Raw cranberries, pistachios | Protects against Aβ-induced cognitive impairment | 5-week-old male rats | Haque et al., 2008 | ||
 HO-1 expression by activation of the Nrf2-ARE pathway HO-1 expression by activation of the Nrf2-ARE pathway |
Bovine aortic endothelial cells | Wu et al., 2006 | |||
| Protects cultured rat cerebellar granule neurons from oxidative stress | Primary cultures of rat cerebellar granule neurons | Schroeder et al., 2009 | |||
| Promotes cleavage of APP into α-CTF and soluble APP-α. | Primary micloglial and neurons cultures from Tg2576 mice | Obregon et al., 2006 | |||
| Cleavage of APP into α-CTF and soluble APP-α with elevated α-secretase cleavage activity and activation of ADAM10 | Mouse brains | Rezai-Zadeh et al., 2008 | |||
| Pterostilbene | Blueberry, eanuts, almonds | Reverses age-related deficits in neuronal and behavioral parameters | Male Fischer 344 rats | Joseph et al., 1999 | It is generally assumed that Pterostilbene can cross the BBB due to its structural similarities to resveratrol. |
| Grapes | Prevents learning and memory deficits | APP/PS1 transgenic mice | Joseph et al., 2003 | ||
 Survival of dopamine-producing neurons Survival of dopamine-producing neurons |
Embryonic dopamin neurons transplanted into the unilaterally dopamin-depleted striatum | McGuire et al., 2006 | Temsamani et al., 2015 | ||
| Improves neuronal signaling, preventing accumulation of proteins tau in the hippocampus of irradiated rats | Rats exposed to 1.5 Gy of 56Fe particles | Poulose et al., 2014 | Andres-Lacueva et al., 2005 | ||
| Delivers antioxidant and signaling modifying capabilities centrally | 19 months old F344 rats | Andres-Lacueva et al., 2005 | |||
 Malondialdehyde and Protein C levels, acetylcholinesterase activity and apoptosis Malondialdehyde and Protein C levels, acetylcholinesterase activity and apoptosis  Glutathione (GSH) levels and GSH-Px activity Glutathione (GSH) levels and GSH-Px activity |
Rats treated with D-galactose | Çoban et al., 2015 | |||
Improved neurological function,  brain infarct volume, and oedema. brain infarct volume, and oedema.  Malondialdehyde and Malondialdehyde and  activity of superoxide dismutase in the ipsilateral hemisphere, with activity of superoxide dismutase in the ipsilateral hemisphere, with  of 4-hydroxynonenal and 8-hydroxyguanosine of 4-hydroxynonenal and 8-hydroxyguanosine |
Male Kunming mice with induced focal cerebral ischemia | Zhou et al., 2015 | |||
| Curcumin | Curry, Worcestershire sauce | Protects neurons against ischemic cell death and ameliorated behavioral deficits | Mongolian gerbils | Wang et al., 2005 | In transgenic APPswe/PS1dE9 mice demonstrated that curcumin, given intravenously for 7 days, crosses the BBB, binds to β-amyloid deposits in the brain and accelerates their rate of clearance. |
| Food additive (E100) |
 Expression of HO-1 Expression of HO-1 |
Cultured hippocampal neurons | Scapagnini et al., 2006 | ||
Reverse chronic stress-induced impairment of hippocampal neurogenesis and  expression of brain-derived neurotrophic factor expression of brain-derived neurotrophic factor |
Chronically stressed rats | Xu et al., 2007 | |||
| Protective function in T-cell-mediated immunity | Male Sprague–Dawley rats | Kou et al., 2013 | Garcia-Alloza et al., 2007 | ||
 HO-1 expression and GSH levels HO-1 expression and GSH levels |
Primary cultures of cerebellar Granule neurons of rats | González-Reyes et al., 2013 | |||
| Disaggregates Aβ as well as prevents fibril and oligomer formation | Alzheimer transgenic APPSw mouse model | Lim et al., 2001 | |||
 GSH and catalase, superoxide dismutase GSH and catalase, superoxide dismutase |
Adult male Wistar albino rats, Male Sprague Dawley rats | Al-Omar et al., 2006; Calabrese et al., 2008 | |||
| Sulforaphane | Broccoli, brussels Sprouts | Activate Nrf2-ARE stress response pathway | Male Sprague Dawley rats and nrf2 –/– mice | Zhao et al., 2007 | Various studies in animal models suggest the ability of Sulforaphane to cross the BBB and to accumulate in cerebral tissues. |
| Cabbage cauliflower | Protects cultured neurons against oxidative stress | ARE–human placental alkaline phosphatase transgenic mice | Kraft et al., 2004 | ||
| Kale, collard greens, horseradish | Protects dopaminergic neurons against mitochondrial toxins | CATH.a cells | Han et al., 2007 | Jazwa et al., 2011 | |
 Spatial learning and memory, and Spatial learning and memory, and  Working memory dysfunction Working memory dysfunction |
Male Sprague Dawley rats | Dash et al., 2009 | Clarke et al., 2011 | ||
 Nrf2 and HO-1 expression Nrf2 and HO-1 expression |
Neonatal hypoxia-ischemia in Sprague–Dawley rat pups | Ping et al., 2010 | |||
| Resveratrol | Red Grapes, Peanut Butter, Dark Chocolate, Itadori Tea | Attenuated beta-amyloid-induced cytotoxicity, apoptotic features, and intracellular ROS accumulation. Beta-amyloid transiently induced activation of NF-κB was suppressed | PC12 cells | Jang and Surh, 2003 | Acute administration of resveratrol by oral gavage using a low dose of 80 μg/kg results in significant accumulation in brain within 4 h. Short term treatment using a concentration of 40 μg/kg by the same route of administration for a period of 15 days also increases resveratrol content in the brain. |
| Blueberries | Neuroprotection against dopaminergic neurons | Organotypic midbrain slice cultures | Okawara et al., 2007 | ||
| Protects cortical neurons from oxidative stress-induced injury | Cultures of cortical neuronal cells isolated from embryos of timed pregnant mice | Zhuang et al., 2003 | |||
| Suppress alcohol-induced cognitive deficits and neuronal apoptosis | Adult male Wistar rats | Tiwari and Chopra, 2013 | |||
 The production of IL-1 beta and TNF-alpha induced by LPS or Aβ in the microglia The production of IL-1 beta and TNF-alpha induced by LPS or Aβ in the microglia |
The mouse microglial cell line BV-2 | Capiralla et al., 2012; Zhong et al., 2012 | Bertelli et al., 1999 | ||
| Resveratrol mimicked oxidizing conditions in neural progenitor cells | Mouse neural progenitor cells | Prozorovski et al., 2008 | Resveratrol being a lipophilic compound can readily cross the BBB via transmembrane diffusion (Lin et al., 2010). | ||
| Protects C. elegans neurons expressing a fragment of the Huntington disease-associated protein huntingtin and mammalian neurons from mutant polyglutamine cytotoxicity | HdhQ111 knock-in mouse model of Huntington disease | Dali-Youcef et al., 2007 | Resveratrol, with its molecular weight of 228 Da (Amri et al., 2012) and lipid soluble properties, should easily cross the BBB. | ||
| Inhibits hypoxia-induced degradation of I kappa B-alpha and phosphorylation of p65 NF-κB protein. These effects were mediated by suppressing the activation of NF-κB, extracellular-signal-regulated kinases (ERK) and JNK/MAPK signaling pathways | In vitro models of the BV-2 microglial cell line and primary microglia | Zhang et al., 2015 |
Author contributions
GC contributed substantially to conception, drafting the article, and final approval; VC contributed substantially to revision for important intellectual content and final approval of the version to be published; SD made substantial contributions to revising the article; GS made substantial contributions to revising the article; AF and NF contributed substantially to revision for important intellectual content and final approval of the version to be published. All the authors gave final approval of the version to be published.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Albani D., Polito L., Batelli S., De Mauro S., Fracasso C., Martelli G., et al. (2009). The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J. Neurochem. 110, 1445–1456. 10.1111/j.1471-4159.2009.06228.x [DOI] [PubMed] [Google Scholar]
- Al-Omar F. A., Nagi M. N., Abdulgadir M. M., Al Joni K. S., Al-Majed A. A. (2006). Immediate and delayed treatments with curcumin prevents forebrain ischemia-induced neuronal damage and oxidative insult in the rat hippocampus. Neurochem. Res. 31, 611–618. 10.1007/s11064-006-9059-1 [DOI] [PubMed] [Google Scholar]
- Alonso A., Zaidi T., Novak M., Grundke-Iqbal I., Iqbal K. (2001). Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. U.S.A. 98, 6923–6928. 10.1073/pnas.121119298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri A., Chaumeil J. C., Sfar S., Charrueau C. (2012). Administration of resveratrol: what formulation solutions to bioavailability limitations? J. Control. Release 158, 182–193. 10.1016/j.jconrel.2011.09.083 [DOI] [PubMed] [Google Scholar]
- Andreadi C. K., Howells L. M., Atherfold P. A., Manson M. M. (2006). Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase- 1 by dietary polyphenols. Mol. Pharmacol. 69, 1033–1040. 10.1124/mol.105.018374 [DOI] [PubMed] [Google Scholar]
- Andres-Lacueva C., Shukitt-Hale B., Galli R. L., Jauregui O., Lamuela-Raventos R. M., Joseph J. A. (2005). Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 8, 111–120. 10.1080/10284150500078117 [DOI] [PubMed] [Google Scholar]
- Ates O., Cayli S., Altinoz E., Gurses I., Yucel N., Sener M., et al. (2007). Neuroprotection by resveratrol against traumatic brain injury in rats Mol. Cell. Biochem. 294, 137–144. 10.1007/s11010-006-9253-0 [DOI] [PubMed] [Google Scholar]
- Babenko O., Kovalchuk I., Metz G. A. (2012). Epigenetic programming of neurodegenerative diseases by an adverse environment. Brain Res. 1444, 96–111. 10.1016/j.brainres.2012.01.038 [DOI] [PubMed] [Google Scholar]
- Barrientos R. M., Kitt M. M., Watkins L. R., Maier S. F. (2015). Neuroinflammation in the normal aging hippocampus. Neuroscience 309, 84–99. 10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S., Zheng W. H., Quirion R. (2000). Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 131, 711–720. 10.1038/sj.bjp.0703626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. A., Sinclair D. A. (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506. 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- Baur J. A., Ungvari Z., Minor R. K., Le Couteur D. G., de Cabo R. (2012). Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 11, 443–461. 10.1038/nrd3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold N. C., Cribbs D. H., Coleman P. D., Rogers J., Head E., Kim R., et al. (2008). Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. U.S.A. 105, 15605–15610. 10.1073/pnas.0806883105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R. G., Lunkenbein S., Ströhle A., Hahn A. (2012). Antioxidants in food: mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 52, 162–171. 10.1080/10408398.2010.499481 [DOI] [PubMed] [Google Scholar]
- Bertelli A. A., Ferrara F., Diana G., Fulgenzi A., Corsi M., Ponti W., et al. (1999). Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: a co-factor in antiinflammatory and anticancer chemopreventive activity. Int. J. Tissue React. 21, 93–104. [PubMed] [Google Scholar]
- Bianco A., Mazzarella G., Turchiarelli V., Nigro E., Corbi G., Scudiero O., et al. (2013). Adiponectin: an attractive marker for metabolic disorders in Chronic Obstructive Pulmonary Disease (COPD). Nutrients 5, 4115–4125. 10.3390/nu5104115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau C. W., Cowley T. R., O'sullivan J., Grehan B., Browne T. C., Kelly L., et al. (2012). The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol. Aging 33, 1005.e1023–1005.e1035. 10.1016/j.neurobiolaging.2011.09.035 [DOI] [PubMed] [Google Scholar]
- Butchart C., Kyle J., McNeill G., Corley J., Gow A. J., Starr J. M., et al. (2011). Flavonoid intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Br. J. Nutr. 106, 141–148. 10.1017/S0007114510005738 [DOI] [PubMed] [Google Scholar]
- Bye N., Zieba M., Wreford N. G., Nichols N. R. (2001). Resistance of the dentate gyrus to induced apoptosis during ageing is associated with increases in transforming growth factor-beta1 messenger RNA. Neuroscience 105, 853–862. 10.1016/s0306-4522(01)00236-6 [DOI] [PubMed] [Google Scholar]
- Calabrese E. J., Bachmann K. A., Bailer A. J., Bolger P. M., Borak J., Cai L., et al. (2007). Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 222, 122–128. 10.1016/j.taap.2007.02.015 [DOI] [PubMed] [Google Scholar]
- Calabrese V., Cornelius C., Mancuso C., Pennisi G., Calafato S., Bellia F. (2008). Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem. Res. 33, 2444–2471. 10.1007/s11064-008-9775-9 [DOI] [PubMed] [Google Scholar]
- Capiralla H., Vingtdeux V., Zhao H., Sankowski R., Al-Abed Y., Davies P., et al. (2012). Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J. Neurochem. 120, 461–472. 10.1111/j.1471-4159.2011.07594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevenini E., Monti D., Franceschi C. (2013). Inflamm-ageing. Curr. Opin. Clin. Nutr. Metab. Care 16, 14–20. 10.1097/MCO.0b013e32835ada13 [DOI] [PubMed] [Google Scholar]
- Chen J., Zhou Y., Mueller-Steiner S., Chen L. F., Kwon H., Yi S., et al. (2005). SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 280, 40364–40374. 10.1074/jbc.M509329200 [DOI] [PubMed] [Google Scholar]
- Cho S. H., Chen J. A., Sayed F., Ward M. E., Gao F., Nguyen T. A., et al. (2015). SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J. Neurosci. 35, 807–818. 10.1523/JNEUROSCI.2939-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. D., Hsu A., Williams D. E., Dashwood R. H., Stevens J. F., Yamamoto M., et al. (2011). Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm. Res. 28, 3171–3179. 10.1007/s11095-011-0500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri S. (2011). From Waddington's epigenetic landscape to small noncoding RNA: some important milestones in the history of epigenetics research. Toxicol. Mech. Methods 21, 252–274. 10.3109/15376516.2011.559695 [DOI] [PubMed] [Google Scholar]
- Çoban J., Doğan-Ekici I., Aydín A. F., Betül-Kalaz E., Doğru-Abbasoğlu S., Uysal M. (2015). Blueberry treatment decreased D-galactose-induced oxidative stress and brain damage in rats. Metab. Brain Dis. 30, 793–802. 10.1007/s11011-014-9643-z [DOI] [PubMed] [Google Scholar]
- Commenges D., Scotet V., Renaud S., Jacqmin-Gadda H., Barberger-Gateau P., Dartigues J. F. (2000). Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 16, 357–363. 10.1023/A:1007614613771 [DOI] [PubMed] [Google Scholar]
- Conti V., Corbi G., Simeon V., Russomanno G., Manzo V., Ferrara N., et al. (2015). Aging-related changes in oxidative stress response of human endothelial cells. Aging Clin. Exp. Res. 27, 547–553. 10.1007/s40520-015-0357-9 [DOI] [PubMed] [Google Scholar]
- Conti V., Izzo V., Corbi G., Russomanno G., Manzo V., De Lise F., et al. (2016). Antioxidant supplementation in the treatment of aging-associated diseases. Front. Pharmacol. 7:24. 10.3389/fphar.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi G., Acanfora D., Iannuzzi G. L., Longobardi G., Cacciatore F., Furgi G., et al. (2008). Hypermagnesemia predicts mortality in elderly with congestive heart disease: relationship with laxative and antacid use. Rejuvenation Res. 11, 129–138. 10.1089/rej.2007.0583 [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C., Christie L. A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Cox K. H., Pipingas A., Scholey A. B. (2015). Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 29, 642–651. 10.1177/0269881114552744 [DOI] [PubMed] [Google Scholar]
- Craft S., Foster T. C., Landfield P. W., Maier S. F., Resnick S. M., Yaffe K. (2012). Session III: mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J. Gerontol. A Biol. Sci. Med. Sci. 67, 754–759. 10.1093/gerona/gls112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs D. H., Berchtold N. C., Perreau V., Coleman P. D., Rogers J., Tenner A. J., et al. (2012). Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9:179. 10.1186/1742-2094-9-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A., Wang M., Lakatta E. G., Ungvari Z. (2008). Inflammation and endothelial dysfunction during aging: role of NF-κB. J. Appl. Physiol. 105, 1333–1341. 10.1152/japplphysiol.90470.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q., Borenstein A. R., Wu Y., Jackson J. C., Larson E. B. (2006). Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am. J. Med. 119, 751–759. 10.1016/j.amjmed.2006.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dali-Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J. (2007). Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann. Med. 39, 335–345. 10.1080/07853890701408194 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. (2009). Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 34, 85–96. 10.1016/j.tibs.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Dash P. K., Zhao J., Orsi S. A., Zhang M., Moore A. N. (2009). Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 460, 103–107. 10.1016/j.neulet.2009.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davinelli S., Bertoglio J. C., Zarrelli A., Pina R., Scapagnini G. (2015). A randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 34, 28–33. 10.1080/07315724.2015.1080108 [DOI] [PubMed] [Google Scholar]
- Davinelli S., Maes M., Corbi G., Zarrelli A., Willcox D. C., Scapagnini G. (2016). Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges. Immun. Ageing 13:16. 10.1186/s12979-016-0070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore E. E., Kang J. H., Breteler M. M., Grodstein F. (2012). Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 72, 135–143. 10.1002/ana.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Kensler T. W. (2005). The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 18, 1779–1791. 10.1021/tx050217c [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., et al. (2002). Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U.S.A. 99, 11908–11913. 10.1073/pnas.172398899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J., Djordjevic A., Adzic M., Mitic M., Lukic I., Radojcic M. B. (2015). Alterations in the Nrf2-Keap1 signaling pathway and its downstream target genes in rat brain under stress. Brain Res. 1602, 20–31. 10.1016/j.brainres.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Donmez G., Wang D., Cohen D. E., Guarente L. (2010). SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142, 320–332. 10.1016/j.cell.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duan W. (2013). Sirtuins: from metabolic regulation to brain aging. Front. Aging Neurosci. 5:36. 10.3389/fnagi.2013.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer D. E., Duennwald M., Markovic P., Wacker J. L., Engemann S., Roark M., et al. (2006). Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum. Mol. Genet. 15, 2743–2751. 10.1093/hmg/ddl210 [DOI] [PubMed] [Google Scholar]
- Enciu A. M., Gherghiceanu M., Popescu B. O. (2013). Triggers and effectors of oxidative stress at blood-brain barrier level: relevance for brain ageing and neurodegeneration. Oxid. Med. Cell. Longev. 2013:297512. 10.1155/2013/297512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farombi E. O., Shrotriya S., Na H. K., Kim S. H., Surh Y. J. (2008). Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 46, 1279–1287. 10.1016/j.fct.2007.09.095 [DOI] [PubMed] [Google Scholar]
- Frank M. G., Barrientos R. M., Biedenkapp J. C., Rudy J. W., Watkins L. R., Maier S. F. (2006). MRNA up-regulation of MHC II and pivotal proinflammatory genes in normal brain aging. Neurobiol. Aging 27, 717–722. 10.1016/j.neurobiolaging.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M., Borrelli L. A., Rozkalne A., Hyman B. T., Bacskai B. J. (2007). Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 102, 1095–1104. 10.1111/j.1471-4159.2007.04613.x [DOI] [PubMed] [Google Scholar]
- Godbout J. P., Johnson R. W. (2004). Interleukin-6 in the aging brain. J. Neuroimmunol. 147:141–144. 10.1016/j.jneuroim.2003.10.031 [DOI] [PubMed] [Google Scholar]
- González-Reyes S., Guzmán-Beltrán S., Medina-Campos O. N., Pedraza-Chaverri J. (2013). J.Curcumin pretreatment induces Nrf2 and an antioxidant response and prevents hemin-induced toxicity in primary cultures of cerebellar granule neurons of rats. Oxid. Med. Cell. Longev. 2013:801418. 10.1155/2013/801418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Yan J., Yang T., Yang X., Bezard E., Zhao B. (2007). Protective effects of green tea polyphenols in the 6- OHDA rat model of Parkinson's disease through inhibition of ROS-NO pathway. Biol. Psychiatry 62, 1353–1362. 10.1016/j.biopsych.2007.04.020 [DOI] [PubMed] [Google Scholar]
- Han J. M., Lee Y. J., Lee S. Y., Kim E. M., Moon Y., Kim H. W., et al. (2007). Protective effect of sulforaphane against dopaminergic cell death. J. Pharmacol. Exp. Ther. 321, 249–256. 10.1124/jpet.106.110866 [DOI] [PubMed] [Google Scholar]
- Hanisch U. K., Kettenmann H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394. 10.1038/nn1997 [DOI] [PubMed] [Google Scholar]
- Haque A. M., Hashimoto M., Katakura M., Hara Y., Shido O. (2008). Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 19, 619–626. 10.1016/j.jnutbio.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Helenius M., Kyrylenko S., Vehviläinen P., Salminen A. (2001). Characterization of aging-associated up-regulation of constitutive nuclear factor-κB binding activity. Antioxid. Redox Signal. 3, 147–156. 10.1089/152308601750100669 [DOI] [PubMed] [Google Scholar]
- Heneka M. T., O'Banion M. K. (2007). Inflammatory processes in Alzheimer's disease. J. Neuroimmunol. 184, 69–91. 10.1016/j.jneuroim.2006.11.017 [DOI] [PubMed] [Google Scholar]
- Innamorato N. G., Rojo A. I., García-Yague A. J., Yamamoto M., de Ceballos M. L., Cuadrado A. (2008). The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 181, 680–689. 10.4049/jimmunol.181.1.680 [DOI] [PubMed] [Google Scholar]
- Jang J. H., Surh Y. J. (2003). Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 34, 1100–1110. 10.1016/S0891-5849(03)00062-5 [DOI] [PubMed] [Google Scholar]
- Jazwa A, Rojo, A. I., Innamorato N. G., Hesse M., Fernández-Ruiz J., Cuadrado A. (2011). Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 14, 2347–2360. 10.1089/ars.2010.3731 [DOI] [PubMed] [Google Scholar]
- Jeong H., Cohen D. E., Cui L., Supinski A., Savas J. N., Mazzulli J. R., et al. (2012). Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 18, 159–165. 10.1038/nm.2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. F., Perkins N. D. (2012). Nuclear factor-κB, p53, and mitochondria: regulation of cellular metabolism and the Warburg effect. Trends Biochem. Sci. 37, 317–324. 10.1016/j.tibs.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Joseph J. A., Denisova N. A., Arendash G., Gordon M., Diamond D., Shukitt-Hale B., et al. (2003). Blueberry supplementation enhances signalling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 6, 153–162. 10.1080/1028415031000111282 [DOI] [PubMed] [Google Scholar]
- Joseph J. A., Shukitt-Hale B., Denisova N. A., Bielinski D., Martin A., McEwen J. J., et al. (1999). Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 19, 8114–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaski J., Aksenova M., Stoyanova A., Butterfield D. A. (2002). Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure activity studies. J. Nutr. Biochem. 13, 273–281. 10.1016/S0955-2863(01)00215-7 [DOI] [PubMed] [Google Scholar]
- Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., et al. (2009). Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 106, 16505–16510. 10.1073/pnas.0908397106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Lin A. (2002). NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227. 10.1038/ni0302-221 [DOI] [PubMed] [Google Scholar]
- Kawashima K., Misawa H., Moriwaki Y., Fujii Y., Fujii T., Horiuchi Y., et al. (2007). Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 80, 2206–2209. 10.1016/j.lfs.2007.01.059 [DOI] [PubMed] [Google Scholar]
- Kennedy D. O., Wightman E. L. (2011). Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2, 32–50. 10.3945/an.110.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. O., Wightman E. L., Reay J. L., Lietz G., Okello E. J., Wilde A., et al. (2010). Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 91, 1590–1597. 10.3945/ajcn.2009.28641 [DOI] [PubMed] [Google Scholar]
- Kim D., Nguyen M. D., Dobbin M. M., Fischer A., Sananbenesi F., Rodgers J. T., et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179. 10.1038/sj.emboj.7601758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Cho J. Y., Kim D. H., Yan J. J., Lee H. K., Suh H. W., et al. (2004). Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intercerebroventricular injection of beta-amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 27, 120–121. 10.1248/bpb.27.120 [DOI] [PubMed] [Google Scholar]
- Kim K. H., Lyu J. H., Koo S. T., Oh S. R., Lee H. K., Ahn K. S., et al. (2011). MyD88 is a mediator for the activation of Nrf2. Biochem. Biophys. Res. Commun. 404, 46–51. 10.1016/j.bbrc.2010.11.051 [DOI] [PubMed] [Google Scholar]
- Kitazawa M., Oddo S., Yamasaki T. R., Green K. N., LaFerla F. M. (2005). Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 8843–8853. 10.1523/JNEUROSCI.2868-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yamamoto M. (2006). Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 46, 113–140. 10.1016/j.advenzreg.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Rapp P. R., Raz N., Small S. A., Sweatt J. D., Tsai L. H. (2012). Mechanisms of age-related cognitive change and targets for intervention: epigenetics. J. Gerontol. A Biol. Sci. Med. Sci. 67, 741–746. 10.1093/gerona/gls110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou M. C., Chiou S. Y., Weng C. Y., Wang L., Ho C. T., Wu M. J. (2013). Curcuminoids distinctly exhibit antioxidant activities and regulate expression of scavenger receptors and heme oxygenase-1. Mol. Nutr. Food Res. 57, 1598–1610. 10.1002/mnfr.201200227 [DOI] [PubMed] [Google Scholar]
- Kraft A. D., Johnson D. A., Johnson J. A. (2004). Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 24, 1101–1112. 10.1523/JNEUROSCI.3817-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian R., Nash T. A., Shidler M. D., Shukitt-Hale B., Joseph J. A. (2010b). Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 103, 730–734. 10.1017/S0007114509992364 [DOI] [PubMed] [Google Scholar]
- Krikorian R., Shidler M. D., Nash T. A., Kalt W., Vinqvist-Tymchuk M. R., Shukitt-Hale B., et al. (2010a). Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 58, 3996–4000. 10.1021/jf9029332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T., Nambara E., McCourt P. (2003). Hormone evolution: the key to signalling. Nature 422:122. 10.1038/422122a [DOI] [PubMed] [Google Scholar]
- Lardenoije R., Iatrou A., Kenis G., Kompotis K., Steinbusch H. W., Mastroeni D., et al. (2015). The epigenetics of aging and neurodegeneration. Prog. Neurobiol. 131, 21–64. 10.1016/j.pneurobio.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Weindruch R., Prolla T. A. (2000). Gene-expression profile of the ageing brain in mice. Nat. Genet. 25, 294–297. 10.1038/77046 [DOI] [PubMed] [Google Scholar]
- Lee J., Jo D. G., Park D., Chung H. Y., Mattson M. P. (2014). Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol. Rev. 66, 815–868. 10.1124/pr.113.007757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legutko A. T., Marichal L., Fievez D., Bedoret A., Mayer H., de Vries L., et al. (2011). Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. Bureau J. Immunol. 187, 4517–4529. 10.4049/jimmunol.1101493 [DOI] [PubMed] [Google Scholar]
- L'Episcopo F., Tirolo C., Testa N., Caniglia S., Morale M. C., Impagnatiello F., et al. (2013). Pluchino S, Marchetti B Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J. Neurosci. 33, 1462–1485. 10.1523/JNEUROSCI.3206-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M., Hao W., Liu Y., Walter S., Mihaljevic I., Rivest S., et al. (2007). Innate immune receptor expression in normal brain aging. Neuroscience 146, 248–254. 10.1016/j.neuroscience.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Liby K. T., Yore M. M., Sporn M. B. (2007). Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 7, 357–369. 10.1038/nrc2129 [DOI] [PubMed] [Google Scholar]
- Lim G. P., Chu T., Yang F., Beech W., Frautschy S. A., Cole G. M. (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21, 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Hron J. D., Peng S. L. (2004). Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21, 203–213. 10.1016/j.immuni.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Chang H. C., Chen T. L., Chang J. H., Chiu W. T., Lin J. W., et al. (2010). Resveratrol protects against oxidized LDL-induced breakage of the blood-brain barrier by lessening disruption of tight junctions and apoptotic insults to mouse cerebrovascular endothelial cells. J. Nutr. 140, 2187–2192. 10.3945/jn.110.123505 [DOI] [PubMed] [Google Scholar]
- Liu G. H., Qu J., Shen X. (2008). NF-kappaB/p65 antagonizes Nrf2– ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 1783, 713–727. 10.1016/j.bbamcr.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Lu T., Pan Y., Kao S. Y., Li C., Kohane I., Chan J., et al. (2004). Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891. 10.1038/nature02661 [DOI] [PubMed] [Google Scholar]
- Lucin K. M., Wyss-Coray T. (2009). Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64, 110–122. 10.1016/j.neuron.2009.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw W. J. (2004). Gene expression profiling in fetal, aged and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem. Res. 29, 1287–1297. 10.1023/b:nere.0000023615.89699.63 [DOI] [PubMed] [Google Scholar]
- Mattson M. P. (2008). Dietary factors, hormesis and health. Ageing Res. Rev. 7, 43–48. 10.1016/j.arr.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. (2012). Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 16, 706–722. 10.1016/j.cmet.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. (2015). What doesn't kill you.… Sci. Am. 313, 40–45. 10.1038/scientificamerican0715-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Cheng A. (2006). Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 29, 632–639. 10.1016/j.tins.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Son T. G., Camandola S. (2007). Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response 5, 174–186. 10.2203/dose-response.07-004.Mattson [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. O., Sortwell C. E., Shukitt-Hale B., Joseph J. A., Hejna M. J., Collier T. J., et al. (2006). Dietary supplementation with blueberry extract improves survival of transplanted dopamine neurons. Nutr. Neurosci. 9, 251–258. 10.1080/10284150601086134 [DOI] [PubMed] [Google Scholar]
- McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J. D. (2006). Dimerization of substrate adaptors can facilitate cullin mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 281, 24756–24768. 10.1074/jbc.M601119200 [DOI] [PubMed] [Google Scholar]
- McNally S. J., Harrison E. M., Ross J. A., Garden O. J., Wigmore S. J. (2007). Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 19, 165–172. 10.3892/ijmm.19.1.165 [DOI] [PubMed] [Google Scholar]
- Menendez J. A., Joven J., Aragonès G., Barrajón-Catalán E., Beltrán-Debón R., Borrás-Linares I., et al. (2013). Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: a new family of gerosuppressant agents. Cell Cycle 12, 555–578. 10.4161/cc.23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milisav I., Poljsak B., Suput D. (2012). Adaptive responses, evidence of cross-resistance and its potential clinical use. Int. J. Mol. Sci. 13, 10771–10806. 10.3390/ijms130910771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S. W., Cho S. H., Zhou Y., Schroeder S., Haroutunian V., Seeley W. W., et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67, 953–966. 10.1016/j.neuron.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra J. R., Lam G., Thummel C. S. (2013). Constitutive activation of the Nrf2/Keap1 pathway in insecticide-resistant strains of Drosophila. Insect Biochem. Mol. Biol. 43, 1116–1124. 10.1016/j.ibmb.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moi P., Chan K., Asunis I., Cao A., Kan Y. W. (1994). Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U.S.A. 91, 9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. J., Liu Z. G. (2011). Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Koyama N., Guillot-Sestier M.-V., Tan J., Town T. (2013). Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 8:e55774. 10.1371/journal.pone.0055774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Evans D. A., Tangney C. C., Bienias J. L., Wilson R. S. (2006). Associations of vegetable and fruit consumption with age related cognitive change. Neurology 67, 1370–1376. 10.1212/01.wnl.0000240224.38978.d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. D., Pistell P. J., Ingram D. K., Johnson W. D., Liu Y., Fernandez-Kim S. O., et al. (2010). High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J. Neurochem. 114, 1581–1589. 10.1111/j.1471-4159.2010.06865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyah V., Mattson M. P. (2015). Neurohormetic phytochemicals: an evolutionary-bioenergetic perspective. Neurochem. Int. 89, 271–280. 10.1016/j.neuint.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Doh S. T., Chan J. Y., Kong A. N., Cai L. (2008). Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br. J. Cancer 99, 2070–2082. 10.1038/sj.bjc.6604703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. D., D'Aigle T., Gowing G., Julien J. P., Rivest S. (2004). Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 24, 1340–1349. 10.1523/JNEUROSCI.4786-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture S. K., Jaiswal A. K. (2012). Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 287, 9873–9886. 10.1074/jbc.M111.312694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon D., Rezai-Zadeh K., Bai Y., Sun N., Hou H., Zeng J., et al. (2006). ADAM10 Activation is required for green tea EGCG-induced α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 281, 16419–16427. 10.1074/jbc.M600617200 [DOI] [PubMed] [Google Scholar]
- Okawara M., Katsuki H., Kurimoto E., Shibata H., Kume T., Akaike A. (2007). Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol. 73, 550–560. 10.1016/j.bcp.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Ou L., Kong L. Y., Zhang X. M., Niwa M. (2003). Oxidation of ferulic acid by momordic charantia peroxidase and related anti-inflammation activity changes. Biol. Pharm. Bull. 26, 1511–1516. 10.1248/bpb.26.1511 [DOI] [PubMed] [Google Scholar]
- Outeiro T. F., Kontopoulos E., Altmann S. M., Kufareva I., Strathearn K. E., Amore A. M. (2007). Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317, 516–519. 10.1126/science.1143780 [DOI] [PubMed] [Google Scholar]
- Pae H. O., Jeong G. S., Jeong S. O., Kim H. S., Kim S. A., Kim Y. C., et al. (2007). Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 39, 267–277. 10.1038/emm.2007.30 [DOI] [PubMed] [Google Scholar]
- Patel P., Lockey R. F., Kolliputi N. (2015). Can inflammation regulate systemic aging? Exp. Gerontol. 67, 1–2. 10.1016/j.exger.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Perkins N. D. (2007). Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62. 10.1038/nrm2083 [DOI] [PubMed] [Google Scholar]
- Perry V. H. (1998). A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J. Neuroimmunol. 90, 113–121. [DOI] [PubMed] [Google Scholar]
- Piantadosi C. A., Withers C. M., Bartz R. R., MacGarvey N. C., Fu P., Sweeney T. E. (2011). Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 286, 16374–16385. 10.1074/jbc.M110.207738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Z., Liu W., Kang Z., Cai J., Wang Q., Cheng N. (2010). Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 343, 178–185. 10.1016/j.brainres.2010.04.036 [DOI] [PubMed] [Google Scholar]
- Poulose S. M., Bielinski D. F., Carrihill-Knoll K. L., Rabin B. M., Shukitt-Hale B. (2014). Protective effects of blueberry- and strawberry diets on neuronal stress following exposure to (56)Fe particles. Brain Res. 1593, 9–18. 10.1016/j.brainres.2014.10.028 [DOI] [PubMed] [Google Scholar]
- Prozorovski T., Schulze-Topphoff U., Glumm R., Baumgart J., Schröter F., Ninnemann O., et al. (2008). Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 10, 385–394. 10.1038/ncb1700 [DOI] [PubMed] [Google Scholar]
- Rabassa M., Cherubini A., Zamora-Ros R., Urpi-Sarda M., Bandinelli S., Ferrucci L., et al. (2015). Low levels of a urinary biomarker of dietary polyphenol are associated with substantial cognitive decline over a 3-year period in older adults: the invecchiare in chianti study. J. Am. Geriatr. Soc. 63, 938–946. 10.1111/jgs.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., Biswas S. K., Kirkham P. A. (2006). Regulation of inflammation and redox signalling by dietary polyphenols. Biochem. Pharmacol. 72, 1439–1452. 10.1016/j.bcp.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Rajendran R., Garva R., Krstic-Demonacos M., Demonacos C. (2011). Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. J. Biomed. Biotechnol. 2011:368276. 10.1155/2011/368276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K., Arendash G. W., Hou H., Fernandez F., Jensen M., Runfeldt M., et al. (2008). Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 1214, 177–187. 10.1016/j.brainres.2008.02.107 [DOI] [PubMed] [Google Scholar]
- Ringman J. M., Frautschy S. A., Teng E., Begum A. N., Bardens J., Beigi M., et al. (2012). Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers. Res. Ther. 4, 43. 10.1186/alzrt146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo A. I., Innamorato N. G., Martín-Moreno A. M., De Ceballos M. L., Yamamoto M., Cuadrado A. (2010). Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia 58, 588–598. 10.1002/glia.20947 [DOI] [PubMed] [Google Scholar]
- Rovillain E., Mansfield L., Caetano C., Alvarez-Fernandez M., Caballero O. L., Medema R. H., et al. (2011). Activation of nuclear factor kappa B signalling promotes cellular senescence. Oncogene 30, 2356–2366. 10.1038/onc.2010.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I. R., Wiseman H., Sanders T. A., Adlercreutz H., Bowey E. A. (2000). Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer 36, 27–32. 10.1207/S15327914NC3601_5 [DOI] [PubMed] [Google Scholar]
- Sakata H., Niizuma K., Yoshioka H., Kim G. S., Jung J. E., Katsu M., et al. (2012). Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 32, 3462–3473. 10.1523/JNEUROSCI.5686-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Hyttinen J. M. T., Kaarniranta K. (2011). AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 89, 667–676. 10.1007/s00109-011-0748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K., Kauppinen A. (2013). Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int. J. Mol. Sci. 14, 3834–3859. 10.3390/ijms14023834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Suuronen T., Kaarniranta K. (2008a). SIRT1 longevity factor suppresses NF-κB-driven immune responses: regulation of aging via NF-κB acetylation? Bioessays 30, 939–942. 10.1002/bies.20799 [DOI] [PubMed] [Google Scholar]
- Salminen A., Lehtonen M., Suuronen T., Kaarniranta K., Huuskonen J. (2008b). Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 65, 2979–2999. 10.1007/s00018-008-8103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Patil J., D'Angelo B., Weber S. G., Mallard C. (2014). NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79, 298–306. 10.1016/j.neuropharm.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw C. L., Guo Y., Yang A. Y., Paredes-Gonzalez X., Ramirez C., Pung D., et al. (2014). The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 72, 303–311. 10.1016/j.fct.2014.07.038 [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. (2005). Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38, 995–1014. 10.1590/S0100-879X2005000700003 [DOI] [PubMed] [Google Scholar]
- Scapagnini G., Butterfield D. A., Colombrita C., Sultana R., Pascale A., Calabrese V. (2004). Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid. Redox Signal. 6, 811–818. 10.1089/ars.2004.6.811 [DOI] [PubMed] [Google Scholar]
- Scapagnini G., Colombrita C., Amadio M., D'Agata V., Sapienza M., Quattrone A., et al. (2006). Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid. Redox Signal. 8, 395–403. 10.1089/ars.2006.8.395 [DOI] [PubMed] [Google Scholar]
- Scapagnini G., Davinelli S., Di Renzo L., De Lorenzo A., Olarte H. H., Micali G., et al. (2014). Cocoa bioactive compounds: significance and potential for the maintenance of skin health. Nutrients 6, 3202–3213. 10.3390/nu6083202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G., Vasto S., Abraham N. G., Caruso C., Zella D., Fabio G. (2011). Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Neurobiology 44, 192–201. 10.1007/s12035-011-8181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer S., Halliwell B. (2012). Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 7, 99–109. 10.1007/s12263-011-0255-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder E. K., Kelsey N. A., Doyle J., Breed E., Bouchard R. J., Loucks F. A. (2009). Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid. Redox Signal. 11, 469–480. 10.1089/ARS.2008.2215 [DOI] [PubMed] [Google Scholar]
- Sheffield L. G., Berman N. E. (1998). Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol. Aging 19, 47–55. [DOI] [PubMed] [Google Scholar]
- Shih P. H., Chan Y. C., Liao J. W., Wang M. F., Yen G. C. (2010). Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J. Nutr. Biochem. 21, 598–605. 10.1016/j.jnutbio.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B. (2012). Blueberries and neuronal aging. Gerontology 58, 518–523. 10.1159/000341101 [DOI] [PubMed] [Google Scholar]
- Sierra A., Gottfried-Blackmore A. C., McEwen B. S., Bulloch K. (2007). Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. 10.1002/glia.20468 [DOI] [PubMed] [Google Scholar]
- Small B. J., Rawson K. S., Martin C., Eisel S. L., Sanberg C. D., McEvoy C. L., et al. (2014). Nutraceutical intervention improves older adults' cognitive functioning. Rejuvenation Res. 17, 27–32. 10.1089/rej.2013.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J. (2011). Green tea polyphenols in drug discovery: a success or failure? Expert Opin. Drug Discov. 6, 589–595. 10.1517/17460441.2011.570750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son T. G., Camandola S., Mattson M. P. (2008). Hormetic dietary phytochemicals. Neuromolecular Med. 10, 236–246. 10.1007/s12017-008-8037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Sudheer A. R., Menon V. P. (2007). Ferulic Acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 40, 92–100. 10.3164/jcbn.40.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Miller K. R., Lopes K. O., Njie E. (2008). Microglial degeneration in the aging brain–bad news for neurons? Front. Biosci. 13, 3423–3438. 10.2741/2937 [DOI] [PubMed] [Google Scholar]
- Streit W. J., Mrak R. E., Griffin W. S. (2004). Microglia and neuroinflammation: a pathological perspective. J. Neuroinflammation 1:14. 10.1186/1742-2094-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R., Ravagna A., Mohmmad-Abdul H., Calabrese V., Butterfield D. A. (2005). Ferulic acid ethyl ester protects neurons against amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J. Neurochem. 92, 749–758. 10.1111/j.1471-4159.2004.02899.x [DOI] [PubMed] [Google Scholar]
- Suzuki K., Koike T. (2007). Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem. Biophys. Res. Commun. 359, 665–671. 10.1016/j.bbrc.2007.05.164 [DOI] [PubMed] [Google Scholar]
- Tafti M., Nishino S., Aldrich M. S., Liao W., Dement W. C., Mignot E. (1996). Major histocompatibility class II molecules in the CNS: increased microglial expression at the onset of narcolepsy in canine model. J. Neurosci. 16, 4588–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temsamani H., Krisa S., Mérillon J. M., Richard T. (2015). Promising neuroprotective effects of oligostilbenes. Nutr. Aging 3, 49–54. 10.3233/NUA-150050 [DOI] [Google Scholar]
- Tetsuka T., Baier L. D., Morrison A. R. (1996). Antioxidants inhibit interleukin-1 induced cyclooxygenase and nitric oxide synthase expression in rat mesanglial cells. Evidence for post-transcriptional regulation. J. Biol. Chem. 271, 11689–11693. [DOI] [PubMed] [Google Scholar]
- Tilstra J. S., Robinson A. R., Wang J., Gregg S. Q., Clauson C. L., Reay D. P., et al. (2012). NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 122, 2601–2612. 10.1172/JCI45785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Chopra K. (2013). Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative-nitrosative stress and inflammatory cascade in the adult rat brain. Neurochem. Int. 62, 861–869. 10.1016/j.neuint.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Tosetti F. D., Noonan M., Albini A. (2009). Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int. J. Cancer 125, 1997–2003. 10.1002/ijc.24677 [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S., Karin M. (2009). Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733. 10.1146/annurev.immunol.021908.132641 [DOI] [PubMed] [Google Scholar]
- Vang O., Ahmad N., Baile C. A., Baur J. A., Brown K., Csiszar A., et al. (2011). What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE 6:e19881. 10.1371/journal.pone.0019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bernhardi R., Eugenín-von Bernhardi L., Eugenín J. (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 7:124. 10.3389/fnagi.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Sun A. Y., Simonyi A., Jensen M. D., Shelat P. B., Rottinghaus G. E., et al. (2005). Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 82, 138–148. 10.1002/jnr.20610 [DOI] [PubMed] [Google Scholar]
- West M. J., Coleman P. D., Flood D. G., Troncoso J. C. (1994). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344, 769–772. [DOI] [PubMed] [Google Scholar]
- Witte A. V., Kerti L., Margulies D. S., Flöel A. (2014). Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 34, 7862–7870. 10.1523/JNEUROSCI.0385-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöll S., Kim S. H., Greten H. J., Efferth T. (2013). Animal plant warfare and secondary metabolite evolution. Nat. Prod. Bioprospect. 3, 1–7. 10.1007/s13659-013-0004-0 [DOI] [Google Scholar]
- Wu C. C., Hsu M. C., Hsieh C. W., Lin J. B., Lai P. H., Wung B. S. (2006). Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 78, 2889–2897. 10.1016/j.lfs.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Wu K, Wang, Z. Z., Liu D, Qi, X. R. (2014). Pharmacokinetics, brain distribution, release and blood-brain barrier transport of Shunaoxin pills. J. Ethnopharmacol. 151, 1133–1140. 10.1016/j.jep.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Xu Y., Ku B., Cui L., Li X., Barish P. A., Foster T. C., et al. (2007). Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 1162, 9–18. 10.1016/j.brainres.2007.05.071 [DOI] [PubMed] [Google Scholar]
- Yao H., Chung S., Hwang J. W., Rajendrasozhan S., Sundar I. K., Dean D. A., et al. (2012). SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 122, 2032–2045. 10.1172/JCI60132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S. M., Johnson R. W. (1999). Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 93, 139–148. [DOI] [PubMed] [Google Scholar]
- Ye S. M., Johnson R. W. (2001). An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 9, 183–192. 10.1159/000049025 [DOI] [PubMed] [Google Scholar]
- Yeung F., Hoberg J. E., Ramsey C. S., Keller M. D., Jones D. R., Frye R. A., et al. (2004). Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380. 10.1038/sj.emboj.7600244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Li H., Liu Q., Liu F., Tang L., Li C., et al. (2011). Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 23, 883–892. 10.1016/j.cellsig.2011.01.014 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yuan L., Zhang Q., Gao Y., Liu G., Xiu M., et al. (2015). Resveratrol attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Int. Immunopharmacol. 28, 578–587. 10.1016/j.intimp.2015.07.027 [DOI] [PubMed] [Google Scholar]
- Zhao J., Moore A. N., Redell J. B., Dash P. K. (2007). Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 27, 10240–10248. 10.1523/JNEUROSCI.1683-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. M., Zong Y., Sun L., Guo J. Z., Zhang W., He Y., et al. (2012). Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. PLoS ONE 7:e32195. 10.1371/journal.pone.0032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang X. M., Ma A., Zhang Y. L., Chen Y. Y., Zhou H., et al. (2015). Orally administrated pterostilbene attenuates acute cerebral ischemia-reperfusion injury in a dose- and time-dependent manner in mice. Pharmacol. Biochem. Behav. 135, 199–209. 10.1016/j.pbb.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Zhuang H., Kim Y. S., Koehler R. C., Doré S. (2003). Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann. N. Y. Acad. Sci. 993, 276–286. discussion: 287–288. 10.1111/j.1749-6632.2003.tb07534.x [DOI] [PubMed] [Google Scholar]